Abstract
If a healthy stable host population at the disease-free equilibrium is subject to the Allee effect, can a small number of infected individuals with a fatal disease cause the host population to go extinct? That is, does the Allee effect matter at high densities? To answer this question, we use a susceptible–infected epidemic model to obtain model parameters that lead to host population persistence (with or without infected individuals) and to host extinction. We prove that the presence of an Allee effect in host demographics matters even at large population densities. We show that a small perturbation to the disease-free equilibrium can eventually lead to host population extinction. In addition, we prove that additional deaths due to a fatal infectious disease effectively increase the Allee threshold of the host population demographics.
Keywords:
1. Introduction
In population models, the Allee effect refers to inverse density dependence at low population sizes. A strong Allee effect occurs in these models when there is a positive equilibrium density, Allee threshold, and the species goes extinct whenever population densities fall below the threshold Citation1,Citation7–11,Citation13–15. The presence of a strong Allee effect in host demographics makes the population vulnerable to extinction as a fatal disease may tip it below the Allee threshold. In a recent paper, Hilker et al. Citation14 showed that in the presence of strong Allee effect in host demographics, a simple susceptible–infected (SI) epidemic model can exhibit stable periodic orbits (by Hopf bifurcation), multiple stationary states, and catastrophic collapses of endemic equilibria. Using a similar SI model with the strong Allee effect, Thieme et al. Citation16 established by mathematical theorems that the transition from population decline to population collapse is mediated by a Hopf bifurcation and a heteroclinic orbit. The SI models of Hilker et al. and Thieme et al. are structurally similar to predator–prey models that have an Allee effect in the prey population and a linear functional response (prey eaten per predator per unit time) Citation2 Citation3 Citation6 Citation10,Citation12–14,Citation17 Citation18. However, most of these studies on the interplay of Allee effects and infectious diseases, especially those in the conservation biology literature, seem to be largely concerned with the role of the Allee effect at small population densities Citation9.
In the present paper, we prove that the presence of an Allee effect in host demographics matters even at large population densities. That is, we show that a small perturbation to the disease-free equilibrium can eventually lead to host population extinction. We focus on the following question. If a healthy stable host population at the disease-free equilibrium is subject to an Allee effect, can a small number of infected individuals with a fatal disease cause the host population to go extinct? To answer this question, we use the SI epidemic model of Hilker et al. and obtain model parameters that lead to host population persistence (with or without infected individuals) and to host extinction. We note that in epidemiological models with frequency-dependent transmission and disease-related mortality, a small number of infected individuals can drive the host population to extinction. In these models, when the strong Allee effect is missing in the host demographics, the transition from host persistence to host extinction occurs ‘gradually’ or ‘slowly’ while the model parameters vary. However, in the presence of a strong Allee effect, the transition from host persistence to extinction is much more abrupt Citation12.
The paper is organized as follows. In Section 2, we introduce the SI model with the strong Allee effect in the demographic equation. In Section 3, we state conditions for population persistence (Theorem 3.3) and extinction (Theorem 3.5). Theorems 3.3 and 3.5 are proved in Sections 4 and 5, respectively. In Section 6, we use specific parameter regimes to illustrate regions of host population persistence (with or without infected individuals) and/or extinction. Section 7 is concerned with the stability of a unique endemic stationary point, and concluding remarks are presented in Section 8.
2. Model equations
To introduce the SI model of Hilker et al. Citation14 with a strong Allee effect in the host demographics, we let P=P(t)≥0 denote the host population at time t≥0. In the presence of an infectious disease, the total population
Following Hilker et al. Citation14, we non-dimensionalize the system Equation(1) by the following change of variables:
To simplify the notation, we drop the ‘prime’ on t′ so that Model Equation(3) reduces to the system of equations
3. Statement of results
In Model Equation(4), we take initial conditions
Theorem 3.1
The solution (p(t), i(t)) of Equations
Equation(4)
and
Equation(5)
satisfies the inequalities
Proof
Clearly, i(t)>0 and then also p(t)<1 for all t>0. To prove that i(t)<p(t) for all t>0, we assume, to the contrary, that there exists a point [ttilde] such that i(t)<p(t) for all 0<t<[ttilde] and . Then,
Model Equation(4) has three disease-free equilibrium points:
If σ≤1, then by the second equation of Model Equation(4)
As in Citation14, we introduce the critical host population density for disease establishment, the disease threshold,
Theorem 3.2
If P T>1, then (1, 0) is locally asymptotically stable and the disease goes extinct.
