Abstract
The basic principle of Doolittle multivariate calibration algorithm (DMCA) was investigated and used for simultaneous quantitative determination of sodium tripolyphosphate (STPP), sodium sulfate (SS), and sodium carbonate (SC) in commercial washing powders with serious overlapping. The process of the Doolittle decomposition algorithm (DDA) is implemented by lower and upper (LU) triangular matrix decomposition which is robust and convenient. Then it was applied to overcome the problem of overlapping spectra of different components in the detergent washing powder. The root mean square error of prediction (RMSEP) for DMCA is 0.084 which confirms the improvement in accuracy, in comparison with K-Matrix (KM) (RMSEP=0.118). It was demonstrated that DDA can avoid matrix inverting, reduce the orders of matrices, and needs a little time for analysis. Therefore, it has bright prospects in chemometrics and it is feasible that the Doolittle algorithm could be applied to the practical determinations in real samples with spectral overlapping.
Introduction
Modern detergents contain several chemical ingredients in different proportions and are complex mixtures to reflect the demands of modern day living in the new millennium. Detergent washing powders involve more than 25 different ingredients such as surfactants, builders (sodium tripolyphosphate [STPP], sodium carbonate [SC], sodium silicate, zeolite), bleachs (perborates, tetra acetyl ethylene diamine, percarbonates), enzymes, and auxiliaries (optical brighteners, fragrance, sodium sulfate [SS]) Citation1. With the growth in complexity of detergent formulations and lack of robust analytical methods, demand for new determination methods has increased in recent years Citation2. STPP is a builder used in detergent powders’ formulation for many years; as is the mixture of STPP with SS which is often used as a builder in cheaper products. SC is called washing soda or sal soda and is effective in oil, grease, and alcohol stain removal Citation3. There are some conventional analytical methods for analysis of washing powder samples, for example extraction by ethanol or classical wet chemistry methods Citation4. Attenuated total reflectance Fourier transform infrared (ATR-FTIR) spectrometry has been introduced as an efficient technique with high signal-to-noise ratio for quantitative determination of ingredients of washing powder samples Citation5. There are several multivariate calibration methods to extract the spectral data for each compound in a mixture with similar spectral characteristics. Partial least-squares (PLS) is a usual tool for multivariate calibration because of the quality of the obtained calibration models, the ease of its application, and the availability of software Citation6. PLS is based on linear relationship between response and intensity of absorption bands Citation7, but this algorithm involves matrix inverting operation and also application of this technique has been restricted with few deviations from linearity.
Doolittle decomposition algorithm (DDA) is a very simple, accurate, and stable numerical analysis algorithm with an easy computational procedure. This algorithm can avoid matrix inverting operation, which is often involved in chemometrics such as K-Matrix (KM), P-Matrix, and PLS methods; reduce the orders of matrixes; speed up the operation of matrixes; and raise the efficiency of computation Citation8–11. However, according to our survey, there are few documents reporting the application of Doolittle algorithm in chemical researches Citation12. In this study, Doolittle algorithm has been developed for simultaneous determination of SS, SC, and STPP in a commercial detergent powder samples by using ATR-FTIR spectroscopy and evaluating the results obtained by multivariate KM and DDA. More reliable results were obtained by DDA algorithm, and the introduction of the Doolittle algorithm will offer more convenience for the solution of a series of chemometrics issues.
Results and discussion
ATR-FTIR spectra and evaluation of Doolittle analysis
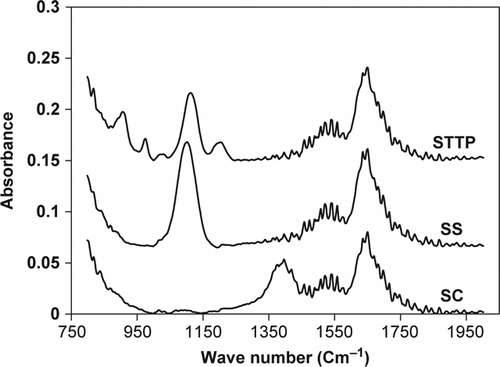
A suitable solvent dissolves the analytical samples without reacting and has the least interference at the spectral region of the analyte. The ATR-FTIR spectra of STPP, SS, and SC using water as the reference () showed severe overlapping in their absorbance bands, and the univariate analysis method cannot be applied to overcome this overlapping and be used to carry out a simultaneous determination of these three components. To evaluate the DDA performance, standard and predicted sample solutions were analyzed. Twenty-five samples of this solution set (sample no. 1–25 in ) were chosen as standard and the 10 other (sample no. 26–35) were used as predicted samples. The compositions of the samples used in calibration and predication sets are summarized in . In the first 14 samples, collinearity could be observed between components’ concentration. This set had been prepared according to the formulation of most washing powders produced by Iranian detergent manufacturers. The other 11 samples were prepared using a fractional factorial design at five concentration levels in order to obtain accurate results in the prediction ability of the method for real samples. Finally, the combination of these two sets made it possible to achieve the aim of method. The ATR-FTIR spectra of 25 standard samples in the spectral region of 800–1500 cm−1 are shown in . The maximum absorbance was observed around 1100 cm−1 spectral region.
