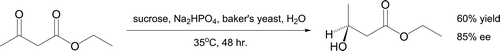Abstract
This article summarizes the wide scope of aqueous organic reactivity from a pedagogical perspective. It is aimed at university instructors with the goal of promoting water as a solvent in academic institutions. Recently, extensive industrial and scholarly research has concerned development of organic reactions in aqueous media. Many examples are now possible in the student laboratory including carbon–carbon/carbon–nitrogen bond-forming reactions and functional group transformations (e.g. oxidations, reductions, and halohydrations). Experiments are summarized from the educational literature (journal articles and laboratory textbooks) and their green features are described. Using water as solvent often promotes significant rate enhancements and operational simplicity – both of importance when training undergraduates during limited laboratory time. Environmental benefits of using water are additionally highlighted to students’ first hand in relation to the Twelve Principles of Green Chemistry.
Introduction
In introductory college courses, undergraduate chemists are often taught that water is a poor solvent in which to attempt organic reactivity. This instruction has its basis in two observations. The vast majority of organic compounds have limited water solubility, and several important reagents (e.g. Grignards, LiAlH4, and thionyl chloride) react with water, thus requiring an anhydrous environment for desired behavior. Yet, H2O is clearly a very high-profile substance (the American Chemical Society celebrated Earth Day in 2008 with the theme “Water: ‘Streaming Chemistry’” Citation1–3). Vigorous effort has shown that many organic reactions can in fact be conducted with water as solvent. Articles have focused on C–C bond-forming processes Citation4, organometallic chemistry Citation5 and stereoselective reactivity Citation6 under aqueous conditions. Lindström has recently organized a comprehensive review of organic reactions in water Citation7. Some transformations are inappropriate for teaching purposes as they employ lengthy reaction times and/or expensive catalysts that are difficult to prepare. However, pedagogical research has developed many student-friendly procedures showcasing water as the reaction medium Citation8. This review, written for university educators, summarizes these experiments and encourages their incorporation into undergraduate curricula at introductory or advanced levels.
Why should students perform reactions in H2O? From a green perspective, water has supreme advantages over organic solvents. It is environmentally benign, abundant, inexpensive and non-flammable. Life itself requires chemical bond formation under aqueous conditions. Additionally, despite reactant insolubility, water can promote pronounced rate enhancements and impressive reaction selectivities with concomitant reduced energy requirements. This is often attributable to hydrophobic effects Citation9 Citation10 where non-polar reactant molecules are forced together in the rate-determining transition state. Exposure to aqueous organic reactivity therefore educates undergraduates about the Twelve Principles of Green Chemistry Citation11 and the current drive to “use safer solvents and reaction conditions” and “increase energy efficiency.”
Experiments highlighted herein utilize water as the sole reaction solvent or as a major co-solvent (at least 50% of composition). Some date from before the green chemistry movement began and are included to illustrate the rich variety of chemistry possible. Reactions are organized under two broad themes: C–C/C–N bond-forming reactions and functional group transformations. Each theme is further sub-divided as follows: C–C/C–N bond-forming reactions are categorized by mechanistic type (nucleophilic addition, transition metal catalysis, pericyclic, radical, and electrophilic substitution). Functional group transformations are delineated by reaction type (oxidation, reduction, halohydration, etherification, and dehydration). Reported student yields or ranges of yields (where known), reaction times, and experimental conditions are highlighted along with other green chemistry features of note.
C–C and C–N bond formations in water
Nucleophilic addition reactions
Alkene synthesis
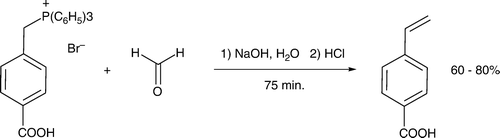
Several pedagogical procedures highlight attack of a nucleophilic carbon atom at an electrophilic center, often leading to alkene and/or alcohol products. Broos et al. Citation12 developed a Wittig reaction in water where 4-carboxybenzyltriphenylphosphonium bromide is deprotonated with sodium hydroxide and stirred with aqueous formaldehyde at room temperature. The product 4-vinylbenzoic acid is isolated in good yield and purity () and recrystallized from aqueous ethanol, engendering another green feature to the experiment.
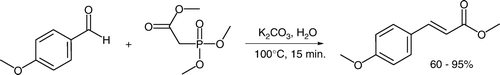
A variation of the Wittig reaction using phosphonate esters as the carbanion source (a Horner–Wadsworth–Emmons or Wittig–Horner reaction) proceeds efficiently in aqueous media Citation13. Rapid one-pot preparation of the sunscreen analog methyl trans-4-methoxycinnamate Citation14 Citation15 and 13 other aromatic cinnamate esters were realized (). These products are purified from ethanol or ethanol:water mixtures. The experiment utilizes potassium carbonate as a weak, environmentally benign base, avoids use of a phase-transfer catalyst Citation16 and forms trans-alkenes in a stereoselective fashion.
