Abstract
In the present work, metals (cadmium, lead, copper, nickel, tin, selenium, and mercury) have been estimated in the Ebro River (Spain) using the zebra mussel (Dreissena polymorpha) as an environmental bio-indicator. In two sequential studies, in 2006 and 2008, concentrations of metals were calculated in water as well as in the shells and fleshes of the zebra mussels. Samples were collected from assorted locations of the river. Metals were determined successfully at trace levels through voltamperometry, a sensitive technique. It has been noted that analysis of bioaccumulators like zebra mussels can be helpful in evaluating metal pollution in water.
Introduction
Zebra mussels (Dreissena polymorpha) are widely used bio-indicators of persistent organic pollutants Citation1, trace metals Citation2, and radionuclides Citation3 in several worldwide ecosystems. They were applied successfully to assess the trace metal contamination in freshwaters. Dreissena polymorpha is a filter-feeding bivalve and is widely spread based on the planktonic larval stage. It is easily collectable species throughout the year having enough biomass for trace analysis. Because of these qualities, it is commonly employed as a bio-indicator for finding out metal contamination in water ecological systems Citation2. Several studies have been reported about using zebra mussel (Dreissena polymorpha) as a bio-indicator in different parts of the world, for instance, in subalpine lakes, Italy Citation2, Lake Erie and Ontario, USA Citation4, Danube River, Vienna Citation5, Bergen Harbor area, Norway Citation6, in Gulf of Olympia, Greece Citation7 and by us in River Ebro (Spain) Citation8.
Several spectroscopic techniques are being used to find out metals in mussel samples. Electrothermal atomic absorption spectroscopy was used for determination of Cd, Co, Cr, Cu, Hg, Ni, Pb, and Zn Citation2. Inductively coupled plasma emission spectroscopy has been used for determination of Ca, Cd, Cr, Cu, Fe, Hg, K, Mg, Mn, Mo, Na, Ni, Pb, Se, Sr, V, and Zn Citation4 Citation9. Cold vapor atomic absorption spectroscopy was utilized for mercury Citation10 while electro thermal atomic absorption spectroscopy for cadmium by using slurries Citation11. Also, cadmium and lead were determined by atomic absorption spectroscopy coupled with graphite furnace Citation12 Citation13. Automated ultrasonic slurry sampling and microwave-assisted digestion was employed to quantify arsenic with electro thermal atomic absorption spectroscopy Citation14. Flame atomic absorption spectrometry and flow injection analysis were employed for finding out cadmium in mussels Citation15. Apart from spectroscopic techniques, a rapid voltammetric method has been developed for the trace determination of copper in fish samples Citation16. In this study, a procedure based on anodic stripping voltamperometry is described and conditions were optimized for determination of Zn, Cd, Pb, Cu, Ni, Fe, Al, Se, Cr, and Sn in mussel samples. Anodic stripping voltammetry is a voltammetric method for quantitative determination of specific ions. The analyte is electroplated on the electrode during a deposition step, and oxidized from the electrode during the stripping step. The current is measured during the stripping step. The oxidation of ions is registered as a peak in the current signal at the potential at which the ions begin to oxidized. The method is simple, sensitive and less time-consuming compared with other techniques in use.
Results and discussion
Zebra mussel (Dreissena polymorpha) is a freshwater bivalve native to the drainage basins of Black and Caspian Sea, spreading along the coasts of eastern USA and Europe. For the first time, they were found in Spain during summer 2001 in Ebro River Citation8. Exterior of zebra mussel is similar to marine mussel. During the biological cycle, the larva produced is scattered with flowing water. It grows rapidly, and can tolerate temperature changes and salinity of water. It is a perfect invader in water ecosystems and reduces quantity of phytoplankton in water rapidly. Its presence increases the rate of deposition of organic matter in sea depths that increases transparency of water. As a bio-accumulator, they can deposit organic matter and pollutants Citation8. The sampling site chosen were entry and exit of Ribaroja dam at Ebro River. The reason for choosing the said site was the fact that in dam when water is stagnant, there will be more bioaccumulation and more metals will be collected by mussels than running water.
