Abstract
We prove the convergence of a family of solutions to a two-dimensional transport equation with a non-local boundary condition modelling the evolution of a population of metastases. We show that when the data of the repartition along the boundary tend to a Dirac mass, then the solution of the associated problem converges and we derive a simple expression for the limit in terms of the solution of a 1D equation. This result permits us to improve the computational time needed to simulate the model.
1. Introduction
In the dynamical evolution of a cancer disease, some cancerous cells can detach from the primary tumour and spread in the organism to form secondary tumours, called metastases. These metastatic tumours can remain very small and beyond the detectable threshold with medical imaging techniques, for instance in the case of breast cancer, yet existence of occult micro-metastases at diagnosis is established Citation12.
A fundamental process in the tumoral growth, called neo-angiogenesis, consists in establishing a vascular network, which ensures to the tumour, supply of nutrients and the possibility to spread metastases in the organism. Thus, a therapeutic approach first proposed by Folkman Citation10 intends to block this process, aiming at starving the tumour by depriving it from nutrient supply. Though, the clinical question of optimal schedules for anti-angiogenic drugs is still open and is of fundamental importance Citation9 Citation15 Citation17.
In this perspective, the use of a mathematical model can lead to an interesting tool for the study in silico of the temporal administration protocols. Various models have been introduced for the evolution of the primary tumour, that can be separated between two classes: mechanistic models like Citation5
Citation13 try to integrate the whole biology of the involved processes and comprise a large number of parameters; on the other hand, phenomenological models aim to describe the tumoral growth without taking into account all the complexity levels (see Citation18 for a review and Citation2
Citation8
Citation11 for examples). In 2000, Iwata et al. Citation14 proposed a phenomenological model for the evolution of the population of metastases, which was then further studied in Citation1
Citation6. This model did not include the angiogenic process in the tumoral growth, hence we combined it with the tumoral model introduced by Hahnfeldt et al. Citation11, which takes into account angiogenesis. The resulting partial differential equation is part of the so-called structured population dynamics (see Citation16 for an introduction to the theory), it is a transport equation with a non-local boundary condition. The population of metastases is represented by a density ρ(t, X) with X being the structuring variable, here two-dimensional with x the size (=number of cells) and θ the so-called ‘angiogenic capacity’. The behaviour of each individual of the population, that is the growth rate G(X) of each metastasis is taken from Citation11 and is designed to take into account for the angiogenic process (see below for its expression). The equation writes
We formulate the biological assumption that the metastases are all born with size 1 and an angiogenic capacity close to a given value θ0. This is translated in the model by considering a density N (repartition along the boundary) very concentrated around the value (1, θ0), for instance with ϵ being a small parameter. In this paper, we demonstrate that the family of solutions
to the problem (1) with data
converges when ϵ goes to zero, to the measure solution
of Equation (1) with the measure boundary data
. Moreover, we derive a simple expression for
involving the solution of a one-dimensional renewal equation. This permits us to simulate only the 1D equation rather than the 2D one in the applications and greatly improves the computational times.
2. Model
In this section, we describe the modelling approach used to take into account angiogenesis in the growth of each tumour taken from Citation11 and its combination with the metastatic model of Citation1 Citation6 Citation14. The mathematical analysis of the resulting model can be found in Citation4.
2.1. The model of tumoral growth under angiogenic control
Let x(t) denote the size (number of cells) of a given tumour at time t Citation11. The growth of the tumour is modelled by a Gompertzian growth rate and the equation is
2.2. Renewal equation for the density of metastasis
We denote and
. We define
and
where b is the maximal reachable size and angiogenic capacity for
solving the system Equation(2)–(3) (see Citation7 for a qualitative study of this ODE system). We consider each tumour to be a particle evolving in Ω with the velocity G. Writing a balance law for the density ρ(t, X), we have
Metastasis do not only grow in size and angiogenic capacity, they are also able to emit new metastasis. We denote by the birth rate of new metastases with size and angiogenic capacity
by metastases of size x and angiogenic capacity θ, and by
the term corresponding to metastases produced by the primary tumour. Expressing the equality between the number of metastases arriving in Ω per unit time (l.h.s in the following equality) and the total rate of new metastases created by both the primary tumour and metastases themselves (r.h.s.), we should have for all t>0
3. Limit 2D–1D
A modelling hypothesis consists in considering that the newly created metastases are all born with the same given vasculature θ0 and not distributed around this value. At least, the distribution N(σ) should be very concentrated around the value , that is
. This is a simplification hypothesis which reduces the complexity of the model and thus its computational cost (see Section 4 for numerical illustrations), but we hope that it does not impoverish the model too much and that this one will still be able to describe the metastatic process. In this case, we would like to know if we can replace the function N by a Dirac mass centred in σ0, in Equation (1). Mathematically, the problem is to determine whether the family of solutions
to the problems
Figure 1. Phase plan of the systems Equation(2) and Equation(3)
. The solution is zero out of the stared characteristics coming from points of the boundary (1, θ) with θ∈[θ0−ϵ, θ0+ϵ]. The values of the parameters are chosen for illustrative purposes and are not realistic ones: a=2, c=5.85, d=0.1, θ0=200, ϵ=100.