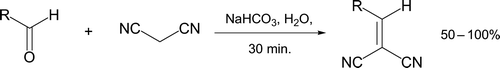
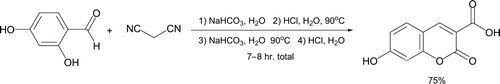
Recently, a Knoevenagel condensation between one of three aldehydes (furfural, 2-naphthaldehyde, or piperonal) and malononitrile was reported under inorganic base-catalyzed conditions in water (, Citation17). No organic solvents are required throughout the short procedure. The reaction proceeds with very intrinsic high atom economy as H2O is the only “wasted” by-product. Fringuelli et al. Citation18 described a related multi-step protocol where consecutive Knoevenagel and Pinner reactions followed by alternating basic and acidic hydrolysis steps leads to coumarin formation ().
A similar multi-step condensation progressing in water is the Hantzsch 1,4-dihydropyridine synthesis Citation19. Using ethanol as a co-solvent, the potent anti-oxidant diludine Citation20 Citation21 is prepared on a multi-gram scale by refluxing ethyl acetoacetate, aqueous ammonia, and aqueous formaldehyde for one hour (). The solid product is easily recrystallized from ethanol.
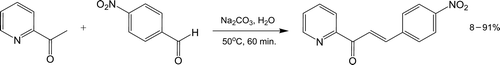
A popular reaction routinely discussed in introductory organic lectures and laboratories is the aldol condensation. Two recent experiments outline aldol reactions from a green perspective under solvent free Citation22 and organocatalytic conditions Citation23. A convenient crossed aldol condensation featuring an aromatic aldehyde and ketone has since been discussed (, Citation24). This stereoselective reaction employing a catalytic amount of sodium carbonate affords a trans-chalcone analog on heating in water, which is readily identifiable by proton NMR.
Alcohol synthesis
A didactic modification of the aldol condensation () is performance of the same reaction at room temperature (, Citation24). Under weakly basic conditions, the initially formed alcohol product (a β-hydroxyketone) is isolated in excellent yield. Undertaking both reactions within the same laboratory session underscores the role heat has in driving the elimination of water and introduces a “discovery” element to the experiment.

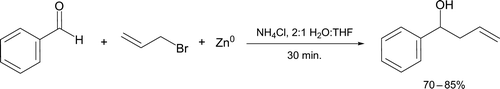
Classical organometallic reagents used in synthesis include those developed by Grignard and Gilman Citation25. It is often stressed to undergraduates that these species must be kept away from moisture (and other protic solvents) until an aqueous acidic work up is performed. Although Grignard reactions are common in the student laboratory, preparation of organometallic can be unreliable and prone to complete failure Citation26. However, Breton and Hughey Citation27 employed an organozinc species formed by reaction of allyl bromide with zinc metal in aqueous THF. This intermediate reacts efficiently with benzaldehyde to form a liquid secondary alcohol (1-phenyl-3-buten-1-ol) in a similar manner to a Grignard reagent (). The reaction mechanism is thought to initially involve an electron transfer from zinc metal to a molecule of allyl halide, forming a radical anion intermediate. This is followed by nucleophilic attack at the electrophilic carbon atom of benzaldehyde Citation28.
Oxazolidinone synthesis
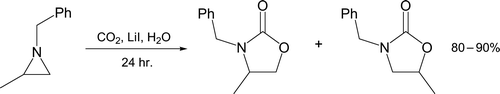
Carbon–nitrogen bond cleavage and formation in water is exemplified by preparation of an oxazolidinone from 1-benzyl-2-methylaziridine (, Citation29). The three-membered ring reacts with carbon dioxide and a variety of iodide salts under pressure to form isomeric products during two laboratory periods. Oxazolidinones are significant as chiral auxiliaries, ligands for metal catalysis, and recently as anti-bacterial agents Citation30–32. Students have an opportunity to probe the effect that different salts have (LiI, NaI, CsI, NH4I) on the ratio of oxazolidinone isomers.
Transition metal-catalyzed reactions
Pd(0)-catalyzed cross-couplings
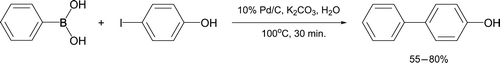
Many transition metal-mediated coupling reactions proceed effectively in an aqueous environment Citation33, a fact exploited by several educators. Palladium0-catalyzed processes have been of particular interest. The Suzuki reaction typically couples an aryl or vinyl halide with a boronic acid or boronic ester under basic conditions in the presence of catalytic Pd0 Citation34. An undergraduate Suzuki reaction was reported in 2001 utilizing Pd(OAc)2 and triphenylphosphine in combination with Na2CO3 as base and aqueous isopropanol as solvent Citation35, where Pd0 is generated in situ. Research indicates many such reactions are possible in pure water Citation36. A Suzuki cross-coupling reaction was designed to synthesize 4-phenylphenol, a biaryl component of important non-steroidal anti-inflammatory drugs (NSAIDs) Citation37. This approach employs water as the sole reaction solvent, features inexpensive palladium on carbon as the active catalyst and solid purification by recrystallization from aqueous methanol ().
Leadbeater has designed similar procedures in a commercial microwave reactor that have been introduced into the undergraduate curriculum Citation38. Additionally, a group project was implemented using an aqueous Suzuki reaction as the focal point Citation39 where students design a research proposal, undertake independent practical work, evaluate their results, and write a journal-style report.
