ABSTRACT
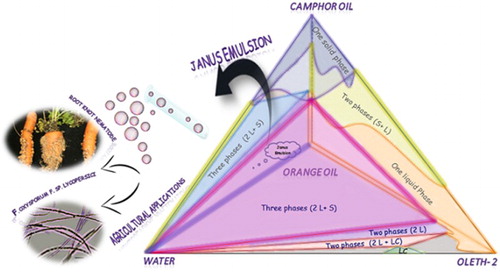
This study investigated the nematicidal and fungicidal activities of the two immiscible camphor and orange oils formulated in Janus emulsion (JE). JE was prepared from camphor and orange oils by a single-step emulsification with different concentrations chosen from an established phase diagram. Camphor oil nematicidal activity was studied against the root knot nematode Meloidogyne incognita, while orange oil fungicidal activity was studied against the wilt fungus Fusarium oxysporum f. sp. lycopersici (Fol). For a comparison study, different concentrations of JE and double emulsions were prepared. Results showed that JE had a significant mortality effect on the second-stage juveniles (J2s) M. incognita with 81% mortality and was able to delay the growth of Fol four and seven days after exposure, while changes in the efficiency of DE were recorded. Prepared JEs were shown to maintain the efficiency and to have slow release profiles for encapsulated oils.
GRAPHICAL ABSTRACT
1. Introduction
Emulsions are among the most important colloidal systems. This can be attributed to their numerous applications in food, agriculture, cosmetics and pharmaceutical industries, where they can be used to transport active materials and to facilitate sustained release ( Citation1, Citation2). Recently, multiple emulsions have attained more commercial and technical importance than conventional emulsions due to their great progress in the application fields such as controlled delivery system for pharmaceutical agents ( Citation3, Citation4).
An emulsion is defined as a system of two immiscible liquids. A multiple emulsion can be formed as droplets of different immiscible liquids are immersed in a third liquid, usually referred to as a host liquid, where different equilibrium topologies are expected to be formed as a water-in-oil-in-water (W/O/W) emulsion or oil-in-water-in-oil (O/W/O) emulsion, or a surface can be divided into two chemically varying regions – Janus emulsion – ((O1 + O2)/W) ( Citation1, Citation3–6). The Janus emulsion (JE) is a multiple emulsion that has two chemically varying regions in an emulsion droplet, next to each other, divided in a way that lowers the interfacial tension and maintains the efficiency of used components ( Citation7). JEs have attracted more attention in research because they can carry different substances and have many applications in different streams, where studies showed that Janus particles arrangement could be utilized for the transport of active materials and for facilitating sustained release ( Citation8).
As JE can be utilized to carry different substances, in this study it has been tested with the use of two immiscible essential oils. Essential oils can be utilized as environmentally safe alternatives to chemicals, where this has been a matter of concern for scientists in order to inhibit the environmental problems associated with the use of chemicals.
Nematicides and fungicides are examples of specific types of chemical pesticides that are used to kill plant’s nematodes and fungal diseases. The use of essential oils has provided a source of biodegradable and easily extractable materials that possess low or no toxicity against birds and mammals, and plays an important role in plants protection. Some studies showed the effect of treating the plant with proper essential oil ( Citation9) where in vitro tests were conducted on root knot nematodes (RKNs) ( Citation10) and on pathogenic fungi ( Citation11, Citation12).
RKNs are plant–parasitic nematodes from the genus Meloidogyne – M. javanica, M. arenaria, M. incognita and M. hapla, which are four major worldwide Meloidogyne species. RKNs are microscopic roundworms that exist in soil in areas with hot climates or short winters ( Citation13). They injure plants by feeding on root cells using their needle-like mouthparts. The root system can become damaged to the point where the plant cannot properly absorb water and nutrients. Plants may appear stunted, having nutrient deficiencies, and may eventually die. Beneath the ground, the roots may have knots or galls on them ( Citation14).
