ABSTRACT
Over the last few years, the green synthesis of nanoparticles (NPs) using plant extracts has emerged as a promising methodology for the fabrication of metallic NPs (especially silver, copper, and gold NPs), as it involves an easy, fast, low-cost, and environmentally friendly bioprocess. However, many factors affect the sizes and morphologies of NPs biosynthesized by this method, including the nature of the plant extract, among others. Therefore, the green synthesis of metal NPs with defined stability, size, and morphology distribution remains under evaluation. In the present study, we propose aqueous extracts from the endemic-medicinal plant Budleja globosa (“Matico”) as an efficient bioproduct for the green synthesis of silver NPs (AgNPs). Experimental results indicate that room temperature, low concentrations of leaf extracts of B. globosa, and silver nitrate salt were sufficient to biosynthesize AgNPs with uniform size (16 nm) and shape distribution (spherical).
GRAPHICAL ABSTRACT
KEYWORDS:
1. Introduction
In recent years, nanotechnology has shown a rapid and successful growth, since it allows known materials to be developed with different properties (Citation1–4). Decreasing the size of any material to nanoscale may change its intrinsic properties. Thus, the properties of a nanostructured material can be quite dissimilar from those of the bulk material, making it suitable for different applications. In particular, metallic nanoparticles (NPs) such as silver and copper NPs (AgNPs and CuNPs) have been applied in a wide variety of fields, including medicine, agriculture, bioengineering, among others (Citation5–9), because they have been proven and recognized as antibacterial and biocide agents (Citation10,Citation11).
Several methods have been used to synthesize NPs, including chemical reduction (Citation12), electrochemical, photochemical (Citation13,Citation14) and physical methods, such as physical vapor condensation (Citation15). At present, the nanomaterials field has generated NPs using green synthesis (Citation1–3, Citation16,Citation17). Green synthesis involves NPs obtained from the mixing of metal salts and natural agents such as vitamins, sugars, plant extracts, biodegradable polymers, and microorganisms (Citation2). When plant extracts are used, they can act as a reducing agent, but also as a stabilizing component for the system (Citation18). In particular, the green synthesis of AgNPs involves the chemical reduction of silver salt solution of AgNO3 by using plant extract. In this process, two phases are recognized: (1) the nucleation phase, where the silver atoms form small nucleuses using high activation energy, (2) and the second phase known as growth phase, in which these small nucleuses are grouped, giving rise to the creation of NPs (Citation18,Citation19). Given the high reduction potential of metal Ag, once the AgNPs are formed, stable particles in stabilizer-free aqueous solutions can be obtained (Citation20–22).
The features of these NPs obtained using plant extracts are influenced by several parameters such as temperature, time of reaction, and pH; however, the nature of biomolecules present in the plant extracts could be the most relevant factor in the bioprocess (Citation2,Citation23,Citation24). Thus, the selected extract is of great importance, as it provides bioreductor agents in the synthesis, such as phenolic compounds, terpenoids, flavonoids, alkaloids, polysaccharides, proteins, enzymes, and amino acids (Citation24,Citation25).
Although there are many studies about the synthesis of silver NPs using plant extracts in the literature, these reports are focused mainly on European, African, and Asiatic plant species. In this scenario, studies about green synthesis with plants from South America are scarce.
In the present work, Buddleja globosa Hope (also known as “Matico”) leaf extract was used to synthetize AgNPs. Buddleja globosa Hope is an abundant medicinal endemic tree species from South America (Citation26), which shows high contents of polyphenols and has a potent antioxidant capacity. In addition, the major biomolecule constituents in infusions have been well described, consisting mainly of verbascoside and luteolin derivatives (Citation27,Citation28).
The aim of the present study was to describe the green synthesis of AgNPs using aqueous B. globosa extracts and to analyze the effectiveness of this bioprocess according to the chemical and physical properties of AgNPs synthesized (e.g. size, shape, surface chemistry, and elemental composition) from different AgNO3 salt and extract concentrations, as well as time of reaction.
