Abstract
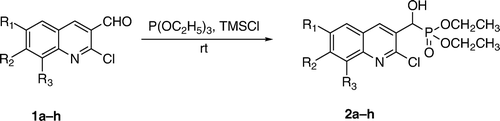
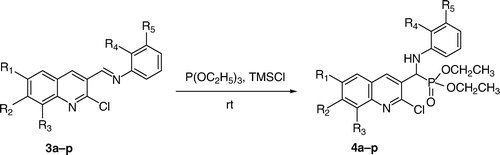
Solvent free, and quantitative yielding synthesis of α-hydroxyphosphonates (2a–h) from 2-chloroquinolin-3-carbaldehyde (1a–h) and α-aminophosphonates (4a–p) from imines (3a–p), obtained from 2-chloroquinoline-3-carbaldehyde by using triethylphosphite in the presence of chlorotrimethylsilane at room temperature in short time.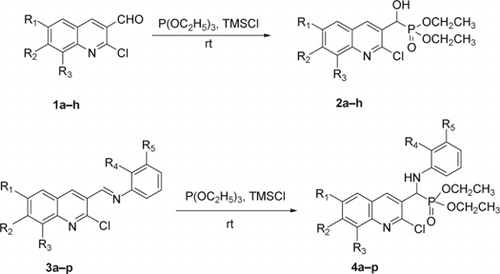
Introduction
Quinolines Citation1–3 are an important class of heterocyclic compounds and have been screened for several biological activities such as bactericidal Citation4, antitumor Citation5, anti-inflammatory Citation6, and antimalarial Citation7. Quinolines such as 2-chloroquinoline-3-carbaldehyde occupy a prominent position as they are key intermediates for further annelation and for various functional group interconversions Citation8 Citation9. It is also reported that organophosphates are potent pesticides which have wide variety of application Citation10. Recently, some new vinyl phosphates have been reported as potent inhibitors of phosphatase Citation11–13 and phosphodiesterase Citation14 Citation15.
There are only a few reports on the synthesis and bioactivity of C–P bonds which have been found to have insecticidal Citation16 and antifungal Citation17 activities. Also α-hydroxyphosphonates Citation18 and α-aminophosphonates are important biologically active compounds Citation19 Citation20. α-Aminophosphonates, due to their structural analogy to amino acids, have been the subject of considerable current interest. They act as peptide mimics Citation21, enzyme inhibitors Citation22 Citation23, antibiotics, and pharmacological agents Citation24 Citation25.
α-Hydroxyphosphonates may serve as precursors for the synthesis of α-amino-phosphonates which are analogs of amino acids. A number of synthetic methods for the synthesis of α-hydroxyphosphonates have been reported Citation26–29. But the disadvantage associated with the existing methodologies is either long reaction times or the requirement of drastic conditions.
In the literature, α-hydroxyphosphonates have been prepared using: quinine catalyst in toluene as solvent Citation30, DBU or n-BuLi in THF Citation31, HCl: ether media in DCM Citation32, LiClO4: diethyl ether solution in the presence of trimethylsilyl chloride (TMSCl) Citation33, toluene and Ti(OiPr)4 Citation34, hydroxyphosphorylation of aldehydes catalyzed by guanidine hydrochloride in water Citation35, BF3.etherate and AlCl3 Citation36, and TFA or TfOH Citation37.
Generally, α-aminophosphonates are prepared in the presence of Lewis acids or bases by the addition of phosphorous nucleophiles to the imines. Lewis acids such as SnCl4, SnCl2, ZrCl4, ZnCl2, and MgBr2 have been used as catalysts for such reactions Citation38–40. Recently, Lewis and Bronsted acids such as LiClO4 Citation41, InCl3 Citation42, lanthanide triflates Citation43, TaCl5–SiO2 Citation44, montmorillonite clay-MW Citation45, Al2O3-MW Citation46, and CF3COOH Citation47 were found to be effective in the preparation of α-aminophosphonates. However, many of these procedures require expensive reagents, long reaction times and suffer from poor yields. These reactions cannot be carried out in one step by the reaction between a carbonyl compounds, an amine and dialkylphosphite because the amine and water present during imine formation can decompose or deactivate the Lewis acid Citation48. In continuation of our work on phosphorus chemistry Citation49–52, herein we wish to report the solvent free synthesis of α-hydroxyphosphonates and α-aminophosphonates at room temperature in quantitative yield.
