?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
In this paper, we study a more general diffusive spatially dependent vaccination model for infectious disease. In our diffusive vaccination model, we consider both therapeutic impact and nonlinear incidence rate. Also, in this model, the number of compartments of susceptible, vaccinated and infectious individuals are considered to be functions of both time and location, where the set of locations (equivalently, spatial habitats) is a subset of with a smooth boundary. Both local and global stability of the model are studied. Our study shows that if the threshold level
the disease-free equilibrium
is globally asymptotically stable. On the other hand, if
then there exists a unique stable disease equilibrium
. The existence of solutions of the model and uniform persistence results are studied. Finally, using finite difference scheme, we present a number of numerical examples to verify our analytical results. Our results indicate that the global dynamics of the model are completely determined by the threshold value
.
1. Introduction
Mathematical and epidemiological models are important tools for analysing various real world phenomena in health science and epidemiology. For infectious diseases, many mathematical and epidemiological models have been studied by researchers to understand the effect of vaccination for controlling the spread of infectious diseases. Diffusive vaccination models are useful models for analysing the impact of vaccination for infectious diseases. Moreover, diffusive vaccination models are useful for getting information about how to control the reasoning individuals.
It is known that vaccine works with the immune system. Evidently as the disease can not provide immunity, so not the vaccination. As a result, most of the diseases have a recovered/immune stage for which vaccination is successful. Some other bacteria can remain in the host without causing any disease. This scenario is called carriage. The following SIS model, a model where recovery is short lived, that is, brings the individuals return to the susceptible class is considerable in this action with vaccination [Citation22]:
where S, V, I are the number of compartments of susceptible individuals, vaccinated individuals and infectious individuals at time t, respectively. a is the recruitment rate of susceptible individuals,
and
are the transmission probabilities of susceptible and vaccinated individuals, the parameter b is the average number of contact partners, n is the vaccination coverage of susceptible individuals, m is the natural death. Since the model monitors population dynamics, it follows that all it's dependent variables and parameters must be non-negative. Further, it is assumed that the prevalent disease does not kill infectious individuals, and treatment does not offer permanent immunity.
Periodic fluctuations occurs in many infectious diseases. Such periodic fluctuations may be driven by extrinsic factors, as reflected in periodic transmission rates, e.g. seasonality [Citation4, Citation20, Citation27], or may be caused by time delays [Citation13], age structure [Citation26], or non-linearity of incidence rates [Citation34]. In the above SIS model, the incidence rate is bilinear, and is given by . The bilinear model generally admits a trivial equilibrium (I = 0) corresponding to the case in which the disease is not present. It also may admit a stable non-trivial equilibrium corresponding to the situation in which the disease is maintained. Wilson and Worcester [Citation34] were the first to consider the more general incidence rate with a factor
and their primarily goal was to investigate the consequences of various assumptions when the laws are not known. In 1969, Severo [Citation28] considered a more general bilinear form
with q<1. Severo [Citation28] also considered a nonlinear recovery rate. Capasso and Serio [Citation7] generalized the incidence rate by considering the bilinear term of the form
with the condition
positive and finite. The model of Capasso and Serio [Citation7] excludes the form
if
Cunningham [Citation33] pointed out that there may exist periodic solutions in a model with an incidence rate
with
In 1986 and 1987 respectively, Liu et. al. [Citation18, Citation19] considered some general incidence rates. They also analysed the conditions under which a Hopf bifurcation occurs for a stable periodic solution and they discussed possible mechanisms for underlying nonlinear incidence rates of the following system
The authors also suggested to consider other forms for the incidence rate and the effects of disease-induced mortality.
In recent years, many other mathematical and epidemiological models have been studied by researchers with different types of interesting incidence rates. Gumel and Moghadas [Citation10] studied the following deterministic epidemic model with non-linear incidence
(1)
(1)
In the above model, the authors introduced the parameter c, the therapeutic treatment coverage of infectious individuals
removed to
compartment. Note that the above model is an SIS model and it was shown that the effectively treated infectious individuals return to the susceptible compartments and behaves similarly. The authors also observed realistically that
from the fact that vaccination can reduce or eliminate the incidence of infection. Also, Gumel and Moghadas [Citation10] analysed the corresponding characteristic equation and studied the local stability of its disease-free and disease equilibria and the optimal vaccine coverage threshold needed for disease control and eradication analytically. In 2014, Buonomo et al. [Citation5] constructed suitable Lyapunov functions and established global stability of disease-free and disease equilibrium of the above system (Equation1
(1)
(1) ) by using LaSalle's invariance principle [Citation16]. The authors also presented optimal vaccination and treatment strategies to minimize both the disease burden and intervention.
