?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
In this paper, we study the stability analysis of latent Chikungunya virus (CHIKV) dynamics models. The incidence rate between the CHIKV and the uninfected monocytes is modelled by a general nonlinear function which satisfies a set of conditions. The model is incorporated by intracellular discrete or distributed time delays. Using the method of Lyapunov function, we established the global stability of the steady states of the models. The theoretical results are confirmed by numerical simulations.
1. Introduction
Chikungunya virus (CHIKV) is an alphavirus and is transmitted to humans by Aedes aegypti and Aedes albopictus mosquitos. The CHIKV attacks the monocytes and causes Chikungunya fever. The humoral response is important to limit the CHIKV progression [Citation33]. In the literature of CHIKV infection, most of the mathematical models have been presented to describe the disease transmission in mosquito and human populations (see e.g. [Citation3–5,Citation31,Citation35,Citation37,Citation38,Citation48]). In a very recent work, Wang and Liu [Citation43] have presented a mathematical model for in host CHIKV infection as
(1)
(1)
(2)
(2)
(3)
(3)
(4)
(4) where S, I, V, and B are the concentrations of uninfected monocytes, infected monocytes, CHIKV particles and B cells, respectively. The uninfected monocytes are created at rate μ and die at rate aS. The uninfected monocytes are attacked by the CHIKV at rate bSV, where b is rate constant of the CHIKV-target incidence. The infected monocytes and free CHIKV particles die are rates
and rV, respectively. An actively infected monocytes produces an average number m of CHIKV particles. The CHIKV particles are attacked by the B cells at rate qBV. The B cells are created at rate η, proliferated at rate cBV and die at rate
.
In recent years, stability analysis of virus dynamics models has become one of the hot topics in virology (see e.g. [Citation1,Citation2,Citation6,Citation24,Citation26,Citation30,Citation32,Citation36,Citation39,Citation42,Citation44,Citation47]). Studying the stability analysis of the models is important for developing antiviral drugs, understanding the virus-host interaction and for predicting the disease progression. Stability of model (Equation1(1)
(1) )–(Equation4
(4)
(4) ) has been studied by Wang and Liu [Citation43].
In system (Equation1(1)
(1) )–(Equation4
(4)
(4) ) it is assumed that the incidence rate between the CHIKV and uninfected monocytes is given by bilinear form. However, such bilinear form is imperfect to depict the dynamical behaviour of the viral infection in detail [Citation27]. Moreover, it is assumed that when the CHIKV contacts the uninfected monocytes it becomes infected and viral producer in the same time. In [Citation34], the authors have described the CHIKV replication cycle as (see Figure ):
Figure 1. CHIKV replication cycle [Citation34].
![Figure 1. CHIKV replication cycle [Citation34].](/cms/asset/8c088b31-314b-4f9f-ad30-b0ecde960b8d/tjbd_a_1503349_f0001_c.jpg)
The virus enters susceptible cells through endocytosis, mediated by an unknown receptor. As the endosome is acidic, conformational changes occur resulting in the fusion of the viral and host cell membranes, causing the release of the nucleocapsid into the cytoplasm, The RNA genome is first translated into the 4nsPs, which together will form the replication complex and assist in several downstream processes (depicted by dashed arrowed line in Figure ). Subsequently, the genome is replicated to its negative-sense strand, which in turn will be used as a template for the synthesis of the 49S viral RNA and 26S subgenomic mRNA. The 26S subgenomic mRNA will be translated to give the structural proteins (C–pE2–6K–E1). After a round of processing by serine proteases, the capsid is released into the cytoplasm. The remaining structural proteins are further modified post-translationally in the endoplasmic reticulum and subsequently in the Golgi apparatus. E1 and E2 associate as a dimer and are transported to the host plasma membrane, where they will ultimately be incorporated onto the virion surface as trimeric spikes. Capsid protein will form the icosahedral nucleocapsid that will contain the replicated 49S genomic RNA before being assembled into a mature virion ready for budding. During budding the virions will acquire a membrane bilayer from part of the host cell membrane.
Therefore, this process may take time period which can be incroporated into the CHIKV model by considering the time delay and latently infeced cells.
The objective of this paper is to propose a CHIKV infection model which improves the model presented in [Citation43] by taking into account (i) two types of infected monocytes, latently infected monocytes and actively infected monocytes, (ii) two types of discrete or distributed time delays (iii) the incidence rate between the CHIKV and the uninfected monocytes is given by a general nonlinear function , where the function Ψ is supposed to satisfy a set of conditions. We investigate the nonnegativity and boundedness of the solutions of the CHIKV dynamics model. We show that the CHIKV dynamics is governed by one bifurcation parameter (the basic reproduction numbers
). We use Lyapunov direct method to establish the global stability of the model's steady states.
