Abstract
The green, mild, and efficient synthesis of 12-alkyl or aryl-8,9,10,12-tetrahydrobenzo[a]xanthen-11-ones has been achieved by a three-component coupling of 2-naphthol, aldehydes, and cyclic1,3-dicarbonyl compounds in the presence of a sulfonic acid functionalized ionic liquid without any solvent under microwave irradiation. The catalyst can be reused at least eight times without any noticeable decrease in catalytic activity.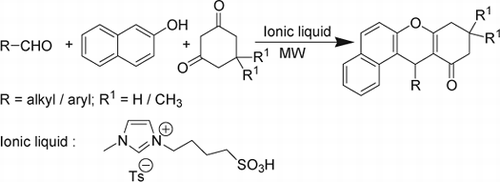
Introduction
Multicomponent reactions (MCR) play an important role in modern synthetic organic chemistry since they produce complex products with several degrees of structural diversity from mixing three or more simple starting materials in one pot Citation1 Citation2. Microwave (MW)-promoted MCR have been attracting research interest from chemists because these reactions exhibit some particular or unexpected reactivities and also because they are significantly useful for green chemistry Citation3–5. In our corresponding investigations, we have reported few MW-promoted multicomponent coupling reactions for various chemical transformation as well as the synthesis of useful heterocyclic compounds Citation6–10.
Xanthenes and benzoxanthenes are important biologically active heterocycles. They possess various biological activities such as anti-inflammatory, antiviral, and antibacterial Citation11–13. Some of them have been used as antagonists for paralyzing the action of zoxazolamine and in photodynamic therapy Citation14–17. Furthermore, these compounds can be used as dyes, as pH-sensitive fluorescent materials for the visualization of biomolecular assemblies, and in laser technologies Citation18–22. A broad utility range has made xanthenes prime synthetic targets, thereby accentuating the need to develop newer synthetic routes for xanthene derivatives. Few methods are available for the synthesis of tetrahydrobenzo[a]xanthen-11-ones under different reaction conditions Citation23–26. Although these methods are quite useful, they suffer from limitations such as the requirement of long reaction times, the use of excess reagents/catalysts, and the use of toxic organic solvents. To avoid these limitations, the discovery of a new and efficient catalyst with high catalytic activity, short reaction time, recyclability, and simple work-up for the preparation of xanthenes under mild and practical conditions is of great interest. Recently we have reported the reaction of 2-naphthol and aldehydes (2:1) to form dibenzoxanthenes in the presence of a catalytic amount of indium chloride in water Citation27.
The application of Brønsted acidic task-specific ionic liquids (TSILs) as catalytic materials is growing continuously in the catalytic field. Combining the useful characteristics of solid acids and mineral acids, TSILs have been used to replace traditional mineral liquid acids, such as hydrochloric acid and sulfuric acid, in chemical reactions. In view of green chemistry, the substitution of harmful liquid acids by reusable TSILs is one of the most promising types of catalyst in chemistry Citation28–32.
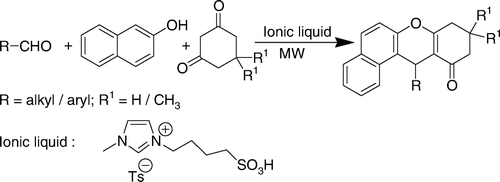
In continuation of our interest in ionic liquid-mediated reactions Citation33, we have explored the utility of a functionalized ionic liquid, 1-butane sulfonic acid-3-methylimidazolium tosylate ([BSMIM]Ts), as a catalyst for the synthesis of 12-alkyl or aryl-8,9,10,12-tetrahydrobenzo[a]xanthen-11-ones under solvent-free conditions (). To the best of our knowledge, this is the first report of a functionalized ionic liquid catalyzed synthesis of 12-alkyl or aryl-8,9,10,12-tetrahydrobenzo[a]xanthen-11-ones under MW irradiation.
