ABSTRACT
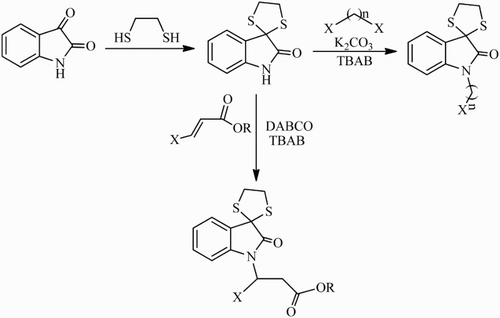
A new series of spiro[[1,3]dithiolane-2,3′-indolines]-2′-one derivatives was synthesized using Michael addition reaction of spiro[[1,3]dithiolane-2,3′-indolin]-2′-one to various α,β-unsaturated esters as well as direct alkylation of this compound with alkyl dihalides. Michael reaction of spiro[[1,3]dithiolane-2,3′-indolin]-2′-one and α,β-unsaturated esters produced related Michael adduct in the presence of tetrabutylammonium bromide and base 1,4-diazabicyclo[2.2.2]octane in good to excellent yields at 80°C under solvent-free conditions. Also, direct alkylation of spiro[[1,3]dithiolane-2,3′-indolin]-2′-one by dihaloalkanes in the presence of base K2CO3 afforded the corresponding products in good yields under the same conditions.
Introduction
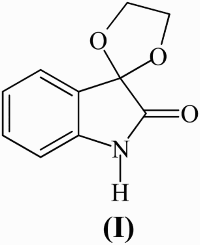
Isatin (1-H indole 2–3 dione) is synthetically a versatile molecule that is employed for the synthesis of a large variety of heterocyclic compounds ( Citation1). Isatin and its derivatives have diversified biological activities, for example, antibacterial ( Citation2), anticonvulsant ( Citation3), antiviral ( Citation4), and antitubercular ( Citation5) activities. Some of the spiro derivatives of isatin are important pharmacophores and exhibit promising biological activities, such as analgesic, fungicidal, and anti-inflammatory activities ( Citation6). Isatin ketals have anxiolytic ( Citation7), psychotropic ( Citation8), and anticonvulsant activities ( Citation9). For instance, spiro[1,3-dioxolane-2,3′-indol]-2′(1′H)-one (I) has sedative, hypnotic, and anesthetic properties ( Citation10).
In contrast to the many examples of the synthesis of different isatin derivative systems, there are only a few reports with regard to the synthesis of isatin thioketal systems. It is surprising that there are no reports on the alkylation of spiro[[1,3]dithiolane-2,3′-indolin]-2′-one 1 to α,β-unsaturated esters by Michael addition reaction or direct alkylation to dihaloalkanes. Herein, in continuation of our interest in the design and discovery of new aza-Michael addition of amides and imides to α,β-unsaturated esters ( Citation11–13), we report the aza-Michael addition of spiro[[1,3]dithiolane-2,3′-indolin]-2′-one to α,β-unsaturated esters and also direct alkylation of this compound by dihaloalkanes under solvent-free conditions ().
Experimental section
Spiro[[1,3]dithiolane-2,3′-indolin]-2′-one and α,β-unsaturated esters were synthesized in our laboratory according to the literature procedures ( Citation10,Citation14). Dihaloalkanes were purchased from Merck and used without further purification. Esters were transferred via syringe. Organic solvents were removed by a rotary evaporator. Structure of the compounds were confirmed by IR, 1H NMR and 13C NMR spectroscopies. The progress of the reactions was followed by TLC using silica-gel SILIG/UV 254 plates. 1H NMR (400 MHz) and 13C NMR (100 MHz) spectra were recorded on a Bruker 400 MHz instrument. FT-IR spectra were recorded on a Perkin-Elmer RX-1 instrument. Elemental analysis for C, H, and N were performed using a Heraeus CHN-O-Rapid analyzer. The melting points were determined in open capillaries with a Stuart Melting Point Apparatus and are uncorrected.
