Abstract
Fe3O4@SiO2/Schiff base complex of metal ions catalyzed the reaction between phenylene-1,2-diamines and 1,2-diketones to produce quinoxalines in aqueous media at room temperature. This eco-friendly method provides several advantages such as mild reaction conditions, good to excellent yields, simple work-up, and nanocatalyst stability. Also, nanocatalyst can be simply recovered by a magnetic field and reused for at least five successive reactions.
1. Introduction
The synthesis of quinoxaline derivatives has been of considerable interest as they possess various biological activities, such as antibacterial (Citation1), anthelmintic (Citation2), antitumor (Citation3), antifungal (Citation1), antidepressant (Citation4), and kinase inhibition agents (Citation5). In addition, quinoxalines have been used for the preparation of various dyes (Citation6) and key intermediates in the synthesis of organic semiconductors (Citation7), dehydro annulenes (Citation8), chemically controllable switches (Citation9), and used in the agricultural field as fungicides, herbicides, insecticides (Citation2). They are also used as anti-inflammatory, anti-protozoal, and anti-HIV (Citation10). The importance and utility of quinoxaline derivatives have led to the development of numerous synthetic routes. The condensation of 1,2-diamines with 1,2-diketones in refluxing ethanol or acetic acid has been used as a useful synthetic route toward quinoxalines (Citation11). For this transformation, several catalysts and reagents have been reported, including a oxidative coupling method (Citation12), ceric(IV) ammonium nitrate (Citation13), sulfamic acid (Citation14), a microwave procedure (Citation15), zeolite (Citation16), POCl3 (Citation17), H6P2W18O62·24H2O (Citation18), CuSO4·5H2O (Citation19), LiBr (Citation20), (NH4)6Mo7O24·4H2O (Citation21), montmorillonite K-10 (Citation22), Zn[L-proline] (Citation23), RuCl2-(PPh3)3-TEMPO (Citation24), polyaniline-sulfate salt (Citation25), Yb(OTf)3 (Citation26), zirconium tetrakis(dodecyl sulfate) (Citation27), o-iodoxybenzoic acid (Citation28), MnO2 (Citation29), ZrO2/Ga2O3/MCM-41 (Citation30), ammonium chloride (Citation31), SbCl3/SiO2 (Citation32), zirconium tetrachloride (Citation33), SnCl2/SiO2 (Citation34), gallium triflate (Citation35), and (NH4)H2PW12O40 (Citation36). However, these methods have many disadvantages, such as low yields, long reaction times, the use of toxic solvents, and harsh reaction conditions. Thus, the development of a new catalyst for the synthesis of quinoxaline derivatives would be highly desirable.
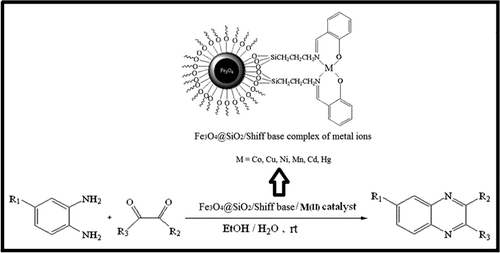
Recently, magnetic core-shell nanostructures have attracted more attention due to their unique magnetic properties. In contrast to most of solid catalysts that their recovery and reusability are very difficult, recovery and thus reusing of core-shell nanostructure magnetic catalysts can be easily carried out under external magnetic field. Therefore, the magnetic core-shell structure composites for catalysis are very well supported (Citation37–39). As part of our program in the development of expedient methods for the synthesis of heterocyclic and biological important compounds, herein, we report the first use of magnetic nanoparticles Fe3O4@SiO2/Schiff base complex of metal ions for the synthesis of quinoxalines under mild reaction conditions ().
2. Results and discussion
In order to optimize the reaction parameters, the reaction between o-phenylenediamine (1 mmol) and benzil (1 mmol) was performed in the presence of 0.03 g Fe3O4@SiO2/Schiff base/Co(II) nanocatalyst at room temperature in the different solvents, such as acetonitrile, chloroform (CDCl3), dichloromethane, ethyl acetate, water, ethanol, methanol, and the different mixture of EtOH/H2O (3/1, v/v). According to the presented results in , the best yield of 2,3-diphenylquinoxaline was formed in the mixture of EtOH/H2O (3/1; , entry 10).
Table 1. Optimization of reaction conditions in the synthesis of 2,3-diphenyl quinoxaline catalyzed by Fe3O4@SiO2/Schiff base complex of Co (II).