2. Materials and methods
2.1. Plant material and extracts preparation
Samples of B. globosa were taken from the botanical garden of Universidad Católica de Temuco (38° 42′ S, 72° 42′ W) in October 2012 (austral Spring season). Aqueous extracts were prepared according to previous published methodology (Citation29).
2.2. Green synthesis of AgNPs
Synthesis of AgNPs was carried out by mixing B. globosa extracts with different aqueous solutions of AgNO3 (Merck Company, Darmstadt, Germany), and the solution was homogenized by stirring at room temperature. The final concentration of AgNO3 used in the synthesis ranged from 0.1 to 10 mM with 5% volume of B. globosa extract.
2.3. UV–Vis-spectroscopy
UV–Vis absorption spectra were measured using a Shimadzu UV-mini 1240 model spectrophotometer. All measurements were performed within the range of 200 –800 nm with a 1 nm interval and with 1 s of integration time.
2.4. X-ray Photoelectron Spectroscopy (XPS)
XPS spectra of AgNPs were measured using a hemispherical analyzer (Physical Electronics 1257 system). For the XPS, a twin anode (Mg and Al) with X-ray source was operated at 400 W of constant power and using Al Kα radiation (1486.6 eV). The samples were placed in a sample stage with an emission angle of 45°. The measurements were carried out by suspending AgNPs on a gold film while gold was served as metallic reference. Au 4f binding energy was 84 eV for samples without any charging effect.
2.5. Fourier Transform Infrared Spectrocopy (FTIR)
FTIR analyses were carried out using a PerkinElmer FTIR spectrometer (FTIR System Spectrum BX). The samples were prepared by using the KBr pellets technique and were analyzed to check the presence of functional groups of the surface chemistry of the reduced AgNPs. The FTIR spectrums were collected at a spatial resolution of 4 cm−1 in the transmission mode, between 4000 and 400 cm−1, respectively.
2.6. Transmission Electron Microscopy (TEM)
TEM was carried out with a FEI Tecnai ST F20 apparatus that operates at 200 kV and it is equipped with an EDS energy dispersive system. Prior to carrying out the TEM measurements, AgNPs obtained from the biosynthesis were purified (by centrifugation) and sonicated. TEM images of AgNPs were processed with the ImageJ software to obtain diameter size distribution of particles.
3. Results and discussion
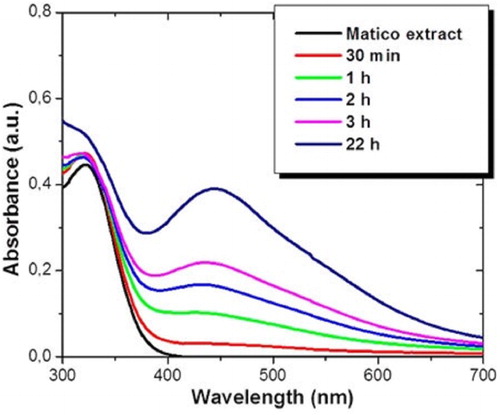
The green synthesis of silver nanoparticles (AgNPs) was carried out by adding plant extract of B. globosa into aqueous solutions of silver nitrate, inducing the reduction of Ag2+ ions into Ag0. This process led to a change in the color of the solution due to the surface plasmon absorption of AgNPs. The biosynthesis of AgNPs was monitored by UV–Vis spectroscopy. shows UV–Vis absorption spectra recorded as a function of time for an aqueous solution of 2 mM concentration of silver nitrate and 5% volume of B. globosa extract. A wide absorption peak localized between 300 nm and 380 nm of wavelength related to B. globosa extract can be observed. After one hour of reaction, the reduction of Ag+ ions into AgNPs shows a minor absorption peak centered at 445 nm of wavelength related to the localized surface plasmon resonance of AgNPs (Citation30). Furthermore, as the reaction time rises, the intensity of this absorption peak also increases. The wavelength absorption of metallic NPs depends on different factors such as particle size, shape and distribution, and also on the dielectric constant of surrounding media. In this case, absorption peaks suggest the formation of spherical AgNPs with 20 nm of average diameter (Citation30). In addition, the increase in the signal intensity with the reaction time indicates a growth in the amount of biosynthesized AgNPs. After 22 hours, the resonance plasmon absorption peak remains unaffected, which confirms the bioprocess was completed. The time reaction is quite efficient in comparison with other plant extracts which need between 24 and 72 hours, or even a week (Citation31,Citation32)
Figure 1. UV–Vis absorption spectrum of AgNPs at different time reaction intervals biosynthesized with 2 mM of AgNO3 and 5% volume of B. globosa extract.
