ABSTRACT
3-(N,N-dimethyldodecylammonium) propanesulfonic acid hydrogen sulfate ([SB3-12][HSO4]), a Brønsted acidic-surfactant-combined ionic liquid, was successfully prepared from cheap materials and applied to catalyze the esterification synthesis of ethyl oleate using oleic acid and ethanol as precursors. The produced condition of oleic acid ethyl ester was optimized including the amount of catalyst, reaction time, molar ratio and content of water. Under optimal condition (ethanol to oleic acid molar ratio, 3:1; amount of the catalyst, 5 wt% (based on the mass of oleic acid); reaction time, 3 h; reaction temperature, 78°C; and content of water, 0.4 wt%), the conversion of oleic acid was over 97%. Due to the reverse micelles formed by [SB3-12][HSO4], the micro-reactor not only promoted the esterification toward the desired side of the ester, but also avoided the hydrolysis of ester. Moreover, the [SB3-12][HSO4] could be reused by a simple decantation separating process and noticeable loss of catalytic activity was not observed even after being recycled for five cycles. The green system offers several advantages, such as excellent yield, efficient catalytic activity, free-solvent and simple operational procedure.
GRAPHICAL ABSTRACT
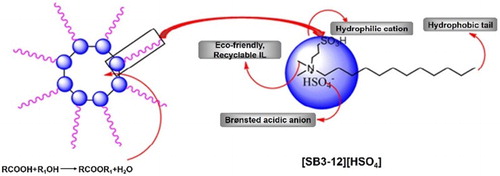
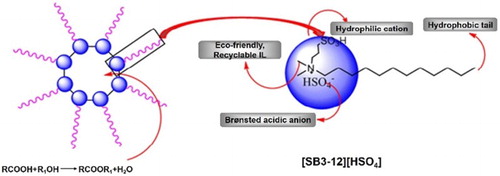
1. Introduction
Esterification of oleic acid with ethanol in the presence of catalytic acids has attracted a lot of attention, since its product ethyl oleate can be applied for the production of biodiesel, be used as solvents for the pharmaceutical industry and serve as lubricant or plasticizer (Citation1). In general, most of the reported acid catalysts are liquid inorganic acids like H2SO4 and solid acids (Citation2,Citation3). However, liquid inorganic acids inevitably have many disadvantages, such as corrosion of equipment, troublesome product separation and environmental pollution (Citation4); and solid acids have also shown some disadvantages, including low activity, easy deactivation and adsorption of products (Citation3,Citation5). To address the drawbacks of both liquid and solid acids, a novel catalytic system, for example, colloidal dispersion or reverse micelles, formed by Brønsted acid–surfactant-combined catalyst, has been reported (Citation6). For example, Kabayashi et al. reported dodecylbenzene sulfonic acid (DBSA)-catalyzed esterification of carboxylic acids and alcohols in water (Citation7,Citation8). In these reactions, DBSA and substrates were able to form emulsion droplets, whose interior was hydrophobic enough to exclude water molecules generated during the reactions. DBSA as a Brønsted acid–surfactant-combined catalyst would self-assemble and made acid catalysts concentrated at the interface of the micelles and thus would enhance the catalytic efficiency. However, DBSA could not be recycled due to the difficulty associated with product separation. As a consequence, the search for novel and green catalysts is still being pursued.
In recent years, Brønsted acidic ionic liquids represent promising catalysts for esterification reaction with excellent yields (Citation9–12). By using the unique merits of the ionic liquids (ILs) to dissolve reactants rather than the product esters, a liquid–liquid biphase will be created when the reaction is complete; consequently, ILs can be reused by decantation of the ester (Citation9–12). For example, Davis and co-workers observed temperature-controlled liquid–solid separation by an alkane sulfonic acid IL as dual solvent-catalyst for esterification (Citation13). Although the system was promising, these results were not satisfactory because the present systems suffered from high content of ILs (20–300 mol%), relatively long reaction time and the need of removing by-product water from the reaction system (Citation10). Surfactant-like ILs, which have amphiphilic nature and are able to form micelle in aqueous solution, represent a novel generation of cationic surfactants with peculiar properties (Citation14–17), and have recently been taped as a new class of IL. Aghabarari et al. described the use of some surfactant–Brønsted acidic-combined ILs based on imidazolium cations as catalysts, which increased the efficiency of transesterification and esterification (Citation18,Citation19). However, ILs containing imidazolium cation are relatively expensive, hindering their industrial applications. Thus, it is necessary to synthesize inexpensive ILs, which can be straightforwardly used (Citation20).
