Abstract
Biginelli compounds 3,4-dihydropyrimidine-2-(1H)-ones are synthesized in high yields via eco-friendly simple reaction procedure using Lactic acid: organocatalyst. The new method reported herein is green and is free of formation of any hazardous by products. The process has significant advantages over other reported methods.
Introduction
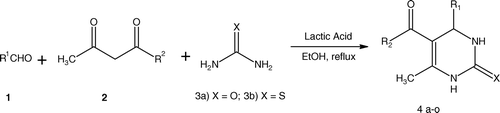
The development of multicomponent reactions Citation1 of biologically significant molecules is of current interest and 3,4-dihydropyrimidine-2-(1H)-ones (for most recent account on Biginelli reaction see Citation2) is one of the leading examples of this type. This scaffold has attracted major attention as ever since it was prepared by Biginelli Citation3 in 1891 via a three-component coupling reaction of urea, active methylene compounds, and an aldehyde. During the past few decades, these Biginelli compounds have been found to be useful as anti-hypertensive agents, anti-carcinogenic agents, calcium channel blockers α-1a-antagonists, neuropeptide Y (NPY) antagonists, anti-inflammatory, and analgesic agents Citation4–10. Of particular interest is the production of monastrol in a single step with high yield, a compound which is being developed as a lead compound for anti-cancer activity Citation11. The prestigious position of these molecules is demonstrated by the large amount of researches reported involving the use of a Lewis acid in the condensation to produce these molecules in more efficient manners; e.g. H2SO4, BF3.Et2O/CuCl, BiCl3, CeCl3.7H2O, Cu(OTf)2, LiBr, Gallium(III) halides, Metal triflimide, p-toluenesulfonic acid, polystyrenesulfonic acid (PSSA), etc. Citation12–20.
Organocatalysts Citation21 are fast replacing the use of metal-based Lewis acids. Keeping in mind the growing interest in developing green processes and procedures in organic synthesis Citation22, organocatalysts are consider to be a more eco-friendly and user-friendly alternative to traditional counterparts. Because of this interest, some examples of organocatalysts used in Biginelli reactions include bakers’ yeast, hydrazine type, oxalic acid, and citric acid Citation23–29. In continuation of our work on new methodologies including Biginelli reactions (17b, 18, 30), we wish to report the efficient use of Lactic acid for the production of 3,4-dihydropyrimidine-2-(1H)-ones.
Results and discussion
It may be noted that Lactic acid catalyzed condensations of an aldehyde, 1,3-dicarbonyl compound and urea gave the corresponding Biginelli compounds in good to excellent yields. Various aromatic, aliphatic, and heterocyclic aldehydes have been employed in this reaction successfully which is testament to the large scope of this catalyst system. Acetylacetone was also used with similar success to provide the corresponding 3,4-dihydropyrimidin-2-(1H)-ones (, entries 13, 14, 15). When urea was replaced with thiourea, the corresponding 3,4-dihydropyrimidin-2-(1H)-thiones were obtained with comparable results. Thus, variations in all three components have been accommodated very comfortably ().
Table 1. Lactic acid mediated synthesis of 3,4-dihydropyrimidin-2-(1H)-ones.
This condensation process is fairly robust and several functionalities such as nitro, chloro, hydroxyl, and methoxy survived during the course of reaction in a single step with high yield. Acid sensitive aldehyde, such as furfural, also worked well without the formation of any side product. Roughly, 25–40 mmol of Lactic acid was found to be sufficient for these reactions. The use of large amount of catalyst was also found to be unfruitful. The use of Lactic acid as an organocatalyst was prompted first by the commercial availability as a cheap reagent and second due to its biodegradability. Unlike conventional Lewis acids during workup, no hazardous waste is produced. The role of the catalyst we believe is to assist in the polarization of the imine intermediate 5 and to activate the active methylene compound 6 via H-bonding ().
Experimental section
Melting points were determined in open capillaries and are uncorrected. Reagent grade chemicals were purchased from commercial source and used as received. IR spectra were recorded in KBr discs on a Perkin–Elmer 240C analyzer. 1H NMR spectra were recorded on a Varian Gemini 300 (300 MHz) spectrometer using tetramethylsilane (TMS) as internal standard. The progress of reaction was monitored by thin layer chromatography (TLC) run on silica gel G (Merck).