General procedure for the addition of spiro[[1,3]dithiolane-2,3′-indolin]-2′-one to α,β-unsaturated esters and dihaloalkanes
To a well-ground mixture of spiro[[1,3]dithiolane-2,3′-indolin]-2′-one 1 (1.0 mmol), tetrabutylammonium bromide (TBAB) (0.5 mmol), base (1.0 mmol, 1,4-diazabicyclo[2.2.2]octane (DABCO) for Michael addition, and K2CO3 for N-alkylation), and α,β-unsaturated esters or dihaloalkanes (1.2 mmol) were added and mixed thoroughly with a glass rod. The resulting mixture was kept in an oil bath at 80°C for the stipulated time ( and ). The progress of the reaction was monitored by TLC. After the completion of the reaction, the mixture was cooled to room temperature and dissolved in chloroform (20 mL). TBAB was recovered by the addition of water (20 mL × 3), then collected, and dried under vacuum. The chloroform layer was washed with water (15 mL × 3). The organic layer was dried over anhydrous Na2SO4. The solvent was removed under reduced pressure and the resulting crude material was purified on a short silica-gel column with ethyl acetate:n-hexane (1:9) as the eluent.
Table 1. Aza-Michael addition of spiro[[1,3]dithiolane-2,3′-indolin]-2′-one 1 to α,β-unsaturated esters 2 under solvent-free conditions.
Table 2. N-alkylation of spiro[[1,3]dithiolane-2,3′-indolin]-2′-one 1 by dihaloalkanes 4 under solvent-free conditions.
Physical and spectroscopic data of isolated products
Methyl 3-(2′-oxospiro[[1,3]dithiolane-2,3′-indolin]-1′-yl) propanoate (3a): Green viscous oil, yield 90%. IR (KBr, cm−1): 3056, 2980, 1735, 1609, 1466, 1187, 1064, 852, 750, 658, 567, 489. 1H NMR (400 MHz, CDCl3): δ ppm 2.63 (t, 2H, J = 7.25 Hz), 3.55–3.61 (m, 5H), 3.77–3.83 (2H, m), 3.91 (t, 2H, J = 7.25 Hz), 6.81 (d, 1H, J = 7.85 Hz), 7.01 (1H, t, J = 7.50 Hz), 7.23 (t, 1H, J = 7.00 Hz), 7.45 (d, 1H, J = 7.45 Hz). 13C NMR (100 MHz, CDCl3): 30.7, 35.2, 39.3, 39.3, 50.8, 107.4, 122.1, 124.5, 125.1, 128.8, 140.6, 170.3, 176.57. Anal. Calcd. For C14H15NO3S2: C, 54.35; H, 4.89; N, 4.53. Found: C, 54.49; H, 5.05; N, 4.72.
Ethyl 3-(2′-oxospiro[[1,3]dithiolane-2,3′-indolin]-1′-yl) propanoate (3b): Green viscous oil, yield 85%. IR (KBr, cm−1): 2963, 2873, 1734, 1612, 1464, 1351, 1262, 1097, 804, 686, 476. 1H NMR (400 MHz, CDCl3): δ ppm 1.13–1.20 (m, 3H), 2.66–2.70 (t, 2H, J = 7.50 Hz), 3.58–3.66 (m, 2H), 3.82–3.85 (m, 2H), 3.87–3.88 (m, 2H), 3.94–3.96 (m, 2H), 6.87 (d, 1H, J = 8.00 Hz), 7.05 (t, 1H, J = 5.20 Hz), 7.25(t, 1H, J = 3.60 Hz) 7.50 (d, 1H, 7.20 Hz). 13C NMR (100 MHz, CDCl3): 15.2, 33.2, 37.5, 41.5, 61.4, 63.2, 109.8, 124.3, 126.7 127.3, 131.0, 143.3, 166.4, 171.8. Anal. Calcd. For C15H17NO3S2: C, 55.70; H, 5.30; N, 4.33. Found: C, 55.78; H, 5.20; N, 4.19.
Butyl 3-(2′-oxospiro[[1,3]dithiolane-2,3′-indolin]-1′-yl) propanoate (3c): Orang viscous oil, yield 90%. IR (KBr, cm−1): 3080, 2949, 1717, 1608, 1486, 1467, 1353, 1165, 752, 686, 442. 1H NMR (400 MHz, CDCl3): δ ppm 0.78 (m, 3H), 1.11–1.24 (m, 2H), 1.39–1.45 (t, 2H, J = 6.80 Hz), 2.60 (t, 2H, J = 4.80 Hz), 3.52–3.57 (m, 2H), 3.76–3.81 (m, 2H), 3.88 (t, 2H, J = 4.80 Hz), 3.92 (t, 2H, J = 2.00 Hz), 6.80 (d, 1H, J = 7.60 Hz), 6.96 (t, 2H, J = 4.80 Hz), 7.18 (t, 1H, J = 7.6 Hz), 7.42 (d, 1H, J = 7.2 Hz). 13C NMR (100 MHz, CDCl3): 14.8, 20.1, 31.5, 33.2, 37.5, 41.2, 63.2, 65.7, 109.8, 124.2, 126.7, 127.2, 131.0, 143.3, 166.4, 171.0. Anal. Calcd. For C17H21NO3S2: C, 58.09; H, 6.02; N, 3.99. Found: C, 58.26; H, 5.91; N, 4.15.
