ABSTRACT
In this research, a new, green and eco-friendly method for the synthesis of spirooxindole derivatives was presented. The reaction was performed at room temperature in the presence of catalytic amounts (4 mol.%) of CuO nanoparticles and products were obtained in high to excellent yields. Low-cost high-efficiency reusable catalyst, short reaction times at room temperature, high to excellent yield and easy purification of products are the main advantages to this protocol. The pronounced advantages of catalyst are expected to create a new pathway for the synthesis of spirooxindoles.
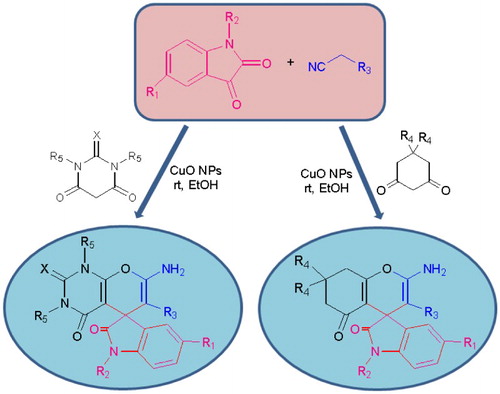
GRAPHICAL ABSTRACT
1. Introduction
Multicomponent reactions appear as a powerful synthetic strategy because of their productivity, simple procedures and facile execution. The flexibility of multicomponent reactions makes them an important type of reaction classes with short reaction times and high to excellent yields (Citation1–4).
Isatin, as a key structure in the synthesis of heterocyclic compounds, has some biological activities (such as anticonvulsant, antidepressant, anti-inflammatory and antimicrobial activities) and effects on the central nervous system (CNS) (Citation5–7). A variety of spirooxindoles containing isatin are important compounds with biological and medicinal properties (Citation8–11). The need to develop synthetic methods for the preparation of these heterocycles has attracted the attention of many scientists. Therefore, different catalysts have been used to synthesize these structures, such as guanidine-functionalized magnetic nanoparticles (Citation12), MgO nanoparticles (Citation13), nickel chloride (Citation14), p-TSA (Citation15, Citation16), HAuCl4·3H2O (Citation17), cesium fluoride (Citation18), l-proline (Citation19), carbon–SO3H (Citation20), hexamethylenetetramine (Citation21), glutathione@Fe3O4 nanoparticles (Citation22) and triphenylphosphine (Citation23).
Copper oxide nanoparticles (CuO NPs) have various applications, such as in gas sensing and field emission (Citation24–26), in antibacterial (Citation27) and antimicrobial activities (Citation28, Citation29), and as reaction catalyst (Citation30–33). Due to high surface area and high efficiency of CuO NPs, we presented a simple synthetic method for the synthesis of spirooxindole derivatives under room temperature, using catalytic amounts of CuO NPs (Scheme 1). Reusability of nanocatalyst, short reaction times and high yield of products under ambient conditions are other merits of this method.
2. Results and discussion
CuO NPs were synthesized using Cu(CH3CO2)2, based on the Tetsuya Kida method (Citation34). The obtained nanoparticles were characterized using FTIR, XRD and SEM techniques.
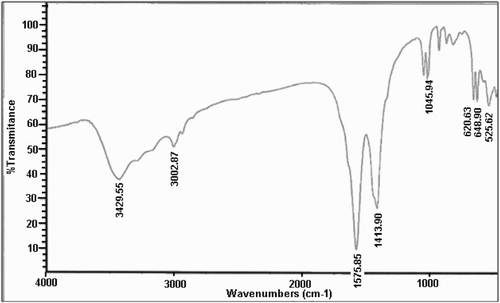
shows the FTIR of CuO NPs. In this spectrum, the peak at about 3429 cm−1 is related to the hydroxyl groups on CuO NPs and the water absorbed by KBr. The absorption peaks at 1576 and 1414 cm−1 are from the bending vibrations of OH combined with copper atoms. Finally, the stretching modes of CuO appeared on 525 and 620 cm−1.
