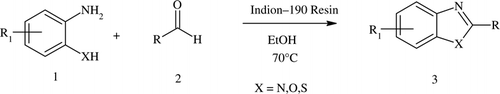Abstract
An efficient and mild protocol has been developed for the preparation of benzimidazoles, benzoxazoles, and benzothiazoles from reactions of aldehydes with o-substituted aminoaromatics in the presence of catalytic amount of Indion 190 resin. Short reaction time, ambient conditions, simple work-up procedure, high yield, easy availability, reusability, and use of an eco-friendly catalyst are some of the striking features of the present protocol.
Introduction
The concept of green chemistry has been playing an important role in recent years for meeting the fundamental scientific challenges of protecting the living environment. One of the thrust areas for achieving this target is to explore alternative reaction conditions and reaction media to accomplish the desired chemical transformation with almost negligible by-products and waste generation as well as elimination of the use of volatile and toxic organic solvents. It is therefore of utmost importance to evolve a simple and effective methodology for the different organic transformations that cover the concept of green chemistry.
Molecules with benzimidazole, benzoxazole, and benzothiazoles moieties are attractive targets for synthesis since they often exhibit diverse and important biological properties. These heterocycles have shown different pharmacological activities such as antibiotic Citation1, antifungal Citation2, antiviral Citation3, anticancer Citation4, antimicrobial Citation5, and antiparkinson Citation6 properties. They have also been used as ligands for asymmetric transformations Citation7. Benzimidazole derivatives are a unique and broad-spectrum class of antirhino/enteroviral agents such as antiulcerative Citation8 and antiallergic Citation9, are effective against the human cytomegalovirus Citation10, and are also efficient selective neuropeptide Y Y1 receptor antagonists Citation11.
A number of methods are in vogue for the synthesis of these heterocycles by using different catalysts such as Pd-catalyzed oxidative cyclization Citation12; ionic-liquid-mediated synthesis Citation13; base-assisted reaction of 1,1-dibromoethanes Citation14, SiO2/ZnCl2 Citation15, ZrOCl2·8H2O Citation16, In(OTF)3 Citation17, polyethylene-glycol-mediated catalysts Citation18, and different heteropolyacid catalysts Citation19, which include condensation of orthoesters Citation20–22, nitriles Citation23, aldehydes Citation24, carboxylic acids Citation25, acid chlorides Citation26, amides Citation27, and esters Citation28 with o-substituted aminoaromatics in the presence of different acids or catalysts; Beckmann rearrangement of o-acylphenol oximes Citation29; photocyclization of phenolic Schiff bases Citation30; and benzimidazole synthesis in solvent-free conditions (15). More recently, Germin et al. Citation31 have developed cleaner protocols for alkoxybenzimidazole synthesis via SNAr reaction, and our group developed benzimidazole, benzoxazole, and benzothiazole from condensation of aldehydes with o-substituted aminoaromatics in the presence of phosphorus trichloride Citation32. However, many of these methods suffer from one or more of the drawbacks such as requirement of strong acidic conditions, long reaction times, low yields, tedious work-up procedures, requirement of excess amounts of reagent, and the use of toxic reagents, catalysts, or solvents. Therefore, there is a strong demand for a highly efficient and environmentally benign method for the synthesis of these heterocycles.
Indion 190 resin possesses unique properties such as physical and chemical stability, no toxicity, no corrosiveness, reusability, environmental compatibility, and selectivity. Indion 190 resin can be handled easily and separated from the reaction mixture by simple filtration, washed with water, dried in oven, and reused as per the experimental convenience Citation33 Citation34. The recovered catalyst has been consecutively reused without any variations in the yield of the products.
Results and discussion
In continuation of our work to develop new methodologies for organic transformations Citation35 Citation36, we now report an efficient and environmentally benign method for the synthesis of benzoxazoles, benzothiazoles, and benzimidazole in excellent yields from o-substituted aminoaromatics and different aldehydes in the presence of Indion 190 resin as catalyst, as shown in .