Recently, many researchers have considered spatial structure as a central factor because it affects the spatial spreading of disease [Citation1, Citation2, Citation6, Citation14, Citation15, Citation24, Citation38, Citation39]. In this paper, we propose a spatially dependent vaccination model which is a diffusive version of the above model (Equation1(1)
(1) ), where we consider the individual movements of all three compartment cells. We strongly believe that our proposed model is a more general and realistic biological and epidemiological model. Throughout the paper, we use the following notation for simplicity:
and
In the following, we present our proposed spatially dependent vaccination model with nonlinear incidence
(2)
(2)
with the following initial values
(3)
(3)
and the zero-flux Neumann boundary conditions
(4)
(4)
where
denotes the outward normal on
The Neumann boundary conditions imply that the populations do not move across the boundary
or the population going out and coming in are equal on the boundary. It is also noted that
are the number of compartments of susceptible individuals, vaccinated individuals and infectious individuals at time t>0 and in location
, respectively. The notion Ω is a spatial habitat in
with a smooth boundary
, Δ is the usual Laplacian Operator, and
and
are the diffusion rates of susceptible, vaccinated and infectious compartments respectively. Since the model monitors dynamics of population, it follows that all its dependent variables and parameters, for examples,
and
must be non-negative as in the non-spatial model (Equation1
(1)
(1) ). We also set the upper bound of c as
, which can be found in the proof of Lemma A.1.
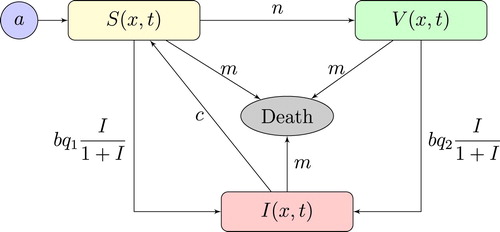
A schematic representation of the model (Equation2(2)
(2) ) is shown in the following Figure .
One of the fundamental issues in the study of infectious diseases via mathematical and epidemiological models is to find the stability of the two constant equilibria, that is, disease-free equilibrium and disease equilibrium. In this paper, we study both local and global stability of our model. Our study shows that if the threshold level the disease-free equilibrium
is globally asymptotically stable. On the other hand, if
then there exists a unique stable disease equilibrium
. The existence of solutions of the model and the uniform persistence results for the model are studied. Finally, using finite difference scheme, we present a number of numerical examples to verify our analytical results. Our results indicate that the global dynamics of the model are completely determined by the threshold value
The paper is organized in the following manner. In Section 2, we present disease-free and disease equilibrium respectively. Moreover, we present basic reproduction number in Section 2. We present our main results in Section 3. In Section 4, we present a number of numerical examples to verify our analytical results using finite difference scheme. Bifurcation results are also supported with parameter varying graphs. In section 5, we present existence and uniqueness of the solution of the system (Equation2(2)
(2) ), local and global steady states along with responsible constraints are presented. Uniform persistence theorems for the model (Equation2
(2)
(2) ) are also highlighted as an interplay of our study. Finally, Section 6 discloses the summary of the results.
2. Preliminaries
For a deep look in the dynamics of the system (Equation2(2)
(2) ), in this section, we will keep an eye on the basic reproduction number, the expected number of secondary cases reproduced by one infected individual in its entire infectious period.
2.1. Disease-free equilibrium
To define the disease-free equilibrium of the system (Equation2
(2)
(2) ), we write the diffusion rates
, since disease-free equilibrium state is not spatially dependent; then
It is noted that for the disease-free equilibrium, we consider the count of compartments of infectious individuals
. Then we find,
This gives the disease-free equilibrium:
(5)
(5)
Let us now find the disease equilibrium of the governing system (Equation2
(2)
(2) ).
2.2. Disease equilibrium
In the case of equilibrium state, we have the disease equilibrium , where the diffusion rates
. Then we write (Equation2
(2)
(2) ) as
(6)
(6)
Here, the number of compartments of infectious individuals
. Then, we find the count of susceptible individuals in the form
(7)
(7)
and the vaccinated individuals
(8)
(8)
Then, for the count of infectious individuals, we get the following polynomial of degree two
(9)
(9)
where
The real positive roots of (Equation9
(9)
(9) ) define the count of infectious individuals
; where the constant term of the quadratic Equation (Equation9
(9)
(9) )
is negative when
.
Thereby, when , we get the unique disease equilibrium
of the model (Equation2
(2)
(2) ).
Now, from (Equation7(7)
(7) ) and (Equation8
(8)
(8) ) we claim that
and similarly for
The proof of these claims are given in Lemma A.1 in Appendix.