Results and discussion
The experimental procedure is very simple. To optimize the reaction conditions, the reaction of β-naphthol, 4-chlorobenzaldehyde, and dimedone was selected as a model to examine the effects of the catalyst (0–20%) and reagents in different reaction conditions under MW irradiation in neat conditions (). The results were evaluated qualitatively through TLC. The best result was achieved by carrying out the reaction with 1:1:1.1 mole ratios of 2-naphthol, 4-chlorobenzaldehyde, and dimedone in the presence of 5 mol% acidic ionic liquid for 10 min (with an installment of 4 min each at a power level of 240 Watts followed by intermittent cooling). The structure of the product was determined from the spectral data. We found that 5 mol% of acid was the optimum amount of catalyst, and increasing the amount of catalyst did not improve the yields while decreasing the amount of catalyst decreased the yield. It should be mentioned that when reactions were carried out in the absence of catalyst for long periods of time (1 h) under the present reaction conditions, the yields of the products were low (<5%).
Table 1. Optimization of the amount of catalyst and MW power on the reaction of 2-naphthol, 4-chlorobenzaldehyde, and dimedone.
After optimizing the reaction conditions, we then examined the generality of the reaction conditions to other substrates using several aromatic and aliphatic aldehydes. The results are summarized in . In all cases, aromatic aldehydes carrying either an electron-withdrawing group or an electron-donating group reacted successfully and gave the products in good to excellent yields. Acid-sensitive substrates such as piperonal and 2-thiophenecarboxaldeyde produced the desired condensation product in good yields (entries 5 and 11). Another advantage of this method is its efficiency for the synthesis of xanthene derivatives from aliphatic aldehydes (entries 12 and 13). However, the reaction conducted with phenol and 1-naphthol instead of 2-naphthol could not afford any product. The work-up procedure is very simple and includes filtration of the mixture to separate the product. After isolation of the product from the water mixture, the water layer containing the acidic ionic liquid was reused after evaporation of the water. We have successfully recovered the catalyst for the model reaction (entry 7, ). The recovered catalyst was used in the next run, and consistent activity was observed for six consecutive cycles. The results are shown in .
Table 2. Synthesis of xanthenes in the presence of acidic iconic liquid.
Table 3. Recyclability of the catalyst.
To expand the scope of the reaction and to generalize the procedure, the replacement of 5,5-dimethyl-1,3-cyclohexanedione with 1,3-cyclohexanedione was examined. To our delight, under the same conditions, the reactions proceeded steadily to afford a series of xanthene-based compounds in good yields.
The efficiency of the present protocol can be realized by comparing some of the results presented here with two recently reported methods, as shown in , which compares reaction time, yields, and reaction conditions. Thus, it is clear from that the present protocol can act as an effective method with respect to time and reaction conditions.
Table 4. Comparison of the present protocol with recently reported methods.
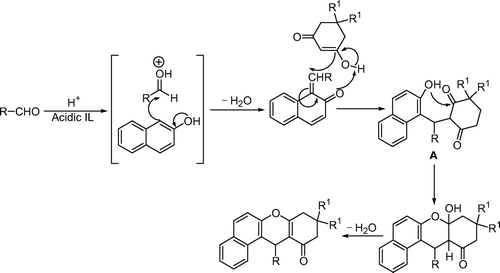
The possible mechanism of this reaction is shown in . The condensation of 2-naphthol with aldehydes under acid catalysis is believed to proceed via ortho-quinone methide intermediates (o-QMs). Then, the trapping of o-QMs with dimedone formed intermediates A, which were cyclized and then dehydrated to give the corresponding xanthene derivatives.
Experimental
Melting points were determined on a glass disk with an electrical bath and are uncorrected. 1H NMR (300 MHz) and 13C NMR (75 MHz) spectra were run in CDCl3 solutions. IR spectra were taken as KBr plates. Elemental analyses were done by Perkin-Elmer autoanalyzer. All liquid reagents were distilled before use. The synthesis of the Brønsted acidic ionic liquid, 1-butane sulfonic acid-3-methylimidazolium tosylate, [BSMIM]Ts was carried out using a method similar to that reported Citation34.