2-Ethylhexyl 3-(2′-oxospiro[[1,3]dithiolane-2,3′-indolin]-1′-yl) propanoate (3d): Red viscous oil, yield 80%. IR (KBr, cm−1): 3056, 2930, 1722, 1609, 1467, 1350, 1185, 1062, 749, 685, 490. 1H NMR (400 MHz, CDCl3): δ ppm 0.81–0.87 (m, 6H), 1.23–1.30 (m, 8H), 1.49–1.50 (m, 1H), 2.72 (t, 2H, J = 7.05 Hz), 3.63–3.69 (m, 2H), 3.85–4.01 (m, 6H), 6.90 (d, 1H, J = 7.80 Hz), 7.07 (t, 1H, J = 7.40 Hz), 7.29 (t, 1H, J = 7.60 Hz), 7.52 (1H, d, J = 7.32 Hz). 13C NMR (100 MHz, CDCl3): 9.7, 12.8, 21.7, 22.4, 27.6, 29.0, 30.9, 35.2, 37.3, 37.5, 39.2, 65.7, 66.1, 107.4, 122.0, 124.4, 125.0, 128.7, 140.9, 170.1, 176.6. Anal. Calcd. For C21H29NO3S2: C, 61.88; H, 7.17; N, 3.44. Found: C, 62.08; H, 7.25; N, 3.66.
Methyl 2-methyl-3-(2′-oxospiro[[1,3]dithiolane-2,3′-indolin]-1′-yl) propanoate (3e): Green viscous oil, yield 78%. IR (KBr, cm−1): 3010, 2936, 1724, 1610, 1468, 1353, 1203, 1092, 752, 687, 490. 1H NMR (400 MHz, CDCl3): δ ppm 1.21–12.4 (m, 3H), 2.97–3.02 (m, 1H), 3.62 (s, 2H), 3.63–3.65 (m, 3H), 3.65–3.70 (m, 1H), 3.85–3.90 (m, 3H), 6.86 (d, 1H, J = 7.60 Hz), 7.08 (t, 1H, J = 7.60 Hz), 7.28 (t, 1H, J = 6.80 Hz), 7.53 (d, 1H, J = 7.60 Hz). 13C NMR (100 MHz, CDCl3): 15.8, 38.7, 41.5, 44.3, 53.2, 63.2, 109.8, 124.4, 126.8, 127.2, 131.1, 143.5, 166.4, 173.0. Anal. Calcd. For C15H17NO3S2: C, 55.70; H, 5.30; N, 4.33. Found: C, 55.92; H, 5.18; N, 4.51.
Butyl 2-methyl-3-(2′-oxospiro[[1,3]dithiolane-2,3′-indolin]-1′-yl) propanoate (3f): Orange viscous oil, yield 65%. IR (KBr, cm−1): 3057, 2959, 1724, 1609, 1467, 1352, 1193, 1091, 750, 686, 490. 1H NMR (400 MHz, CDCl3): δ ppm 0.86 (t, 3H, J = 7.3 Hz), 1.22 (d, 3H, J = 7.02 Hz), 1.26 (t, 2H, J = 3.50), 1.45–1.52 (m, 1H), 3.97–3.02 (m, 1H), 3.62–3.68 (m, 2H), 3.84–3.87 (dd, 2H, J1 = 2.30 Hz, J2 = 7.40 Hz), 3.89–3.93 (m, 3H), 3.95–3.98(m, 1H), 6.86 (d, 1H, J = 7.85 Hz), 7.08 (t, 1H, J = 7.50 Hz), 7.29 (t, 1H, J = 7.65 Hz), 7.35(d, 1H, J = 35 Hz). 13C NMR (100 MHz, CDCl3): 12.6, 13.8, 17.9, 28.6, 29.4, 36.9, 39.3, 39.4, 42.2, 63.8, 107.7, 122.2, 124.6, 127.0, 128.8, 141.4, 173.4, 177.2. Anal. Calcd. For C18H23NO3S2: C, 59.15; H, 6.34; N, 3.83. Found: C, 58.99; H, 6 .52; N, 4.02.