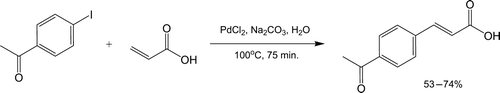
The closely related Heck reaction Citation40 couples an aryl halide with an electron-deficient alkene in the presence of Pd0. Refluxing a mixture of 4-iodoacetophenone and acrylic acid with palladium(II) chloride in aqueous Na2CO3 leads to stereoselective formation of trans-4-acetylcinnamic acid (, Citation41).
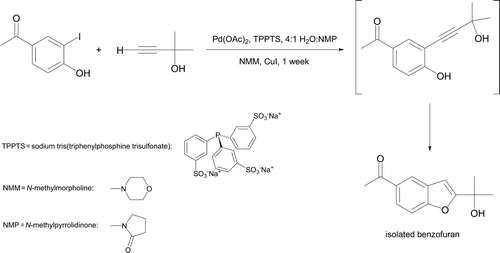
A Pd0-catalyzed Sonogashira reaction Citation42 between an aryl halide and a terminal alkyne in 4:1 water:N-methylpyrrolidinone as solvent has been described Citation43. This approach uses palladium(II) acetate with a water-solubilizing ligand (TPPTS) and a reaction time of one week at room temperature. Interestingly, the initial product cyclizes under the reaction conditions to form a benzofuran derivative (). Benzofuran rings are components of many biologically active substances, both synthetic and natural Citation44. This represents a significantly greener methodology toward benzofuran synthesis than more traditional approaches of heating reactants in pyridine or dimethylformamide as solvent Citation45 Citation46. Similarly, Harper et al. Citation47 utilized a water-soluble Pd(0) complex to catalyze reaction between diethyl phosphite and iodobenzene generating diethyl phenylphosphonate and a new carbon–phosphorus bond.
Ru(III) catalysis
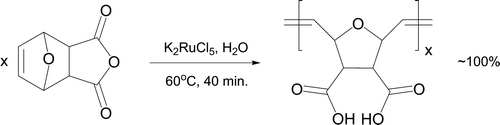
Ring-Opening Metathesis Polymerization (ROMP) reactions proceed readily and near quantitatively in water if the appropriate catalyst is selected Citation48. Ruthenium(III) salts, such as RuCl3 and K2RuCl5 are often utilized Citation49. The Diels–Alder adduct of furan and maleic anhydride (exo-7-oxabicyclo[2.2.l]hept-5-ene-2,3-dicarboxylic anhydride) undergoes an aqueous ROMP reaction in 40 minutes (). A functionalized polymer with high carboxyl content is formed that is soluble in polar solvents and characterized by IR, proton NMR, DSC and molecular weight measurements. The cis:trans polymeric ratio can be determined by 13C NMR. Water is thought to behave as a co-catalyst by dramatically decreasing the initiation period required for reaction.
Pericyclic reactions
A 1,3-dipolar cycloaddition
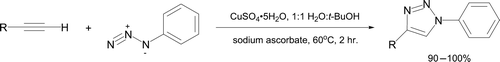
“Click chemistry” is a process-driven approach to organic synthesis involving many green principles of pedagogical importance Citation50. “Click” reactions must proceed under simple conditions (e.g. in water or the absence of solvent) with high yields/stereospecificities and generation of easily removed, benign by-products. Products of “click” reactions must be physiologically stable and purified by straightforward methods, such as recrystallization. A 1,3-dipolar cycloaddition between terminal alkynes and phenyl azide (, Citation51) fulfills many such criteria. These Cu(I)-catalyzed reactions are undertaken with water:t-butanol as solvent, easily monitored by TLC, require no chromatographic purification and very high yielding. Nearly all the 1,2,3-triazoles precipitate as solids, allowing different students to synthesize different “clicked” products.
A Diels–Alder cycloaddition
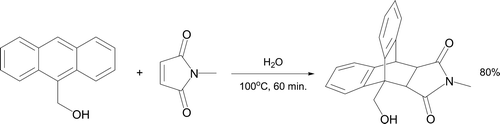
Aqueous Diels–Alder reactions have been a focus of attention for almost 30 years. Rideout and Breslow reported a large rate of acceleration on reacting anthracene-9-methanol with N-ethylmaleimide in water compared to other solvents Citation9. This was ascribed as a hydrophobic effect and extended to other reactions having a negative volume of activation. Design of an undergraduate experiment utilizing N-methylmaleimide as the dienophile took place (, Citation52). Diels–Alder reactions are highly atom efficient and exceptional examples of environmentally friendly chemistry. This procedure illustrates how a greener solvent can be used both for its benign characteristics and ability to significantly enhance the rate of an important carbon–carbon bond-forming reaction.
Hetero Diels–Alder cycloadditions
Two published experiments highlight hetero Diels–Alder reactions in aqueous environments Citation53 Citation54 which also benefit from the hydrophobic effect. In the first example, aqueous glyoxylic acid is heated and stirred with cyclopentadiene and copper(II) sulfate for three hours (). The initial Diels–Alder adduct rearranges to form a racemic mixture of lactones in moderate yield with the endo product predominating over the exo (65:35). 2D NMR can be used to determine the nature of the major lactone by interpretation of NOESY spectra.