In this work, a Brønsted acidic ionic liquid, 3-(N,N-dimethyldodecylammonium) propanesulfonic acid hydrogen sulfate ([SB3-12][HSO4]), was synthesized with surfactant properties, possessing a hydrophobic tail, a hydrophilic cation and a Brønsted acidic counter ions (see ). [SB3-12][HSO4] was demonstrated to assemble reverse micelles in the mixtures of oleic acid and ethanol to promote esterification reaction. The formed micelles had a hydrophilic interior; thus, the product esters generated during the reaction were found to be effectively excluded from the reverse micelles, and water molecules generated during the reaction could automatically enter the interior of the reverse micelles. The auto-separation of water from ester avoided the reverse hydrolysis of the formed ester. Furthermore, self-aggregation of [SB3-12][HSO4] would concentrate acid catalysts at the interface of the reverse micelles. With these two advantages, the esterification equilibrium would shift toward the desired side of the ester (Citation21) (see ). An efficient and environmentally friendly catalyst, Brønsted acid–surfactant-combined ionic liquid, was demonstrated for the synthesis of ethyl oleate considering the high catalytic activity and its easy reusability.
2. Results and discussions
2.1. TGA result
To ascertain the thermal degradation of [SB3-12][HSO4], TGA was performed and the TGA curve for [SB3-12][HSO4] is shown in .
A multi-step weight loss process was observed from 50°C to 600°C. From 50°C to 220°C, the observed weight loss of 10% was related to the removal of water and organic solvent. From 220°C to 600°C, the weight loss of 87% was ascribed to the decomposition of the [SB3-12]+ and HSO4−. The weight of [SB3-12][HSO4] was sharply decreased at above 220°C, Therefore, the thermal decomposition temperature of [SB3-12][HSO4] of 220°C is stable enough to be a catalyst in ethyl oleate synthesis.
2.2. Catalytic performances of [SB3-12][HSO4] for ethyl oleate synthesis
2.2.1. Effect of the catalyst amount on the oleic acid conversion
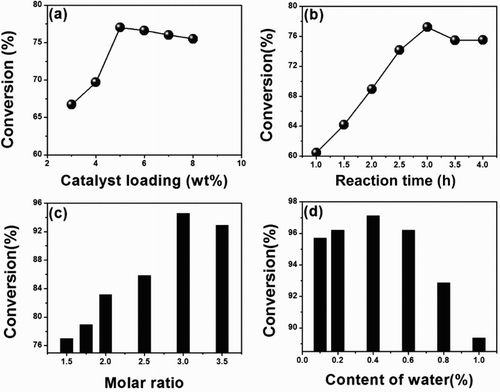
(a) shows the effect of the [SB3-12][HSO4] amount on the oleic acid conversion (based on the mass of oleic acid). When the amount of [SB3-12][HSO4] was at 3% and 4%, the conversion of oleic acid was increased, probably because the catalyst amount was low and the number of available active sites was not sufficient to promote the reaction of oleic acid and ethanol. When the amount of the catalyst was increased continuously, water was produced during esterification as hydrophilic substance and reverse micelles were formed from the hydrophobic reactants in the presence of a certain amount of catalyst. Water molecules automatically entered the reverse micelle interiors due to the system hydrophobicity, and the catalyst was existing at the interface of reverse micelles to promote the reaction. Moreover, the catalyst increased the number of acid sites available; thus, the conversion of oleic acid reached a maximum as the amount of the catalyst was at 5%. However, the conversion of oleic acid was decreased when the amount of catalyst was increased from 6% to 7% and 8%. This is likely due to an excessive amount of the catalyst, although further increases in the amount of catalyst could result in some degree of catalyst aggregation and decrease the number of acidic sites available (Citation22,Citation23). Similar results have also been reported in the literature about the surfactant–Brønsted acidic-combined catalyst (Citation18,Citation19,Citation24). Therefore, the optimum mass ratio of the catalyst to oleic acid was found to be 5%.