The method was optimized before analysis. The sensitivity of voltamperometry varies from metal to metal, as shown in . In 2007, similar work had been carried out for judging metals in water but in different times of the year (January, February, November, and December) Citation8. In the present work, metal concentrations in zebra mussel (Dreissena polymorpha), taken from Ebro River in January, March, July, and October, were measured by voltamperometry and the results are shown in . Technique was found to be more sensitive for lead, zinc, and cadmium whereas rather less sensitivity was found for mercury, tin, and iron. , , and show results at entry point of Ribaroja dam in flesh, sludge, and water in that order while , , and show results at exit point of the dam.
Figure 1. Metals in zebra mussels (Dreissena polymorpha) by voltamperometry technique in Ebro River in different times of year. 1=zinc (mg/kg), 2=cadmium (mg/kg), 3=lead (mg/kg), 4=copper (mg/kg), 5=nickel (mg/kg), 6=iron (g/kg), 7=aluminum (g/kg), 8=tin (mg/kg), 9=selenium (mg/kg), 10=mercury (mg/kg), 11=chromium (mg/kg).
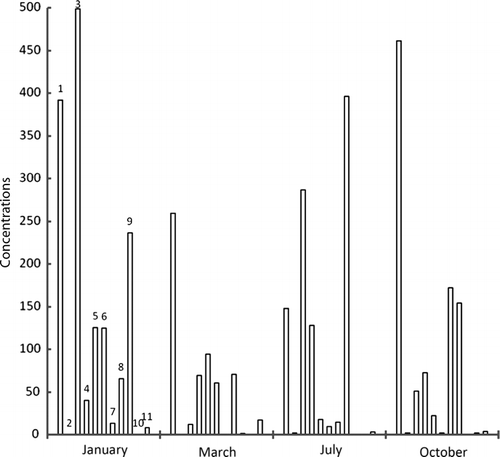
Figure 2a. Concentrations of metals in flesh samples of zebra mussels (Dreissena polymorpha) by voltamperometry technique taken from entry point of dam at Ebro River in different times of year; units=mg/kg, 1=cadmium, 2=lead, 3=copper, 4=nickel, 5=tin, 6=selenium, 7=mercury, 8=chromium.
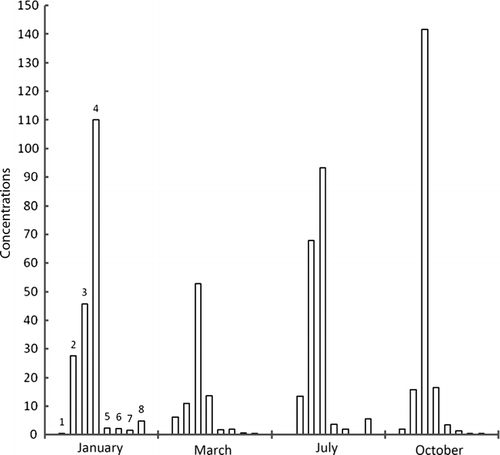
Figure 2b. Concentrations of metals in flesh samples of zebra mussels (Dreissena polymorpha) by voltamperometry technique taken from exit point of dam at Ebro River in different times of year; units=mg/kg, 1=cadmium, 2=lead, 3=copper, 4=nickel, 5=tin, 6=selenium, 7=mercury, 8=chromium.
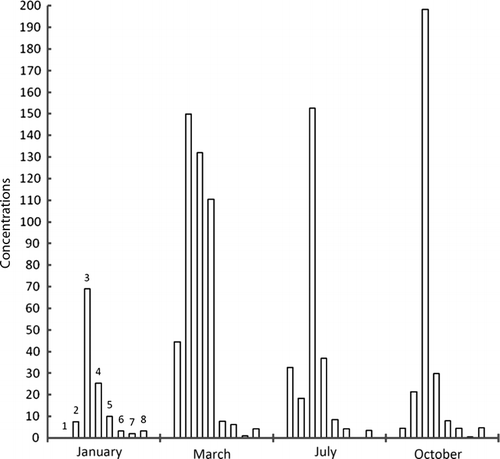
Figure 3a. Concentrations of metals in sludge samples taken from entry point of dam at Ebro River collected along zebra mussel colonies in different times of year; units=mg/kg, 1=cadmium, 2=lead, 3=copper, 4=nickel, 5=tin, 6=selenium, 7=mercury, 8=chromium.