Fusarium oxysporum is a fungal plant pathogen that causes the most economical damage to agricultural crops, especially on tomato, and it is usually accompanied by RKN infestations. F. oxysporum causes vascular wilt disease by infecting the plants through their roots and grow internally through the cortex to the stele, which in turn plugs the vascular system and results in plant stunting that leads to its death. Fusarium wilt caused by F. oxysporum f. sp. lycopersici (Fol) is known worldwide as one of the most harmful diseases that can affect tomato plants ( Citation15).
Camphor oil nematicidal activity has been investigated in several studies dealt with studying the effect of plant extracts on Meloidogyne spp. Camphor oil (0.5 mg/ml) showed 31% mortality of second-stage juveniles (J2s) of M. incognita after 72 h of exposure ( Citation10). In another study, camphor oil in an inhibitory concentration of 0.097 mg/ml estimated to kill 100% M. incognita population ( Citation16).
Several in vitro studies were carried out to determine the antifungal activity of some essential oils against many plant pathogenic fungi. Orange oil showed an inhibitory effect against Fol in a study aimed to investigate effects of essential oils against 12 pathogenic fungi ( Citation17).
Mixing oils together (by encapsulating oils in micro-granules) showed that some combinations of oils lost effectiveness upon mixing ( Citation18); in our study, we report that encapsulating oils in JE maintain effectiveness of used oils.
This paper reports JE to host more than one oil and provides suitable solutions for some agricultural problems keeping the effectiveness at a high level. It is a suitable formula that allows the existence of more than one oil together without being mixed, thus providing their individual effects without undue loss of efficiency that might be associated with physical mixing ( Citation6, Citation18).
2. Materials and methods
2.1. Materials
Surfactants: Polyoxyethylene (20) sorbitan mono-oleate, polysorbate-80 (Tween®80), polyoxyethylene (2) oleyl ether, oleth-2 (Brij®93) (ICI). Oils: camphor oil (d-camphor flowers) (BDH laboratory), unfolded orange oil (Firmenich). Dyes: trypan blue (Sigma Aldrich), 1-[4-(phenylazo)phenylazo]-2-naphthol (Sudan®III) (Hopkin & Williams).
2.2. Phase diagram establishment
The titration method at room temperature (25°C) ( Citation19) was used to determine the ternary phase diagram for a system composed of camphor oil, orange oil, distilled water and oleth-2 surfactant. The region of solubility was determined by adding water to a pre-weighed mixture of oil (camphor or orange)/surfactants; the sample was shacked and centrifuged at 3000 rpm for 5 min. Finally, the turbidity and clarity points were observed and then the three-dimensional ternary phase diagram was constructed.
2.3. Emulsion preparation and observation
As immiscible essential oils should be used to prepare Janus particles (both oils in same globule), the solubility test for the selected orange and camphor essential oils was performed; it involved preparing a large number of samples by mixing different composition of the two oils and observing their miscibility, followed by centrifugation to separate the used oils ( Citation6).
JE samples were tested with traces of water soluble dye (trypan blue) and orange oil soluble dye (Sudan®III) to be able to identify emulsion composition using the microscope (inverted microscope, MEIJI Techno), and to figure out Janus globules. To ensure that the used dye does not color both oils, different percentages from selected oils were mixed with trace amounts of Sudan®III dye and were then centrifuged to report dye solubility in each oil.
Non-ionic surfactants were used to examine JE. Used percentages of non-ionic surfactants () were within the approved maximum percentage in the agricultural application field (5% for non-ionic surfactants) ( Citation20, Citation21). Oleth-2 surfactant was used with JE, while in the prepared double emulsions (DEs) two surfactants were needed (oleth-2 and polysorbate-80). Distilled water served as control.
Table 1. Description of emulsions percentage composition and their pH values.
Several emulsions were prepared from different composition of surfactant, oil and water (). The prepared emulsions included one original concentrated formula. The concentrated formula was diluted with water with certain percentages that maintains Janus formula upon dilution.
JE was prepared in a single-step emulsification technique, where all the constituents of the emulsions (oils, water and one surfactant) were added at the same time and mixed together on a common vibrator equipment ( Citation22).
