Abstract
Heteropolyacid (H14 [NaP5W30O110])/SiO2 nano particles were synthesized by a micro emulsion technique. The morphology and size of these nano particles were characterized using, transmission electron microscopy and X-ray diffraction. In a designed photo reactor, the photo degradation of methyl orange as common azo dye pollutants in the environment was selected as a test reaction to estimate the catalytic activity of the synthesized nano particles and found to be excellent.
Introduction
Heteropolyacids belong to a large class of metal–oxygen cluster anions Citation1 and can be formed by a self-assembly process. In recent years, heteropolyacids have been studied extensively as acid and redox catalysts in many reactions Citation2 Citation3. These catalysts are green and harmless to the environment with respect to corrosiveness, safety, quantity of waste, and separability. Other key green aspects of solid heteropolyacid catalysts are related to their synthesis in an aqueous process and achievements of successful practical applications including: (i) water-tolerant acid catalysis; (ii) catalysis in pseudo liquid phase; (iii) solid-phase catalysis; (iv) bi-functional catalysis in combination with noble metals; and (v) green processes in bi-phase systems Citation4.
Along this line, there are several large-scale industrial processes utilizing heteropolyacid catalysts as oxidative and acid catalysts both in solid state and in solution Citation5. However, there is a need to develop and generate new catalysts of these compounds that are more active for applications as solid acid catalysts.
Recently, because of the unique properties of nano particles Citation6–8, the application of them as catalysts has attracted much attention. As the particle size decreases, the relative number of surface atoms increases, and thus the activity increases. In addition, nanometer-sized particles may show unique properties for several applications Citation9–11. In recent years, interest has focused mainly on the synthesis of nano catalysts such as the Keggin nano catalysts Citation12 Citation13. However, the synthesis and catalytic activity of the Preyssler nano catalyst has not been studied extensively and its photocatalytic activity has been largely overlooked.
A Pryessler acid is a highly acidic catalyst with excellent catalytic activity in a variety of acid-catalyzed reactions Citation14. The catalyst consists of an anion with a formula of [NaP5W30O110]14 − which has an unusual five-fold symmetry achieved by fusion of five {PW6O22} groups. The central sodium ion lies not on the equator of the anion but in a plane roughly defined by oxygen atoms of the phosphate groups. The presence of the sodium cation reduces the overall anion symmetry from D5h to C5v Citation15.
In continuation of our work with Preyssler catalysts, it was of great interest to synthesize a nano Preyssler anion and use this nano catalyst in photocatalytic reactions. Photocatalysis by solid heteropolyacids is a new branch in the field of photocatalytic chemistry. The oxidation of alcohols to aldehydes or ketones Citation16 Citation17, the functionalization of alkanes to form alkenes or ketones Citation18, dimerization of alkenes Citation19, and degradation of all kinds of aqueous organic pollutants for advanced oxidation processes Citation20–22 are catalyzed by heteropolyacids.
We report in this study, for the first time, the synthesis of nano structured Preyssler catalysts supported on silica, having various morphologies which can be controlled by the synthetic conditions. In addition to continuation of our work on the catalytic behavior of heteropolyacids Citation23 and extending the applications of Preyssler catalyst to nanotechnology and photocatalytic reactions, we investigated photo degradation of methyl orange as a model of azo dyes under UV light irradiation using hydrogen peroxide as an oxidant.
Results and discussions
Silica-supported Preyssler nano structures were obtained through a micro emulsion method. Although this procedure has been reported previously, this method has never been reported for the synthesis of Preyssler nano structures with different morphologies.
The obtained nano structures were characterized by transmission electron microscopy (TEM) as shown in . The micrographs show various morphologies under different synthetic conditions.
Figure 1. TEM images of the synthesized nano structures (the amorphous background structures are part of the TEM grid). (A) S=3, time = 30 h; (B) S=3, time = 12 h; (C) S=3, time = 18 h.