In order to find the optimum reaction conditions for the condensation reaction, preliminary efforts were mainly focused on the evaluation of different solvents and catalyst. The reaction has been carried out between o-phenylenediamine and benzaldehyde in the presence of different catalysts and solvents at different temperatures, and results are shown in and , respectively.
Table 1. Preparation of 2-phenylbenzimidazole using various catalysts.a
The results mentioned in clearly indicate the effective use of Indion 190 resin for the preparation of 2-phenylbenzimidazole.
Table 2. Effect of solvents on preparation of 2-phenylbenzimidazole.a
The results presented in demonstrate the effective use of ethanol as a solvent for preparation of 2-phenylbenzimidazole in the presence of Indion 190 resin as a catalyst.
Thus, we used Indion 190 resin as a catalyst in the present work. In order to elucidate the role of Indion 190 resin as catalyst, a controlled reaction was conducted using o-phenylenediamine and benzaldehyde in ethanol in the absence of catalyst. This resulted in the formation of trace amount of the fused product after 24 h at 70°C temperature. However, reaction with the same substrate using 10%/weight of Indion 190 resin at 70°C in ethanol afforded the products in quantitative yield in 4 h. Lower temperatures required more time for the completion of the reaction in the presence of Indion 190 resin catalyst. In the absence of Indion 190 resin, the reaction was slow and required drastic conditions with unsatisfactory yields. This could be overcome with the use of Indion 190 resin at 70°C. It is postulated that Indion 190 resin plays a complex role in accelerating the condensation reaction and thus promotes the formation of products.
We observed that catalyst concentration also plays an important role in catalyzing the formation of benzimidazole, benzoxazole, and benzothiazole. A model reaction was performed between o-phenylenediamine and benzaldehyde with various amounts of Indion 190 resin, and it was found that the better yield was obtained at 10%/weight catalyst (). At a higher amount of catalyst, the yield of the corresponding product decreases because of an increase in the acidity of the reaction medium.
Table 3. Optimization of the amount of Indion 190 resin for the preparation 2-phenylbenzimidazole.a
The generality of the procedure was evaluated by the reaction of different o-aminophenol, o-aminothiophenol, and o-phenylenediamine with different aldehydes ().
Table 4. Synthesis of 2-substituted benzimidazole, benzoxazole, and benzothiazoles.a
As shown in , aromatic, aliphatic, and unsaturated aldehydes reacted with different o-substituted amino aromatics without any significant difference in the reaction time to give the corresponding 2-substituted benzimidazole, benzothiazole, and benzoxazole in good yield. The method has the ability to tolerate other functional groups such as methoxy, ester, halides, and olefins. The products were synthesized in good to excellent yields and characterized by FT-IR, 1H NMR, and physical constant. Physical and spectral data of known compounds are in agreement with those reported in the literature (12–19).
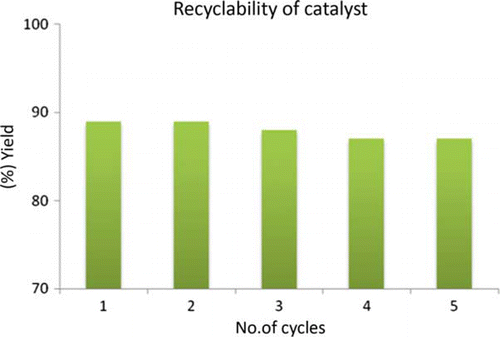
The reusability of the catalyst is important for the large-scale operation and industrial point of view. Therefore, the recovery and reusability of Indion 190 resin was examined. The catalyst was separated and reused after washing with water and drying at 100°C. The reusability of the catalyst was investigated in the reaction of o-phenylenediamine with benzaldehyde (). The results illustrated in showed that the catalyst can be used five times with consistent yield. The color of the catalyst remains same even after the fifth cycle.
Experimental
All commercial reagents were used as received without purification and all solvents were of reagent grade. The reaction was monitored by thin layer chromatography (TLC) using 0.25 mm E. Merck silica gel 60 F254 precoated plates, which were visualized with UV light. Melting points were taken in open capillaries. The IR spectra were recorded on a PerkinElmer 257 spectrometer using KBr discs. 1H NMR spectra were recorded on a VXR-300 MHz instrument using tetramethylsilane as an internal standard.
