ABSTRACT
Tin exchanged montmorillonite K10 (SnII-Mont K10) was prepared by ion exchange between SnCl2 and montmorillonite K10. The SnII-Mont K10 was characterized by X-ray diffraction, scanning electron microscope and energy-dispersive X-ray spectroscopy. The synthesized SnII-Mont K10 was used as a recoverable solid catalyst for synthesis of 3-methyl-4-arylmethylene isoxazole-5(4H)-ones via one-pot multicomponent cyclocondensation of hydroxylamine hydrochloride, ethyl acetoacetate and benzaldehyde derivatives in water under ultrasound irradiations. The yields of products were obtained 87–96%. The remarkable advantages of this method are a low-cost and eco-friendliness catalyst, rapid completion of the reactions, and avoidance of using organic solvents, excellent yield and mild conditions.
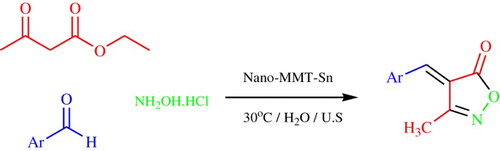
GRAPHICAL ABSTRACT
Introduction
One-pot multicomponent reactions have recently been discovered to be a powerful synthetic tool for the synthesis of heterocyclic compounds, since the products are formed in one-pot and the diversity can be achieved simply by varying each component. The simplicity of a one-pot procedure, the possible structural variations, the atom economy and convergent character, operational simplicity and the very large number of accessible organic compounds are among the described advantages of multicomponent reactions ( Citation1, Citation2). One such multicomponent reaction that belongs to the latter category is the isoxazole derivatives synthesis.
Isoxazole and isoxasolone derivatives are important organic heterocycles, because of their widespread pharmacological activities such as fungicidal ( Citation3), analgesic ( Citation4), antimicrobial ( Citation5), antioxidant ( Citation6), nematicidal ( Citation7), anti-inflammatory compounds ( Citation8) and hypoglycemic activity ( Citation9). In recent studies, several reports on the synthesis of isoxasolones heterocycles involves the one-pot reactions of an aldehyde, hydroxylamine hydrochloride and a β-ketoester using various catalytic substrates have been presented ( Citation10–17). Due to the importance of isoxazole heterocycles, we decided to explore an efficient, simple and fast synthesis of isoxasolones using Tin exchanged montmorillonite K10 as a heterogeneous nanocatalyst under ultrasound irradiation.
In recent years, ultrasound irradiation has been introduced as a green technology for different types of chemical reactions. Ultrasonic energy leads to cavitations, namely, formation, growth and implosive collapse of bubbles in a liquid during a very short period of time. This induces intense local heating with very short lifetimes, which promotes organic reactions to occur. Therefore, chemical transformations under ultrasound irradiation are more advantageous comparing to conventional methods in view of its milder reaction condition, high yields in shorter reaction time, improved selectivity, simple experimental procedure and energy conservation ( Citation18, Citation19).
Recent progresses in fine chemical synthesis demonstrate that the field is promising with the newly developed catalysts and methods. Montmorillonite has attracted attention in heterogeneous catalytic processes because of their surface area in the range of 750 m2/g, high structural charge (up to 1000 meq/kg) and catalytic activities. Montmorillonite is one of the most intensively explored as a heterogeneous support for organic reactions due to its physical and chemical properties ( Citation20). To enhance the acidity property and high catalytic activities, SnCl2 can be used with montmorillonite to be widely used as a powerful catalyst for various organic transformations under mild conditions ( Citation21, Citation22).
However, there are no examples of the use of SnII-Mont K10 as a catalyst for the synthesis of 3-methyl-4-arylmethylene isoxazole-5(4H)-ones under sonication. Therefore, we like to report, for the first time, a new efficient, and clean methodology for the synthesis of 3-methyl-4-arylmethylene isoxazole-5(4H)-ones via a three component condensation of an aromatic aldehyde, ethyl acetoacetate, hydroxylamine hydrochloride, and SnII-Mont K10 as a heterogeneous catalyst under ultrasonic irradiation, which is an efficient preparation 3-methyl-4-arylmethylene isoxazole-5(4H)-one ().