Figure 3. Factors on oleic acid conversion (based on the mass of oleic acid): (a) catalyst loading, reaction conditions: n (ethanol): n (oleic acid) = 1.5:1, 78°C, 3 h; (b) reaction time, reaction condition: n (ethanol): n (oleic acid) = 1.5:1, 5 wt% catalyst (based on mass of oleic acid), 78°C; (c) amount of ethanol, reaction condition: 5 wt% catalyst (based on mass of oleic acid), 3 h, 78°C; (d) water content on the catalytic activity, reaction condition: n (ethanol): n (oleic acid) = 1.5:1, 5 wt% catalyst (based on mass of oleic acid), 3 h, 78°C.
2.2.2. Effect of reaction time on the oleic acid conversion
(b) shows the effect of reaction time on the conversion of oleic acid. The results indicate that the conversion was increased with increasing the reaction time, and reached a maximum of 77.2% with a reaction time of 3 h. After this time, the conversion was decreased slightly. This observation indicates that esterification is an equilibrium reaction and the ester product can react with water formed during the reaction to reduce slightly the conversion of oleic acid after 3 h (Citation18,Citation19,Citation25,Citation26).
2.2.3. Effect of the amount of ethanol on the oleic acid conversion
An excess of reactant ethanol is necessary for the esterification of oleic acid with ethanol, because more reactants would shift the reaction equilibrium toward the product ethyl oleate. (c) shows the effect of the amount of ethanol on the esterification of oleic acid with ethanol. The highest conversion of oleic acid was 94.6% with an ethanol to oleic acid mole ratio of 3:1, 5 wt% (based on the mass of oleic acid), a reaction time of 3 h and reaction temperature, 78°C. A further increase of this mole ratio resulted in a decrease in the conversion of oleic acid, probably because oleic acid and catalyst became excessively diluted with excess ethanol.
2.2.4. Effect of the amount of water on the oleic acid conversion
Water is a by-product of the esterification; a little amount of water in the oleic acid/ethanol/[SB3-12][HSO4] system is easy to form reverse micelles to catalyze the esterification reaction (Citation27). Thus, the effect of water amount on the esterification was investigated. Some water had been added to the mixture under the optimum reaction conditions and the results are given in (d).
The catalytic activity was observed to be related to the water content. The conversion was increased gradually when the water content was increased from 0.1% to 0.4%. The conversion was increased to 97.1% at a water content of 0.4%. However, the conversion was decreased gradually when the water content was increased from 0.6% to 1.0%. The conversion was significantly reduced to 89.4% when 1.0% water was added. The results were ascribed to the formation of reverse micelles in the presence of a little water. Once the reverse micelles with a hydrophilic interior assembled by [SB3-12][HSO4] were formed, the reverse micelles served as micro-reactors to enhance the esterification and micro-separator to automatically separate water produced during the reaction from ethyl oleate favoring the ester formation. The separation of water from ethyl oleate not only prevented hydrolysis of ethyl oleate, but also rapidly promoted the equilibrium to shift toward the product ethyl oleate. However, excessive amounts of water would inhibit the proceeding of esterification (Citation21,Citation28).
Based on the aforementioned results, the best activity of [SB3-12][HSO4] reached 94.6% conversion. The esterification of oleic acid with ethanol in the presence of [SB3-12][HSO4] could be smoothly performed in the mild conditions, and the product ethyl oleate could be isolated conveniently with high conversion. However, the same reaction catalyzed by 12-tungstophosporic acid supported on zirconia only 88% of oleic acid conversion (4 h, 100°C) (Citation1). Therefore, [SB3-12][HSO4] was an efficient catalyst of the synthesis of ethyl oleate.
2.2.5. Analysis of the catalytic mechanism of esterification
The higher catalytic activity of [SB3-12][HSO4] could be related to its surfactant properties that could self-assemble the reverse clusters in hydrophobic reagents. The reverse clusters increased the interaction between the hydrophobic oleic acid and the Brønsted acidic sites at the interface of the clusters, which could catalyze the esterification more efficiently. Moreover, because of the hydrophobic property of the cluster externally and hydrophilicity of the cluster in the interior, the produced water could automatically enter the cluster interior while the ethyl oleate product was removed from the cluster. With progressing the reaction, the clusters were enlarged to form inverse micelles. The separation of water from the ester promoted the reaction equilibrium to shift toward the desired side of the ester (Citation21,Citation28).
