Abstract
Imines constitute a class of therapeutic agents that possess a broad spectrum of pharmacological activity. The conventional method for synthesis of imines by nucleophilic addition of amines to ketones or aldehydes requires long reaction times along with the use of organic solvents and a glacial acetic acid catalyst. We report the synthesis of Schiff's bases of isonicotinic acid hydrazide by novel, green routes using sonication, stirring, and microwave irradiation, respectively. Initial results are reported and indicate that by employing greener methods under aqueous conditions, high yields and shorter reaction times can be achieved.
Introduction
The goal of green chemistry is to develop environmentally friendly synthetic reactions and processes that avoid the use of reagents (reaction should be catalytic and reagent is reusable), avoid use of solvents if possible, and minimize formation of byproducts Citation1. Green chemistry Citation2 can be defined as carrying out chemical activities, including chemical design, manufacture, use, and disposal such that hazardous substances will not be used and generated. Green chemistry involves the use of microwave technology, sonochemistry, phase transfer catalysis, ionic liquids, and many more techniques.
Imines are functional groups or chemical compounds containing a carbon nitrogen double bond which have varied biological activities, such as anticonvulsant, antidepressant, analgesic, antiinflammatory, antiplatelet, antimalerial, antimicrobial, antimycobacterial, vasodilators, and antiviral activity Citation3–6. The preparation of imines has been carried out by refluxing the mixture of amines and the carbonyl compounds in organic solvent under azeotropic conditions in order to separate the water formed Citation7. An attempt was made to synthesize Schiff's bases of isonicotinic acid hydrazide by the green route techniques of stirring, microwave irradiation and sonication Citation8.
Results and discussion
The results of the spectral studies (UV, IR, 1H NMR) of the synthesized Schiff's bases of isonicotinic acid hydrazide are as given below.
(1a) Benzylidene isonicotinoyl hydrazone
UV data – λ max–302 nm
IR-cm−1 (KBR): 3199, 3026, 1689, 1598, 1552, 1355, 1411, 763, 686.
1H NMR (DMSO) ppm: 9.44–9.53 (2H), 8.78–8.81 (2H), 8.37 (1H), 7.43–7.89 (5H), 7.43 (1H).
(1b) Salicylidene isonicotinoyl hydrazone
UV data – λ max–290 nm.
IR-cm−1 (KBR): 3199, 3180, 3026, 1679, 1626, 1552, 1390, 1355, 1161, 999.
1H NMR (DMSO) ppm: 11(1H), 8.6–8.9 (2H), 8.8 (1H), 7.8–8 (2H), 7.6 (1H), 7.4–7.8 (1H), 6.8–7 (2H), 7.3 (1H), 2.5.
(1c) p -anisalidene isonicotinoyl hydrazone
UV data – λ max–320 nm.
IR-cm−1 (KBR): 3031, 2873, 1681, 1660, 1514, 1477, 1371, 1315, 740.
1H NMR (DMSO) ppm: 8.8 (2H), 8.35 (1H), 7.63–7.70 (2H), 7.4 (1H), 6.84–6.89 (2H), 3.78 (3H), 2.5.
(1d) Cinnamylidene isonicotinoyl hydrazones
UV data – λ max–326 nm.
IR-cm−1 (KBR): 3242, 3035, 1647, 1625, 1544, 1361, 910.
1H NMR studies (DMSO) ppm: 8.68 (2H), 8.16 (1H), 7.78(2H), 7.28–7.4 (5H), 7.01(1H), 6.92–6.97(2H), 2.5.
(1e) 4-hydroxy benzylidene isonicotinoyl hydrazone
UV data – λ max–323 nm.
IR spectra cm−1(KBR): 3288, 3257, 3031, 1666, 1600, 1550, 1415, 1377, 1164.
1H NMR (DMSO) ppm: 11.5–12 (1H), 8.5–8.8 (2H), 8.3–8.4(1H), 8 (1H), 7.7–7.9 (2H), 7.5(2H), 6.8–7(2H).
The comparative data with results of the synthesis of isonicotinoyl hydrazones by conventional and green route methods are given in Tables .
Table 1. Benzylidene isonicotinoyl hydrazone (1a).
Table 2. Salicylidene isonicotinoyl hydrazone (1b).
Table 3. p-anisalidene isonicotinoyl hydrazone (1c).
Table 4. Cinnamylidene isonicotinoyl hydrazones (1d).
Table 5. 4-hydroxy benzylidene isonicotinoyl hydrazone (1e).