Figure 2. ATR-FTIR spectra of 25 different three components standard solutions with concentration range of 0.25– 2.89, 0.20–2.49, and 0.2912–1.2010 g/100 g for STPP, SC, and SS, respectively (water as the reference).
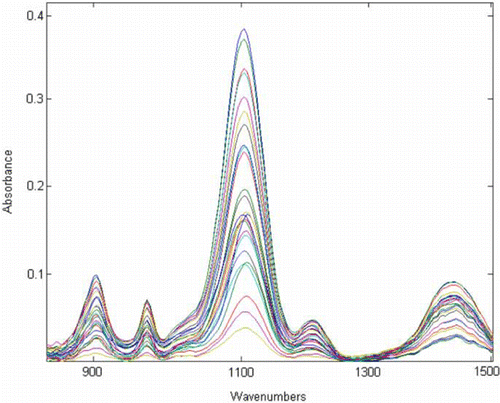
Table 1. Composition of the calibration (sample 1–25) and predication sample (26–35).
The process of solving the concentration matrix by Doolittle
The calibration concentration matrix C25×3 was gained according to the concentration ratios given in .
The absorbance matrix A25×86 was obtained from the spectral data of 25 calibration samples. According to Lambert–Beer law
Solving the following equation
Y is turn out; with another equation
Through calculating the matrix B gotten previously and the absorbency matrix A 10×86 of the real samples, the matrix C 10×3 of the concentrations of real samples is obtained. The process is as follows. The number of rows of the real samples matrix is the number of unknown samples and the number of columns of the real samples matrix is the number of components. The absorbance matrix A 10×86 of 10 real samples was obtained from the spectra data of the samples. The number of rows of the absorbance matrix is the number of unknown samples and the number of columns of the absorbency matrix is the number of wavelengths. B is the sensitivity coefficient matrix. Again, according to Lambert–Beer Law
Solving the following equation
Y is turn out, with another equation
Comparing Doolittle and KM
The spectral data were also processed by KM which has been used widely in multi-calibration in chemometrics. shows the results of Doolittle algorithm and KM. The general applicability of the proposed method and prediction samples was tested and evaluated by calculating two statistical parameters, the square of the correlation coefficient (R 2) and root mean square error predication (RMSEP; ). It is demonstrated that the error of Doolittle multivariate calibration is less small. It is more worthwhile to mention that Doolittle algorithm can avoid matrix inversion and improve sensitivity by reducing the orders of matrixes, speeding up the operation of matrixes and raising the efficiency of computation especially when carrying out a great amount of calculation. Doolittle multivariate calibration is superior to and more convenient than other calibrations.
Table 2. Quantitative analysis of STTP, SS, and SC applied in predication (samples no. 26–35) set by DMCA and KM models (g per 100 g).
Table 3. Statistical parameters of the applied models.
Recovery study
Washing powders are complex formulations containing not only STPP, SS, and SC but also several other ingredients. Nine synthetic mixture samples, which also contain carboxy methyl cellulose (CMC), sodium silicate, and sodium hydroxide besides the three proposed components, were analyzed and the recovery percentages were calculated. The results are shown in . The recovery established by Doolittle multivariate calibration algorithm (DMCA) were: 95.306–110.200%, 97.568–110.307%, and 94.090–105.494% for STPP, SS, and SC, respectively. The good agreement between the obtained results (using DDA), and the known values is an indication of the successful applicability of the proposed method for simultaneous determination of STPP, SS, and SC in complex samples.
Table 4. Recovery study results.
Also, the precision of the method was tested by calculating the standard deviation (SD) and relative standard deviation (RSD) of the results obtained for sample nos. 1, 3, 7, and 9 of (see ).
Table 5. Precision for synthetic mixtures (n=3).
Experimental
Apparatus and software
A FTIR spectrometer (Magna 550, Nicolet, Madison,WI, USA) equipped with a DTGS detector, an Ever-Glo source, and a CsI beam splitter was applied. The ATR-FTIR spectra were obtained by a 45° ZnSe cell. The data obtained from WINFIRST software were exported in ASCII format, and the data treatment was done with MATLAB for windows (Mathworks, Version 7.4).
Chemical reagents
STPP, SS, SC, and NaOH were of analytical grade and were acquired from Merck. Liquid sodium silicate with a purity of 45.5% and CMC with a purity of 65% were supplied by Clariant and Tolypers Co., respectively. Distilled water was used as the solvent during the procedure.
FTIR spectrometry
In order to provide the standard and predicting samples, 35 aqueous solutions of STPP, SS, and SC were prepared in a 100 ml beaker, by distilled water. The concentration range for STPP, SS, and SC was 0.2579–2.8913, 0.2029–2.4935, and 0.2912–1.2010 (g per 100 g), respectively. The compositions of these samples are shown in . Nine synthetic mixture samples were also prepared in the same way while different amounts of other ingredients of Iranian washing powders (CMC, Sodium silicate, and sodium hydroxide) were also added to each mixture.
