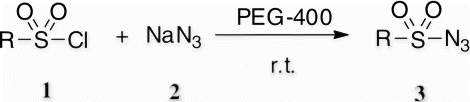Abstract
Sulfonyl azides have efficiently been synthesized via a convenient and environmentally benign procedure, in which sulfonyl chlorides undergo nucleophilic substitution reaction with sodium azide in PEG-400 under mild conditions. The sulfonyl azides were obtained in 84–97% isolated yields.
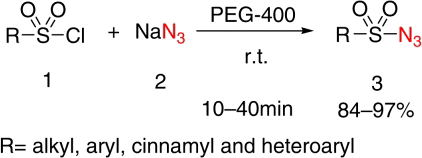
Introduction
Sulfonyl azides are very valuable reagents in organic chemical transformations such as the preparation of α-diazocarbonyl compounds Citation1, the hydro-hydrazination or/and hydroazidation of olefins Citation2 Citation3, the aziridination of olefins Citation4, the radical amination Citation5 Citation6, and metal-catalyzed coupling reactions Citation7. Due to a wide range of applications, there are many methods available for the preparation of sulfonyl azides. For example, sulfonyl azides were prepared by reacting sulfonyl anhydrides Citation8, α-disulfones Citation9, or 1-sulfonylbenzotriazole Citation10 with sodium azide. These procedures may suffer from the unavailability of starting materials or their difficulty in preparation. Additionally, diazotization of sulfonyl hydrazides with NO+ has also been employed but still requires the availability of the hydrazides Citation11. However, the most practical laboratory methods for preparing sulfonyl azides by nucleophilic substitution reaction of sulfonyl chlorides with sodium azide in various solvents are such as alcohol/H2O, acetone/H2O, DME/H2O, and so on Citation3 Citation12–17. Since nucleophilic substitution reactions of sulfonyl chloride involve a nonpolar organic compound and a polar ionic salt, sodium azide, the heterogenous reactions are often troublesome because the polar and nonpolar reagents are often not soluble in a single solvent system. Consequently, to improve the yields and to facilitate the product isolation, the nucleophilic displacement reactions are carried out under phase-transfer catalysis conditions Citation18 Citation19. However, these methodologies often suffer from complex procedures, long reaction times, and low yields. Thus, there is a great demand for the development of new convenient and eco-friendly synthetic methods toward assessing sulfonyl azides.
In the recent years, polyethylene glycols (PEGs) have attracted great interest and have been explored as a novel, powerful, eco-friendly reaction medium for various organic transformations Citation20–25 due to their relatively inexpensive, thermally stable, readily recyclable, and biodegradable. In a continuation of our work Citation20 to explore PEG as an efficient and eco-friendly reaction medium, we report here a convenient and practical synthesis of sulfonyl azides by using sodium azide in PEG-400 at room temperature ().
Results and discussion
Initially, we examined the effectivity of PEG-400 for the model reaction of 4-Tosyl chloride and sodium azide (Entry 4, ). In a typical experimental procedure, a screening of different solvents (CH3CN, THF, CH2Cl2, toluene, and so on) for the model reaction revealed that PEG-400 was the most active reaction medium.