2. CHIKV model with discrete time delays
We propose the following latent CHIKV dynamics model with general incidence rate taking into account two discrete time delays:
(5)
(5)
(6)
(6)
(7)
(7)
(8)
(8)
(9)
(9) where L and I are the concentrations of latently infected monocytes and actively infected monocytes, respectively. The parameter λ is the latent to active transmission rate constant. A fraction
of infected cells is assumed to be latently infected monocytes and the remaining ρ becomes actively infected monocytes, where
. Here,
is the time between CHIKV entry an uninfected monocyte to become latently infected monocyte (such monocyte contains the CHIKV but is not producing it), and
is the time between CHIKV entry an uninfected monocyte to become actively infected monocyte (such monocyte produces the CHIKV particles). The probability of latently and actively infected monocytes surviving to the age of
and
are represented by
and
respectively, where
and
are constants. The incidence of new infections of uninfected monocytes occurs at a rate
, which includes the rate of contacts between CHIKV and uninfected monocytes as well as the probability of cell entry per contact [Citation42].
We consider the following initial conditions:
(10)
(10) where
and C is the Banach space of continuous functions mapping the interval
into
with norm
Then the uniqueness of the solution for t>0 is guaranteed [Citation25].
The function Ψ is assumed to satisfy the following:
Assumption 2.1
Ψ is continuously differentiable,
for all
for all
for all S>0.
Assumption 2.2
is a decreasing function of V for all
Remark 2.1
Assumption 2.1(iii) implies that
(11)
(11) From 2.2 we get
(12)
(12) From 2.1(ii) and 2.2 we obtain
which yields
(13)
(13)
2.1. Preliminaries
In this subsection we investigate the nonnegativity and boundedness of the solutions of model (Equation5(5)
(5) )–(Equation9
(9)
(9) ).
Lemma 2.1
The solutions of system (Equation5(5)
(5) )–(Equation9
(9)
(9) ) with the initial states (Equation10
(10)
(10) ) are nonnegative and ultimately bounded.
Proof.
From Equations (Equation5(5)
(5) ) and (Equation9
(9)
(9) ) we have
and
. Thus,
and
for all
. Moreover, for
we have
By recursive argument, we get
and
for all
Next, we establish the boundedness of the model's solutions. Let where
. The nonnegativity of the model's solution implies that
which yields
. Let us define
then
Since
, then
It follows that
,
. Let us define
Then
,
, and
,
and
. Therefore,
and
are ultimately bounded.
Lemma 2.1 reveal that the following positively invariant set contains omega limit sets of system (Equation5(5)
(5) )–(Equation8
(8)
(8) ):
2.2. The existence of steady states
The basic reproduction number of system (Equation5(5)
(5) )–(Equation9
(9)
(9) ) is given by
where
and
Lemma 2.2
For system (Equation5(5)
(5) )–(Equation9
(9)
(9) ), assume that Assumptions 2.1–2.2 are satisfied, then there exists a threshold parameter
such that if
then the CHIKV-free steady state
is the only steady state for the system. If
then the system has a unique endemic steady state
.
Proof.
Let be any steady state of the system (Equation5
(5)
(5) )–(Equation9
(9)
(9) ) satisfying the following equations:
(14)
(14)
(15)
(15)
(16)
(16)
(17)
(17)
(18)
(18) From Equations (Equation14
(14)
(14) )–(Equation18
(18)
(18) ) we obtain
(19)
(19) Substituting Equation (Equation19
(19)
(19) ) into Equation (Equation17
(17)
(17) ) we have
(20)
(20) Applying Assumption A1, we have V =0 is one of the solutions of Equation (Equation20
(20)
(20) ), which gives a CHIKV-free steady state
. If
then from Equations (Equation14
(14)
(14) ) and (Equation20
(20)
(20) ) we have
which implies
Then, from Equation (Equation18
(18)
(18) ) we have
We define a function
as
Now we get a value of V such that
(21)
(21) Let
, then Equation (Equation21
(21)
(21) ) becomes
Let
we have
and
Then
gives a unique
. It follows that
Clearly,
From Assumption 2.1,
then
Therefore, if
, then
and there exists a
such that
From Equations (Equation17
(17)
(17) ) and (Equation18
(18)
(18) ) we obtain
Let
in Equation (Equation14
(14)
(14) ) and define a function
as
By Assumption 2.1, we have
and
Since
is a strictly decreasing function of S, then there exists a unique
such that
Thus an endemic steady state
exists when
2.3. Global stability
The following theorems investegate the global stability of the CHIKV-free and endemic steady states of system (Equation5(5)
(5) )–(Equation8
(8)
(8) ) by constructing suitable Lyapunov functionals. We define
Clearly,
and
for x>0. Denote
.