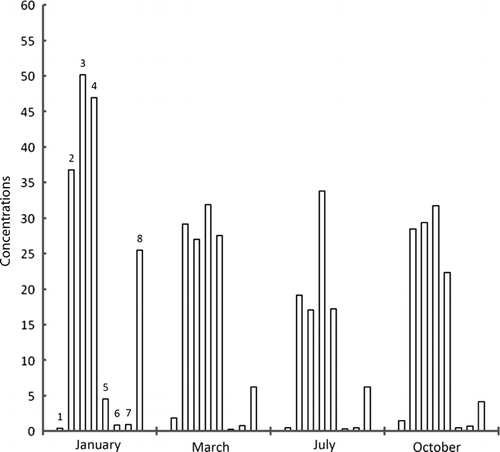
Figure 3b. Concentrations of metals in sludge samples taken from exit point of dam at Ebro River collected along zebra mussel colonies in different times of year; units=mg/kg, 1=cadmium, 2=lead, 3=copper, 4=nickel, 5=tin, 6=selenium, 7=mercury, 8=chromium.
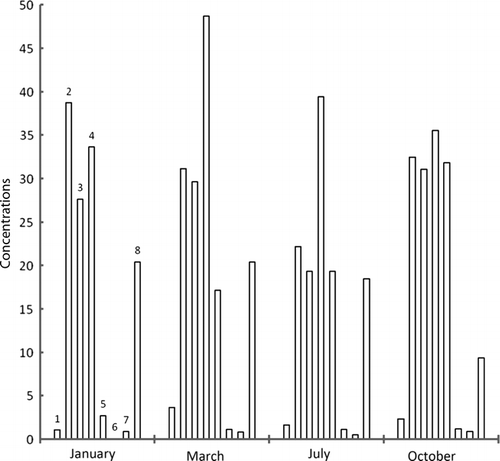
Figure 4a. Concentrations of metals in water samples taken from entry point of dam at Ebro River collected along zebra mussel colonies in different times of year; units=mg/kg, 1=cadmium, 2=lead, 3=copper, 4=nickel, 5=tin, 6=selenium, 7=mercury, 8=chromium.
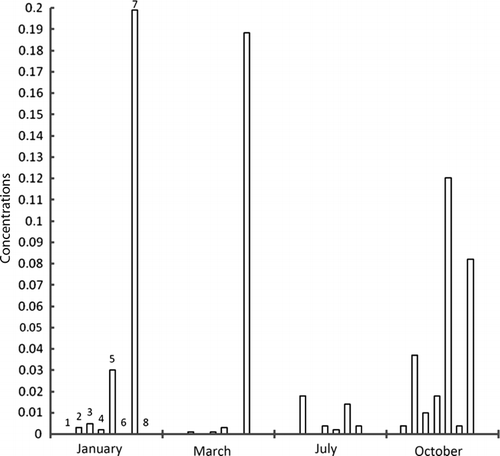
Figure 4b. Concentrations of metals in water samples taken from exit point of dam at Ebro River collected along zebra mussel colonies in different times of year; units=mg/kg, 1=cadmium, 2=lead, 3=copper, 4=nickel, 5=tin, 6=selenium, 7=mercury, 8=chromium.
Maximum accumulation of cadmium has taken place in March at exit point of dam. Same was the case with lead, nickel, and selenium. In general, concentration of metals, except copper, lessens as the year passes on, however, mercury has concentrated. The metal concentrations were higher in winter since these species accumulate metal for their physiological activities in cold. Afterwards, as the time goes on, metal concentration decreases through natural excretory processes. Maximum zinc concentration was found in December and January while cadmium in November and December. It supports the idea of metal build-up for physiological activities in cold but after winter, unload metals. Lead has the same tendency but not prominent. Metals like lead, mercury, tin, and chromium have wide range of applications in industry and in agriculture; therefore, they come to the sea in huge quantities. Study of analytes like zebra mussels will allow us to note metal contamination in sea, during specific times of year.