Further dilution for JE was performed to examine their nematicidal and fungicidal effects. Diluted emulsions with low essential oils percentages were tested since some previous studies reported that essential oils can have a phytotoxic effect on crops seedlings ( Citation23). Diluted camphor oil emulsion, diluted orange oil emulsion, concentrated and diluted DEs (prepared in two-step emulsification technique where oils, water and two surfactants, one with low and one with high hydrophilic–lipophilic balance (HLB) value, were used) and surfactant samples were prepared as well for comparison study.
2.4. Nematicidal and fungicidal effects
2.4.1. Nematode culture
A population of RKN, M. incognita, was isolated from infected roots of diseased eggplants grown in an open-field area in Jerash upland/Jordan (32°15′N, 35°53′E, 460 m). The isolate of nematode was determined as M. incognita using morphological and molecular techniques ( Citation24).
The nematode culture was maintained by being subcultured on cucumber plants (CV Super Samara) in the growth chamber at 20°C at the Faculty of Agriculture/University of Jordan. Inocula of the cultures were used for further studies.
2.4.1.1. Testing for the hatching of second-stage Juveniles of RKNs M. incognita
Different emulsions formulations were prepared () and used to examine hatching of J2s of RKN from eggmasses. Three replicates were performed for each treatment and three eggmasses per replicate were used. Three eggmasses of RKN species were handpicked from galled eggplant roots and placed in a Syracuse glass dishes (1.5 cm in diameter). The Syracuse dishes were placed in a plastic firmly closed Petri dish (9 cm in diameter) to avoid essential oil evaporation.
Each Syracuse dish contained a total of 1 ml of each prepared treatment. Surfactant solutions and distilled water served as a control. Syracuse dishes (3 dishes/ treatment) were left at room temperature (25°C). The hatched J2s were counted after four and seven days using a dissecting microscope.
2.4.1.2. Testing the mortality of second-stage Juveniles of RKNs M. incognita
Mortality experiments were carried out on second-stage juveniles. Freshly hatched J2s from handpicked eggmasses placed in water and incubated at room temperature were used. A total of 30 hatched J2s were placed in each Syracuse dish (1.5 cm in diameter) containing 0.5 ml of the prepared formulations (). Three replicates were performed and Syracuse dishes were left at room temperature (25°C). Numbers of dead J2s were counted after two, four and seven days of exposure using a dissecting microscope and mortality percentages were calculated.
Temporary mounts of exposed J2s to different treatments were prepared and examined using a compound microscope (Leica DM 750) to asses if there were any morphological changes on intestine.
2.4.2. Fungal material and inoculum preparation
An isolate of Fusarium oxysporum f. sp. lycopersici (Fol) was obtained from the stem of wilted tomato plants grown under plastic house conditions in the Jordan valley was used. The morphology and the molecular characterization of the isolated fungus were determined, Fol identity was verified at the morphological level using a diagnostic Key ( Citation25) and at the DNA level using species-specific primers ( Citation26), detailed isolation and identification methods are mentioned somewhere else ( Citation27).
To obtain cultures of the fungal isolate, a disc was taken from a stock culture and was placed on potato dextrose agar (PDA) and incubated at 25°C for five days to obtain fungal growth. Subsequently, sub culturing was performed to be used for further studies.
To examine JE inhibitory effect on Fol, testing for its growth on PDA media plates was performed. Fungal discs were soaked for 3 h in order to compare JE inhibitory effect with different prepared formulations (). After soaking, soaked Fusarium discs were transferred to a new PDA media plate. Mycelium morphological changes accompanied fungal composition were tested using a compound microscope.
2.4.2.1. Testing the growth of Fusarium oxysporum f. sp. lycopersici
To examine the JE inhibitory effect on Fol, testing for its growth on PDA media plates was performed. Fungal discs were soaked for 3 h in order to compare the JE inhibitory effect with different prepared formulations ().
