Abstract
The corrosion inhibition of Neem (Azadirachta indica (AZI)) leaves extract as a green inhibitor of zinc corrosion in H2SO4 has been studied using the gravimetric method. The results of the study reveal that the different concentrations of the AZI extract inhibit zinc corrosion and that inhibition efficiency of the extract varies with concentration and temperature.
Keywords:
Introduction
Corrosion is the destruction of material resulting from exposure and interaction with the environment. It is a major problem that must be confronted for safety, environment, and economic reasons Citation1. Several efforts have been made using corrosion preventive practices and the use of green corrosion inhibitors is one of them Citation2. The use of green inhibitors for the control of corrosion of metals Citation3 and alloys which are in contact with aggressive environment is an accepted and growing practice Citation4 Citation5. Large numbers of organic compounds are under study to investigate their corrosion inhibition potential. All these studies have revealed that organic compounds, especially those with N, S, and O, show significant inhibition efficiency. However, most of these compounds are not only expensive, but also toxic to living beings Citation6. It is needless to point out the importance of cheap and safe inhibitors of corrosion.
Plant extracts and organic species have therefore become important as an environmentally acceptable, readily available, and renewable source for wide range of inhibitors Citation7–12. They are the rich sources of ingredients which have very high inhibition efficiency Citation6 and are hence termed “Green Inhibitors” Citation10. Green corrosion inhibitors Citation13 are biodegradable and do not contain heavy metals or other toxic compounds. The successful use of naturally occurring substances to inhibit the corrosion of metals in acidic and alkaline environment have been reported by some research groups Citation14–20 to mention but a few. Research efforts to find naturally organic substances or biodegradable organic materials to be used as effective corrosion inhibitors of a wide number of metals has been one of the key areas in our research group Citation21.
Azadirachta indica (AZI) – the Neem plant
Our focus in this paper is on Neem (Azadirachta indica (AZI)), which is more specifically the extract from Neem leaves. Several studies have been carried out and have remained focused on the Neem plant parts for a their various pharmacological activities (anti-inflammatory, anti-pyretic, analgesic, immunostimulant, anti-fertility, anti-carcinogenic, anti-malarial, and hepatoprotective) Citation22–24 and medicinal properties Citation24 to mention but a few. The Neem extract has been only very occasionally involved in environmental engineering and environmental chemistry research with the analysis of the adsorption of Pb(II) from aqueous solution by AZI leaf powder by Bhattacharyya and Sharma Citation25, the adsorption and corrosion inhibitive properties of AZI in acid solutions Citation26 and the study of copper corrosion inhibition by AZI leaves extract in 0.5M sulfuric acid by Citation27. AZI has been well known in India and its neighboring countries for more than 2000 years as one of the most versatile medicinal plants having a wide spectrum of biological activity Citation24. Neem is an evergreen tree, cultivated in various parts of the Indian subcontinent. Neem has been extensively used in ayurveda Citation22, unani, and homoeopathic medicine and has become a cynosure of modern medicine. The Sanskrit name of the Neem tree is “Arishtha” meaning “reliever of sickness” and hence is considered as “Sarbaroganibarini” Citation24. The tree is still regarded as a “village dispensary” in India. The importance of the Neem tree has been recognized by the US National Academy of Sciences, which published a report in 1992 entitled “Neem – a tree for solving global problems.”
Chemical investigation on the products of the Neem tree was extensively undertaken in the middle of the twentieth century. Nimbin was the first bitter compound isolated from Neem oil, and thereafter more than 135 compounds have been isolated from different parts of Neem and several reviews have also been published on the chemistry and structural diversity of these compounds which are divided into two major classes: isoprenoids Citation28 and others. Neem extracts contain significant amounts of water soluble, electrochemically active compounds, as well as high concentrations of alkaloids, fatty acids, and nitrogen and oxygen-containing compounds. Neem is bitter in taste. The bitterness is due to an array of complex compounds called “triterpenes” or more specifically “limonoids.” Nearly 100 protolimonoids, limonoids or tetranortriterpenoids, pentanortriterpenoids, hexanortriterpenoids, and some non-terpenoid constituents have been isolated from various parts of the Neem tree Citation29 Citation30; still more are being isolated. The most important bioactive principal is azadirachtin; at least 10 other limonoids possess insect growth in regulating activity Citation31 Citation32. Neem fruits, seeds, oil, leaves, bark, and roots have such uses as general antiseptics, anti-microbials, treatment of urinary disorders, diarrhea, fever and bronchitis, skin diseases, septic sores, infected burns, hypertensions, and inflammatory diseases. Neem oil and its isolates – nimbidin, nimbidiol, and nimbin – inhibit fungal growth on humans and animals Citation33. Neem leaf extracts and teas can treat malaria and the anti-malarial action is attributable to gedunin, a limonoid. Given the wide spectrum of chemical species present in Neem leaves and their respective multifaceted chemical and biological properties, we therefore articulated that channeling Neem leaf extract for yet another use into green inhibition of corrosion studies may yield some interesting results. All the more, Neem extract has been only very occasionally involved in environmental engineering and environmental chemistry research with the analysis of the adsorption of Pb(II) from aqueous solution by Neem leaf powder by Bhattacharyya and Sharma Citation25, the adsorption and corrosion inhibitive properties of AZI in acid solutions Citation26 and the study of copper corrosion inhibition by AZI leaves extract in 0.5M sulfuric acid by Valek and Martinez Citation27.