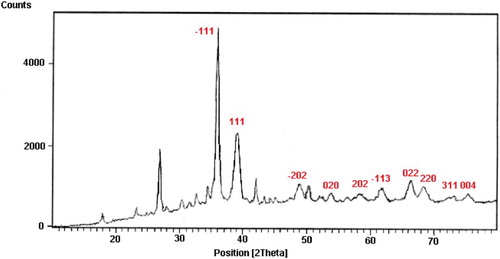
Crystallinity of CuO NPs was evaluated by the XRD method. From the XRD pattern of CuO NPs in , it is clear that main diffraction peaks can be indexed to a crystalline structure of CuO NPs. The size of synthesized nanoparticles calculated from the Debye–Scherrer equation based on the XRD pattern shows that the nano copper oxides have mean sizes of about 18.55 nm.
In addition, the morphology of CuO NPs was investigated by the SEM technique. shows that CuO NPs were prepared through the precipitation method and this result confirmed the particle size obtained from the XRD pattern.
After the preparation and characterization of nanocatalyst, the optimization of the reaction conditions (catalyst amounts and solvent) was investigated using a model reaction containing isatin (1 mmol), malononitrile (1 mmol) and dimedone (1 mmol) in the presence of CuO NPs at room temperature.
For evaluation of the effect of catalyst amounts on the yield of product, the model reaction was performed in the presence of a different quantity of catalyst. Based on the results obtained from , it is found that 4 mol.% of CuO NPs is the best quantity for obtaining the highest yield (entry 4).
Table 1. Optimization of the catalyst amount.
Additionally, the solvent effect on the yield and time of reaction was studied using model reaction in the presence of 4 mol.% (0.003 g) of catalyst and different organic solvents. The obtained results showed that EtOH had more effect on the yield of reaction and can be used as efficient solvent (entry 3) ().
Table 2. Study of solvent effect on the yield of 4a.
After the optimization of catalyst and solvent, for evaluation of nanocatalyst efficiency, a variety of spirooxindoles were synthesized at optimum conditions using malononitrile or ethylcyanoacetate, 1,3-diketones such as dimedone, barbituric acid, 1,3-dimethyl barbituric acid, 2-thiobarbituric acid and also 1,2-diketo compounds such as isatin and three of isatin derivatives as substrates. The results and details are summarized in . As shown in this table, good to excellent yields were achieved at room temperature and short reaction times. Also, the yields of products from the reaction of ethylcyanoacetate gave corresponding spirooxindoles in lower yields and longer reaction times than malononitrile (4c, 4f). This is due to less reactivity of ethylcyanoacetate. On the other hand, the low yield of 4k product in longer time is from lower reactivity of 5-bromoisatine. Also, steric hindrance of N,N-dimethyl barbituric acid in addition to low reactivity of 5-bromoisatine leads to low yield of 4n after 20 min.
Table 3. Synthesis of spirooxindole derivatives in the presence of CuO NPs.
In addition, turn over frequency (TOF) and turnover number (TON) values were calculated and results are depicted in . Results exhibited high efficiency (due to high turnover frequencies) and greater stability (high turnover numbers) of catalyst.
Presented methodology is a chromatography free process. In fact, products were purified only with recrystallization from ethanol and this is the most important advantage of this process. All obtained pure products were characterized using physical and spectroscopic data and were consistent in comparison with authentic samples.
Finally, we studied the reusability of the CuO NPs in the reaction between isatin, malononitrile and dimedone to give 4a product. After every cycle of reaction, nanocatalyst was separated, washed with EtOH and dried and then reused in new reaction. Results in show that CuO NPs have high efficiency even after reused for three times. In addition, a slight, gradual decrease in the catalyst activity is observed because of the clogging of some catalyst active sites with organic reactants during the reaction process.
Table 4. Evaluation of reusability of CuO NPs.
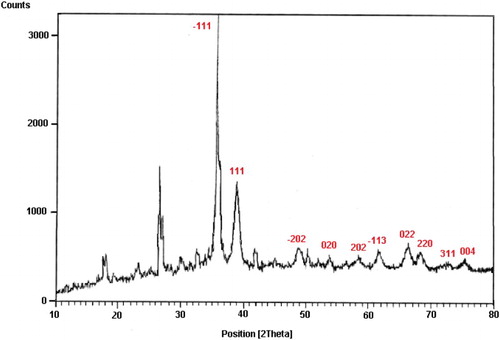
Reused catalyst was characterized using the XRD technique (). As can be seen from , the XRD pattern of recycled catalyst is the same as the initial nanocatalyst with no considerable changes. It is concluded that the structure and performance of catalyst is stable under reaction conditions after three times recycling.
