Abstract
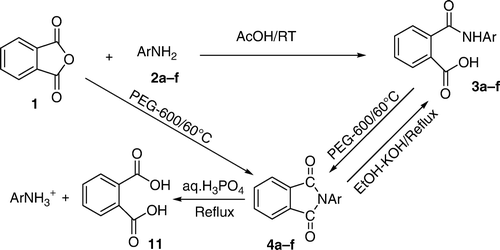
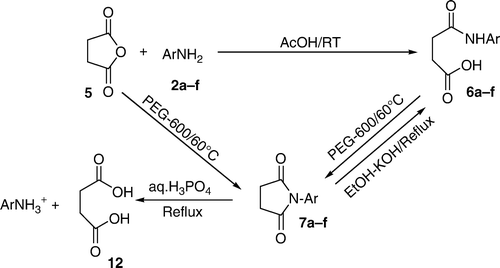
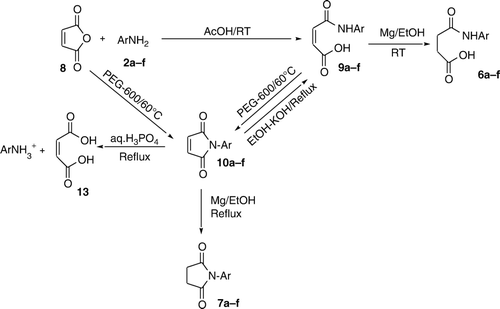
Phthalic anhydride 1 was reacted with aromatic primary amines 2 in acetic acid at room temperature to yield mono acid mono amide derivatives 3. The latter were each transformed into the corresponding imides 4 by heating in the green solvent PEG-600 at 60°C high yields and in high purity in short reaction times involving a dehydrative ring closure. The above reaction of 1 with 2 in acetic acid was extended to succinic anhydride 5 and maleic anhydride 8 resulting in open chain compounds 6 and 9, respectively. The latter were cyclized, once again in the green solvent PEG-600, to the corresponding imides, i.e. succinimides 7 and maleimides 10, respectively. Each of the imides 4, 7, and 10 could be reconverted to the corresponding open chain compounds 3, 6, and 9 by heating in ethanolic KOH. Simple hydrolysis of imides 4, 7, and 10 with aq.H3PO4 yielded the corresponding dicarboxylic acids 11, 12, and 13, respectively. Treatment of maleic amide 9 and maleicimide 10 with Mg in EtOH under reflux gave the corresponding succinic amide 6 and succinicimide 7, respectively, by the chemo selective reduction of the double bond without touching the amide/carbonyl groups.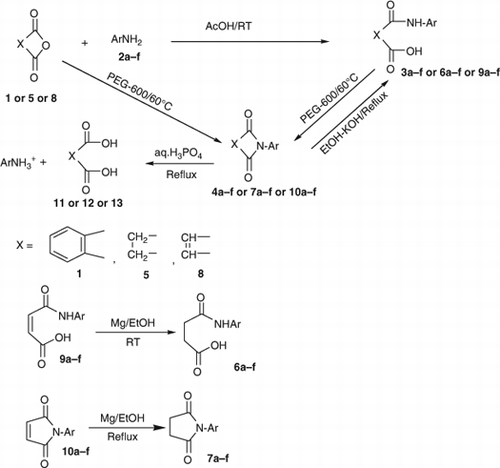
Introduction
Phthalic anhydride is a valuable petrochemical yielding a variety of commercially important products/intermediates Citation1 Citation2. Its most important reaction is with nucleophiles Citation3–9. Phthalic anhydride reacts with a variety of nucleophiles such as nitrogen nucleophiles Citation3 Citation4, oxygen Citation5 Citation6, carbon nucleophiles Citation7 Citation8, and so on. The nature of the product obtained in these reactions Citation9 depends on the nature of the nucleophiles, reaction conditions, and so on. Although the reactions of phthalic anhydride with amines have been widely studied Citation3 Citation4, no simple and clear cut study seems to have been done as far as formation of open chain or cyclic products are concerned. Gustav and coworkers reported Citation10 the preparation N- substituted phthalimides by the condensation of aromatic amines with phthalic anhydride in glacial acetic acid without isolating any open chain compound. We now wish to report another simple, efficient, and eco-friendly approach to N-substituted imides taking the advantage of PEG-600 as the reaction medium.