Results and discussion
Characterization of montmorillonite SnII-Mont K10
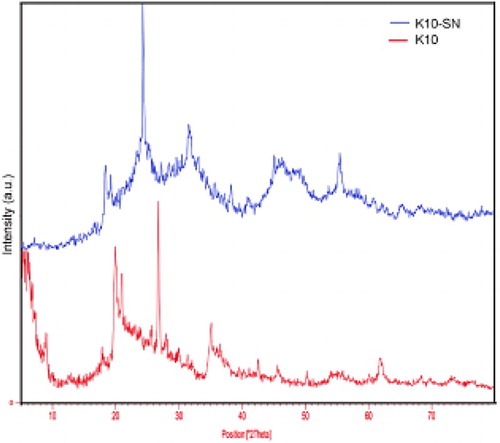
Montmorillonite K10 supported SnII composite (SnII-Mont K10) was prepared by the ion-exchange process. In order to analyze the catalyst, it was studied by powder X-ray diffraction (XRD) which the obtained diffractogram is reported in . As shown in this figure, in montmorillonite K10, the positions and relative intensities of all the peaks coincide well with the XRD pattern that are mainly in the amorphous form. When Sn cations are exchanged for other cations in the montmorillonite, the frontier between the interlayer of SnII-Mont K10 is different from that of the interlayer of MMT. It is indicated that the Sn cation increases the distance between the sheets and alter the montmorillonite structures (d-spacing MMT – Sn = 11.84 and d-spacing MMT = 9.86). Also, the shifting of the (8.96) peak towards a lower shift peak (7.46) suggests the intercalation of Sn into the clay interlayer.
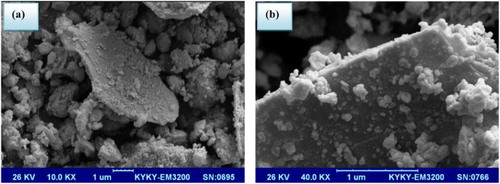
Furthermore, the scanning electron microscope (SEM) micrograph of the neat K10 and modified clay was shown in ((a),(b)), respectively. It is clear that the layered structure of MMT after modification is similar to that of the parent clay.
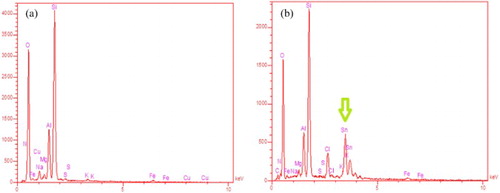
The EDX spectra of K10 clay and SnII-Mont K10 are shown in . In the spectrum of montmorillonite in , the three sharp peaks were observed which are related to Al, Si and O elements. However, in the SnII-Mont K10 in (b), in addition to above-mentioned peaks, a peak was observed which verify the Sn element. The content of Sn in the clays is 21.43 Wt %.
Preparation of 3-methyl-4-arylmethylene isoxazole-5(4H)-ones
We are reporting here an efficient sonochemical SnII-Mont K10 catalyzed for the synthesis of isoxasolones. The addition of SnII-Mont K10 accelerated this organic reaction which leads to great results. Using 4-methoxy-benzaldehyde, ethyl acetoacetate and hydroxylamine hydrochloride as the model substrates in water, this condensation was studied with SnII-Mont K10 catalyst under ultrasound irradiation. Thereafter, the reaction was evaluated by varying the concentrations of catalyst (0.006–0.02 g). shows the yield of isoxazolone against catalyst amount data. The use of 0.01 g of SnII-Mont K10 in the reaction mixture was sufficient for achieving the best yield (entry 5). Also, it was observed that the reaction in the presence of catalyst and ultrasound irradiation with the power of 90 W leaves the best result as the obtained product has 96% isolated yield during 20 min (entry 11). This shows that both the catalyst as well as ultrasound energy has an important role in the completion of this reaction. The combination of ultrasonic irradiation and heterogeneous catalyst is a standard technology used in this context. Ultrasound provides an unusual mechanism due to the immense temperatures (up to 5000 K) and high pressures (up to 1000 bar) and the extraordinary heating rate generated by the cavitation phenomenon in this medium ( Citation18).