After soaking, soaked Fusarium discs were transferred to a new PDA media plate. Mycelium morphological changes accompanied fungal composition were tested using a compound microscope.
3. Results and discussion
3.1. Phase diagram establishment
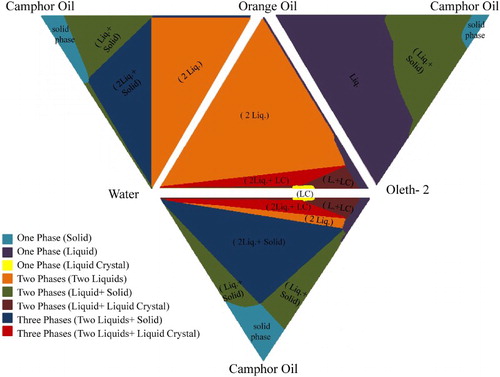
Four established ternary phase diagrams served as a mapping for the components used. The two-phase area from the established 3-D phase diagram was used to prepare the optimum formula of JE. The areas of the three phases were not used since they can cause unwanted destabilization effects in the emulsion (detailed information about each constructed phase diagram can be found in the supporting information). represents the 3-D ternary phase diagram, where all designed phase diagrams corners and edges of camphor oil, orange oil, water and oleth-2 were matched.
3.2. Emulsion preparation and observation
Different samples were prepared in the two-phase region (). Kinetically stable JE was obtained (low movement/separation for particles under microscope) (see Figure 7 in the supporting information) and the encapsulated globules were orange and camphor oil with water surrounding them. As the JE formula is described by (O1 + O2)/W, our emulsion can be referred to as orange oil + camphor oil/water.
From the established 3-D phase diagram (), dilution for concentrated JE was possible, where it is shown clearly that by diluting concentrated JE composition toward water corner, a two-phase system was still present. Different percentages were tested, the optimum formula was chosen upon the observed globules of JE, and the overall stability time of the emulsion.
The optimum composition for diluted Janus formula was camphor oil 3%, orange oil 1.5%, oleth-2 3.5%, and water 92% (Figure 8, supporting information).
As a result, encapsulation for orange and camphor oil in the Janus formula was obtained, and the concentrated Janus formula approved further water dilution tolerance. The stability of the different prepared emulsions was studied for the prepared percentages of concentrated and diluted JE and DE, where they were left to stand at room temperature (25°C). The destabilization study was limited to visual eye observation of layers separation.
The interfacial tension between emulsion components gives a indication on emulsion formation, where the interfacial free energies depend on the relative numbers for the interfacial tensions ( Citation22); the present contribution is concerned with the system, water/camphor oil/orange oil.
As recorded in the literature the interfacial tension between orange oil and water can be increased or decreased within the range of 4–8 mJ/m2 at 25°C due to the interfacial aging (which is an indication of interface instability that can be caused by several chemical reactions of oxidation and reduction or by adsorption) ( Citation28).
Interfacial tension between camphor oil and water was not recorded yet since when small bits of camphor oil are floated on water, they try to dissolve and reduce surface tension in that region, but due to the irregular sizes and shapes, and the rough edges of camphor oil water pushes with uniform surface tension against camphor edges and create a circular motion for camphor on water surface ( Citation29) and to the best of our knowledge the interfacial tension between camphor oil and orange oil was not recorded as well.
As a result, the interfacial tension will not give a true indication for the emulsion formation due to the interfacial aging, but since different topologies were formed, this indicates that the difference in the interfacial free energies were sufficient to form the different topologies of double or triple emulsion configuration (see supporting information, Figure 6).
Using the ternary phase diagram to demonstrate phase separation, concentrated and diluted JE tends to transfer to one-phase regions; one oil phase and pure water. Diluted Janus 1 and diluted Janus 2 emulsions are from the same two-phase area, so they tend to separate in the same way. Since low concentration of non-ionic surfactant oleth-2 was used in JE, the major instability recorded was creaming due to flocculation of oil droplets ( Citation30).