Results and discussion
Treatment of phthalic anhydride 1 with aniline (2a, i.e. 2, Ar = Ph) in acetic acid at RT for about 15–20 minutes resulted in the formation of N-phenylphthalamidic acid (3a, i.e. 3, Ar = Ph). Its structure has been established on the basis of spectral and analytical data. Thus, its IR (KBr) spectrum showed absorption of 3335 cm−1 assignable to the free NH/OH stretching vibration whereas the bonded OH and the bonded NH stretching vibrations appeared as a broad peak 3100 cm−1. The strong, sharp absorption at 1720 cm−1 in the IR spectrum was assigned to acid carbonyl group whereas the one at 1640 cm−1 to the amide carbonyl group. Its 1H NMR in DMSO–d6 showed peaks at Δ 7.3–8.0 (m, 9H, all aromatic protons), 10.1 (s, 1H, −NH), 12.6 (s, 1H, −OH, D2O exchangeable). Its mass spectrum (CIMS) showed the molecular ion peak at m/z 242 corresponding to a molecular mass of 241 when recorded in the Q + 1 mode.
The above reaction was found to be general one and was extended to substituted anilines 2 and the products thus obtained were assigned structure 3 on the basis of spectral and analytical data () ().
Table 1. Characterization data, reaction time, and yields of 3/6/9a–f in acetic acid.
When the above product 3a (i.e. 3, Ar = Ph) was heated in PEG-600 at 60°C for about six–eight hrs, there resulted in the formation of previously reported Citation10 cyclized product N-phenylphthalimide 4a (i.e. 4, Ar = Ph). Although this compound is known in literature, it has been characterized in our work on the basis of spectral data. Thus, its IR (KBr) spectrum showed the absence of any absorption in the NH or OH region and showed a strong absorption at 1660 cm−1 assigned to the imide carbonyl group. Its 1H NMR in DMSO–d6 showed peaks at Δ 7.4–8.0 (m, all aromatic protons, i.e. 9H). Its mass spectrum (CIMS) showed the molecular ion peak at m/z 224 corresponding to a molecular mass of 223 when recorded in the Q + 1 mode.
The above reaction has been found to be general one and has been extended to substituted anilines 2 and the products thus obtained were assigned structure 4 on the basis of spectral and analytical data ().
Table 2. Characterization data, reaction time, and yields of 4/7/10a–f in PEG-600.
When the above cyclized products 4 were heated in ethanolic KOH under reflux for two–five hrs, there resulted in the formation of corresponding open chain compounds 3 whereas in aq.H3PO4 under reflux for three–five hrs, there resulted in the formation of phthalic acid 11.
Reaction of 1 with 2 in PEG-600 at 60°C for about 3–4 hrs resulted in the formation of cyclic products 4 that were found to be identical with the ones obtained earlier in the step-wise route (i.e. 1 + 2 → 3 → 4). The products were formed in good yields and no side products were detected. In the absence of PEG-600, the conversion of reactants to products was very slow and the yields were also very low. Polyethylene glycol (PEG-600) has been applied here as an efficient reaction medium and as a green solvent for the preparation of N-substituted imides. It is a biologically acceptable inexpensive polymer and is eco-friendly. Numerous examples of its use as an eco- friendly green solvent in organic syntheses have been reported Citation8.
Treatment of succinic anhydride 5 with anilines 2 in acetic acid at RT for about 10–15 minutes similarly resulted in the formation of mono acid mono amide derivatives of succinic anhydride 6. When these products 6 were heated in PEG-600 at 60°C for about 4–6 hrs, there resulted in the formation of N-substituted succinimides 7 with the loss of elements of water. These cyclized products 7 could also be obtained by the direct reaction of succinic anhydride 5 with anilines 2 in PEG-600 at 60°C for about 3–4 hrs which were found to be identical with the ones obtained earlier in the step-wise route (i.e. 5 + 2 → 6 → 7). When these cyclized compounds 7 were heated in ethanolic KOH under reflux for about three–six hrs, there resulted in the formation of corresponding open chain compounds 6 whereas in aq.H3PO4 under reflux for about three–eight hrs, there was formed succinic acid 12 and their structures have been established on the basis of spectral and analytical data () ().