Table 1. Optimization of reaction conditions in view of catalyst amount and max power intensity.a
The model reaction for preparation of product 4 under ultrasound irradiations at 30°C was examined using a variety of solvents and the results are summarized in . As seen in , the best results were obtained for H2O in the presence of ultrasound irradiations (entry 2). In the absence of solvent the yields reduced significantly (entry 6) and using solvents such as EtOH, MeOH and DCM was completely unsuccessful (entries 3–5). Therefore, H2O was chosen as solvent of this system. The application of ultrasound in this reaction has advantages such as shorter reaction times, milder reaction condition and higher yields in comparison with the classical method.
Table 2. Screening of solvent effect on model reaction under ultrasound irradiation at 30°C.a
After optimization of the reaction conditions, we studied the scope of this method, particularly with regard to library construction. We have extended the procedure using a series of aromatic aldehydes with both electron-donating and electron-withdrawing substituents. Most importantly, aromatic aldehydes carrying either electron-withdrawing or electron-donating substituents reacted very well to give the corresponding isoxazoles with excellent yields and high purity. The results are summarized in .
Table 3. SnII-Mont catalyzed synthesis of 3-methyl-4-arylmethylene isoxazole-5(4H)-ones under sonication.
Then, we compared our catalytic data with that found in the literature. Comparison of the results shows a better catalytic activity of SnII-Mont K10 to the synthesis of isoxazoles ().
Table 4. Comparison of SnII-Mont K10 with some other catalysts for the synthesis of 4a.
To explore the advantages of SnII-Mont K10 as a catalyst for the synthesis of the model compound 4a, we compared results reported in the literature for this reaction mediated by other catalysts (). It is clear from this table that SnII-Mont K10 is an efficient catalyst which could be useful in the synthesis of isoxazoles.
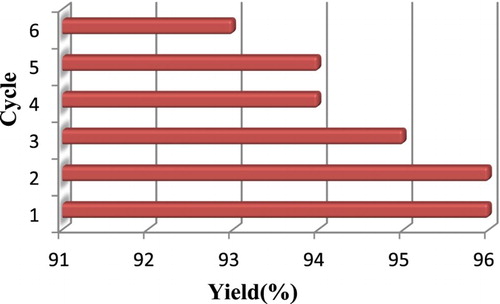
Next, we have investigated the reusability of SnII-Mont K10 ultrasound irradiation. In this process, the model reaction was again studied under optimized conditions. After the completion of the reaction, heterogeneous catalysts were separated by filtration, and reused after dilution with ethyl acetate for the model reaction. As indicated in , recycled catalyst showed no efficiency loss with respect to reaction time and yield after six consecutive runs.
Mechanisms of the reaction
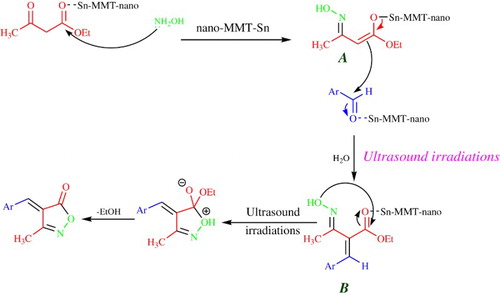
A possible mechanism for the one-pot cyclocondensation of aromatic aldehyde, ethyl acetoacetate and hydroxylamine hydrochloride, in the presence of SnII-Mont K10, is shown in . At first, the tin-exchanged clay acts as a Lewis acid and increases the electrophilic character of the carbonyl groups in ethyl acetate. Also, SnII-Mont K10 is given the high Lewis acidity of Sn as well as its ability to produce acid media from coordinated water molecules that this process is accelerated via sonication in the layered structure of MMT. Then the nucleophilic attack of the amino group of hydroxylamine hydrochloride occurs at the activated carbonyl carbon of ethyl acetoacetate to result oxime intermediate A. The catalyst then activates the carbonyl group of oxime intermediate and the nucleophilic attack occurs at the carbonyl carbon of the aromatic aldehyde which is also activated by SnII-Mont K10 under sonication. Then, cyclization of intermediate B is done for the synthesis of 3-methyl-isoxazole-5(4H)-one under ultrasound irradiation. In a heterogeneous solid/liquid system, adsorption of intermediate oxime (A) is improved on the SnII-Mont K10 surface from the effects of catalyst surface and also ultrasonic irradiation (10b, c, 12c).
