Abstract
The inhibitory effect of 3-[(4-amino-2-methyl-5-pyrimidinyl) methyl]-5-(2-hydroxyethyl)-4-methyl thiazolium chloride hydrochloride (thiamine hydrochloride) or vitamin B1 hydrochloride on acid corrosion of copper in 2.5 M nitric acid (HNO3) solution was investigated using a weight loss method. Thiamine hydrochloride inhibited the corrosion of copper in 2.5 M HNO3 solutions. The inhibition efficiency increased with increasing concentration of thiamine hydrochloride but decreased with increasing exposure. Adsorption of thiamine hydrochloride molecules on copper surface was found to obey Langmuir adsorption isotherm with standard free energy of adsorption (Δ) –30.55 kJ mol−1. The inhibition mechanism was characterized from trends of inhibition efficiency, thermodynamic, and kinetic parameters.
Introduction
Studies on acid corrosion and inhibition of copper in nitric acid solution are generating a lot of interest because nitric acid solution is the corrosive solution of choice for both the chemical dissolution and electroplating processes used in the fabrication of electronic devices and copper is susceptible to corrosion in nitric acid solution Citation1 Citation2.
Numerous inorganic and organic compounds have been reported as corrosion inhibitors for metals in different media, but the toxic nature of some of them limits their application. Attention has been focused on the need to design and develop green or non-toxic corrosion inhibitor to replace toxic ones for a sustainable development. Amino acids Citation3–5, phthalazine derivatives Citation6, caffeine Citation7, imidazole derivatives Citation8, and guanidine Citation9 have been reported as nontoxic corrosion inhibitors for copper in acid media and their inhibitive action was found to depend on molecular structures, the nature of the metal surface, types of ions in the electrolytes, ability to form complexes, and the extent of adsorption on the metal surface. We have also reported the inhibitive and adsorption properties of 3-[(4-amino-2-methyl-5-pyrimidinyl) methyl]-5-(2-hydroxyethyl)-4-methyl thiazolium chloride hydrochloride or thiamine hydrochloride, a naturally occurring substance and its derivative on mild steel Citation10–12 and aluminum Citation13 in HCl solutions using weight loss and hydrogen evolution techniques. The literature on nontoxic corrosion inhibitors for copper in nitric acid solution is not in a large number in comparison with that for copper in both HCl and H2SO4 solutions. However, to the best of our knowledge there is no reported work in the open literature on the inhibitive and adsorption properties of AMMPTC on copper in nitric acid solutions.
This paper is a follow-up effort on our previous work Citation10–12 on the development of nontoxic (green) corrosion inhibitors of metals that do not contain heavy metals and organic phosphates. Thiamine hydrochloride, nontoxic organic compound consists of pyrimidine ring and thiazollium ring bridged by methylene group is already known for its complex formation owing to its coordination sites Citation14. It is of interest to note that thiamine hydrochloride contains sulfur atom and many organic compounds containing sulfur atom have been developed as copper corrosion inhibitors for different industrial application Citation15 owing to the ability of S atom to be strongly adsorbed on copper surface. Therefore, thiamine hydrochloride may be a potential nontoxic corrosion inhibitor for copper in nitric acid solution. The aim of this work is to investigate the inhibitive effect of thiamine hydrochloride on the acid corrosion of copper in 2.5 M HNO3 solution and its adsorption characteristics using weight loss technique.
Results and discussion
The results obtained are presented in Figures and Tables for different concentrations of thiamine hydrochloride in 2.5 M HNO3 solutions from gravimetric measurements.
Figure 1. Variation of weight loss with thiamine hydrochloride concentration for copper in 2.5 M HNO3 at 30°C.