Indeed, in this case, the eigenvalues of the Jacobian matrix at (1, 0), −r(1−u) and , are both negative.
In the rest of the paper, we consider the case where 0<P
T<1. We first state conditions for persistence of the infected population of Model Equation(4).
Theorem 3.3
If
Remark 3.4 (a) Let
Figure 1. Condition Equation(8) hold where u<y
cr
<P
T and the horizontal line, i=((σ−1)/σ)(1−P
T), is below the maximum value of the p-nullcline, i=(r/α)(1−p)(p−u)p.
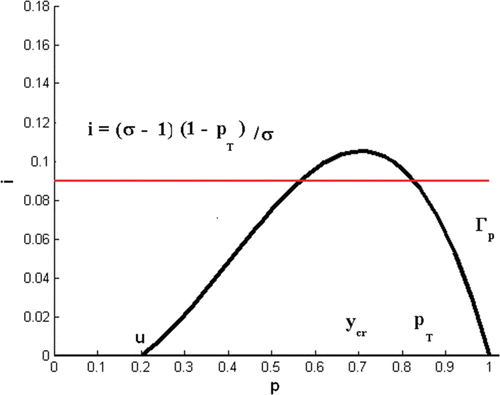
In Model Equation(4), all trajectories (p(t), i(t)) with p(0)<u lead to host population extinction. When 0<P
T<1, Theorem 3.3 asserts that in the presence of disease infection, the Allee threshold is effectively increased to
. That is, due to disease-induced death, the host population size needs to be larger to ensure persistence of the infected population Citation10.
In Model Equation(4), host population extinction is possible with 0<P
T<1 and
. To illustrate this, we next consider the case where instead of EquationEquation (7)
, we have
Theorem 3.5
If
and
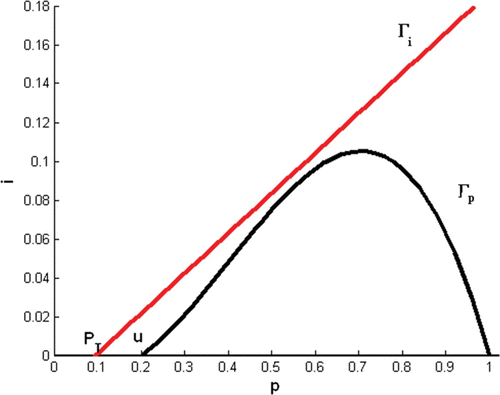
Condition Equation(12) holds whenever the p-
, Γ
p
, is below the i-
, Γ
i
, of Model Equation(4)
().
Theorems 3.3 and 3.5 are proved in Sections 4 and 5, respectively. In Section 6, we give numerical examples and discuss the conclusion of these theorems in terms of persistence and extinction of a healthy population in which a small but fatal disease was introduced.
Figure 2. Condition Equation(12) holds where P
T<u<1 and the p-nullcline is below the i-nullcline.
4. Proof of Theorem 3.3
To prove Theorem 3.3, we first establish two auxiliary results, Lemmas 4.1 and 4.2.
Lemma 4.1
Assume that 0<P
T<1. Then, for any
there exists a small enough
and a function
such that if
Proof
We assume that
We next claim that
If in the above proof i(0) is equal to ϵ, then becomes a function
which depends only on ϵ and
. In the next lemma, we restate this case where instead of t=0 we take any initial time t=t
1.