Experimental
Materials and methods
Chemicals such as hydroxylamine hydrochloride, ethyl acetoacetate, aldehyde derivatives, montmorillonit-K10, SnCl2, ethanol, methanol and DCM were purchased from Fluca and Merck chemical companies in high purity. All of the materials were of commercial reagent grade. The products are characterized by comparison of their spectral data (13C and 1H-NMR, IR, UV and Rf) and physical data with authentic samples. 1H and 13C NMR spectra were recorded on an Avance BRUKER (DRX- 400 MHz) in CDCl3 as solvent. UV–VIS spectra were taken by a double beam Perkin-Elmer 550S spectrophotometer in the range of 200–400 nm, using chloroform as the solvent. IR spectra were determined on a Nicolet Magna series FTIR 550 spectrometer using KBr pellets. The purity determination of the substrates and reaction monitoring were accomplished by thin layer chromatography (TLC) on silica gel polygram SILG/UV 254 plates. A Bandelin Sonorex Super 10P Ultrasonic Bath (water) (with a frequency of 35 kHz and a nominal power 100 W) was used. The melting points were determined by a Yanagimoto micro melting point apparatus. Structures were characterized using a Holland Philips Xpert XRD diffractometer (CuK, radiation, λ = 0.154056 nm), at a scanning speed of 2°/min from 10° to 100° (2θ). The surface morphology of montmorillonite based materials was analyzed by field emission SEM (KYKY-EM3200).
Preparation of SnII-Mont K10
Montmorillonite SnII-Mont K10 was prepared according to the reported procedure in the literature ( Citation21). In short, a mixture containing SnCl2·2H2O (0.19 g) and montmorillonite clay (0.8 g) in distilled water (10 mL) was stirred at room temperature for 36 h. The product was centrifuged and the modified clay was washed with copious amounts of deionized water until the discarded filtrate was free from chloride ions (checked by 0.1 M AgNO3). Finally, the prepared catalyst was dried at 110°C for 12 h.
General procedure for the preparation of 3-methyl-4-arylmethylene isoxazole-5(4H)-ones
To a 50 mL round-bottomed flask was added sequentially aromatic aldehyde (1 mmol), ethyl acetoacetate (1 mmol), hydroxylamine hydrochloride (1 mmol), SnII-Mont K10 (0.01 g) and distilled water (5 mL). The reaction was irradiated under sonication at 30°C. The precipitate is gradually formed during the reaction. After completion of the reaction (monitored by TLC; petroleum ether-ethyl acetate 1:1), the precipitate was filtered at the pump and dried in oven. Then, the mixture was dissolved in ethyl acetate and the catalyst was separated by filtration. The solvent was removed under reduced pressure. The crude product was crystallized from EtOH to afford the pure product in high yield.
Conclusion
In this study, we have investigated the application of heterogeneous SnII-Mont K10 for the synthesis of isoxazole compounds that offer 3-methyl-4-arylmethylene isoxazole-5(4H)-ones under ultrasound irradiation, in high yields and high purity maintaining the advantage of the one-pot approach. The present methodology offers advantages such as mild reaction conditions, reduced reaction times, simple manipulation and economic viability of the catalyst, compared with conventional methods.
Supplement_Material.docx
Download MS Word (29.4 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
Majid Ahmadzadeh received his B.Sc. degree in Chemistry from the University of Zabol, in 2009 and then his M.Sc. degree in Organic chemistry from the Semnan university in 2012. He received his Ph.D. degree in Organic Chemistry from the University of Kashan (Kashan, Iran) in 2017 which included Preparation of pyranopyrazole and isoxazolone hetrocycles using modified montmorillonite nanocatalysts under the supervision of Dr Javad Safari.
Zohre Zarnegar received her BS (2002) in Pure Chemistry from University of Kashan (Iran). Next, she studied her MS (2008) in Organic Chemistry at the Lorestan University. She performed her MS research in the field of nanochemistry which included synthesis of novel dendrimers under the supervision of Prof. Mohsen Adeli. She obtained her PhD degree in Organic Chemistry at University of Kashan (2014) which included development of magnetic nanoparticles and their application in hostguest systems and some of the organic reactions under the supervision of Dr Javad Safari. She added a postdoctoral stage from the Iran Nanotechnology Initiative Council (INIC) in 2016.