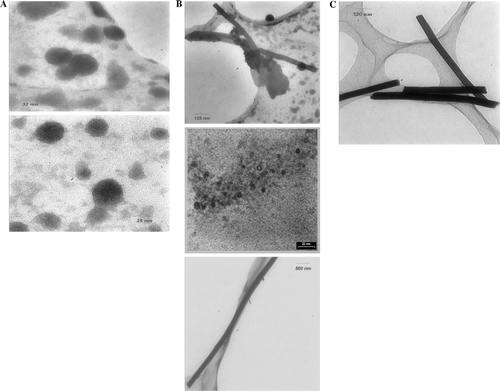
The morphology of the product was found to be strongly dependent on the reaction conditions such as concentration and reaction time. In addition, in the same reactions under specific reaction conditions, only spherical nano particles could be obtained. The sizes and morphology of products were controlled by changing the water to surfactant, sodium bis (2-ethylhexyl) sulfosuccinate molar ratio (S) and reaction time. For a short time, the tubular structure prevails, whereas the spherical shapes dominated at longer times.
The spherical particles with size of about 30 nm were obtained at molar ratio = 3 and 30 h, while the tubular morphology were obtained at molar ratio = 3 and various times (12–30 h). The molar ratio has been studied in various ranges and the results showed that higher molar ratios are unfavorable. TEM investigation with samples prepared at various times indicated that always different structures were formed, which are well known as the spherical and tubular structures. When the concentration was kept at S=3 and reaction time was raised to 30 h, the spherical structures only were obtained (A). A mixture of nano wire and nano spherical structures were obtained at S=3 and 12 h (B). This figure shows 30 nm spheres and some nano wires with diameters even smaller than the spheres. In the lower left image, the dark round structures are 3 nm in diameter and in the lower right image the wire structures are 100 nm in diameter. These images show typical results. As we can see, the diameter of most of the spheres and wires were 3 nm and 100 nm, respectively. We can suggest that, at first a mixture of nano spheres (30 nm) and nano wires (<30 nm) have been formed and gradually the diameter of the most spheres have decreased to 3 nm and for nano wires increased to 100 nm. The reason is not clear for us now, but can be attributed to the particular conditions and time. When the reaction time was 18 h (S=3), the fraction of nano wire increased evidently, so the nano wires with diameter of about 100 nm were only obtained (C).
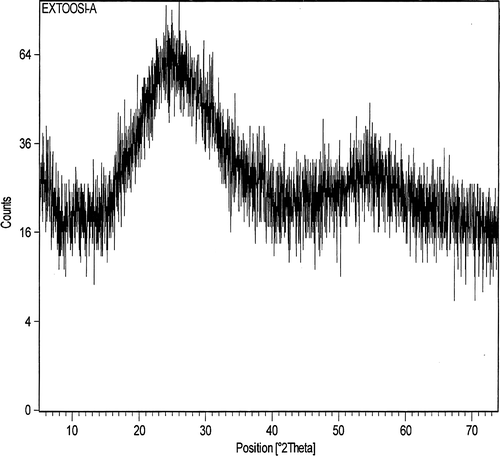
The reason is not yet clear, but is not surprising; shape changes of particles have been observed in other synthetic methods of nano particles Citation24 Citation25 . One explanation for the shape changes can be attributed to the meta stable states, which could spontaneously change into an equilibrium state under reaction conditions, which is in agreement with the observations Citation24 . shows powder X-ray diffraction (XRD) patterns of the synthesized samples. The patterns of the spherical products contain a broad peak centered at 52 A°. Analogous diffraction patterns have been observed for other synthesized samples.