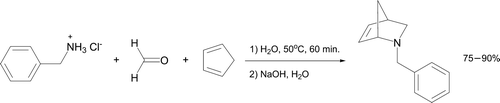
A similar one-pot protocol has very recently been reported, where the iminium ion formed from benzylamine hydrochloride and aqueous formaldehyde is reacted in situ with cyclopentadiene (). The product 2-azanorbornene is isolated in excellent yield after extraction as a pale yellow oil suitable for IR and NMR analyses.
Radical reactions
Oxidative biaryl synthesis
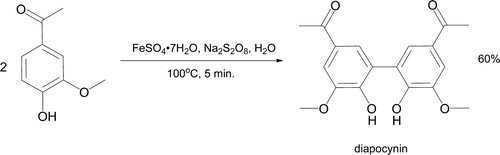
The aromatic compound apocynin (4-hydroxy-3-methoxyacetophenone) has been oxidized to diapocynin in 60% yield on heating with iron(II) sulfate and sodium peroxydisulfate in water for five minutes (, Citation55 Citation56). A sulfate radical anion (SO4 •–) generates a carbon-based radical ortho to the hydroxy group in apocynin and coupling occurs to form the product, which may have anti-oxidative and anti-inflammatory properties Citation57. Mak adopted a similar approach during preparation of racemic 1,1′-bi-2-naphthol from 2-naphthol Citation58. In this case the oxidant is iron(III) chloride (). The racemic product is subsequently resolved by treatment with (-)-N-benzylcinchonidinium chloride to isolate solid (S)-BINOL and (R)-BINOL after acidic hydrolysis. These two compounds and their derivatives are useful chiral ligands for asymmetric catalysis Citation59.
Electrophilic substitution reactions
Dipyrromethane preparation
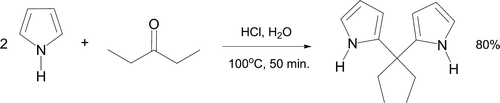
A one-pot preparation of meso-diethyl-2,2′-dipyrromethane via an electrophilic aromatic substitution mechanism has been documented Citation60. The reaction involves refluxing 3-pentanone with two equivalents of pyrrole in aqueous HCl for 50 minutes (). This green synthesis involves little product purification as the dipyrromethane separates as large crystals from the aqueous medium and does not require recrystallization. Dipyrromethanes are important intermediates in both natural and artificial syntheses of tetrapyrrolic macrocycles. Pyrrole rings are particularly well known as porphyrin components Citation61.
Aromatic nitration
Jones-Wilson et al. reported an experiment where the amino acid tyrosine is nitrated under standard conditions (HNO3/H2SO4) in water to form 3-nitrotyrosine (, Citation62). Tyrosine represents a naturally occurring and non-toxic aromatic reactant with the added benefit of being water soluble, and the product is recrystallized from water after washing with ethyl acetate.
Functional group transformations in water
Oxidation reactions
Epoxide synthesis
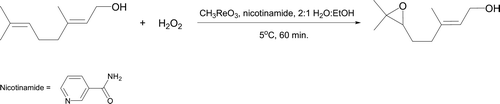
The terpene geraniol ((E)-3,7-dimethylocta-2,6-dien-1-ol) reacts smoothly with hydrogen peroxide in the presence of catalytic methyltrioxorhenium and nicotinamide to form 6,7-epoxygeraniol (, Citation63). This epoxidation is performed in aqueous ethanol and exhibits good atom economy with water as the only by-product. Other green advantages are use of 3% hydrogen peroxide (rather than the “normal” 30% solution) and nicotinamide (derived from a commercial vitamin tablet) instead of pyridine. The experiment represents an impressive alternative to alkene epoxidations undertaken with m-chloroperoxybenzoic acid (MCPBA) which are considerably less atom efficient and typically require chlorinated solvents.
The strong and versatile oxidizing agent, Oxone (potassium peroxymonosulfate, 2KHSO5 •KHSO4 •K2SO4) Citation64 has several applications in the organic teaching laboratory. In the presence of acetone it generates dimethyldioxirane which is the active epoxidant of cyclohexene, norbornylene, and β-pinene Citation65. Near quantitative yields are achieved after a 30-minute reaction time under basic conditions in a water:acetone solvent ().
Carboxylic acid synthesis
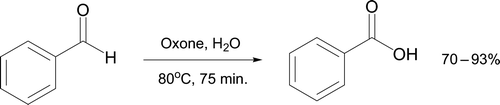
Gandhari et al. Citation66 have also employed Oxone for oxidation of aromatic aldehydes to carboxylic acids. Benzoic acid is synthesized from benzaldehyde in excellent yield on heating with Oxone in water with no organic co-solvent present (). The product is recrystallized from water making this a particularly green oxidation approach, eliminating use of harsh and toxic oxidizing agents (KMnO4, K2Cr2O7) in strongly acidic media. Five other benzaldehyde derivatives (2-Cl, 4-Cl, 4-NO2, 4-Br and 3-OCH3) also react under such conditions utilizing aqueous ethanol as solvent.