Javad Safari was born in Kashan, Iran in 1965. He obtained his B.Sc. degree in Chemistry at the University of Kashan in 1989. He received his M.Sc and Ph.D. degrees in Organic Chemistry at the University of Isfahan in 1992 and 2002. He is teaching as associate professor of Chemistry at the University of Kashan. His research interests focus on heterocyclic chemistry, spectroscopy, catalysis and organic methodology.
Additional information
Funding
References
- (a) Brahmachari, G.; Banerjee, B. Facile and One-pot Access to Diverse and Densely Functionalized 2-amino-3-cyano-4H-pyrans and Pyran-annulated Heterocyclic Scaffolds via an Eco-friendly Multicomponent Reaction at Room Temperature Using Urea as a Novel Organo-catalyst. ACS Sustainable Chem. Eng. 2014, 2, 411−422; (b) Solhy, A.; Elmakssoudi, A.; Tahir, R.; ed Karkouri, M.; Larzek, M.; Bousmina, M.; Zahouily, M. Clean Chemical Synthesis of 2-amino-chromenes in Water Catalyzed by Nanostructured Diphosphate Na2CaP2O7. Green Chem. 2010,12, 2261–2267. doi: 10.1021/sc400312n
- (a) Ramazani, A.; Asiabi, P.A.; Aghahosseini, H.; Gouranlou, F. Review on the Synthesis and Functionalization of SiO2 Nanoparticles as Solid Supported Catalysts. Curr. Org. Chem. 2017, 21, 908–922; (b) Khoobi, M.; Ma’mani, L.; Rezazadeh, F.; Zareie, Z.; Foroumadi, A.; Ramazani, A.; Shafiee, A. One-pot Synthesis of 4H-benzo[b]pyrans and Dihydropyrano[c]chromenes Using Inorganic – Organic Hybrid Magnetic Nanocatalyst in Water. J. Mol. Catal. A Chem. 2012,359, 74–80; (c) Tabatabaei Rezaei, S.J.; Shamseddin, A.; Ramazani, A.; Mashhadi Malekzadeh, A.; Azimzadeh Asiabi, P. Palladium Nanoparticles Immobilized on Amphiphilic and Hyperbranched Polymer-functionalized Magnetic Nanoparticles: An Efficient Semi-heterogeneous Catalyst for Heck Reaction. Appl. Organomet. Chem. 2017, in press. DOI:10.1002/aoc.3707; (d) Fardood, S.T.; Ramazani, A.; Golfar, Z.; Joo, S.W. Green Synthesis of Ni-Cu-Zn Ferrite Nanoparticles Using Tragacanth Gum and Their Use as An Efficient Catalyst for the Synthesis of Polyhydroquinoline Derivatives. Appl. Organomet. Chem. 2017, in press. DOI: 10.1002/aoc.3823; (e) Hasanpour, Z.; Maleki, A.; Hosseini, M.; Gorgannezhad, L.; Nejadshafiee, V.; Ramazani, A.; Haririan, I.; Shafiee, A.; Khoobi, M. Efficient Multicomponent Synthesis of 1,2,3-triazoles Catalyzed by Cu(II) Supported on PEI@Fe3O4 MNPs in a Water/PEG300 System. Turk. J. Chem. 2017,41, 294–307; (f) Taran, J.; Ramazani, A.; Aghahosseini, H.; Gouranlou, F.; Tarasi, R.; Khoobi, M.; Joo, S.W. One-pot Three-component Syntheses of α-aminophosphonates from a Primary Amine, Quinoline-4-Carbaldehyde and a Phosphite in the Presence of MCM-41@PEI as an Efficient Nanocatalyst. Phosphorus, Sulfur, Silicon. Relat. Elem. 2017,192, 776–781. doi: 10.2174/1385272820666161021103415