General experimental procedure for the synthesis of 3,4-dihydropyrimidin-2-(1H)-ones
A mixture of aldehyde (2 mmol), 1,3-dicarbonyl compound (2 mmol), urea (3 mmol), and Lactic acid 82–85% (25–40 mmol) was stirred in ethanol. The resulting mixture was refluxed for duration (). After completion (as followed by TLC), excess ethanol was evaporated under reduced pressure. The residues were treated with cold water (30 ml). The crude product thus obtained was filtered and recrystallized from ethanol to afford 3,4-dihydropyrimidin-2(1H)-one.
Physical and spectral data
5-Ethoxycarbonyl-4-(phenyl)-6-methyl-3,4-dihydropyrimidin-2(1H)-thione (entry 1): m.p. 208–09°C(208–10; ref. 13); IR (KBr): 3414, 3227, 3104, 2939, 1704, 1651,1589; 1HNMR (DMSO-d6) δ 8.87 (s, 1H), 7.72 (s, 1H), 7.23–7.32 (m, 5H), 5.14 (s, 1H), 3.97 (q, 2H), 2.24 (s, 3H), 1.09 (t, 3H).
5-Ethoxycarbonyl-4-(4-chlorophenyl)-6-methyl-3,4-dihydropyrimidin-2(1H)-thione (entry 2): m.p. 192–94°C(192–95; ref. 13); IR (KBr): 3415, 3256, 3111, 2988, 1701, 1649, 1487; 1H NMR (DMSO-d6) δ 9.1 (s, 1H), 8.68 (s, 1H), 7.38 (d, 2H), 7.24 (d, 2H), 5.14 (d, 1H), 3.97 (q, 2H), 2.24 (s, 3H), 1.08 (t, 3H).
5-Ethoxycarbonyl-6-methyl-4-(2-thienyl)-3,4-dihydropyrimidin-2(1H)-thione, (entry 3): m.p. 214–16°C(215–17; ref. 17); IR (KBr): 3423, 3243, 1651, 1555 cm−1; 1H NMR (DMSO-d 6): δ 10.39 (s, 1H), 9.67 (s, 1H), 7.41 (d, J=4.2 Hz, 1H), 7.00–6.85 (m, 2H), 5.39 (s, 1H), 4.06 (q, J=6.8 Hz, 2H), 2.29 (s, 1H), 1.16 (t, J=6.8 Hz, 3H). Anal. found: C, 51.14; H, 4.89; N, 9.83. C12H14N2O2S2 requires C, 51.02; H, 5.00; N, 9.93%.
5-Ethoxycarbonyl-6-methyl-4-phenyl-3,4-dihydropyrimidin-2(1H)-one, (entry 5): m.p. 201–02°C(202–03; ref. 13); IR (KBr): 3412, 3229, 1710, 1639 cm−1; 1H NMR (DMSO-d 6): δ 9.18 (s, 1H), 7.73 (s, 1H), 7.20–7.30 (m, 5H), 5.14 (s, 1H), 3.98 (q, J=7.2 Hz, 2H), 2.24 (s, 3H), 1.06 (t, J=7.2 Hz, 3H). Anal. found: C, 64.67; H, 6.13; N, 10.83. C14H16N2O3 requires C, 64.62; H, 6.15; N, 10.77%.
4-(4-Chlorophenyl)-5-ethoxycarbonyl-6-methyl-3,4-dihydropyrimidin-2(1H)-one, (entry 6): m.p. 212–13°C(210–12; ref. 16); IR (KBr): 3420, 3242, 1708, 1645 cm−1; 1H NMR (DMSO-d 6): δ 9.20 (s, 1H), 7.76 (s, 1H), 7.40 (d, J=9.0 Hz, 2H), 7.26 (d, J=9.0 Hz, 2H) 5.16 (s, 1H), 3.95 (q, J=7.1 Hz, 2H), 2.19 (s, 3H), 1.10 (t, J=7.1 Hz, 3H). Anal. found: C, 57.13; H, 5.09; N, 9.44. C14H15ClN2O3 requires C, 57.05; H, 5.13; N, 9.50%.
5-Ethoxycarbonyl-6-methyl-4-(4-nitrophenyl)-3,4-dihydropyrimidin-2(1H)-one, (entry 7): m.p. 208–10°C(207–10; ref. 17); IR (KBr): 3415, 3236, 1715, 1675 cm−1; 1H NMR (DMSO-d 6): δ 9.28 (s, 1H), 8.26 (d, J=8.7 Hz, 2H), 7.80 (s, 1H), 7.70 (d, J=8.7 Hz, 2H) 5.26 (s, 1H), 3.93 (q, J=7.0 Hz, 2H), 2.25 (s, 3H), 1.09 (t, J=7.0 Hz, 3H). Anal. found: C, 55.14; H, 4.95; N, 13.69. C14H15N3O5 requires C, 55.08; H, 4.92; N, 13.77%.