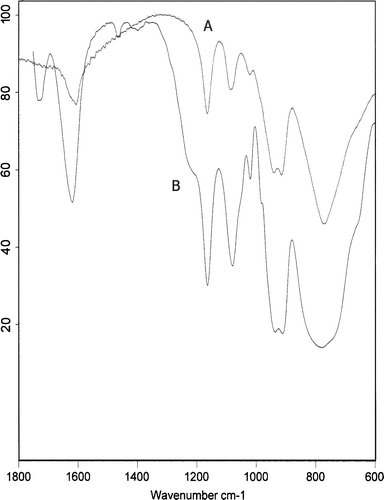
The heteropolyacid H14[NaP5W30O110] in the SiO2 nano particles was confirmed by infrared spectroscopy as shown in . The asymmetric stretching frequency of the terminal oxygen is observed at 960 cm−1 and the P–O asymmetric stretching frequency is noted at 1080 and 1165 cm−1. The prominent P–O bands at 960, 1080, and 1165 cm−1 are consistent with a C5V symmetry anion. These bands demonstrate that H14[NaP5W30O110] is preserved in the HPA/SiO2 nano particles. In addition, the protonated water of H14[NaP5W30O110] also remained in the nano particles at 1730 cm−1. It could be confirmed that the heteropolyacid H14[NaP5W30O110] was successfully immobilized into the SiO2 nano particles since the heteropolyacid does not react with tetraethoxysilane or with water in the micro emulsion, but it can remain in the silica nano particles without appreciable change of the structures (the silica particles were not large enough to cause a scattering baseline).
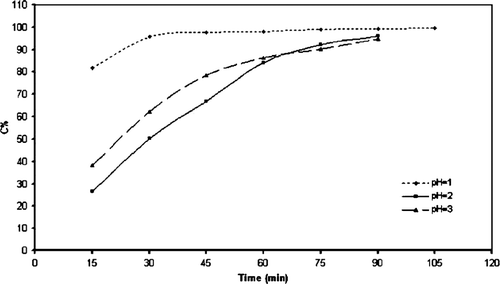
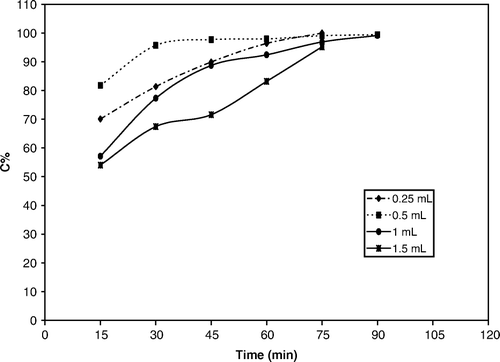
The photo degradation of methyl orange, in a designed photo reactor in our laboratory was performed as a test reaction to estimate the catalytic activity of these nano structures. We designed a photo reactor and checked the intensity changes of UV band in methyl orange in a photocatalytic reaction. The degree of methyl orange decolorization was used as a measurement for photocatalytic activity. To optimize the reaction conditions, Preyssler acid as bulk form (pure catalyst dissolved in solution) was used as a standard catalyst and the photodegradation of methyl orange solution were analyzed in the pH value of 1.0, 2.0, and 3.0. The effect of the pH value on the photocatalytic decolorization of methyl orange solution is shown in . The degree of methyl orange decolorization is indicative of its photodegradation and the excellent photocatalytic activity of the Preyssler catalyst. The relationship between the degradation degree of methyl orange and the pH value of the solution indicate that a low pH value can facilitate the decolorization reaction. These results were obtained with the initial concentration of 6.4×10−5 mol/L and 1×10−5 mole for methyl orange and catalyst, respectively. Obviously, the lower pH was favorable for the photodegradation of this azo dye by the Preyssler catalyst. shows the results for the effect of hydrogen peroxide on the photocatalytic degradation of methyl orange using the Preyssler acid. This figure shows that the best efficiency in a shorter time can be reached when the quantity is only 0.5 mL. We can see in that decolorization is 80% after 15 minutes and >95% after 30 minutes. At 1.5 mL, the degradation degree decreased. This result suggests an inhibition effect at higher hydrogen peroxide concentration. At the optimum conditions (pH = 1 and H2O2=0.5 mL) a similar study for photodegradation of methyl orange was carried out for various nano catalyst amounts (spherical nano structures, S=3, time = 30 h) for a fixed concentration of methyl orange.
Figure 5. Effect of the hydrogen peroxide addition on the photocatalytic decolorization of the methyl orange solution.