- Santos, M.M.M.; Faria, N.; Iley, J.; Coles, S.J.; Hursthouse, M.B.; Martins, M.L.; Moreira, R. Reaction of Naphthoquinones with Substituted Nitromethanes. Facile Synthesis and Antifungal Activity of Naphtho[2,3-d]isoxazole-4,9-diones. Bioorg. Med. Chem. Lett. 2010, 20, 193–195. doi: 10.1016/j.bmcl.2009.10.137
- Kano, H.; Adachi, I.; Kido, R.; Hirose, K. Isoxazoles. XVIII. Synthesis and Pharmacological Properties of 5-aminoalkyl- and 3-aminoalkylisoxazoles and Related Derivatives. J. Med. Chem. 1967, 10, 411–418. doi: 10.1021/jm00315a028
- Saikh, F.; Das, J.; Ghosh, S. Synthesis of 3-methyl-4-arylmethylene Isoxazole-5(4H)-ones by Visible Light in Aqueous Ethanol. Tetrahedron Lett. 2013, 54, 4679–4682. doi: 10.1016/j.tetlet.2013.06.086
- Padmaja, A.; Rajasekhar, C.; Muranikrishna, A.; Padmavathi, V. Synthesis and Antioxidant Activity of Oxazolyl/Thiazolylsulfonylmethyl Pyrazoles and Isoxazoles. Eur. J. Med. Chem. 2011, 46, 5034–5038. doi: 10.1016/j.ejmech.2011.08.010
- Srinivas, A.; Nagaraj, A.; Reddy, C.S. Synthesis and In Vitro Study of Methylene-bis-tetrahydro[1,3]thiazolo[4,5-c]isoxazoles as Potential Nematicidal Agents. Eur. J. Med. Chem. 2010, 45, 2353–2358. doi: 10.1016/j.ejmech.2010.02.014
- Karabasanagouda, T.; Adhikari, A.V.; Girisha, M. Synthesis of Some New Pyrazolines and Isoxazoles Carrying 4-methylthiophenyl Moiety as Potential Analgesic and Anti-inflammatory Agents. Indian J. Chem. 2009, 48B, 430–437.
- Kang, Y.Y.; Shin, K.J.; Yo, K.H.; Seo, K.J.; Hong, C.Y.; Lee, C.S.; Park, S.Y.; Kim, D.J.; Park, S.W. Synthesis and Antibacterial Activity of New Carbapenems Containing Isoxazole Moiety. Bioorg. Med. Chem. Lett. 2000, 10, 95–99. doi: 10.1016/S0960-894X(99)00646-0
- (a) Maddila, S.N.; Maddila, S.; van Zyl, W.E.; Jonnalagadda, S.B. Ag/SiO2 as a Recyclable Catalyst for the Facile Green Synthesis of 3-methyl-4-(phenyl)methylene-isoxazole-5(4H)-ones. Res. Chem. Intermed. 2016, 42, 2553–2566; (b) Kiyani, H.; Darbandi, H.; Mosallanezhad, A.; Ghorbani, F. 2-Hydroxy-5-sulfobenzoic Acid: An Efficient Organocatalyst for the Three-component Synthesis of 1-amidoalkyl-2-naphthols and 3,4-disubstituted isoxazol-5(4H)-ones. Res. Chem. Intermed. 2015,41, 7561–7579; (c) Setamdideh, D. DOWEX(R)50WX4/H2O: A Green System for a One-pot and Three-component Synthesis of Isoxazol-5(4H)-one derivatives. J. Mex. Chem. Soc. 2015,59, 191–197; (d). Patil, M.S.; Mudaliar, C.; Chaturbhuj, G.U. Sulfated Polyborate Catalyzed Expeditious and Efficient Three Component Synthesis of 3-methyl-4-(hetero)arylmethylene Isoxazole-5 (4H)-ones. Tetrahedron Lett. 2017,58, 3256–3261. doi: 10.1007/s11164-015-2167-2
