Abstract
Hydrotalcite containing magnesium and aluminium (Mg–Al HT) with a molar ratio of Mg(II)/Al(III) = 2.5 has been prepared by a co-precipitation method using the effluent of a Friedel–Crafts acylation reaction. The HT was calcined at 500°C and reconstructed with deionized water. The synthesized HT was characterized by XRD and FT-IR spectroscopy and was successfully used as a catalyst in the Knoevenagel reaction of aldehydes and active methylene compounds. The catalyst was found to be reusable.
Hydrotalcites (HTs) have recently received much attention in view of their potential usefulness as adsorbents, anion exchangers, and most importantly, as basic catalysts Citation1–3. HTs are anionic clays, which can be obtained from natural sources as well as synthesized under different conditions. Among HTs, Mg–Al HTs have received maximum attention. They can be prepared by different methods. They are commonly prepared by the treatment of an aqueous solution containing bivalent and trivalent metal ions in different mole ratios with an aqueous alkali. It is reported that the M2 +/M3 + mole ratios ranging from 2 to 4 give good crystalline HTs Citation4. HTs can be used in the as-synthesized form or after different treatments. C–C bond formation reactions are very important in organic synthesis, and base catalyzed carbanion mediated reactions still dominate this area. Mg–Al HTs are basic in nature and can be used to catalyze base catalyzed organic reactions, such as aldol condensation Citation5 Citation6, Michael addition Citation7, and Knoevenagel reaction Citation8.
Due to increased awareness toward the environment, effluent treatment has gained tremendous importance in the chemical industry, particularly in industries utilizing reactions with high E-factors. Friedel–Crafts acylation reactions involve the use of large amounts of Bronsted or Lewis acids and have high E-factors, making this type of reaction environmentally unfriendly. The effluent from a Friedel–Crafts acylation reaction contains a large concentration of metal ions, e.g. Al3 + ions when AlCl3 is used as a catalyst. Therefore, we targeted the use of Al3 + ions from the effluent of a typical Friedel–Crafts acylation reaction in the preparation of Mg–Al HT and subsequently using the HT in a typical base catalyzed reaction, such as the Knoevenagel reaction. This would decrease the environmental load of the Friedel–Crafts reaction and makes a valued addition to the reaction. With a similar objective we previously reported the use of rice husk silica in the preparation of solid acid catalysts Citation9.
In this paper, we describe the preparation of Mg–Al HT with a molar ratio of Mg(II)/Al(III) = 2.5 from the Al3 + containing effluent of a typical Friedel–Crafts acylation reaction. The acetylation of anisole was first carried out using acetyl chloride in the presence of anhydrous AlCl3 as a catalyst, as per the procedure reported in the literature Citation10. The effluent generated after breaking the reaction and isolating the product methoxyacetophenone was clarified with charcoal and the Al3 + content in the solution was estimated by Atomic Absorption Spectroscopy. The HT was then prepared by adding a requisite amount of Mg(NO3)2.6H2O to the effluent containing Al3 + ions and following the reported procedure Citation1. The HT was calcined in a furnace at 500°C and again reconstructed using deionized water under an inert atmosphere.
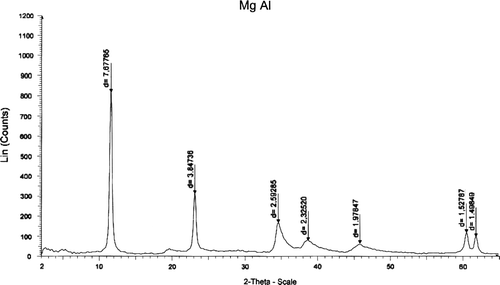
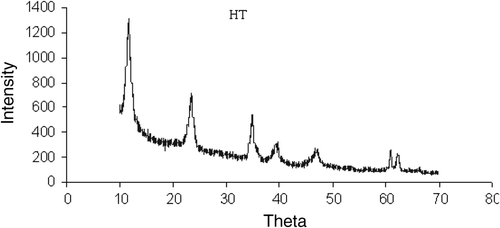
The reconstructed HT was characterized by XRD and FT-IR techniques. It was observed that, the XRD pattern of the HT () was the same as was reported previously 11. The XRD shows that the (003) plane contains the Mg/Al/OH layers, while the (006) plane contains exclusively the anionic layers. The basal spacing is 7.87 ?, corresponding to the HT phase. The XRD of as-synthesized Mg–Al HT () is given for comparison with that of the reconstructed Mg–Al HT.
