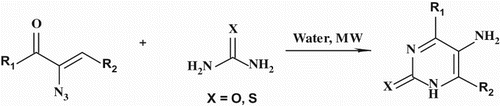ABSTRACT
An efficient and ecofriendly method for the synthesis of disubstituted 5-aminopyrimidines from vinyl azides and urea or thiourea was developed. This reaction proceeds under microwave irradiation conditions in the presence of water as a solvent. The remarkable features of this new protocol are high conversion, short reaction times, cleaner reaction profiles and straightforward procedure.
GRAPHICAL ABSTRACT
1. Introduction
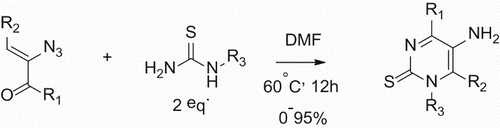
Heterocyclic molecules are of biological interest due to their potential physical and chemical properties ( Citation1). Among these, the pyrimidine compounds occupy a unique position in pharmaceutical chemistry, as they are components of nucleic acids. Pyrimidines are correlated with various therapeutic activities, e.g. anti-HIV ( Citation2), anti-tubercular ( Citation3), antitumor ( Citation4), antineoplastic ( Citation5), anti-inflammatory ( Citation6), diuretic ( Citation7), antimalaria ( Citation8), and cardiovascular ( Citation9). These compounds also have diverse applications such as bactericidal ( Citation10), fungicidal ( Citation11), and analgesics ( Citation12). It is worthy to note that only a few direct synthesis of 5-aminopyrimidines have been described in the literature. One of the most common methods used for the preparation of 5-aminopyrimidines employs the reduction of 5-nitropyrimidines with multiple steps ( Citation13), or vinyl azides using base ( Citation14) or toxic solvents (Scheme 1) ( Citation15). In this regard, the development of a facile and green synthetic method to access the disubstituted 5-aminopyrimidine is needed.
Scheme 1. Previous procedure developed by Yu and coworkers for the preparation of 5-aminopyrimidines.
Vinyl azide is a key synthon for the formation of nitrogen-containing heterocycles (azaheterocycles) ( Citation16). Especially, it has been reported that vinyl azide was employed with various transition metals in pyrrole synthesis ( Citation15).
In continuation of our research program focusing on the preparation of heterocyclic and therapeutic agents ( Citation17) and the development of more ecofriendly process and synthesis ( Citation18), we decided to optimize the synthesis of disubstituted 5-aminopyrimidines from vinyl azide and urea or thiourea in water, under microwave irradiations (Scheme 2).
2. Results and discussion
2.1. Optimization of reaction conditions
First, to optimize the reaction conditions, the coupling of 2-azido-3-(4-bromophenyl)-1-phenylprop-2-en-1-one 1 with urea was selected as the model reaction (). Initially, we found out that the use of a two-fold molar excess of urea is the good ratio as described by Yu and coworkers ( Citation15). The use of the excess of urea was necessary to ensure complete formation of the product and also for the compensation of the decomposed urea to CO2 + NH3. As shown in , different solvents were tested (entries 1–7). Starting from MeOH or CH3CN (entries 1 and 2), low yields were obtained. When toluene or dioxane was employed, increased yields were observed, 53% and 46%, respectively (entries 5 and 6). Moreover, the use of dimethylformamide (DMF) or tetrahydrofuran (THF) led to the described product with good yields. However, we were pleased to find that water was the most effective solvent with a highly improved chemical yield of 97%, despite the quite poor solubility of substrates, in only 10 min of microwave irradiation (entry 7).
Table 1. Optimization of reaction conditions.a
2.2. Typical procedure for the synthesis of 5-amino-6-(4-bromophenyl)-4-phenyl-1H-pyrimidine-2-one 3a
To a 10-mL glass microwave vial, a mixture of vinyl azide 1 (0.25 mmol) and urea 2 (0.5 mmol) in water (5 mL) was added. The mixture was heated in a microwave for 10 min at 160 W (120°C). After completion of the reaction, the mixture was cooled and then the crude was filtered. The crude product was purified by recrystallization (EtOH/Water (4/1)) to afford 5-amino-6-(4-bromophenyl)-4-phenyl-1H-pyrimidine-2-one 3a. As known the heating azides, or mixtures which contain azides, result in a rapid decomposition of the material, and often in an explosion. This process releases a large volume of gases. In our case, we have worked on a small amount of azides to avoid any problem. In our cases, we did not meet any difficulty.
2.3. Extension of the methodology
Having in hands the optimal reaction condition, the scope of the reaction was studied using a series of vinyl azides and urea or thiourea. The vinyl azides were readily prepared according to the literature ( Citation19).
The presence of an electron-donor group (CH3 or OCH3) on the ring of R1 or R2 decreased the reactivity of vinyl azides towards urea or thiourea (, entries 3, 5, 10, 13, 15, and 20). However, yields were higher than those observed by Yu and coworkers. When R1 or R2 consisted in an electron-withdrawing group such as Cl, Br, and F, the reactivity of the corresponding vinyl azides was increased and higher yields were achieved (entries 1, 2, 4, 6–8, 11, 12, 14, and 16–18). The use of vinyl azide bearing a thienyl at the R2 position (, entries 9 and 19) gave lower yield than the other products.
