Abstract
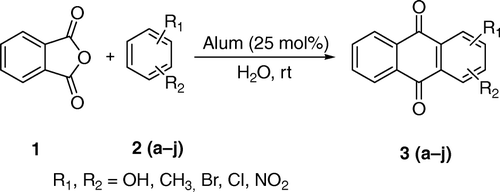
Alum (KAl(SO4)2·12H2O) performs as a novel catalyst for the synthesis of anthraquinone derivatives from phthalic anhydride and substituted benzenes in good to excellent yields (70–96%) using water as a solvent at ambient temperature. Several solvents were examined for this reaction; however, in terms of reaction yield and time, water was found to be the optimum solvent. The remarkable advantages offered by this method are an inexpensive and easily available catalyst, a simple procedure, mild conditions, and much faster (60–120 min) reactions.
Keywords:
Introduction
Anthraquinones are important members of the organic family. Their structure is observed in some synthetic dyes and in many naturally occurring substances, such as pigments, vitamins, and enzymes Citation1–3. The quinone compounds occupy an important place among the different classes of anti-tumor agents Citation4. The hydroxylated 9,10-anthraquinones are widely found in nature and are known to display various pharmacological activities Citation5. Furthermore, the anthraquinones of the Rubiaceae family exhibit some interesting in vivo biological activities, such as anti-microbial Citation6, anti-fungal Citation7, hypotensive, analgesic Citation8, anti-malarial Citation9 Citation10, anti-oxidant Citation11, anti-leukemic, and mutagenic functions Citation12 Citation13.
The synthesis of anthraquinone derivatives currently is of great interest. There are various methods that have been reported for the synthesis of anthraquinones. The most common ones including the intramolecular condensation of aryl and o-aroylbenzoic acid produce anthraquinone derivatives using fuming sulfuric acid Citation14, benzoyl chloride and concentrated sulfuric acid Citation15 Citation16, benzoyl chloride and zinc chloride Citation17, and POCl3/P2O3Cl4 Citation18. It has been found that Friedel–Crafts reactions between phthalic anhydride and substituted benzenes in the presence of a eutectic mixture of aluminum chloride and sodium chloride (2:1) melt have been used for the preparation of various anthraquinones Citation19 Citation21. Other catalysts, such as AlCl3/H2SO4 Citation21, montmorillonite clay and AlCl3/NaCl melt Citation22, montmorillonite K10 clay, and thin layer chromatography (TLC) grade silica gel Citation23 as heterogeneous catalyst have also been used for this transformation. These methods suffer from some disadvantages, such as long reaction times, use of toxic solvent, the reaction conditions are usually quite severe, some reagents are commercially unavailable, and the desired product was afforded from two steps with low yields. There still remains the need for a simple and efficient process for the synthesis anthraquinones derivatives.
In recent year, organic reactions in aqueous media have received considerable attention. The fact is that water is the inexpensive, most abundant, non-toxic, and environmentally friendly solvent. It exhibits unique reactivity and selectivity, which is different from those in conventional organic solvents Citation24. In this respect, the development of water-tolerant catalysts has rapidly become an area of intense research. However, there is not any report for the synthesis of anthraquinone derivatives in aqueous media catalyzed by alum. These findings prompted us to investigate the synthesis of anthraquinone derivatives in aqueous media. Alum (KAl(SO4)2·12H2O) was found to be effective in the synthesis of cis-isoquinolic acids Citation25, mono- and di-substituted 2,3-dihydroquinazolin-4(1H)-ones Citation26, dihydropyrimidines via Biginelli reaction Citation27, coumarins Citation28, 1,3,4-oxadiazoles Citation29, dibenzoxanthenes Citation30, 1,5-benzodiazepines Citation30, trisubstituted imidazoles Citation30 etc. However, there are no examples of the use of alum as a catalyst for the synthesis of anthraquinone derivatives.
Results and discussion
As a continuation of our research devoted to the development of green organic chemistry by performing organic transformations with water as the reaction medium Citation31–37, herein, we have developed an efficient and green methodology for the synthesis of anthraquinone derivatives from phthalic anhydride and substituted benzene using alum (25 mol%) as an inexpensive catalyst in water, which makes use of milder conditions over the reported procedure as depicted in ; the methodology developed is simple with good to excellent yields of products.
We first compared the catalyst effect on different solvents for the synthesis of 2-methylanthraquinone as summarized in . In a typical experiment, the reaction of phthalic anhydride 1 and toluene 2g in water was carried out in the presence of alum (KAl(SO4)2·12H2O) to afford the corresponding 2-methylanthraquinone 3g in 92% yield. The reaction proceeded rapidly at ambient temperature with 25 mol% of catalyst (which is highly water soluble). The reaction was completed within 60 min (, entry 10) and no remarkable change in the yield of product was observed up to 90 min (, entry 11). We kept the catalyst constant and used different solvents, such as CH2Cl2, CH3CN, THF, CHCl3, DMSO, DMF, MeOH, EtOH, and dioxane; all of which afforded a very low yield of product (, entries 1–9). Varying the amount of catalyst did not improve the yield (, entries 12–15). These results suggest that water is the best solvent for the synthesis of 2-methylanthraquinone; it may be due to the greater solubility of the catalyst in water compared with organic solvent.