Results and discussion
We have reported the synthesis of α-hydroxyphosphonates Citation51 from 2-chloroquinolin-3-carbaldehyde at reflux temperature in toluene; while at reflux temperature, TMSCl was added. To add TMSCl at the reflux temperature is not ecofriendly because it emits gases during addition. Azizi et al. Citation33 reported that for the same system at room temperature costly moisture sensitive reagents such as LiClO4 and diethyl ether media could be used. To overcome these difficulties we have developed a new method for the synthesis of α-hydroxyphosphonate from 2-chloroquinolin-3-carbaldehyde. Herein we wish to report the newer method which is economically viable, solvent free, and carried out at room temperature in quantitative yield.
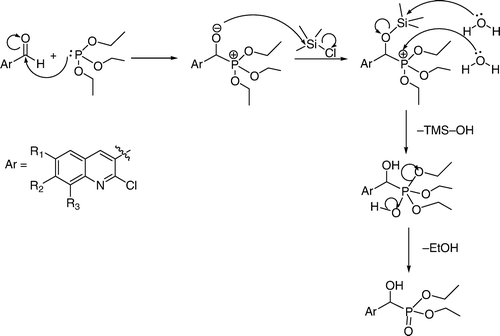
To the mixture of triethylphosphite and 2-chloroquinolin-3-carbaldehyde (1a–h) TMSCl was added in stirring at room temperature. During addition, we observed that the reaction was exothermic (temperature rise from 25 to 40°C). 2-Chloroquinolin-3-carbaldehyde has two reaction centers: chlorine at the second and a formyl group at the third position of quinoline ring. However, the formyl group has higher reactivity in the presence of TMSCl. TMSCl reacts with the formyl group to generate a carbonium ion and a TMS protected hydroxyl group. Triethylphosphite attacks the generated carbonium ion and the mentioned compound rearranges to α-trimethylsilyloxyphophonate. By adding methanol, unreacted TMSCl was reacted with methanol and generates gaseous HCl which yielded desired product (2a–h) (, ). Through this method, there was significant improvement in the yields of the products. The reported yields of α-hydroxyphosphonates in the presence of solvent and at higher temperature were 76–83% Citation51. The yields of the α-hydroxyphosphonates using this new process are now in the range of 95–97%. Here we have synthesized eight compounds by applying the same procedure and obtained each in quantitative yield. All the synthesized compounds are characterized by spectral analysis, physical constants, and compared with their authentic.
Table 1. TMSCl facilitated synthesis of α-hydroxyphosphonates.
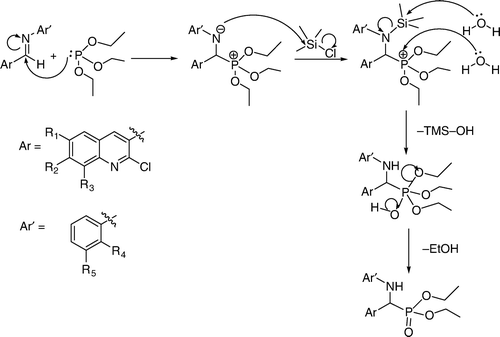
Previously we have synthesized α-aminophosphonates Citation52 containing highly bioactive quinoline moiety in two steps. In the first step, imines of 2-chloroquinoline-3-carbaldehyde were synthesized and converted to α-aminophosphonates using TMSCl and triethylphosphite in acetonitrile at reflux in the next step. Herein we report a newer method which is economically viable, solvent free and carried out at room temperature in quantitative yield. Imines (3a–p) () were prepared at room temperature from derivatives of 2-chloroquinoline-3-carbaldehyde and 3-fluoroaniline or 2-methylaniline in ethanol using a catalytic amount of acetic acid in excellent yields and were characterized by mass spectra. α-Aminophosphonates (4a–p) (, ) were then prepared in quantitative yields by reacting imines (3a–p) with triethylphosphite in the presence of TMSCl at room temperature. After completion of the reaction, the excess TMSCl was removed using methanol. The reported yields of α-aminophosphonates in the presence of solvent and at higher temperature were 89–93% Citation52. The yields of the α-aminophosphonates using this new process are now in the range of 95–98%. Here we have synthesized 16 compounds by applying the same procedure and obtained each in quantitative yield. All the compounds synthesized were unequivocally characterized on the basis of analytical data.
Table 2. TMSCl facilitated synthesis of α-aminophosphonates.
The mechanism of the formation of α-hydroxyphosphonates and α-aminophosphonates in the presence of TMSCl have shown in and , respectively.