To prove the produced water could enter the inverse micelles, the micropolarity of the reverse micelle was studied by UV–visible spectroscopy. The shift in the visible absorption maximum λmax of methyl orange (MO) is a sensitive spectra of the local environment about micelles (Citation29–31). The λmax of MO will shift to long wavelengths with increasing the water content in the micelles’ interior (Citation32), indicating great polarity. shows the visible absorption spectra of MO in the reaction system with different reaction times. When the reaction was controlled for 1, 2 and 3 h in the optimum conditions, the visible absorption reached maximum of MO was 402, 412 and 420 nm, respectively. The shift to long wavelengths in the visible absorption maximum clearly demonstrates that the resultant water entered the interior of reverse micelles.
Figure 4. UV spectra of MO in the reaction system with different reaction times ((a) 1 h λmax = 402 nm; (b) 2 h, λmax = 412 nm; (c) 3 h, λmax = 420 nm) at 298 K, [MO] = 1 × 10−5 M.
![Figure 4. UV spectra of MO in the reaction system with different reaction times ((a) 1 h λmax = 402 nm; (b) 2 h, λmax = 412 nm; (c) 3 h, λmax = 420 nm) at 298 K, [MO] = 1 × 10−5 M.](/cms/asset/ad7f7af8-503d-4f9a-86dd-6e4130a76561/tgcl_a_1342001_f0004_c.jpg)
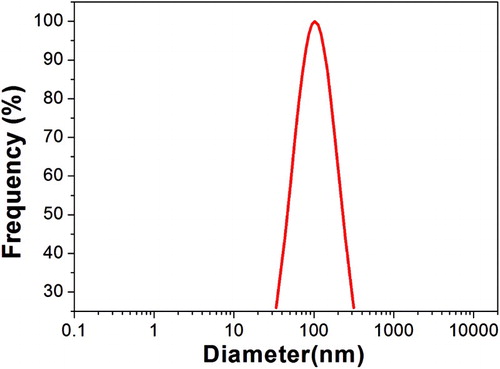
Ionic liquid [SB3-12][HSO4] was insoluble in oleic acid. When ethanol was added, the mixture was stirred until transparent and homogeneous. The size and size distributions of the possible micelles in the mixture were then measured by dynamic light scattering (DLS). No size was detected, suggesting no formation of reverse micelles at the start of the reaction. The average size and shape of micelles formed from [SB3-12][HSO4] at the end of reaction in the optimum conditions were confirmed by DLS and transmission electron microscope (TEM), respectively. shows the sizes of micelles distributed in range of 35–300 nm in diameter. demonstrates that the shape of the formation of micelles by [SB3-12][HSO4] was mostly spherical. The reverse micelles behaved as micro-separators in the reaction, which automatically separate the produced water from the product ethyl oleate.
2.2.6. Reusability of [SB3-12][HSO4] for the esterification
One of the most important factors in designing a new catalyst is its reusability. Thus, the recycling performance of this Brønsted acid–surfactant-combined IL was investigated by performing five runs under optimized conditions. After completion of the reaction, the reaction mixture was centrifuged, a biphasic system was formed and the ethyl oleate of the upper phase was separated by simple decantation. [SB3-12][HSO4] that remained at the lower phase was then washed with diethyl ether to remove ethyl oleate and used for the next cycle after removing water and ethanol. The recovered activities of [SB3-12][HSO4] for esterification were investigated (). It was revealed that the catalyst remained almost stable after five runs and gave a relatively high conversion of oleic acid of 88.9%. In order to determine the leaching of ionic liquid in reuse of [SB3-12][HSO4] during the esterification, the amount of sulfur leaching of [SB3-12][HSO4] after five cycles was measured by elemental analysis. The result showed that about 5% sulfur was leached. The performance indicated that [SB3-12][HSO4] afforded outstanding activity and desirable stability in the esterification of oleic acid with ethanol.