Research objectives
Due to its position in the electrochemical series, zinc with an oxidation potential of 0.76 eV is much prone to corrosion, especially in acidic medium. The corrosion of Zn in acidic medium is believed to occur according to “Hydrogen evolution Mechanism.” In the present study, we are trying to study corrosion of zinc and the inhibition of the corrosion process by AZI extract. To the best of our knowledge, nothing has been specifically reported on the use of AZI extract for the inhibition of zinc corrosion in acidic medium. AZI leaves are often used in the medicinal and pharmaceutical industry. An additional beneficial use of Neem leaves to curb corrosion of zinc would surely imply the successful utilization of this powerful and versatile natural resource in the metallurgical, materials science, and chemical engineering industries.
The present study therefore seeks to investigate the inhibitive properties of AZI leaves extract for zinc corrosion using a gravimetric technique in an acidic media (H2SO4 acid) with and without the extract at two temperatures. Any positive result would help reduce the economic cost of corrosion control as well as decrease the subsequent environmental threats from inhibitor usage because Neem leaves extract is non-toxic and biodegradable.
Results and discussion
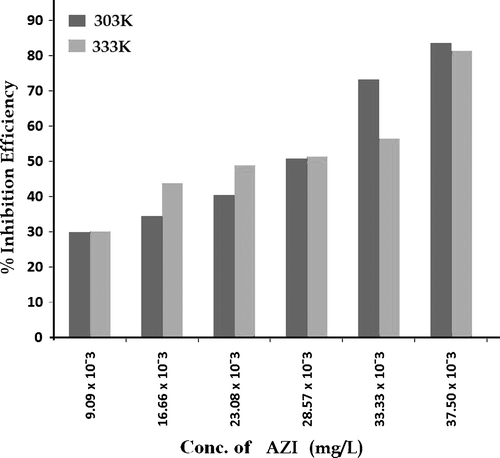
shows values of corrosion rate (CR) of zinc in all the concentrations of H2SO4 studied and it shows that CR increases with increase in H2SO4 concentration. shows the CR for the corrosion of zinc at 2.0N H2SO4 in the absence and presence of AZI extract at 303 and 333 K. Addition of increasing concentration of the inhibitor generally retards the CR of zinc in the solutions. This is clearly seen from the decreasing change in mass loss taking place (A and B) at a particular acid concentration corresponding with an increase in inhibitor concentration. shows values of inhibition efficiency of the different concentrations of AZI extract at 303 and 333 K.
Figure 1. Variation of mass loss change with increase in concentration of AZI leaves extract for various acid concentrations at the two temperatures investigated.
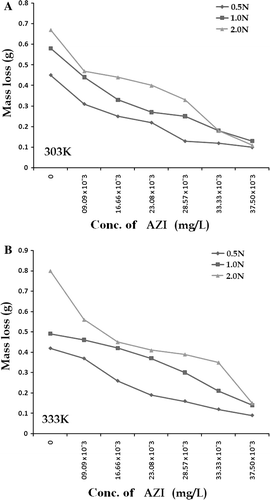
Table 1. Corrosion rate for the corrosion of zinc in H2SO4 at 303 K.
Table 2. Corrosion rates for the corrosion of zinc in 2N H2SO4 solutions containing AZI extract.
Table 3. Inhibition efficiency (%I) for 2.0N H2SO4
Effect of concentration
From and , it is found that the rate of corrosion of zinc is affected by concentration of H2SO4, temperature, and concentration of AZI extract. The rate of zinc corrosion increases as the concentration of H2SO4 increases and also increases as the temperature is increased (). Analysis and interpretation of trends in show that corrosion increases as the concentration of the acid increases confirming that the rate of corrosion of zinc in H2SO4 increases with concentration. The mass loss taking place and recorded at the different concentrations of the AZI extract are lower than that of the blank solution (for the 2.0N H2SO4) indicating that different concentrations of the AZI extract retard the corrosion of zinc. It is supposed to be due to adsorption of AZI extract on the surface of Zn. This hypothesis shall be verified in a future study with details of the electrochemical studies reported and discussed therein.
