Abstract
Battery technology is an important anthropogenic source of the heavy metals which are highly threatening to human health. A category of rechargeable lithium batteries that is of great interest is the set of batteries where the cathode material is a lithium iron phosphate (LiFePO4). LiFePO4 is an environmentally friendly and safe lithium-ion battery cathode material, but it has a key limitation, and that is its extremely low-electronic conductivity, a problem that can be greatly overcome by zinc-doping LiFePO4. For the first time to our knowledge, a low-temperature method, that is advantageous both economically and technologically, for the synthesis of a zinc-doped LiFePO4 is presented. Since the method appears to be applicable for synthesizing various zinc-doped LiFePO4 compounds with the general formula LiFe1−x Zn x PO4 (0<x<1), it is very promising for the production of a green cathode material for lithium-ion batteries.
Introduction
The main threats to human health from heavy metals are associated with exposure to lead, cadmium, and mercury Citation1. Rechargeable nickel–cadmium batteries are where cadmium compounds are currently mainly used Citation1, lead-acid cells are a main family of batteries Citation2, and some small batteries represent potential sources of mercury Citation3. It is, therefore, appropriate to say that battery technology has been in need of improvement as for environmental friendliness and safety.
Lithium-ion batteries were introduced by the Sony Corporation in the early 1990s Citation4. They are of great technological importance because of their usage in personal electronic devices, such as cellular phones, notebook computers, laptops, and high-end desktop computers. Also, advanced rechargeable lithium batteries have, in principle, the capability of satisfying all of the performance requirements of an electric vehicle Citation5. A category of rechargeable lithium batteries that is of great interest is the set of batteries where the cathode material is a lithium iron phosphate (LiFePO4) Citation6. LiFePO4 is an environmentally friendly and safe Citation6. It has a high-lithium intercalation voltage (~3.5 V relative to lithium metal), high-theoretical capacity (170 mA h g−1), low raw materials cost, and stability when used with common electrolyte systems Citation6. Yet, it also has a key limitation, and that is its extremely low-electronic conductivity Citation6. A feasible way to enhance the intrinsic conductivity is doping alien cations of elements such as Mn, Mg, Ti, and Zr Citation7. Zinc is the most electronegative transition metal in nature, and zinc-doping LiFePO4, therefore, can improve the electronic conductivity of LiFePO4 most greatly. Experimental studies on electrochemical behavior of zinc-doped LiFePO4 for lithium battery positive electrode have shown not only that zinc-doping can increase the conductivity of LiFePO4, but also that the conductivity of zinc-doped LiFePO4 varies with Zn:Fe ratio Citation8.
Lithium battery cathode materials are often synthesized by using “solid-state” synthetic methods. In this synthetic path, exact stoichiometric amounts of starting materials are well mixed, ground, and then left to react at high temperatures. Although the compounds of interest are achieved, two main problems stay unsolved. First of all, the solid-state synthesis is often costly, due especially to the required high heat. As for the other problem, due to large particle size, the surface areas of the compounds obtained by using the solid-state synthesis are relatively small. The alternative method used for the synthesis of lithium battery cathode materials is a set of “wet” synthetic methods. This is a low-temperature synthetic method. In wet methods, the precursors react in solutions. Since the problem of very high temperatures does not exist in this synthetic path, it is much more economically feasible. Also, the products have larger surface areas compared to those of the solid-state methods.