Table 1. Synthesis of sulfonyl azides from sulfonyl chlorides using PEG-400 as an efficient reaction medium.a
To investigate the generality and scope of the reaction, various sulfonyl chlorides were subjected to the reaction conditions and no additional catalyst and solvent were required. The results are summarized in . As presented in , all aryl and aliphatic sulfonyl chlorides gave sulfonyl azides in excellent yields in 10–40 minutes. Aryl sulfonyl chlorides containing both electron-donating, such as methyl, methoxyl, and electron-withdrawing groups, like nitro, acetamido, underwent the conversion smoothly. Aryl sulfonyl chlorides with electron-withdrawing groups such as NO2 required slightly more long time (Entries 8–10, ). With more sterically hindered sulfonyl chlorides, satisfactory yields were still obtained from the nucleophilic substitution (Entries 5, 6, and 10, ). 2-Nitrobenzenesulfonyl chloride took the longest time caused by the electronic and steric hindrance effect (Entry 10, ). The presence of various functional groups such as halides, nitro, acetamino, and methoxyl on the aryl sulfonyl chlorides was tolerated (Entries 7–13, ). Trans-β-styrenesulfonyl chloride and 2-thiophenesulfonyl chloride have also been successfully converted into their corresponding sulfonyl azides in high yields (Entries 14 and 15, ). In short, the products were all formed in excellent yields and no side products were detected. The structures of all products were identified by their physical and spectral data. Infrared spectra of all compounds have strong characteristic band at 2120–2160 cm−l (N3), 1310–1370 cm−l, and 1100–1170 cm−l (SO2).
Experimental
All reagents were purchased from commercial sources unless otherwise stated. Petroleum ether/ethyl acetate (8:1) (TLC) was carried out on silica gel 60 F254 precoated plates (0.20–0.25 mm thickness) and visualized with UV light (254 nm). Melting points were determined with X-6 (Beijing Fukai Co. Ltd.) melting point apparatus and were uncorrected. 1H NMR and 13C NMR (600 and 150 MHz, respectively) spectra were recorded in CDCl3. 1H NMR chemical shifts are reported in ppm (δ) relative to tetramethylsilane (TMS) with the solvent resonance employed as the internal standard (CDCl3, δ 7.26 ppm). Data are reported as follows: chemical shift, multiplicity (s = singlet, d = doublet, t = triplet, and m = multiplet), coupling constants (Hz), and integration. 13C NMR chemical shifts are reported in ppm from TMS with the solvent resonance as the internal standard (CDCl3, δ 77.0 ppm). Compounds 1l Citation27, 1m Citation27, and 1n Citation28 were prepared by following the reported methods.
Typical procedure for synthesis of sulfonyl azides
A typical experimental procedure is as follows: a mixture of sulfonyl chloride (2 mmol) and NaN3 (2.4 mmol) in PEG-400 (2 mL) was vigorously stirred at room temperature for the appropriate time () until TLC indicated total disappearance of sulfonyl chloride. After completion, the reaction mixture was poured into water and extracted with dry ether. The organic layer was removed under reduced pressure and afforded pure sulfonyl azides in excellent yield [Caution: Sufficient care has to be exercised while treating organic azides because of their explosive nature]. The crude products were generally sufficient purity to be used without further purification. The pure compounds can also be obtained by flash silica gel column chromatography with petroleum ether/ethyl acetate (8:1) or crystallized from methanol. The PEG-400 was recovered from the aqueous layer and reused without loss of activity.
Characterization data of selected known compounds and new compound
The products are all known except 3m and were identified by comparing their physical and spectral data with literature values. Spectral data for selected and new compounds are described in the following subsections.
1-Butanesulfonyl azide (3a)
Pale yellow liquid. 1H NMR (CDCl3): δ H 0.99 (t, J=7.4 Hz, 3H), 1.48–1.57 (m, 2H), 1.88–1.93 (m, 2H), 3.32 (t, J=7.9 Hz, 2H). 13C NMR (CDCl3): δ C 13.4, 21.3, 25.3, 55.7. IR (KBr), v|cm−1: 3306, 2965, 2877, 2378, 2136, 1467, 1364, 1242, 1198, 1159, 1100, 1079, 917, 794, 735.
Phenylmethanesulfonyl azide (3b)
Colorless solid, m.p. 53–54 °C. 1H NMR (CDCl3): δ H 4.53 (s, 2H), 7.43–7.48 (m, 5H). 13C NMR (CDCl3): δ C 61.9, 126.6, 129.3, 129.9, 130.9. IR (KBr), v|cm−1: 3436, 3294, 2979, 2137, 1599, 1496, 1456, 1407, 1355, 1270, 1179, 1159, 1136, 1031, 884, 793, 748.
