Abstract
Various di- and mono-substituted quinazolinones were synthesized by one-pot condensation of isatoic anhydride, aldehyde and amine, or ammonium carbonate using [bmim]HSO4 as catalyst in aqueous medium. Water as a reaction media, recyclability of catalyst, shorter reaction time, and good yields (70–90%) are some of the salient features of the developed protocol.
Introduction
2,3-Dihydroquinazolinone derivatives have been reported to possess diverse pharmacological activities, such as anti-tumor activity, diuretic properties, herbicidal activity, and plant growth regulation ability. They are also reported to posses the ability to inhibit enzymes of biological importance (e.g. Metalloenzymes) Citation1 Citation2. Several methods are described in the literature for the preparation of these important classes of compounds. Sharma and Kaur reported the synthesis of 2,3-dihydroquinazolin-4(1H)-ones via condensation of anthranilamide with aldehyde or ketone in presence of para-toluene sulfonic acid as catalyst under reflux conditions Citation3. Moore et al. observed formation of quinazolinone on refluxing anthranilamide with benzil in acetic acid by using ZnCl2 as catalyst Citation4. Shi and co-workers carried out reductive cyclization of ortho-nitrobenzamides with aldehydes and ketones to obtain corresponding 2,3-dihydroquinazolin-4(1H)-ones in good yields Citation5. Ozaki et al. synthesized various 2,3-dihydroquinazolin-4(1H)-one derivatives starting from different anthranilic acid derivatives in a multi-step procedure Citation6. Various quinazolinone derivatives can also be synthesized by the reaction of isatoic anhydride with Schiff's bases Citation7. Quinazolin-4(3H)-ones can be reduced to corresponding dihydroquinazolinones by using sodium borohydride or sodium cyanoborohydride in acetic acid Citation8. Recently, Salehi et al. reported one-pot three-component condensation of isatoic anhydride, aldehyde, and amine catalyzed by acidic reagents, such as silica sulfuric acid, montmorillonite K-10, and para-toluenesulphonic acid for the synthesis of 2,3-dihydroquinazolin-4(1H)-ones Citation9–11. Lewis acid catalysts, such as Gallium (III) triflate Citation12 and Zinc (II) perfluorooctanoate Citation13 have also been used for catalyzing one-pot reaction for synthesis of quinazolinones.
Although many procedures are reported in the literature for the synthesis of 2,3-dihydroquinazolin-4(1H)-one derivatives, most of them suffer from drawbacks like low yields, multistep procedures, harsh reaction conditions, and longer reactions times. Thus, development of a simple, convenient, and efficient method for the synthesis of 2,3-dihydroquinazolin-4(1H)-ones is highly desirable.
Over the past decade utility of ionic liquids in catalysis and as neoteric solvent for various synthetic processes has been well recognized by the chemists all over the world. Due to their chemical properties, such as recyclability, negligible vapor pressure, ability to dissolve wide range of substrates and catalyst, thermal stability, and so on, they have been considered as viable alternatives to the conventional volatile organic solvents Citation15–18. Protic and Bronsted acidic ionic liquids, in particular, have received increasing attention for carrying out the organic transformations as they can replace volatile organic solvents as well as highly acidic catalytic systems Citation19 Citation20. Use of these ionic liquids have been found to be advantageous as they can be easily recycled, are easy to synthesize, and usually involve simple reaction processes as compared with most of the traditional methods.
Results and discussion
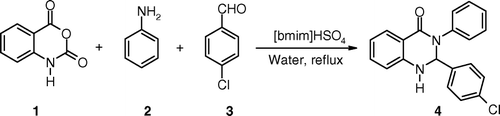
In order to exploit potential of ionic liquids for the synthesis of quinazolinone derivatives, one-pot three-component condensation of isatoic anhydride, aldehydes, and amines in presence of various Protic and Bronsted acidic ionic liquids as catalyst was investigated. para-Chlorobenzaldehyde and aniline were chosen as the model substrates for condensation with isatoic anhydride to synthesize corresponding quinazolinone derivative 4 (). Water was chosen as a reaction media since the use of water as a solvent offers several environmental benefits. Further in many reactions significant rate enhancements were observed when reactions were carried out in water compared to other organic solvents Citation21–23. This acceleration has been attributed to many factors including the hydrophobic effect, enhanced H-bonding in the transition state, and cohesive energy density of water.
Various Protic and Bronsted acidic ionic liquids namely, 1-butyl-3-methylimidazolium hydrogensulfate [bmim]HSO4, 1-butyl-3-methylimidazolium dihydrogenphosphate [bmim]H2PO4, 1-methylimidazolium trifluoroacetate [Hmim]Tfa, and 1-methylimidazolium-p-toluenesulfonate [Hmim]Tsa were screened as catalyst (25 mol% with respect to aldehyde). [bmim]HSO4 was found to be efficient catalyst as compared with other ionic liquids ().