LiFePO4 has originally been synthesized using the solid-state path by heating lithium carbonate (Li2CO3), ammonium dihydrogenphospate (NH4H2PO4), and iron oxalate dihydrate (FeC2O4.2H2O) in nitrogen or argon gas at 600–850°C Citation6. Using the same sources for lithium, phosphate, and iron, and using zinc oxide (ZnO) as the source for zinc, a zinc-doped LiFePO4 material has also been prepared by a solid-state route by Liu et al. Citation7. Iron (II) sulfate has been used in the past as a starting material for high-temperature synthesis of both LiFePO4 Citation9 and zinc-doped LiFePO4 Citation10. The proposed reaction is as follows:
Results and discussion
X-ray diffraction analysis
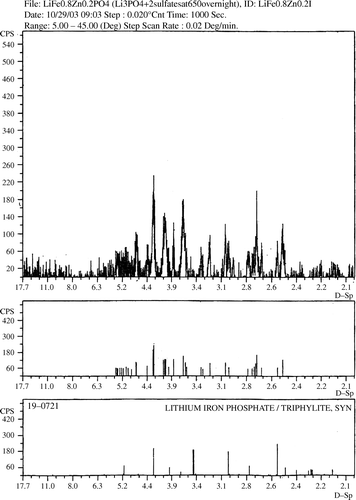
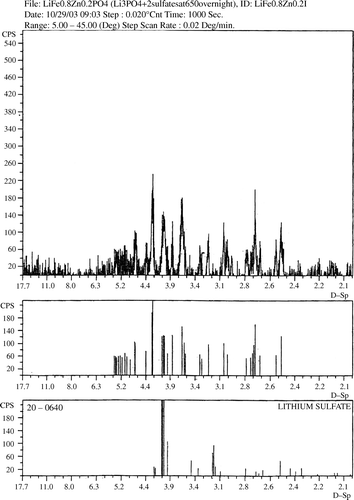
The X-ray powder diffraction analysis of the zinc-doped product showed that the prepared sample was amorphous. In order to make the compound crystalline, the prepared sample was heated to higher temperatures. The sample showed meaningful crystallinity only after being heated at 650°C for 24 hours () Citation11. The X-ray powder pattern showed that the crystalline phase is isostructural with LiFePO4. It also shows some extra diffraction peaks that we were able to assign to some Li2SO4 impurities (). lists the interplanar spacings of our product versus those of LiFePO4 Citation12 and Li2SO4 Citation13.
Table 1. The interplanar spacings of our product versus those of LiFePO4 and Li2SO4.
Fourier Transform Infrared (FTIR) spectroscopy
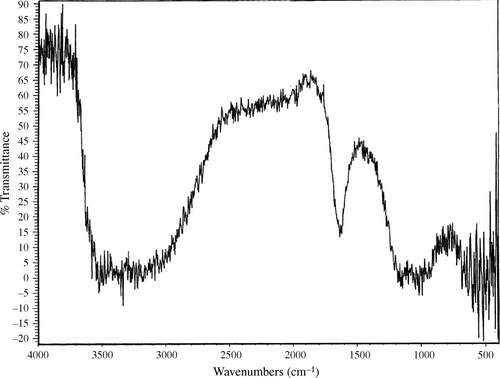
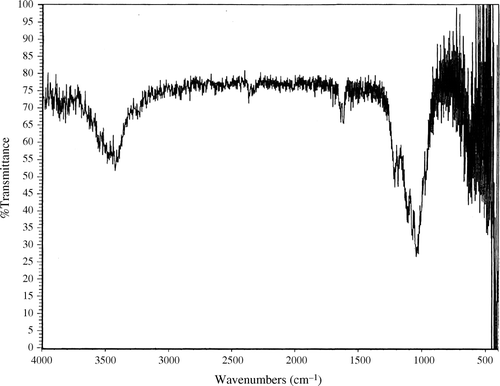
In order to confirm the results of the X-ray powder diffraction analysis, Fourier Transform Infrared (FTIR) spectra of both the sample heated to 110°C for three hours and the one heated to 650°C were obtained ( and ). Both spectra show a wide peak between 1000 and 1100 cm−1. That peak corresponds to vibrations due to PO4 3− ions Citation14. However, a sharper peak is found in both spectra at 1100 cm−1 exactly. That peak corresponds to vibrations due to SO4 2− ions Citation14. However, a sharper peak is found in both spectra at 1100 cm−1 exactly. The peaks observed at 1600 and 3500 cm−1 are both due to vibrations in water molecules Citation14. This is water adsorbed by the prepared sample due to exposure of the sample to the atmosphere.