Theorem 2.1
Suppose that and 2.1–2.2 are satisfied, then
is GAS.
Proof.
Define
Note that,
for all S,L,I,V,B>0 and
. Calculating
along the trajectories of (Equation5
(5)
(5) )–(Equation9
(9)
(9) ) we get
(22)
(22) Simplifying Equation (Equation22
(22)
(22) ) we get
Using
,
and inequality (Equation12
(12)
(12) ) we get
(23)
(23) From Assumption 2.1 we have, if
then
for all
Also,
if and only if
and
Let
and
be the largest invariant subset of
. The solutions of system (Equation5
(5)
(5) )–(Equation9
(9)
(9) ) converge to
[Citation25]. Each element in
satisfies
Then from Equations (Equation7
(7)
(7) )–(Equation8
(8)
(8) ) we have
. By the LaSalle's invariance principle,
is GAS.
Theorem 2.2
Suppose that and 2.1–2.2 are satisfied, then
is GAS.
Proof.
We have
for all S,L,I,V,B>0 and
. Calculating
along the trajectories of (Equation5
(5)
(5) )–(Equation9
(9)
(9) ) we get
(24)
(24) Simplifying Equation (Equation24
(24)
(24) ) we get
Applying
we obtain
From Lemma 2.2 we have
then
and
(25)
(25) Using the following equalities:
we get
(26)
(26) Using Assumption 2.1(ii) and inequality (Equation13
(13)
(13) ), we get
. Let
and
be the largest invariant subset of
. It can be verified that
if and only if
,
, and
(27)
(27) For each element of
we have
, then
, which gives
. From Equation (Equation27
(27)
(27) ) we get
,
. Then
contains a single point that is
. It follows from LaSalle's invariance principle,
is GAS.
3. CHIKV model with delay-distributed
We consider the following latent CHIKV dynamics model with distributed delays:
(28)
(28)
(29)
(29)
(30)
(30)
(31)
(31)
(32)
(32) Here,
is probability that uninfected monocyte contacted by CHIKV at time
survived τ time units and becomes latently infected monocyte at time t, where
is limit superior of this delay, and
is probability that uninfected monocyte contacted by the CHIKV at time
survived τ time units and becomes actively infected monocyte at time t, where
is limit superior of this delay. The probability distribution functions
and
are assumed to satisfy
(33)
(33) where, ι is a positive number. Let
Then,
i=1,2.
The initial conditions for system (Equation28(28)
(28) )–(Equation32
(32)
(32) ) is the same as given by (Equation10
(10)
(10) ) where
.
3.1. Preliminaries
Lemma 3.1
The solutions of system (Equation28
(28)
(28) )–(Equation32
(32)
(32) ) with the initial conditions (Equation10
(10)
(10) ) are nonnegative and ultimately bounded.
Proof.
From Lemma 2.1 we have and
for all
Moreover, one can show that for
From Equation (Equation28
(28)
(28) ), we have
. Let
then
It follows that
,
,
, and
, where
,
and
are defined previously. This shows the ultimate boundedness of
and
.
3.2. The existence of steady states
Lemma 3.2
For system (Equation28(28)
(28) )–(Equation32
(32)
(32) ), assume that Assumptions 2.1–2.2 are satisfied, then there exists a threshold parameter
such that if
then the CHIKV-free steady state
is the only steady state for the system. If
then the system has a unique endemic steady state
.
Proof.
Let be any steady state of the system (Equation28
(28)
(28) )–(Equation32
(32)
(32) ) satisfying the following equations:
(34)
(34)
(35)
(35)
(36)
(36)
(37)
(37)
(38)
(38) The proof can be completed in the same line as the proof in Lemma 2.2. The basic reproduction number is defined as
where
3.3. Global Stability
Theorem 3.1
Suppose that and Assumtions 2.1–2.2 is satisfied then
is GAS.
Proof.
Let
Calculating
along the trajectories of (Equation28
(28)
(28) )–(Equation32
(32)
(32) ) we get
Then, we have
(39)
(39) From Remark 2.1 we have, if
then
for all
Also,
if and only if
Then
is GAS.
Theorem 3.2
Suppose that and 2.1–2.2 are satisfied, then
is GAS.
Proof.
Define a Lyapunov functional as
Calculating
along the trajectories of (Equation28
(28)
(28) )–(Equation32
(32)
(32) ) we get
Applying
we obtain
Using the steady state conditions for
:
then
and
(40)
(40) Applying the following equalities:
we get
(41)
(41) Clearly,
, and similar to the proof of Theorem 2.2 it can be easily show that
occurs at
. It follows from LaSalle's invariance principle,
is GAS.