Table 3. Characterization data, reaction time, and yields of 4/7/10a–f in PEG-600.
Treatment of maleic anhydride 8 with anilines 2 in acetic acid at RT for about 10–15 minutes resulted in the formation of mono acid mono amide derivatives of maleic anhydride 9. When these products 9 were heated in PEG-600 at 60°C for about 4–6 hrs, there resulted in the formation of N-substituted maleimides 10 with the loss of elements of water. These cyclized products 10 could also be obtained by the direct reaction of maleic anhydride 8 with anilines 2 in PEG-600 at 60°C for about 3–4 hrs, which were found to be identical with the ones obtained earlier in the step-wise route (i.e. 8 + 2 → 9 → 10). When these cyclized compounds 10 were heated in ethanolic KOH under reflux for two–five hrs, there resulted in the formation of corresponding open chain compounds 9 whereas in aq.H3PO4 under reflux for two–five hrs, there was formed maleic acid 13.
Thus, the behavior of succinic anhydride 5 and maleic anhydride 8 was very similar to that of phthalic anhydride 1.
Treatment of 10 with Mg turnings in ethanol under reflux for about two–four hrs, followed by the simple processing resulted in the formation of corresponding 7 identical with the ones obtained earlier in the step-wise route ( i.e. 5 + 2 → 6 → 7). Similarly, treatment of 9 with Mg turnings in ethanol at room temperature for about 1–3 hrs, followed by the simple processing resulted in formation of corresponding 6 that were found to be identical with the ones obtained earlier in the step-wise route (i.e. 5 + 2 → 6). Thus, Mg turnings in ethanol reduce chemo selectively the double bonds of 9 and 10 without touching the amide or imide carbonyl groups.
All the above reactions are summarized in the Schemes .
Experimental
Melting points are uncorrected and were determined in open capillary tubes in sulfuric acid bath. TLC were run on silica gel—G and visualization was done using iodine or UV light. IR spectra were recorded using Perkin-Elmer 1000 instrument in KBr pellets. 1H NMR spectra were recorded in CDCl3/DMSO–d6using TMS as internal standard with 400 MHz spectrometer. Mass spectra were recorded on Agilent-LCMS instrument under CI conditions and given by Q + 1 value only.
3b: −IR (KBr): 1651 cm−1 (−C = O of amide group), 1722 cm−1 (−C = O of acid group), 3131 cm−1 (broad, −NH of amide group), 3321 cm−1 (−OH of acid group); 1H-NMR (400 MHz, CD3OD/TMS): Δ 2.35 (s, 3H, −CH3), 7.04–8.5 (m, 8H corresponds to aromatic protons), 10.2 (s, 1H, −NH), 12.8 (s, 1H, −COOH).
3c: −IR (KBr): 1636 cm−1 (−C = O of amide group), 1700 cm−1 (−C = O of acid group), 3056 cm−1 (broad, −NH of amide group), 3270 cm−1 (−OH of acid group); 1H-NMR (400 MHz, CD3OD/TMS): Δ 2.30 (s, 3H, −CH3), 6.9 − 8.31 (m, 8H corresponds to aromatic protons), 10.2 (s, 1H, −NH), 12.8 (s, 1H, −COOH).
3d: −IR (KBr): 1637 cm−1 (−C = O of amide group), 1720 cm−1 (−C = O of acid group), 3131 cm−1 (broad, −NH of amide group), 3324 cm−1 (−OH of acid group); 1H-NMR (400 MHz, CD3OD/TMS): Δ 7.25–8.5 (m, 8H corresponds to aromatic protons), 9.5 (s, 1H, −NH), 11.8 (s, 1H, −COOH).
3e: −IR (KBr): 1637 cm−1 (−C = O of amide group), 1722 cm−1 (−C = O of acid group), 3120 cm−1 (broad, −NH of amide group), 3321 cm−1 (−OH of acid group); 1H-NMR (400 MHz, CD3OD/TMS): Δ 3.73 (s, 3H, −OCH3), 7.04–8.5 (m, 8H corresponds to aromatic protons), 10.1 (s, 1H, −NH), 12.3 (s, 1H, −COOH).