Additionally, we have carried out the leaching test through the filtration method. In this test, two experiments were designed. In first reaction, 4 mol.% of CuO NPs was added to a mixture of isatin (1 mmol), malononitrile (1 mmol) and dimedone (1 mmol) in EtOH (2 ml). The mixture was stirred for 4 min at room temperature. After that, reaction mixture was dissolved in acetone and the catalyst was separated. Isolated yield of reaction was about 50%. Another reaction was carried out in the same manner as that of the first one. In the second test, the catalyst was separated after 4 min and the reaction was continued with filtrate for another 4 min at room temperature. After the separation of product, isolated yield was 50% and no increasing was observed in the yield of product.
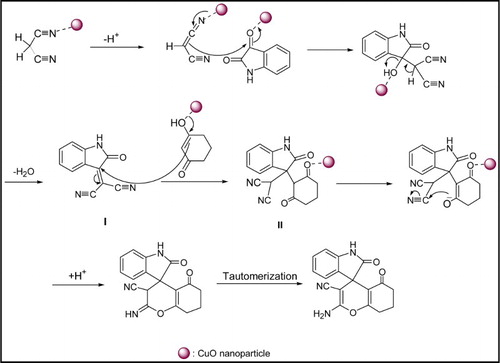
A proposed mechanism for the synthesis of spirooxindoles in the presence of CuO NPs is presented in Scheme 2. As can be seen in this scheme, in the first step, malononitrile was activated by CuO NPs and then attacked as a nucleophile to the carbonyl group of isatin (chelated to CuO NPs). After condensation reaction, resulted intermediate (I) was obtained and addition of 1,3-dicarbonyl compound to I through Michael addition reaction generated intermediate of II. In the last step, the cyclization and tautomerization leads to a spirooxindole derivative.
The efficiency of CuO NPs has been compared with some of the other catalysts applied for the synthesis of spirooxindole derivatives. Results were listed in . As can be seen from this table, reactions catalyzed with CuO NPs (as a high efficient eco-friendly catalyst) showed shorter reaction times along with higher yields of products.
Table 5. Comparison of the efficiency of CuO NPs with other catalysts for the synthesis of 4a and 4j.
3. Experimental section
3.1. Materials and methods
The products were isolated and characterized by physical and spectral data. The FTIR spectra were recorded on FTIR Magna 550 apparatus using KBr plates. 1H NMR and 13C NMR spectra were recorded with a Bruker Avance DRX-400 spectrometer at 400 and 100 MHz, respectively. Powder X-ray diffraction (XRD) was carried out on a Philips diffractometer of X’pert Company with monochromatized Cu Kα radiation (λ = 1.5406 Å). The morphology of products was studied by electron scanning microscopy (SEM; model: LEO 1455VP).
3.2. Synthesis of CuO NPs
CuO NPs were prepared through a precipitation reaction between (25 mmol) Cu(CH3COO)2 in (100 mmol) NaOH dissolved in ethanol at 78°C for 1 h. The obtained brown nanoparticles were separated by filtration and washed with ethanol and finally dried at 100°C for 12 h (Citation34).
3.3. General procedure for the synthesis of spirooxindoles
A mixture of isatin derivative (1 mmol), malononitrile or ethyl cyanoacetate (1 mmol) and 1,3-dicarbonyl compound (1 mmol) in the presence of 4 mol.% of CuO NPs was stirred in 2 ml ethanol at room temperature. The progress of the reaction was monitored by TLC (n-hexane/ethyl acetate, 2:1 ratio). After the completion of the reaction, resulted mixture was resolved in acetone and filtered for the separation of nanocatalyst. Then, the product was obtained by evaporation of solvent from filtrate and for further purification, was recrystallized from EtOH to obtain pure products.
4. Conclusion
In conclusion, a mild, safe and efficient method for the preparation of spirooxindoles was presented. The process has proved to be very effective, with a high-performance catalyst and easy to operate. It is also very clean in that the products require no further purification and the scale up of this method is easy.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
Leila Moradi was born in Qom, Iran. She received her M.Sc. degree in Organic Chemistry from the university of Kashan, Kashan Iran under the supervision of Professor Hossein Naeimi in 2004. She obtained her Ph.D. degree in the same University, under the supervision of Professor Hossein Naeimi and Professor Ali Mohajeri, in 2009. She is a Professor Assistant in department of organic chemistry, faculty of chemistry, of Kashan University. Her research interests include organic synthesis, carbon nantubes, Functionalization and application of carbon nanotubes, synthetic methodology and applications of nano-heterogeneous catalysts in multicomponent reactions.