Adipic acid (1,6-hexanedioic acid) is an important chemical necessary for synthesis of Nylon 6,6, a polymer often made by students in undergraduate laboratories. Adipic acid is prepared industrially via vigorous oxidation of cyclohexanol or cyclohexanone with nitric acid Citation67, generating nitrous oxide as an ozone-depleting agent. A greener approach to adipic acid synthesis has been described Citation68. Cyclohexene is oxidized by hydrogen peroxide using catalytic sodium tungstate and a phase-transfer catalyst (Aliquat 336) in water (). The environmentally benign feature is underscored by recycling the aqueous reaction mixture for subsequent runs. Adipic acid crystallizes on cooling the reaction mixture and is readily recrystallized from water in good to excellent yields.
Alcohol oxidation
A similar set of conditions is used to oxidize five secondary alcohols to corresponding ketones (, Citation69). Both solid and liquid carbonyl products are isolated by either vacuum filtration or ether extraction/evaporation with high purity (typically 94–98%). In the former cases washing solid ketones with water is all the purification needed.
Reduction reactions
Alcohol preparation using sodium borohydride
Hudak and Sholes reported an undergraduate experiment involving cyclohexanone reduction with sodium borohydride in 1986 Citation70. Although commonly used in alcohol solvents, NaBH4 is soluble and stable enough in aqueous alkali to effectively reduce many aldehydes and ketones. A revision made by Zaczek (, Citation71) is more energy efficient (a 15-minute reaction time in ice compared to a 30-minute reflux) and has an environmental benefit of using less basic solution and less ether for extraction.
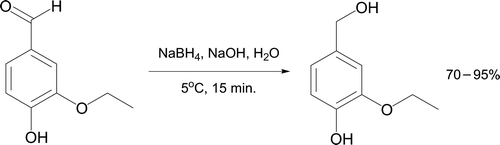
A microscale NaBH4 reduction of vanillin (4-hydroxy-3-methoxybenzaldehyde) to vanillyl alcohol in 1-M aqueous NaOH has since been described Citation72. More recently Miles et al. Citation73 outlined reduction of ethyl vanillin under related conditions (). The product ethyl vanillyl alcohol is converted to Methyl Diantilis (3-ethoxy-4-hydroxybenzyl methyl ether) which has found use in shampoos and fragrances Citation74.
Alcohol preparation using baker's yeast
It is possible to reduce the ketone group in ethyl acetoacetate in a stereoselective and chemoselective fashion to generate (S)-ethyl 3-hydroxybutanoate (, Citation75 Citation76). This profiles use of enzymes in organic synthesis by adding baker's yeast to a fermenting aqueous sugar solution of the β-ketoester in the presence of Na2HPO4. Incubation at 35°C leads to product formation with, after ether extraction, a reported ee of 85% Citation77. The achiral reducing agent NaBH4 would form ethyl 3-hydroxybutanoate as a racemate.
Halohydration reactions
Alkene halohydration
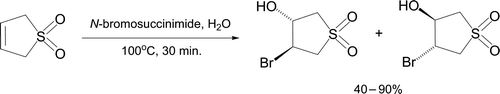
Bromohydration of the heterocycle 3-sulfolene is conveniently achieved in water using N-bromosuccinimide as a controlled source of Br2 Citation78, representing a simple example of an often-discussed reaction. The white solid product forms in good to excellent yield on heating for 30 minutes () and is readily recrystallized from pure water. The bromohydrin trans configuration is apparent from the coupling constant of protons at C3 and C4 (J=3 Hz).
Halolactone synthesis
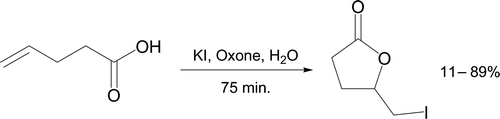
Crouch et al. Citation79 proposed a discovery-oriented experiment, where 4-pentenoic acid is reacted with aqueous potassium iodide and Oxone at room temperature (). Molecular iodine is generated which forms an iodonium ion from the alkene. Subsequent Markovnikov attack of an oxygen atom at the more highly substituted carbon leads to a five-membered iodolactone, which is challenging to predict. Absence of an O–H absorption in the product IR spectrum rules out formation of an iodohydrin and indicates a carboxylic acid is no longer present. The proton NMR unambiguously indicates nucleophilic attack at the tertiary carbon of the iodonium ion.
An etherification reaction
Williamson ether synthesis via phase-transfer catalysis
Preparation of an ether under phase-transfer catalytic conditions was reported by Hill and Corredor Citation80. shows the Williamson synthesis of benzyl butyl ether by this approach. The reaction proceeds in good yield and utilizes aqueous sodium hydroxide as base in the presence of tetrabutyl ammonium salts to facilitate phase transfer.
A dehydration reaction
Acetal synthesis
Although seemingly counterintuitive, a dehydration reaction (generating water) is achievable under aqueous conditions Citation81. Reaction of benzaldehyde with pentaerythritol and catalytic HCl leads to cyclic acetal formation (), known as a benzal. The process proceeds in water due to product insolubility in the aqueous medium. The equilibrium product is removed by precipitation (driving reaction to completion) and the two remaining hydroxyl groups in pentaerythritol do not react with a second equivalent of benzaldehyde to generate the dibenzal. Temperature control is important (above 35°C leads to increased dibenzal formation, and pentaerythritol precipitates from solution below 35°C). Opportunities exist to highlight use of acetals as protecting groups for aldehydes/ketones and optimization of reaction conditions to maximize yield of the desired product.