Lemma 4.2
Assume that 0<P T<1 and
Proof
[Proof of Theorem 3.3] In view of Lemma 4.2, we need only to bound i(t) from below in intervals , where
and t
1>0. We may further assume that (t
1, t
2) is a maximal interval for which
so that
. Then,
for some values t smaller than t
1. Let t
0 be the largest value of t, t<t
0, with the property that i(t) is monotonically decreasing from t
0 to t
1. Then,
Note that the way was determined,
may be viewed as a function
(of ϵ only). We next observe that
5. Proof of Theorem 3.4
We denote by the union of
Hence,
Consider next the case when equality holds in EquationEquation (12) with
. Then,
and
have one point of intersection,
, where they are tangent to each other. As easily computed, the eigenvalues λ
i
of the Jacobian matrix at Q are λ1=0 and
. Hence, Q is a saddle point, so that no trajectory (p(t), i(t)) which is above
, for some t=[ttilde], can converge to Q as
. It follows, as illustrated in the phase diagram in , that any trajectory (p(t), i(t)) with
, where
is sufficiently small and δ2>0, must cross successively
and
and then, as in the case considered above,
as
. In particular,
for some finite time t
δ.
Under the condition Equation(12) with ϵ sufficiently small, the i-nullcline intersects the p-nullcline at two points Q
1 and Q
2, where
and
are small enough, so that, by continuity, the corresponding trajectory
with
, where
, is sufficiently small and δ2>0 satisfies
, and hence
as
.
Remark 5.1 The assumption that ϵ in condition Equation(12) is small is essential. shows an example where the two points of intersection of the nullclines, Q
1 and Q
2, are not sufficiently close, and in this case, the solution (p(t), i(t)) converges to Q
2 as
.
Figure 3. In Model Equation(4), Γ
i
(straight line) is tangent to Γ
p
(cubic) and a solution (circles) with initial condition (1, 0.0001) converges to (0, 0) as t→∞. Here, α=r=0.016667, d=0.00482, σ=1.241, and u=0.2.
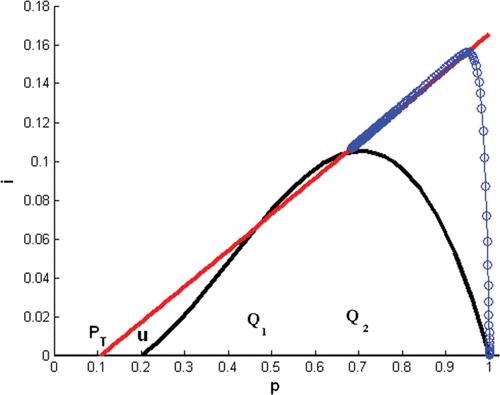
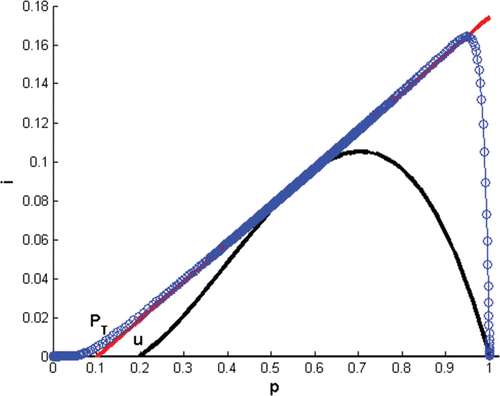
Figure 4. Phase plane of Model Equation(4) with two interior positive steady points and a solution (circles) with initial condition (1, 0.0001) converges to an interior fixed point as t→∞. Here, α=r=0.016667, d=0.00482, σ=1.23, and u=0.2.
6. Extinction and persistence
We are interested in the following question regarding system Equation(4). Given a healthy stable population with p(0)=1 but subject to the Allee effect in the host demographics, can a small number of individuals infected with a fatal disease cause the host population to go extinct? If this is the case no matter how small the initial number of infected individuals, i(0), then we say that the model parameters are in the host extinction phase; otherwise we say that the parameters are in the host persistence phase.
We shall, for illustration, focus on the parameters u, the Allee threshold, and the infection parameter σ, keeping all the other parameters fixed. When 0<P
T<1, under conditions Equation(7) and Equation(8)
, Theorem 3.3 asserts that if
for some
(and, in particular, if p(0)=1), for any small number of infected individuals i(0), the inequality Equation(9)
holds. Thus, (u, σ) is a point of persistence of the infected population and, hence (by Theorem 2.1), also of the host population. On the other hand, Theorem 3.5 asserts that (u, σ) is a point of host population extinction if
and condition Equation(12)
holds.