Zeynab Ataei was born in 1990 in Roudbar, Iran. She received her B.Sc. degree in pure Chemistry from Guilan University, Rasht, Iran (2012) and her M.Sc. degree in Organic Chemistry at University of Kashan, Kashan, Iran (2016) under supervision of Dr. Leila Moradi. Her research field was the synthesis of spirooxindoles through multicomponent reaction using nano-heterogeneous catalysts.
Additional information
Funding
References
- Domling, A. Angew. Chem. Int. Ed. 2000, 39, 3168–3210. doi: 10.1002/1521-3773(20000915)39:18<3168::AID-ANIE3168>3.0.CO;2-U
- Black, D.A.; Arndtsen, B.A. Org. Lett. 2004, 6, 1107–1110. doi: 10.1021/ol036462+
- Dhawan, R.; Arndtsen, B.A. J. Am. Chem. Soc. 2004, 126, 468–469. doi: 10.1021/ja039152v
- Dghaym, R.D.; Dhawan, R.; Arndtsen, B.A. Angew. Chem. Int. Ed. 2001, 40, 3328–3330. doi: 10.1002/1521-3757(20010903)113:17<3328::AID-ANGE3328>3.0.CO;2-M
- Bhrigu, B.; Pathak, D.; Siddiqui, N.; Alam, M.S.; Ahsan, W. Int. J. Pharm. Sci. Drug. Res. 2010, 2, 229–235.
- Raj, V. Int. J. Curr. Pharm. Res. 2012, 4, 1–9.
- Pandeya, S.N.; Smitha, S.; Jyti, M.; Sridhar, S.K. Acta. Pharm. 2005, 55, 27–46.
- Bhaskar, G.; Arun, Y.; Balachandran, C.; Saikumar, C.; Perumal, P.T. Eur. J. Med. Chem. 2012, 51, 79–91. doi: 10.1016/j.ejmech.2012.02.024
- Wu, G.; Ouyang, L.; Liu, J.; Zeng, S.; Huang, W.; Han, B.; Wu, F.; He, G.; Xiang, M. Mol. Divers. 2013, 17, 271–283. doi: 10.1007/s11030-013-9432-3
- Galliford, C.V.; Scheidt, K.A. Angew. Chem. Int. Ed. 2007, 46, 8748–8758. doi: 10.1002/anie.200701342
- Pavlovska, T.L.; Redkin, R.G.; Lipson, V.V.; Atamanuk, D.V. Mol. Divers. 2016, 20, 299–344. doi: 10.1007/s11030-015-9629-8
- Alemi Tameh, F.; Safaei-Ghomi, J.; Mahmoudi-Hashemi, M.; Shahbazi Alavi, H. RSC Adv. DOI:10.1039/C6RA08458C
- Karmakar, B.; Nayak, A.; Banerji, J. Tetrahedron Lett. 2012, 53, 5004–5007. doi: 10.1016/j.tetlet.2012.07.030
- Zhang, X.-N.; Li, Y.-X.; Zhang, Z.-H. Tetrahedron 2011, 67, 7426–7430. doi: 10.1016/j.tet.2011.07.002
- Ahadi, S.; Khavasi, H.R.; Bazgir, A. Chem. Pharm. Bull. 2008, 56, 1328–1330. doi: 10.1248/cpb.56.1328
- Ghahremanzadeh, R.; Fereshtehnejad, F.; Yasaei, Z.; Amanpour, T.; Bazgir, A. J. Heterocycl. Chem. 2010, 47, 967–972. doi: 10.1002/jhet.399
- Kidwai, M.; Jahan, A.; Mishra, N.K. Appl. Catal. A: General 2012, 425-426, 35–43. doi: 10.1016/j.apcata.2012.02.043
- Wagh, Y.B.; Tayade, Y.A.; Padvi, S.A.; Patil, B.S.; Patil, N.B.; Dalal, D.S. Chin. Chem. Lett. 2015, 26, 1273–1277. doi: 10.1016/j.cclet.2015.06.014
- Li, Y.; Chen, H.; Shi, C.; Shi, D.; Ji, S. J. Comb. Chem. 2010, 12, 231–237. doi: 10.1021/cc9001185