Benzenesulfonyl azide (3c)
Colorless solid, m.p. 13–14 °C. 1H NMR (CDCl3): δ H 7.63 (t, J=7.5, J=7.9 Hz, 2H), 7.74 (t, J=7.5 Hz, 1H), 7.97 (d, J=7.9 Hz, 2H). 13C NMR (CDCl3): δ C 127.5, 129.7, 134.8, 138.5. IR (KBr), v|cm−1: 3273, 3069, 2921, 2128, 1732, 1583, 1449, 1373, 1313, 1170, 1087, 1020, 930, 753.
4-Toluenesulfonyl azide (3d)
Colorless solid, m.p. 22–23 °C. 1H NMR (CDCl3): δ H 2.48 (s, 3H), 7.41 (d, J=8.1 Hz, 2H), 7.84 (d, J=8.3 Hz, 2H). 13C NMR (CDCl3): δ C 21.7, 127.5, 130.3, 135.6, 146.2. IR (KBr), v|cm−1: 3273, 3067, 2926, 2127, 1595, 1494, 1450, 1371, 1308, 1167, 1121, 1086, 1018, 814, 748.
2,4,6-Trimethylbenzene-1-sulfonyl azide (3e)
Tan liquid. 1H NMR (CDCl3): δ H 2.3 (s, 3H), 2.67 (s, 6H), 7.02 (s, 2H). 13C NMR (CDCl3): δ H 21.1, 22.7, 132.2, 133.3, 139.9, 144.6. IR (KBr), v|cm−1: 3276, 2981, 2924, 2382, 2122, 1602, 1566, 1455, 1366, 1291, 1191, 1166, 1051, 965, 854, 745.
2,4,6-Triisopropylbenezensulfonyl azide (3f)
Colorless solid, m.p. 42–43 °C. 1H NMR (CDCl3): δ H 1.26 (d, J=7.1 Hz, 6H), 1.30 (dd, J=6.6, J=6.8 Hz, 12H), 2.91–2.96 (m, 1H), 4.22–4.26 (m, 2H), 7.22 (s, 2H). 13C NMR (CDCl3): δ C 23.4, 24.3, 24.7, 29.7, 29.8, 34.3, 124.1, 124.3, 139.3, 150.4, 150.9, 155.6. IR (KBr), v|cm−1: 3435, 3055, 2961, 2930, 2870, 2121, 1598, 1462, 1434, 1385, 1378, 1364, 1350, 1261, 1175, 1104, 1059, 889, 802, 740.
4-Methoxysulfonyl azide (3g)
Colorless solid, m.p. 50–51 °C. 1H NMR (CDCl3): δ H 3.91 (s, 3H), 7.05 (d, J=8.9 Hz, 2H), 7.90 (d, J=8.9 Hz, 2H). 13C NMR (CDCl3): δ C 55.9, 114.8, 128.6, 129.9, 164.6. IR (KBr), v|cm−1: 3273, 3096, 2984, 2129, 1590, 1495, 1369, 1318, 1266, 1187, 1163, 1110, 1085, 1020, 832, 805, 744.
4-Nitrobenzenesulfonyl azide (3h)
Tan solid, m.p. 100–101 °C. 1H NMR (CDCl3): δ H 8.17 (d, J=8.8 Hz, 2H), 8.46 (d, J=8.8 Hz, 2H). 13C NMR (CDCl3): δ H 124.9, 128.9, 143.8, 151.0. IR (KBr), v|cm−1: 3107, 2920, 2143, 1606, 1531, 1377, 1350, 1311, 1178, 1160, 1085, 854, 769, 744.