The photodegradation of methyl orange solution with various amounts of nano catalyst were analyzed including: 5×10−6, 1×10−5, 1.5×10−5, and 3×10−5 moles (the weight ratio of catalyst to tetraethoxysilane was fixed and with control of tetraethoxysilane amounts, the moles of the Preyssler were calculated). The first-order constants are listed in . It is inferred from this study that the rate increases with an increase in the amount of catalyst, obviously due to the higher number of photocatalytically active sites. The UV results for the best conditions are shown in A. This figure shows that methyl orange solution can be degraded under UV light in the presence of Preyssler nano catalyst.
Figure 6. (A) Photo degradation of methyl orange in the presence of Preyssler nano catalyst, (elapsed time from top to bottom: t=0, 15, 30, 35, 40, 45, 50). (B) Decolorization percentage versus time.
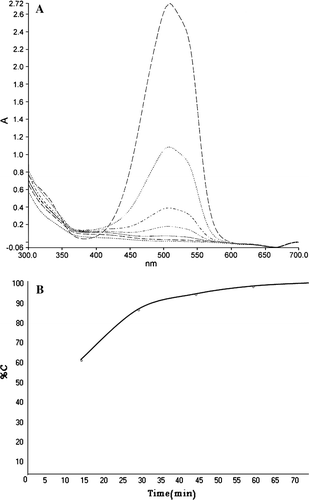
Table 1. The rate constants for photodegradation of methyl orange in different amounts of the nano catalyst.
After 40 minutes, the total absorption of methyl orange decreased and 95% decolorization was obtained. The photocatalytic decolorization of methyl orange solution versus time is shown in B. Our data indicate that the decolorization increases as the time is increased. This figure shows 60% decolorization after 15 minutes, 86% after 30 minutes, and 95% after 40 minutes. Hence, the nano catalyst appears to perform similarly to the bulk catalyst. In the bulk form, the catalyst is fully available to the reaction, while in the case of the nanostructured form, most of the catalyst may be buried within the nano particles being unavailable. Thus, a small amount of surface catalyst performs as well as a larger amount of dissolved catalyst. Degradation of methyl orange in the presence of nano wires exhibited a similar trend. There are several factors that can affect the photocatalytic activity. Literature in photocatalysis research reveals that in the most cases the photocatalytic activity can be strongly dependent on the crystallographic structure, morphology, and size of the particles Citation26 Citation27. It has also been shown that the activity or inactivity strongly depends on the preparation conditions Citation28. It is also worthwhile to mention that other factors such as the substrate nature, polarity, loading percentage, support type, doping with transition metal ions, surface depositing of noble metal clusters, and coupling with other semiconductors may strongly influence the photocatalytic activity Citation29–33 .
As a control experiment series, we studied the photo degradation reaction in the absence of nano catalyst, oxidant, and UV-illumination. The decolorization reaction of methyl orange in the absence of UV-illumination had no effect and the degree of degradation did not change significantly. Without the nano catalyst, the reaction is very slow and without hydrogen peroxide the reaction will not progress.
Experimental section
Chemicals and instruments
Sodium bis (2-ethylhexyl) sulfosuccinate, cyclohexane, tetraethoxysilane, sodiumtungstate (Na2WO4.2H2O), orthophosphoric acid, potassium chloride, hydrochloric acid, hydrogen peroxide, and methyl orange were purchased from commercial sources. Preyssler acid was prepared according to our earlier work Citation1. The particle size and shape of nano structures were observed by TEM (LEO 912 AB). FT-IR spectra were recorded with a Brucker scientific spectrometer (solid sample, KBr pellets).The XRD profiles of the samples were obtained using a PW 3710-Philips powder diffractometer. The absorbance of the methyl orange solution was measured using UV-Vis specterophotometry (Perkin Elmer Lambda 25).