- Rao, B.M.; Reddy, G.N.; Reddy, T.V.; Devi, B.L.A.P.; Prasad, R.B.N.; Yadav, J.S.; Reddy, B.V.S. Tetrahedron Lett. 2013, 54, 2466–2471. doi: 10.1016/j.tetlet.2013.02.089
- Wang, G.-D.; Zhang, X.-N.; Zhang, Z.-H. J. Heterocycl. Chem. 2013, 50, 61–65. doi: 10.1002/jhet.994
- Jamatia, R.; Gupta, A.; Pal, A.K. RSC Adv. DOI: 10.1039/C5RA27552K
- Riyaz, S.; Naidu, A.; Dubey, P.K. Lett. Org. Chem. 2012, 9, 101–105. doi: 10.2174/157017812800221771
- Zhou, K.; wang, R.; Xu, B.; Li, Y. Nanotechnology 2006, 17, 3939–3943. doi: 10.1088/0957-4484/17/15/055
- Mishra, S.; Santra, S.; Hajna, A. RSC. Adv. 2015, 5, 91326–91330. doi: 10.1039/C5RA18350B
- Vidyasagar, C.C.; Naik, Y.A.; Venkatesh, T.G.; Viswanatha, R. Powder Technol. 2011, 214, 337–343. doi: 10.1016/j.powtec.2011.08.025
- Das, D.; Bikash, B.; Nath, C.; Phokon, P.; Kumar, S. Colloids. Surf. B: Biointerfaces 2013, 101, 430–433. doi: 10.1016/j.colsurfb.2012.07.002
- Ren, G.; Hu, D.; Chang, E.W.C.; Reip, P.; Allaker, R. Int. J. Antimicrob. Agents. 2009, 33, 587–590. doi: 10.1016/j.ijantimicag.2008.12.004
- EL-Trass, A.; EL-Shamy, H.; EL-Mehasseb, I.; EL-Kemary, M. Appl. Surf. Sci. 2012, 258, 2997–3001. doi: 10.1016/j.apsusc.2011.11.025
- Zarei Ahmady, A.; Keshavarz, M.; Kardani, M.; Mohtasham, N. Orient. J. Chem. 2015, 31, 1841–1846. doi: 10.13005/ojc/310369
- Ayoman, E.; Hossini, G.; Haghighi, N. Int. J. Nanosci. Nanotechnol. 2015, 11, 63–70.
- Chaudhary, G.R.; Bansal, P.; Kaur, N.; Mehta, S.K. Rsc. Adv. 2014, 4, 49462–49470. doi: 10.1039/C4RA07620F
- Nasrollahzadeh, M.; Mohammad Sajadi, S.; Rostami-Vartooni, A.; Mamand Hussin, S. J. Colloid. Interface. Sci. 2016, 466, 113–119. doi: 10.1016/j.jcis.2015.12.018
- Kida, T.; Oka, T.; Nagano, M.; Ishiwata, Y.; Zheng, X. J. Am. Ceram. Soc. 2007, 90, 107–110. doi: 10.1111/j.1551-2916.2006.01402.x
- Dabiri, M.; Bahramnejad, M.; Baghbanzadeh, M. Tetrahedron. 2009, 65, 9443–9447. doi: 10.1016/j.tet.2009.08.070
- Raghuvanshi, D.S.; Singh, K.N. J. Heterocycl. Chem. 2010, 47, 1323–1327. doi: 10.1002/jhet.451
- Zhu, S.-L.; Ji, S.-J.; Zhang, Y. Tetrahedron. 2007, 63, 9365–9372. doi: 10.1016/j.tet.2007.06.113
- Mahmoud, A.F.; Abd El-Latif, F.F.; Ahmed, A.M. Chin. J. Chem. 2010, 28, 91–96. doi: 10.1002/cjoc.201090041
- Chandam, D.R.; Mulik, A.G.; Patil, D.R.; Deshmukh, M.B. Res. Chem. Intermed. DOI: 10.1007/s11164-015-2093-3
- Li, Y.; Chen, H.; Shi, C.; Shi, D.; Ji, S. J. Comb. Chem. 2010, 12, 231–237. doi: 10.1021/cc9001185
- Mobinikhaledi, A.; Foroughifar, N.; Bodaghi Fard, M.A. Synth. Commun. 2011, 41, 441–450. doi: 10.1080/00397911003587507