2-Nitrobenzenesulfonyl azide (3j)
Tan solid, m.p. 68–71 °C. 1H NMR (CDCl3): δ H 7.83 (ddd, 1H), 7.88 (ddd, 1H), 7.92 (dd, 1H), 8.20 (dd, 1H). 13C NMR (CDCl3): δ H 125.4, 131.7, 132.7, 133.0, 135.7. IR (KBr), v|cm−1: 3320, 3100, 2923, 2381, 2157, 1594, 1550, 1438, 1363, 1315, 1261, 1194, 1145, 1120, 967, 853, 755, 737.
4-Bromobenzenesulfonyl azide (3k)
White solid, m.p. 78–79 °C. 1H NMR (CDCl3): δ H 7.76 (d, J=8.4 Hz, 2H), 7.82 (d, J=8.6 Hz, 2H). 13C NMR (CDCl3): δ C 128.9, 130.3, 133.1, 137.5. IR (KBr), v|cm−1: 3246, 3094, 2923, 2148, 1571, 1470, 1392, 1376, 1168, 1083, 1064, 1008, 819, 770, 732.
4-Acetamidobenzenesulfonyl azide (3l)
White solid, m.p. 107–108 °C. 1H NMR (CDCl3): δ H 2.25 (s, 1H), 7.78 (d, J=9.0 Hz, 2H), 7.79 (brs, 1H), 7.89 (d, J=8.8 Hz, 2H). 13C NMR (CDCl3): δ C 24.7, 119.6, 129.6, 129.0, 132.7, 143.9, 168.9. IR (KBr), v|cm−1: 3303, 3264, 3185, 3112, 2130, 2120, 1676, 1585, 1534, 1405, 1365, 1315, 1265, 1165, 1086, 839, 752, 707.
3-Chloro-4-acetamidobenzenesulfonyl azide (3m)
White solid, m.p. 95–97 °C. 1H NMR (CDCl3): δ H 2.32 (s, 3H), 7.85 (dd, J=2.2, J=9.0 Hz, 1H), 7.87 (brs, 1H), 7.97 (d, J=2.2 Hz, 1H), 8.74 (d, J=8.8 Hz, 1H). 13C NMR (CDCl3): δ C 25.1, 120.9, 122.6, 127.5, 128.3, 133.2, 140.3, 168.5. IR (KBr), v|cm−1: 3401, 3119, 3070, 2340, 2134, 1712, 1575, 1505, 1392, 1375, 1306, 1171, 1098, 857, 837, 778, 745.
Trans-β-styrenesulfoyl azide (3n)
White solid, m.p. 31–32 °C. 1H NMR (CDCl3): δ H 6.94 (d, J=15.3 Hz, 1H), 7.45–7.47 (m, 2H), 7.50 (m, 1H), 7.53–7.55(m, 2H), 7.70 (d, J=15.3 Hz, 1H). 13C NMR (CDCl3): δ C 123.2, 126.8, 128.9, 129.4, 129.6, 129.8, 131.3, 132.2. IR (KBr), v|cm−1: 3292, 3065, 2345, 2130, 1725, 1610, 1576, 1495, 1450, 1368, 1180, 1154, 1107, 1074, 975, 863, 821, 749.
2-Thiophenesulfonyl azide (3o)
Pale yellow, m.p. 30–31 °C. 1H NMR (CDCl3): δ H 7.21 (dd, J=3.9, J=4.9 Hz, 1H), 7.80 (dd, J=1.4, J=4.9 Hz, 1H), 7.21 (dd, J=1.4, J=3.9 Hz, 1H). 13C NMR (CDCl3): δ C 128.0, 134.7, 135.1, 138.2. IR (KBr), v|cm−1: 3271, 3101, 2129, 1754, 1601, 1504, 1400, 1378, 1345, 1167, 1094, 1019, 857, 757, 746.
Conclusion
In summary, we have disclosed a simple, mild, and efficient method for the synthesis of sulfonyl azides. Compared to the previously reported methods, this protocol offers several advantages including exceedingly mild conditions, operational simplicity, more environmentally benign, short reaction time, and higher reaction yield. Further investigations on the application of PEG-400 on other catalytically synthetic reactions will be reported in due course.