Conclusions
Water is a viable solvent for many organic reactions undergraduates learn in introductory and advanced courses. Reproducible, safe procedures illustrate utility of aqueous media and (very often) the practical simplicity afforded. Most experiments can be completed in a single three-hour laboratory period. Indeed, considering reactivity and operative mechanisms possible, one could envisage development of a synthetic laboratory curriculum where no organic solvents are used before product purification. Some experiments require only water as both reaction solvent and recrystallization medium Citation66 Citation68 Citation78, or produce solids not requiring recrystallization Citation17 Citation49 Citation52 Citation60. However, it remains important that students learn to handle organic solvents and the risks associated with them as part of their chemical training. Institutional integration of several experiments highlighting water as a solvent (and rotation of such experiments from year to year) would suffice as an introduction to the field.
Although benefits of water are conspicuously apparent, there is a notable drawback. Despite being an exceptionally safe solvent, water is often more challenging to purify on reaction completion than many organic alternatives, due to its relatively high-boiling point. Any by-products or impurities must be removed as aqueous waste streams will eventually reach aquifers, with attendant risk of human exposure Citation82. Indeed, Blackmond et al. have asserted “water is only a truly green solvent if it can be directly discharged to a biological effluent treatment plant” Citation83. Significantly, students should be taught that there is no single “ideal solvent” and that much research continues to develop new media that improve upon existing technologies Citation84 Citation85. Related green chemistry principles Citation11 need addressing in the context of each reaction undertaken, as implementing water alone does not render a process environmentally friendly. Several reactions incorporate excess reagents Citation13 Citation19 Citation66 leading to reduced experimental atom economies. High temperatures are required for long times in some cases Citation66 Citation68 Citation69 and many reactions do not employ catalytic species. Introducing water as the solvent of choice in the undergraduate organic curriculum is simply “a step in the green direction” Citation23.
References
- Moore , J.W. J. Chem. Educ . 2008 , 85 , 171 .
- Tomasik , J.H. 2008 . J. Chem. Educ. , 85 : 185 – 187 .
- Jacobsen , E.K. 2008 . J. Chem. Educ. , 85 : 188 – 190 .
- Li , C-J. 2005 . Chem. Rev. , 105 : 3095 – 3165 .
- Chan , T.H. 2004 . Can. Chem. News. , 56 : 18 – 19 .
- Lindström , U.M. 2002 . Chem. Rev. , 102 : 2751 – 2772 .
- Lindström , U.M. Organic Reactions in Water: Principles, Strategies and Applications ; Blackwell : Oxford , 2007 .
- Greener Education Materials for Chemists (GEMs) Web site . http://greenchem.uoregon.edu/gems.html (accessed January 20, 2009) .
- Rideout , D.C. and Breslow , R. J. 1980 . Am. Chem. Soc. , 102 : 7816 – 7817 .
- Breslow , R. J. 2006 . Phys. Org. Chem. , 19 : 813 – 822 .
- Anastas , P.T. ; Warner , J.C. Green Chemistry: Theory and Practice ; Oxford University Press : New York , 1998 ; p 30 .
- Broos , R. ; Tavernier , D. ; Anteunis , M. J. Chem. Educ . 1978 , 55 , 813 .
- Cheung , L.L.W. , Lin , R.J. , McIntee , J.W. and Dicks , A.P. 2005 . Chem. Educator , 10 : 300 – 302 .
- Breton , G.W. and Belk , M.K. 2004 . Chem. Educator , 9 : 27 – 29 .
- Stabile , R.G. and Dicks , A.P. 2004 . J. Chem. Educ. , 81 : 1488 – 1491 .
- Mayo , D.W. , Pike , R.M. and Trumper , P.K. 1999 . Microscale Organic Laboratory , 4th ed , 271 – 273 . Wiley : NY .
- Esteb , J.J. , Fravel , B. , Magers , J. , McNulty , L. , O'Reilly , S. and Wilson , A.M. 2007 . Chem. Educator , 12 : 324 – 326 .
- Fringuelli , F. , Piermatti , O. and Pizzo , F. 2004 . J. Chem. Educ. , 81 : 874 – 876 .
- Norcross , B.E. , Clement , G. and Weinstein , M. 1969 . J. Chem. Educ. , 46 : 694 – 695 .
- Abdalla , A.E. , Tirzite , D. , Tirzitis , G. and Roozen , J.P. 1999 . Food Chem. , 66 : 189 – 195 .
- Olek , R.A. , Ziolkowski , W. , Kaczor , J.J. , Greci , L. , Popinigis , J. and Antosiewicz , J. 2004 . J. Biochem. Mol. Biol. , 37 : 416 – 421 .
- Cave , G.W.V. and Raston , C.L. 2005 . J. Chem. Educ. , 82 : 468 – 469 .
- Bennett , G.D. 2006 . J. Chem. Educ. , 83 : 1871 – 1872 .
- Crouch , R.D. , Richardson , A. , Howard , J.L. , Harker , R.L. and Barker , K.H. 2007 . J. Chem. Educ. , 84 : 475 – 476 .
- McMurry , J. 2008 . Organic Chemistry , 7th edn , 345 – 348 . Belmont, CA : Thomson Higher Education .