3f: −IR (KBr): 1639 cm−1 (−C = O of amide group), 1719 cm−1 (−C = O of acid group), 3128 cm−1 (broad, −NH of amide group), 3321 cm−1 (−OH of acid group); 1H-NMR (400 MHz, CD3OD/TMS): Δ 7.41–8.35 (m, 8H corresponds to aromatic protons), 9.5 (s, 1H, −NH), 11.8 (s, 1H, −COOH).
6a: −IR (KBr): 1664 cm−1 (−C = O of amide group), 1698 cm−1 (−C = O of acid group), 3193 cm−1 (broad, −NH of amide group), 3313 cm−1 (−OH of acid group); 1H-NMR (400 MHz, CD3OD/TMS): Δ 2.50–2.61 (t, 4H, −CH2−CH2) 7.00–7.64 (m, 5H corresponds to 5H aromatic protons), 8.1 (s, 1H, −NH), 11.2 (s, 1H, −COOH).
6b: −IR (KBr): 1660 cm−1 (−C = O of amide group), 1698 cm−1 (−C = O of acid group), 3188 cm−1 (broad, −NH of amide group), 3307 cm−1 (−OH of acid group); 1H-NMR (400 MHz, CD3OD/TMS): Δ 2.3 (s, 3H, CH3 2.50–2.61 (t, 4H, −CH2−CH2) 7.04 − 7.52 (m, 4H corresponds to aromatic protons), 8.1 (s, 1H, −NH), 11.2 (s, 1H, −COOH).
6c: −IR (KBr): 1645 cm−1 (−C = O of amide group), 1695 cm−1 (−C = O of acid group), 3160 cm−1 (broad, −NH of amide group), 3296 cm−1 (−OH of acid group); 1H-NMR (400 MHz, CD3OD/TMS): Δ 2.35 (s, 3H, CH3) 2.50–2.61 (t, 4H, −CH2−CH2) 6.88 − 7.52 (m, 4H corresponds to aromatic protons), 8.1 (s, 1H, −NH), 11.2 (s, 1H, −COOH).
6d: −IR (KBr): 1659 cm−1 (−C = O of amide group), 1699 cm−1 (−C = O of acid group), 3181 cm−1 (broad, −NH of amide group), 3296 cm−1 (−OH of acid group); 1H-NMR (400 MHz, CD3OD/TMS): Δ 2.50–2.61 (t, 4H, −CH2−CH2), 7.25–7.58 (m, 4H corresponds to aromatic protons), 8.1 (s, 1H, −NH), 11.2 (s, 1H, −COOH).
6e: −IR (KBr): 1658 cm−1 (−C = O of amide group), 1695 cm−1 (−C = O of acid group), 3182 cm−1 (broad, −NH of amide group), 3300 cm−1 (−OH of acid group); 1H-NMR (400 MHz, CD3OD/TMS): Δ 2.50 − 2.61 (t, 4H, −CH2−CH2), 3.73 (s, 3H, −OCH3), 6.75–7.53 (m, 4H corresponds to aromatic protons), 8.1 (s, 1H, −NH), 11.2 (s, 1H, −COOH).
6f: −IR (KBr): 1658 cm−1 (−C = O of amide group), 1697 cm−1 (−C = O of acid group), 3176 cm−1 (broad, −NH of amide group), 3305 cm−1 (−OH of acid group); 1H-NMR (400 MHz, CD3OD/TMS): Δ 2.50 − 2.61 (t, 4H, −CH2−CH2), 7.41–7.53 (m, 4H corresponds to aromatic protons), 8.1 (s, 1H, −NH), 11.2 (s, 1H, −COOH).
9a: −IR (KBr): 1662 cm−1 (−C = O of amide group), 1710 cm−1 (−C = O of acid group), 3062 cm−1 (broad, −NH of amide group), 3312 cm−1 (−OH of acid group); 1H-NMR (400 MHz, CDCl3/TMS): Δ 6.51 − 6.80 (d, 2H, alkene protons), 7.00–7.64 (m, 5H corresponds to aromatic protons), 8.1 (s, 1H, −NH), 11.2 (s, 1H, −COOH).