Acknowledgements
We are grateful for financial support from the Chinese Academy of Sciences (Hundreds of Talents Program).
References
- Ye T , McKervey MA. Organic Synthesis with .alpha.-Diazo Carbonyl Compounds . Chem. Rev . 1994 ; 94 : 1091 . doi: 10.1021/cr00028a010
- Waser J , Nambu H , Carreira EM. Cobalt-Catalyzed Hydroazidation of Olefins: Convenient Access to Alkyl Azides . J. Am. Chem. Soc . 2005 ; 127 : 8294 . doi: 10.1021/ja052164r
- Waser J , Gaspar B , Nambu H , Carreira EM . Hydrazines and Azides via the Metal-Catalyzed Hydrohydrazination and Hydroazidation of Olefins . J. Am. Chem. Soc . 2006 ; 128 : 11693 . doi: 10.1021/ja062355+
- Ruppel JV , Jones JE , Huff CA , Kamble RM , Chen Y , Zhang XP. A highly effective cobalt catalyst for olefin aziridination with azides: hydrogen bonding guided catalyst design . Org. Lett . 2008 ; 10 : 1995 . doi: 10.1021/ol800588p
- Masterson DS , Shckleford JP. Free-Radical Azidation with γ-15N-Labeled Phenylsulfonyl Azide . Synlett . 2007 ; 8 : 1302 . doi: 10.1055/s-2007-977427
- Panchaud P , Chabaud L , Landais Y , Ollivier C , Renaud P , Zigmantas S. Radical Amination with Sulfonyl Azides: A Powerful Method for the Formation of C-N Bonds . Chem. Eur. J . 2004 ; 10 : 3606 . doi: 10.1002/chem.200400027
- Ruppel JV , Kamble RM , Zhang XP. Cobalt-Catalyzed Intramolecular C–H Amination with Arylsulfonyl Azides . Org. Lett . 2007 ; 9 : 4889 . doi: 10.1021/ol702265h
- Laurent N , Lafont D , Boullanger P , Mallet JM. An alternative high yielding and highly stereoselective method for preparing an α-Neu5NAc-(2,6)-d-GalN3 building block suitable for further glycosylation . Carbohydr. Res . 2005 ; 340 : 1885 . doi: 10.1016/j.carres.2005.05.007
- Farng L-PO , Kice JL. Substitution reactions of alkanesulfonyl derivatives: direct substitution vs. elimination-addition mechanisms in substitution reactions of alkyl .alpha.-disulfones . J. Am. Chem. Soc . 1981 ; 103 : 1137 . doi: 10.1021/ja00395a024
- Katritzky A , Widyan K , Gyanda K. Synthesis of Sulfonyl Azides . Synthesis . 2008 ; 8 : 1201 . doi: 10.1055/s-2008-1072568
- Stefane B , Kocevar M , Polanc S. Nitrosation with Sodium Hexanitrocobaltate(III) . J. Org. Chem . 1997 ; 62 : 7165 . doi: 10.1021/jo9703820
- El-Sayed , RA. Review on the chemistry of sulfonohydrazides and sulfonoazides . Phosphorus, Sulfur Silicon Relat. Elem . 2004 ; 179 : 237 . doi: 10.1080/10426500490274673
- Andersen NG , Ramsden PD , Che D , Parvez M , Keay BA. A Novel Resolution Procedure for the Preparation of P-Stereogenic Phosphine Oxides . Org. Lett . 1999 ; 1 : 2009 . doi: 10.1021/ol991174s
- Harmon RE , Wellman G , Gupta SK. Reaction of arylsulfonyl azides with N-methylindole . J. Org. Chem . 1973 ; 38 : 11 . doi: 10.1021/jo00941a003
- Leffler JE , Tsuno Y. Some Decomposition Reactions of Acid Azides . J. Org. Chem . 1963 ; 28 : 902 . doi: 10.1021/jo01039a004