Typical procedure for the synthesis of silica-supported Preyssler nano particles
To a solution of sodium bis (2-ethylhexyl) sulfosuccinate as surfactant in cyclohexane (0.2 M) was added a solution of Preyssler acid in a specified amount of water. The molar ratio of water to surfactant was selected to be 3, 5, and 7. Tetraethoxysilane was then added into the microemulsion phase. After mixing for various amounts of time (8, 12, 18, 25, and 30 h) at room temperature, dispersed Preyssler acid/SiO2 nano structures were centrifuged (15,000 rpm) and the particles were rinsed with acetone (four times) and dried in a vacuum oven.
Typical procedure for photo catalytic degradation of methyl orange
The photo reactor was designed in our laboratory. In a typical reaction, a 250 mL Pyrex glass was equipped with a magnetic stirrer, 150 mL of a 6.4×10−5 mol/L methyl orange solution, hydrogen proxide (0.5 mL), and catalyst (1×10−5 mol). The mixture was stirred and purged with nitrogen for 1 h, and then it was irradiated under the high pressure of mercury lamp (125 W) as UV light source. The temperature in the glass reactor was kept 25±2°C by the circulating water. At given irradiation time intervals, liquid samples were taken from the mixture, and the absorbance of the methyl orange solution measured with a UV-Vis spectrophotometer. The degree of methyl orange decolorization was calculated according to the equation: C%=(A0–A)/A0×100, where C is the degree of decolorization, A0 is the initial absorbance of the methyl orange solution, and A is the absorbance of the methyl orange solution after photo catalysis. The nano catalyst can be easily separated by filtration and can be recycled in reaction. After four times, the degree of decolorization decreased only by 7%.
Conclusions
This work presents, for the first time, a pathway for the synthesis of Preyssler nano structures with different morphologies. The formation of different morphologies is strongly dependant on the reaction conditions. It was shown that the morphologies are mainly governed by the meta stable state of the catalyst particles, which is related to the molar ratio of water to surfactant, the temperature, and the reaction time. We observed that all of the synthesized nano structures exhibit excellent photo catalytic activity when exposed in UV irradiation. The remarkable degradation of methyl orange in the presence of this nano catalyst indicates that the treatments of other organic pollutants could be performed in the presence of this catalyst, in order to obtain a perfect photodegradation degree. This catalytic activity can also be extended to the other catalytic reactions.
References
- Souchay , P. 1969 . Ions Mineraux Condenses , Paris : Masson .
- Okuhara , T. ; Mizuno , N. ; Misono , M. Appl. Catal. A: Gen . 2001 , 222 , 63 – 77 .
- Corma , A. Chem. Rev . 1995 , 95 , 559 – 614 .
- Misono , M. Sur. Chem. Catal . 2000 , 3 , 471 – 475 .
- Misono , M. Chem. Com . 2001 , 13 , 1141 – 1152 .
- Rodriguez , J.A. ; Drorak , T.J ; Sambasivan , S. ; Fischer , D. J. Phys. Chem. B . 2000 , 104 , 319 – 328 .
- Gorla , C.R. ; Emanetoglu , N.W. ; Liang , S. ; Mago , W.E. ; Lu , Y. ; Wraback , M. ; Shen , H. J. Appl. Phys . 1999 , 85 , 2595 – 2602 .
- Lee , J.H. ; Song , W.C. ; Yi , J.S. ; Yang , K.J. ; Han , W.D. ; Hwang , J. Thin Solid Films 2003 , 431 , 349 – 355 .
- Alivisatos , A.P. Science 1996 , 271 , 933 – 937 .
- Gilbert , B. ; Huang , F. ; Zhang , H. ; Waychunas , G.A. ; Banfield , J.F. Science 2004 , 305 , 651 – 654 .
- Michalet , X. ; Pinaud , F.F. ; Bentolia , L.A. ; Tsay , J.M. ; Doose , S. ; Li , J.J. ; Sundaresan , G. ; Wu , A.M. ; Gambhir , S.S. ; Weiss , S. Science 2005 , 307 , 528 – 534 .
- Sawant , D.P. ; Vinu , A. ; Jacob , N.E. ; Lefebvre , F. ; Halligudi , S.B . J. Catal . 2005 , 235 , 341 – 352 .