Figure 7. Reusability of [SB3-12][HSO4] for esterification. Reaction conditions: n (ethanol): n (oleic acid) = 1.5:1; catalyst = 5wt% (based on the mass of oleic acid), 3 h, 78°C.
![Figure 7. Reusability of [SB3-12][HSO4] for esterification. Reaction conditions: n (ethanol): n (oleic acid) = 1.5:1; catalyst = 5wt% (based on the mass of oleic acid), 3 h, 78°C.](/cms/asset/8c85a44d-f109-4352-a458-6b14ca89ed86/tgcl_a_1342001_f0007_b.gif)
In comparison with enzymatic synthesis of ethyl oleate, native and immobilized lipase was highly active with a conversion of 78.6% in a 7-h reaction. However, owing to inactivation or desorption of lipase, the catalyst was not reused effectively (Citation33). Therefore, [SB3-12][HSO4] showed high activity and excellent re-use potential.
3. Experimental section
3.1. Materials and methods
N,N-Dimethyldodecylamine (97%) and 3-propanesultone were purchased from the Aladdin-Reagent Co. Ltd. Other chemicals (AR) were obtained from Tianjin Guangfu Fine Chemical Research Institute Co., Ltd. All the chemicals were used as received without any further treatment.
The melting points were determined using a MP300 melting point apparatus. FT-IR spectra were recorded using a PerkinElmer Spectrum RX1 spectrophotometer in the range from 400 to 4000 cm−1. The 1H NMR and 13C NMR spectra were measured by using a Bruker 400 MHz spectrometer in CD3OD. The thermal decomposition profiles were collected through DTG-60 simultaneous Thermal Analyzer (10°C min−1 heating rate under nitrogen). The diameter of the reaction system was recorded by a NanoBroke 173Plus particle analyzer using DLS. The UV–vis molecular absorbance data were collected on T6 UV–vis spectrometer at room temperature, and the gas chromatography (GC) analysis was performed on an Agilent model 7890A GC coupled to a hydrogen ion FID. Elemental analyses were recorded on Elementar Vario EL cube. The shape of micelles was confirmed by 200 kV Tecnai G2 TEM.
3.2. Catalyst preparation and characterization
3.2.1. Characterization of the 3-(N,N-dimethyldodecylammonium) propanesulfonate
The 3-(N,N-dimethyldodecylammonium) propanesulfonate was synthesized according to the following procedure: N,N-dimethyldodecylamine (10 mL, 0.06 mol) was slowly added to a solution of 1,3-propanesultone (5.0 mL, 0.06 mol) in 15 mL 1,2-dichloroethane. After stirring for 3 h at 55°C, acetone was added and then the reaction mixture was filtered to obtain the white precipitate. Subsequently, the precipitate was recrystallized in acetone-95% ethanol solution, and then dried under a vacuum at 60°C for 10 h, yielding 3-(N,N-dimethyldodecylammonium) propanesulfonate([SB3-12]). Standard characteristics are listed as follows with Mp: 235.3–237.1°C; FT-IR(KBr): 3448, 2918, 2852, 1654, 1468, 1196, 1046 and 722; 1H NMR (400 MHz, CD3OD): δ 0.92(t, 3H, CH3–C–), 1.31 (s, 18H, –CH2–), 1.78 (s, 2H, C–CH2C–N), 2.17–2.21 (pent, 2H, N–C–CH2–C–S), 2.87 (t, 2H, CH2–S), 3.11 (s, 6H, N–CH3), 3.33–3.35 (pent, 2H, N–CH2), 3.51–3.54 (pent, 2H, –CH2–C–C–S); 13C NMR (400 MHz, CD3OD): δ13.02, 18.53, 22.32, 25.55, 26.04, 29.33, 31.66, 32.29, 47.40–47.83, 49.86, 61.62, 62.49 and 64.25.