2.3. Basic reproduction number
The Jacobian matrix of the linearized model (Equation2(2)
(2) ) at
is:
with eigenvalues
and
. Since all the model parameters are positive, it can be easily observed that
. Thus, the equilibrium
is locally asymptotically stable provides
. Hence, by the definition of basic reproduction number [Citation3],
of (Equation2
(2)
(2) ) is
(10)
(10)
For the sake of comprehension and clarity, we state our key results in the following section.
3. Main results
Theorem 3.1
Assume that . Then for any given initial data
, system (Equation2
(2)
(2) )–(Equation4
(4)
(4) ) has a unique solution
on
and further the solution semiflow
, has a global compact attractor in
.
Theorem 3.2
When
, the disease-free equilibrium
of the system (Equation2
(2)
(2) ) is locally asymptotically stable;
When
, the disease equilibrium
of the system (Equation2
(2)
(2) ) is locally asymptotically stable.
Theorem 3.3
If , then the disease-free equilibrium
of system (Equation2
(2)
(2) ) is globally asymptotically stable.
Theorem 3.4
If , then the disease equilibrium
of system (Equation2
(2)
(2) ) is globally asymptotically stable if c = 0 or when the integral
is non-positive or is dominated by negative values in the responsible Lyapunov function.
Remark 3.1
See the last part of the proof of Theorem 3.4 in Section 5. The result in [Citation5] that corresponds to Theorem 3.4, and on whose proof the proof of Theorem 3.4 is based, simply requires
Theorem 3.5
Assume that . If
, then there exists a constant
such that for any
with
, we have
The proofs of the Theorems 3.1–3.5 are formulated through a series of steps in the Section 5.
At this stage, first we want to justify all the key results by considering several numerical examples.
4. Examples and applications
For numerical verification for our analytic work, we choose finite-difference method based on Crank-Nicolson implicit time difference [Citation8, Citation17].
We can nicely observe the simulation part of the model (Equation2(2)
(2) ) by using some graphical presentations. We take the initial conditions as:
and the boundary condition is:
Let us assume the diffusion rates
.
Example 4.1
Let set the system parameters as followings:
Then the formula (Equation10
(10)
(10) ) gives us the basic reproduction number as
. Of course, Theorem 3.3 ensures that, these values of parameters lead us to the disease-free equilibrium results as shown in Figure .
Figure 2. Disease free equilibrium of the model (Equation2(2)
(2) ) with time and spatial domain.
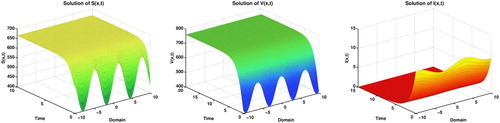
From the formula (Equation5(5)
(5) ), we can calculate our analytic values of disease-free equilibrium
and compare with the graphical interpretations to be accepted.
Example 4.2
Now let the system parameters are:
Then the formula (Equation10
(10)
(10) ) gives us the basic reproduction number as
which ensures by Theorem 3.4 that, these values of parameters leads us to the disease equilibrium results as shown in Figure .
Figure 3. Disease equilibrium of the model (Equation2(2)
(2) ) with time and spatial domain.
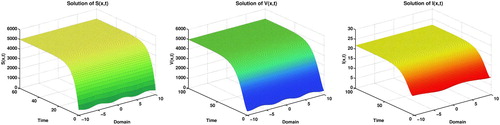
4.1. Parameter bifurcation observations
Now we are interested to know how the system (Equation2(2)
(2) ) responses for different values of the system parameters.
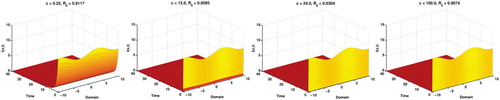
From these Figures (Figure ), we clearly see that the disease is being extincted faster as c is increasing. But when c is more than then c has no valuable effect for the disease for this parametric setup and more interestingly we get a cusp at
.
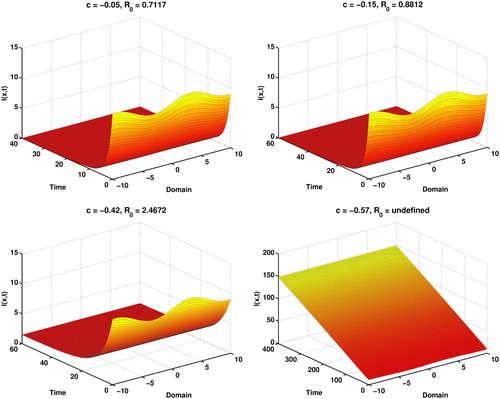
Though, in our system (Equation2(2)
(2) ) we assumed c to be non-negative anyhow; but if the disease causing environment still predominates, then we may consider c to be negative, for example,
. And in that scenario, we get the following results,
Figure shows that, if c is negative i.e. in disease causing environment when basic reproduction number , then it is a disease-free equilibrium while
reveals disease equilibrium. We also see the infection is increasing in a constant rate very roughly when
is undefined in the case of
.