- (a) Liu, Q.; Hou, X. One-pot Three-component Synthesis of 3-methyl-4-arylmethylene-Isoxazol-5(4H)-ones Catalyzed by Sodium Sulfide. Phosphorus Sulfur Silicon Relat. Elem. 2012, 187, 448–453; (b) Vekariya, R.H.; Patel, K.D.; Patel, H.D. Fruit Juice of Citrus Limon as a Biodegradable and Reusable Catalyst for Facile, Eco-friendly and Green Synthesis of 3,4-disubstituted isoxazol-5(4H)-ones and dihydropyrano[2,3-c]-pyrazole Derivatives. Res. Chem. Intermed. 2016,42, 7559–7579; (c) Kiyani, H.; Kanaani, A.; Ajloo, D.; Ghorbani, F.; Vakili, M. N-bromosuccinimide (NBS)-promoted, Three Component Synthesis of α,β-unsaturated isoxazol-5(4H)- ones, and Spectroscopic Investigation and Computational Study of 3-methyl-4-(thiophen-2-ylmethylene)isoxazol- 5(4H)-one. Res. Chem. Intermed. 2015,41, 7739–7773. doi: 10.1080/10426507.2011.621003
- (a) Mirzazadeh, M.; Mahdavinia, G.H. Fast and Efficient Synthesis of 4-Arylidene-3-phenylisoxazol-5-ones. E- J. Chem. 2012, 9, 425–429; (b) Pawar, G.T.; Gadekar, S.P.; Arbad, B.R.; Lande, M.K. Modification, Characterization, and Catalytic Application of Mesolite for One Pot Synthesis of 3-methyl-4-arylmethylene-isoxazol-5(4H)-ones. Bull. Chem. React. Eng. Catal. 2017,12, 32–40; (c) Kiyani, H.; Ghorbani, F. Expeditious Green Synthesis of 3,4-disubstituted isoxazole-5(4H)-ones Catalyzed by Nano-MgO. Res. Chem. Intermed. 2016,42, 6831–6844; (d) Safari, J.; Ahmadzadeh, M.; Zarnegar, Z. Sonochemical Synthesis of 3-methyl-4-arylmethylene Isoxazole-5(4H)-ones by Amine-modified Montmorillonite Nanoclay. Catal. Comm. 2016,86, 91–95. doi: 10.1155/2012/562138
- (a) Zhang, Y.Q.; Ma, J.J.; Wang, C.; Li, J.C.; Zhang, D.N.; Zang, X.H.; Li, J. One-pot Synthesis of 3-methyl-4-arylmethylene-isoxazol-5(4H)-ones. Chin. J. Org. Chem. 2008, 28, 141–144; (b) Chavan, A.P.; Pinjari, A.B.; Mhaske, P.C. An Efficient Synthesis of 4-arylmethylidene-3-substituted-isoxazol-5(4H)-ones in Aqueous Medium. J. Heterocycl. Chem. 2015,52, 1911–1915; (c) Setamdideh, D. One-pot Green Synthesis of Isoxazol-5(4H)-one Derivatives by Dowex1-x8OH in Water. J. Serb. Chem. Soc. 2016,81, 971–978.
- (a) Kiyani, H.; Ghorbani, F. Sodium Saccharin as a Clean and Efficient Catalyst for the Synthesis of 4-arylidene-3methylisoxazol-5(4H)-ones via One-pot Three-component Reaction in Aqueous Medium. Heterocycl. lett. 2013, 3, 359–369; (b) Kiyani, H.; Ghorbani, F. Efficient Tandem Synthesis of a Variety of Pyranannulated Heterocycles, 3,4-disubstituted isoxazol-5(4H)-ones, and α,β-unsaturated Nitriles Catalyzed by Potassium Hydrogen Phthalate in Water. Res. Chem. Intermed. 2015,41, 7847–7882.
- (a) Kiyani, H.; Ghorbani, F. Synthesis of Arylmethylideneisoxazol-5(4H)-ones in Water Catalyzed by Sodium Citrate. Heterocycl. lett. 2013, 3, 359–369; (b) Kiyani, H.; Ghorbani, F. Boric Acid-catalyzed Multi-component Reaction for Efficient Synthesis of 4H-isoxazol-5-ones in Aqueous Medium. Res. Chem. Intermed. 2015,41, 2653–2664.