Dipentyl 2-(2′-oxo-1′,2′-dihydrospiro[[1,3]dithiolane-2,3′-inden]-1′-yl) succinate (3g): Red viscous oil, yield 70%. IR (KBr, cm−1): 3055, 2929, 1717, 1608, 1458, 1467, 1350, 1145, 1096, 750, 687, 490. 1H NMR (400 MHz, CDCl3): δ ppm 0.78 (t, 3H, J = 7.20 Hz), 0.85 (t, 3H, J = 6.90 Hz), 1.08–1.22 (m, 4H), 1.24–1.35 (m, 4H), 1.49–1.63 (m, 4H), 2.93–3.02 (dd, 1H, J1 = 9.70 Hz, J2 = 20.18 Hz), 3.36–3.42 (dd, 1H, J1 = 6.65 Hz, J2 = 16.75 Hz), 3.62–3.70 (m, 2H), 3.85–3.93 (m, 2H), 4.07 (m, 3H), 4.11–4.21 (m, 1H), 5.31 (t, 1H, J = 7.00 Hz), 6.86 (d, 1H, J = 7.90 Hz), 7.09 (t, 1H, J = 8.20 Hz), 7.29 (t, 1H, J = 7.55 Hz), 7.55 (d, 1H, J = 7.20 Hz). 13C NMR (100 MHz, CDCl3): 12.8, 21.1, 21.2, 26.9, 27.0, 28.6, 32.6, 39.3, 39.4, 49.7, 64.3, 65.3, 107.9, 122.4, 124.7, 124.9, 128.8, 140.5, 167.5, 169.3, 176.5. Anal. Calcd. For C24H33NO5S2: C, 60.10; H, 6.93; N, 2.92. Found: C, 60.02; H, 7.11; N, 2.79.
Dihexyl 2-(2′-oxo-1′,2′-dihydrospiro[[1,3]dithiolane-2,3′-inden]-1′-yl) succinate (3h): Red viscous oil, yield 65%. IR (KBr, cm−1): 3057, 2931, 1734, 1610, 1467, 1354, 1174, 1066, 994, 749, 686, 498. 1H NMR (400 MHz, CDCl3): δ ppm 0.80 (m, 3H, J = 7.00 Hz), 0.85–0.89 (m, 3H), 1.15–1.21 (m, 4H), 1.25–1.37 (m, 8H), 1.53–1.58 (m, 2H), 1.63–1.71 (m, 2H), 2.96–3.02 (dd, 1H, J1 = 8.80 Hz, J2 = 16.75 Hz), 3.36–3.42 (dd, 1H, J1 = 6.60 Hz, J2 = 16.80 Hz), 3.64–3.70 (m, 2H), 3.87–3.92 (m, 2H), 4.07 (t, 2H, J = 11.60 Hz), 4.17 (t, 2H, J = 9.75 Hz), 5.30 (t, 1H, J = 6.90 Hz), 6.86 (d, 1H, J = 8.15 Hz), 7.10 (t, 1H, J = 7.55 Hz), 7.28 (t, 1H, J = 7.80 Hz), 7.55 (d, 1H, J = 7.45 Hz). 13C NMR (100 MHz, CDCl3): 12.8, 21.3, 21.4, 24.3, 24.4, 27.3, 27.4, 30.2, 30.4, 32.6, 39.2, 39.3, 49.6, 64.3, 64.5, 74.3, 107.8, 122.3, 124.7, 128.8, 132.4, 140.4, 167.41, 169.2, 176.4. Anal. Calcd. For C26H37NO5S2: C, 61.51; H, 7.35; N, 2.76. Found: C, 61.23; H, 7.21; N, 2.90.
Dioctyl 2-(2′-oxospiro[[1,3]dithiolane-2,3′-indolin]-1′-yl) succinate (3i): Red viscous oil, yield 63%. IR (KBr, cm−1): 3057, 2928, 1734, 1610, 1467, 1353, 1175, 1099, 748, 686, 496. 1H NMR (400 MHz, CDCl3): δ ppm 0.84 (m, 6H), 1.15 (s, 4H), 1.24 (s, 16H), 1.53–1.58 (m, 3H), 1.64–1.69 (m, 1H), 2.96–3.02 (dd, 1H, J1 = 7.65 Hz, J2 = 16.57 Hz), 3.36–3.40 (dd, 1H, J1 = 6.65 Hz, J2 = 11.10 Hz), 3.62–3.69 (m, 2H), 3.86–3.92 (m, 2H), 4.03–4.09 (m, 2H), 4.15–4.21 (m, 2H), 5.30 (t, 1H, J = 6.90 Hz), 6.86 (d, 1H, J = 11.20 Hz), 7.09 (t, 1H, J = 7.40 Hz), 7.28 (t, 1H, J = 6.95 Hz), 7.55 (d, 1H, J = 7.45 Hz). 13C NMR (100 MHz, CDCl3): 13.0, 21.5, 24.6, 24.7, 27.2, 27.4, 28.0, 28.6, 30.6, 30.7, 32.6, 39.3, 39.4, 49.7, 64.3, 65.3, 107.6, 122.4, 124.7, 128.8, 132.5, 140.5, 167.5, 169.3, 176.5. Anal. Calcd. For C30H45NO5S2: C, 63.91; H, 8.04; N, 2.48. Found: C, 63.77; H, 7.53; N, 2.71.