Table 1. Solvent effect for synthesis of 3-methylantraquinone 3g a.
With optimal conditions in hand, we then reacted various benzene derivatives with phthalic anhydride to give the corresponding anthraquinone derivatives in good to excellent yields (, entries 3 (a–j)). In a similar fashion, a variety of substituted benzene with electron donating substituents reacts well under the reaction conditions giving the corresponding anthraquinone derivatives with high yields in short reaction time (, entry 3 (a–g)). In contrast, by using substituted benzene with electron withdrawing groups, the corresponding anthraquinone derivatives were produced slowly, and the reaction required long reaction time (, entry 3 (h–j)).
Table 2. Synthesis of anthraquinone derivatives from phthalic anhydride with substituted benzenes in the presence of alum in water.
All the reactions were completed within 60–120 min. The obtained yields of anthraquinone derivatives were 70–96%.The structures of the products were confirmed by IR, 1H NMR, mass spectrometry, and elemental analysis.
Experimental
Reactions were monitored by TLC (silica, 80:Citation20 ethyl acetate:hexane). IR spectra were recorded on Perkin–Elmer FTIR spectrophotometer in KBr disc. 1H NMR spectra were recorded on a 400 MHz Varian–Mercury Plus spectrometer and are reported as parts per million (ppm) downfield from tetramethylsilane as internal standard. The following abbreviations are used: singlet (s), doublet (d), triplet (t), multiplet (m), and broad (br). Mass spectra were taken with a Waters Micromass-Quattro-II mass spectrometer. All chemicals were obtained from commercial suppliers and were used without purification.
General procedures
Phthalic anhydride (1 mmol), substituted benzene (1.1 mmol), and water (5 mL) were mixed in a 25-mL single-neck round-bottom flask, and to this alum 25 mol% was added. The reaction mixture was stirred at room temperature for the appropriate time (, entries 3 (a–j)) and the progress of the reaction was monitored by TLC. After completion of the reaction, the mixture was extracted with ethyl acetate (2×10 mL). The combined organic layer was dried over anhydrous Na2SO4 and evaporated under reduced pressure; the crude material was purified by column chromatography over silica gel to afford products 3 (a–j) with high purity.
The spectral data of some representative anthraquinone derivatives
2-Methylanthraquinone (3g)
C15H10O2, yellow solid; IR (KBr, cm−1): 2950, 2900, 1669, 1593, 1326, and 1291; 1H NMR (400 MHz, CDDL3): δ2.4–2.8 (3H, s) and 7–8.5 (7H, m); EIMS (m/z,%): 222 [M + 1].
2-Chloroanthraquinone (3i)
C14H7ClO2, pale yellow; IR (KBr, cm−1): 1680, 1582, 1300, and 1280; 1H NMR (400 MHz, CDDL3): δ7.42–7.98 (7H, m); EIMS (m/z,%): 245 [M + 1].
Conclusion
In conclusion, we have developed an efficient and convenient green synthesis for the preparation of anthraquinone derivatives from commercially available phthalic anhydride and substituted benzene using the inexpensive, non-toxic, and easily available alum catalyst. The method offers several advantages including high yield of products, short reaction times, and ease of work up procedure.
Acknowledgements
We gratefully acknowledge the funding support received for this project from the University Grants Commission (WRO), Pune, India.
References
- Thomson , R.H. 1957 . Naturally Occurring Quinones , 309 – 312 . London : Buttetworths .
- Fieser , L.F. and Fieser , M. 1961 . Advanced Organic Chemistry , 845 – 851 . New York : Reinhold .
- Fieser , L.F. ; Fieser , M. Topics in Organic Chemistry ; Reinhold: New York, 1963; Chapters 1 and 9 181 293 .
- Valderrama , J.A. , Gonza'lez , M.F. , Pessoa-Mahana , D. , Tapia , R.A. , Fillion , H. , Pautet , F. , Rodriguez , J.A. , Theoduloz , C. and Schmeda-Hirschmann , G. 2006 . Bioorg. Med. Chem , 14 ( 14 ) : 5003 – 5011 .
- Shi , Y.Q. , Fukai , T. , Sakagami , H. , Kuroda , J. , Miyaoka , R. , Tamura , M. , Yoshida , N. and Nomura , T. 2001 . Anticancer Res , 21 ( 4A ) : 2847 – 2853 .