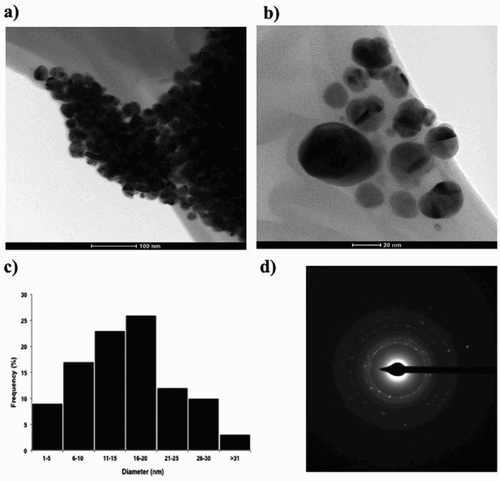
In order to confirm the size and shape of the AgNPs biosynthesized, Transmission electron microcopy (TEM) was carried out. (a, b) shows TEM images of AgNPs biosynthesized. Most of the NPs analyzed were spherical shapes and showed some levels of agglomeration. The size of the AgNPs ranged from 1 nm to 53.5 nm (n = 100), and the average diameter size was 16.37 ± 8.35 nm ((c)). Electron diffraction pattern (SAED) from multiple silver NPs is shown in (d). The ring-like diffraction pattern indicates the crystalline nature of the AgNPs. The diffraction rings can be indexed on the basis of FCC structure of silver in which rings arise from the reflection of (111), (200), (220), and (311) lattice planes, respectively. Similar SAED patterns have been found for AgNPs biosynthesized from other kinds of natural extracts (Citation33,Citation34).
Figure 2. Transmission electron microscope (TEM) images of AgNPs synthetized with B. globosa extracts; (a) a random field view of AgNPs (scale bar representing 100 nm), (b) a high magnification image of spherical AgNPs (scale bar representing 20 nm), (c) a histogram of diameter size distribution of AgNPs (n = 100) with an average diameter of 16 nm, and (d) Electron diffraction pattern (SAED) from multiple silver NPs.
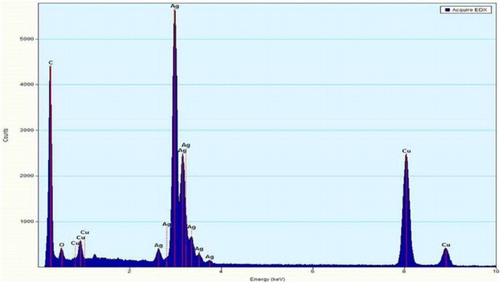
shows energy dispersion X-ray spectra of AgNPs, which reveals the presence of Ag, C, O, and Cu. However, no signal of N from AgNO3 was observed. C and O signals come from organic compounds of B. globosa extract, suggesting that some rests of the extract are capping the AgNPs. The signal of Cu comes from the Cu TEM grid where the AgNPs samples were mounted.
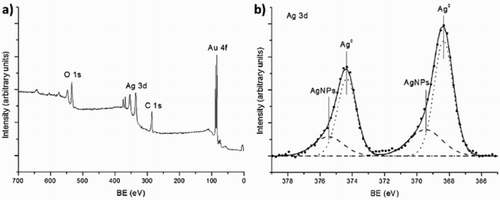
XPS measurements were performed in order to study the oxidation state of AgNPs. (a) shows the survey spectrum of AgNPs biosynthesized with 2 mM concentration of AgNO3 and 5% volume of B. globosa extract. The only elements present in the spectrum were O, C, Ag, and Au. This last element comes from the substrate (for further details see experimental section). It should be noticed that nitrogen was not detected from AgNO3, expected at ∼400 eV, indicating the formation of AgNPs, and this result was consistent with energy dispersion X-ray measurements. (b) shows a high resolution spectrum from Ag 3d band. This spectrum can be fitted by two doublets. The first one was placed at 368.3 and 374.3 eV, which correspond with the binding energy of metallic Ag (Citation35). The second one was placed at 369.5 and 375.5 eV, a binding energy higher than 368.8 eV expected for AgNO3 (Citation35), which confirms that silver nitrate was not present in the sample. In addition, it has been observed that the presence of this peak at higher binding energy was related with the formation of AgNPs (Citation36).