As concentrated and diluted JEs showed better kinetic stability, this emphasis the idea of lowering the interfacial tension between used oils by encapsulating them in one globule next to each other ( Citation31). As low HLB and high HLB surfactants were used in concentrated and diluted DE, this supposed to enhance particles’ stability but emulsions tend to separate after 3 min of their formation, which proves that using two surfactants in the emulsion can partially enhance kinetic stability, but emulsions’ topologies play a significant role in their kinetic stability.
Evaporation studies of emulsions were mentioned in the supplementary information as it plays an essential role in emulsions science, especially in the effectiveness of the application in different areas ( Citation32).
3.3. Nematicidal and fungicidal effects
3.3.1. Nematode culture
The prepared JEs were further tested against the RKN M. incognita. Results showed that concentrated JE caused a total inhibition of hatching of second-stage juveniles of the RKN (J2s), while few J2s hatched when the eggmasses were exposed to camphor oil emulsion alone, and to diluted Janus 1 emulsion.
Concentrated JE was as effective as camphor oil in causing high mortality of J2s of the RKN; however, the two diluted Janus concentrations have cidal activities but less than their camphor oil.
3.3.1.1. Effect of JE on hatching of second-stage Juveniles of RKNs M. incognita
Results showed that treatment of eggmasses of M. incognita with concentrated JE caused a total inhibition of J2s hatching, even after seven days of exposure while hatching occurred in all other emulsions four days after exposure and increased seven days after exposure.
The lowest hatching four and seven days post treatment was recorded in those eggmasses exposed to camphor oil emulsion followed by diluted Janus 1 emulsion and concentrated DE. The number of hatched J2s in orange oil emulsion was high and did not differ significantly than control. The hatching of J2s in both surfactants was high and did not differ significantly than control (). Our observations indicated that the size of the fresh egg was smaller than those exposed to JE, where J2s with empty internal regions were seen inside the egg but hatching process did not occur.
Table 2. Effect of treatments on hatching of J2s from eggmasses of M. incognita at different exposure times.
3.3.1.2. Effect of JE on mortality of second-stage Juveniles of RKNs M. incognita
After two days of exposure, concentrated JE and camphor oil reported to have the highest significant mortality percentage since 40% J2s of M. incognita died (). Significantly a fewer number of J2s were killed when exposed to the two diluted Janus concentrations than those J2s exposed to either concentrated Janus or camphor emulsion. On the other hand, the DEs whether concentrated or diluted did not cause any kill of J2s after two days of exposure. Similarly, the control treatments whether water or surfactant did not kill any J2s.
Table 3. Effect of treatments on mortality of J2s of M. incognita at different exposure times.
After four days of exposure, diluted Janus 1 and concentrated JE showed 62% and 55% dead J2s, respectively. Exposure of J2s to diluted Janus 2 and camphor oil emulsion reported 47% and 44% dead J2s, respectively. Results also showed that concentrated DE, orange oil and diluted DE have the lowest mortality effect on J2s.
After seven days of exposure results indicated that diluted Janus 1 and concentrated JE recorded the highest significant mortality effect on J2s with an average of 84% and 69% dead J2s, respectively, compared to concentrated DE and diluted DE that showed 38% and 3.93% dead J2s, respectively. Dead J2s were observed in control treatments and this might be due to the lack of host (food) for the obligatory parasitism of this nematode.
Regarding morphological changes for J2s, J2s exposed to diluted DE, surfactants, orange oil and diluted Janus 2 did not differ significantly than distilled water treatment (). While camphor, diluted JE and concentrated JE exhibited changes in the intestinal regions, where vacuoles appeared in some intestinal region of J2s exposed to concentrated JE, deterioration of the intestine was observed in J2s of RKN exposed to camphor oil emulsion and diluted Janus 1 emulsion.
By studying the effect of the used emulsions acidity on RKN, increasing acidity did not affect the hatching or the survival of J2s as indicated in a study for chemical fertilizers effect on RKN. The study reported that decreasing the pH affected adversely juveniles of M. incognita hatching and survival ( Citation33), and in this study, results showed that J2s survived at a pH ranged from 3.79 to 7.10 ().