Table 1. Effect of ionic liquids in the one-pot synthesis of 4.a
To optimize the amount of catalyst and optimum time for the reaction, experiments were planned in succession where the reactions were carried out at room temperature, under reflux conditions, in the absence of catalyst, and with varying amount of catalyst. The results are summarized in . No product formation was observed without reflux at room temperature. In the absence of catalyst under reflux conditions some product formation was observed (, entry 2) but the yields were poor. The yields were improved in presence of catalyst [bmim]HSO4. The catalyst was found to be effective even at concentration of 5 mol%.
Table 2. Effect of amount of [bmim]HSO4 in the one-pot synthesis of 4.a
In order to generalize the procedure and to study the effect of substituents on the product formation, variety of aldehydes and amines were screened under optimized conditions. The results obtained are shown in . Quinazolinones were synthesized in 70–90% yields from various aromatic aldehydes and amines. Aliphatic amines also afforded good yields of the corresponding quinazolinone derivatives.
Table 3. One-pot synthesis of 2,3-disubstituted-2,3-dihydroquinazolin-4(1H)-ones catalyzed by [bmim]HSO4.a
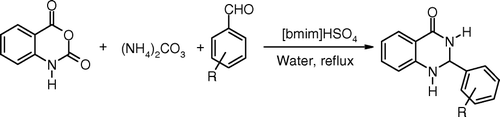
Ammonium salts have been used as the source of ammonia in the synthesis of various nitrogen containing heterocyclic compounds. Synthesis of 2-substitued-2,3-dihydroquinazolin-4(1H)-ones was attempted via one-pot condensation of isatoic anhydride, aromatic aldehydes, and ammonium carbonate as a source of ammonia by using above developed protocol (). Various 2-substituted-2,3-dihydroquinazolin-4(1H)-one derivatives were synthesized in 70–85% yield within 3–4 h ().
Table 4. One-pot synthesis of 2-substituted-2,3-dihydroquinazolin-4(1H)-ones catalyzed by [bmim]HSO4.a
The recyclability of [bmim]HSO4 was tested in the reaction of para-chlorobenzaldehyde and aniline with isatoic anhydride in aqueous medium under same experimental conditions. The aqueous medium along with catalyst obtained after separation of product was reused again and found to give comparable yields of the product even after reusing for three times as shown in .
Table 5. Recycling of catalyst.a
Experimental
Ionic liquids are prepared as per the procedures reported in the literature Citation15–20. Isatoic anhydride, ammonium carbonate, amines, and aldehydes were purchased from Sd Fine Chemicals and Aldrich, and were used as received. The products were analyzed by 1H-NMR and mass spectroscopy techniques. 1H-NMR was recorded on a JEOL (300 MHz) instrument using DMSO-d6 as solvent. A mass spectrum was recorded on Schimadzu QP-2010 spectrophotometer. Elemental analysis was performed by using Thermo finnigan CHN-analyzer.
Typical procedure
In a typical experiment, to a mixture of aldehyde (5 mmol), isatoic anhydride (5.5 mmol), and amine (5 mmol) or ammonium carbonate (7 mmol) in H2O (10 mL) taken in a 50 mL of round-bottomed flask was added 5 mol% of [bmim]HSO4. Reaction mixture was heated under reflux for specified time. After completion of reaction, the product was separated by filtration and purified by column chromatography. The products are analyzed by 1H-NMR and mass spectroscopy techniques. In recyclability study, the filtrate obtained after separation of crude product by filtration (i.e. aqueous extract containing IL and traces of reactants) was reused as it is without any purification for next reaction involving same reactants.
Most of the synthesized compounds are know and their physical data, NMR-spectra, and mass spectra were found identical with those of authentic samples. New compounds were analyzed by 1H-NMR, 13C-NMR, mass spectra, and elemental analysis and the data obtained as given below:
-
2-(4-methoxyphenyl)-3-(1-butyl)-2,3-dihydroquinazolin-4(1H)-one (, entry 8). 1 H-NMR (300 MHz, DMSO-d 6 ): Δ = 0.82 (3H, t), 1.22 (2H, m), 1.45 (2H, m), 2.48 (2H, m), 3.69 (3H, s), 5.76 (1H, s), 6.58–6.64 (2H, m), 6.86–6.89 (2H, m), 7.13–7.59 (3H, m), 7.60–7.62 (1H, m). 13 C NMR (DMSO-d 6 ): Δ = 14.13, 20.00, 29.93, 44.30, 55.54, 70.15, 99.99, 114.25, 114.65, 115.44, 117.43, 127.80, 127.90, 133.48, 133.58, 146.77, 159.70, 162.64. MS (ESI, 80 eV) m/z (%): 311.2 (100) Anal. calcd. for C19H22N2O2: C, 73.5; H, 7.1; N, 9.0. Found C, 73.4; H, 7.2; N, 9.1.