![Figure 1. Phase plan of the systems Equation(2) and Equation(3). The solution is zero out of the stared characteristics coming from points of the boundary (1, θ) with θ∈[θ0−ϵ, θ0+ϵ]. The values of the parameters are chosen for illustrative purposes and are not realistic ones: a=2, c=5.85, d=0.1, θ0=200, ϵ=100.](/cms/asset/db098b77-ae9e-4de4-a3e9-94edf67d81e3/tjbd_a_568071_o_f0001g.jpg)
and gives a simple expression for the limit. For ,
or
, we will denote
the set of continuous linear forms on the Banach space of bounded continuous functions on 𝒪. We denote
the set of continuous functions with values in
, the continuity being taken in the sense of the weak-* topology. We give now the definition of weak solution to the problem (1) when N is a measure on
.
Definition 3.1
[(Weak solution) . We say that a measure
is a weak solution of the problem (1) if for all
with
The proof of the theorem requires the following technical lemma.
Lemma 3.2
Let
be a sequence going to zero,
and
be a sequence of functions of
such that
. Then
Proof
We compute, for t∈[0, T] and :
We establish now the theorem of this paper.
Theorem 3.3
[(Convergence)] Let G being defined by Equations
Equation(2)
and
Equation(3)
,
and
given by Equation
Equation(6)
. Let
be the weak solution of Equation
Equation(7)
. Then
Proof
| • |
Step 1. Simplification of the problem. Let | ||||
| • |
Step 2. Convergence for the sequence
| ||||
| • |
Step 3. Back to weak solutions. For a general data | ||||
| • |
Step 4. Back to ρ
k
. Denoting also ρ
k
the measure on Ω with density ρ
k
and in the same way | ||||
Remark 3.4
[(Uniqueness for EquationEquation (8))] In the proof of the previous theorem, we did not need to address the question of uniqueness of solutions to the problem Equation(8)
. However, there is uniqueness and it can be proved by the standard method of establishing existence of regular solutions to the adjoint problem. Indeed here the adjoint problem for a measure data
and a source term in
writes
Remark 3.5
[(Linear density)] To model directly the situation where all the metastases are born with the same angiogenic capacity θ0, we could consider that the metastases evolve on the one-dimensional curve and model the number of metastases via a linear density
. Then the number of metastases on the curve between the points
and
would be given by
, since
. Comparing this approach to the previous one where, after passing to the limit
, the number of metastases between X
1 and X
2 is
(from formula Equation(10)
), the analogy would be to identify
and thus this last quantity would solve the problem (17). In the linear density approach, it would yet not be possible to derive a simple equation on ρ1 since
has not a simple expression comparing with
which gives EquationEquation (4)
in the 2D modelling approach.
4. Numerical illustration
In Citation3, we developed a numerical scheme to simulate the problem (1). It is a Lagrangian scheme based on the method of characteristics which consists of discretizing the boundary with M points and simulating the equation along each characteristic curve coming from the boundary, after having straightened it. Remark that since the initial condition is zero, the solution only lives in the red part of and we only discretize the red part of the boundary . In , we illustrate the numerical convergence of this scheme. We took as a reference solution the numerical solution with M=100 and dt=0.02, and plotted the relative errors of the total number of metastases at the end of the simulation, that is:
, where
is the numerical solution with discretization parameters dt and M and ρref is the reference solution. We observe that the error in dt is much more important than the error in M. For example, for dt=0.1, the difference between the error with M=10 and M=100 is less than 10−5. This is due to the fact that, for the values of the parameters that we use in practical situations (the ones of , taken from Citation11), the characteristics are very concentrated around a particular one. We choose as a good approximation the values dt=0.1 and M=10.