General experimental procedure
A mixture of o-substituted aminoaromatics (0.1 mol), aldehydes (0.1 mol), Indion 190 resin (10%/weight), and ethanol (5 mL) was stirred at 70°C for 4 h. After completion of the reaction, as monitored by TLC, the reaction mixture was filtered hot to separate out the catalyst, and the catalyst residue was washed with ethanol and water, dried, and reused. Filtrate was evaporated under reduced pressure to get the crude product that was crystallized by using isopropyl alcohol.
Representative spectral data
2-(1,3-Benzothiazol-2-yl)-5-(diethyl amino)phenol (, entry t): IR (KBr, cm–1): 2875, 1630, 1618, 1456, 1342, 1135, 812, 743. 1H NMR (300 MHz) δ=1.21 (t, 6H), 3.41 (q, 4H), 6.27 (s, 1H), 7.26–7.28 (d, 1H, J=8.0 Hz), 7.29–7.31 (d, 1H, J = 8.8, 2.0 Hz), 7.44–7.46 (d, 1H, J=8.8, 2.0 Hz), 7.80–7.86 (d, 2H, J=8.0, 2.0 Hz), 12.56 (s, 1H). LC-MS (299.4, 98. 67%).
Conclusion
In conclusion, Indion 190 resin was found to be a mild and efficient catalyst for the formation of benzoxazoles, benzothiazoles, and benzimidazoles. The use of this inexpensive, easily available, and reusable catalyst makes this protocol practical, environment-friendly, and economically attractive. The simple work-up procedure, mild reaction conditions, short reaction times, high yields of products, and nontoxic nature of the catalyst are other advantages of the present method.
Acknowledgements
The authors are greatly thankful to the Indian Institute of Technology, Mumbai, India, for recording the 1H NMR and mass spectra.
References
- Evans D.A. ; Sacks C.E. ; Kleschick W.A. ; Taber T.R. J. Am. Chem. Soc . 1979 , 101 , 6789 – 6791 .
- Yamato M. J. Pharm. Soc. Jpn . 1992 , 112 , 81 – 99 .
- Song X. ; Vig B.S. ; Lorenzi P.L. ; Drach J.C. ; Townsend L.B. ; Amidon G.L. J. Med. Chem . 2005 , 48 , 1274 – 1277 .
- Kumar D. ; Jacob M.R. ; Reynolds M.B. ; Kerwin S.M. Bioorg. Med. Chem . 2002 , 10 , 3997 – 4004 .
- Yildiz-Oren I. ; Yalcin I. ; Aki-Sener E. ; Ucarturk N. Eur. J. Med. Chem . 2004 , 39 , 291 – 298 .
- Benazzou A. ; Boraund T. ; Dubedat P. ; Boireau J.M. ; Stutzmann C. Eur. J. Pharmcol . 1995 , 284 , 299 – 307 .
- Figge A. ; Altenbach H.J. ; Brauer D.J. ; Tielmann P. Tetrahedron Asymmetr . 2002 , 13 , 137 – 144 .
- Scott L.J. ; Dunn C.J. ; Mallarkey G. ; Sharpe M. Drugs 2002 , 62 , 1503 – 1538 .
- Nakano H. ; Inoue T. ; Kawasaki N. ; Miyataka H. ; Matsumoto H. ; Taguchi T. ; Inagaki N. ; Nagai H. ; Satoh T. Bioorg. Med. Chem . 2000 , 8 , 373 – 380 .
- Zhu Z. ; Lippa B. ; Drach J.C. ; Townsend L.B. J. Med. Chem . 2000 , 43 , 2430 – 2437 .
- Zarrinmayeh H. ; Nunes A.M. ; Ornstein P.L. ; Zimmerman D.M. ; Arnold M.B. ; Schober D.A. ; Gackenheimer S.L. ; Bruns R.F. ; Hipskind P.A. ; Britton T.C. ; Cantrell B.E. ; Gehlert D.R. J. Med. Chem . 1998 , 41 , 2709 – 2719 .