4. Numerical simulations
In order to confirm our theoretical results, we carry out some numerical simulations for systems (Equation5(5)
(5) )– (Equation8
(8)
(8) ).
We choose the function Ψ as
Then we have
(42)
(42)
(43)
(43)
(44)
(44)
(45)
(45)
(46)
(46) where
Assume that
Now, we have to verify Assumptions A1–A2. Clearly,
for all
Moreover, for S>0 and V >0 we have
Since
then
and
for all
Thus, Assumption 2.1 holds true. We have
for all
which shows that Assumption 2.2 also holds true. Thus, the global stability results shown in Theorems 2.1–2.2 are guaranteed for model (Equation42
(42)
(42) )–(Equation46
(46)
(46) ). The parameter
for model (Equation42
(42)
(42) )–(Equation46
(46)
(46) ) is given by
From the above analysis, we perform the numerical results for system (Equation42
(42)
(42) )–(Equation46
(46)
(46) ) with parameters values given in Table . All computations are executed by MATLAB.
Table 1. The data of system (Equation42 (42)
(42)  (42) )–(Equation46
(42) )–(Equation46 (46)
(46)  (46) ).
(46) ).
Firstly, the effect of parameter b on the qualitative behaviour of the system will be discussed below. We assume . We consider three different initial conditions as
IC1:
and
IC2:
and
IC3:
and
We use two sets of values of parameter b to get the following:
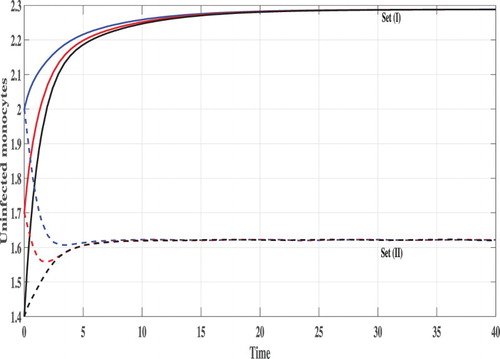
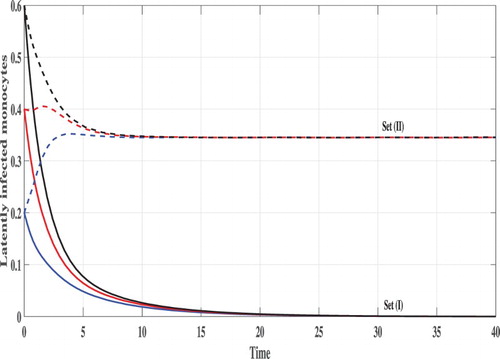
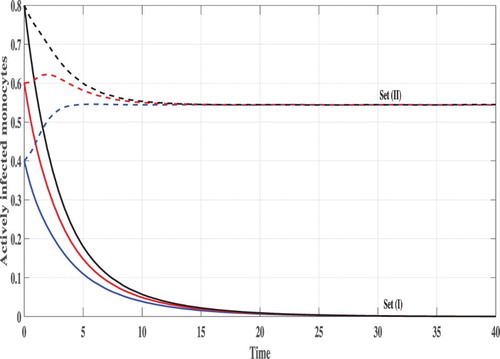
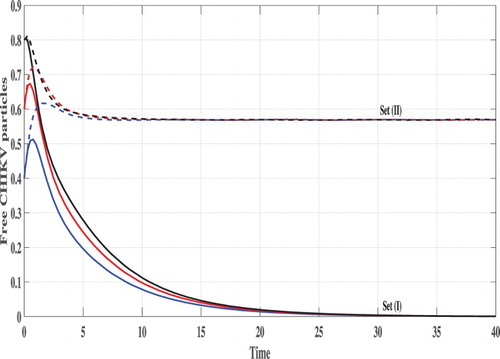
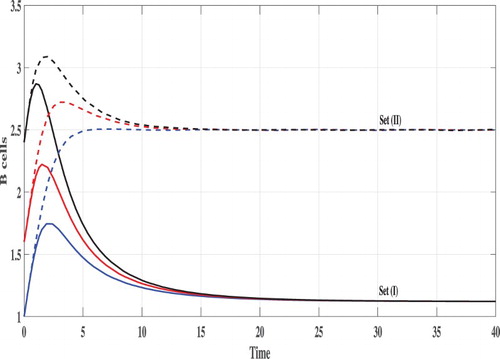
Set (I): We choose Using these data, we compute
From Figures , we can see that, the concentration of uninfected monocytes are increasing and tends to its normal value (the case when there is no CHIKV)
, while the concentrations of latently infected monocytes, actively infected monocytes and the CHIKV paticles are decreasing and tend to zero. As a result the proliferation rate of the B cells i.e. cBV will tend to zero and then the concentration of the B cells tends to its normal value (the case when there is no CHIKV)
. Thus, the solutions of the system eventually lead to the CHIKV-free steady state
for all the three initial conditions IC1–1C3. This result is consistent with the result of Theorem 2.1 that
is GAS. In this case, the CHIKV will be eliminated from the body.