Figure 2. Convergence of the numerical scheme for the 2D model (problem (1)), with ϵ=100. The values of the parameters for the growth velocity field G are from [11] and correspond to mice data: a=0.192, c=5.85, d=0.00873, θ 0=625. For the metastasis parameters, we used: m=0.001 and α=2/3. Total simulation time T=50. (a) Relative error in function of M, for various timesteps dt. (b) Relative error in function of dt, for various values of the discretization parameter of the boundary M.
![Figure 2. Convergence of the numerical scheme for the 2D model (problem (1)), with ϵ=100. The values of the parameters for the growth velocity field G are from [11] and correspond to mice data: a=0.192, c=5.85, d=0.00873, θ 0=625. For the metastasis parameters, we used: m=0.001 and α=2/3. Total simulation time T=50. (a) Relative error in function of M, for various timesteps dt. (b) Relative error in function of dt, for various values of the discretization parameter of the boundary M.](/cms/asset/02d11529-9abb-4147-af07-c05f85460cad/tjbd_a_568071_o_f0002g.jpg)
Because the equation is two-dimensional simulating the equation can have a high-computational cost, especially for large times (). Thanks to Theorem 3.3 if we make the biological assumption that all the metastases are born with an angiogenic capacity close to the value θ0, then the metastasis density is close to ρ and the total number of metastases at time t is close to
, with n being the solution of Equation (11), by applying the formula Equation(10)
with the test function
to obtain the total mass of the measure
. Thus we only have to simulate Equation (11), which with our scheme consists in simulating along the only characteristic coming from the point (1, θ0). The convergence stated in Theorem 3.3 is illustrated in . The relative difference for the total number of metastases at the end of the simulation, between the simulation in 1D and the one in 2D for various values of ϵ is plotted. That is, if T is the end time of the simulation:
Figure 3. Relative difference between the 1D simulation and the 2D one, for 5 values of ϵ: 100, 50, 10, 1 and 0.1. The values of the parameters are the same than in and the timestep used is dt=0.1. (a) Convergence when ϵ goes to zero, for T=15 and T=100. The value of M used for the 2D simulations is M=10. (b) Convergence when ϵ goes to zero, with respect to M (M=10, 50, 100), for T=50. The three curves are almost all the same.
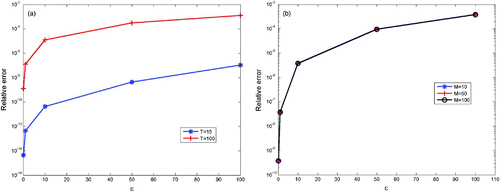
Table 1. Computational times on a personal computer of various simulations in 1D and 2D.
In are given various computational times on a personal computer for the simulation in 2D and in 1D. The simulations were performed with the same parameters as in and for the 2D simulations we used and M=10 points of discretization of the boundary.
We observe that simulating in 1D improves greatly the computational times, especially for the large time simulations. Since the evolution of a cancer disease can be very slow, it is important to be able to simulate the model for large times (say, more than a year in the human case). Here the times are in days and we see that, thanks to the convergence of Theorem 3.3, the numerical method for simulating the model is improved in terms of the computational cost.
5. Conclusion
We proved the convergence of the family of the solutions to the problem Equation(7)
, to the one of the problem Equation(8)
, and established a simple expression for the limit in term of the solution of a 1D equation. This is of great importance in view of the applications since we can simulate only the 1D equation and thus highly improve the computational cost. The model is now ready to be a useful tool with two main possible applications.
First it can be used as a diagnostic tool, to refine the actual classifications like TNM (for Tumour, lymph Nodes and Metastases) or SBR (Scarff, Bloom, Richardson) which deal only with the visible metastases. Indeed, identifying the parameters m and α for a given patient could determine the metastatic aggressiveness of its cancer. A fundamental problem in this direction that needs to be addressed is the mathematical parameter identifiability (inverse problem). Efficient numerical methods have also to be developed to achieve practical parameter identification, which will permit us to confront the model to real data in order to study its validity as a phenomenological model.
The second main application of the model is its use in the rationalization of the temporal administration protocols for an anti-angiogenic drug alone as well as in combination with a cytotoxic drug. Finding the optimal schedule for these issues is still a clinical open question. The associated optimization problems through the model have to be solved both at theoretical and numerical levels.