Effect of temperature
hence shows mass loss plots for the corrosion of zinc in the presence of different concentration of the AZI extract at 303 K (A) and 333 K (B), respectively. Comparing A and B, it is found that at a fixed concentration of the inhibitor and a fixed acid concentration, the mass loss taking place at 333 K is in most of the instances higher than that occurring 303 K indicating that the inhibition efficiency of AZI extract decreases with increase in temperature. The decrease may be due to competition between forces of adsorption and desorption. These very same competing forces of adsorption and desorption may also actually explain the occasional discrepancies in mass loss change observed in . From , it can also be seen that inhibition efficiency of AZI extract varies with its concentration. Optimum value of inhibition efficiency (83.58%) was obtained at an extract concentration of 37.50 mg/L, while the least value was obtained at an extract concentration of 9.09 mg/L. shows the variation of inhibition efficiency against the different concentrations of AZI extract at both 303 and 333 K. The significant difference between values of inhibition efficiency of AZI extract obtained at 303 and 333 K for especially the higher concentrations of the extract suggests that the mechanism of adsorption of the inhibitor on the zinc surface is by physical adsorption. For a physical adsorption mechanism, inhibition efficiency of an inhibitor decreases with temperature while for a chemical adsorption mechanism, values of inhibition efficiency increase with temperature Citation34 Citation35.
Experimental
Preparation of stock solution of Neem Azadirachta indica (AZI) leaves extract
Stock solution of the AZI leaves extract was prepared by boiling 0.5 kg of air-dried Neem leaves in deionized water and left overnight. The contents of the extraction process were then mixed in a graduated cylinder, filtered, and the resulting solution was kept in a refrigerator at low temperatures of 2°C in order to prevent the contents from being altered due to the chemical, physical, and biological reactions it might otherwise undergo Citation36.
Specimen preparation
Rectangular specimen sheets of zinc were mechanically pressed cut to form different coupons, each of dimension 5.0×2.5×0.04 cm. Each coupon was degreased by washing with ethanol, dried in acetone, and preserved in a dessicator. All reagents used for the study were Analar grade and double distilled water was used for their preparation. Specimens containing a small hole of 2 mm diameter near the upper edge were used for the determination of CR. The working surfaces of the zinc coupons were carefully and lightly polished with grade P600 SiC polishing paper in order to remove the oxide layer and eliminate the reactions that would have otherwise taken place with the acid and this zinc oxide layer.
Calculation of inhibition efficiency (%I) and degree of surface coverage (θ)
The mass loss method was employed for a room temperature (303 K) and 333 K. The temperature for each run of the experiments was kept constant using a thermostat. In this procedure, the mass loss of the metal in uninhibited (with no AZI extract) and inhibited solutions were monitored and recorded. About 50 mL of test solutions were analyzed. From these data, the inhibition efficiency (%I) and degree of surface coverage (θ) were calculated Citation37 using Equations Equation1 and Equation2, respectively:
Conclusion
From the present study, it is found that AZI leaves extract can be used as an inhibitor for zinc corrosion in H2SO4 medium. While the green inhibitor molecule most supposedly acts by being adsorbed on zinc surface, the overall inhibition is provided by a synergistic effect. It has also been found that the inhibitive action of AZI leaves extract is basically controlled by temperature and the concentration of the inhibitor. A probable sequel to the present study would be to perform in-depth chemical and analytical investigations using techniques like NMR or IR spectroscopy together with electrochemical studies so as to depict which are the active components of the AZI leaves extract involved in the corrosion inhibition reaction, and also elucidate the corrosion inhibition mechanism.
Acknowledgements
The authors are grateful to Dr. V.K. Agarwal (Chairman) of the Institute of Engineering and Technology (IET) Group of Institutions, Alwar, India, for providing us the opportunity to establish a Computational and Green Chemistry Research Laboratory at IET whereat burgeoned the idea to carry out the present work. Special thanks also go to Ms Vanessa Jumnoodoo (Department of Chemical & Environmental Engineering, University of Mauritius) for her insight into the further chemical and analytical tests that have to be done to elucidate the corrosion inhibition reaction mechanisms.
References
- Thompson , N.G. , Yunovich , M. and Dunmire , D. 2007 . Cost of Corrosion and Corrosion Maintenance Strategies . Corrosion Rev. , 25 : 247 – 262 .