Set (II): We take Then, we compute
It means that, the system has two positive steady states
and
where
is GAS. Figures – show that the concentration of uninfected monocytes are decreasing, while the concentrations of latently infected monocytes, actively infected monocytes and the CHIKV paticles are increasing. The increasing of the CHIKV paticles will increase the proliferation rate of the B cells and then the concentration of the B cells are also increased. The states of the system converge to the steady state
for all the three initial conditions IC1–IC3. This confirms the result of Theorem 2.2.
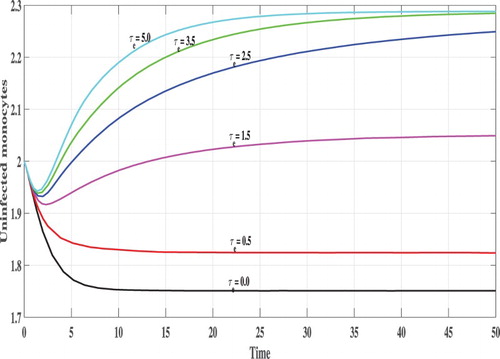
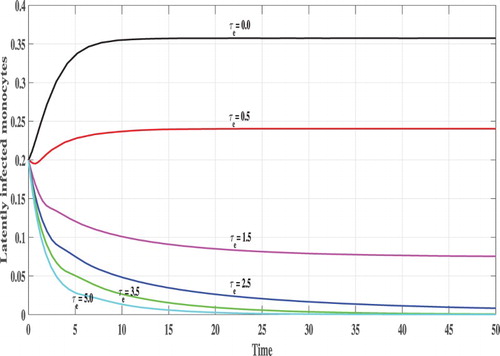
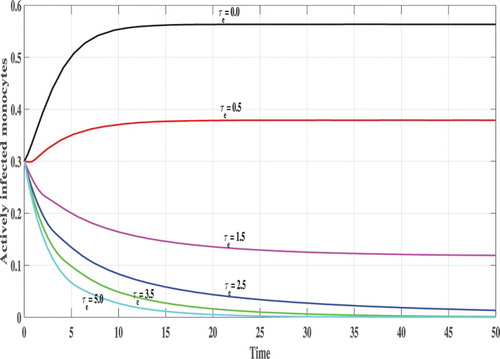
Next, we investigate the effect of time delays on the stability of the steady states. We vary the parameter and fix b=2.5. The following initial conditions are used:
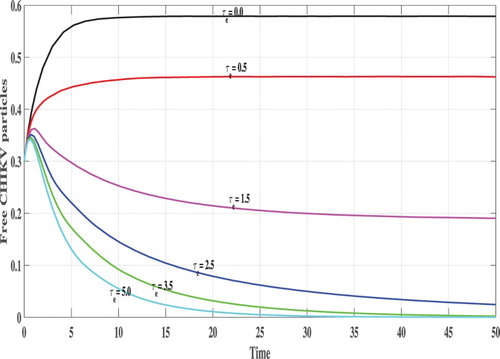
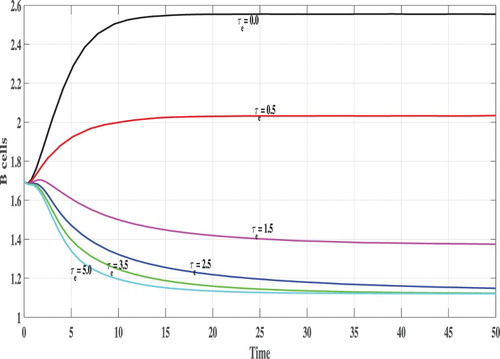
In Figures , we show the evolution of the states of system S, L, I, V and B with respect to the time. We can see that, for lower values of
e.g.
, 0.5, 1.0 and
the corresponding values of
satisfy
and the trajectory of the system tends to the steady states
. This confirms the results of Theorem 2.2 that
is GAS. On the other hand, when
becomes higher e.g.
and
then
, and the system has one positive steady state
which confirms the results of Theorem 2.1 that
is GAS. In this case, the CHIKV particles are cleared from the body.
Let be the critical value of the parameter
, such that
From Table we obtain
. The value of
for different values of
are listed in Table . We can observed that as
is increased then
is decreased. Moreover, we have the following cases:
if
, then
exists and it is GAS,
if
, then
is GAS. It is clearly seen that, an increasing in time delay will stabilize the system around
. In terms of biology, the time delay has a similar effect as the antiviral treatment which can be used to eliminate the CHIKV. We observe that, sufficiently large periods of delay reduce CHIKV replication and also clear the virus.