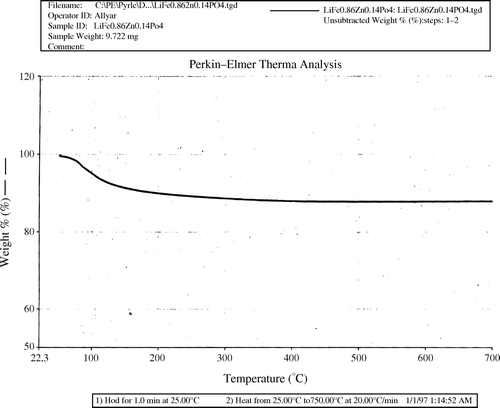
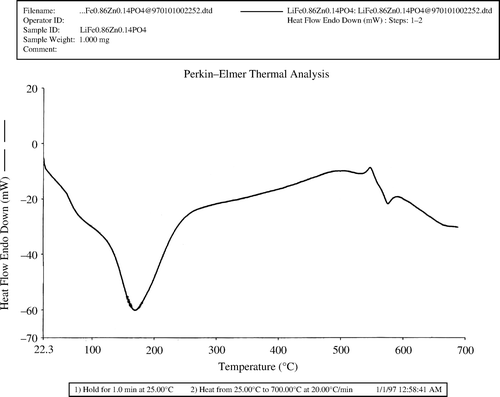
TGA and DTA analysis
In order to check if the original product is the same as the one available after being heated at 650°C, Thermogravimetric Analyzer (TGA) along with Differential Thermal Analyzer (DTA) were used. The thermal gravimetric diagram shows only one weight loss that starts at about 80°C (). This weight loss is due to the endothermic thermal event observed by differential thermal analysis (). This event is attributed to the loss of water. This is well shown by FTIR Spectroscopy where vibrations peaks due to water molecules are greatly diminished when the sample is heated to 650°C. The change is a decrease in mass during the temperature increase of the sample (from 75 to 125°C). The differential thermal diagram shows one endothermic peak in that temperature range. That peak indicates the loss of water. The DTA also shows an exothermic peak at around 540°C (). The X-ray powder diffraction results show that this exothermic peak corresponds to the process of the crystallization of our product.
Lithium-7 solid-state nuclear magnetic resonance (NMR) spectroscopy
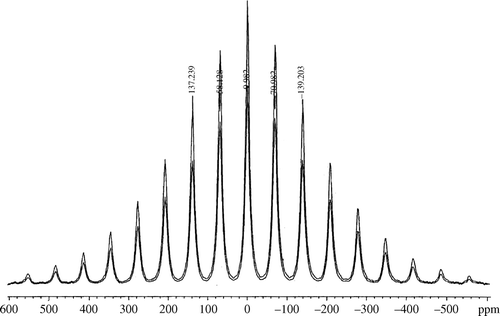
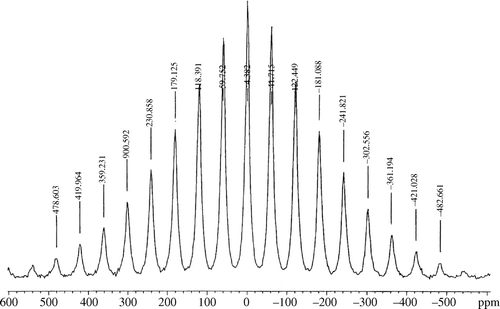
The Li-7 Magic Angle Spinning Nuclear Magnetic Resonance (MAS-NMR) spectrum of undoped LiFePO4 is available in the literature Citation15. The Li-7 MAS-NMR spectra of our doped product were obtained and compared with the literature results ( and ).
In each spectrum, the Li-7 magnetic resonance shift was found at −0.982 ppm relative to 0.01 M LiCl (aq). and show the Li-7 MAS-NMR spectra obtained at two different spinning frequencies in order to differentiate between spinning sidebands, due to the inability to average-out all anisotropic interactions, and the Li-7 resonance shift. The Li-7 magnetic resonance shift in and is in agreement with the literature. It is noteworthy that the Li-7 NMR signal for Li2SO4 appears at about 0 ppm relatively to 0.01 M LiCl (aq).Therefore, it is not possible at ambient temperature to distinguish the Li2SO4 impurity in the solid-state NMR spectrum from the material of interest.
Elemental analysis
Another characterization technique that confirms the formation of a zinc-doped LiFePO4 material, as well as the coexistence of some lithium sulfate in our product, is elemental analysis. The technique was applied for both the original product (i.e. the one obtained at 110°C), as well as the one heated at 650°C. The results of the analysis are listed in . The data in along with the fact that the molar ratio of iron to zinc in the desired product is 1–x to x lead us to the composition results listed below:
Table 2. Results from elemental analysis.