Typical procedure for the synthesis of 12-benzo[1,3]dioxol-5-yl-9,9-dimethyl-8,9,10,12-tetrahydrobenzo[a]xanthen-11-one (entry 5, Table 1)
To a mixture of 2-naphthol (288 mg, 2 mmol), piperonal (300 mg, 2 mmol), and dimedone (308 mg, 2.2 mmol), acidic ionic liquid (39 mg, 5 mol%) was added. The mixture was irradiated under MW in a domestic MW oven at 240 W (20% power) for 14 min (with a brief interval after every 4 min of heating) as required to complete the reaction. The mixture, after being cooled to room temperature, was poured into crushed ice (20 g) and stirred for 5–10 min. The solid separated was filtered under suction (water aspirator), washed with ice-cold water (20 mL), and then recrystallized from hot ethanol to afford pure product (637 mg, 80%) as white powder. Mp 190–191°C; IR (KBr) 2954, 2875, 1643, 1479, 1375 cm–1; 1H NMR (300 MHz, CDCl3) ▵ 7.98 (d, J=8.1 Hz, 1H), 7.75 (t, J=7.5 Hz, 2H), 7.46–7.24 (m, 3H), 6.84 (d, J=7.8 Hz, 1H), 6.78 (s, 1H), 6.60 (d, J=7.8 Hz, 1H), 5.82 (s, 1H), 5.77 (s, 1H), 5.63 (s, 1H), 2.55 (s, 2H), 2.27 (s, 2H), 1.10 (s, 3H), 0.98 (s, 3H); 13C NMR (75 MHz, CDCl3) ▵ 196.8, 163.6, 147.5, 147.3, 145.6, 138.7, 131.3, 131.2, 128.7, 128.3, 126.8, 124.8, 123.5, 121.6, 117.5, 116.9, 114.1, 108.8, 107.8, 100.6, 50.8, 41.2, 34.2, 32.1, 29.1, 27.1; Anal. found C 78.23%, H 5.41%. Cald for C26H22O4, C 78.37%, H 5.57%.
This procedure was followed for the synthesis of all xanthene derivatives listed in . The catalyst was recovered almost quantitatively after removal of water under reduced pressure. The catalyst was reused in the reaction, and it showed the same activity as fresh catalyst without any loss of its activity. After eight recycles, the catalyst still had a high activity and gave the desired product in excellent yield (82%, for entry 7, ).
Conclusions
Brønsted acidic ionic liquid [BSMIM]Ts is an efficient catalyst for the preparation of 12-alkyl or aryl-8,9,10,12-terahydrobenzo[a]xanthen-11-one derivatives. The notable advantages of this procedure are (a) simple operation; (b) excellent yields; (c) fast reaction; (d) general applicability; (e) reusability of catalyst; and (f) above all, green synthesis avoiding toxic reagents and solvents. Thus it provides a better and more practical alternative to existing methodologies for synthesis of 12-alkyl or aryl-8,9,10,12-tetrahydrobenzo[a]xanthen-11-ones. Further studies on the application of the recent methodology to the synthesis of biologically active compounds are under investigation.
Acknowledgements
A.H. is pleased to acknowledge the financial support from DST (Grant No. SR/FTP/CS-107/2006). A.M. acknowledges financial support from CSIR (Grant No. 01(2251)/08/EMR-II). D.K. thanks CSIR for his fellowship.
References
- Zhu , J. ; Bienayme , H. Multicomponent Reactions , 1st ed. ; Wiley-VCH : Wenheim , 2005 .
- Ugi , I. Pure Appl. Chem. 2001 , 73 , 187 – 191 .
- Polshettiwar , V. ; Varma , R.S. Acc. Chem. Res. 2008 , 41 , 629 – 639 .
- Roberts , B.A. ; Strauss , C.R. Acc. Chem. Res. 2005 , 38 , 653 – 661 .
- Kappe , C.O. Angew. Chem. Int. Ed. 2004 , 43 , 6250 – 6284 .
- Ranu , B.C. ; Hajra , A. ; Dey , S.S. ; Jana , U. Tetrahedron 2003 , 59 , 813 – 819 .
- Ranu , B.C. ; Samanta , S. ; Hajra , A. Synlett 2002 , 987 – 989 .
- Ranu , B.C. ; Hajra , A. Tetrahedron 2001 , 57 , 4767 – 4773 .
- (a) Ranu , B.C. ; Hajra , A. ; Jana , U. Synlett 2000 , 75 – 76 ; (b) Ranu, B.C.; Hajra, A.; Jana, U. Tetrahedron Lett. 2000, 41, 531–533 .