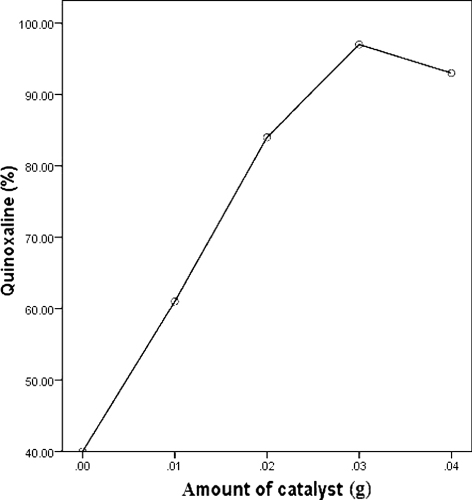
The reaction was also studied in the presence of different amount of the catalyst in EtOH/H2O (3/1, v/v), but in the all of investigated cases, the yields did not improve even in higher reaction times (, entries 11–14 and ). In order to elucidate the role of the nanocatalyst, a control reaction was conducted using benzil and o-phenylenediamine in the absence of nanocatalyst. Quinoxaline was formed after 12 h with a 40% yield (, entry 14).
In order to study the catalytic activity of metal ions, we examined the condensation of benzil with 1,2-phenylenediamines to evaluate the efficiency of Fe3O4@SiO2/Schiff base complex of metal ions as catalyst ().
Table 2. The effect of metal ions on the preparation of 2,3-diphenylquinoxaline after 40 min.
The catalytic activity of various Lewis acids was found to be of the order Co(II)>Cu(II)>Ni(II)>Mn(II)>Cd(II)>Hg(II) and indicate that the nanocatalyst performance is better for Fe3O4@SiO2/Schiff base complex of Co(II).
To recognize the generality and the scope of our method, various aromatic and aliphatic 1,2-diamines were reacted with benzil in the presence of Fe3O4@SiO2/Schiff base/Co(II) (0.03 g) at room temperature (). All the reactions with substituted 1,2-phenylenediamines proceeded very cleanly and no undesirable side reactions were observed. Results in show that electron-donating groups at the phenyl ring of 1,2-diamine favored the formation of product (, entries 7–11). However, aliphatic 1,2-diamines afforded the corresponding quinoxaline derivatives in slightly lower yields and longer reaction times (, entries 20 and 21). In contrast, electron-withdrawing groups such as nitro, benzoyl, and chloro gave slightly lower yields (, entries 12–18). Substrate bearing a strong electron-withdrawing NO2 group gave lower yield even after longer reaction times (, entries 13–15). On the other hand, electron-donating substituents associated with aromatic 1,2-diketone decreased the product yields and the effect is contrary with electron-withdrawing groups (, entries 2, 8, 15, and 17). Therefore, our results demonstrate that Fe3O4@SiO2/Schiff base complex of Co(II) is a very effective nanocatalyst for the one-pot condensations of o-phenylenediamine derivatives and α-diketones to form quinoxalines in excellent yields.
Table 3. Synthesis of quinoxaline derivatives catalyzed by Fe3O4@SiO2/Schiff base complex of Co(II) at room temperaturea
Comparison of the efficiency of Fe3O4@SiO2/Schiff base/Co(II) in the formation of 6-nitro-2,3-diphenylquinoxaline from the reaction of 4-nitrobenzene-1,2-diamine and benzil with those of several reported acid catalyst in the literature, indicates that this reaction is completed, in the most cases, in shorter time with higher yield in green media with simple work-up ().
Table 4. Literature results for the synthesis of 6-nitro-2,3-diphenylquinoxaline at room temperature.
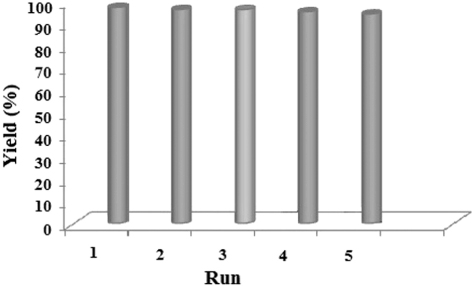
Recycling of catalyst and reducing the amount of catalyst are important aspects of green chemistry. According to this topic, reusability of nanocatalyst was tested in the reaction of benzil with o-phenylenediamine in EtOH/H2O (3/1, v/v). After five times recycling, the nanocatalyst showed no significant change on reactivity and yields slightly decreased with increasing number of cycles of the reaction (97%, 96%, 96%, 95%, and 94%; ).
3. Experimental
3.1. General
Solvents, reagents, and chemicals were obtained from Merck (Germany) and Fluka (Switzerland) chemical companies in high purity. The NMR spectra were recorded on a Bruker Avance DPX-250 MHz spectrometer in CDCl3 using tetramethylsilane as an internal reference. Melting points were determined by Buchi Melting Point B-545 electrical melting point apparatus and quantitative elemental analysis (CHN) were obtained by reported on a Flash EA instrument. The quinoxaline derivatives were characterized by their melting points and comparison with literature values.
3.2. General procedure for Schiff base complex of metal ions functionalized magnetite and silica nanoparticles
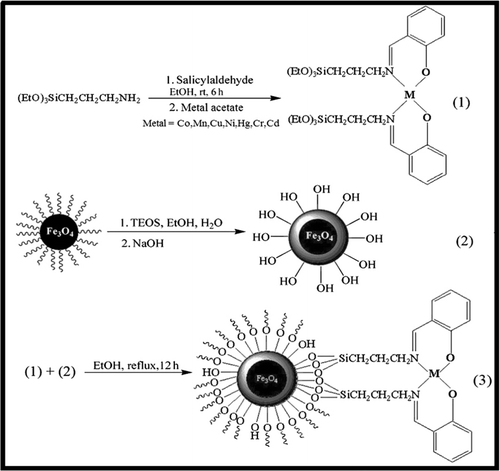
According to a modified Stober method (Citation40), to the solution of 3-aminopropyl (trimethoxy) silane (1 mmol, 0.176 g) in 25 mL ethanol was added dropwise the stoichiometric amount of salicylaldehyde (1 mmol, 0.122 g) in ethanol (25 mL). The yellow obtained solution, due to imine formation, was stirred at room temperature for 6 h. The resulting Schiff base ligand, as the bright yellow precipitate, was separated by filtration and washed with ethanol (5 mL), and then dried in vacuum. The crude product was recrystallized from ethanol to obtain the pure product in 98% yield (0.271 g). Then, to the solution of the Schiff base ligand (2 mmol) in ethanol (25 mL) was added metal acetates (1 mmol) and the mixture was refluxed and the progress of the reaction was monitored by thin layer chromatography (TLC). After completion of the complex formation, the crude product as a colored solid was filtered and washed with ethanol (2 mL × 5 mL). Then the pure product was obtained by recrystallization from ethanol. Then, Fe3O4@SiO2 (2 g) was added to the solution of Schiff complex of metal ions (1 mmol) in ethanol (10 mL) and the resultant mixture was under reflux for 12 h. The solvent was removed and the resulting solid was dried at 80°C overnight. The product was washed with ethanol and water to remove unreacted species and dried at 80°C for 6 h ().
3.3. General procedure for the preparation of quinoxalines
To a mixture of 1,2-diamine (1 mmol), 1,2-diketone (1 mmol), and 0.03 g of Fe3O4@SiO2/Schiff base/Co(II) in a 50 mL round-bottomed flask, EtOH/H2O [12 mL, 3/1 (v/v)] was added, and the mixture was stirred at room temperature. The progress of the reaction was monitored by TLC. After completion of the reaction, ethyl acetate was added to the mixture and the insoluble catalyst was separated by magnetic field. The filtrate was dried on anhydrous sodium sulfate and concentrated with a rotary evaporator under reduced pressure to give the pure product.
3.4. Spectral data of new compounds
Yellow oil; 1H-NMR (250 MHz, CDCl3): δ = 1.45 (t, 6H, CH3), 3.07 (q, 4H, CH2), 7.33–7.68 (m, 2H, CH), 7.97–8.01(m, 2H, CH). 13C-NMR (63 MHz, CDCl3): δ = 12.6, 28.4, 128.5, 128.6, 141.0, 157.3. Anal. Found (%): C, 76.82; H, 7.41; N, 14.93. Calc. for C12H14N2 (%): C, 77.47; H, 7.52; N, 15.05 (, entry 3).
Pale yellow oil, 1H-NMR (250 MHz, CDCl3): δ = 1.37 (t, 6H, CH3), 2.55 (s, 3H, CH3), 3.06 (m, 4H, CH2), 7.49 (d, 1H, CH), 7.78 (s, 1H, CH), 7.96 (d, 1H, CH). 13C-NMR (63 MHz, CDCl3): δ = 12.2, 12.3, 21.3, 27.9, 27.9, 127.0, 127.6, 130.5, 138.5, 155.9, 156.7. Anal. Found (%): C, 77.32; H, 7.85; N, 13.78. Calc. for C13H16N2 (%): C, 78.01; H, 7.99; N, 13.99 (, entry 10).
Acknowledgment
Authors gratefully acknowledge the financial support of this work by the research council of University of Shiraz.
References
- Badran, M.M.; Moneer, A.A.; Refaat, H.M.; El-Malah, A.A. J. Chin. Chem. Soc. 2007, 54, 469–478.
- Sakata, G.; Makino, K.; Karasawa, Y. Heterocycles. 1988, 27, 2481–2515.10.3987/REV-88-397
- Hazeldine, S.T.; Polin, L.; Kushner, J.; White, K.; Bouregeois, N.M.; Crantz, B.; Palomino, E.; Corbett, T.H.; Horwitz, J.P. J. Med. Chem. 2002, 45, 3130–3137.10.1021/jm0200097
- Sarges, R.; Howard, H.; Browne, R.R.C.; Label, L.A.; Seymour, P.A. J. Med. Chem. 1990, 33, 2240–2254.10.1021/jm00170a031
- Kim, Y.B.; Kim, Y.H.; Park, J.Y.; Kim, S.K. Bioorg. Med. Chem. Lett. 2004, 14, 541–544.10.1016/j.bmcl.2003.09.086
- Brock, E.D.; Lewis, D.M.; Yousaf, T.I.; Harper, H.H. The Procter and Gamble Company, USA. World Patent WO9951688, 1990.
- Dailey, S.; Feast, J.W.; Peace, R.J.; Sage, I.C.; Till, S.; Wood, E.L. J. Mater. Chem. 2001, 11, 2238–2243.10.1039/b104674h
- Sascha, O.; Rudiger, F. Synlett. 2004, 6, 1509–1512.
- Crossley, M.J.; Johnston, L.A. Chem. Commun. 2002, 9, 1122–1123.10.1039/b111655j
- Hui, X.; Desrivot, J.; Bories, C.; Loiseau, P.M.; Franck, X.; Hocquemiller, R.; Fidadere, B. Bioorg. Med. Chem. Lett. 2006, 16, 815–820.10.1016/j.bmcl.2005.11.025
- Brown, D.J. Quinoxalines Supplement II, the Chemistry of Heterocyclic Compounds; Wiley: Hoboken, NJ, 2004; pp 1–92.
- Antoniotti, S.; Donach, E. Tetrahedron. Lett. 2002, 43, 3971–3973.10.1016/S0040-4039(02)00715-3
- More, S.V.; Sastry, M.N.V.; Yao, C.F. Green. Chem. 2006, 8, 91–95.10.1039/b510677j
- Darabi, H.R.; Mohandessi, S.; Aghapoor, K.; Mohsenzadeh, F. Catal. Commun. 2007, 8, 389–392.10.1016/j.catcom.2006.06.033
- Zhao, Z.; Wisnoski, D.D.; Wolkenberg, S.E.; Leister, W.H.; Wang, Y.; Lindsley, C.W. Tetrahedron. Lett. 2004, 45, 4873–4876.10.1016/j.tetlet.2004.04.144
- Venugopal, D.; Subrahmanyam, M. Catal. Commun. 2001, 2, 219–224.10.1016/S1566-7367(01)00037-1
- Venkatesh, C.; Singh, B.; Mahata, P.K.; Junjappa, H. Org. Lett. 2005, 7, 2169–2172.10.1021/ol0505095
- Heravi, M.M.; Bakhtiari, K.; Bamoharram, F.F.; Tehrani, M.H. Monatsh. Chem. 2007, 138, 465–467.10.1007/s00706-007-0594-5
- Heravi, M.M.; Tahe, S.; Bakhtiari, K.; Oskooie, H.A. Catal. Commun. 2007, 8, 211–214.10.1016/j.catcom.2006.06.013
- Hasaninejad, A.; Zare, A.; Mohammadizadeh, M.R.; Shekouhy, M. Green. Chem. Lett. Rev. 2010, 3, 143–148.10.1080/17518251003619192
- Hasaninejad, A.; Zare, A.; Mohammadizadeh, M.R.; Karami, Z. J. Iran. Chem. Soc. 2009, 6, 153–158.10.1007/BF03246514
- Huang, T.; Wang, R.; Shi, L.; Lu, X. Catal. Commn. 2008, 9, 1143–1147.10.1016/j.catcom.2007.10.024
- Heravi, M.M.; Tehrani, M.H.; Bakhtiari, K.; Oskooie, H.A. Catal. Commun. 2007, 8, 1341–1344.10.1016/j.catcom.2006.11.026
- Robinson, R.S.; Taylor, R.J.K. Synlett. 2005, 6, 1003–1005.
- Srinivas, C.; Kumar, C.N.S.S.P.; Rao, V.J.; Palaniappan, S. J. Mol. Catal. A: Chem. 2007, 265, 227–230.10.1016/j.molcata.2006.10.018
- Wang, L.; Liu, J.; Tian, H.; Qian, C. Synth. Commun. 2004, 34, 1349–1357.10.1081/SCC-120030683
- Hasaninejad, A.; Zare, A.; Zolfigol, M.A.; Shekouhy, M. Synth. Commun. 2009, 39, 569–579.10.1080/00397910802406737
- Heravi, M.M.; Bakhtiari, K.; Tehrani, M.H.; Javadi, N.M.; Oskooie, H.A. ARKIVOC. 2006, xvi, 16–22.10.3998/ark.5550190.0007.g02
- Raw, S.A.; Wilfred, C.D.; Taylor, R.J.K. Org. Biomol. Chem. 2004, 2, 788–796.10.1039/b315689c
- Ajaikumar, S.; Pandurangan, A. Appl. Catal. A: Gen. 2009, 357, 184–192.10.1016/j.apcata.2009.01.021
- Darabi, H.R.; Tahoori, F.; Aghapoor, K.; Taala, F.; Mohsenzadeh, F. J. Braz. Chem. Soc. 2008, 1646–1652.10.1590/S0103-50532008000800028
- Darabi, H.R.; Aghapoor, K.; Mohsenzadeh, F.; Taala, F.; Asadollahnejad, N.; Badiei, A. Catal. Lett. 2009, 133, 84–89.10.1007/s10562-009-0161-2
- Aghapoor, K.; Darabi, H.R.; Mohsenzadeh, F.; Balavar, Y.; Daneshyar, H. Transit. Metal. Chem. 2010, 35, 49–53.10.1007/s11243-009-9294-9
- Darabi, H.R.; Aghapoor, K.; Mohsenzadeh, F.; Jalali, M.R.; Talebian, Sh.; Ebadi-Nia, L.; Khatemifar, E.; Aghaee, A. Bull. Korean. Chem. Soc. 2011, 32, 213–218.10.5012/bkcs.2011.32.1.213
- Cai, J.J.; Zou, J.P.; Pan, X.Q.; Zhang, W. Tetrahedron. Lett. 2008, 49, 7386–7390.10.1016/j.tetlet.2008.10.058
- KunKuma, V.; Prabhavathi Devi, B.L.A.; Giri, B.Y.; Prasad, R.B.; Sai Prasad, P.S. Eur. J. Chem. 2011, 2, 495–498.10.5155/eurjchem.2.4.495-498.413
- Deng, Y.H.; Qi, D.W.; Deng, C.H.; Zhang, X.M.; Zhao, D.Y. J. Am. Chem. Soc. 2008, 130, 28–29.10.1021/ja0777584
- Xu, X.Q.; Deng, C.H.; Gao, M.X.; Yu, W.J.; Yang, P.Y.; Zhang, X.M. Adv. Mater. 2006, 18, 3289–3293.10.1002/adma.200601546
- Shao, D.D.; Xu, K.K.; Song, X.J.; Hu, J.H.; Yang, W.L.; Wang, C.C. J. Colloid. Interface. Sci. 2009, 336, 526–532.10.1016/j.jcis.2009.02.061
- Esmaeilpour, M.; Sardarian, A.R.; Javidi, J. Appl. Catal. A. Gen. 2012, 445–446, 359–367.10.1016/j.apcata.2012.09.010
- Aly, M.M.; Al-Shatti, N.I. Transition. Met. Chem. 1998, 23, 361–369.10.1023/A:1006985824030
- Mason, T.J. Chem. Soc. Trans. 1893, 63, 1284–1293.10.1039/ct8936301284
- Dobrodei, A.N.; El'tsov, A.V. J. Gen. Chem. 1999, 69, 1658–1668.
- Ghsoh, B.; Mukherjee, S.; Jha, S. Synthesis. 1985, 3, 313–321.
- Niknam, K.H.; Saberi, D.; Mohagheghnejad, M. Molecules. 2009, 14, 1915–1926.10.3390/molecules14051915