It was found that in the flesh samples taken from the dam exit point, metal concentration was high because of holding water in dam that made metal build-up suitable. Although, absorption and desorption went on simultaneously, but the samples got in March, have higher values because of high rates of absorption of metals. The sludge samples taken from the entry point of the dam have highest values in January. Metal concentration in water was highest in October because of activities taking place in summer. However, metal content in waters near to sediments was almost constant indicating an establishment of dynamic equilibrium (absorption–desorption).
Experimental
Reagents, standards, and instruments
All reagents used in this work were of high grade purity purchased from either Merck or Sigma-Aldrich. Double distilled water was used to prepare standards and samples. Stock solutions of metals were prepared by dissolving appropriate quantity of pure metal or anhydrous metal salt of high purity in distilled water. The standards were prepared by successive dilution of stock solutions. Anodic stripping voltametry was performed on a VA-Computrace (Model-757) equipped with GOES-646 processor. Mercury electrode was used for potential measurements, whereas silver/silver chloride electrode and platinum electrode were employed as reference electrode, respectively.
Sampling sites and timing
Samples were collected by different locations along the river (exit and entry points of Ribaroja dam), and at different depths, and in different times of year. Annual range of water parameters is shown in .
Table 1. Annual range of different water parameters collected by various depth levels.
Sample preparation
The zebra mussel colonies were drawn out alive (together with associated soil and water) from Ebro River. The three components (zebra mussels, sludge of moist soil, and water) were separated. Afterwards, mussels were soaked in distilled water for few hours and then in dilute HCl (1:100). They were spread over a filter paper and dried. Rind was separated with plastic tweezers. Comprehensive washing was carried out to remove all types of impurities like eggs and mussels in the embryonic process, since they can cause interruption during analysis. Flesh and shells were separated. Shells were crushed with mortar and flesh with turmix.
Shells were heated in china dish at 110oC for 2 hours to remove moisture. Organic content was removed by calcining at 550oC for 2 hours in controlled temperature furnace. Material gained after calcination was digested with 5 mL nitric acid (1:1) for 10–15 min and then diluted to 100 mL after filtration.
Flesh was also treated like shell sample, except that 1 g calcium carbonate was added to each 10 g of flesh sample to avoid metal loss during calcination. After digestion with 5 mL nitric acid (1:1), sample was preserved.
Analysis of metals
Electroanalytical system was calibrated for zinc, cadmium, mercury, lead, copper, nickel, selenium, iron, aluminum, chromium and tin, one by one, and then respective samples of each metal were run. Detection limits, linear range and percentage recovery of each metal were got by using synthetic samples ().
Table 2. Analytical parameters (detection limits, linear range, and percentage recovery) of voltamperometry.
Conclusions
Metals, nutritious as well as pollutants, were determined by voltamperometry in zebra mussel (Dreissena polymorpha), and collected at entry and exit points of Ribaroja dam located at Ebro River (Spain). The study has shown that metals like copper, cadmium, zinc, chromium, iron, selenium and mercury varies in water; in different parts of years depending on different factors like temperature, retention time in dam, and absorption capacity of zebra mussel. Voltamperometry is a good source to see variations in metal concentration in water that will help to explore outcomes of metal pollution in watery systems.
Acknowledgements
The authors would like to thank the members of “Grupo Especial de Actividades Subacuáticas de la Guardia Civil” for diving in the Ebro River and collecting the mussels for this study; the Spanish Electric Company “Endesa” for financial help for this project and also the Arágon Regional Government for financing our research group E 75.
References
- Binelli , A. ; Provini , A. DDT is still a problem in developed countries: the heavy pollution of Lake Maggiore . Chemosphere 2003 , 52 , 717 – 723 .