3.2.2. Characterization of the 3-(N,N-dimethyldodecylammonium) propanesulfonic acid hydrogen sulfate
3-(N,N-dimethyldodecylammonium) propanesulfonic acid hydrogen sulfate ([SB3-12][HSO4]) was prepared by mixing the 3-(N,N-dimethyldodecylammonium) propanesulfonate with an equal mole sulfuric acid solutions (98%) stirring for 3 h at 80°C. The mixed solution was dried under vacuum at 100°C, then washed repeatedly with diethyl ether to remove unreacted materials and dried in a vacuum at 50°C again. A light yellow viscous liquid was finally obtained. Standard characteristics are listed as follows with FT-IR(KBr): 3415, 2927, 2844, 1630, 1456, 1220, 1046, 1028, 886, 720 and 585. 1H NMR (400 MHz, CD3OD): δ 0.79 (t, 3H, CH3–C–1.20–1.31 (s, 18H, –CH2–), 1.67 (s, 2H, C–CH2C–N), 2.08–2.10 (pent, 2H, N–C–CH2–C–SO3), 2.78 (t, 2H, CH2–S), 2.98 (s, 6H, N–CH3), 3.20 (pent, 2H, N–CH2), 3.39 (pent, 2H, –CH2–C–C–S); 13C NMR (400 MHz, CD3OD): δ13.01, 18.49, 22.10, 22.32, 26.02, 28.81–29.33, 31.66, 46.96–48.24, 49.86, 62.38 and 64.80.
3.3. General procedure for ethyl oleate synthesis
A certain molar ratio of oleic acid and ethanol, and a certain amount of [SB3-12][HSO4] were added to a round bottom flask fitted with a reflux condenser. The reaction mixture was continuously stirred using a magnetic stirrer in an oil-bath for a length of reaction time at 78°C. The effects of the catalyst amount, reaction time and the amount of ethanol on the oleic acid conversion were investigated. An emulsion was then obtained showing the surfactant property of [SB3-12][HSO4]. After completion of the reaction, the reactor was cooled down to room temperature, and two phases were formed. Specifically, [SB3-12][HSO4] stayed at the bottom of the flask, which was immiscible with ethyl oleate of the upper phase. The reaction scheme for the ethyl oleate synthesis is shown in . Thus, the ethyl oleate could be simply separated by decantation, and the [SB3-12][HSO4] was easily recycled after removing water and ethanol. The content of the reaction product (ethyl oleate) was measured by GC, and the conversion of oleic acid was calculated, based on the acid value, in the following equation:(1) where a1 is the acid value (mg KOH g−1) of oleic acid, and a2 is the acid value (mg KOH g−1) of the upper phase after removing water and ethanol in a vacuum at 70°C. The acid value (the mass of KOH in milligrams, which is required to neutralize 1 g chemical material) was determined by a KOH in ethanol solution titration using phenolphthalein as indicator. All reported data were averages of the experiments performed several times, and the average error for the determination was less than 3%.
4. Conclusion
In conclusion, the Brønsted acidic–surfactant-combined ionic liquid [SB3-12][HSO4] has been synthesized successfully and demonstrated to be an efficient and reusable catalyst for esterification of oleic acid with ethanol with a conversion of oleic acid of 94.6% under the optimized reaction conditions. By controlling the water content, the conversion can reach even 97.1%. More importantly, reverse micelles formed by [SB3-12][HSO4] in the reaction system not only prevent the micro-reactor hydrolysis of ethyl oleate, but also rapidly promote the equilibrium reaction toward ethyl oleate. Therefore, the esterification catalyzed by the Brønsted acidic–surfactant-combined ionic liquid [SB3-12][HSO4] should be promising for practical production as a green process; we are proceeding to investigate catalytic effects on other condensation reactions by Brønsted acid–surfactant-combined ionic liquid.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
Yongjun Zheng received his Ph.D. from the Graduate University of Chinese Academy of Science in 2009. He is now a teacher in the Anyang Institute of Technology. His research interest focuses on the area of ionic liquid and micellar catalysis.
Yong Zheng received his Ph.D. from the University of Chinese Academy of Sciences in 2013. He is now a teacher in the Anyang Institute of Technology. His research interest focuses on the area of ionic liquid.
Shuang Yang obtained her master’s degree from the China University of Mining and Technology in 2006. His research focuses on the area of organic chemistry.
Zhanhu Guo is the Director of Integrated Composites Laboratory in the Chemical and Biomolecular Engineering Department, University of Tennessee. His research interest focuses on novel catalysts.