Table 2. The values of steady states, 
 for model (Equation42
for model (Equation42 (42)
(42)  (42) )–(Equation46
(42) )–(Equation46 (46)
(46)  (46) ) with different values of
(46) ) with different values of 
 .
.
5. Conclusion and discussion
In this paper, we have studied two within-host CHIKV dynamics models with antibody immune response. The incidence rate between the CHIKV and the uninfected monocytes has been modelled by a general nonlinear function Ψ. We have incorporated intracellular discrete or distributed time delays into the model. We have considered two types of infected monocytes, latently infected monocytes (such monocytes contain the CHIKV but are not producing it) and the actively infected monocytes (such monocytes are producing the CHIKV). We have shown that, the solutions of each model are nonnegative and ultimate bounded which ensure the well-posedness of the model. We have derived the basic reproduction number for the model. Sufficient conditions on Ψ and
are established which guarantee the existence and global stability of the two steady states, CHIKV-free steady state
and endemic steady state
. We have investigated the global stability of the steady states of the model by using Lyapunov method and LaSalle's invariance principle. We have conducted numerical simulations with a specific choice of the function Ψ and have shown that both the theoretical and numerical results are consistent.
The results show that, for the CHIKV infection model, the time delay has a significant effect on both global asymptotic properties of and global asymptotic properties of
. This is due to the dependence of the parameter
on the time delay. For model (Equation42
(42)
(42) )–(Equation46
(46)
(46) )
is given by
which is a decreasing function in both
and
. It has a clear meaning that, if the length of the delay is large, infected monocytes may die before it spreads CHIKV due to the small survival probability in the delay period between the infection phase and the CHIKV-producing phase. Clearly,
, where
is the basic reproduction number of system (Equation42
(42)
(42) )–(Equation46
(46)
(46) ) without delay. It follows that, the system with delay is more stabilizable abound
than that without delay.
Acknowledgments
This article was funded by the Deanship of Scientific Research (DSR) at King Abdulaziz University, Jeddah. The authors, therefore, acknowledge with thanks DSR for technical and financial support.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- A. Alshorman, X. Wang, J. Meyer, and L. Rong, Analysis of HIV models with two time delays, J. Biol. Dyn. 11(S1) (2017), pp. 40–64. doi: 10.1080/17513758.2016.1148202
- C. Connell McCluskey and Y. Yang, Global stability of a diffusive virus dynamics model with general incidence function and time delay, Nonlinear Anal. 25 (2015), pp. 64–78. doi: 10.1016/j.nonrwa.2015.03.002
- Y. Dumont and F. Chiroleu, Vector control for the chikungunya disease, Math. Biosci. Eng. 7 (2010), pp. 313–345. doi: 10.3934/mbe.2010.7.313
- Y. Dumont and J.M. Tchuenche, Mathematical studies on the sterile insect technique for the chikungunya disease and aedes albopictus, J. Math. Biol. 65(5) (2012), pp. 809–854. doi: 10.1007/s00285-011-0477-6
- Y. Dumont, F. Chiroleu, and C. Domerg, On a temporal model for the chikungunya disease: Modeling, theory and numerics, Math. Biosci. 213 (2008), pp. 80–91. doi: 10.1016/j.mbs.2008.02.008
- A.M. Elaiw, Global properties of a class of HIV models, Nonlinear Anal. 11 (2010), pp. 2253–2263. doi: 10.1016/j.nonrwa.2009.07.001
- A.M. Elaiw, Global properties of a class of virus infection models with multitarget cells, Nonlinear Dyn. 69(1–2) (2012), pp. 423–435. doi: 10.1007/s11071-011-0275-0
- A.M. Elaiw, Global stability analysis of humoral immunity virus dynamics model including latently infected cells, J. Biol. Dyn. 9(1) (2015), pp. 215–228. doi: 10.1080/17513758.2015.1056846
- A.M. Elaiw and N.A. Almuallem, Global dynamics of delay-distributed HIV infection models with differential drug efficacy in cocirculating target cells, Math. Methods Appl. Sci. 39 (2016), pp. 4–31. doi: 10.1002/mma.3453
- A.M. Elaiw and N.H. AlShamrani, Global properties of nonlinear humoral immunity viral infection models, Int. J. Biomath. 8(5) (2015). Article ID 1550058. doi: 10.1142/S1793524515500588
- A.M. Elaiw and N.H. AlShamrani, Global stability of humoral immunity virus dynamics models with nonlinear infection rate and removal, Nonlinear Anal. 26 (2015), pp. 161–190. doi: 10.1016/j.nonrwa.2015.05.007
- A.M. Elaiw and N.H. AlShamrani, Stability of a general delay-distributed virus dynamics model with multi-staged infected progression and immune response, Math. Methods Appl. Sci. 40(3) (2017), pp. 699–719. doi: 10.1002/mma.4002
- A.M. Elaiw and N.H. AlShamrani, Stability of latent pathogen infection model with adaptive immunity and delays, J. Integr. Neurosci 2018. DOI:10.3233/JIN-180087.