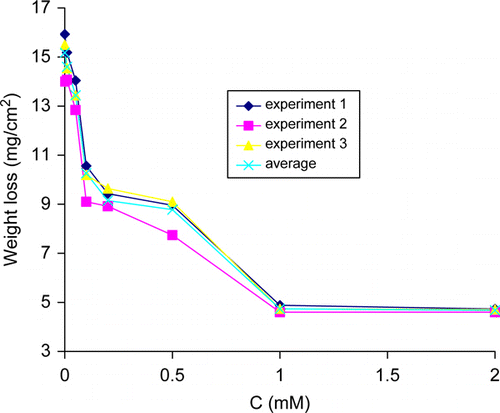
Table 1. Percentage inhibition efficiency, corrosion rate, and surface coverage of thiamine of thiamine hydrochloride on copper at 30°C 30 min immersion period.
The weight losses of copper versus concentration of thiamine hydrochloride for 30 min immersion time at 30°C are presented in .
In the addition of thiamine hydrochloride resulted in low material loss (mg/cm2) as compared with that of control at 30°C. The weight losses (mg/cm2) of copper coupon in the presence of 0.01, 0.05, 0.1, 0.2, 1, and 2 mM concentrations of thiamine hydrochloride decreased by a factor of 0.96, 0.89, 0.68, 0.58, 0.31, and 0.30, this in reference to the blank (control, ), respectively. These results implied that thiamine hydrochloride inhibited the corrosion of copper in 2.5 M HNO3 solution at these concentrations at 30°C for 30 min immersion period. The percentage inhibition efficiency values are listed in for triplicate specimens and were precise to ±4% and this indicates good reproducibility.
These results are indicative of the inhibition of the dissolution of copper in HNO3 solution, with the maximum inhibition efficiency values of 69.9% at 2 mM thiamine hydrochloride at 30°C. The inhibitory activity of thiamine hydrochloride with respect to acid dissolution of copper in HNO3 solution may be associated with their adsorbability due to the presence of adsorption centers of S, N, and O in thiamine hydrochloride. A similar view has been reported previously Citation13 on the behavior of thiamine hydrochloride on aluminum surface in HCl solution. It is evident in that the corrosion rate decreased with increasing concentration of the inhibitor in the acid solutions and the percentage inhibition efficiency values increase with increasing inhibitor concentration. This indicates that the inhibition is due to the adsorption of inhibitor's molecule onto copper surface and the inhibitor acts as an adsorption inhibitor. Previously, similar views have been put forward on the inhibition of acidic corrosion of metals Citation16–20.
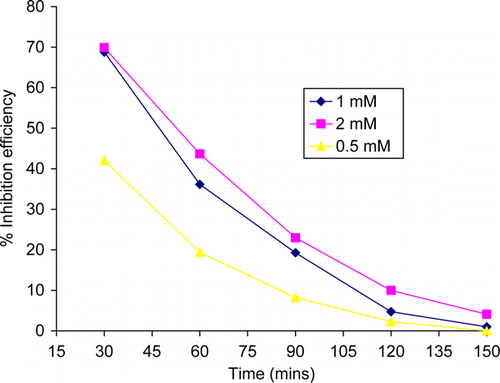
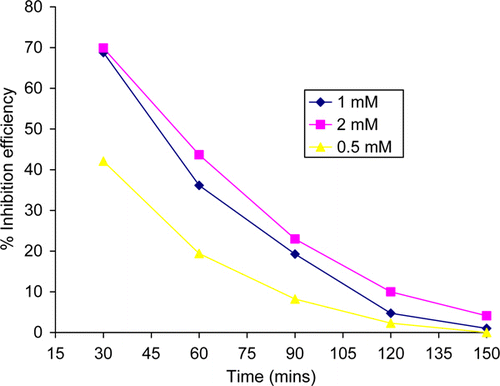
The effect of immersion time on inhibition efficiency is shown in . As seen in , the inhibition efficiency decreases with increase in immersion time. The decrease in the value of percentage inhibition efficiency with immersion time may be ascribed to increase in desorption of the inhibitor molecules from metal surface with increasing immersion time.
Figure 2. Variation of percentage inhibition efficiency of thiamine hydrochloride with immersion period.