Experimental
Conventional method
Synthesis of Schiff's bases of isoniazid by conventional method Citation9
Reaction
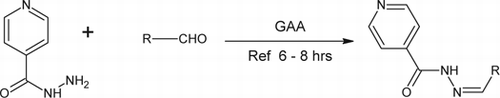
Procedure
To a solution of isoniazid (0.01 M) in ethanol, various aldehydes (0.01 M) each in ethanol, were added with intermittent shaking. To this mixture, glacial acetic acid (GAA) was added dropwise with shaking and then refluxed for 6–8 hrs. The completion of reaction was monitored by thin layer chromatography (TLC) (CHCl3:Methanol 8:2). The reaction mixture was concentrated and the residue obtained washed with water and dried. The crude product obtained on re-crystallization from alcohol gave the pure hydrazones of isonicotinic acid hydrazine (INH) (1a–1e). The synthesized compounds were characterized by their melting point and by spectral data (UV, IR, 1H NMR).
Green route methods
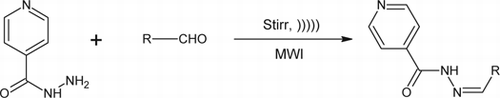
To a solution of isoniazid (0.01 M) in water, various aldehydes (0.01 M) were added. For reaction by stirring, the reaction mixture was stirred until the completion of the reaction (TLC, CHCl3:Methanol 8:2). For the sonication method, the reaction mixture was sonicated Citation10 in an ultrasonic bath until the completion of the reaction (TLC, CHCl3:Methanol 8:2). The microwave synthesis Citation11–13 was performed by irradiating the reaction mixture under microwave at the power level 3 (240 W, 35% irradiation) until the completion of the reaction (TLC, CHCl3:Methanol 8:2). The reaction mixture was filtered and the residue obtained was washed with water and dried. The crude product obtained on re-crystallization from alcohol gave the pure hydrazones of INH shown in (1a–1e). The synthesized compounds were characterized by their melting point and by spectral data (UV, IR, 1H NMR).
Table 6. Isonicotinoyl hydrazones (1a–1e).
Conclusion
The conventional reaction for the synthesis of Schiff's bases of isonicotinic acid hydrazide requires longer reaction times (360–420 min reflux) for the completion of the reaction. The reaction also involves the use of ethanol as solvent and GAA as a catalyst. On the other hand, the reactions carried out employing green route methods, such as sonication, microwave irradiation and by stirring using water as a solvent required no catalyst. In addition, the time span required for the completion of the reaction is less than that of the conventional method. The reaction by the stirring method required 60–120 min while the sonication method required 40–80 min for the completion of reaction. The reaction involving microwave irradiation required very short reaction times (7–8 min for the completion of the reaction). The green route methods required simple workup procedures, i.e. simple filtration to isolate the products and also gave comparatively improved yields as compared to the conventional method.
The results of the physicochemical characterization of the synthesized products by chromatographic and spectroscopic studies suggested that the products obtained by both conventional and green route methods were comparable in chemical composition.
References
- Ahluwalia , V.K. ; Kidwani , M. New Trends in Green Chemistry ; Anamaya : New Delhi , 2006 ; 2 ; pp 3 – 4 .
- Albini , A. ; Fagnoni , M . Green Chem . 2004 , 6 , 1 – 6 .
- Wikipedia “Imine” Page , http://en.wikipedia.org/wiki/Imines (accessed April 7, 2009) .
- Rollas , S. ; Kucukguzel , S.G. Mol . 2007 , 12 , 1910 – 1939 .
- Piato , A.L. ; Detanico , B.C. ; Jesus , J.F. ; Lhullier , F.L. ; Nunes , D.S. ; Elisabetsky , E. J. Ethnopharmacol . 2008 , 118 , 300 – 304 .
- Lidija , B.R. Acta. Pharm . 2004 , 54 , 157 – 162 .
- Hardman , J.G. Goodman & Gillman's The Pharmacological Basis of Therapeutics , 10th ed .; McGraw Hill : New Delhi , 2001 ; pp 235 , 401 , 765 , 1224 .
- Cocco , M.T. ; Congiu , C. ; Onnis , V. ; Pusceddu , M.C. ; Schivo , M.L. ; Logu , A.D. Euro. J. of Med. Chem . 1999 , 34 , 1071 – 1076 .
- Sriram , D. ; Yogeeswari , P. ; Madhu , K. Bioorg. and Med. Chem. Lett . 2005 , 15 , 4502 – 4505 .
- Guzen , K.P. ; Guarezemini , A.S. ; Orfao , A.T.G. ; Cella , R. ; Pereira , C.M.P. ; Stefani , H.A. Tetrahedron Lett . 2007 , 48 , 1845 – 1848 .
- Samanta , S.K. ; Kylanlahti , I. ; Yli- Kauhaluoma , J. Bioorg. and Med. Chem. Lett . 2005 , 15 , 3717 – 3719 .
- Harju , K. ; Kylanlahti , I. ; Paananen , T. ; Polamo , M. ; Nielsen , J. ; Yli-Kauhaluoma , J. J. Comb. Chem . 2006 , 8 3 , 344 – 349 .
- Mehta , H. ; Yogeshwari , P. ; Sriram , D. ; Sridharan , I. J. Zhejiang Univ. Sci. B . 2007 , 8 1 , 45 – 55 .