9b: −IR (KBr): 1634 cm−1 (−C = O of amide group), 1701 cm−1 (−C = O of acid group), 3096 cm−1 (broad, −NH of amide group), 3286 cm−1 (−OH of acid group); 1H-NMR (400 MHz, CDCl3/TMS): Δ 2.35 (s, 3H, −CH3), 6.51 − 6.80 (d, 2H, alkene protons), 7.04–7.52- (m, 4H corresponds to aromatic protons), 8.1 (s, 1H, −NH), 11.2 (s, 1H, −COOH).
9c: −IR (KBr): 1634 cm−1 (−C = O of amide group), 1698 cm−1 (−C = O of acid group), 3086 cm−1 (broad, −NH of amide group), 3277 cm−1 (−OH of acid group); 1H-NMR (400 MHz, CDCl3/TMS): Δ 2.35 (s, 3H, −CH3), 6.51–6.80 (d, 2H, alkene protons), 6.88–7.52 (m, 4H corresponds to aromatic protons), 8.1 (s, 1H, −NH), 11.2 (s, 1H, −COOH).
9d: −IR (KBr): 1660 cm−1 (−C = O of amide group), 1700 cm−1 (−C = O of acid group), 3110 cm−1 (broad, −NH of amide group), 3297 cm−1 (−OH of acid group); 1H-NMR (400 MHz, CDCl3/TMS): Δ 6.51–6.80 (d, 2H, alkene protons), 7.25–7.58 (m, 4H corresponds to aromatic protons), 8.1 (s, 1H, −NH), 11.2 (s, 1H, −COOH).
9e: −IR (KBr): 1643cm−1 (−C = O of amide group), 1708 cm−1 (−C = O of acid group), 3096 cm−1 (broad, −NH of amide group), 3295 cm−1 (−OH of acid group); 1H-NMR (400 MHz, CDCl3/TMS): Δ 3.73 (s, 3H, −OCH3), 6.51–6.80 (d, 2H, alkene protons), 6.75 − 7.53 (m, 4H corresponds to aromatic protons), 8.1 (s, 1H, −NH), 11.2 (s, 1H, −COOH).
9f: −IR (KBr): 1665 cm−1 (−C = O of amide group), 1710 cm−1 (−C = O of acid group), 3188 cm−1 (broad, −NH of amide group), 3302 cm−1 (−OH of acid group); 1H-NMR (400 MHz, CDCl3/TMS): Δ 6.51–6.80 (d, 2H, alkene protons), 7.41–7.53 (m, 4H corresponds to aromatic protons), 8.1 (s, 1H, −NH), 11.2 (s, 1H, −COOH).
4b: −IR (KBr): 1713 cm−1 (−C = O of amide group); 1H-NMR (400 MHz, CDCl3/TMS): Δ 2.35 (s, 3H, −CH3), 7.04–8.5 (m, 8H corresponds to 8H aromatic protons).
4c: −IR (KBr): 1713 cm−1 (−C = O of amide group ); 1H-NMR (400 MHz, CDCl3/TMS): Δ 2.30 (s, 3H, −CH3), 6.9 − 8.31 (m, 8H corresponds to aromatic protons).
4d: −IR (KBR): 1714 cm−1 (−C = O of amide group); 1H-NMR (400 MHz, CD3OD/TMS): Δ 7.25–8.5 (m, 8H corresponds to aromatic protons).
4e: −IR (KBr): 1710 cm−1 (−C = O of imide group); 1H-NMR (400 MHz, CDCl3/TMS): Δ 3.73 (s, 3H, −OCH3), 7.04–8.5 (m, 8H corresponds to aromatic protons).
4f: −IR (KBr): 1714 cm−1 (−C = O of imide group); 1H-NMR (400 MHz, CDCl3/TMS): Δ 7.41–8.35 (m, 8H corresponds to aromatic proton).
7a: −IR (KBr): 1702 cm−1 (−C = O of amide group); 1H-NMR (400 MHz, CDCl3/TMS): Δ 2.80–2.91 (s, 4H, −CH2-CH2), 7.00–7.64 (m, 5H corresponds to aromatic protons).
7b: −IR (KBr): 1705 cm−1 (−C = O of imide group); 1H-NMR (400 MHz, CDCl3/TMS): Δ 2.3 (s, 3H, −CH3), 2.80–2.91 (s, 4H, −CH2−CH2), 7.04–7.52(m, 4H corresponds to aromatic protons).