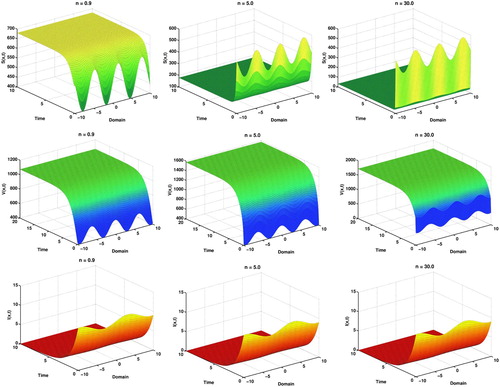
Here, in Figure , we clearly observe the impacts of vaccination coverage parameter n over susceptible and vaccinated
individuals. Susceptible
count converges to a minimum level and vaccinated
count increases to a maximum level as n growing large. But infectious
count remains approximately same for each cases.
5. Auxiliary results and proofs
5.1. Existence and uniqueness of solution
In this portion, we prove the existence and uniqueness of the solution of the system (Equation2(2)
(2) ) by learning the algorithm partially from a similar study of Xu et al. [Citation36].
Let us denote the subset of with vectors
as
and
be a Banach space with the supremum norm
. Also we define
then
is a strongly ordered space. Suppose that
is the
semigroups associated with
and
subject to the Neumann boundary conditions, respectively. Then it follows that for any
and
where,
are the Green functions associated with
, subject to the Neumann boundary conditions, respectively. It then follows from [Citation29] that the function
is compact and strongly positive. Particularly,
is a strongly continuous semigroup.
If is the generator of
, then
is a semigroup generated by the operator
which is defined on
. Now for any
, let us define
by:
Using these operators, we can write (Equation2
(2)
(2) )–(Equation4
(4)
(4) ) as the following integral equation
where,
It can also be rewritten as the following abstract differential equation
(11)
(11)
where,
and
.
Since is local Lipschitz continuous on
, it then follows that for any
, (Equation11
(11)
(11) ) admits a unique noncontinuous mild solution
such that
for all t in its maximum interval of existence. Moreover, it follows from ([Citation35], Corollary 2.2.5) that
is a class solution of (Equation2
(2)
(2) ) with Neumann boundary conditions (Equation4
(4)
(4) ) for all
. Further, by the scalar parabolic maximum principle, we see from the equation in (Equation2
(2)
(2) ) that
and
are all non-negative. Therefore, we obtain the following basic result on solution of the governing system (Equation2
(2)
(2) )–(Equation4
(4)
(4) ).
Lemma 5.1
For any initial value function , system (Equation2
(2)
(2) )–(Equation4
(4)
(4) ) has a unique solution
on
with
and
, where
.
Next, we show that the solution of the system (Equation2(2)
(2) )–(Equation4
(4)
(4) ) with the initial value function
actually exists globally, that is,
. To this end, we need the following result ([Citation21], Lemma 5.1).
Consider the following reaction-diffusion equation
(12)
(12)
where
and
are positive constants.
Lemma 5.2
The system (Equation12(12)
(12) ) admits a unique positive steady state
which is globally attractive in
.
Now we are ready to produce the proof of the Theorem 3.1.
Proof of Theorem 3.1.
By Lemma 5.1, the system (Equation2(2)
(2) )–(Equation4
(4)
(4) ) has a unique solution
on
and
for any
and
.
Now, let define the total population
(13)
(13)
and recall the primary assumption of Theorem 3.1 statement:
. Then
(14)
(14)
It follows from Lemma 5.2 that
is a global attractor for the reaction-diffusion Equation (Equation14
(14)
(14) ).
By (Equation14(14)
(14) ), for any
, we see that there exist some
such that
Now, according to (Equation13
(13)
(13) ), as the first equation of (Equation2
(2)
(2) ) is local Lipschitz continuous on
, it can easily be said that, for any
, there exist some
such that
Then by the similar argument as above, we also show that there are
, independent of the choice of
, and
, such that
Therefore, the non-negative solution of (Equation2
(2)
(2) )–(Equation4
(4)
(4) ) is ultimately bounded with respect to the maximum norm. This means that the solution semiflow
defined by
, is point dissipative. In view of [Citation35],
is compact for any
. Thus, [Citation11] implies that
, has a global compact attractor in
.
This completes the proof.
5.2. Analysis of local steady states
In this section, we want to explain the local stability of the equilibria for the system (Equation2(2)
(2) ). Thus we consider the proof of our second result, Theorem 3.2.