Figure 4. X-ray photoelectron spectroscopy (XPS) measurement of AgNPs biosynthesized with 2 mM of AgNO3 and 5% volume of B. globosa extract in aqueous solution. (a) XPS survey. (b) High resolution spectrum from Ag 3d band.
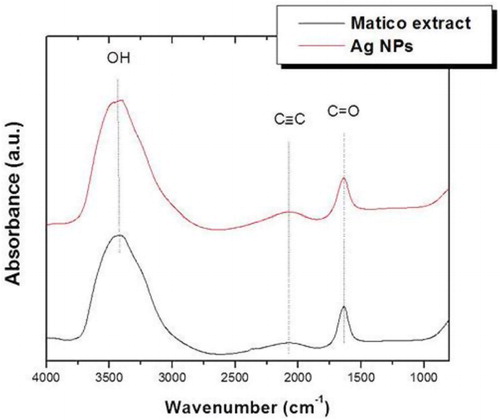
In order to study the formation mechanism of AgNPs, FTIR measurements were performed. shows the FTIR absorbance spectra of AgNPs biosynthesized with B. globose extract. This measurement confirms the presence of some functional groups capping the AgNPs. In particular, three main peaks were observed at 3464, 2083, and 1636 cm–1. The broad band around 3464 cm−1 was due to OH stretching vibrations of phenols and carboxylic groups present in the extract. It can also correspond to N-H stretching vibration of NH2 groups; however, the presence of nitrogen was discarded by XPS and EDS measurements. The absorbance band at 2083 cm−1 corresponds to alkyne groups of phytoconstituents of the extract and the band at 1636 cm−1 can be associated to C = O stretching vibrations. Results are similar to other Ag NPs synthetized by different plant extracts (Citation37).
Figure 5. Fourier transform infrared (FTIR) spectra of B. globosa extract and biosynthesized Ag NPs.
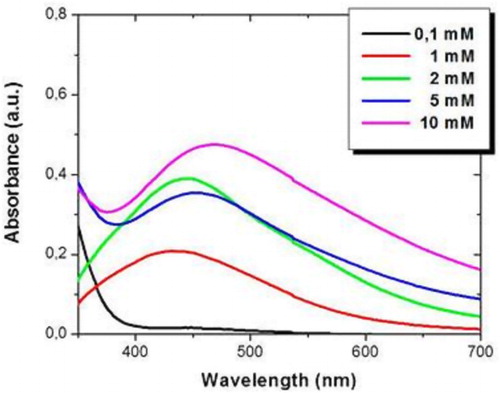
The properties of AgNPs biosynthesized by B. globosa extract are very sensitive to different parameters, such as AgNO3 and extract concentration, as well as the temperature of the process and the Ph of the solution. shows the absorbance spectrum of AgNPs fabricated with different concentration of AgNO3 after three hours of reaction, fixing 5% volume of B. globosa extract. A broad absorption band in the range of 420–480 nm of wavelength was observed for all the concentration of AgNO3 used, which was characteristic of localized surface plasmon resonance of AgNPs. In addition, as the concentration of silver salt was increased, the intensity of the absorption peak also increased, suggesting that the amount of AgNPs rises. Furthermore, a red shift in the absorption band was observed from 432 nm with 1 mM of AgNO3 to 467 nm with 10 mM of AgNO3. The red shift was caused by quantum size effects, indicating a large size distribution given by agglomerated AgNPs, which was in agreement with TEM images. Moreover, for higher concentration of AgNO3, the absorption bands were wider, suggesting a smaller diameter size of AgNPs.