Tao Zhang is studying in the Department of Chemical and Environmental Engineering, Anyang Institute of Technology. He is mainly investigating esterification.
Haixiang Song received his Ph.D. from the Beijing Institute of Technology in 2005. Her research has focused on polymer nanomaterials.
Qian Shao is the professor of the College of Chemical and Environmental Engineering, Shandong University of Science & Technology. Her research interest focuses on novel catalysts.
Additional information
Funding
References
- Oliveira, C.F.; Dezaneti, L.M.; Garcia, F.A.C.; de Macedo, J.L.; Dias, J.A.; Dias, S.C.L.; Alvim, K.S.P. Appl. Catal. A: Gen. 2010, 372 (2), 153–161. doi: 10.1016/j.apcata.2009.10.027
- Melo Júnior, C.A.R.; Albuquerque, C.E.R.; Carneiro, J.S.A.; Dariva, C.; Fortuny, M.; Santos, A.F.; Egues, S.M.S.; Ramos, A.L.D. Ind. Eng. Chem. Res. 2010, 49 (23), 12135–12139. doi: 10.1021/ie100501d
- Harmer, M.A.; Sun, Q. Appl. Catal. A: Gen. 2001, 221 (1–2), 45–62. doi: 10.1016/S0926-860X(01)00794-3
- Zhang, L.; Cui, Y.; Zhang, C.; Wang, L.; Wan, H.; Guan, G. Ind. Eng. Chem. Res. 2012, 51 (51), 16590–16596. doi: 10.1021/ie302419y
- Ishihara, K.; Hasegawa, A.; Yamamoto, H. Angew. Chem. Int. Ed. 2001, 40 (21), 4077–4079. doi: 10.1002/1521-3773(20011105)40:21<4077::AID-ANIE4077>3.0.CO;2-1
- Li, J.; Tang, Y.; Wang, Q.; Li, X.; Cun, L.; Zhang, X.; Zhu, J.; Li, L.; Deng, J. J. Am. Chem. Soc. 2012, 134 (45), 18522–18525. doi: 10.1021/ja308357y
- Manabe, K.; Iimura, S.; Sun, X.-M.; Kobayashi, S. J. Am. Chem. Soc. 2002, 124 (40), 11971–11978. doi: 10.1021/ja026241j
- Manabe, K.; Sun, X.-M.; Kobayashi, S. J. Am. Chem. Soc. 2001, 123 (41), 10101–10102. doi: 10.1021/ja016338q
- Xie, C.; Li, H.; Li, L.; Yu, S.; Liu, F. J. Hazard. Mater. 2008, 151 (2–3), 847–850. doi: 10.1016/j.jhazmat.2007.11.113
- Gui, J.; Cong, X.; Liu, D.; Zhang, X.; Hu, Z.; Sun, Z. Catal. Commun. 2004, 5 (9), 473–477. doi: 10.1016/j.catcom.2004.06.004
- Joseph, T.; Sahoo, S.; Halligudi, S.B. J. Mol. Catal. A: Chem. 2005, 234 (1-2), 107–110. doi: 10.1016/j.molcata.2005.03.005
- Li, Y.; Hu, S.; Cheng, J.; Lou, W. Chinese. J. Catal. 2014, 35 (3), 396–406. doi: 10.1016/S1872-2067(14)60005-X
- Cole, A.C.; Jensen, J.L.; Ntai, I.; Tran, K.L.T.; Weaver, K.J.; Forbes, D.C.; Davis, J.H. J. Am. Chem. Soc. 2002, 124 (21), 5962–5963. doi: 10.1021/ja026290w
- Borissova, M.; Palk, K.; Koel, M. J. Chromatogr. A. 2008, 1183 (1–2), 192–195. doi: 10.1016/j.chroma.2007.12.077
- Crescenzo, A.D.; Aschi, M.; Canto, E.D.; Giordani, S.; Demurtas, D.; Fontana, A. Phys. Chem. Chem. Phys. 2011, 13 (23), 11373–11383. doi: 10.1039/c1cp20140a