- Rahman , M. ; Roy , A. ; Majee , A. ; Hajra , A. J. Chem. Res. 2009 , 178 – 179 .
- Poupelin , J.P. ; Saint-Ruf , G. ; Foussard-Blanpin , O. ; Narcisse , G. ; Uchida-Ernouf , G. ; Lacroix , R. Eur. J. Med. Chem. 1978 , 13 , 67 – 71 .
- Lambert , R.W. ; Martin , J.A. ; Merrett , J.H. ; Parkes , K.E.B. ; Thomas , G.J. PCT Int. Appl. WO 9,706,178, 1997 .
- Hideo , T. ; Teruomi , J. Jpn. Patent 56,005,480, 1981 .
- Buu-Hoi , N.P. ; Saint-Ruf , G. ; De , A. ; Hieu , H.T. Chim. Ther. 1972 , 7 , 83 – 86 .
- Saint-Ruf , G. ; Hieu , H.T. ; Poupelin , J.P. Naturwissenschaften 1975 , 62 , 584 .
- Ion , R.-M. Prog. Catal. 1997 , 6 , 55 – 76 .
- Ion , R.M. ; Planner , A. ; Wiktorowicz , K. ; Frackowiak , D. Acta Biochim. Pol. 1998 , 45 , 833 – 845 .
- Banerjee , A. ; Mukherjee , A.K. Stain Technol. 1981 , 56 , 83 – 85 .
- Menchen , S.M. ; Benson , S.C. ; Lam , J.Y.L. ; Zhen , W.-G. ; Sun , D.-Q. ; Rosenblum , B.B. ; Khan , S.H. ; Taing , M. US Patent 6,583,168, 2003 .
- Knight , C.G. ; Stephens , T. Biochem. J. 1989 , 258 , 683 – 687 .
- Sirkencioglu , O. ; Talinli , N. ; Akar , A. J. Chem. Res. 1995 , 502 .
- Ahmad , M. ; King , T.A. ; Ko , D.-K. ; Cha , B.H. ; Lee , J. J. Phys. D: Appl. Phys. 2002 , 35 , 1473 – 1476 .
- Das , B. ; Laxminarayana , K. ; Krishnaih , M. ; Srinivas , Y. Synlett 2007 , 3107 – 3112 .
- Li , J. ; Tang , W. ; Lu , L. ; Su , W. Tetrahedron Lett. 2008 , 49 , 7117 – 7120 .
- Wang , R.-Z. ; Zhang , L.-F. ; Cui , Z.-S. Synth. Commun. 2009 , 39 , 2101 – 2107 .
- Khurana , J.M. ; Mangoo , D. Tetrahedron Lett. 2009 , 50 , 4777 – 4780 .
- Urinda , S. ; Kundu , D. ; Majee , A. ; Hajra , A. Heteroatom Chem. 2009 , 20 , 232 – 234 .
- Cole , A.C. ; Jensen , J.L. ; Ntai , I. ; Tran , K.L.T. ; Weaver , K.J. ; Forbes , D.C. ; Davis , J.H. Jr J. Am. Chem. Soc. 2002 , 124 , 5962 – 5963 .
- Fang , D. ; Zhou , X.-L. ; Ye , Z.-W. ; Liu , Z.-L. Ind. Eng. Chem. Res. 2006 , 45 , 7982 – 7984 .
- Leng , Y. ; Wang , J. ; Zhu , D. ; Ren , X. ; Ge , H. ; Shen , L. Angew. Chem. Int. Ed. 2009 , 48 , 168 .
- Chakraborti , A.K. ; Roy , S.R. ; Kumar , D. ; Chopra , P. Green Chem. 2008 , 10 , 1111 – 1120 .
- Chakraborti , A.K. ; Roy , S.R. J. Am. Chem. Soc. 2009 , 131 , 6902 .
- Ranu , B.C. ; Dey , S.S. ; Hajra , A. Tetrahedron 2003 , 59 , 2417 – 2421 .
- Du , Z. ; Li , Z. ; Deng , Y. Synth. Commun. 2005 , 35 , 1343 – 1349 .