Abstract
A simple and efficient synthesis of 5-arylidene-2,4-thiazolidinediones by the Knoevenagel condensation of aldehydes with 2,4-thiazolidinedione in the presence of 2-hydroxy ethylammonium propionate under solvent-free conditions is described. The attractive features of this procedure are operational simplicity, high yields, short reaction times, easy to prepare the catalyst, mild and environmentally benign reaction conditions, and recycle of the catalyst.
Introduction
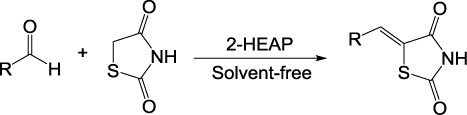
2,4-Thiazolidinedione and its derivatives exhibit a variety of pharmacological activities (Citation1, Citation2). Thus, the synthesis of 2,4-thiazolidinedione derivatives is currently of much importance. The most common method for the synthesis of 5-arylidine-2,4-thiazolidinediones is Knoevenagel condensation between aromatic aldehydes and 2,4-thiazolidinedione. It is reported that such condensation can be accelerated by sodium acetate in acetic acid (Citation3–Citation5). Catalysts like piperidine (Citation6–Citation9), piperidinium acetate (Citation10–Citation13), glycine and sodium carbonate (Citation14), ammonium acetate (Citation15), KAl(SO4)2·12H2O (Citation16), and baker's yeast (Citation17) have also been reported for the synthesis of 5-arylidene-2,4-thiazolidinediones. Various alternative methods were also reported for the synthesis of 5-arylidene-2,4-thiazolidinediones using solvent-free microwave irradiation (Citation18–Citation19). Knoevenagel condensation of various aromatic aldehydes with 2,4-thiazolidinedione has been carried out in polyethylene glycol-300 (Citation20). Also, Kumar and co-workers (Citation21) reported the synthesis of 5-arylidene-2,4-thiazolidinedione derivatives using aldonitrones in polyethylene glycol. However, many of these methodologies are associated with several shortcomings such as low to moderate yields, long reaction times, tedious work-up procedures, requirement of special apparatus, use of environmentally unfavorable solvents, requirement of excess of catalysts, and difficulty in recovery and reusability of the catalysts. The use of ionic liquids as catalysts and solvents for chemical reactions offers many advantages from an environmental perspective (Citation22–Citation24). Recently, synthesis of 5-arylidene-2,4-thiazolidinediones by Knoevenagel condensation catalyzed by ionic liquids [bnmim]Cl (Citation25) and C3[min]2·2[Br-] (Citation26) has been reported. However, ionic liquids with imidazolium as the cation are relatively expensive, which hinders their industrial applications. Therefore, the discovery of a new, easily available catalyst with high catalytic activity for the preparation of 5-arylidene-2,4-thiazolidinediones under mild and environmentally benign conditions is still necessary. Elimination of volatile organic solvents in organic synthesis is an important goal in green chemistry. Solvent-free conditions for organic synthesis have increased rapidly in recent years (Citation27). Herein, we report a simple and efficient synthesis of 5-arylidene-2,4-thiazolidinediones using easily available, cheap ionic liquid 2-hydroxy ethylammonium propionate as catalyst under solvent-free conditions ().
Results and discussion
In order to optimize the reaction conditions, the reaction of benzaldehyde and 2,4-thiazolidinedione has been considered as standard model reaction. Reactions were carried out simply by mixing 2,4-thiazolidinedione with benzaldehyde in the presence of a catalytic amount of ionic liquid under solvent-free conditions. During the reaction process, a solid product spontaneously formed. After cooling, the solid product was diluted with water. The separated solid was suction filtered, washed with water, and recrystallized from hot ethanol to obtain the pure product. The recovery of ionic liquid was made by vacuum distillation of the filtrate.
We screened three different ionic liquids 2-hydroxy ethylammonium formate (2-HEAF), 2-hydroxy ethylammonium acetate (2-HEAA), and 2-hydroxy ethylammonium propionate (2-HEAP) for the model reaction. All the results are listed in . In an initial study, the effect of reaction temperature on the yield was examined using 20 mol% 2-hydroxy ethylammonium propionate as catalyst under solvent-free conditions. The results show that the higher reaction temperature, the more efficiently the reaction could proceed. The best result was obtained at 70°C for 3 minutes under solvent-free conditions. Raising the reaction temperature from 70 to 80°C did not increase the yield.