- Teimouri A , Chermahini AN , Emami M. Synthesis, characterization, and DFT studies of a novel azo dye derived from racemic or optically active binaphthol . Tetrahedron . 2008 ; 64 : 11776 . doi: 10.1016/j.tet.2008.09.104
- Reagan MT , Nickon A. The photolysis of sulfonyl azides in isopropyl alcohol . J. Am. Chem. Soc . 1968 ; 90 : 4096 . doi: 10.1021/ja01017a032
- Kumar SM. Comvenient Preparation of Aryl Sulfonyl Azides Under Triphasf Catalytic Conditions . Synth. Commun . 1987 ; 17 : 1015 . doi: 10.1080/00397918708063962
- Hasegawa K , Arai S , Nishida A. Synthesis of α-diazo-β-hydroxyesters through a one-pot protocol by phase-transfer catalysis: application to enantioselective aldol-type reaction and diastereoselective synthesis of α-amino-β-hydroxyester derivatives . Tetrahedron . 2006 ; 62 : 1390 . doi: 10.1016/j.tet.2005.11.028
- For recent some examples of the use of PEG in organic synthesis see ref. (20–25) Zhao J , Wei S , Ma X , Shao H. A mild and environmentally benign method for the synthesis of glycals in PEG-600/H2O . Green Chem . 2009 ; 11 : 1124 doi: 10.1039/b821681a
- Rupesh K , Preeti C , Surendra N , Ramesh C. Polyethylene glycol as a non-ionic liquid solvent for Michael addition reaction of amines to conjugated alkenes . Green Chem . 2006 : 8 : 356 . doi: 10.1039/b517397c
- Suryakiran N , Reddy TS , Ashalatha K , Lakshman M , Venkateswarlu Y. Facile polyethylene glycol (PEG-400) promoted synthesis of β-ketosulfones . Tetrahedron Lett . 2006 ; 47 : 3853 . doi: 10.1016/j.tetlet.2006.03.181
- Das B , Krishnaiah M , Balasubramanyam P , Veeranjaneyulu P , Kumar DN. A remarkably simple N-formylation of anilines using polyethylene glycol . Tetrahedron Lett . 2008 ; 49 : 2225 . doi: 10.1016/j.tetlet.2008.02.050
- Zhu DJ , Chen JX , Xiao HL , Liu MC , Ding JC , Wu HY. Efficient and Expeditious Synthesis of Di- and Trisubstituted Thiazoles in PEG Under Catalyst-Free Conditions . Synth. Commun . 2009 ; 39 : 2895 . doi: 10.1080/00397910802691874
- Liang J , Lv J , Fan JC , Shang ZC. Polyethylene Glycol as a Nonionic Liquid Solvent for the Synthesis of N-Alkyl and N-Arylimides . Synth. Commun . 2009 ; 39 : 2822 . doi: 10.1080/00397910802474966
- McManus SP , Smith MR , Abramovitch RA , Offor MN. J. Thermolysis of sulfonyl azides bearing nucleophilic neighboring groups. A search for anchimeric assistance . Org. Chem . 1984 ; 49 : 683 . doi: 10.1021/jo00178a022
- Ilies MA , Vullo D , Pastorek J , Scozzafava A , Ilies M , Caproiu MT , Pastorekova S , Supuran CT. Carbonic Anhydrase Inhibitors. Inhibition of Tumor-Associated Isozyme IX by Halogenosulfanilamide and Halogenophenylaminobenzolamide Derivatives . J. Med. Chem . 2003 ; 46 : 2187 . doi: 10.1021/jm021123s
- Culbertson BM , Dietz , S. Some aromatic vinyl sulphonyl chlorides . J. Chem. Soc. C . 1968 , 992 . doi: 10.1039/j39680000992