Didecyl 2-(2′-oxospiro[[1,3]dithiolane-2,3′-indolin]-1′-yl)succinate (3j): Red viscous oil, yield 65%. IR (KBr, cm−1): 3050, 2854, 1734, 1610, 1467, 1260, 1173, 1058, 748, 685, 496. 1H NMR (400 MHz, CDCl3): δ ppm 0.80 (m, 6H), 1.05 (s, 8H), 1.19 (s, 16H), 1.45–1.50 (m, 8H), 2.90–2.95 (dd, 1H, J1 = 4.90 Hz, J2 = 11.80 Hz), 3.28–3.35 (dd, 1H, J1 = 8.45 Hz, J2 = 14.75 Hz), 3.55 (t, 4H, J = 6.65 Hz), 3.95–4.03 (m, 4H), 5.23 (t, 1H, J = 6.75 Hz), 6.79 (d, 1H, J = 9.45 Hz), 7.05 (t, 1H, J = 7.55 Hz), 7.21 (t, 1H, J = 6.80 Hz), 7.48 (d, 1H, J = 7.35 Hz). 13C NMR (100 MHz, CDCl3): 13.1, 21.6, 24.7, 24.8, 27.3, 27.4, 28.3, 28.4, 28.5, 28.6, 30.8, 31.7, 32.7, 39.3, 39.4, 49.7, 61.9, 64.3, 65.3, 107.9, 122.4, 124.8, 128.9, 132.5, 140.5, 167.5, 169.4, 176.6. Anal. Calcd. For C34H53NO5S2: C, 65.87; H, 8.62; N, 2.26. Found: C, 65.59; H, 8.78; N, 2.60.
1′-(2-Bromoethyl)spiro[[1,3]dithiolane-2,3′-indolin]-2′-one (5a): White solid, yield 70%, m.p. 55°C. IR (KBr, cm−1): 3057, 2915, 1717, 1609, 1464, 1357, 1157, 1083, 747, 539, 440. 1H NMR (400 MHz, CDCl3): δ ppm 3.57 (t, 2H, J = 7.05 Hz), 3.63–3.71 (m, 2H), 3.86–3.94 (m, 2H), 4.09 (t, 2H, J = 7.05 Hz), 6.88 (d, 1H, J = 7.85 Hz), 7.11 (t, 1H, J = 7.40 Hz), 7.31 (t, 1H, J = 7.80 Hz), 7.55 (1H, d, J = 7.35 Hz). 13C NMR (100 MHz, CDCl3): 26.1, 39.4, 40.9, 61.0, 107.4, 122.4, 124.7, 125.0, 128.9, 140.9, 176.8. Anal. Calcd. For C12H12BrNOS2: C, 43.64; H, 3.66; N, 4.24. Found: C, 43.85; H, 3.72; N, 4.60.
1′-(2-Chloroethyl)spiro[[1,3]dithiolane-2,3′-indolin]-2′-one (5b): White solid, yield 82%, m.p. 55°C. IR (KBr, cm−1): 3059, 2918, 1717, 1608, 1487, 1359, 1164, 1082, 667, 747, 444. 1H NMR (400 MHz, CDCl3): δ ppm 3.63–3.71 (m, 2H), 3.75 (t, 2H, J = 6.60 Hz), 3.86–3.92 (m, 2H), 4.03 (t, 2H, J = 6.55 Hz), 6.89 (d, 1H, J = 7.80 Hz), 7.11 (t, 1H, J = 7.55 Hz), 7.31 (t, 1H, J = 7.70 Hz), 7.55 (d, 1H, J = 7.45 Hz). 13C NMR (100 MHz, CDCl3): 39.2, 39.4, 41.1, 61.0, 107.5, 122.4, 124.7, 125.0, 128.9, 141.2, 177.0. Anal. Calcd. For C12H12ClNOS2: C, 50.43; H, 4.23; N, 4.90. Found: C, 50.66; H, 3.97; N, 4.73.