3.3.2. Fungal culture
3.3.2.1. Effect of JE on the growth of Fusarium oxysporum f. sp. lycopersici
Results showed that the treatment of Fol with concentrated DE caused a total inhibition of Fusarium growth four days of incubation and even after seven days of exposure. Partial growth of the fungus resulted upon exposure to concentrated JE, where results showed that there was no significant difference between concentrated JE and concentrated DE growth within four days of exposure, but after seven days of exposure concentrated JE was only able to delay the fungal growth while concentrated DE inhibited its growth.
Orange oil, diluted Janus 2 and diluted Janus 1 emulsions showed a medium inhibitory effect for growth after four and seven days of exposure, where after seven days average fungal growth of 75%, 76% and 77% was shown ().
Table 4. Effect of treatments on growth of F. oxysporum f. sp. lycopersici exposed for 3 h.
Orange oil indicated to have a slightly higher inhibitory effect than camphor oil after four days of exposure (36% and 45% growth, respectively) and seven days of exposure (75% and 81% growth, respectively). After four and seven days of exposure the fungal Fol exposed to camphor oil, polysorbate-80, diluted double and oleth-2 emulsions did not differ significantly from control.
Mycelium morphological changes accompanied fungal composition after seven days of exposure for orange oil emulsion, concentrated JE, concentrated DE and control, where after seven days of exposure to orange oil, diluted Janus 1 and diluted Janus 2, and polysorbate-80, Fusarium mycelium had purple color, where Fol colonies are usually fast growing, mycelium is colorless at first and with age it becomes pale pink or purplish ( Citation15, Citation34, Citation35). Irregular growth for Fusarium was observed upon exposure to diluted DE and oleth-2 sample emulsions.
Morphological changes of mycelium accompanied fungal growth (caused from the penetration of the emulsion to the mycelium), which in turn inhibited its growth. Concentrated DE (instead of JE) recorded the total inhibition effect against fungal growth. This result can be discussed upon the topology of Janus and DEs. JE globules contain both oils next to each other, which cause the two oils to be released gradually at a time; hence oils (camphor and orange oil) can be released in the same manner. The first oil to penetrate the mycelium affects its growth. Concentrated JE, diluted Janus 2 and diluted Janus 1 could only delay the growth of the fungus after seven days of exposure compared to distilled water; this can indicate the slow release of the two oils at one time from Janus globules.
Concentrated DE contains water/orange oil/camphor oil/water globules topology. It contains orange oil surrounding camphor oil; hence, orange oil (higher fungicidal effect than camphor oil) will have the first penetration into the mycelium and inhibit their growth. There was no growth of Fol exposed to concentrated DE within four and seven days; this indicates that all orange percentages in the emulsion were released and entered the mycelium at once and inhibited its growth. The effect of growth inhibition from the orange oil release did not show up in the diluted DE upon the exposure of the fungus, which indicates changes in the effectiveness of used oils, whereby in JE all formulations showed same effect, and this enhances the efficiency of Janus formulation use when a slow release of emulsion contents in recommended.
4. Conclusion
Immiscible camphor and orange oils were used in Janus droplets as they have well-known nematicidal and fungicidal activities that allow their use in further agricultural studies. The behavior of camphor and orange oils with water and Oleth-2 surfactant was studied and mapped in a 3-D phase diagram to be able to prepare the optimum formula of JE from a suitable area. For a comparison study, different concentrations of JE and double ordinary emulsions (DEs) were prepared. Essential oils efficiencies within the different prepared formulas were tested on hatching and mortality of M. incognita J2s and on inhibiting the growth of Fol. Hatching in vitro studies indicated that JEs had the highest nematicidal effect against J2s hatching inhibition. The same results were observed on J2s mortality, where JEs caused a high percentage of killing for J2s four and seven days after exposure and were more effective than DEs. Studies on the wilt fungus showed that the inhibiting effect of concentrated DE on the growth of Fol was not maintained in the diluted DE, while JEs showed same inhibition effects. This demonstrates the changes in the composition and efficiency of DEs while for JE the efficiency for the encapsulated oils was maintained.