Figure 6. UV–Vis absorbance spectrum of AgNPs synthesized with different concentrations of AgNO3, fixing 5% of B. globosa extract.
B. globosa has been demonstrated as an efficient bioproduct to synthetize AgNPs with spherical shape. In the present study, the biosynthesized AgNPs showed similar properties in comparison with other AgNP fabricated by plant extracts with equal antioxidant capacity (Citation20,Citation21, Citation24). However, the efficient time reaction and the possibility to modify the main properties of AgNPs by changing the AgNO3 concentrations suggest that this bioprocess can be implemented to obtain AgNPs for catalyst and antimicrobial applications.
4. Conclusions
One-step green synthesis of stable silver NPs using B. globosa leaves extract at room temperature was reported in this work. Silver NPs were successfully biosynthesized by this simple, fast, cost effective, eco-friendly, and efficient method, which excludes external stabilizers or reducing agents. In this process, the main polyphenols present in plant extracts of matico, such as verbascoside and/or luteolin may be related with the chemical reduction of silver ions Ag2+ to Ag0, allowing the nucleation and growth of silver NPs with spherical shape and an average diameter of 16 nm. In addition, they also act as stabilizing agents of NPs. The properties of the final silver NPs including the average diameter, dispersion and optical properties can be easily controlled by changing the main parameters in the process, such as silver nitrate concentration.
Acknowledgements
The authors also gratefully thank Dr Rodrigo Espinoza for TEM measurements.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
Dr. Erico R. Carmona was born in Chile. He received the B.E. degree in Biology in 2005 from Universidad Arturo Prat, Iquique, Chile. In addition, he received the Ph.D. degree in Environmental Science in 2009 from Universidad Autónoma de Barcelona, Spain. Since 2011 he is titular researcher at Universidad Católica de Temuco, Chile, and since 2015 he is part of Núcleo de Investigación e Bioproductos y Materiales Avanzados (BIOMA) from Universidad Católica de Temuco, Chile. He is the author and co-author of more than 20 international pre-review papers. His main areas of research are the study of toxic and genotoxic potential of metal based nanoparticles in whole organisms.
Dr. Noelia Benito was born in Spain in 1984. She received the B.E. degree in Physics in 2009 from Universidad Autónoma de Madrid, Spain. In addition, she received the M.E. and Ph.D. in Advanced Materials and Nanotechnology from Universidad Autónoma de Madrid, Spain in 2010 and 2014, respectively. Since 2014 she is post-doctoral researcher at Facultad Ciencias Físicas y Matemáticas of Universidad de Chile, Chile. She is the author and co-author of more than 10 international pre-review papers. Her main areas of research interest are surface science and thin films.
Tanya Plaza was born in Chile in 1987. He received the B.E. degree in veterinarian in 2013 from Universidad Católica de Temuco, Chile. Since 2014 she has been working in different research projects as laboratory technician including Education Tonalli LTDA. and FONDECYT 11150322.
Dr. Gonzalo Recio-Sánchez was born in Spain in 1986. He received the B.E. degree in Physics in 2009 from Universidad Autónoma de Madrid, Spain. In addition, he received the M.E. and Ph.D. in Advanced Materials and Nanotechnology from Universidad Autónoma de Madrid, Spain in 2010 and 2013, respectively. Since 2014 he is Professor at Departamento de Ciencias Matemáticas y Físicas of Universidad Católica de Temuco, and since 2016 he is the director of Núcleo de Investigación en Bioproductos y Materiales Avanzados (BIOMA) at Universidad Católica de Temuco. He is the author and co-author of more than 20 international pre-review papers. His main areas of research interest are optoelectronic and photonic properties of nanostructures and nanomaterials.