- A.M. Elaiw and A.A. Raezah, Stability of general virus dynamics models with both cellular and viral infections and delays, Math. Methods Appl. Sci. 40(16) (2017), pp. 5863–5880. doi: 10.1002/mma.4436
- A.M. Elaiw and S.A. Azoz, Global properties of a class of HIV infection models with Beddington-DeAngelis functional response, Math. Methods Appl. Sci. 36 (2013), pp. 383–394. doi: 10.1002/mma.2596
- A.M. Elaiw, I.A. Hassanien, and S.A. Azoz, Global stability of HIV infection models with intracellular delays, J. Korean Math. Soc. 49(4) (2012), pp. 779–794. doi: 10.4134/JKMS.2012.49.4.779
- A.M. Elaiw, A. Raezah, and A.S. Alofi, Effect of humoral immunity on HIV-1 dynamics with virus-to-target and infected-to-target infections, AIP Adv. 6(8) (2016). Article ID 085204. doi: 10.1063/1.4960987
- A.M. Elaiw, N.H. AlShamrani, and K. Hattaf, Dynamical behaviors of a general humoral immunity viral infection model with distributed invasion and production, Int. J. Biomath. 10(3) (2017). Article ID 1750035. doi: 10.1142/S1793524517500358
- A.M. Elaiw, A.A. Raezah, and K. Hattaf, Stability of HIV-1 infection with saturated virus-target and infected-target incidences and CTL immune response, Int. J. Biomath. 10(5) (2017). Article ID 1750070. doi: 10.1142/S179352451750070X
- A.M. Elaiw, N.H. AlShamrani, and A.S. Alofi, Stability of CTL immunity pathogen dynamics model with capsids and distributed delay, AIP Adv. 7 (2017). Article ID 125111.
- A.M. Elaiw, A. Raezah, and A. Alofi, Stability of a general delayed virus dynamics model with humoral immunity and cellular infection, AIP Adv. 7(6) (2017). Article ID 065210. doi: 10.1063/1.4989569
- A.M. Elaiw, E. Kh. Elnahary, and A.A. Raezah, Effect of cellular reservoirs and delays on the global dynamics of HIV, Adv. Difference Equ. 2018 (2018), p. 85. doi: 10.1186/s13662-018-1523-0
- A.M. Elaiw, A.A. Raezah, and B.S. Alofi, Dynamics of delayed pathogen infection models with pathogenic and cellular infections and immune impairment, AIP Adv. 8 (2018). Article ID 025323.
- L. Gibelli, A. Elaiw, M.A. Alghamdi, and A.M. Althiabi, Heterogeneous population dynamics of active particles: Progression, mutations, and selection dynamics, Math. Models Methods Appl. Sci. 27 (2017), pp. 617–640. doi: 10.1142/S0218202517500117
- J.K. Hale and S.M. Verduyn Lunel, Introduction to Functional Differential Equations, Springer Verlag, New York, 1993.