- Tondo, D.W.; Leopoldino, E.C.; Souza, B.S.; Micke, G.A.; Costa, A.C.O.; Fiedler, H.D.; Bunton, C.A.; Nome, F. Langmuir. 2010, 26 (20), 15754–15760. doi: 10.1021/la102391e
- Drinkel, E.; Souza, F.D.; Fiedler, H.D.; Nome, F. Curr. Opin. Colloid Interface Sci. 2013, 18 (1), 26–34. doi: 10.1016/j.cocis.2013.01.001
- Aghabarari, B.; dorostkar, N.; Ghiaci, M.; Amini, S.G.; Rahimi, E.; Martinez-Huerta, M.V. J Taiwan Inst Chem Eng. 2014, 45 (2), 431–435. doi: 10.1016/j.jtice.2013.08.003
- Aghabarari, B.; Dorostkar, N.; Martinez-Huerta, M.V. Fuel Process. Technol. 2014, 118, 296–301. doi: 10.1016/j.fuproc.2013.10.003
- Dong, F.; Zhenghao, F.; Zuliang, L. Catal. Commun. 2009, 10 (8), 1267–1270. doi: 10.1016/j.catcom.2009.02.003
- Qiu, Y.; Sun, H.; Ma, Z.; Xia, W. J. Mol. Catal. A: Chem. 2014, 392, 76–82. doi: 10.1016/j.molcata.2014.04.031
- Han, M.; Yi, W.; Wu, Q.; Liu, Y.; Hong, Y.; Wang, D. Bioresour. Technol. 2009, 100 (7), 2308–2310. doi: 10.1016/j.biortech.2008.10.046
- Liang, X.; Gong, G.; Wu, H.; Yang, J. Fuel. 2009, 88 (4), 613–616. doi: 10.1016/j.fuel.2008.09.024
- Hong, S.-G.; Kim, H.S.; Kim, J. Langmuir. 2014, 30 (3), 911–915. doi: 10.1021/la404189e
- He, L.; Qin, S.; Chang, T.; Sun, Y.; Gao, X. Catal. Sci. & Technol. 2013, 3 (4), 1102. doi: 10.1039/c2cy20714a
- Zhang, L.; Xian, M.; He, Y.; Li, L.; Yang, J.; Yu, S.; Xu, X. Bioresour. Technol. 2009, 100 (19), 4368–4373. doi: 10.1016/j.biortech.2009.04.012
- Igarashi, T.; Yagyu, D.; Naito, T.; Okumura, Y.; Nakajo, T.; Mori, Y.; Kobayashi, S. Appl. Catal. B: Environ. 2012, 119–120 (0), 304–307. doi: 10.1016/j.apcatb.2012.03.001
- Gang, L.; Xinzong, L.; Eli, W. New J. Chem. 2007, 31 (3), 348–351. doi: 10.1039/b615448d
- Zhu, D.M.; Schelly, Z.A. Langmuir. 1992, 8 (1), 48–50. doi: 10.1021/la00037a011
- Zheng, Y.; Eli, W. J. Disper. Sci. Technol. 2009, 30 (5), 698–703. doi: 10.1080/01932690802553890
- Zheng, Y.; Meng, F.; Liu, M. J. Disper. Sci. Technol. 2015, 36 (11), 1607–1611. doi: 10.1080/01932691.2014.982285
- Gao, Y.; Li, N.; Zheng, L.; Zhao, X.; Zhang, S.; Han, B.; Hou, W.; Li, G. Green Chem. 2006, 8 (1), 43–49. doi: 10.1039/B510902G
- Foresti, M.L.; Ferreira, M.L. Catalysis Today. 2005, 107–108, 23–30. doi: 10.1016/j.cattod.2005.07.053

![Figure 2. TGA curves for [SB3-12][HSO4].](/cms/asset/5694aecf-b7b7-4755-ae49-d227bf5f6f69/tgcl_a_1342001_f0002_b.gif)
![Figure 6. TEM images of the oleic acid/[SB3-12][HSO4]/ethanol system.](/cms/asset/adae3b5f-2542-4919-91d6-5c4a37f3dd0c/tgcl_a_1342001_f0006_b.gif)
![Figure 8. Different stages of esterification catalyzed by [SB12-3][HSO4].](/cms/asset/8e7ebd13-c8e0-44b0-a33e-c813a399df20/tgcl_a_1342001_f0008_c.jpg)