4-(3-Chlorophenyl)-5-ethoxycarbonyl-6-methyl-3,4-dihydropyrimidin-2(1H)-one, (entry 8): m.p. 192–93°C(192–193; ref. 18); IR (KBr): 3416, 3230, 1706, 1642 cm−1; 1H NMR (DMSO-d 6): δ 9.02 (s, 1H), 7.50 (s, 1H) 7.16–7.35 (m, 4H), 5.20 (s, 1H), 4.02 (q, J=7.2 Hz, 2H), 2.29 (s, 3H), 1.12 (t, J=7.2 Hz, 3H). Anal. found: C, 57.16; H, 5.15; N, 9.39. C14H15ClN2O3 requires C, 57.05; H, 5.13; N, 9.50%.
5-Ethoxycarbonyl-4-(4-methoxylphenyl)-6-methyl-3,4-dihydropyrimidin-2(1H)-one, (entry 9): m.p. 200–01°C(199–201; ref. 13); IR (KBr): 3414, 3241, 3116, 2954, 1708, 1645, 1512; 1H NMR (DMSO-d6) δ 9.13 (s, 1H), 7.66 (s, 1H), 7.13 (d, 2H), 6.86 (d, 2H), 5.08 (d, 1H), 3.97 (q, 2H), 3.72 (s, 3H), 2.23 (s, 3H), 1.09 (t, 3H).
5-Ethoxycarbonyl-6-methyl-4-(isopropyl)-3,4-dihydropyrimidin-2(1H)-one, (entry 10): m.p. 194–95°C(194–95; ref. 17); IR (KBr): 3416, 3239, 1704, 1651 cm−1; 1H NMR (DMSO-d 6): δ 8.67 (s, 1H), 6.38 (s, 1H), 4.28 (s, 1H), 4.12 (q, J=7.3 Hz, 2H), 2.27 (s, 3H), 1.80 (m, 1H), 1.26 (t, J=7.1 Hz, 3H), 0.94 (d, J=6.5 Hz, 3H), 0.85 (d, J=6.5 Hz, 3H). Anal. found: C, 60.98; H, 5.72; N, 10.08. C14H16N3O4 requires C, 60.87; H, 5.80; N, 10.14%.
4-Butyl-5-(ethoxycarbonyl)-6-methyl-3,4-dihydropyrimidin-2(1H)-one, (entry 11): m.p. 156–58°C(156–58; ref. 17); IR (KBr): 3240, 1715, 1653 cm−1; 1H NMR (DMSO-d 6): δ 9.01 (s, 1H), 7.51 (s, 1H), 5.12 (s, 1H), 3.97 (q, J=6.8 Hz, 2H), 2.26 (s, 3H), 1.41–1.22 (m, 9H), 1.11 (t, J=6.8 Hz, 3H).
5-(Ethoxycarbonyl)-4-(2-furfuryl)-6-methyl-3,4-dihydropyrimidin-2(1H)-one, (entry 12): m.p. 204–05°C(204–05; ref. 13); IR (KBr): 3252, 1705, 1663 cm−1; 1H NMR (DMSO-d 6): δ 9.15 (s, 1H), 7.71 (s, 1H), 7.52 (s, 1H), 6.11 (d, J=2.9 Hz, 1H), 5.62 (d, J=2.8 Hz, 1H), 5.12 (s, 1H), 3.91 (q, J=7.3 Hz, 2H), 2.22 (s, 1H), 1.13 (t, J=7.3 Hz, 3H).
5-Acetyl-6-methyl-4-phenyl-3,4-dihydropyrimidin-2(1H)-one, (entry 13): m.p. 210–11°C(209–12; ref. 16); IR (KBr): 3241, 1715, 1643 cm−1; 1H NMR (DMSO-d 6): δ 9.20 (s, 1H), 7.77 (s, 1H), 7.35–7.25 (m, 5H), 5.25 (s, 1H), 2.24 (s, 3H), 2.07 (s, 3H).