Additional information
Funding
References
- Logeswari, P.; Silambarasan, S.; Abraham, J. J. Saudi Chem. Soc. 2015, 19 (3), 311–317. doi: 10.1016/j.jscs.2012.04.007
- Kharissova, O.V.; Dias, H.R.; Kharisov B.I.; Pérez, B.O.; Pérez, V.M. J. Trends Biotechnol. 2013, 31 (4), 240–248. doi: 10.1016/j.tibtech.2013.01.003
- Zorzi, G.K.; Carvalho, E.L.S.; Von Poser, G.L.; Teixeira, H.F. Rev. Bras. Farmacogn. 2015, 25 (4), 426–436. doi: 10.1016/j.bjp.2015.07.015
- Sanna, V.; Roggio, A.M.; Pala, N.; Marceddu, S.; Lubinu, G.; Mariani, A.; Sechi, M. Int. J. Biol. Macromol. 2015, 72, 531–536. doi: 10.1016/j.ijbiomac.2014.08.053
- Recio-Sánchez, G.; Namura, K.; Suzuki, M.; Martín-Palma, R.J. Nanoscale Res. Lett. 2014, 9 (1), 1–6. doi: 10.1186/1556-276X-9-487
- Khalil, M.M.; Ismail, E. H.; El-Baghdady, K. Z.; Mohamed, D. Arab. J. Chem. 2014, 7 (6), 1131–1139. doi: 10.1016/j.arabjc.2013.04.007
- Albertos, P.; Romero-Puertas, M.C.; Tatematsu, K.; Mateos, I.; Sánchez-Vicente, I.; Nambara, E.; Lorenzo, O. Nat. Commun. 2015, 6, 8669.
- Nasrollahzadeh, M.; Atarod, M.; Jaleh, B.; Gandomirouzbahani, M. Ceram. Int. 2016, 42 (7), 8587–8596. doi: 10.1016/j.ceramint.2016.02.088
- Rostami-Vartooni, A.; Nasrollahzadeh, M.; Alizadeh, M. J. Alloys Compd. 2016, 680, 309–314. doi: 10.1016/j.jallcom.2016.04.008
- Naddeo, J.J.; Ratti, M.; O’Malley, S.M.; Griepenburg, J.C.; Bubb, D.M.; Klein, E. A. Adv. Sci. Eng. Med. 2015, 7 (12), 1044–1057. doi: 10.1166/asem.2015.1811
- Marin, S.; Mihail Vlasceanu, G.; Elena Tiplea, R.; Raluca Bucur, I.; Lemnaru, M.; Minodora Marin, M.; Mihai Grumezescu, A. Curr. Trends Med. Chem. 2015, 15 (16), 1596–1604. doi: 10.2174/1568026615666150414142209
- Wang, H.; Qiao, X.; Chen, J.; Ding, S. Colloids Surf. A. 2005, 256 (2), 111–115. doi: 10.1016/j.colsurfa.2004.12.058
- Khaydarov, R.A.; Khaydarov, R.R.; Gapurova, O.; Estrin, Y.; Scheper, T. J. Nanopart. Res. 2009, 11 (5), 1193–1200. doi: 10.1007/s11051-008-9513-x
- Zaarour, M.; El Roz, M.; Dong, B.; Retoux, R.; Aad, R.; Cardin, J.; Mintova, S. Langmuir. 2014, 30 (21), 6250–6256. doi: 10.1021/la5006743
- Simchi, A.; Ahmadi, R.; Reihani, S.S.; Mahdavi, A. Mater. Des. 2007, 28 (3), 850–856. doi: 10.1016/j.matdes.2005.10.017
- Veerasamy, R.; Xin, T.Z.; Gunasagaran, S.; Xiang, T.F.W.; Yang, E.F.C.; Jeyakumar, N.; Dhanaraj, S. A J. Saudi Chem. Soc. 2011, 15 (2), 113–120. doi: 10.1016/j.jscs.2010.06.004
- Dubey, S.P.; Dwivedi, A.D.; Lahtinen, M.; Lee, C.; Kwon, Y.N.; Sillanpaa, M. Spectrochim. Acta, Part A. 2013, 103, 134–142. doi: 10.1016/j.saa.2012.11.021