We illustrate the regions of persistence versus extinction in in the case
Figure 5. Region of host persistence with no infected individuals (disease extinction) is denoted by ‘open squares’, region of disease persistence is denoted by ‘open circles,’ and region of host extinction is denoted by ‘asterisks’ in (u, σ)-plane with initial condition (p, i)=(1, 0.0001). Here, α=0.1, d=0.25, r=0.2, u∈(0, 0.5), and σ>1.
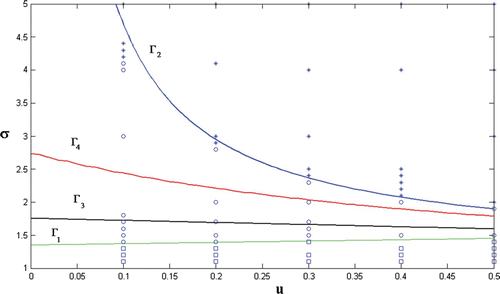
Theorem 3.3 asserts that the region between Γ3 and Γ2 is a region of persistence of the infected population. Theorem 3.5 asserts the region for some small η>0 is a region of extinction of the host population. Points in regions between Γ1 and Γ3 and between Γ2 and Γ4 may be either points of disease population extinction or persistence. Simulations in with initial condition
show points of host population persistence without infected individuals by open squares, points of disease persistence by open circles, and points of host population extinction by asterisks. Note that by Theorem 3.2, every point below Γ1 is a state of host population persistence with no infected individuals.
It follows from that if we increase the infection rate (respectively, the Allee threshold) while all the other parameters are kept fixed at their current values, then it is possible for the system parameters to shift from host population persistence phase to host population extinction phase. In , stability questions (equilibrium versus oscillation) are not considered.
7. Stability of the unique endemic equilibrium
Let
Theorem 7.1
If 0<u<P
T<1, then Model
Equation(4)
has a unique stationary point
Proof
Note that is a stationary point with ī>0 if and only if
is a zero of the function
This may only occur for x in the interval P
T< x<1. Since f
Equation(1)<0, f(P
T)>0, and
if u<x<1, f(x) has precisely one zero
in this interval. The slope of the i-nullcline at
is clearly larger than the slope of the p-nullcline, that is,
Hilker et al.
Citation14 showed that Model Equation(4) is capable of exhibiting an interior periodic orbit via a Hopf bifurcation of the interior stationary points.
8. Conclusion
Many plant and animal species are known to exhibit the Allee effect Citation9. Examples of species that exhibit the Allee effect and suffer from fatal diseases include the endangered African wild dog Lycaon pictus Citation4 Citation5 Citation8 and the island fox Urocyon littoralis Citation1 Citation7. Studies of systems that exhibit the Allee mechanism seem to be focused on the role of the Allee effect at small population densities. However, in combination with an infectious disease, we prove that a small perturbation to the disease-free equilibrium can lead to the catastrophic extinction of the host population. That is, a small number of infected individuals can lead to host population extinction. To prove this result, we use the relative position of a disease threshold to the Allee threshold and host population carrying capacity and obtained verifiable conditions that guarantee the persistence (with or without infected individuals) or extinction of the host population of the SI model of Hilker et al. Citation14. In the presence of the Allee effect in the host demographics, population models which exhibit multistability and transitions from host population persistence (with or without infected individuals) to extinction become much more abrupt. Mathematical proofs of these bifurcation phenomena and how they impact host population extinctions at high densities are open questions.
Also, we prove that when there is an Allee effect in the host demographics and a fatal disease invades the host population, then the Allee threshold is effectively increased. Deredec and Courchamp Citation10 reported a similar result. At large population densities, the Allee effect and infectious diseases can work together to drive otherwise stable healthy host populations to extinction even at large population densities. Thus, understanding the interplay between the Allee effect and disease epidemic models may have important implications in conservation biology, ecology, and epidemiology.
With view to the effects of migration on populations of endangered species, for example, the endangered African wild dog, it would be interesting to investigate how the extinction threshold of one population is influenced by the collective migrations of several populations with different Allee thresholds.