- (a) Liu, Q.; Zhang, Y.N. One-pot Synthesis of 3-methyl-4-arylmethylene-isoxazol-5(4H)-ones Catalyzed by Sodium Benzoate in Aqueous Media: A Green Chemistry Strategy. Bull Korean Chem. Soc. 2011, 32, 3559–3560; (b) Vaidya, S.P.; Shridhar, G.; Ladage S.; Ravishankar L. A Facile Synthesis of Isoxazolone Derivatives Catalyzed by Cerium Chloride Heptahydrate in Ethyl Lactate as a Solvent: A Green Methodology. Curr. Green Chem. 2016,3, 160–167. doi: 10.5012/bkcs.2011.32.10.3559
- (a) Kiyani, H.; Ghorbani, F. Potassium Phthalimide as Efficient Basic Organocatalyst for the Synthesis of 3,4-disubstituted Isoxazol-5(4H)-ones in Aqueous Medium, J. Saudi Chem. Soc. 2017, 21, S112–S119; (b) Rikani, A.B.; Setamdideh, D. One-pot and Three-component Synthesis of Isoxazol-5(4H)-one Derivatives in the Presence of Citric Acid. Orient Chem. 2016,32, 1433–1437. doi: 10.1016/j.jscs.2013.11.002
- (a) Zarnegar, Z.; Safari, J. A Highly Efficient Magnetic Solid Acid Catalyst for Synthesis of 2,4,5-trisubstituted Imidazoles Under Ultrasound Irradiation. Ultrason. Sonochem. 2013, 20, 740–746; (b) Zarnegar, Z.; Safari, J.; Mansouri Kafroudi, Z. Co3O4–CNT Nanocomposites: A Powerful, Reusable, and Stable Catalyst for Sonochemical Synthesis of Polyhydroquinolines. New J. Chem. 2015,39, 1445–1451. (c) Liu, Q.; Ai, H.; Li, Z. Potassium Sorbate as an Efficient and Green Catalyst for Knoevenagel Condensation. Ultrason. Sonochem. 2011,18, 477–479; (d) Liu, Q.; Ai, H.M. Sodium Benzoate as a Green, Efficient, and Recyclable Catalyst for Knoevenagel Condensation. Synth. Commun. 2012,42, 3004–3010; (e) Liu, Q.; Ai, H.-M.; Feng, S. Ultrasound-assisted Synthesis of Acylals from Aldehydes Using Mg(CH3SO3)2–HOAC. Synth. Commun. 2012,42, 122–127. doi: 10.1016/j.ultsonch.2012.10.004
- (a) Ramazani, A.; Rouhani, M.; Joo, S.W. Novel, Fast and Efficient One-pot Sonochemical Synthesis of 2-aryl-1,3,4-oxadiazoles. Ultrason. Sonochem. 2014, 21, 262–267; (b) Ramazani, A.; Rouhani, M.; Joo, S.W. Catalyst-free Sonosynthesis of Highly Substituted Propanamide Derivatives in Water. Ultrason. Sonochem. 2016,28, 393–399; (c) Rouhani, M.; Ramazani, A.; Joo, S.W. Ultrasonics in Isocyanide-based Multicomponent Reactions: A New, Efficient and Fast Method for the Synthesis of Fully Substituted 1,3,4-oxadiazole Derivatives Under Ultrasound Irradiation. Ultrason. Sonochem. 2015,22, 391–396; (d) Ahankar, H.; Ramazani, A.; Ślepokura, K.; Lis, T.; Joo, S.W. Synthesis of Pyrrolidinone Derivatives from Aniline, an Aldehyde and Diethyl Acetylenedicarboxylate in an Ethanolic Citric Acid Solution Under Ultrasound Irradiation. Green Chem. 2016,18, 3582–3593. doi: 10.1016/j.ultsonch.2013.06.009
- Zarnegar, Z.; Alizadeh, R.; Ahmadzadeh, M.; Safari, J. C–N Bond Formation in Alicyclic and Heterocyclic Compounds by Amine-modified Nanoclay. J. Mol. Struct. 2017, 1144, 58–65. doi: 10.1016/j.molstruc.2017.05.004
- Lei, Z.; Ma, G.; Jia, C. Montmorillonite (MMT) Supported Tin (II) Chloride: An Efficient and Recyclable Heterogeneous Catalyst for Clean and Selective Baeyer–Villiger Oxidation with Hydrogen Peroxide. Catal. Commun. 2007, 8, 305–309. doi: 10.1016/j.catcom.2006.05.045
- Breen, C.; Molloy, K.C.; Quill, K. Mossbauer Spectroscopic and Thermogravimetric Studies of Tin-clay Complexes. Clay Miner. 1992, 27, 445–455. doi: 10.1180/claymin.1992.027.4.05