Data statistical analysis
Data obtained from egg hatching experiments were analyzed by using ANOVA ( Citation36). Means were separated using the Duncan multiple range test (DMRT) at a P = .05. Mortality data for J2s were corrected according to Schneider–Orelli’s formula.
Supplementary_Material.doc
Download MS Word (1.2 MB)Acknowledgements
Also, the authors would extend their gratitude to HMSCR (Hamdi Mango Center for Scientific Research) and the Faculty of Agriculture at the University of Jordan for providing a suitable well-equipped working environment.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
Mona Sharar is currently a PhD student at Humboldt University of Berlin and a research assistant in Bundesanstalt für Materialforschung und -prüfung (BAM). The author received her MSc degree in Chemistry from the University of Jordan, Amman, in 2014, where her research direction was toward green chemistry to eliminate the generation of hazardous substances.
Ayat Bozeya is currently a PhD student at the University of Jordan, Amman and a researcher in Hamdi Mango Center. She is a physical chemist, her research direction is concerned with colloidal chemistry and emulsification techniques.
Luma Al-Banna is an Associate professor at the University of Jordan, Amman. She received her PhD and master's degree in plant pathology/Nematology from University of California, Davis, in 1996. Her research is concerned with plant pathology/Nematology.
Abeer Al-Bawab is a professor of Physical Chemistry at the University of Jordan, Amman. She received her PhD and master's degree in Physical Chemistry from Clarkson University (CU) – New York, USA, in 1997. Her research is concerned with colloidal chemistry and emulsification techniques.
ORCID
Abeer Al-Bawab http://orcid.org/0000-0003-2131-1791
Additional information
Funding
References
- Al Bawab, A.; Bozeya, A.; Hasinovic, H.; Friberg, S.E. Arab. J. Chem. 2015, 8, 246–254. doi: 10.1016/j.arabjc.2011.10.005
- Friberg, S.E.; Al Bawab, A.; Bozeya, A.; Aikens, P. J. Colloid Interface Sci. 2009, 336, 345–351. doi: 10.1016/j.jcis.2009.03.036
- Perro, A.; Reculusa, S.; Ravaine, S.; Bourgeat-Lami, E.; Duguet, E. J. Mater. Chem., 2005, 15, 3745–3760. doi: 10.1039/b505099e
- Dluska, E.; Markowska, A. Chem. Eng. Process. 2009, 48, 438–445. doi: 10.1016/j.cep.2008.06.005
- Pashley, R.M.; Karaman M.E. Applied Colloid and Surface Chemistry; Wiley: Chichester, West Sussex, 2004; pp 1–11, 79–90, 127.
- Guzowski, J.; Garstecki, P.; Korczyk, P. Pol. Acad. Sci. 2010, 44, 01–224.
- Hasinovic, H. A One-step Process to Prepare Triple Janus Emulsions. Doctoral Dissertation, Ray University, 2012.
- Andreas, W.; Axel, M. Chem. Rev. 2013, 113, 5194–5261. doi: 10.1021/cr300089t
- El-Zemity, S.; Ahmed, S. J. Pest Control Environ. Sci. 2005, 13, 61–72.
- Al-Banna, L.; Darwish, R.; Aburjai, T. Phytopathol. Mediterr. 2003, 42, 123–128.
- Cosic, J.; Vrandecic, K.; Postic, J.; Jurkovic, D.; Ravlic, M. Poljoprivreda. 2010, 16, 25–28.
- Vasudera, N.; Sharma, T. Pharmaceut. Drug Res. 2012, 1, 1–7.
- Abu-Gharbieh, W.I. Root-Knot Nematodes, Meloidogyne Species, in Jordan, 2nd ed.; University of Jordan Publication: Amman, 1994; 1–60 ( in Arabic).