- Uchida , S. ; Mizuno , N . Coord. Chem. Rev . 2007 , 251 , 2537 – 2546 .
- (a) Bamoharram , F.F. ; Heravi , M.M. ; Roshani , M. ; Gahangir , M. ; Gharib , A. Appl. Catal . 2006 , 302 , 42 – 47 ; (b) Bamoharram , F.F. ; Roshani , M. ; Alizadeh , M.H. ; Razavi , H. ; Moghayadi , M. J. Braz. Chem. Soc. 2006 , 17 , 505 – 509 ; (c) Bamoharram , F.F. ; Heravi , M.M. ; Roshani , M. ; Gharib , A. ; Gahangir , M. J. Mol. Catal . 2006 , 252 , 90 – 95 ; (d) Bamoharram , F.F. ; Heravi , M.M. ; Roshani , M. ; Tavakoli , N. J. Mol. Catal. 2006 , 252 , 219 – 225 ; (e) Bamoharram , F.F. ; Heravi , M.M. ; Roshani , M. ; Akbarpour , M. J. Mol. Catal . 2006 , 255 , 193 – 198 ; (f) Bamoharram , F.F. ; Heravi , M.M. ; Roshani , M. ; Gahangir , M. ; Gharib , A. J. Mol. Catal. 2007 , 271 , 126 – 130 ; (g) Bamoharram , F.F. ; Heravi , M.M. ; Roshani , M. ; Gharib , A. ; Gahangir , M. J. Chin. Chem. Soc . 2007 , 54 , 1017 – 1020 ; (h) Heravi , M.M. ; Bakhtiari , K. ; Daroogheha , Z. ; Bamoharram , F.F. Catal. Com . 2007 , 8 , 1991 – 1994 ; (i) Heravi , M.M. ; Motamedi , R. ; Bamoharram , F.F. ; Seify , N. Catal. Com . 2007 , 8 , 1467 – 1471 ; (j) Hekmatshoar , R. ; Heravi , M. ; Sadjadi , M.S. ; Oskooie , H. ; Bamoharram , F.F. Catal. Com. 2008 , 9 , 837 – 841 ; (k) Heravi , M.M. ; Bakhtiari , K. ; Zadsirjan , V.F. ; Bamoharram , F.F. ; Heravi , O.M. Biorg. Med. Chem. Lett. 2007 , 17 , 4262 – 4265 ; (l) Heravi , M.M. ; Bakhtiari , K. ; Daroogheha , Z. ; Bamoharram , F.F. J. Mol. Catal . 2007 , 273 , 99 – 101 ; (m) Heravi , M.M. ; Zadsirjan , V. ; Bakhtiari , K. ; Oskooie , H. ; Bamoharram , F.F . Catal. Com . 2007 , 8 , 315 – 318 ; (n) Heravi , M.M. ; Derikvand , F. ; Ranjbar , L. ; Bamoharram , F.F. J. Mol. Catal. 2007 , 261 , 156 – 159 ; (o) Heravi , M.M. ; Khorasani , M. ; Derikvand , F. ; Oskooie , H. ; Bamoharram , F.F. Catal. Com. 2007 , 8 , 1886 – 1890 ; (p) Heravi , M.M. ; Sadjadi , S. ; Oskooie , H. ; Hekmat Shoar , R. ; Bamoharram , F.F. Catal.Com . 2008 , 9 , 504 – 507 ; (q) Heravi , M.M. ; Sajadi , S. ; Oskooie , H.A. ; Hekmat Shoar , R. ; Bamoharram , F.F. Molecules 2007 , 12 , 255 – 262 ; (r) Hekmat Shoar , R. ; Sajadi , S. ; Heravi , M.M. ; Bamoharram , F.F. Molecules 2007 , 12 , 2223 – 2228 ; (s) Heravi , M.M. ; Sadjadi , S. ; Oskooie , H. ; Hekmat Shoar , R. ; Bamoharram , F.F. Catal. Com . 2008 , 9 , 470 – 474 .