Adsorption isotherms and characteristics of adsorption process
A detailed mechanism for inhibition is not possible without an understanding of the adsorption process at the metal/electrolyte interface. To clarify the nature of adsorption, the fits of various adsorption isotherms to the empirical data were tested. For organic corrosion inhibitors inhibiting through adsorption mode, as in this case, the degree of surface coverage, θ can be determined from the value of inhibition efficiency with θ=I%/100.
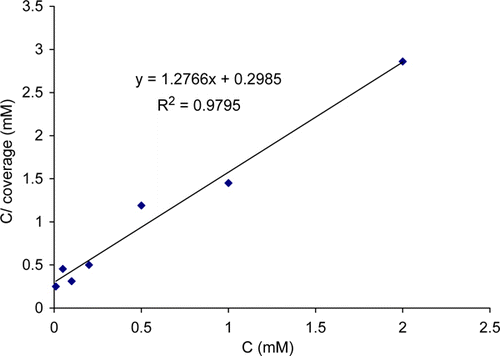
The experimental data were applied to different adsorption isotherm equations and found to fit the Langmuir adsorption isotherm Citation19,Citation21–23 with correlation coefficient of 0.980 (), which may be formulated as equation Citation1:
Figure 3. Langmuir adsorption plot for copper in 2.5 M HNO3 solution containing thiamine hydrochloride at 30°C.
The K is related to the standard free energy of adsorption by the equation:
The value of standard free energy of adsorption, Δ was obtained from the values of K. The value for Δ
for the inhibition of acid corrosion of copper by thiamine hydrochloride was found to be –30.55 kJ mol–1. The negative value of adsorption free energy suggests the spontaneity of the adsorption of thiamine hydrochloride molecules and the stability of the adsorbed layer on copper surface.
The adsorption process is adjudged physical adsorption due to the value of Δ that was below –40 kJ mol–1. There is a general concession that adsorption with Δ
values below –40 kJ mol–1 are physical in nature Citation11
Citation12
Citation18
Citation19
Citation25–29.
Kinetic model
The kinetic model was employed to give further insight into the inhibition properties of AMMPTC. The effect of temperature on the corrosion rate of copper in nitric acid solution in the absence and presence of 0.5–2 mM thiamine hydrochloride was studied at 30, 35, 40, and 45±0.2°C by gravimetric measurements.
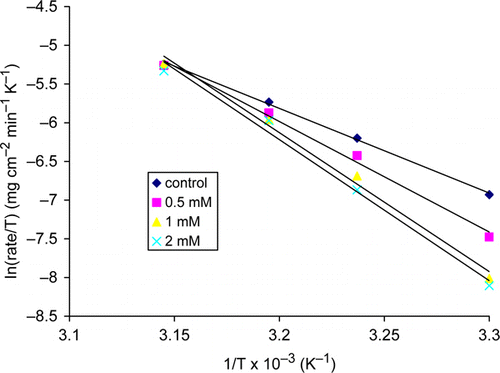
shows the dependence of thiamine hydrochloride efficiency on temperature when different concentrations of thiamine hydrochloride were added. Inhibitor efficiency decreased as the temperature increased and this indicates physisorption of thiamine hydrochloride at the copper surface. Desorption of inhibitor molecules with increased temperature is usually observed in cases of physisorption. According to Noor Citation30 and the reference there in, the kinetic – thermodynamic model represent the modified form of Langmuir adsorption isotherm; log (θ/1-θ)=log K /+y log C, reformulated by including the heat of adsorption parameter as follows:
Figure 5. Plot of log (θ/1 – θ) versus 1/T at concentrations of 0.5, 1, and 2 mM thiamine hydrochloride.