5-Acetyl-6-methyl-4-(4-methoxyphenyl)-3,4-dihydropyrimidin-2(1H)-one, (entry 14): m.p. 190–91°C(191–93; ref. 18); IR (KBr): 3415, 3232, 1700, 1598 cm−1; 1H NMR (DMSO-d 6): δ 9.15 (s, 1H), 7.67 (s, 1H), 7.21 (d, J=8.3 Hz, 2H), 6.82 (d, J=8.3 Hz, 2H) 5.16 (s, 1H), 3.67 (s, 3H), 2.22 (s, 3H), 2.10 (s, 3H). Anal. found: C, 64.77; H, 6.06; N, 10.65. C14H16N2O3 requires C, 64.62; H, 6.15; N, 10.77%.
5-Acetyl-6-methyl-4-(4-nitrophenyl)-3,4-dihydropyrimidin-2(1H)-one, (entry 15): m.p. 235–38°C(235–38; ref. 17); IR (KBr): 3241, 3120, 2983, 2960, 1724, 1701, 1650, 1513, 1460, 1375, 1280, 1261, 1225, 1180, 1092, 984, 785, 693 cm−1.
Conclusion
In summary, the present method disclosed here employs Lactic acid and is an efficient, one-pot, single-step procedure for preparation of 3,4-dihydropyrimidin-2-(1H)-ones in excellent yields. The reaction time is dramatically reduced to 2.5–4 hours in contrast to reported procedure involving longer reaction time. The process is less hazardous as the use of low boiling solvents such as acetonitrile is avoided. In addition, the process involved mild reaction conditions and simple work up. The present study describes the first ever use and catalytic activity of Lactic acid in the synthesis of 3,4-dihydropyrimidin-2-(1H)-ones.
Acknowledgements
Authors thank the Council of Scientific and Industrial Research, New Delhi, India for financial support for this research project. Also Indian National Science Academy (INSA), New Delhi, India is thanked for additional financial support.
References
- (a) Sheldon , R.A. Chem. Ind. (London) 1997 , 12 – 17 ; (b) Clark , J.H. Chemistry of Waste Minimization ; Chapman and Hall : London , 1995 ; (c) Clark , J.H. ; Macquarrie , D.J. Chem. Soc. Rev 1996 , 25 , 303 – 310 .
- Saini , A. ; Kumar , S. ; Sandhu , J.S. Indian J. Chem. Soc . 2007 , 84 , 959 – 970 .
- (a) Biginelli , P. Chem. Ber . 1891 , 24 , 1317 – 1319 ; (b) Biginelli , P. Chem. Ber . 1891 , 24 , 2962 – 2965 ; (c) Biginelli , P . Gazz. Chim. Ital. 1889 , 19 , 212 – 215 ; (d) Biginelli , P. Gazz. Chim. Ital. 1893 , 23 , 360 – 413 .
- Grover , G.J. ; Dzwonczyk , S. ; McMullen , D.M. ; Normadinam , C.S. ; Sleph , P.G. ; Moreland , S.J. J. Cardiovasc. Pharmaco . 1995 , 26 , 282 – 291 .
- Kato , T. Japn. Kokai Tokkyo Koho JP . 1984 , 59 , 190 – 974 ; Chem. Abstr . 1985 , 102 , 132067 .
- (a) Atwal , K.S. ; Moreland , S. Biorg. Med. Chem. Lett . 1991 , 1 , 291 – 294 ; (b) Atwal , K.S. ; Rovnyak , G.C. ; Kinball , S.D. ; Floyd , D.M. ; Moreland , S. ; Swanson , B.N. ; Gougoutas , J.Z. ; Schwartz , J. ; Smillie , K.M. ; Mallay , M.F . J. Med. Chem . 1990 , 33 , 2629 – 2635 .
- Kappe , C.O. Eur. J. Med. Chem . 2000 , 35 , 1043 – 1052 .
- Atwal , K.S. ; Swanson , B.N. ; Unger , S.E. ; Floyd , D.M. ; Moreland , S. ; Hedberg , A. ; Reilly , B.C. J. Med. Chem . 1991 , 34 , 806 – 811 .
- Bozing , D. ; Benko , P. ; Petocz , L. ; Szecsey , M. ; Toempe , P. ; Gigler , G. ; Gacsalyi I. ; Gyertyan , I. (EGIS Gyogyszergyar) Eur. Pat. Appl. EP . 1991 , 409 , 233 ; Chem. Abstr . 1991 , 114 , 247 .
- Sadanandam , Y.S. ; Shetty , M.M. ; Diwan , P.V. Eur. J. Med. Chem . 1992 , 27 , 87 – 92 .