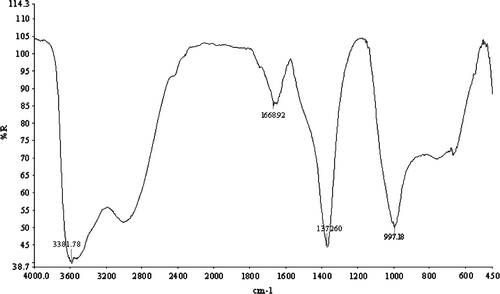
The FT-IR data are useful to identify the presence of the anions in the interlayer of brucite-like sheets and also to determine the orientation of the anions. In the FT-IR of the spectrum () of the reconstructed Mg–Al HT, the absorption at 3581 cm−1 corresponded to the H-bonding stretching vibration of the OH groups in the brucite layer. A shoulder present at around 3000 cm−1 was due to the H-bonding between H2O and the anions in the interlayer. A peak at 1668 cm−1 corresponded to H2O bending ().
To check the catalytic activity of the reconstructed Mg–Al HT prepared from the effluent, a Knoevenagel reaction of benzaldehyde Citation1 with malononitrile, ethyl cyanoacetate, and nitromethane was carried out ().
Scheme 1. Reaction of aromatic aldehydes with active methylene compounds in the presence of Mg-Al HT.
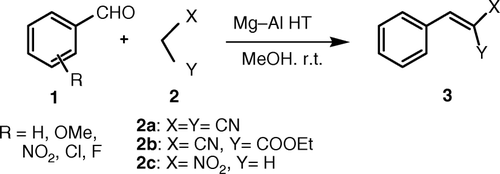
The reaction was carried out at room temperature using different solvents such as methanol, toluene, water, and DMF. It was observed that the reaction in methanol gave excellent yields. In the absence of the catalyst, very low yield of the product was obtained. When the reaction was carried out using the HT (Mg/Al = 2.5) prepared by conventional method [using Al (NO3)3.9H2O] and compared with the HT (Mg/Al = 2.5) prepared from the effluent, it was observed that the yields of benzylidiene malononitrile obtained were 94% and 96%, respectively. The HT is a heterogeneous catalyst and could easily be separated from the reaction mixture by filtration. The recovered catalyst was used for successive runs in reaction of benzaldehyde with malononitrile to test its reusability (). The catalyst was then used in a series of reactions with three different active methylene compounds; malononitrile was found to be the best donor in this series of reactions (Entries 1 − 3, ). Under the optimized conditions, a series of benzaldehydes were reacted with malononitrile to obtain the corresponding products (Entries 4–9, ).
Table 1. Reusability of reconstructed Mg/Al HT.
Table 2. Knoevenagel reaction of aldehydes with active methylene compounds in the presence of reconstructed Mg–Al HT at room temperature.
In conclusion, Mg–Al HT can be prepared using Al3 + ion containing effluent of an AlCl3 catalyzed Friedel–Crafts acylation reaction and can be effectively used to catalyze Knoevenagel reactions. This will be a value-added step toward decreasing the E-factor of the Friedel–Crafts reaction.
Procedure
A Friedel–Crafts acylation reaction of anisole with acetyl chloride was carried out as per the procedure reported in the literature Citation10. The effluent obtained after isolating the product was clarified by animal charcoal. The Al3 + content in the effluent was determined by Atomic Absorption Spectroscopy. Mg(NO3)2.6H2O (2.38 g, 9.30 mmol) was added to the effluent containing Al3 + (0.50 g, 3.7 mmol) and the solution was diluted up to 20 ml, added dropwise using a dropping funnel, with an aqueous solution (20 ml) containing NaOH (1.04 g, 26 mmol) and Na2CO3 (0.79 g, 7.4 mmol), taken in a two-necked round bottom flask and the solution was stirred (magnetic stirring). The solution was heated at 80°C for 15 h, cooled to room temperature and filtered. The precipitate was washed with hot distilled water several times until the filtrate was neutral (pH paper). The solid was dried in an electric oven at 353 K in air.