Proof of Theorem 3.2.
By linearizing the system (Equation2(2)
(2) ) at
, we get
where,
Then, we can obtain the following characteristic polynomial
where, λ is the eigenvalue which determines temporal growth,
is the
identity matrix and
is the wave-number [Citation24]. Then, we have
(15)
(15)
Now, it is clear that
It follows from
that
is locally asymptotically stable.
In the following, we prove the second part of the theorem. Linearizing the system (Equation2(2)
(2) ) at
, we obtain
where,
Then we obtain the following characteristic equation
(16)
(16)
where,
Now, let us take
then we can get
These lead us to the following conclusion
By the Routh-Hurwitz criterion, we know that all eigenvalues of (Equation16
(16)
(16) ) have negative real parts. It means that the disease equilibrium
of system (Equation16
(16)
(16) ) is locally asymptotically stable when
.
5.3. Global stability analysis
In this section, we investigate the global stability of the two constant equilibria in the case of a bounded domain Ω in which
is an arbitrary positive solution of the system (Equation2
(2)
(2) ). First, let us consider the following shortcuts for convenience
In case of global analysis, we consider the Lyapunov functional and the results varies with basic reproduction number. We stated two important results in the earlier Section 2.
At this phase, we are in stable setting to establish the Theorem 3.3 as long as the basic reporduction number .
Proof of Theorem 3.3.
Let define a Lyapunov function as
where,
Calculating the time derivative of
along the solution of (Equation2
(2)
(2) ) gives
Then from (Equation2
(2)
(2) ), we can write
But, as
, we can write
By Green's formula and Neumann boundary conditions (Equation4
(4)
(4) ), we get
(17)
(17)
Similarly,
(18)
(18)
Again, by Green's formula and the Neumann boundary conditions (Equation4
(4)
(4) ), we have the Green's first identity as
which implies
(19)
(19)
By the same arguments, we also can write
(20)
(20)
(21)
(21)
Then using the above arguments, we have
(22)
(22)
Recall the Equation (EquationA6
(A6)
(A6) ) which is described in the proof of Theorem A.1 (Appendix)
(23)
(23)
Since c>0, then using (Equation23
(23)
(23) ) the last integral of (Equation22
(22)
(22) ) satisfies
Hence,
whenever
.
And, when ; we calculate,
and vice-versa. Consequently, the singleton
is the greatest compact invariant set in
. Then, LaSalle's invariance principle [Citation12] refers to
; which means, whenever
, the disease-free equilibrium
is globally asymptotically stable. This establishes Theorem 3.3.
In a similar manner, it is stated that the disease equilibrium of (Equation2(2)
(2) ) is globally asymptotically stable and the proof is prescribed as follows:
Proof of Theorem 3.4.
Let us define a Lyapunov function as
where,
Calculating the time derivative of
along the solution of (Equation2
(2)
(2) ) gives
Then from (Equation2
(2)
(2) ), it can written as
(24)
(24)
Note that from (Equation6
(6)
(6) ), we have
and by substituting these in (Equation24
(24)
(24) ) yields
For writing convenience, let assume,
such that
Applying the Green's formula and zero Neumann boundary conditions, we obtain
(25)
(25)
We know the arithmetic mean is greater than or equal to the geometric mean. Consequently, for all
, we find
Moreover, if either c = 0 or
then
and the result is immediately proved. Rewrite
in the following form
For c>0, it is remarked that the outcome of the integral
can be either negative or non-negative depending on the sign of
and these two different scenarios are
| Case (a): |
| ||||
| Case (b): |
| ||||
When Case (a) is true for all , or for at-least large
or
, the situation is clearly in favour and the result is well established.
But for Case (b) to be true, the integral function coincides with our expected result if the rest part of (Equation25
(25)
(25) ) equates or dominates on
for all
, or for at-least large t or
.
Hence, the Equation (Equation25(25)
(25) ) reveals that,
for
. Since the above inequalities become equalities whenever
and hence
for
. Now, LaSalle's invariance principle [Citation12] refers to
which means, when
, the disease equilibrium
is globally asymptotically stable. This concludes the proof.
5.4. Uniform persistence
By linearizing the third equation of system (Equation2(2)
(2) ) at
, the disease-free equilibrium, we get the followings:
(26)
(26)
Then referring the arguments as in the proof of ([Citation6], Theorem 2.2), ([Citation24], Theorem 2), ([Citation12], Theorem 4.2), ([Citation21], Theorem 2.11), ([Citation32], Theorem 3.4), ([Citation36], Theorem 3.2), ([Citation29], Theorem 4.2); Yang et al. [Citation37] established the uniform persistence result for the respective system through the following procedure.