7c: −IR (KBr): 1705 cm−1 (−C = O of imide group); 1H-NMR (400 MHz, CDCl3/TMS): Δ 2.35 (s, 3H, −CH3), 2.80–2.91 (s, 4H, −CH2−CH2), 6.88–7.52 (m, 4H corresponds to aromatic protons).
7d: −IR (KBr): 1707 cm−1 (−C = O of imide group); 1H-NMR (400 MHz, CDCl3/TMS): Δ 2.80–2.91 (s, 4H, −CH2−CH2), 7.25–7.58 (m, 4H corresponds to aromatic protons).
7e: −IR (KBr): 1707 cm−1 (−C = O of imide group); 1H-NMR (400 MHz, CDCl3/TMS): Δ 2.80 − 2.91 (s, 4H, −CH2−CH2), 3.73 (s, 3H, −OCH3), 6.75–7.53 (m, 4H corresponds to aromatic protons).
7f: −IR (KBr): 1708 cm−1 (−C = O of imide group); 1H-NMR (400 MHz, CDCl3/TMS): Δ 2.80–2.91 (s, 4H, −CH2−CH2), 7.41–7.53 (m, 4H corresponds to aromatic protons).
10a: −IR (KBr): 1678 cm−1 (−C = O of imide group); 1H-NMR (400 MHz, CDCl3/TMS): Δ 6.51 − 6.80 (d, 2H, alkene protons), 7.00– − 7.64 (m, 5H corresponds to aromatic protons).
10b: −IR (KBr): 1685 cm−1 (−C = O of imide group); 1H-NMR (400 MHz, CDCl3/TMS): Δ 2.35 (s, 3H, −CH3), 6.51 − 6.80 (d, 2H, alkene protons), 7.04–7.52 (m, 4H corresponds to aromatic protons).
10c: −IR (KBr): 1685 cm−1 (−C = O of imide group); 1H-NMR (400 MHz, CDCl3/TMS): Δ 2.35 (s, 3H, −CH3), 6.51–6.80 (d, 2H, alkene protons), 6.88–7.52 (m, 4H corresponds to aromatic protons).
10d: −IR (KBr): 1708 cm−1 (−C = O of imide group); 1H-NMR (400 MHz, CDCl3/TMS): Δ 6.51–6.80 (d, 2H, alkene protons), 7.25–7.58 (m, 4H corresponds to aromatic protons).
10e: −IR (KBr): 1702 cm−1 (−C = O of imide group); 1H-NMR (400 MHz, CDCl3/TMS): Δ 3.73 (s, 3H, −OCH3), 6.51 − 6.80 (d, 2H, alkene protons), 6.75–7.53 (m, 4H corresponds to aromatic protons).
10f: −IR (KBr): 1707 cm−1 (−C = O of imide group); 1H-NMR (400 MHz, CDCl3/TMS): Δ 6.51–6.80 (d, 2H, alkene protons), 7.41–7.53 (m, 4H corresponds to aromatic protons).
General procedure for the preparation of 3/6/9 in AcOH from 1/5/8
To a solution of the anhydride 1/5/8 (10 mM) in acetic acid (10–15 ml) at RT was added a solution of substituted aniline (11 mM) in acetic acid (2–3 ml) at RT. The reaction mixture was stirred well for 20 minutes to 4 hrs, when a colorless crystalline solid separated out which was filtered, washed with little amount of acetic acid (2–3 ml), then dried. This product was recrystallized from ethanol to obtain pure 3/6/9.
General procedure for the preparation of 4/7/10 in PEG-600 from 3/6/9
A solution of 3/6/9 (10 mM) in PEG-600 was heated at 60°C for 3–4 hrs. Then reaction mixture was cooled to room temperature and poured into ice-cold water. The separated solid was filtered washed with water (8–10 ml), then dried. This product was recrystallized from acetic acid to obtain pure 4/7/10.
General procedure for the preparation of 4/7/10 in PEG-600 from 1/5/8
A solution of the anhydride 1/5/8 (10 mM) in PEG-600 was added a solution of substituted aniline (11 mM) in PEG-600 was heated at 60°C for 3–4 hrs. The reaction mixture was cooled to room temperature and poured into ice-cold water. The separated solid was filtered, washed with water (8–10 ml), then dried. This product was and recrystallized from acetic acid to obtain pure 4/7/10.