Table 1. Effect of different reaction conditions on ionic liquids catalyzed synthesis of 5-benzylidene-2,4-thiazolidinedione.Footnotea
Furthermore, to determine the appropriate concentration of the catalyst, we investigated the model reaction at different concentrations of catalyst like 10, 15, 20, and 25 mol% at 70°C, and the product formed in 86, 93, 93, and 94% yield, respectively. This indicates that 15 mol% of 2-hydroxy ethylammonium propionate is sufficient for the best result.
Subsequently, other catalysts, 2-hydroxy ethylammonium formate and 2-hydroxy ethylammonium acetate, were screened at 70°C (, entries 11 and 12), and the results show that 2-hydroxy ethylammonium propionate provided the highest yield (, entry 9). The investigated ionic liquids had the same cation, the results indicated that the anion of ionic liquids influenced the reaction rate. It is noteworthy that in the absence of a catalyst under the reaction conditions, no product formation was observed after 60 min (, entry 13). This result indicates that the catalyst exhibits a high catalytic activity in this transformation.
Encouraged by these results, to show the generality and scope of this new protocol, we have extended this protocol to a variety of aldehydes. Considering the reaction time and yield of product, 2-hydroxy ethylammonium propionate was selected as the optimum catalyst to promote the synthesis of 5-arylidene-2,4-thiazolidinediones, and the results () clearly demonstrate that 2-hydroxy ethylammonium propionate is an excellent catalyst. Several functionalities present in the aryl aldehydes such as halogen, methoxy, and nitro group were tolerated. In all the cases, the corresponding 5-arylidene-2,4-thiazolidinediones were obtained in good to excellent yield after 3–65 min. All the products are known compounds and can be confirmed by comparing their physical constants and spectral data with reported results in literature.
Table 2. 2-Hydroxy ethylammonium propionate-catalyzed synthesis of 5-arylidene-2,4-thiazolidinediones.Footnotea
Finally, the reusability of 2-hydroxy ethylammonium propionate was investigated by stirring benzaldehyde (20 mmol), 2,4-thiazolidinedione (20 mmol), and 2-hydroxy ethylammonium propionate (3 mmol) under solvent-free conditions at 70°C (). The recovered 2-hydroxy ethylammonium propionate can be reused at least five additional times in subsequent reactions with only negligible loss of catalytic activity. Compared with the traditional catalysts, the easy recycling performance is also an attractive property of the ionic liquids for the environmental protection and economic reasons.
Table 3. Recycling of the 2-hydroxy ethylammonium propionate for the synthesis of 5-benzylidene-2,4-thiazolidinedione.Footnotea
In order to show the merits of present method, the efficiency of 2-hydroxy ethylammonium propionate was compared with that of other ionic liquids reported earlier in the synthesis of 5-arylidene-2,4-thiazolidinediones by Knoevenagel condensation of aldehydes with 2,4-thiazolidinedione. The data summarized in clearly demonstrate that 2-hydroxy ethylammonium propionate is effective catalyst for the synthesis of 5-arylidene-2,4-thiazolidinediones. The present catalyst seems to be more beneficial from the economical and accessibility viewpoint (2-hydroxy ethylammonium propionate is easy to prepare and very inexpensive compared with [bnmim]Cl and C3[min]2·2[Br−]).
Table 4. Comparison of ionic liquids used as catalysts for the synthesis of 5-arylidene-2,4-thiazolidinediones in condensation of benzaldehyde with 2,4-thiazolidinedione.
Experimental
FT-IR spectra were obtained on a Nexus 470 spectrophotometer. Nuclear magnetic resonance (NMR) spectra were recorded on a Bruker Avance III 400 with tetramethylsilane as internal standard. Melting points were determined on a melting point apparatus. 2,4-Thiazolidinedione was prepared according to the literature method (Citation7). 2-Hydroxy ethylammonium formate, 2-hydroxy ethylammonium acetate, and 2-hydroxy ethylammonium propionate were easily prepared from cheaply available acid and ethanolamine by a simple acid–base neutralization reaction (Citation28, Citation29).
General procedure for the synthesis of 5-arylidene-2,4-thiazolidinediones
A mixture of 2,4-thiazolidinedione (10 mmol), aldehyde (10 mmol), and 2-hydroxy ethylammonium propionate (1.5 mmol) was stirred at 70°C for the specified time. The progress of the reaction was monitored by thin layer chromatography (ethyl acetate/petroleum ether = 1/3 as eluent). After completion of reaction, the reaction mixture was cooled to room temperature and diluted with water. The separated solid was suction-filtered and dried. It was further purified by crystallization from ethanol. The filtrate containing the ionic liquid was then evaporated to dryness under reduced pressure, and the resulting catalyst was reused directly for the next run.