- K. Hattaf, N. Yousfi, and A. Tridane, Mathematical analysis of a virus dynamics model with general incidence rate and cure rate, Nonlinear Anal. Real World Appl. 13(4) (2012), pp. 1866–1872. doi: 10.1016/j.nonrwa.2011.12.015
- G. Huang, Y. Takeuchi, and W. Ma, Lyapunov functionals for delay differential equations model of viral infections, SIAM J. Appl. Math. 70(7) (2010), pp. 2693–2708. doi: 10.1137/090780821
- D. Huang, X. Zhang, Y. Guo, and H. Wang, Analysis of an HIV infection model with treatments and delayed immune response, Appl. Math. Model. 40(4) (2016), pp. 3081–3089. doi: 10.1016/j.apm.2015.10.003
- X. Li and S. Fu, Global stability of a virus dynamics model with intracellular delay and CTL immune response, Math. Methods Appl. Sci. 38 (2015), pp. 420–430. doi: 10.1002/mma.3078
- B. Li, Y. Chen, X. Lu, and S. Liu, A delayed HIV-1 model with virus waning term, Math. Biosci. Eng. 13 (2016), pp. 135–157. doi: 10.3934/mbe.2016.13.135
- X. Liu and P. Stechlinski, Application of control strategies to a seasonal model of chikungunya disease, Appl. Math. Model. 39 (2015), pp. 3194–3220. doi: 10.1016/j.apm.2014.10.035
- S. Liu and L. Wang, Global stability of an HIV-1 model with distributed intracellular delays and a combination therapy, Math. Biosci. Eng. 7(3) (2010), pp. 675–685. doi: 10.3934/mbe.2010.7.675
- K.M. Long and M.T. Heise, Protective and pathogenic responses to Chikungunya virus infection, Curr. Trop. Med. Rep. 2(1) (2015), pp. 13–21. doi: 10.1007/s40475-015-0037-z
- F.M. Lum and L.F.P. Ng, Cellular and molecular mechanisms of Chikungunya pathogenesis, Antiviral Res. 120 (2015), pp. 165–174. doi: 10.1016/j.antiviral.2015.06.009
- C.A. Manore, K.S. Hickmann, S. Xu, H.J. Wearing, and J.M. Hyman, Comparing dengue and chikungunya emergence and endemic transmission in A. aegypti and A. albopictus, J. Theoret. Biol. 356 (2014), pp. 174–191. doi: 10.1016/j.jtbi.2014.04.033
- C. Monica and M. Pitchaimani, Analysis of stability and Hopf bifurcation for HIV-1 dynamics with PI and three intracellular delays, Nonlinear Anal. 27 (2016), pp. 55–69. doi: 10.1016/j.nonrwa.2015.07.014
- D. Moulay, M. Aziz-Alaoui, and M. Cadivel, The chikungunya disease: Modeling, vector and transmission global dynamics, Math. Biosci. 229 (2011), pp. 50–63. doi: 10.1016/j.mbs.2010.10.008
- D. Moulay, M. Aziz-Alaoui, and H.D. Kwon, Optimal control of chikungunya disease: Larvae reduction, treatment and prevention, Math. Biosci. Eng. 9 (2012), pp. 369–392. doi: 10.3934/mbe.2012.9.369
- A.U. Neumann, N.P. Lam, H. Dahari, D.R. Gretch, T.E. Wiley, T.J Layden, and A.S. Perelson, Hepatitis C viral dynamics in vivo and the antiviral efficacy of interferon-alpha therapy, Science 282 (1998), pp. 103–107. doi: 10.1126/science.282.5386.103
- P.K. Roy, A.N. Chatterjee, D. Greenhalgh, and Q.J.A. Khan, Long term dynamics in a mathematical model of HIV-1 infection with delay in different variants of the basic drug therapy model, Nonlinear Anal. 14 (2013), pp. 1621–1633. doi: 10.1016/j.nonrwa.2012.10.021
- X. Shi, X. Zhou, and X. Son, Dynamical behavior of a delay virus dynamics model with CTL immune response, Nonlinear Anal. 11 (2010), pp. 1795–1809. doi: 10.1016/j.nonrwa.2009.04.005
- H. Shu, L. Wang, and J. Watmough, Global stability of a nonlinear viral infection model with infinitely distributed intracellular delays and CTL imune responses, SIAM J. Appl. Math. 73(3) (2013), pp. 1280–1302. doi: 10.1137/120896463
- Y. Wang and X. Liu, Stability and Hopf bifurcation of a within-host chikungunya virus infection model with two delays, Math. Comput. Simul. 138 (2017), pp. 31–48. doi: 10.1016/j.matcom.2016.12.011
- L. Wang, M.Y. Li, and D. Kirschner, Mathematical analysis of the global dynamics of a model for HTLV-I infection and ATL progression, Math. Biosci. 179 (2002), pp. 207–217. doi: 10.1016/S0025-5564(02)00103-7
- K. Wang, A. Fan, and A. Torres, Global properties of an improved hepatitis B virus model, Nonlinear Anal. 11 (2010), pp. 3131–3138. doi: 10.1016/j.nonrwa.2009.11.008
- X. Wang, S. Tang, X. Song, and L. Rong, Mathematical analysis of an HIV latent infection model including both virus-to-cell infection and cell-to-cell transmission, J. Biol. Dyn. 11(S2) (2017), pp. 455–483. doi: 10.1080/17513758.2016.1242784
- J. Wang, J. Lang, and X. Zou, Analysis of an age structured HIV infection model with virus-to-cell infection and cell-to-cell transmission, Nonlinear Anal. 34 (2017), pp. 75–96. doi: 10.1016/j.nonrwa.2016.08.001
- L. Yakob and A.C. Clements, A mathematical model of chikungunya dynamics and control: The major epidemic on Reunion Island, PLoS One 8 (2013), p. e57448. doi: 10.1371/journal.pone.0057448