Sample 110°C Li1.52Fe0.842Zn0.160P0.942
Sample 650°C Li1.70Fe0.833Zn0.165P1.09.
Expressing the subscripts by one significant figure, the elemental composition of the analyzed material for Li, Fe, Zn, and P is Li2Fe0.8Zn0.2P. The subscripts of 0.8, 0.2, and 1 for Fe, Zn, and P are numerically equal to the molar ratios of the starting materials FeSO4.7H2O, ZnSO4.6H2O, and lithium phosphate (Li3PO4), which were also expressed by one significant figure. The elemental composition Li2Fe0.8Zn0.2P is the same as the one of zinc-doped lithium iron phosphate (LiFe0.8Zn0.2PO4; for Li, Fe, Zn, and P) except for an excess of lithium. This observation verifies both the production of a zinc-doped LiFePO4 compound with the formula LiFe0.8Zn0.2PO4 and the existence of Li2SO4 in the product. It is also important to note that as the Li2SO4 byproduct is produced in the aqueous phase and, consequently, a significant fraction of it ends up in the filtrate, the Li2SO4 impurity in the product does not account for the entire Li2SO4 (aq) produced.
Product purification
This work has been aimed at developing a low-temperature method for the synthesis of zinc-doped LiFePO4. As the product was originally obtained in the form of a precipitate, the entire precipitate was what went through characterization. The purification of the zinc-doped LiFePO4 can be achieved by mixing the precipitate with hot water followed by the filtration-based removal of the impurity as aqueous Li2SO4 in the filtrate. We were very careful during the cleaning process of our prepared product, making sure to avoid any treatment that may alter or change the identity or originality of our prepared sample. Although product purity is important, at this early stage, the focus was more on the type of raw product our technique can lead to, than on yield or purity.
Experimental
Materials
The starting materials used for the synthesis were Li3PO4 from Aldrich Chemical Co., ferrous sulfate crystals (FeSO4.7H2O) from Baker and Adamson Chemical Co., and zinc sulfate crystals (ZnSO4.6H2O) from Mallinckrodt Chemical Co.
Preparation
In the wet synthesis of LiFe0.8Zn0.2PO4, centimolar amounts of Li3PO4 (MW=115.79 g/mol), FeSO4.7H2O (MW=278.01 g/mol), and ZnSO4.6H2O (MW=269.54 g/mol) are used in the molar ratios of 1 Li3PO4:0.8 FeSO4.7H2O:0.2 ZnSO4.6H2O. Using deciliter volumes of de-ionized water, the Li3PO4 sample is mixed with de-ionized water with continuous stirring, while water is brought to boiling, and the hydrated iron and zinc sulfate samples are dissolved together in another aliquot of de-ionized water. Then the solution is added to the Li3PO4 suspension all at once. The reaction mixture is stirred and heated continuously for about two hours to have the iron and zinc ions precipitated (as LiFe0.8Zn0.2PO4) completely (as the proposed reaction equation predicts based on the used molar ratios of the starting materials). In about two hours, the heat is removed. After half an hour, the mixture is filtered using the suction filtration method. The green precipitate is heated in an oven at 110°C for three hours. The product (that is, the final product) is amorphous. In order to crystallize it for the purpose of characterization, some of the prepared sample was heated at 650°C for 24 hours.
Characterization
In order to characterize the product, the following characterization techniques and procedures were applied.
X-ray diffraction
X-ray powder patterns were obtained for both the sample heated at 110°C and the one heated at 650°C using a SCINTAG automated PAD-X diffractometer utilizing monochromatic CuK@1pha radiation. The diffraction patterns were taken in the range of 5<2θ<45 degrees. The 2θ step size was 0.02 degrees and the count time was one second. Quartz was used as an internal standard.
Fourier Transform Infrared (FTIR) spectroscopy
FTIR spectra of both the sample heated at 110°C and the one heated at 650°C were collected using a Nicolet Magna 760 spectrometer. Both spectra were collected with a resolution of 4 cm−1 and 64 scans per sample in the mid-IR region of 4000–400 cm−1. Potassium bromide was used as the reference.