1′-(5-Bromopentyl)spiro[[1,3]dithiolane-2,3′-indolin]-2′-one (5c): Orange solid, yield 87% m.p. 64°C. IR (KBr, cm−1): 3056, 2951, 1733, 1606, 1469, 1359, 1201, 1070 753, 601, 454. 1H NMR (400 MHz, CDCl3): δ ppm 1.25–1.54 (m, 4H), 1.76–1.90 (m, 2H), 3.45 (t, 2H, J = 5.40 Hz), 3.64–3.70 (m, 2H), 3.73 (t, 2H, J = 6.45 Hz) 3.86–3.93 (m, 2H), 6.86 (d, 1H, J = 7.75 Hz), 7.09 (t, 1H, J = 7.50 Hz), 7.26–7.32 (m, 1H), 7.54 (d, 1H, J = 7.30 Hz). 13C NMR (100 MHz, CDCl3): 24.7, 28.4, 30.3, 32.0, 38.2, 39.4, 61.1, 107.5, 122.1, 124.6, 125.2, 129.8, 141.3, 177.0. Anal. Calcd. For C15H18BrNOS2: C, 48.39; H, 4.87; N, 3.76. Found: C, 48.12; H, 4.76; N, 3.67.
1′-(6-Bromohexyl)spiro[[1,3]dithiolane-2,3′-indolin]-2′-one (5d): Red solid, yield 95%, m.p. 66°C. IR (KBr, cm−1): 3027, 2930, 1705, 1609, 1465, 1353, 1144, 1090, 747, 552, 441. 1H NMR (400 MHz, CDCl3): δ ppm 1.34–1.50 (m, 4H), 1.66–1.73 (qui, 2H, J = 7.25 Hz), 1.80–1.87 (qui, 2H, J = 7.50 Hz), 3.38 (t, 2H, J = 6.70 Hz), 3.62–3.71 (m, 4H), 3.86–3.94 (m, 2H), 6.81 (d, 1H, J = 7.80 Hz), 7.08 (t, 1H, J = 7.55 Hz), 7.29 (t, 1H, J = 7.05 Hz), 7.35 (d, 1H, J = 7.40 Hz). 13C NMR (100 MHz, CDCl3): 24.7, 25.9, 26.5, 31.3, 32.7, 38.9, 39.3, 61.0, 107.4, 121.9, 124.4, 125.1, 128.7, 141.4, 176.8. Anal. Calcd. For C16H20BrNOS2: C, 49.74; H, 5.22; N, 3.63. Found C 49.93, H 5.35, N 3.50.
1′-(6-Chlorohexyl)spiro[[1,3]dithiolane-2,3′-indolin]-2′-one (5e): Red solid, yield 88%, m.p. 66°C. IR (KBr, cm−1): 3058, 2934, 1714, 1609, 1467, 1351, 1153, 899, 748. 1H NMR (400 MHz, CDCl3): δ ppm 1.23–1.28 (m, 2H), 1.46–1.54 (m, 2H), 1.70 (qui, 2H, J = 7.50 Hz), 1.88 (qui, 2H, J = 6.40 Hz), 3.38 (t, 2H, J = 6.70 Hz), 3.61–3.71 (m, 4H), 3.86–3.94 (m, 2H), 6.81 (d, 1H, J = 7.80 Hz), 7.08 (t, 1H, J = 7.50 Hz), 7.29 (t, 1H, J = 8.00 Hz), 7.54 (d, 1H, J = 7.30 Hz). 13C NMR (100 MHz, CDCl3): 24.2, 25.3, 28.6, 30.4, 32.4, 38.9, 39.4, 61.1, 107.5, 122.0, 124.6, 125.3, 128.9, 141.4, 176.9. Anal. Calcd. For C16H20ClNOS2: C, 56.20; H, 5.90; N, 4.10. Found: C, 55.93; H, 5.80; N, 4.28.
Results and discussion
In line with our interest in the aza-Michael addition reaction of isatin ketal derivatives to α,β-unsaturated esters (Citation13), in this project we decided to synthesize some novel isatin thioketal derivatives by applying solvent-free conditions. Therefore, the aza-Michael addition of spiro[[1,3]dithiolane-2,3′-indolin]-2′-one 1 to n-butyl acrylate 2c in the presence of organic salt TBAB and different bases such as pyridine, K2CO3, Na2CO3, KOH, NaOH, and DABCO was investigated as a model reaction to evaluate their capabilities for access to the best base ().