- Clough , S. ; Goldman , E. ; Williams , S. ; George , B. J. Chem. Educ . 1986 , 63 , 176 .
- Breton , G.W. ; Hughey , C.A. J. Chem. Educ . 1998 , 75 , 85 .
- Li , C-J. 1996 . Tetrahedron , 52 : 5643 – 5668 .
- Wallace , J.R. , Lieberman , D.L. , Hancock , M.T. and Pinhas , A.R. 2005 . J. Chem. Educ. , 82 : 1229 – 1230 .
- Zappia , G. , Cancelliere , G. , Gacs-Baitz , E. , Delle Monache , G. , Misiti , D. , Nevola , L. and Botta , B. 2007 . Curr. Org. Synth. , 4 : 238 – 307 .
- Yeom , C-E. , Kim , H.W. , Shin , Y.J. and Kim , B.M. 2007 . Tet. Lett. , 48 : 9035 – 9039 .
- Renslo , A.R. , Luehr , G.W. and Gordeev , M.F. 2006 . Bioorg. Med. Chem. , 14 : 4227 – 4240 .
- Genet , J-P. , Darses , S. and Michelet , V. 2008 . Pure Appl. Chem. , 80 : 831 – 844 .
- Miyaura , N. and Suzuki , A. 1995 . Chem. Rev. , 95 : 2457 – 2483 .
- Callam , C.S. and Lowary , T.L. 2001 . J. Chem. Educ. , 78 : 947 – 948 .
- Franzén , R. and Xu , Y. 2005 . Can. J. Chem. , 83 : 266 – 272 .
- Aktoudianakis , E. , Chan , E. , Edward , A.R. , Jarosz , I. , Lee , V. , Mui , L. , Thatipamala , S.S. and Dicks , A.P. 2008 . J. Chem. Educ. , 85 : 555 – 557 .
- Leadbeater , N.E. and McGowan , C.B. 2006 . Clean, Fast Organic Chemistry – Microwave Assisted Laboratory Experiments , Matthews, NC : CEM .
- Novak , M. , Wang , Y-T. , Ambrogio , M.W. , Chan , C.A. , Davis , H.E. , Goodwin , K.S. , Hadley , M.A. , Hall , C.M. , Herrick , A.M. , Ivanov , A.S. , Mueller , C.M. , Oh , J.J. , Soukup , R.J. , Sullivan , T.J. and Todd , A.M. 2007 . Chem. Educator , 12 : 1 – 5 .
- Heck , R.F. 1982 . Org. React. , 27 : 345 – 390 .
- Cheung , L.L.W. , Aktoudianakis , E. , Chan , E. , Edward , A.R. , Jarosz , I. , Lee , V. , Mui , L. , Thatipamala , S.S. and Dicks , A.P. 2007 . Chem. Educator , 12 : 77 – 79 .
- Sonogashira , K. , Tohda , Y. and Hagihara , N. 1975 . Tet. Lett. , 16 : 4467 – 4470 .
- (a) Gilbertson , R. ; Doxsee , K. ; Succaw , G. ; Huffmann , L.M. ; Hutchison , J.E. In Greener Approaches to Undergraduate Chemistry Experiments ; Kirchhoff , M. , Ryan , M.A. American Chemical Society : Washington, DC , 2002 ; pp 4 – 7 ; (b) Doxsee , K.M. ; Hutchison , J.E. Green Organic Chemistry – Strategies, Tools and Laboratory Experiments ; Pacific Grove, CA : Brooks-Cole , 2004 ; pp 189 – 196 .
- McCallion , G.D. 1999 . Curr. Org. Chem. , 3 : 67 – 76 .
- Castro , C.E. ; Stephens , R.D. J. Org. Chem . 1963 , 28 , 2163 .
- Schneiders , G.E. and Stevenson , R. 1980 . Synth. Commun. , 10 : 699 – 705 .
- Harper , B.A. , Rainwater , J.C. , Birdwhistell , K. and Knight , D.A. 2002 . J. Chem. Educ. , 79 : 729 – 731 .
- Novak , B.M. and Grubbs , R.H. 1988 . J. Am. Chem. Soc. , 110 : 7542 – 7543 .
- Viswanathan , T. and Jethmalani , J. 1993 . J. Chem. Educ. , 70 : 165 – 167 .
- Kolb , H.C. , Finn , M.G. and Sharpless , K.B. 2001 . Angew. Chem. Int. Ed. , 40 : 2004 – 2021 .
- Sharpless , W.D. , Wu , P. , Hansen , T.V. and Lindberg , J.G. 2005 . J. Chem. Educ. , 82 : 1833 – 1836 .
- Huffmann , L.M. ; McKenzie , L.C. ; Hutchison , J.E. Diels-Alder Reaction in Water . http://greenchem.uoregon.edu/PDFs/GEMsID84.pdf (accessed January 20, 2009) .
- Augé , J. and Lubin-Germain , N. 1998 . J. Chem. Educ. , 75 : 1285 – 1287 .
- Sauvage , X. and Delaude , L. 2008 . J. Chem. Educ. , 85 : 1538 – 1540 .