Representative spectral data
(5Z)-5-Benzylidene-2,4-thiazolidinedione (,entry 1)
IR (KBr) νmax/cm−1: 3135,3029,1737,1690,1603. 1H NMR (DMSO-d6, 400 MHz): δ = 7.49–7.62 (m, 5H, ArH), 7.80 (s, 1H, CH), 12.64 (s, 1H, NH).
(5Z)-5-(4-Methylbenzylidene)-2,4-thiazolidinedione (,entry 2)
IR (KBr) νmax/cm−1: 3163,3049,1736,1687,1599. 1H NMR (DMSO-d6, 400 MHz): δ = 2.35 (s, 3H, CH3), 7.34 (d, J = 8.0 Hz, 2H, ArH), 7.49 (d, J = 8.0 Hz, 2H, ArH), 7.75 (s, 1H, CH), 12.58 (s, 1H, NH).
(5Z)-5-(4-Methoxybenzylidene)-2,4-thiazolidinedione (,entry 3)
IR (KBr) νmax/cm−1: 3229,3033,1732,1697,1589. 1H NMR (DMSO-d6, 400 MHz): δ = 3.82 (s, 3H, OCH3), 7.09 (d, J = 8.8 Hz, 2H, ArH), 7.55 (d, J = 8.8 Hz, 2H, ArH), 7.74 (s, 1H, CH), 12.52 (s, 1H, NH).
(5Z)-5-(4-Fluorobenzylidene)-2,4-thiazolidinedione (,entry 4)
IR (KBr) νmax/cm−1: 3131,3041,1729,1697,1589. 1H NMR (DMSO-d6, 400 MHz): δ = 7.36–7.68 (m, 4H, ArH), 7.81 (s, 1H, CH), 12.64 (s, 1H, NH).
Conclusion
In conclusion, we have described a simple, efficient, and cleaner methodology for the synthesis of 5-arylidene-2,4-thiazolidinedione derivatives by Knoevenagel condensation of different aldehydes with 2,4-thiazolidinedione using 2-hydroxy ethylammonium propionate as catalyst under solvent-free conditions. The attractive features of this procedure are the operational simplicity, the high yields, the short reaction times, easy to prepare the catalyst, mild and environmentally benign reaction conditions, and recycle of the catalyst, all of which make it a useful and attractive strategy for the synthesis of 5-arylidene-2,4-thiazolidinedione derivatives.
Acknowledgements
This research work was financially supported by the Graduate Student Innovative Foundation of South-Central University for Nationalities.
References
- Oguchi, M.; Wada, K.; Honma, H.; Tanaka, A.; Taneko, T.; Sakakibara, S.; Ohsumi, J.; Serizawa, N.; Fujiwara, T.; Horikoshi, H.; Fujita, T. J. Med. Chem. 2000, 43, 3052–3066. 10.1021/jm990522t
- Malamas, M.S.; Sredy, J.; Gunawan, I.; Mihan, B.; Sawicki, D.R.; Seestaller, L.; Sullivan, D.; Flam, B.R. J. Med. Chem. 2000, 43, 995–1010. 10.1021/jm990476x
- Lo, C.P.; Shropshire, E.Y.; Croxall, W.J. J. Am. Chem. Soc. 1953, 75, 4845–4846. 10.1021/ja01115a516
- Ibrahim, M.A.; Abdel-Hamed, M.A.; El-Gohary, N.M. J. Braz. Chem. Soc. 2011, 22, 1130–1139. 10.1590/S0103-50532011000600019
- Li, X.F.; Feng, Y.Q.; Zhang, W.H.; Wang, D.H. Transactions of Tianjin Univer. 2003, 9, 228–230.
- Xia, Z.; Knaak, C.; Ma, J.; Beharry, Z.M.; McInnes, C.; Wang, W.; Kraft, A.S.; Smith, C.D. J. Med. Chem. 2009, 52, 74–86. 10.1021/jm800937p
- Pattan, S.R.; Kekare, P.; Dighe, N.S.; Bhawar, S.B.; Nikalje, A.; Pati, A.; Hole, M.B. Asian J. Research Chem. 2009, 2, 123–126.