TGA and DTA
Thermal gravimetric analysis (TGA) and differential thermal analysis (DTA) diagrams of the sample heated at 110°C were obtained using a TG 7/DT 7 Perkin Elmer Analyzer in flowing nitrogen with heating from 22.3 to 700°C at a rate of 10°C/min.
Lithium-7 solid-state nuclear magnetic resonance (NMR) spectroscopy
The Li-7 MAS-NMR spectra were acquired on a Varian Unity Pulse 300 solids spectrometer equipped with a XC-5 MAS probe from Doty Scientific. Samples were packed in the ambient in either silicon nitride or zirconia rotors with Aurum or Kel-F caps. MAS data for each sample were acquired at two spinning speeds with at least a difference of 1000 Hz to determine the isotropic shift with reference to 0.01 M LiCl (aq). Spinning speeds are provided in the appropriate figure caption.
Elemental analysis
Both the sample heated at 110°C and the one heated at 650°C were given to Schwarzkopf Microanalytical Laboratory, Inc. in Woodside, New York (http://www.schwarzkopfmicrolab.com/) for their mass percentages determination for iron, lithium, phosphorus, and zinc.
Conclusion
In order to substitute the environmentally friendly lithium-ion battery cathode material LiFePO4 with an environmentally and technologically similar advantageous material which has a higher electronic conductivity, a LiFe0.8Zn0.2PO4 was synthesized using, for the first time, a synthetic method which is low-temperature and is, therefore, in comparison to the high-temperature method, expected to be resulting in a product with a relatively large surface area. The developed synthetic method has also the advantage of costing less as far as heating is the concern (as instrumentally applying more heat requires a higher thermal budget). Following purification, the compound can be electrochemically tested and evaluated as a green cathode material. With the iron-to-zinc molar ratio in the produced zinc-doped LiFePO4 being the same as the one in the quantities of the iron and zinc starting materials, the method appears to be applicable for synthesizing various zinc-doped LiFePO4 compounds with the general formula LiFe1−x Zn x PO4 (0<x<1). This makes the method very promising for the low-temperature production of a high-electronic-conductivity green cathode material for lithium-ion batteries.
Acknowledgements
We would like to thank all the staff of the Long Island University (Brooklyn Campus) chemistry preparation rooms for their great cooperation in providing us with the chemicals used in this work. Becky Gee thanks Professor Ruth Stark and Dr. Hsin Wang (Department of Chemistry, College of Staten Island, City University of New York) for use of their solid-state NMR instrumentation.
References
- Järup , L. Br. Med. Bull . 2003 , 68 , 167 182 .
- Alper , J. Chem. Mag. (ACS) 2002 , Autumn , 11 14 .
- Risher , J.F. ; De Rosa , C.T. J. Environ. Health 2007 , 70 , 9 16 .
- Thomas , J. Nat. Mater . 2003 , 2 , 705 706 .
- Nazri , G. MRS Bull . 2002 , 27 , 628 631 .
- Chung , S. ; Bloking , J.T. ; Chiang , Y. Nat. Mater . 2002 , 1 , 123 128 .
- Liu , H. ; Cao , Q. ; Fu , L.J. ; Li , C. ; Wu , Y.P. ; Wu , H.Q. Electrochem. Commun . 2006 , 8 , 1553 1557 .
- Shenouda , A.Y. ; Liu , H.K. J. Alloys Compd . 2009 , 477 , 498 503 .
- Xu , C.B. , Lee , J. and Teja , A.S. 2008 . J. Supercrit. Fluids , 44 : 92 – 97 .
- Ni , J.F. , Zhou , H.H. , Chen , J.T. and Zhang , X.X. 2005 . Mater. Lett , 59 : 2361 – 2365 .
- Santoro , R.P. and Newnham , R.E. 1967 . Acta Crystallogr. , 22 : 344 – 347 .
- 19-0721 . JCPDS-International Centre for Diffraction Data ; 1996 .
- 20-0640 JCPDS-International Centre for Diffraction Data 1996
- Nyquist , R.A. and Kagel , R.O. 1971 . Infrared Spectra of Inorganic Compounds , New York and London : Academic Press .
- Tucker , M.C. , Doeff , M.M. , Richardson , T.J. , Fiones , R. , Cairns , E.J. and Reimer , J.A. 2002 . J. Am. Chem. Soc , 124 : 3832 – 3833 .