- Dasari , M.S. , Richards , K.M. , Alt , M.L. , Crawford , C.F.P. , Schleiden , A. , Ingram , J. , Hamidou , A.A.A. , Williams , A. , Chernovitz , P.A. , Luo , R. , Sun , G.Y. , Luchtefeld , R. and Smith , R.E. 2008 . J. Chem. Educ. , 85 : 411 – 412 .
- van den Worm , E. ; van den Berg , A.J.J. ; Kemeling , G.M. ; Beukelman , C.J. ; Halkes , S.B.A. ; Labadie , R.P. ; van Dijk , H. Isolation, Characterization and Activity of Diapocynin, an Apocynin Metabolite . Chapter 5 . http://igitur-archive.library.uu.nl/dissertations/1957866/c5.pdf (accessed January 20, 2009) .
- Klees , R.F. , DeMarco , P.C. , Salazsnyk , R.M. , Ahuja , D. , Hogg , M. , Antoniotti , S. , Kamath , L. , Dordick , J.S. and Plopper , G.E. 2006 . J. Biomed. Biotechnol. , 2006 : 1 – 10 .
- Mak , K.K.W. 2004 . J. Chem. Educ. , 81 : 1636 – 1640 .
- Pu , L. 1998 . Chem. Rev. , 98 : 2405 – 2494 .
- Sobral , A.J.F.N. 2006 . J. Chem. Educ. , 83 : 1665 – 1666 .
- Lee , C-H. , Li , F. , Iwamoto , K. , Dadok , J. , Bothner-By , A.A. and Lindsey , J.S. 1995 . Tetrahedron , 51 : 11645 – 11672 .
- Jones-Wilson , T.M. and Burtch , E.A. 2005 . J. Chem. Educ. , 82 : 616 – 617 .
- (a) Goodwin , T.E. J. Chem. Educ . 2004 , 81 , 1187 – 1190 ; (b) Hicks , A.R. ; Davis , B.L. ; Dill , W.M. ; Rogers , C. ; Goodwin , T.E. Development of a Green Epoxidation Experiment for the Introductory Organic Laboratory . http://web.clark.edu/nfattaleh/classes/212/Lab/212W08GeraniolEpoxLab.pdf (accessed January 20, 2009) .
- Marcotullio , M.C. , Epifano , F. and Curini , M. 2003 . Trends Org. Chem. , 10 : 21 – 34 .
- Broshears , W.C. , Esteb , J.J. , Richter , J. and Wilson , A.M. 2004 . J. Chem. Educ. , 81 : 1018 – 1019 .
- Gandhari , R. , Maddukuri , P.P. and Vinod , T.K. 2007 . J. Chem. Educ. , 84 : 852 – 854 .
- Sato , K. , Aoki , M. and Noyori , R. 1998 . Science , 281 : 1646 – 1647 .
- (a) Reed , S.M. ; Hutchison , J.E. J. Chem. Educ . 2000 , 77 , 1627 – 1629 ; (b) Doxsee , K.M. ; Hutchison , J.E. Green Organic Chemistry – Strategies, Tools and Laboratory Experiments ; Brooks-Cole: Pacific Grove, CA, 2004 ; pp 135 – 141 .
- Hulce , M. and Marks , D.W. 2001 . J. Chem. Educ. , 78 : 66 – 67 .
- Hudak , N.J. and Sholes , A.H. 1986 . J. Chem. Educ. , 63 : 161
- Zaczek , N.M. J. Chem. Educ . 1986 , 63 , 909 .
- Fowler , R.G. 1992 . J. Chem. Educ. , 69 : A43 – A46 .
- Miles , W.H. and Connell , K.B. 2006 . J. Chem. Educ. , 83 : 285 – 286 .
- Ochsner , P.A. Perfumes Containing Benzyl Ethers . US Patent 4,657,700, April 14 , 1987 .
- Williamson , K.L. , Minard , R.D. and Masters , K.M. 2007 . Microscale and Macroscale Organic Experiments , 5th ed , 785 – 791 . New York, NY : Houghton Mifflin .
- Pohl , N. , Clague , A. and Schwarz , K. 2002 . J. Chem. Educ. , 79 : 727 – 728 .
- Seebach , D. , Sutter , M.A. , Weber , R.H. and Züger , M.F. 1984 . Org. Synth. , 63 : 1 – 9 .
- Greenberg , F.H. 1985 . J. Chem. Educ. , 62 : 638
- Crouch , R.D. , Tucker-Schwartz , A. and Barker , K. 2006 . J. Chem. Educ. , 83 : 921 – 922 .
- Hill , J.W. and Corredor , J. 1980 . J. Chem. Educ. , 57 : 822
- Collard , D.M. , Jones , A.G. and Kriegel , R.M. 2001 . J. Chem. Educ. , 78 : 70 – 72 .
- Doxsee , K.M. and Hutchison , J.E. 2004 . Green Organic Chemistry – Strategies, Tools and Laboratory Experiments , 74 Pacific Grove, CA : Brooks-Cole .
- Blackmond , D.G. , Armstrong , A. , Coombe , V. and Wells , A. 2007 . Angew. Chem. Int. Ed. , 46 : 3798 – 3800 .
- Clark , J.H. and Tavener , S.J. 2007 . Org. Process Res. Dev. , 11 : 149 – 155 .
- Jessop , P.G. 2007 . Can. Chem. News , 59 : 16 – 18 .