Scheme 2. Aza-Michael addition of spiro[[1,3]dithiolane-2,3′-indolin]-2′-one 1 to n-butyl acrylate 2c as model reaction.
![Scheme 2. Aza-Michael addition of spiro[[1,3]dithiolane-2,3′-indolin]-2′-one 1 to n-butyl acrylate 2c as model reaction.](/cms/asset/55ea5483-55f6-456f-9688-fb1e0cf83a12/tgcl_a_1250958_f0002_b.gif)
This investigation showed that DABCO is a suitable base for this reaction and the best results were obtained when DABCO was applied as a base at 80°C. To select the best solvent for the model reaction in the absence of TBAB and in the presence of base DABCO, next we tested various solvents in the reaction media. This study showed that in the presence of a variety of solvents such as CH2Cl2, CHCl3, DMSO, CH3OH, EtOH, CH3CO2Et, n-hexane, and water, no considerable progress was observed for the model reaction and we obtained the best yields in the presence of TBAB. In interpretation of this successfully, we can think that, in our reaction, organic salt TBAB plays the role of a high polar solvent and accelerates the reaction by dissolving all the organic (thioketal, ester, dihaloalkane, and DABCO) and inorganic (K2CO3) reactants. Also, we optimized the amounts of TBAB and DABCO. Herein, the best conditions were obtained as spiro[[1,3]dithiolane-2,3′-indolin]-2′-one (1 mmol), α,β-unsaturated esters (1.2 mmol), TBAB (0.5 mmol), and DABCO (1 mmol). Therefore, with this established optimum conditions, we were keen to explore the scope of the reaction with respect to various α,β-unsaturated esters and spiro[[1,3]dithiolane-2,3′-indolin]-2′-one, the results of which are depicted in .
It is seen from results of that the reactions proceeded smoothly and afforded the corresponding products with isolated yields ranging from 50% to 90% in 120–150 min. It has been observed that the bulkiness of the alkoxy group (–OR) of acrylic esters did not affect significantly the yields and reaction times under model reaction conditions (, entries 1–4). It is important to note that when fumaric esters were used as the Michael acceptor, the reaction proceeded slowly (, entries 7–10). Surprisingly, we observed that the best results were achieved by carrying out the reaction with acrylic esters, despite containing only one carbonyl electron-withdrawing group, as the Michael acceptor. The results in show that the steric effects of substitutes on β-carbon atom are more important than their electronic effects. These effects showed that fumaric esters were more sensitive to the size of alkoxy groups than acrylic esters (, entries 9 and 10). Also, when methyl and butyl methacrylate were used as Michael acceptors, the yields were lower than that obtained using acrylic esters (, entries 5 and 6). It can be attributed to the steric hindrance of methyl at the α-position of these esters.
With these precedents and with the aim of preparing other new derivatives of spiro[[1,3]dithiolane-2,3′-indolin]-2′-one, we decided to carry out N-alkylation of this compound by dihalogenoalkyl groups (). Therefore, the alkylation of isatin thioketal 1 to 1,6-dibromohexane 4d was tried as a model reaction ().
Scheme 3. Direct N-alkylation reaction of spiro[[1,3]dithiolane-2,3′-indolin]-2′-one 1 with 1,6-dibromohexane 4d (as a model reaction).
![Scheme 3. Direct N-alkylation reaction of spiro[[1,3]dithiolane-2,3′-indolin]-2′-one 1 with 1,6-dibromohexane 4d (as a model reaction).](/cms/asset/d8cd90c1-f907-407d-90ef-4a99551122d0/tgcl_a_1250958_f0003_b.gif)
In the presence of TBAB and different organic and inorganic bases, the results for the model reaction indicated that K2CO3 was the best choice. Also, we proceeded this reaction in the absence of TBAB and in different solvents. The reaction failed in most of conventional organic solvents such as CH2Cl2, CHCl3, DMSO, CH3OH, EtOH, CH3CO2Et, n-hexane as well as water, even upon prolonged heating at the boiling point. Therefore, with the best reaction conditions in hand (K2CO3 (1 mmol), TBAB (0.5 mmol), spiro[[1,3]dithiolane-2,3′-indolin]-2′-one (1 mmol), and dihaloalkane (1 mmol)), we next considered the scope of the reaction by employing various dihaloalkanes with isatin thioketal 1 ().
From , it is clear that, generally, the reactions produced the corresponding products in good yields and change in the length of chain (CH2)n did not have a considerable effect on the reaction yields.