-
2-(4-methoxyphenyl)-3-(4-chlorophenyl)-2,3-dihydroquinazolin-4-(1H)-one (, entry 6). 1 H-NMR (300 MHz, DMSO-d 6 ): Δ = 3.67 (3H, s), 6.23 (1H, s), 6.67–6.75 (2H, m), 6.83(2H, d, J=9 Hz), 7.23–7.57 (8H, m). 13 C NMR (DMSO-d 6 ): Δ = 55.50, 72.65, 99.99, 114.18, 115.25, 115.45, 117.93, 128.42, 128.49, 128.63, 128.96, 130.56, 132.62, 134.32, 139.99, 147.26, 159.65, 162.91. MS(ESI, 140 eV) m/z (%): 365 (100), 367 (35) Anal. calcd. for C21H17ClN2O2: C, 69.1; H, 4.7; N, 7.6. Found C, 69.3; H, 4.7; N, 7.4.
Conclusions
In conclusion the ionic liquid [bmim]HSO4 proved to be a useful catalyst for one-pot three-component synthesis of 2,3-disubstituted as well as 2-substituted-2,3-dihydroquinazolinones. The developed protocol offers an efficient, mild, and eco-friendly route for the synthesis of dihydroquinazolinone derivatives. Aqueous reaction medium, high yields, recyclability of catalyst, cheap, and commercially available starting materials, ease of work up procedure are some of the salient features of the developed protocol.
Acknowledgements
This work was financially supported by the Defense Research and Development Organization, (ERIP/ER/0503495/M/01) New Delhi, India. N.B.D., S.V.B., A.R.D., and D.G.R. gratefully acknowledge CSIR, New Delhi for offering senior research fellowship.
References
- Hamel , E. ; Lin , C.M. ; Plowmn , J. ; Wang , H. ; Lee , K. ; Paull , K.D. Biochem. Pharmacol . 1996 , 51 , 53 59 .
- Hour , M. ; Huang , L. ; Kuo , S. ; Xia , Y. ; Bastow , K. ; Nakanishi , Y. ; Hamel , E. ; Lee , K. J. Med. Chem . 2000 , 43 , 4479 4487 .
- Sharma , S.D. ; Kaur , V. Synthesis 1989 , 9 , 677 680 .
- Moore , J.A. ; Sutherland , G.J. ; Sowerby , R. ; Kelly , E.G. ; Palermo , S. ; Webster , W. J. Org. Chem . 1969 , 34 , 887 892 .
- Shi , D. ; Rong , L. ; Wang , J. ; Zhuang , Q. ; Wang , X. ; Hu , H. Tetrahedron Lett . 2003 , 44 , 3199 3201 .
- Ozaki , K. ; Yamada Y. ; Oine , T. ; Ishizuka , T. ; Iwasawa , Y. J. Med. Chem . 1985 , 28 , 568 576 .
- Rao , V.B. ; Ratnam , C.V. Indian J. Chem. Sect. B Org. Chem. Incl. Med. Chem . 1979 , 18 , 409 412 .
- Li , S.W. ; Nair , M.G. ; Edwards , D.M. ; Kisliuk , R.L. ; Gaumont , Y. ; Dev , I.K. ; Duch , D.S. ; Hmphrys , J. ; Smith , G.K. ; Ferone , R. J. Med. Chem . 1991 , 34 , 2746 2754 .
- Salehi , P. ; Dabiri , M. ; Zolfigol , M.A. ; Baghbanzadeh , M. Synlett 2005 , 7 , 11545 1157 .
- Salehi , P. ; Dabiri , M. ; Baghbanzadeh , M. ; Bahramnejad , M. Synth. Commun . 2006 , 36 , 2287 2292
- Baghbanzadeh , M. ; Salehi , P. ; Dabiri , M. ; Kozehgary , G. Synthesis 2006 , 2 , 344 348 .
- Chen , J. Wu , D. ; He , F. ; Liu , M. ; Wu , H. ; Ding , J. ; Su , W. Tetrahedron Lett . 2008 , 49 , 3814 3818 .
- Wang , L.M. ; Hu , L. ; Shao , J.H. ; Yu , J. ; Zhang , L. J. Flu. Chem . 2008 , 129 , 1139 1145 .
- CAS No. 63384-42-9 Spectral data compared with that obtained from WSS; Wiley Subscription Services, Inc., US .
- Chowdhury , S. ; Mohan , R.S. ; Scott , J.L. Tetrahedron 2007 , 63 2363 2389 .
- Muzart , J. Adv. Synth. Catal . 2006 , 348 , 275 295 .
- Jain , N. ; Kumar , A. ; Chauhan , S. ; Chauhan , S.M.S. Tetrahedron 2005 , 61 , 1015 1060 .
- Zhao , H. ; Malhotra , S.V. Aldrichimica Acta 2002 , 35 3 , 75 83 .
- Greaves , T.L. ; Drummond , C.J. Chem. Rev . 2008 , 108 1 , 206 237 .
- Zhao , G. ; Jiang , T. ; Gao , H. ; Han , B. ; Juang , J. ; Sun , D. Green Chem . 2004 , 6 , 75 77 .
- Breslow , R. Acc. Chem. Res . 1991 , 24 , 159 164 .
- Gajewski , J.J. Acc. Chem. Res . 1997 , 30 , 219 225 .
- Pratt , L.R. ; Pohrille , A. Chem. Rev . 2002 , 102 , 2671 2692 .
- Smith , T.A.K. ; Stephen , H. Tetrahedron 1957 , 1 , 38 44 .