Experimental
2-Chloroquinoline-3-carbaldehydes were prepared in the laboratory by the reported method Citation53. Triethylphosphite and chlorotrimethylsilane were procured from Lancaster; methanol and N,N-dimethylformamide (DMF) were procured from S.D. Fine-chem.
All melting points were determined in open capillaries on Kumar's melting point apparatus. The products were characterized by their spectral data. 1H NMR spectra were recorded on Varian Gemini in CDCl3 at 400 MHz using TMS as an internal standard. IR spectra were recorded on a Perkin–Elmer FTIR using KBr discs. Mass spectra were recorded on Micromass Quatrro-II using electrospray Ionization technique, showing (m + 1) peak as a molecular ion peak. The test for the purity of products and the progress of the reactions was accomplished by TLC on Merck silica gel plates.
General procedure
(2a) Diethyl (2-chloro-quinolin-3-yl) (hydroxy) methylphosphonate
To the mixture of 2-chloroquinoline-3-carbaldehyde (0.95 gm, 5 mmol), triethylphosphite (1.66 gm, 10 mmol), and chlorotrimethylsilane (1.08 gm, 10 mmol) were added dropwise and stirred at room temperature. The progress of the reaction was monitored by TLC using hexane:ethyl acetate (7:3) as the solvent system. After completion of the reaction (25 min), the reaction mixture was dissolved in methanol for the quenching of excess TMSCl and removal of the residual silyl ester linkages. This methanolic solution was concentrated to get crude product. Further purification was achieved by dissolving the crude compound in dichloromethane and precipitated by hexane. The solid obtained was stirred for 15 min and filtered, washed with hexane and dried at 40°C (1.56 gm, yield 96%, m.p. 124–126°C).
IR (KBr), cm −1 : 3246 (−OH); 1218 (−P = O); 1033 (−P–O–C). 1 H NMR (CDCl 3 ), δ ppm: 1.2 (t, 3H, O–CH2–CH3); 1.3 (t, 3H, O–CH2–CH3); 2.0 (s, 1H, −CH–OH); 4.0 (m, 4H, O–CH2–CH3 and O–CH2–CH3); 5.6 (d, 1H, −CH–P = O); 7.5 (t, 1H, Ar–H, C6); 7.7 (t, 1H, Ar–H, C7); 7.8 (d,1H, Ar–H, C5); 8.0 (d, 1H, Ar–H, C8); 8.6 (s, 1H, Ar–H, C4). ES–MS: m/z 330 (m + 1) and 331.9 (m + 3).
Elemental analysis: C14H17ClNO4P calculated: C: 51.00%, H: 5.20%, N: 4.25%; found: C: 51.027%, H: 5.393%, N: 4.35%.
(4a) Diethyl (3-fluorophenylamino) (2-chloroquinolin-3-yl)methylphosphonate
To a mixture of N-((2-chloroquinolin-3-yl-methylene)-3-fluorobenzenamine (1.12 gm, 4 mmol) and triethylphosphite (1.66 gm, 10 mmol) was added TMSCl (1.08 gm, 10 mmol). The progress of the reaction was monitored by TLC using hexane:ethyl acetate (8:2) as the solvent system. After the completion of the reaction, the reaction mixture was dissolved in methanol for the quenching of excess TMSCl and removal of the residual silyl ester linkages. This methanolic solution was concentrated to get crude product. The solid was further recrystallized from DMF and water mixture, dried in an oven at 50°C for 8.0 h (dry wt.=1.59 gm, yield 96%).
IR (KBr): 3311 cm−1 (−NH); 1234 cm−1 (−P = O); 1032 cm−1 (−P–O–C). 1 H NMR (CDCl 3 , δ ppm): 1.05 (t, 3H, O–CH2 –CH3, J=8 Hz); 1.35 (t, 3H, O–CH2–CH3, J=8 Hz); 3.7 (m, 1H, O–CH2–CH3); 3.9 (m, 1H, O–CH2–CH3); 4.2 (m, 2H, O–CH2–CH3); 5.4 (d, 1H, –NH–CH–P = O, J=24 Hz); 6.3–6.5 (m, 3H, Ph–H, C2, C4, C6); 7.0 (dd, 1H, Ph–H, C5, J=8 Hz); 7.5 (t, 1H, Quinolin-H, C5, J=8 Hz); 7.69 (t, 1H, Quinolin-H, C6, J=8 Hz); 7.75 (d, 1H, Quinolin-H, C7, J=8 Hz); 7.99 (d, 1H, Quinolin-H, C8, J=8 Hz); 8.34 (d, 1H, Quinolin-H, C4, J=8 Hz). ES–MS: m/z 423.1 (m + 1) and 425.1 (m + 3).