- Khan, S. Screening of Tomato Cultivars Against Root Knot Nematodes and Their Biological Management. Doctoral Dissertation, University of Agriculture, Faisalabad, Pakistan, 2009.
- Agrios, G.N. Plant Pathology, 5th ed.; Elsevier Academic Press: San Diego, CA, 2005; pp 12–200.
- Barbosa, P.; Lima, A.S.; Vieira, P.; Dias, L.S.; Tinoco, M.T.; Barroso, J.G.; Pedro, L.G.; Figueiredo, A.C.; Mota, M. J. Nematol. 2010, 42, 8–16.
- Pattnaik, S.; Subramanyam, V.R.; Kole, C.R. Microbios. 1996, 86, 237–46 ( Abstract).
- Rodriguez-Kabana, R.; Simmons, L. Fungicidal Herbicidal and Nematicidal Activities of Essential Oils in Slow-Release Formulations; Auburn University and Albama Agricultural Experiment station: Auburn, Alabama, 2005; pp 28–31.
- Al Bawab, A.; Barber, J.L.; Friberg, S.E.; Aikens, P. J. Dispers. Sci. Technol., 1998, 19, 399–420. doi: 10.1080/01932699808913182
- United States Environmental Protection Agency. Herbicides. http://www.epa.gov/caddis/ssr_herb_int.html (accessed Nov 12, 2013).
- Curran, W.S. Adjuvant for Enhancing Herbicide Performance. http://extension.psu.edu/pests/weeds/control/adjuvants-for-enhancing-herbicide-performance (accessed Nov 12, 2013).
- Hasinovic, H.; Friberg, S.E. J. Colloid Interface Sci. 2011, 361, 581–586. doi: 10.1016/j.jcis.2011.05.069
- Meyer, S.L.F.; Lakshman, D.K.; Zasada, I.A.; Vinyard, B.T.; Chitwood, D.J. Herltechnology. 2008, 18, 631–638.
- Bader, Y.M. Investigating the Suppressive Effects of Entomopathogenic Nematodes on Root-Knot Nematodes Attacking Cucumber. PhD Dissertation, Faculty of Agriculture; University of Jordan, Amman, Jordan, 2013.
- Booth, C. The Genus Fusarium. Common Wealth Agriculture Bureaux: Kew, Surrey, 1971.
- Yoder, W.; Christianson, L. Fungal Genet. Biol. 1998, 23, 68–80. doi: 10.1006/fgbi.1997.1027
- Akash, M.; AL- Banna, L.; Al-awaida, W.; Hidmi, T. Res. Crops. 2014, 15, 423–431. doi: 10.5958/2348-7542.2014.00132.6
- Arneodo, C.; Baszkin, A.; Benoit, J.P.; Thies, C. Colloids Surf. 1989, 34, 159–169. doi: 10.1016/0166-6622(88)80094-5
- Bhantnagar, V.P. A Complete Course in ISC Physics; Pitambar: New Delhi, 2009.
- Schick, M.J. Nonionic Surfactants Physical Chemistry; Madison Avenue: New York, 1987.
- Jiao, J.; Burgess, D.J. Multiple Emulsion Stability: Pressure Balance and Interfacial Film Strength; Wiley: Hoboken, NJ, 2008.
- Al Bawab, A.; Odeh, F.; Bozeya, A.; Aikens, P.A.; Friberg, S.E. Flavour Fragr. J. 2009, 24, 155–159. doi: 10.1002/ffj.1926
- Habash, S.S.; Al-Banna, L. J. Nematol, 2010, 43, 95–100.
- Pitt, J.I.; Hocking, A.D. Fungi and Food Spoilage, 3rd ed.; Springer: NY, USA. 2009, p 519.
- Duschatzky, C.; Martinez, A.; Almeida, N.; Bonivardo, S. J. Essent. Oil Res. 2004, 16, 621–626. doi: 10.1080/10412905.2004.9698812
- Little, T.M.; Hills, F.J. Agricultural Experimentation: Design and Analysis; New York: John Wiley & Sons, 1978.