References
- Barbolosi , D. , Benabdallah , A. , Hubert , F. and Verga , F. 2009 . Mathematical and numerical analysis for a model of growing metastatic tumors . Math. Biosci. , 218 : 1 – 14 .
- Barbolosi , D. and Iliadis , A. 2001 . Optimizing drug regimens in cancer chemotherapy: A simulation study using a PKPD model . Comput. Biol. Med. , 31 : 157 – 172 .
- Benzekry , S. 2010 . “ Mathematical and numerical analysis of a model for anti-angiogenic therapy in metastatic cancers ” . submitted for publication Available at http://hal.archives-ouvertes.fr/hal-00518110/fr/
- Benzekry , S. 2011 . Mathematical analysis of a two-dimensional population model of metastatic growth including angiogenesis . J. Evol. Equations , 11 : 187 – 213 .
- Billy , F. , Ribba , B. , Saut , O. , Morre-Trouilhet , H. , Colin , T. , Bresch , D. , Boissel , J. , Grenier , E. and Flandrois , J. 2009 . A pharmacologically based multiscale mathematical model of angiogenesis and its use in investigating the efficacy of a new cancer treatment strategy . J. Theoret. Biol. , 260 : 545 – 562 .
- Devys , A. , Goudon , T. and Laffitte , P. 2009 . A model describing the growth and the size distribution of multiple metastatic tumors . Discrete Contin. Dyn. Syst. Ser. , B 12 : 731 – 767 .
- d'Onofrio , A. and Gandolfi , A. 2004 . Tumour eradication by antiangiogenic therapy: Analysis and extensions of the model by Hahnfeldt et al. (1999) . Math. Biosci. , 191 : 159 – 184 .
- d'Onofrio , A. , Ledzewicz , U. , Maurer , H. and Schttler , H. 2009 . On optimal delivery of combination therapy for tumors . Math. Biosci. , 222 : 13 – 26 .
- Ebos , J. M.L. , Lee , C. R. , Cruz-Munoz , W. , Bjarnason , G. A. , Christensen , J. G. and Kerbel , R. S. 2009 . Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis . Cancer Cell , 15 : 232 – 239 .
- Folkman , J. 1972 . Antiangiogenesis: New concept for therapy of solid tumors . Ann. Surg. , 175 : 409 – 416 .
- Hahnfeldt , P. , Panigraphy , D. , Folkman , J. and Hlatky , L. 1999 . Tumor development under angiogenic signaling: A dynamical theory of tumor growth, treatment, response and postvascular dormancy . Cancer Res. , 59 : 4770 – 4775 .
- Harris , J. , Morrow , M. and Norton , L. 1997 . “ Malignant tumors of the breast ” . In Cancer: Principles and Practice of Oncology , 5 , Edited by: DeVita , J. V. , Hellman , S. and Rosenberg , S. 1557 – 1602 . Philadelphia : Lippincott-Raven .
- Hinow , P. , Gerlee , P. , McCawley , L. J. , Quaranta , V. , Ciobanu , M. , Wang , S. , Graham , J. M. , Ayati , B. P. , Claridge , J. , Swanson , K. R. , Loveless , M. and Anderson , A. R. 2009 . A spatial model of tumor-host interaction: Application of chemotherapy . Math. Biosci. Eng. , 6 : 521 – 545 .
- Iwata , K. , Kawasaki , K. and Shigesada , N. 2000 . A dynamical model for the growth and size distribution of multiple metastatic tumors . J. Theoret. Biol. , 203 : 177 – 186 .
- Paez-Ribes , M. , Allen , E. , Hudock , J. , Takeda , T. , Okuyama , H. , Vinals , F. , Inoue , M. , Bergers , G. , Hanahan , D. and Casanovas , O. 2009 . Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis . Cancer Cell , 15 : 220 – 231 .
- Perthame , B. 2007 . “ Transport Equations in Biology, Frontiers in Mathematics ” . Basel : Birkhaser .
- Riely , G. J. , Rizvi , N. A. , Kris , M. G. , Milton , D. T. , Solit , D. B. , Rosen , N. , Senturk , E. , Azzoli , C. G. , Brahmer , J. R. Sirotnak , F. M. 2009 . Randomized phase II study of pulse erlotinib before or after carboplatin and paclitaxel in current or former smokers with advanced non-small-cell lung cancer . J. Clin. Oncol. , 27 : 264 – 270 .
- Swan , G. W. 1990 . Applications of optimal control theory in biomedicine . Math. Biosc. , 101 : 237 – 284 .