- Muller , A. ; Peters , F . Chem. Rev . 1998 , 98 , 239 – 272 .
- Fox , M.A. ; Cardona , R. ; Gaillard , E. J. Am. Chem. Soc . 1987 , 109 , 6347 – 6354 .
- Hill , C.L. J. Am. Chem. Soc . 1985 , 107 , 5148 – 5157 .
- Renneke , R.F. ; Hill , C.L. J. Am. Chem. Soc . 1986 , 108 , 3528 – 3529 .
- Yamase , T. ; Usami , T. J. Chem.Soc. Dalton Trans . 1988 , 183 – 190 .
- Mylonas , A. J. Mol. Catal . 1994 , 92 , 261 – 267 .
- Hu , C. ; Yue , B. ; Yamase , T. Appl. Catal . 2000 , 194 , 99 – 107 .
- Guo , Y. ; Hu , C. J. Mol. Catal . 2007 , 262 , 136 – 148 .
- (a) Heravi , M.M. ; Bakhtiari , K. ; Bamoharram , F.F. Catal. Com . 2006 , 7 , 373 – 376 ; (b) Heravi , M.M. ; Bakhtiari , K. ; Bamoharram , F.F. Catal. Com. 2006 , 7 , 499 – 501 ; (c) Heravi , M.M. ; Derikvand , F. ; Bamoharram , F.F. J. Mol. Catal . 2005 , 242 , 173 – 175 ; (d) Alizadeh , M.H. ; Razavi , H. ; Bamoharram , F.F. ; Daneshvar , K. J. Mol. Catal . 2003 , 206 , 89 – 93 ; (e) Heravi , M.M. ; Bakhtiari , K. ; Javadi , N.M. ; Bamoharram , F.F. J. Mol. Catal . 2006 , 264 , 50 – 52 ; (f) Heravi , M.M. ; Derikvand , F. ; Bamoharram , F.F. J. Mol. Catal . 2006 , 263 , 112 – 114 ; (g) Heravi , M.M. ; Rajabzadeh , G. ; Bamoharram , F.F. ; Seifi , N. J. Mol. Catal . 2006 , 256 , 238 – 241 ; (h) Heravi , M.M. ; Ranjbar , L. ; Derikvand , F. ; Bamoharram , F.F. Catal. Com . 2007 , 8 , 289 – 291 .
- Bartsch , K. ; Leonhardt , A. Carbon 2004 , 42 , 1731 – 1736 .
- Leyva , A.G. ; Troiani , H.E. ; Curiale , C.J. ; Sanchez , R.D. ; Levy , P. Physica B . 2007 , 398 , 344 – 347 .
- Kamat , P. Chem. Rev . 1993 , 93 , 267 – 300 .
- Balazs , N. ; Mogyorosi , K. ; Sranko , D.F. ; Pallagi , A. ; Alapi , T. ; Oszko , A. ; Dombi , A. ; Sipos , P. Appl. Catal. B: Environmental 2008 , 84 , 356 – 362 .
- Martin , S.T. , Morrison , C.L. and Hoffmann , M.R. 1994 . J. Phys. Chem. , 98 : 13695 – 13704 .
- Lv , K. and Xu , Y.M. 2006 . J. Phys. Chem. B. , 110 : 6204 – 6212 .
- Carriazo , D. , Addamo , M. , Marci , G. , Martin , C. , Palmisano , L. and Rives , V. 2009 . Appl. Catal. A: Gen. , 365 : 172 – 179 .
- Zheng , J.Y. , Yu , H. , Li , X.J. and Zhang , S.Q. 2008 . Appl. Surf. Sci. , 254 : 1630 – 1635 .
- Yang , Y. , Li , X.J. , Chen , J.T. and Wang , L.Y.J. 2004 . Photochem. Photobiol. A. , 163 : 517 – 522 .
- Yu , H. , Li , X.J. , Zheng , S.J. and Xu , W. 2006 . Mater. Chem. Phys. , 97 : 59 – 63 .