- Mayer , T.U. ; Kapoor , T.M. ; Haggarty , S.J. ; King , R.W. ; Schreiber , S.I. ; Mitchison , T.J. Science 1999 , 286 , 971 – 977 .
- Kappe , C.O. Eur. J. Med. Chem . 2000 , 35 , 1043 – 1052 .
- Hu , E.H. ; Sidler , D.R. ; Dolling , U.H. J. Org. Chem . 1998 , 63 , 3454 – 3457 .
- Ramalinga , K. ; Vijayalakshmi , P. ; Kaimala , T.N.B. Synlett . 2001 , 863 – 865 .
- Bose , D.S. ; Fatima , L. ; Mereyala , H.B. J. Org. Chem . 2003 , 68 , 587 – 590 .
- Paraskar , A.S. ; Dewkar , G.K. ; Sudalai , A. Teterahedron Lett . 2003 , 44 , 3305 – 3308 .
- (a) Maiti , G. ; Kundua , P. ; Guin , C. Tetrahedron Lett . 2003 , 44 , 2757 – 2758 ; (b) Baruah , P.P. ; Gadhwal , S. ; Prajapati , D. ; Sandhu , J.S. Chem. Lett . 2002 , 1038 – 1039 .
- Saini , A. ; Kumar , S. ; Sandhu , J.S. Indian J. Chem . 2007 , 46B , 1886 – 1889 .
- Suzuki , I. ; Suzumura , Y. ; Takeda , K. Teterahedron Lett . 2006 , 47 , 7861 – 7864 .
- (a) Bose , A.K. ; Manhas , M.S. ; Pednekar , S. ; Ganguly , S.N. ; Dang , H. ; He , W. ; Mandadi , A. Teterahedron Lett . 2005 , 46 , 1901 – 1903 (b) Polshettiwar , V. ; Varma , R.S. Teterahedron Lett 2007 48 7343 – 7346 .
- (a) Ramasastry , S.S.V. ; Zhang , H. ; Tanaka , F. ; Barbas III , C.F. J. Am. Chem. Soc . 2003 , 125 , 10808 – 10809 ; (b) Carlone , A. ; Cabrera , S. ; Marigo , M. ; Jorgensen , K.A. . Angew Chem. Int. Ed . 2007 , 46 , 1101 – 1104 ; (c) Zu , L. ; Li , H. ; Xie , H. ; Wang , J. ; Jiang , W . J. Am. Chem. Soc . 2007 , 129 , 10886 – 10894 ; (d) Brogan , A.P. ; Dickerson , T.J. ; Janda , K.O. Chem. Commun . 2007 , 4952 – 4954 ; (e) Martin , N.J.A. ; Ozores , L. ; List , B. J. Am. Chem. Soc. 2007 , 129 , 8976 – 8977 ; (f) Li , G. ; Liang , Y. ; Antilla , J.C. J. Am. Chem. Soc. 2007 , 129 , 5830 – 5831 .
- (a) List , B. Chem. Rev . 2007 , 107 , 5413 – 5415 ; (b) Alexakis , A. , CHIMIA 2007 , 61 , 212 – 214 .
- Kumar , A. ; Maurya , R.M. Tetrahydron Lett . 2007 , 48 , 4569 – 4571 .
- Suzuki , I. ; Iwata , Y. ; Takeda , K. Tetrahedron Lett . 2008 , 49 , 3238 – 3241 .
- Sangshetti , J.N. ; Kokare , N.D. ; Shinde , D.B. J. Het. Chem . 2008 , 45 , 1191 – 1193 .
- Rame , E. ; Kotra , V. ; Bansal , N. ; Varala , R. ; Adapa , S.R. RASAYAN J. Chem . 2008 , 1 , 188 – 190 .
- Tu , S. ; Fang , F. ; Miao , C. ; Jiang , H. ; Feng , Y. ; Shi , D. ; Wang , X. Tetrahedron Lett . 2003 , 44 , 6153 – 6155 .
- Debache , A. ; Boumoud , B. ; Amimour , M. ; Belfaitah , A. ; Rhouatia , S. ; Carboni , B. Tetrahedron Lett . 2006 , 47 , 5697 – 5699 .
- Mabry , J. ; Ganem , B. Tetrahedron Lett . 2006 , 47 , 55 – 56 .
- Gohain , M. ; Prajapati , D. ; Sandhu , J.S. Syntlett . 2004 , 235 – 238 .