- Pang Y. ; Hua W. Tetrahedron Lett . 2009 , 50 , 6680 – 6683 .
- Yadav A.K. ; Kumar M. ; Yadav T. ; Jain R. Tetrahedron Lett . 2009 , 50 , 5031 – 5034 .
- Shen W. ; Kohn T. ; Fu Z. ; Jiao X. ; Lai S. ; Sahmitt M. Tetrahedron Lett . 2008 , 49 , 7284 – 7286 .
- Jacob R.G. ; Dutra L.G. ; Radatz C.S. ; Mendes S.R. ; Perin G. ; Lenardao E. Tetrahedron Lett . 2009 , 50 , 1495 – 1497 .
- Baltork I.M. ; Khosropour A.R. ; Hojati S.F. Catal. Commun . 2007 , 8 , 1865 – 1870 .
- Trivedi R. ; De S.K. ; Gibbs R.A. J. Mol. Catal. A Chem . 2006 , 245 , 8 – 11 .
- Mukhopadhyay C. ; Tapaswi P.K. Tetrahedron Lett . 2008 , 49 , 6237 – 6240 .
- Heravi M.M. ; Sadjadi S. ; Oskoose H.A. ; Shoar R.H. Catal. Commun . 2008 , 9 , 504 – 507 .
- Villemin D. ; Hammadi M. ; Martin B. Synth. Commun . 1996 , 26 , 2895 – 2899 .
- Doise M. ; Dennin F. ; Blondeau D. ; Sliwa H. Tetrahedron Lett . 1990 , 31 , 1155 – 1156 .
- Jenkins G.L. ; Knevel A.M. ; Davis C.S. J. Org. Chem . 1961 , 26 , 274.
- Hein D.W. ; Alheim R.J. ; Leavitt J.J. J. Am. Chem. Soc . 1957 , 79 , 427 – 429 .
- Salehi P. ; Dabiri M. ; Zolfigol M.A. ; Otokesh S. ; Baghbanzadeh M. Tetrahedron Lett . 2006 , 47 , 2557 – 2560 .
- So Y.H. ; Heeschen J.P. J. Org. Chem . 1997 , 62 , 3552 – 3561 .
- Nadaf R.N. ; Siddiqui S.A. ; Daniel T. ; Lahoti R.J. ; Srinivasan K.V. J. Mol. Catal. A Chem . 2004 , 214 , 155 – 159 .
- Terashima M. ; Ishii M.A. Synthesis 1982 , 484 – 485 .
- Chakraborti A.K. ; Rudrawar S. ; Kaur G. ; Sharma L. Synlett 2004 , 1533 – 1536 .
- Bhawal B.M. ; Mayabhate S.P. ; Likhite A.P. ; Deshmukh A.R. Synth. Commun . 1995 , 25 , 3315 – 3321 .
- Chen Y. ; Zeng D.X. J. Org. Chem . 2004 , 69 , 5037 – 5040 .
- Germin H. ; Harris C.S. ; Vautier M. ; Warin N. Tetrahedron Lett . 2010 , 51 , 554 – 556 .
- Padalkar V.S. ; Phatangare K.R. ; Gupta V.D. ; Patil V.S. ; Umape P.G. ; Sekar N. Mater. Res. Bull (communicated, manuscript no. MRB-10-642) .
- Chaskar A. ; Yewale S. ; Langi B. ; Deokar H. J. Kor. Chem. Soc . 2009 , 53 , 422 – 426 .
- Chaskar A. ; Jaffer F. ; Langi B. ; Yewale S. ; Bodkhe A. J. Kor. Chem. Soc . 2009 , 53 , 224 – 228 .
- Padalkar V.S. ; Patil V.S. ; Phatangare K.R. ; Umape P.G. ; Sekar N. Synth. Commun . 2011 , 41 6 , 925 – 938 .
- Phatangare K.R. ; Padalkar V.S. ; Patil V.S. ; Gupta V.D. ; Umape P.G. ; Sekar N. Synth. Commun ., in press .