Table 2. Reactions of various vinyl azides with urea or thiourea.a
In conclusion, we have developed a simple, fast, efficient, and ecofriendly method for the synthesis of disubstituted 5-aminopyrimidine-2(1H)-ones or 5-aminopyrimidine-2(1H)-thiones, from readily available α-azidovinyl ketones and urea or thiourea under microwave irradiation, in water as non-toxic and environmentally benign green solvent in mild conditions to obtain the corresponding products in good yields. The reaction is realized by 1,4-Michael addition and subsequent rearrangement. This method is very attractive and can contribute to reduce environmental problems.
Supplement_Material.docx
Download MS Word (15.9 KB)Acknowledgment
The authors express their gratitude to Jouf University for the research funds and its support of this work: Project number 37/380.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
Dr Oussama Dehbi received his Ph.D. in organic chemistry from the University of Orleans in France in December 2012. Actually, he is an assistant professor and researcher in the College of Science and Arts, Al Qurayyat, Jouf University, KSA. His research focuses on the design and synthesis of new drug and studies of their structure–activity relationship and also the development of a new ecofriendly method for the synthesis of organic compounds.
Dr Esam A. Ishak received his Ph.D. in organic chemistry from Al Azhar University in Egypt in 2003. Actually, he is an assistant professor and researcher in the College of Science and Arts, Al Qurayyat, Jouf University, KSA. He is a researcher at the Olive Research Center at Jouf University. His research focuses on the synthesis of heterocyclic compounds using green chemistry methodologies and assessing their biological activity as antibacterial and antifungal reagents. Also, it focuses on the design and synthesis of new drug and the studies of their structure–activity relationship.
Dr Mohammed A. Bakht received his Ph.D. in pharmaceutical chemistry in 2009. He is currently working as an assistant professor in the Department of Pharmaceutical Chemistry in the College of Pharmacy, Prince Sattam Bin Abdulaziz University, KSA. His research interest is in the synthesis of potential organic scaffold utilizing new green chemistry technics and studying their pharmacological activities. He has few novel green solvents and catalysts to his name. He has also used ultrasound technology for the synthesis purposes.
Dr Mohammed H. Geesi received his Ph.D. in organic chemistry from the University of Southampton, UK, in 2004. Geesi is currently a researcher and assistant professor in the College of Arts and Science, Prince Sattam Bin Abdulaziz University. His research focuses on the development and synthesis of new heterocycles using green, facile and low-cost processing methods.
Dr Mohammed B. Alshammari was born in Saudi Arabia. He received his B.Sc. and M.Sc. degrees in Chemistry from King Saud University, Saudi Arabia. He received his Ph.D. degree from Cardiff University, UK, in 2013 under the supervision of Professor Keith Smith. His research is focused on the use of organolithium reagents as intermediates in organic synthesis. Currently, he is working as an assistant professor of Organic Chemistry at Prince Sattam Bin Abdulaziz University, Saudi Arabia.
Dr Vincent Chagnault received his Ph.D. in organic chemistry from the University of Poitiers in France in 2006. Actually, he is an assistant professor in University of Amiens and researcher in (Laboratoire de glycochimie, des Antimicrobiens et des Agroressources, UMR 7378 CNRS). His research focuses on the design and synthesis of new carbohydrate chemistry and the studies of their structure–activity relationship.
Dr Abdellah Kaiba received his Ph.D. in physical chemistry from the University of Bordeaux in France. Abdellah is an assistant professor and researcher in the Department of Physics, College of Science and Humanities Studies, Prince Sattam Bin Abdulaziz University, Saudi Arabia. His research focuses on the development of new organometalic compounds and catalysts.
Dr Saïd Lazar received his Ph.D. in bioorganic chemistry from the University of Orléans in France in 1990. In 1994, he obtained his habilitation to direct research (HDR) at the University of Orléans. Actually, he is a professor of biochemistry in University of Hassan II-Casablanca (Morocco) and researcher in Laboratory of Biochemistry, Environment and Agri-Food, URAC 36. Dr Saïd Lazar does research in Bioorganic Chemistry, Catalysis and Biochemistry. His current project is “Industrial waste management”.
Dr Yassine Riadi received his Ph.D. in organic pharmaceutical chemistry from the University of Orleans in France in 2013. Yassine is an assistant professor and researcher in the College of Pharmacy, Prince Sattam Bin Abdulaziz University. His research focuses on the development of new green catalysts using facile and low-cost processing methods and their use in the synthesis of new drugs and compounds.
Additional information
Funding
References
- Abe Brown, R.C.D. J. Chem. Soc. Perkin Trans., 1998, 1, 3293. doi: 10.1039/a805801f
- Noriyuki, K.; Hitoshi, M.; Shionogi & Co. Ltd., