ABSTRACT
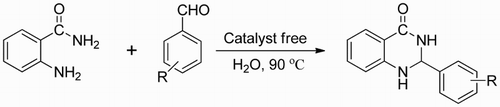
A simple and environmentally benign method is described for the synthesis of 2,3-dihydroquinazolin-4(1H)-ones by direct cyclocondensation of 2-aminobenzamide with aromatic aldehydes using water as the reaction medium. The parameters such as temperature, substrate molar ratio and reaction time were examined to establish the optimal synthetic process. The present procedure has advantages of low cost, mild reaction conditions, simple workup process, simply purification, excellent yields and no use of catalyst and hazardous organic solvents.
GRAPHICAL ABSTRACT
Introduction
2,3-Dihydroquinazolin-4(1H)-one derivatives belong to an important class of nitrogen heterocycles ( Citation1) that attracted much attention because these quinazolinone scaffolds as core unit for a wide spectrum of nature plant alkaloids and pharmacological compounds such as evodiamine, febrifugine, isofebrifugine, metolazone, quinethazone, afloqualone, raltitrexed and nolatrexed. Moreover, these derivatives exhibit various potential biological and pharmaceutical activities including anticonvulsant ( Citation2), antiparkinsonian ( Citation3), antihypertensive ( Citation4), antibacterial ( Citation5), anticancer ( Citation6), diuretic ( Citation7), as well as monoamine oxidase inhibition ( Citation8) and plant growth regulation ( Citation9). As intermediates, 2,3-dihydroquinazolin-4(1H)-ones can easily be oxidized to their quinazolin-4(3H)-one analogues ( Citation10, Citation11), which also include important biological active compounds, and are significant privileged structures in pharmaceutical synthesis ( Citation12).
Based on the importance of those compounds numerous classical synthetic methods have been described for the synthesis of 2,3-dihydroquinazolin-4(1H)-ones, and one of the simplest and direct methods is the cyclocondensation of 2-aminobenzamide with aromatic aldehydes using various acids as catalyst, such as CuCl2 ( Citation10), ZrCl4 ( Citation13), TiCl4/Zn ( Citation14), NH4Cl ( Citation15), trichloroacetic acid ( Citation16), succinimide-N-sulfonic acid ( Citation17), 2-morpholinoethanesulfonic acid ( Citation18), p-TSA ( Citation19). In recent years, widely recyclable catalysts, such as ionic liquids ( Citation20–23), metal-CNTs ( Citation24, Citation25), nanocrystalline sulfated zirconia ( Citation26) and PEG-400 ( Citation27) have also been used for the preparation of these derivatives on account of the growing demand for the development of more sustainable and environmental-friendly compounds. However these methodologies involve at least one of the following imperfections: environmentally harmful solvent, expensive reagent, sensitive catalyst, a cumbersome preparation process for the required catalyst, tedious workup conditions or liberating hazardous HF during recycling.
In addition, the potential of water as a solvent has become highly interested in devising a method for organic synthesis, not only because of water's economic and environmental characteristics ( Citation28), but also due to its unique reactivity and selectivity ( Citation29). Moreover, water as a solvent in many reactions can enhance rates of reaction and facilitate product recovery ( Citation30, Citation31). Furthermore, water has advantages of non-volatility, nontoxicity and perfect safety compared with organic solvents. However, as an environmental-friendly and economically affordable reaction medium, water has been rarely used in the procedures preparing 2,3-dihydroquinazolin-4(1H)-one derivatives via direct condensation of 2-aminobenzamide with aldehydes.
Thus, as part of our continuing research work in green chemistry and enzyme promiscuity, we are interested in developing an efficient biocatalytic route for the synthesis of 2,3-dihydroquinazolin-4(1H)-ones. It was discovered unexpectedly that the cyclization reaction of 2-aminobenzamide and 4-methylbenzaldehyde could be performed in water under catalyst-free conditions.
Experimental
All reagents were obtained from commercial suppliers and used without further purification unless otherwise noted. Melting points were obtained on a WRS-1B Digital Melting Point Apparatus. The NMR spectra were recorded on a Bruker 400 MHz instrument using DMSO-d6 as the solvent. Chemical shifts (δ) were expressed in ppm with tetramethylsilane as the internal standard, and coupling constants (J) were reported in Hz.
A mixture of 2-aminobenzamide (1 mmol) and aldehyde (1.5 mmol) in 10 mL of water was stirred in a round bottomed flask at 90°C in a preheated oil bath as shown in Scheme 1. After completion of the reaction, the mixture was cooled to room temperature, and the water-insoluble desired product was precipitated. The crude products were obtained directly by filtration and washed with 50% cold ethanol. Finally, the solid product was recrystallized from EtOH (80%).
Results and discussion
Based on the above results, 2-aminobenzamide and 4-methylbenzaldehyde were selected as the model compounds to optimize the reaction conditions. Initially, the model reaction was performed at different temperatures in order to achieve the desired yield at the lowest possible temperature. As shown in , the yield of the product increased accordingly with the temperature below 90°C, and the yield of 71% gained at 90°C was remarkably higher than that of other temperatures. And the yield dramatically decreased when the temperature was further increased to the reflux temperature, which was probably because of the more side reactions at excessively high temperature. Consequently, 90°C was selected as the optimal temperature for the follow-up studies. Next, the effects of the molar ratio of reactants on the yield were investigated, and a better result was obtained when the molar ratio of 2-aminobenzamide to 4-methylbenzaldehyde was 1:1.5 ().
Table 1. The effect of temperature on the model reaction.a
Table 2. The effect of molar ratio on the model reaction.a
With the optimized reaction conditions in hand, some other reactants were used to expand this catalyst-free cyclocondensation reaction to show the generality of the green procedure for the synthesis of 2,3-dihydroquinazolin-4(1H)-ones. The results are summarized in , various benzaldehyde derivatives with electron-donating or electron-withdrawing groups reacted with 2-aminobenzamide under the optimized reaction conditions. In general, the reaction could proceed smoothly and was not influenced by substituents of the aromatic ring, and the corresponding products of 2,3-dihydroquinazolin-4(1H)-ones were obtained in good to excellent yields (67–92%). In fact, it was difficult to dissolve the reactants in the reaction medium, namely, water, so adequate stirring was necessary for a better yield. However, the reaction was not successful when caproaldehyde (or hypnone) was employed instead of aldehyde under the optimized conditions (data not shown).
Table 3. Investigation of the reactant scope of the cyclocondensation reaction for synthesis of 2,3-dihydroquinazolin-4(1H)-one derivatives.a
Conclusions
In conclusion, a simple, green and catalyst-free synthetic method has been described for the synthesis of 2,3-dihydroquinazolin-4(1H)-ones via the cyclocondensation of 2-aminobenzamide with aromatic aldehydes in water. Simplified operational process, easy post-treatment process and excellent yields are the remarkable advantages of this synthetic strategy. More importantly, this reaction could be performed efficiently under catalyst-free conditions using water as the reaction medium. To the best of our knowledge, this is the first example for the synthesis 2,3-dihydroquinazolin-4(1H)-ones under catalyst-free conditions and in water.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplemental data and research materials
Supplemental data for this article can be accessed at the Taylor & Francis website, http://dx.doi.org/10.1080/17518253.2015.1109145.
Supplemental_Material.doc
Download MS Word (2.2 MB)Additional information
Funding
References
- Erlanson, D.A.; McDowell, R.S.; O'Brien, T. J. Med. Chem. 2004, 47, 3463–3482.
- Obniska, J.; Kami-Ski, K. Acta Pol. Pharm. 2006, 63, 101–108.
- Gangwal, N.A.; Kothawade, U.R.; Galande, A.D.; Pharande, D.S.; Dhake, A.S. Indian J. Heterocycl. Chem. 2001, 10, 291–294.
- Kumar, A.; Tyagi, M.; Srivastava, V.K. Indian J. Chem. Sect. 2003, 42, 2142–2145.
- Kung, P.-P.; Casper, M.D.; Cook, K.L.; Wilson-Lingardo, L.; Risen, L.M.; Vickers, T.A.; Ranken, R.; Blyn, L.B.; Wyatt, J.R.; Cook, P.D.; Ecker, D.J. J. Med. Chem. 1999, 42, 4705–4713.