Reconstruction of Mg–Al HT
The HT was calcinated in an electric furnace at 500°C for 6–7 h, transferred to a Schlenk flask (250 ml), cooled to 473 K, and kept under vacuum for 1 h. Deionized water (15 ml) was added to the flask under an inert atmosphere, to avoid the CO2 contamination, and the mixture was kept stirring at room temperature for 10 h. The solution was allowed to settle. The excess water was removed by filtration under inert atmosphere and dried.
General procedure for the Knoevenagel reaction
To a 100 ml round bottom flask, benzaldehdye (10 mmol), active methylene compound (10 mmol), Mg–Al HT (0.5 g), and methanol (10 ml) were added. The mixture was stirred at room temperature till complete consumption of the reactants (TLC). The solution was filtered and the catalyst washed with hot methanol (2×5 ml). The filtrate was concentrated, poured in cold water, and the solid filtered. The product was purified by crystallization from absolute ethanol and dried at 60°C.
Spectroscopic data for selected compounds
2-Benzylidene malononitrile (Entry 1)
IR (KBr) ν: 3038, 2225, 1598 cm−1. 1H NMR (CDCl3, 300 MHz) δH: 7.54–7.64 (m, 3H), 7.91 (d, 2H), 8.10 (s, 1H) ppm. GC-MS: 154 (base peak), 127, 103, 76, 51.
Ethyl-2cyano-3-phenylacrylate (Entry 2)
IR (KBr) ν: 3027, 2219, 1720, 1602 cm−1. 1H NMR (CDCl3, 300 MHz) δH: 1.40 (t, 3H), 4.39 (q, 2H), 7.53 (d, 2H), 7.98(d, 2H), 8.26 (s, 1H) 8.30 (s, 1H) ppm. GC-MS: 201 (base peak), 172, 156, 128, 102, 77, 51.
2-(4-Chlorobenzylidene)malononitrile (Entry 7)
IR (KBr) ν: 3038, 2230, 1585 cm−1. 1H NMR (CDCl3, 300 MHz) δH: 7.26 (s, 1H), 7.52 (d, 2H), 7.74 (s, 1H), 7.85 (d, 2H) ppm. GC-MS: 188, 161, 153 (base peak), 126, 99, 75, 50.
2-(4-Fluorobenzylidene)malononitrile (Entry 8)
IR (KBr) ν: 3038, 2230, 1593 cm−1. 1H NMR (CDCl3, 300 MHz) δH: 7.24 (d, 2H), 7.76 (s, 1H), 7.96 (d, 2H), 7.99 (s, 1H) ppm. GC-MS: 172 (base peak), 145, 121, 94, 75, 50.
Acknowledgements
The authors thank the CSIR, New Delhi, for a Research Grant (No. 01 (2056)/06/EMR-II). Siddheshwar W. Kshirsagar thank the CSIR for providing Senior Research Fellowship.
References
- Cavani , F. ; Trifiro , F. ; Vaccari , A. Catal. Today 1991 , 11 , 173 301 .
- Di Cosimo , J.I. ; DIéz , V.K. ; Xu , M. ; Iglesia , E. ; ApesteguIá , C.R. J. Catal . 1998 , 178 , 499 510 .
- Evans , D.G. ; Duan , X. Chem. Commun . 2006 , 5 , 485 496 .
- Reichle , W.T. Sol. States Ionics 1986 , 22 , 135 141 .
- Rao , K.K. ; Gravelle , M. ; Valente , J.S. ; Figueras , F. J. Catal . 1998 , 173 , 115 121 .
- Kantam , M.L. ; Choudary , B.M. ; Reddy , C.V. ; Rao , K.K. ; Figueras , F. Chem. Commun . 1998 , 1033 1034 .
- Choudary , B.M. ; Kantam , M.L. ; Reddy , C.V. ; Rao , K.K. ; Figueras , F. J. Mol. Catal. A Chem . 1999 , 146 , 279 284 .
- Ebitani , K. ; Motokura , K. ; Mori , K. ; Mizugaki , T. ; Kaneda , K. J. Org. Chem . 2006 , 71 , 5440 5447 .
- Shinde , A.B. ; Shrigadi , N.B. ; Samant , S.D. J. Chem. Technol. Biotechnol . 2003 , 78 , 1234 1238 .
- Vogel , A.I. Text Book of Practical Organic Chemistry , 4th ed 1978 773 774 .
- Climent , M.J. ; Corma , A. ; Iborra , S. ; Velty , A. J. Catal. 2004 , 221 , 474 482 .