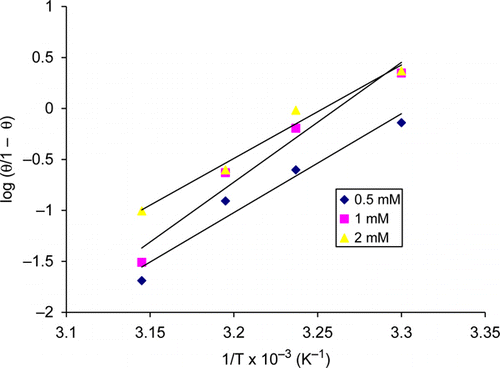
Table 2. Heat of adsorption, Q ad and the correlation coefficients for thiamine hydrochloride on copper surface in 2.5 M HNO3.
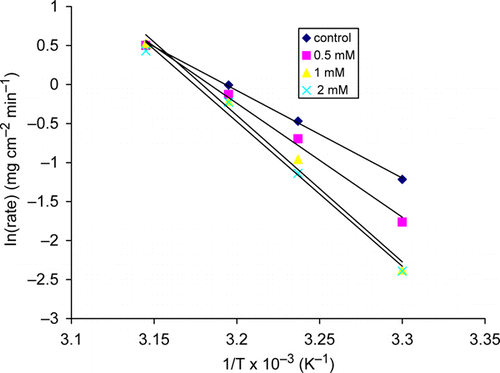
and depict the fitting of the corrosion data to Arrhenius and Errying equations for inhibited and uninhibited copper in 2.5 M HNO3 solution. The activation parameters obtained from and are listed in .
Table 3. Corrosion activation parameters and the correlation coefficients for copper in 2.5 M HNO3 with and without thiamine hydrochloride.
The plot of ln(rate) versus 1/T and ln(rate/T) versus 1/T gave straight lines with slopes of –E a and (–H*/R), respectively. The intercepts are A and (lnR/Nh)+ΔS*/R) for the Arrhenius and Eyring transition state equations, respectively.
The linear regression coefficients in show that the relationship between ln(rate) and 1/T, and ln (rate/T) and 1/T can be described by the Arrhenius equation and Eyring transition state equations, respectively. The activation parameters; Ea, ΔH* and ΔS* for the corrosion process were determined and presented in .The free energy change, ΔG* for the corrosion process was calculated using the relationship ΔG*=ΔH* – TΔS* and the results obtained given in . The values of pre-exponential factor and apparent activation energy are higher in the presence of the inhibitor. The values of pre-exponential factor and apparent activation for the unhibited solution are 5.76×1015 gm−2h−1 and 93.10 kJ mol−1, respectively, while 2.39×1020 to 1.3×1025 gm−2 h−1 and 121.16–155.88 kJ mol−1 are obtained for pre-exponential factor and apparent activation energy, respectively, for the inhibited solutions. This result suggests that both the increase in apparent activation energy and pre-exponential factor leads to an increase in energy barrier for the corrosion of copper in HNO3 solution. Foudal et al. Citation31 has reported similar trend in the value of apparent activation energy for copper corrosion in HNO3 acid solution in the presence of phthalimide derivatives.
In uninhibited and inhibited solutions the values of E a are larger than for the corresponding values of ΔH* with 2.66 kJ mol–1; this is in agreement with the well-known equation for a unimolecular reaction, E a – ΔH*=RT Citation32 Citation33. The positive values of ΔG* in the absence and presence of AMMPTC show that the activated complex was not stable both in the absence and presence of the inhibitor. As presented in Table 3, ΔG* becomes more positive with increasing concentration of the inhibitor. The implies that in the presence of the inhibitor the activated complex becomes less stable as compared to its absence.
The mechanism of inhibition
Corrosion inhibition of copper in nitric acid solution by thiamine hydrochloride can be explained on the basis of adsorption of its molecule on the metal surface. In acid solutions, thiamine hydrochloride exists as cationic species by being protonated at the sulfur atom, since such compounds are known to be protonated at the sulfur atom Citation11 Citation12. The charge molecules adsorb on the charge metal surface, thereby reducing the surface area that is available for the attack of the aggressive ions from the acid solutions.