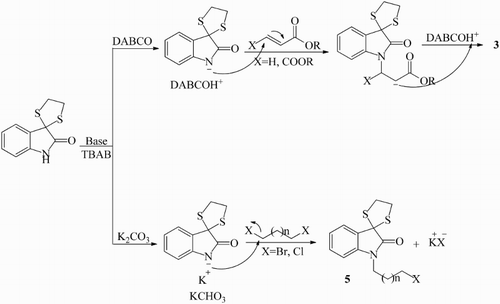
We suggested two suitable mechanisms for the production of compounds 3 and 5. These mechanisms explain the role of TBAB and bases DABCO and K2CO3 in these reactions ().
Conclusions
In summary, we synthesized novel derivatives of isatin thioketal using a new, facile, and efficient method under solvent-free conditions. The reaction between spiro[[1,3]dithiolane-2,3′-indolin]-2′-one and α,β-unsaturated esters was successful in the presence of organic salt TBAB and basic catalyst DABCO at 80°C. Also, spiro[[1,3]dithiolane-2,3′-indolin]-2′-one and alkyl dihalides reacted successfully in the presence of TBAB and inorganic base K2CO3 at 80°C. We also observed that in the first reaction, lower yields of products and longer reaction time were obtained when fumaric esters were used as the Michael acceptor. It is considerable that both the bases used in the reactions are readily available, and also facile procedures would make the method practical and useful to synthetic chemists.
Supplementary_Material.docx
Download MS Word (24.7 MB)Acknowledgments
The authors are grateful to the laboratories of Tehran and Tabriz University as well as the University of Mohaghegh Ardabili for the product analysis.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
Dr Imanzadeh is professor in University of Mohaghegh Ardabili.
Dr Banaei is Assistant Professor in Payame Noor University.
Mr Fathi is M.Sc. student in Payame Noor University.
Ms Soltanzadeh is Ph.D. student in University of Mohaghegh Ardabili.
References
- Mondal, P.; Banerjee, M.; Jana, S.; Bose, A. Pharm. Chem. 2010, 2, 169–172.
- Verma, R.S.; Nobles, W.L. J. Pharm. Sci. 1975, 64, 881–882. doi: 10.1002/jps.2600640539
- Popp, F.D.; Parson, R.; Donigan, B.E. J. Pharm. Sci. 1980, 69, 1235–1237. doi: 10.1002/jps.2600691035
- Pandeya, S.N.; Sriram, D.; Nath, G.; Declercq, E. Eur. J. Pharm. Sci. 1999, 9, 25–31. doi: 10.1016/S0928-0987(99)00038-X
- Tran, V.H.; Nguyen, Q.D.; Le, N.V.; Le, T.T. Tap. Chi. Douc. Hoc. 1999, 8, 15–7.
- Mashelkar, C.U.; Rane, M.D. Indian J. Chem. (Sec-B). 2005, 44B, 1452–1455.
- Geronikaki, A.; Babaev, E.; Deharden, J.; Deharden, W. Bioorg. Med. Chem. 2004, 12, 6559–6568. doi: 10.1016/j.bmc.2004.09.016
- Zhunghietu, G. Rev. Roum. Chim. 2001, 46, 517–520.
- Rajopadhye, M.; Popp, F.D. J. Med. Chem. 1988, 31, 1001–1005. doi: 10.1021/jm00400a018
- Zapata-Sudo, G.; Pontes, L.B.; Gabriel, D.; Mendes, T.C.F.; Riberio, N.M.; Pinto, A.C.; Trachez, M.M.; Sudo, R.T. Pharmacol. Biochem. Behav. 2007, 86, 678–685. doi: 10.1016/j.pbb.2007.02.013
- Imanzadeh, G.H.; Kazemi, F.; Zamanloo, M.; Mansoori, Y. Ultrason. Sonochem. 2013, 20, 722–728. doi: 10.1016/j.ultsonch.2012.09.003
- Soltanzadeh, Z.; Imanzadeh, G.H.; Noroozi-Pesyan, N.; Şahin, E.; Hooshmand, H. Tetrahedron. 2016, 72, 1736–1741. doi: 10.1016/j.tet.2016.02.024
- Imanzadeh, G.H.; Soltanizadeh, Z.; Khodayari, A.; Zamanloo, M.; Mansoori, Y.; Salehzadeh, J. Chin. J. Chem. 2012, 30, 891–899. doi: 10.1002/cjoc.201100351
- Vogel, A. Vogel’s Textbook of Practical Organic Chemistry, 4th ed.; Longman Press: London, 1978.