Setting , we get
(27)
(27)
Now substituting
and the values of
into (5.4) we obtain the principal eigenvalue of (Equation26
(26)
(26) )
corresponding to which there is the unique positive eigen-function
.
Thus, observing this equation we can claim the following lemma:
Lemma 5.3
The principal eigenvalue, has the same sign as
.
To claim the uniform persistence of the system (Equation2(2)
(2) )–(Equation4
(4)
(4) ), we now establish the following lemma and theorem using the similar arguments from [Citation37].
Lemma 5.4
If is the solution of the system (Equation2
(2)
(2) )–(Equation4
(4)
(4) ) with
, then
for any
, we always have
and
. Furthermore, we have
and
if there exists some
such that
is not true, then
.
Proof.
From the system (Equation2(2)
(2) ), it is clear that
and
in
for any
. Then,
Now applying ([Citation21], Lemma 1) and the comparison principle, we get
Then there exists a
such that
Consequently, the second equation of system (Equation2
(2)
(2) ) follows that
Finally, from the third equation of the system (Equation2
(2)
(2) ), we can write
By the strong maximum principle and the Hopf boundary Lemma [Citation25], this validates the second part.
After the completion of the above arguments, we obtain the results for disease persistence as described in Theorem 3.5 in Section 2. Now, it is time to produce the last result, Theorem 3.5 when the disease are persisting.
Proof of Theorem 3.5.
Let us assume that and also suppose
and
From Lemma 5.4, for any
, we get
, that is,
.
Let define and
be the omega limit set of the orbit
. Now, first, let us claim that
Since , we have
. Hence,
. From the first equation of system (Equation2
(2)
(2) ), we know that
uniformly for
. Hence
. It follows from Lemma 5.3 that
when
. By the continuity of
, there exists a sufficiently small positive number
such that
.
Let us now claim that is a uniform weak repeller for
in the sense that
Suppose, by contradiction, there exists
such that
Then there exists
such that
and
, for all
and
. Therefore,
satisfies
By Lemma 5.3, we conclude that
is the strongly positive eigenfunction corresponding to
. It follows from
for all
and
that there exists
such that
. Clearly,
is a solution of the following system
According to the comparison principle, we can obtain
This implies that
is unbounded, which is a contradiction.
Define a continuous function by
It is easy to see that
. Moreover, we conclude that if
or
and
, then
for all
. Thus,
is a generalized distance function for the semiflow
. It follows from the above discussion that any forward orbit of
in
converges to
. It is obvious that
is isolated in
and
. Further, there is no cycle in
from
to
. Applying ([Citation30], Theorem 3), there exists a
such that
Therefore,
Then by Lemma 5.4(i), the proof of this theorem is established.
Since Theorem A.1 from appendix proves existence of global solution for the system (Equation2(2)
(2) ) with distinct diffusion rates, the persistence theorem is also true for the system (Equation2
(2)
(2) ) where the diffusion rates
are not identical and we describe the following statement as a remark.
Remark 5.1
If , then there exists a constant
such that for any
with
, we have
6. Conclusion
In this manuscript, a spatially dependent vaccination model is proposed for infectious diseases. We have studied analytic inter-locution of disease-free equilibrium, disease equilibrium, basic reproduction number, existence and uniqueness of the solution of the corresponding system, local stability, global stability and uniform persistence theorem for the system. We present a number of numerical examples to verify our analytical results. It is shown that the numerical solution of the system corresponds to the analytical results. Our study may help to predict the upcoming probable results of treatments via vaccination and therapy against malignant diseases.
Acknowledgments
The author is grateful to the anonymous referees for their valuable comments and constructive suggestions to get the final version of the manuscript. The author M. Kamrujjaman research was partially supported by the University Grant Commission (UGC), year 2019-2020, Bangladesh.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- S. Ahmed, M. Kamrujjaman, and S. Rahman, Dynamics of a viral infectiology under treatment, J. Appl. Anal. Comput. 10(5) (2020), pp. 1800–1822.
- M.S. Alam, M. Kamrujjaman, and M.S. Islam, Parameter sensitivity and qualitative analysis of dynamics of ovarian tumor growth model with treatment strategy, J. Appl. Math. Phys. 8 (2020), pp. 941–955.
- R.M. Anderson and R.M. May, Infectious Diseases of Humans, Oxford University Press, London, 1991.
- J. L. Aron and I. B. Schwartz, Seasonality and period-doubling bifurcations in an epidemic model, J. Theor. Biol. 110 (1984), pp. 665–679.
- B. Buonomo, D. Lacitignola, and C. Vargar-De-Leon, Qualitative analysis and optimal control of an epidemic model with vaccination and treatment, Math. Comput. Simulation 100 (2014), pp. 88–120.