The value of Δ obtained (−30.55 kJ mol−1) is in support of electrostatic interaction between the charge inhibitor and the charge metal surface (that is physisorption mechanism).
Experimental
Material preparation
The chemical structure of the compound of interest, 3-[(4-amino-2- methyl-5-pyrimidinyl) methyl]-5-(2-hydroxyethyl)-4-methyl thiazolium chloride hydrochloride or thiamine hydrochloride is presented below: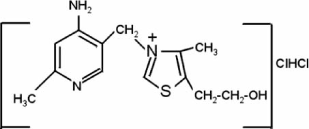
The thiamine hydrochloride (CAS 67 – 03 – 8) and HNO3 were analytical grade reagents and used as received.
Rectangular specimens in sizes of 5×2×0.04 cm were cut from copper sheet (98.61% Cu) for weight loss measurements. The coupons were used for the study as received without further polishing, but were degreased in absolute ethanol, dried in acetone, and stored in a moisture free desiccators before their use in corrosion studies. Doubly distilled water was used for the preparation of all the solutions. Additive concentrations of 0.01, 0.05, 0.1, 0.2, 0.5, 1, and 2 mM were prepared in the electrolyte solution (2.5 M HNO3).
Weight loss determination
The procedure for weight loss determination was similar to that reported previously Citation11 Citation13. The pre-weighed copper coupons were immersed in 50 ml of 2.5 M HNO3 solutions (in open beakers) without and with different concentrations (0.01–2 mM) of the additive at 30°C. The variation of weight loss was monitored at 30 min interval progressively for 120 min per coupon at 30°C. Each reading reported is an average of three experimental readings recorded to the nearest 0.0001 g on a Mettler digital analytical balance.
The effect of temperature on the corrosion rate of copper coupon in 2.5 M HNO3 for 30 min immersion period at 35, 40, and 45±0.2°C was also studied with 0.5, 1, and 2 mM thiamine hydrochloride. The temperature was thermostatically controlled using water thermostat with precision of ±0.2°C. Corrosion rate (r s ), and percentage inhibition (% I) were calculated using Equations Citation6 and Citation7:
Conclusions
Based on the results obtained, the following conclusions can be drawn:
The studied compound thiamine hydrochloride can be used as nontoxic corrosion inhibitor for copper corrosion in HNO3 solution.
The inhibition efficiency of thiamine hydrochloride increases with increasing inhibitor concentration over the concentration range 0.01–2 mM and decreases with increasing temperature.
The inhibitive action of the inhibitor was attributed to adsorption of its molecule on to copper surface in the acidic solution and the data fit well into Langmuir adsorption isotherm.
The value of Δ was negative and this confirmed the spontaneity of the adsorption process on the surface of copper in nitric acid solution. The corrosion activation parameters (E
a
, ΔH*, ΔS*, and ΔG* were higher in the presence of thiamine hydrochloride than in the absence of thiamine hydrochloride.
References
- Sherif , E.M. and Park , S.-M. 2006 . Corros. Sci. , 48 : 4065 – 4079 .
- Fiala , A. , Chibani , A. , Darchen , A. , Boulkamh , A. and Djebbar , K. 2007 . Appl. Surf. Sci. , 253 : 9347 – 9356 .
- Scendo , M. 2008 . Corros. Sci. , 50 : 1584 – 1592 .
- Zhang , D. , Gao , L.-X. and Zhou , G.-D. 2005 . J. App. Electrochem. , 35 : 1081 – 1085 .
- Zhang , D. , Cai , Q. , He , Q.-R. , Gao , X.-M. and Zhou , G.-D. 2008 . Mater. Chem. Phys. , 112 : 353 – 358 .
- Abd El-Maksoud , S.A. 2004 . Electrochim. Acta , 49 : 4205 – 4212 .
- Fallavena , T. , Antonow , M. and Goncalves , R.S. 2006 . Appl. Surf. Sci. , 253 : 566 – 571 .