Elemental analysis: C20H21ClFN2O3P calculated: C: 56.81%, H: 5.01%, N: 6.63%; found: C: 56.72%, H: 4.95%, N: 6.65%.
Conclusion
In conclusion, a new methodology was developed for the synthesis of α-hydroxyphosphonates 2a–h from 2-chloroquinolin-3-carbaldehyde 1a–h by using triethylphosphite in the presence of TMSCl at room temperature in quantitative yields. Also a new methodology was developed for the synthesis of α-aminophosphonate (4a–p) derivatives from imines of 2-chloroquinoline-3-carbaldehydes for the first time using TMSCl at room temperature. All the reactions were performed under mild reaction conditions, shorter reaction times and in quantitative yields ( and ). The methodology developed will be of much use to combinatorial chemist.
Acknowledgements
Authors are thankful to the Head, Department of Chemistry, Dr. B.A.M. University, Aurangabad for providing laboratory facilities.
References
- Elderfield , R. 1952 . Heterocycl . Compd. , 4 : 125 – 129 .
- Meth-Cohn , O. and Narine , B. 1978 . Tetrahedron , 19 : 2045 – 2048 .
- Ali M.M. Tasneem K.C. Rajanna K.C. Saiprakash P.K. Synletters 2001 2 251 253 and references cited therein
- Patel , H.V. , Vyas , K.V. and Fernandes , P.S. 1990 . Indian J. Chem. , 29(B) : 836 – 842 .
- Sukhova , N.M. , Lidak , M. , Zidermane , A. , Pelevina , I.S. and Voronia , S.S. 1989 . Khim. Farm. Zh. , 23 : 1226 – 1229 .
- Dillard , R.D. , Pavey , D.E. and Benslay , D.N. 1973 . J. Med. Chem. , 16 : 251 – 253 .
- Craig , J.C. and Person , P.E. 1971 . J. Med. Chem. , 14 : 1221 – 1222 .
- Meth-Cohn O. Heterocycles 1993 35 539 557 and references cited therein
- Rajendran , S.P. , Manonmoni , M. and Vijaya-Lakshmi , S. 1994 . Org. Prep. Proced. Int. , 26 : 383 – 385 .
- Engel R. In Handbook of Organophosphorus Chemistry New York Marcel Dekker 1992
- Hang , S.B. , Mullins , T.S. , Shim , H. and Raushal , F.M. 1997 . Biochemistry , 36 : 9022 – 9028 .
- Berggren , M.M. , Burns , L.A. , Abraham , R.T. and Powis , G. 1993 . Cancer Res. , 53 : 1862 – 1866 .
- Cao , X.D. , Moran , E.J. , Siev , D. , Lio , A. , Ohashi , C. and Mjalli , A.M. 1995 . Bioorg. Med. Chem. Lett. , 5 : 2953 – 2958 .
- Widlanski , T.S. , Myer , J.K. , Stec , B. , Holtz , K.M. and Kantroewitz , E.R. 1997 . Chem. Biol. , 4 : 489 – 498 .
- Stowell , J.K. and Widlanski , T.S. 1995 . J. Org. Chem. , 60 : 6930 – 6936 .
- Maurer F. Riebel H.J. Hammann I. Behrenz W. Homeyer B. Ger. Offen 2533601 1977
- Molodykh , Z.V. , Aleksandrova , I.A. , Belyalov , R.U. , Gazizor , T.K. and Reznik , V.S. 1990 . Khim. Farm. Zh. , 24 : 136 – 139 .
- Drake G.L. Calamari T.A. In Industrial Uses of Phosphonates (Review) Hilder Brand R.L. Boca Raton, FL CRC Press 1983 Chapter 7
- Fields , S.C. 1999 . Tetrahedron , 55 : 12237 – 2273 .
- Yokomatsu , T. , Yoshida , Y. and Shibuya , S. 1994 . J. Org. Chem. , 59 : 7930 – 7933 .
- Kafarski , P. and Lejczak , B. 1991 . Phosphorus, Sulfur, Silicon Relat. Elem. , 63 : 193 – 215 .