- R.S. Cantrell and C. Cosner, Spatial Ecology Via Reaction-Diffusion Equations, John Wiley and Sons. Ltd., 2003.
- V. Capasso and G. Serio, A generalization of the Kermack-McKendrick deterministic epidemic model, Math. Biosci. 42 (1978), pp. 41–61.
- L.C. Evans, Partial Differential Equations, AMS, Providence, 1998.
- W. Fitzgibbon, C. Martin, and J. Morgan, Uniform bounds and asymptotic behavior for a diffusive epidemic model with criss-cross dynamics, J. Math. Anal. Appl. 184 (1994), pp. 39–414.
- A.B. Gumel and S.M. Moghadas, A qualitative study of a vaccination model with non-linear incidence, App. Math. Comput. 143 (2003), pp. 409–419.
- J. Hale, Asymptotic Behavior of Dissipative Systems, American Mathematical Society, Providence, 1988.
- D. Henry, Geometric Theory of Semilinear Parabolic Equations, Springer, New York, 1981.
- H. W. Hethcote, H. W. Stech, and P. Van Den Driessche, Nonlinear oscillations in epidemic models, SIAM J. Appl. Math. 40(1) (1981), pp. 1–9.
- Z. Hossine, A.A. Megla, and M. Kamrujjaman, A short review and the prediction of tumor growth based on numerical analysis, Adv. Res. 19(1) (2019), pp. 1–10.
- J.I. Ira, Md. Shahidul Islam, J.C. Misra, and Md. Kamrujjaman, Mathematical modelling of the dynamics of tumor growth and its optimal control, Int. J. Ground Sediment & Water. (2020) in press.
- J.P. LaSalle, The Stability of Dynamical System. Regional Conference Series in Applied Mathematics, SIAM, Philadelphia, 1976.
- R.J. LeVeque, Numerical Methods for Conservation Laws, Birkhauser, Boston, Basel, Berlin, 1998.
- W.M. Liu, H.W. Hetchote, and S.A. Levin, Dynamical behavior of epidemiological models with non-linear incidence rates, J. Math. Biol. 25 (1987), pp. 359–380.
- W. Liu, S.A. Levin, and Y. Iwasa, Influence of nonlinear incidence rates upon the behavior of SIRS epidemiological models, J. Math. Biol. 23 (1986), pp. 187–204.
- W. P. London and J. A. Yorke, Recurrent outbreak of measles, chickenpox and mumps: I. seasonal variation in contact rates, Am. J. Epidemiol. 98 (1973), pp. 453–468.
- Y. Lou and X.-Q. Zhao, A reaction-diffusion malaria model with incubation period in the vector population, J. Math. Biol. 62 (2011), pp. 543–568.
- M. Martcheva, An Introduction to Mathematical Epidemiology, Springer, 2015.
- J. Morgan, Global existence for semilinear parabolic systems, SIAM J. Math. Anal. 20 (1989), pp. 1128–1144.
- J.D. Murray, Mathematical Biology, I and II, 3rd ed., Springer, New York, 2002.
- M.H. Protter and H.F. Weinberger, Maximum Principles in Differential Equations, Springer-Verlag, New York, 1984.
- D. Schenzle, An age-structured model of pre- and post-vaccination measles transmission, IMA J. Math. Appl. Med. Biol. 1(2) (1984), pp. 169–91.
- I. B. Schwartz, Multiple stable recurrent outbreaks and predictability in seasonally forced nonlinear epidemic models, J. Math. Biol. 21 (1985), pp. 347–361.
- N. C. Severo, Generalizations of some stochastic epidemic models, Math. Biosci. 4 (1969), pp. 395–402.
- H.L. Smith, Monotone Dynamical Systems: An Introduction to the Theory of Competitive and Cooperative Systems: Mathematical Surveys and Monographs, Amer. Math. Soc., 1995.
- H.L. Smith and X. Zhao, Robust persistence for semi-dynamical systems, Nonlinear Anal. TMA 47 (2001), pp. 6169–6179.
- J. Smoller, Shock Waves and Reaction-Diffusion Equations, Springer Science & Business Media, Vol. 258, 2012.
- W. Wang and X.-Q. Zhao, A nonlocal and time-delayed reaction-diffusion model of dengue transmission, SIAM. Appl. Math. 71 (2011), pp. 147–168.
- P. J. Wangersky and W.J. Cunningham, Time lag in Prey-Predator population models, Ecology 38 (1979), pp. 136–139.
- E. B. Wilson and J. Worcester, The law of mass action in epidemiology, Proc. Natl. Acad. Sci. U. S. A. 31(1) (1945), pp. 24–31.