- Sittie , A.A. , Lemmich , E. , Olsen , C.E. , Hviid , L. , Kharazmi , A. , Nkrumah , F.K. and Christensen , S.B. 1999 . Planta Med , 65 ( 3 ) : 259 – 261 .
- Rath , G. , Ndonzao , M. and Hostettmann , K. 1995 . Int. J. Pharmacog , 33 ( 2 ) : 107 – 114 .
- Younos , C. , Rolland , A. , Fleurentin , J. , Lanhers , M. , Misslin , R. and Mortier , F. 1990 . Planta Med , 56 ( 5 ) : 430 – 434 .
- Adwankar , M.K. and Chitnis , M.P. 1982 . Chemotherapy , 28 ( 4 ) : 291 – 293 .
- Koumaglo , K. , Gbeassor , M. , Nikabu , O. , de Souza , C. and Werner , W. 1992 . Planta Med , 58 ( 6 ) : 533 – 534 .
- Tripathi , Y.B. , Sharma , M. and Manickam , M. 1997 . Indian J. Biochem. Biophys , 34 ( 3 ) : 302 – 306 .
- Chang , P. and Chen , C. 1995 . Chin. Pharm. J. (Taipei) , 47 ( 4 ) : 347 – 353 .
- Ismail , N.H. , Ali , A.M. , Aimi , N. , Kitajima , M. , Takayama , H. and Lajis , N.H. 1997 . Phytochemistry , 45 ( 8 ) : 1723 – 1725 .
- Fieser , L.F. 1941 . Org. Synth. Coll Vol. 1 , 353 – 358 .
- Clar , E. 1948 . Chem. Ber , 81 ( 1 ) : 52 – 63 .
- Clar , E. J. Chem. Soc . 1949 , 2440 2442 .
- Clar , E. 1948 . Chem. Ber , 81 ( 2 ) : 169 – 179 .
- Sami , E.S. and Saleh , R. 2001 . Molecules , 6 : 279 – 286 .
- Horii , Z. , Hakusui , H. and Momose , T. 1968 . Chem. Pharm. Bull , 16 : 1262 – 1265 .
- Horii , Z. , Momose , T. and Tamura , Y. 1965 . Chem. Pharm. Bull , 13 : 797 – 803 .
- Dhruba , B.R. , Patil , V.B. and Rama Rao , A.V . 1976 . Indian . J. Chem , 14B : 622
- Hossein , N. and Roozbeh , N. 2008 . Chin. J. Catal , 29 ( 1 ) : 86 – 90 .
- Singh R. Geetanjali J. Serb. Chem. Soc 2005 70 7 937 942
- Nirada , D. and Ganguly , M. 2008 . Indian J. Chem , 47B : 153 – 154 .
- Li C-J. Chan T-H. Wiley Hoboken, NJ 2007 Organic Reactions in Aqueous Media , 2nd ed
- Demko , Z.P. and Sharpless , K.B. 2001 . J. Org. Chem , 66 : 7945 – 7950 .
- Li , C.J. 2005 . Chem. Rev , 105 : 3095 – 3166 .
- Narayan , S. , Muldoon , J. , Finn , M.G. , Fokin , V.V. , Kolb , H.C. and Sharpless , K.B. 2005 . Angew. Chem. Int. Ed , 44 : 3275 – 3279 .
- Lindstrom , U.M. 2002 . Chem. Rev Vol. 102 , 2751 – 2772 .
- Wei , C. and Li , C.J. 2003 . J. Am. Chem. Soc , 125 : 9584 – 9585 .
- Azizian , J. ; Mohammadi , A.A. ; Karimi , A.R. ; Mohammadizadeh , M.R. J. Org. Chem. 2005 , 70 , 350 352 Azizian, J.; Mohammadi, A.A.; Karimi, A.R.; Mohammadizadeh, M.R. Appl. Catal. 2006, 300, 85–88.
- Dabiri , M. , Salehi , P. , Otokesh , S. , Baghbanzadeh , M. , Kozehgary , G. and Mohammadi , A.A. 2005 . Tetrahedron Lett , 46 : 6123 – 6126 .
- Dabiri , M. , Baghbanzadeh , M. , Kiani , S. and Vakilzadeh , Y. 2007 . Monatsh. Chem , 138 : 997 – 999 .
- Dabiri , M. , Baghbanzadeh , M. and Bahramnejad , M. 2007 . Monatsh. Chem , 138 : 1253 – 1255 .
- Dabiri , M. , Baghbanzadeh , M. , Nikcheh , M.S. and Arzroomchilar , E. 2008 . Bioorg. Med. Chem. Lett , 18 : 436 – 438 .
- Mahajan , D. , Nagvi , T. , Sharma , R.L. and Kapoor , K.K. 2008 . Aust. J. Chem , 61 : 159 – 162 .
- Mohammadi , A.A. , Mivechi , M. and Kefayati , H. 2008 . Monatsh. Chem , 139 : 935 – 937 .