Acknowledgements
The authors thank the referees for useful comments and suggestions that improved our manuscript. This research has been supported in part by the Mathematical Biosciences Institute of The Ohio State University, Department of Homeland Security, DIMACS and CCICADA of Rutgers University, and the National Science Foundation under grant DMS 0931642 and 0832782.
References
- Angulo , E. , Roemer , G. W. , Berec , L. , Gascoigne , J. and Courchamp , F. 2007 . Double Allee effects and extinction in island fox . Conserv. Biol , 21 : 1082 – 1091 .
- Bazykin , A. 1998 . “ Nonlinear Dynamics of Interacting Populations ” . In World Scientific Series on Nonlinear Analysis: Series A , Vol. 22 , River Edge, NJ : World Scientific .
- Berezovsky , F. , Novozhilov , S. and Karev , G. 2007 . Population models with singular equilibrium . Math. Biosci , 208 : 270 – 299 .
- Burrows , R. , Hofer , H. and East , M. I. 1995 . Population dynamics, intervention and survival in African wild dogs (Lycaon pictus) . Proc. R. Soc. B Biol. Sci , 262 : 235 – 245 .
- Clifford , D. L. , Mazet , J. A.K. , Dubovi , E. J. , Garcelon , D. K. , Coonan , T. J. , Conrad , P. A. and Munson , L. 2006 . Pathogen exposure in island fox (Urocyon littoralis) populations: Implications for conservation management . Biol. Conserv , 131 : 230 – 243 .
- Conway , E. D. and Smoller , I. A. 1986 . Global analysis of a system of predator–prey equations . SIAM J. Appl. Math , 46 : 630 – 642 .
- Courchamp , F. , Clutton-Rock , T. and Grenfell , B. 1999 . Inverse density dependence and the Allee effect . Trends Ecol. Evol , 14 : 405 – 410 .
- Courchamp , F. , Clutton-Rock , T. and Grenfell , B. 2000 . Multipack dynamics and the Allee effect in the African wild dog, Lycaon pictus . Anim. Conserv , 3 : 277 – 285 .
- Courchamp , F. , Berec , L. and Gascoigne , J. 2008 . Allee Effect in Ecology and Conservation , New York : Oxford University Press .
- Deredec , A. and Courchamp , F. 2006 . Combined impacts of Allee effect and parasitism . Oikos , 112 : 667 – 679 .
- Franke , J. and Yakubu , A.-A. 2008 . Disease-induced mortality in density dependent discrete-time SIS epidemic models . J. Math. Biol , 57 ( 6 ) : 755 – 790 .
- Hilker , F. M. 2010 . Population collapse to extinction: The catastrophic combination of parasitism and Allee effect . J. Biol. Dyn , 4 : 86 – 101 .
- Hilker , F. M. , Langlais , M. , Petrovski , S. V. and Malchow , H. 2007 . A diffusive SI model with Allee effect and application to FIV . Math. Biosci , 206 : 61 – 80 .
- Hilker , F. M. , Langlais , M. and Malchow , H. 2009 . The Allee effect and infectious diseases: Extinction, multistability, and the (dis-)appearance of oscillations . Am. Nat , 173 ( 1 ) : 72 – 88 .
- Shreiber , S. J. 2009 . Allee effects, extinctions and chaotic transients in simple population models . Theor. Popul. Biol , 64 : 201 – 209 .
- Thieme , H. R. , Dhirasakdanon , T. , Han , Z. and Trevino , R. 2009 . Species decline and extinction: Synergy of infectious disease and Allee effect? . J. Biol. Dyn , 3 ( 2–3 ) : 305 – 323 .
- van Voorn , G. L.K. , Hemerik , L. , Boer , M. P. and Kooi , B. W. 2007 . Heteroclinic orbits indicate overexploitation in predator–prey systems with a strong Allee effect . Math. Biosci , 209 : 451 – 469 .
- Wang , J. , Shi , J. and Wei , J. 2011 . Predator–prey system with strong Allee effect in prey . J. Math. Biol , 62 : 291 – 331 .