- Anuradha , K. , Vimala , R. , Narayanasamy , B. , Arockia Selvi , J. and Rajendran , S. 2008 . Corrosion Inhibition of Carbon Steel in Low Chloride Media by an Aqueous Extract of Hibiscus Rosa-Sinensis . Linn. Chem. Eng. Comm. , 195 : 352 – 366 .
- Valdez , B. , Cheng , J. , Flores , F. , Schorr , M. and Veleva , L. 2003 . Application of Vapour Phase Corrosion Inhibitors for Silver Corrosion Control in the Electronics Industry . Corrosion Rev. , 21 : 445 – 458 .
- Taylor , S.R. and Chambers , B.D. 2007 . The Discovery of Non-Chromate Corrosion Inhibitors for Aerospace Alloys Using High-Throughput Screening Methods . Corrosion Rev. , 25 : 571 – 590 .
- Khaled , K.F. 2008 . New Synthesized Guanidine Derivative as a Green Corrosion Inhibitor for Mild Steel in Acidic Solutions . Int. J. Electrochem. Sci. , 3 : 462 – 475 .
- Bothi Raja , P. and Sethuraman , M.G. 2008 . Natural Products as Corrosion Inhibitor for Metals in Corrosive Media – A Review . Mater Lett. , 62 : 113 – 116 .
- Rajendran , S. , Amalraj , A.J. , Joice , M.J. , Anthony , N. , Trivedi , D.C. and Sundaravadivelu , M. 2004 . Corrosion Inhibition by the Caffeine – Zn2+ System . Corrosion Rev. , 22 : 233 – 248 .
- Mesbah , A. , Juers , C. , Lacouture , F. , Mathieu , S. , Rocca , E. , François , M. and Steinmetz , J. 2007 . Inhibitors for Magnesium Corrosion: Metal Organic Frameworks . Solid State Sci. , 9 : 322 – 328 .
- Okafor , P.C. , Osabor , V.I. and Ebenso , E.E. 2007 . Eco-friendly Corrosion Inhibitors: Inhibitive Action of Ethanol Extracts of Garcinia Kola for the Corrosion of Mild Steel in H2SO4 Solutions . Pigment and Resin Technol. , 36 : 299 – 305 .
- Lebrini , M. , Traisnel , M. , Lagrenée , M. , Mernari , B. and Bentiss , F. 2008 . Inhibitive Properties, Adsorption and a Theoretical Study of 3,5-Bis(n-pyridyl)-4-Amino-1,2,4-Triazoles as Corrosion Inhibitors for Mild Steel in Perchloric Acid . Corrosion Sci. , 50 : 473 – 479 .
- Radoj(i( , I. , Berkovi( , K. , Kova( , S. and Vorkapi(-Fura( , J. 2008 . Natural Honey and Black Radish Juice as Tin Corrosion Inhibitors . Corrosion Sci. , 50 : 1498 – 1504 .
- Refaey , S.A.M. , Abd El Malak , A.M. , Taha , F. and Abdel-Fatah , H.T.M. 2008 . Corrosion and Inhibition of Cu-Zn Alloys in Acidic Medium by Using Isatin . Int. J. Electrochem. Sci. , 3 : 167 – 176 .
- Sharma , S.K. ; Mudhoo , A. ; Khamis , E. Green Corrosion Inhibitors: An overview of Recent Research . J. Corrosion Sci. Eng. (Electronic Journal) 2009 , 11 , 1 – 25 .
- Abdel-Gaber , A.M. ; Khamis , E. ; Abo-Eldahab , H. ; Adeel , S. Mater. Chemistry and Phys . 2008 , 109 , 297 – 305 .
- Khamis , E. , Hefnawy , A. and El-Demerdash , A.M. 2007 . Acid Cleaning of Steel Using Arghel Echo Friendly Corrosion Inhibitor . Materialwissenschaft und Werkstofftechnik , 38 : 227 – 232 .
- Umoren , S.A. and Ebenso , E.E. 2008 . Studies of Anti-corrosive Effect of Raphia Hookeri Exudates Gum – Halide Mixtures for Aluminium Corrosion in Acidic Medium . Pigment and Resin Technol. , 37 : 173 – 182 .
- Okafor , P.C. and Ebenso , E.E. 2007 . Inhibitive Action of Carica Papaya Extracts on the Corrosion of Mild Steel in Acidic Media and their Adsorption Characteristics . Pigment and Resin Technol. , 36 : 134 – 140 .