- J. Wu, Theory and Applications of Partial Functional Differential Equations, Springer, New York, 1996.
- Z. Xu, Y. Xu, and Y. Huang, Stability and traveling waves of a vaccination model with nonlinear incidence, Comput. Math. App. 75 (2018), pp. 561–581.
- Y. Yang and S. Zhang, Dynamics of a diffusive vaccination model with nonlinear incidence, Comput. Math. Appl. 75(12) (2018), pp. 4355–4360.
- Y. Yang, J. Zhou, and C.-H. Hsu, Threshold dynamics of a diffusive SIRI model with nonlinear incidence rate, J. Math. Analy. App. 478(2) (2019), pp. 874–896.
- Y. Yang, L. Zou, J. Zhou, and C.-H. Hsu, Dynamics of a waterborne pathogen model with spatial heterogeneity and general incidence rate, Nonlinear Anal. Real World Appl. 53 (2020), p. 103065.
Appendix
Lemma A.1
For disease equilibrium , we claim that
when,
and
.
Proof.
Recall the disease-free equilibrium:
(A1)
(A1)
For endemic equilibrium, similarly we also recall the equations which counts
and
, respectively such that
(A2)
(A2)
(A3)
(A3)
Now, from (EquationA1
(A1)
(A1) ) and (EquationA2
(A2)
(A2) )
Then,
is equivalent to
Hence, we consider
where
and
is defined later in (EquationA5
(A5)
(A5) ). Thus, this condition indicates the inequality as
Next, it is time to show that
Similarly, from (EquationA1
(A1)
(A1) ), (EquationA2
(A2)
(A2) ) and (EquationA3
(A3)
(A3) ), we obtain
which yields
(A4)
(A4)
Introducing an inequality
and using the relation
, from the equation (EquationA4
(A4)
(A4) ), it is easy to show that
Finally, from the third equation of system (Equation6
(6)
(6) ), we get
Therefore,
(A5)
(A5)
Hence the proof is completed.
Now we are going to state and prove the Theorem 3.1 for distinct diffusion coefficients:
Theorem A.1
For any given initial data , system (Equation2
(2)
(2) )–(Equation4
(4)
(4) ) has a unique solution
on
and further the solution semiflow
, has a global compact attractor in
.
Proof.
By Lemma 5.1, the system (Equation2(2)
(2) )–(Equation4
(4)
(4) ) has a unique solution
on
and
for any
and
.
We want now to find the upper bound of that will be enough to complete the proof [Citation9, Citation23, Citation31]. First, we assume the following
We claim that Σ is invariant [Citation31]. To see this, we set
, where
Then successively, if
then
where
is defined in Lemma A.1 and
. Which implies,
(A6)
(A6)
Again if
then
Hence,
(A7)
(A7)
Now, we take
such that
Therefore,
(A8)
(A8)
Which proves that Σ is invariant [Citation9, Citation23, Citation31].
Therefore, the non-negative solutions of (Equation2(2)
(2) )–(Equation4
(4)
(4) ) are ultimately bounded with respect to the maximum norm. This means that the solution semiflow
defined by
, is point dissipative. In view of [[Citation35], Corollary 2.2.6],
is compact for any
. Thus, [[Citation11], Theorem 3.4.8] implies that
, has a global compact attract in
.
This completes the proof.
Glossary of Notation
| Ω | = | Bounded spatial habitat |
| = | Smooth boundary of bounded spatial habitat Ω | |
| = | Set of real numbers | |
| = | Set of ordered n-tuples of real numbers | |
| = | Basic reproduction number | |
| = | Disease-free equilibrium | |
| = | Disease equilibrium | |
| N | = | Total population |
| S | = | Number of susceptible individuals |
| V | = | Number of vaccinated individuals |
| I | = | Number of infectious individuals |
| a | = | Recruitment rate of susceptible individuals |
| b | = | Average number of contact partners |
| = | Transmission probability of susceptible individuals | |
| = | Transmission probability of vaccinated individuals | |
| m | = | Natural death |
| n | = | Vaccination coverage of susceptible individuals |
| c | = | Therapeutic treatment coverage of infected individuals |
| t | = | Time |
| = | Column vector or element of | |
| = | Nonlinear incidence rate | |
| = | ||
| = | ||
| = | Diffusion rates | |
| Δ | = | Laplacian Operator |
| ω | = | Outward normal to the boundary |
| J | = | Jacobian matrix |
| λ | = | Eigenvalue |
| = | Banach space | |
| = | Arbitrary norm | |
| = | Supremum norm | |
| Γ | = | Green function |
| = | Generator set | |
| Φ | = | Solution semiflow |
| V | = | Lyapunov function |