- Stupnisek-Lisac , E. , Gazivoda , A. and Madzarac , M. 2002 . Electrochim. Acta , 47 : 4189 – 4194 .
- Khaled , K.F. 2008 . Appl. Surf. Sci. , 255 : 1811 – 1888 .
- Abiola , O.K. and Oforka , N.C. 2002 . J. Corros. Sci. Eng. , 3 : 1 – 8 .
- Abiola , O.K. and Oforka , N.C. 2004 . Mater. Chem. Phys. , 83 : 315 – 322 .
- Abiola , O.K. 2006 . Corros. Sci. , 48 : 3078 – 3090 .
- Abiola , O.K. 2007 . Corros. Mater. , 32 : 10 – 15 .
- Adeyemo , A. , Kolawole , G.A. and Jimoh , W. 1986 . J. Coord. Chem. , 15 : 103 – 107 .
- Zhang , D. , Gao , L.-X. and Zhou , G.-D. 2004 . Corros. Sci. , 46 : 3031 – 3040 .
- Fouda , A.S. , Gouda , M.M. and Abd EI-Rahman , S.L. 2000 . Bull. Korean Chem. Soc. , 21 : 1085 – 1089 .
- Bansiwal , A. , Anthony , A. and Mathur , S.P. 2000 . Br. Corros. J. , 35 : 301 – 303 .
- Kumar , T. , Vishwanatham , S. and Udayabhanu , G. 2004 . Corros. Eng. Sci. Technol. , 39 : 327 – 332 .
- Abiola , O.K. , Oforka , N.C. , Ebenso , E.E. and Nwinuka , N.M. 2007 . Anti-Corros. Meth. Mater. , 54 : 219 – 224 .
- Okafor , P.C. , Ikpi , M.E. , Uwah , I.E. , Ebenso , E.E. , Ekpe , U.J. and Umoren , S.A. 2008 . Corros. Sci. , 50 : 2310 – 2317 .
- Abd El Rehim , S.S. ; Ibrahim , M.A.M. ; Khalid , K.F. Mater. Chem. Phys . 2001 , 70 , 268 – 273 .
- Abdel-Gaber , A.M. ; Abd-El-Nabey , B.A. ; Sidahmed , I.M. ; El-Zayady , A.M. ; Saadawy , M. Corros. Sci . 2006 , 48 , 2765 – 2779 .
- Tang , L. ; Li , X. ; Si , Y. ; Mu , G. ; Liu , G. Mater. Chem. Phys . 2006 , 95 , 29 – 38 .
- Ebenso , E.E. , Ekpe , U.J. , Umoren , S. , Jackson , E. , Abiola , O.K. and Oforka , N.C. 2006 . J . Appl. Polym. Sci. , 100 : 2889 – 2894 .
- Okafor , P.C. and Ebenso , E.E. 2007 . Pigment Resin Technol. , 36 : 134 – 140 .
- Donahue , F.M. and Nobe , K. 1965 . Electrochem. Soc. , 112 : 886 – 891 .
- Khamis , E. , Bellucci , F. , Latanision , R.M. and El-Ashry , E.S.H. 1991 . Corrosion , 47 : 677 – 686 .
- Bilgic , S. and Sahin , M. 2001 . Mater. Chem. Phys. , 70 : 290 – 275 .
- Okafor , P.C. and Zheng , Y.G. 2009 . Corros. Sci. , 51 : 761 – 768 .
- Noor , E.A. 2005 . Corros. Sci. , 47 : 33 – 55 .
- Fouda , A.S. , Abd El-Aal , A. and Kandil , A.B. 2006 . Desalination , 201 : 216 – 223 .
- Bentiss , F. , Bouanis , M. , Mernari , B. , Traisnel , M. , Vezin , H. and Lagrenée , M. 2007 . Appl. Surf. Sci. , 253 : 3696 – 3704 .
- Noor , E.A. and Al-Moubaraki , A.H. 2008 . Mater. Chem. Phys. , 110 : 145 – 154 .