General procedure for the preparation of 3/6/9 from 4/7/10
A solution of N-substituted imide 4/7/10 in ethanolic KOH was refluxed for 2–3 hrs. The reaction mixture was cooled to room temperature and poured into ice-cold water, acidified with acetic acid. The separated solid was filtered, washed with ethanol (2–3 ml) and then dried. This product was recrystallized from alcohol to obtain pure 3/6/9.
General procedure for the preparation of 11/12/13 from 4/7/10
A solution of N-substituted imide 4/7/10 in aq.H3PO4 was refluxed for three–five hrs and the reaction mixture was cooled to room temperature. The solid that separated was filtered, washed with water (8–10 ml) followed by ethanol (2–4 ml) then dried to obtain corresponding acids 11/12/13.
General procedure for the preparation of 6 from 9
To a suspended solution of 9 (10 mM) in ethanol (15–20 ml) at RT was added a solution of Mg in ethanol (5–10 ml) at RT for about 1–3 hrs. The reaction mixture was stirred well until a clear solution obtained and this solution was poured into ice-cold water. The separated solid was filtered, washed with water (8–10 ml) followed by little amount of alcohol (2–5 ml), then dried. This product was recrystallized from ethanol to obtain pure 6.
General procedure for the preparation of 7 from 10
To a suspended solution of 10 (10 mM) in ethanol (15–20 ml) at RT was added a solution of Mg in ethanol (5–10 ml).The reaction mixture was refluxed for about two–four hrs and this solution was poured into ice-cold water. The separated solid was filtered, washed with water (8–10 ml) followed by little amount of alcohol (2–5 ml), then dried. This product was recrystallized from ethanol to obtain pure 7.
Conclusion
We have developed a simple and efficient protocol for the synthesis of N-substituted imides from anhydrides using PEG-600 as a solvent. The mildness and eco-friendly nature of the synthesis, short reaction time, and excellent yields are notable advantages of this protocol. The present method has many obvious advantages compared to those reported in literature, including simplicity of the methodology, ease of product isolation, no use of catalyst, and being environmentally benign.
Acknowledgements
Authors are thankful to the authorities of Jawaharlal Nehru Technological University Hyderabad, for providing laboratory facilities and for constant encouragement.
References
- Kirk-Orthmer's Encyclopedia of Chemical Technology , 3rd ed John Wiley : New York , 1973 ; Vol. 15 , 738 .
- The Merck Index , 9th ed Merck, NJ : Whitehouse Station , 1976 ; 959 .
- Pushkin , N.A. , et al. Bull. Soc. Chim. Roy. Yugoslav. 1933 , 4 , 23 – 30 ; CA 1934, 28, 2687 .
- Feist , S. ; Feist , K. ; Schultz , J. Arch. Pharm . 1934 , 272 , 785 – 792 ; CA 1935, 29, 792 .
- Waldmann , H. and Schubert , H. 1951 . Chem. Ber. , 84 : 139 – 144 .
- Dubey , P.K. , Mohiuddin , S.M.G. and Ramesh , D. 1997 . Asian J. Chem. , 9 : 379 – 387 .
- Wang , C.H. , Isensee , R. , Griffith , A.M. and Christiansen , B.E. 1947 . J. Amer. Chem. Soc. , 69 : 1909 – 1911 .
- Wilson , J.M. J. Chem. Soc . 1951 , 2297 – 2299 .
- Mohiuddin , S.M.G. Reactions of Phthalic Anhydride with Certain Nucleophiles . M.Phil. Thesis, J.N.T. University, Hyderabad , 1994 .
- Gustav , V. ; Artus , V. Ber . 1942 , 75B , 1558 – 1568 ; CA 1944, 38, 1221 .
- Gupta , G. and Wagh , S.B. 2006 . Indian J. Chem. , 45 : 697
- Mary , L.S. , Florence , L.S. and Elizabeth , P.S. 1928 . J. Am. Chem. Soc. , 50 : 47
- Heilbron , I. ; Bunbury , H.M. Dictionary of Organic Compounds (Chinese) ; Scientific Press : China , 1996 ; 292 .
- Searle , N.E. (E. I. du pont de Nemours & Co.). US Patent 2,444,536 ; Chem. Abstr . 1948 , 42 , 7340 .