- Mittal, A.K.; Chisti, Y.; Banerjee, U. C. Biotechnol. Adv. 2013, 31 (2), 346–356. doi: 10.1016/j.biotechadv.2013.01.003
- Baker, C.; Pradhan, A.; Pakstis, L.; Pochan, D.J.; Shah, S.I. J. Nanosci. Nanotechnol. 2005, 5 (2), 244–249. doi: 10.1166/jnn.2005.034
- Recio-Sánchez, G.; Lagos, C.; Benito-Gómez, N.; García, A.; Marcos R.; Carmona, E. R. Mater. Lett. 2016, 183, 255–260. doi: 10.1016/j.matlet.2016.07.115
- Tajbakhsh, M.; Alinezhad, H.; Nasrollahzadeh, M.; Kamali, T. A. J. Alloys and Compd. 2016, 685, 258–265. doi: 10.1016/j.jallcom.2016.05.278
- Atarod, M.; Nasrollahzadeh, M.; Sajadi, S.M. J. Colloid Interface Sci. 2016, 462, 272–279. doi: 10.1016/j.jcis.2015.09.073
- Harne, S.; Sharma, A.; Dhaygude, M.; Joglekar, S.; Kodam, K.; Hudlikar, M. Colloids Surf. B. 2012, 95, 284–288. doi: 10.1016/j.colsurfb.2012.03.005
- Rajan, R.; Chandran, K.; Harper, S.L.; Yun, S.I.; Kalaichelvan, P.T. Ind. Crops. Prod. 2015, 70, 356–373. doi: 10.1016/j.indcrop.2015.03.015
- Rauwel, P.; Rauwel, E.; Ferdov, S.; Singh, M.P. Adv. Mater. Sci. Eng. 2015, 2015, 624394.
- Estomba, D.; Ladio, A.; Lozada, M. J. Ethnopharmacol. 2006, 103, 109–119. doi: 10.1016/j.jep.2005.07.015
- Backhouse, N.; Delporte, C.; Apablaza, C.; Farías, M.; Goïty, L.; Arrau, S.; Negrete, R.; Castro C.; Miranda H. J. Ethnopharmacol. 2008, 119 (1), 160–165. doi: 10.1016/j.jep.2008.06.022
- Backhouse, N.; Rosales, L.; Apablaza, C.; Goïty, L.; Erazo, S.; Negrete, R.; Theodoluz, C.; Rodriguez, J.; Delporte, C. J. Ethnopharmacol. 2008, 116 (2), 263–269. doi: 10.1016/j.jep.2007.11.025
- Carmona, E.R.; Escobar, B.; Obando, V. Fresenius Environ. Bull. 2016, 25, 5758–5764.
- Mogensen, K.B.; Kneipp, K. J. Phys. Chem. C. 2014, 118 (48), 28075–28083. doi: 10.1021/jp505632n
- Dipankar, C.; Murugan, S. Colloids Surf. B. 2012, 98, 112–119. doi: 10.1016/j.colsurfb.2012.04.006
- Khalil M.; Ismail E.H.; El-Baghdady K.Z.; Mohamed D. Arab. J. Chem. 2014, 7 (6), 1131–1139. doi: 10.1016/j.arabjc.2013.04.007
- Song, J.Y.; Kim, B.S. Bioprocess. Biosyst. Eng. 2009, 32 (1), 79. doi: 10.1007/s00449-008-0224-6
- Parashar, V.; Parashar, R.; Sharma, B.; Pandey, A.C. Dig. J. Nanomater. Biostruct. 2009, 4 (1), 45–50.
- Ferraria, A.M.; Carapeto, A.P.; do Rego, A.M.B. Vacuum. 2012, 86 (12), 1988–1991. doi: 10.1016/j.vacuum.2012.05.031
- Busby, Y.; Pireaux, J.J. J. Electron Spectrosc. Relat. Phenom. 2014, 192, 13–18. doi: 10.1016/j.elspec.2013.12.010
- Ahmed, S.; Ahmad, M.; Swami, B.L.; Ikram, S. J. Radiat. Res. Appl. Sci. 2016, 9 (1), 1–7. doi: 10.1016/j.jrras.2015.06.006