- Allen , M.C. , Fuhrer , W. , Tuck , B. , Wade , R. and Wood , J.M. 1989 . J. Med. Chem. , 32 : 1652 – 1661 .
- Giannousis , P.P. and Bartlett , P.A. 1987 . J. Med. Chem. , 30 : 1603 – 1609 .
- Atherton , F.R. , Hassal , C.H. and Lambert , R.W. 1986 . J. Med. Chem. , 29 : 29 – 40 .
- Baylis E. K. Campbell C. D. Dingwall J. J. Chem. Soc., Perkin Trans 1984 I 2845 2853
- Lerner , R.A. , Benkovic , S.J. and Schultz , P.G. 1991 . Science , 252 : 659 – 667 .
- Groger , H. and Hammer , B. 2000 . Chem. Eur. J. , 6 : 943 – 948 .
- Olah , G.O. and Wu , A.H. 1991 . J. Org. Chem. , 56 : 902 – 904 .
- Jeanmaire , T. , Hervaud , Y. and Boutevin , B. 2002 . Phosphorus, Sulfur, Silicon Relat. Elem. , 177 : 1137 – 1145 .
- Smaardijk , A.A. , Noorda , S. , van Bolhuis , F. and Wynberg , H. 1985 . Tetrahed. Lett. , 26 : 493 – 496 .
- Oscar , P. and Backvall , J-E. 2003 . J. Org. Chem. , 68 : 4815 – 4818 .
- Goldman W. Soroka M. Synthesis 2006 3019 3024
- Azizi , N. and Saidi , M.R. 2003 . Phosphorus, Sulfur, Silicon Relat. Elem. , 178 : 1255 – 1259 .
- Yokomatsu T. Yamagishi T. Shibuya S. J. Chem. Soc. Perkin Trans I 1997 1527 1533
- Heydari , A. , Arefi , A. , Khaksar , S. and Tajbakhsh , M. 2006 . Catal. Commun. , 7 : 982 – 984 .
- Pudovic , A.N. , Zimin , M.G. , Sobanov , A.A. and Musina , A.A. 1976 . Zh. Obshch. Khim. , 46 : 1455 – 1461 .
- Nifant'ev , E.E. , Kukhareva , T.S. , Popkova , T.N. and Davydocchkina , O.V. 1986 . Zh. Obshch. Khim. , 56 : 304 – 309 .
- Kudzin Z.H. Lyzwa P. Luczak J. Andrijewski G. Synthesis 1997 44 46
- Yadav J.S. Reddy B.V.S. Sarita Raj K. Bhaskar Reddy K. Prasad A.R. Synthesis 2001 2277 2780
- Lee S.G. Park J.H. Kang J. Lee J.K. Chem. Commun. 2001 1698 1699
- Saidi M.R. Azizi N. Synletters 2002 1347 1349
- Ranu , B.C. , Hajra , A. and Jana , U. 1999 . Org. Lett. , 1 : 1141 – 1143 .
- Qian , C. and Huang , T. 1998 . J. Org. Chem. , 63 : 4125 – 4128 .
- Chandrasekher , S. , Prakash , S.J. , Jagadeshwar , V. and Narsihmulu , C. 2001 . Tetrahed. Lett. , 42 : 5561 – 5563 .
- Yadav J.S. Reddy B.V.S. Madan C. Synletters 2001 1131 1133
- Kaboudin , B. and Nazari , R. 2001 . Tetrahed. Lett. , 42 : 8211 – 8213 .
- Akiyama T. Sanada M. Fuchibe K. Synletters 2003 1463 1464
- Zon , J. 1981 . Pol. J. Chem. , 55 : 643 – 646 .
- Mane , A.S. , Chavan , V.P. , Karale , B.K. , Hangarge , R.V. , Gaikwad , M.S. and Shingare , M.S. 2002 . Synthetic Commun. , 32 ( 17 ) : 2633 – 2636 .
- Chavan , V.P. , Mane , A.S. and Shingare , M.S. 2001 . Indian J. Chem. , 40B : 339 – 341 .
- Pokalwar R.U. Hangarge R.V. Maske P.V. Shingare M.S. Arkivoc 2006 xi 196 204
- Pokalwar , R.U. , Hangarge , R.V. , Madje , B.R. , Ware , M.N. and Shingare , M.S. 2008 . Phosphorus, Sulfur, Silicon Relat. Elem. , 183 : 1461 – 1470 .
- Meth-Cohn O. Narine B. Tarnowski B. J. Chem. Soc., Perkin Trans I 1981 1520 1530