- Hour, M.-J.; Huang, L.-J.; Kuo, S.-C.; Xia, Y.; Bastow, K.; Nakanishi, Y.; Hamel, E.; Lee, K.-H. J. Med. Chem. 2000, 43, 4479–4487.
- Harlie, A. Parish, J.; Gilliom, R.D. J. Med. Chem. 1982, 25, 98–102.
- Gupta, R.C.; Nath, R.; Shanker, K.; Bhargava, K.P.; Kishore, K. J. Indian Chem. Soc. 1979, 56, 219–220.
- Hamel, E.; Lin, C.M.; Plowman, J.; Wang, H.-K.; Lee, H.-H.; Pad, K.D. Biochem. Pharmacol. 1996, 51, 53–59.
- Abdel-Jalil, R.J.; Voelter, W.; Saeed, M. Tetrahedron Lett. 2004, 45, 3475–3476.
- Romero, A.H.; Salazar, J.; López, S.E. Synthesis. 2013, 45, 2043–2050.
- Liu, J.-F.; Lee, J.; Dalton, A.M.; Bi, G.; Yu, L.; Baldino, C.M.; McElory, E.; Brown, M. Tetrahedron Lett. 2005, 46, 1241–1244.
- Abdollahi-Alibeik, M.; Shabani, E. Chin. Chem. Lett. 2011, 22, 1163–1166.
- Shi, D.; Rong, L.; Wang, J.; Zhuang, Q.; Wang, X.; Hu, H. Tetrahedron Lett. 2003, 44, 3199–3201.
- Shaabani, A.; Maleki, A.; Mofakham, H. Synth. Commun. 2008, 38, 3751–3759.
- Karimi-Jaberi, Z.; Zarei, L. Acta Chim. Slov. 2013, 60, 178–183.
- Ghashang, M.; Mansoor, S.S.; Aswin, K. Res. Chem. Intermed. 2013, 41, 1–14.
- Labade, V.B.; Shinde, P.V.; Shingare, M.S. Tetrahedron Lett. 2013, 54, 5778–5780.
- Xua, B.L.; Chen, J.P.; Qiao, R.Z.; Fu, D.C. Chin. Chem. Lett. 2008, 19, 537–540.
- Davoodnia, A.; Allameh, S.; Fakhari, A.R.; Tavakoli-Hoseini, N. Chin. Chem. Lett. 2010, 21, 550–553.
- Chen, J.; Su, W.; Wu, H.; Liu, M.; Jin, C. Green Chem. 2007, 9, 972–975.
- Shaterian, H.R.; Aghakhanizadeh, M. Res. Chem. Intermediat. 2013, 40, 1655–1668.
- Wang, J.; Zong, Y.; Fu, R.; Niu, Y.; Yue, G.; Quan, Z.; Wang, X.; Pan, Y. Ultrason. Sonochem. 2014, 21, 29–34.
- Safari, J.; Gandomi-Ravandi, S. J. Mol. Catal. A:Chem. 2014, 390, 1–6.
- Safari, J.; Gandomi-Ravandi, S. C. R. Chim. 2013, 16, 1158–1164.
- Abdollahi-Alibeik, M.; Shabani, E. J. Indian Chem. Soc. 2013, 11, 351–359.
- Parthasaradhi, Y.; Rakhi, C.; Suresh, S.; Tangenda, S.J. Eur. J. Med. Chem. 2013, 4, 462–466.
- Li, C.-J. Chem. Rev. 2005, 105, 3095–3166.
- Jiang, B.; Cao, L.-J.; Tu, S.-J.; Zheng, W.-R.; Yu, H.-Z. J. Comb. Chem. 2009, 11, 612–616.
- Otto, S.; Engberts, J.B.F.N. Org. Biomol. Chem. 2003, 1, 2809–2820.
- Pratt, L.R.; Pohorille, A. Chem. Rev. 2002, 102, 2671–2692.
- Safaei, H.R.; Shekouhy, M.; Shafiee, V.; Davoodi, M. J. Mol. Liq. 2013, 180, 139–144.
- Paramasivam Sivaguru, K.P.; Madheswaran Kiruthiga, P.V.; Lalitha, A. J. Iran. Chem. Soc. 2015, 12, 95–100.