- Camusso , M. ; Balestrini , R. ; Binelli , A. Use of zebra mussel (Dreissena polymorpha) to assess trace metal contamination in the largest Italian subalpine lakes . Chemosphere 2001 , 44 , 263 – 270 .
- Fraysse , B. , Baudin , J. , Garnier-laplace , J. , Adam , C. and Boudou , A. 2002 . Effects of Cd and Zn waterborne exposure on the uptake and depuration of Co-57, Ag-110 and Cs-134 by the Asiatic clam (Corbicula fluminea) and the zebra mussel (Dreissena polymorpha)-whole organism study . Environ. Poll. , 118 : 297 – 306 .
- Rutzke , M. , Gutenmann , W. , Lisk , D. and Mills , E. 2000 . Toxic and nutrient element concentrations in soft tissues of zebra and quagga mussels from Lakes Erie and Ontario . Chemosphere , 40 : 1353 – 1356 .
- Gundacker , C. 1999 . Tissue-specific heavy metal (Cd, Pb, Cu, Zn) deposition in a natural population of the zebra mussel Dreissena polymorpha pallas . Chemosphere , 38 : 3339 – 3356 .
- Andersen , V. , Maage , A. and Johannessen , P. 1996 . Heavy metals in blue mussels (Mytilus edulis) in the Bergen Harbor area, western Norway . Bull. Environ. Contam. Toxicol. , 57 : 589 – 596 .
- Antoniou , V. , Zantopoulus , N. and Tsitsamis , S. 1994 . Heavy metal concentrations in mussels from the Gulf of Olympias-Chalkidiki, Greece . J. Environ. Sci. Health , A31 : 55 – 65 .
- Anzano , J. , Lasheras , R. , Bonilla , B. , Bonilla , A. , Lanaja , J. , Cia , I. , Peribañez , J. and Anwar , J. 2007 . Use of Zebra Mussel (Dreissena polymorpha) to assess trace metals in river water . J. Sci. Res. , 37 : 9 – 13 .
- Rodríguez , F. and Pampín , M. 1994 . Determination of iron, copper and zinc in tinned mussels by inductively-coupled plasma-atomic emission-spectrometry (ICP-AES) . Fresen. J. Anal. Chem. , 348 : 390 – 395 .
- Capelo , J. , Lavilla , I. and Bendicho , C. 2000 . Chemometric approach for the optimization of a flow injection manifold applied to the determination of total Hg in seafood by cold vapour AAS . Atom. Spectros. , 21 : 229 – 233 .
- Capelo , J. , Lavilla , I. and Bendicho , C. 1998 . Ultrasound-assisted extraction of cadmium from slurried biological samples for electrothermal atomic absorption spectrometry . J. Anal. Atom. Spectrom. , 13 : 1285 – 1290 .
- Lavilla , I. , Capelo , J. and Bendicho , C. 1999 . Determination of cadmium and lead in mussels by electrothermal atomic absorption spectrometry using an ultrasound-assisted extraction method optimized by factorial design . Fresen. J. Analyt. Chem. , 363 : 283 – 288 .
- Petersen , A. and Mortensen , G. 1994 . Trace-elements in shellfish on the Danish market . Food Addit. Contam. , 11 : 365 – 373 .
- Santos , C. , Moreno , F. , Lavilla , I. and Bendicho , C. 2000 . Total as in seafood as determined by transverse heated electrothermal atomic absorption spectrometry-longitudinal Zeeman background correction: An evaluation of automated ultrasonic slurry sampling, ultrasound-assisted extraction and microwave-assisted digestion methods . J. Anal. Atom. Spectrom. , 15 : 987 – 994 .
- Yebra , M. , Enríquez , M. and Cespón , R. 2000 . Preconcentration and flame atomic absorption spectrometry determination of cadmium in mussels by an on-line continuous precipitation-dissolution flow system . Talanta , 52 : 631 – 636 .
- Jugade , R. and Joshi , A. 2005 . Adsorptive cathodic stripping voltammetric determination of copper in fish samples . J. Indian Chem. Soc. , 82 : 842 – 844 .