- El-Etre , A.Y. 2006 . Khillah Extract as Inhibitor for Acid Corrosion of SX 316 Steel . Appl. Surf. Sci. , 252 : 8521 – 8525 .
- Umoren , S.A. , Ogbobe , O. , Ebenso , E.E. and Ekpe , U.J. 2006 . Effect of Halide Ions on the Corrosion Inhibition of Mild Steel in Acidic Medium Using Polyvinyl Alcohol . Pigment and Resin Technol. , 35 : 284 – 292 .
- Bendahou , M.A. , Benadellah , M.B.E. and Hammouti , B.B. 2006 . A Study of Rosemary Oil as a Green Corrosion Inhibitor for Steel in 2M H3PO4 . Pigment and Resin Technol. , 35 : 95 – 100 .
- Sharma , S.K. , Chaudhary , A. and Singh , R.V. 2008 . Gray Chemistry verses Green Chemistry: Challenges and Opportunities . RASAYAN J. Chem. , 1 : 68 – 92 .
- Subapriya , R. and Nagini , S. 2005 . Medicinal Properties of Neem Leaves: A Review . Curr. Med. Chem. Anti Can. Agents , 5 : 149 – 156 .
- Dasgupta , T. , Banerjee , S. , Yadava , P.K. and Rao , A.R. 2004 . Chemopreventive Potential of Azadirachta Indica (Neem) Leaf Extract in Murine Carcinogenesis Model Systems . J. Ethnopharmacol. , 92 : 23 – 36 .
- Biswas , K. , Chattopadhyay , I. , Banerjee , R.K. and Bandyopadhyay , U. 2002 . Biological Activities and Medicinal Properties of Neem (Azadirachta indica) . Curr. Sci. , 82 : 1336 – 1345 .
- Bhattacharyya , K.G. and Sharma , A. 2004 . Adsorption of Pb(II) from Aqueous Solution by Azadirachta Indica (Neem) Leaf Powder . J. Hazard. Mater. , 113 : 97 – 109 .
- Oguzie , E.E. 2006 . Adsorption and Corrosion Inhibitive Properties of Azadirachta Indica in Acid Solutions . Pigment and Resin Tech. , 35 : 334 – 340 .
- Valek , L. and Martinez , S. 2007 . Copper Corrosion Inhibition by Azadirachta Indica Leaves Extract in 0.5M Sulphuric Acid . Mater. Lett. , 61 : 148 – 151 .
- Roy , M.K. , Kobori , M. , Takenaka , M. , Nakahara , K. , Shinmoto , H. , Isobe , S. and Tsushida , T. 2007 . Antiproliferative Effect on Human Cancer Cell Lines after Treatment with Nimbolide Extracted from an Edible Part of the Neem Tree (Azadirachta indica) . Phytother. Res. , 21 : 245 – 250 .
- Sawchuk , M.J. An environmentally friendly insecticide for conifer seed orchards . MS Thesis, Department of Biological Sciences, Simon Fraser University , 1994 , 1 – 58 .
- Koul , O. , Isman , M.B. and Ketkar , C.M. 1990 . Properties and Uses of Neem, Azadirachta indica . Can. J. Bot. , 68 : 1 – 11 .
- Saxena , R.C. and Kidiavai , E.L. 1997 . Neem Seed Extract Spray Applications as Low-Cost Inputs for Management of the Flower Thrips in the Cowpea Crop . Phytoparasitica , 25 : 99 – 110 .
- Schmutterer , H. 1995 . The Neem Tree Azadirachta Indica A. Juss. and Other Meliaceous Plants: Source of Unique Natural Products for Integrated Pest Management, Medicine, Industry and Other Purposes , Germany : VCH, Weinheim .
- Suresh , G.V. , Gopalakrishnan , G. and Masilamani , S. 2007 . Neem for Plant Pathogenic Fungal Control: The Outlook in the New Millennium. Neem: Today and in the New Millennium , 183 – 207 . Springer : The Netherlands .
- Ebenso , E.E. Bull Electrochem . 2003 , 19 , 209 – 216 .
- Ebenso , E.E. Bull Electrochem . 2004 , 20 , 551 – 559 .
- Sliwka-Kaszynska , M. , Kot-Wasik , A. and Namiesnik , J. 2003 . Preservation and Storage of Water Samples . Crit. Rev. Environ. Sci. Tech. , 33 : 31 – 44 .
- Eddy , N.O. and Ebenso , E.E. 2008 . Adsorption and Inhibitive Properties of Ethanol Extracts of Musa Sapientum Peels as a Green Corrosion Inhibitor for Mild Steel in H2SO4 . AJPAC , 2 : 46 – 54 .