For each of these real samples, after grinding and homogenizing, 1.000 g was used for analysis. Each weighed sample was diluted to a final weight of 25 g with distilled water. Spectra of standard solution and real sample with air background do not contain any useful information in IR signals, thus the spectrum of distilled water was set as background, obtained under the same experimental conditions as the samples and standards (). According to our previous experiences, the ATR-FTIR spectra of standard samples were obtained in 750–2000 cm–1 spectral region Citation5.
Principles of Doolittle multivariate calibration
Doolittle algorithm
Doolittle algorithm is a mathematical method based on decomposition of matrix A (a nonsingular matrix with rank n) into two matrix, such as lower and upper triangular matrix. The decomposition process is as follows Citation13–18.
The matrix can be expressed as:
where L is a lower triangular matrix and U is an upper triangular matrix.
The first step
The second step
l i2 =(a i2 –l i1 ×u 12)/u 22,(i=3,…,n) and so on, until the (k-1)th step
The kth step
The nth step
The solution of equation (A·x=b) can be obtained by solving L·y=b and U·x=y.
Conclusion
A new method based on mid-IR reflectance spectrometry was proposed for the nondestructive quantitative determination of SS, SC, and STPP, with the Doolittle multivariate calibration being compared to PLS. Doolittle multivariate calibration improved the sensitivity of the results by the triangle decomposing of the matrixes and avoiding matrix inverting. The investigations demonstrated that the Doolittle multivariate calibration results are more robust. Therefore, the Doolittle algorithm is sufficient in multivariate calibration and shows good capability in analyzing the mixed systems with noticeable spectral overlapping. Obviously, Doolittle multivariate calibration will have a bright prospect in analyzing systems which are difficult to separate. While such a calibration method is still based on linear model and the system has its linear range, for non-linear systems, it cannot provide satisfactory results.
References
- Hoyt , J.L. , Sones , E.L. and Sooter , A.J. 2000 . Separation and Quantitative Determination of Active Ingredients in Detergent Formulations , 74601 Ponca City , Oklahoma : Continental Oil Company, Research and Development Department .
- Wilsch-Irrgans , A. , Sanli , O. , Harer , J. and Jauman , G. 2003 . Tenside. Surf. Det. , 40 : 187
- Henry Hancox , MD. 1854 . A Case of Death from the Absorption of Washing Soda . The Lancet Journal. , 64 : 434 – 435 .
- ASTM Method D501–89 , Standard Test Method for Sampling and Chemical Analysis of Alkaline Detergents . 2005 American Society for Testing and Material , Philadelphia , PA .
- Khanmohammadi , M.R. , Ashori , A. , Kargosha , K. and Bagheri Garmarudi , A. 2007 . J. Surfact. Deterg. , 10 : 81 – 86 .
- Franco , V.G. , Per′In , J.C. , Mantovani , V.E. and Goicoechea , H.C. 2006 . Monitoring Substrate and Products in a Bioprocess with FTIR Spectroscopy Coupled to Artificial Neural Networks Enhanced with a Genetic-Algorithm-Based Method for Wavelength Selection . Talanta. , 68 : 1005 – 1012 .
- Hemmateenejad , B. , Miri , R. , Akhonda , R. and Shamsipur , M. 2002 . Chemom. Intell. Lab. Syst. , 64 : 91 – 99 .
- Ding , Y.P. 2005 . Anal. Sci. , 21 : 327 – 330 .
- Chen , N.Y. , Ding , Y.P. , Li , G.Z. and Ye , C.Z. 2002 . Comput. Appl. Chem. , 19 : 717
- Brown , C.W. , Lynch , P.F. and Obermski , R.J. 1982 . Anal. Chem. , 54 : 1472 – 1479 .
- Li , Q.Y. , Wang , N.C. and Yi , D.Y. 1995 . Modern Numerical Analysis , 170 – 185 . Beijing : Science Publication .
- Ding , Y.P. , Mu , T. and Wu , Q. 2007 . Chemom. Intell. Lab. Syst. , 88 : 167 – 169 .
- Qamber , I.S. 1999 . Relia. Eng. Syst. Saf. , 64 : 359
- Abbasbandy , S. , Ezzati , R. and Jafarian , A. 2006 . J. Appl. Math. Comput. , 172 : 633
- Kurdi , A.A. and Kincaid , D.R. 2006 . J. Comput. Appl. Math. , 185 : 391
- Kaya , D. and Wright , K. 2005 . J. Appl. Math. Comput. , 163 : 179
- Mittal , R.C. and Al-Kurdi , A. 2002 . J. Comput. Math. Appl. , 43 : 131 – 155 .
- Agensin , K.E. 1986 . Numerical Analysis Introduction , Shanghai : Shanghai Science and Technology Publication .