- Sonawane, L.V.; Bari, S.B. Int. J. Biol. Chem. 2011, 5, 68–74. 10.3923/ijbc.2011.68.74
- Bruno, G.; Costantino, L.; Curinga, C.; Maccari, R.; Monforte, F.; Nicolo, F.; Ottana, R.; Vigorita, M.G. Bioorg. Med. Chem. 2002, 10, 1077–1084. 10.1016/S0968-0896(01)00366-2
- Zidar, N.; Tomasic, T.; Sink, R.; Rupnik, V.; Kovac, A.; Turk, S.; Patin, D.; Blanot, D.; Martel, C.C.; Dessen, A.; Premru, M.M.; Zega, A.; Gobec, S.; Masic, L.P.; Kikelj, D. J. Med. Chem. 2010, 53, 6584–6594. 10.1021/jm100285g
- Cantello, B.C.C.; Cawthorne, M.A.; Cottam, G.P.; Duff, P.T.; Haigh, D.; Hindley, R.M.; Lister, C.A.; Smith, S.A; Thurlby, P.L. J. Med. Chem. 1994, 37, 3977–3985. 10.1021/jm00049a017
- Mahalle, S.; Ligampalle, D.; Mane, R. Heteroatom Chem. 2009, 20, 151–156. 10.1002/hc.20528
- Wu, Y.; Karna, S.; Choi, C.H.; Tong, M.; Tai, H.H.; Na, D.H.; Jang, C.H.; Cho, H. J. Med. Chem. 2011, 54, 5260–5264. 10.1021/jm200390u
- Chowdhy, M.M.; Michael, D.; Mingas, P.; White, A.J.P.; William, D.J. J. Chem. Soc., Perkin Trans. 1, 2000, 3495–3504. 10.1039/b004312p
- Metwally, N.H.; Rateb, N.M.; Zohdi, H.F. Green Chem. Lett. Rev. 2011, 4, 225–228. 10.1080/17518253.2010.544330
- Shelke, K.F.; Sapkal, S.B.; Kakade, G.K.; Sadaphal, S.A.; Shingate, B.B.; Shingare, M.S. Green Chem. Lett. Rev. 2010, 3, 17–21. 10.1080/17518250903478345
- Pratap, U.R.; Jawale, D.V.; Waghmare, R.A.; Lingampalle, D.L.; Mane, R.A. New J. Chem. 2011, 35, 49–51. 10.1039/c0nj00691b
- Yang, D.H.; Yang, B.Y.; Chen, B.C.; Chen, S.Y. Org. Prep. Proced. Int. 2006, 38, 81–85. 10.1080/00304940609355982
- Yang, B.Y.; Yang, D.H. J. Chem. Res. 2011, 35, 238–239. 10.3184/174751911X13025104502362
- Mahalle, S.R.; Netankar, P.D.; Bondge, S.P.; Mane, R.A. Green Chem. Lett. Rev. 2008, 1, 103–106. 10.1080/17518250802139881
- Kumar, D.; Narwal, S.; Sandhu, J.S. Int. J. Med. Chem. 2013, 4.
- Shi, F.; Gu, Y.; Zhang, Q.; Deng, Y. Catal. Surv. Asia 2004, 8, 179–186. 10.1023/B:CATS.0000038536.55980.f3
- Suresh; Sandhu, J.S. Green Chem. Lett. Rev. 2011, 4, 289–310. 10.1080/17518253.2011.572294
- Suresh; Sandhu, J.S. Green Chem. Lett. Rev. 2011, 4, 311–320. 10.1080/17518253.2011.572295
- Shelke, K.F.; Sapkal, S.B.; Madje, B.R.; Shingate, B.B.; Shingare, M.S. Bull. Catal. Soc. India 2009, 8, 30–34.
- Jawale, D.V.; Pratap, U.R.; Lingampalle, D.L.; Mane, R.A. Chin. J. Chem. 2011, 29, 942–946. 10.1002/cjoc.201190192
- Tanaka, K.; Toda, F. Chem. Rev. 2000, 100, 1025–1074. 10.1021/cr940089p
- Cota, I.; Gonzalez-Olmos, R.; Iglesias, M.; Medina, F. J. Phys. Chem. B 2007, 111, 12468–12477. 10.1021/jp073963u
- Bicak, N. J. Mole. Liq. 2005, 116, 15–18. 10.1016/j.molliq.2004.03.006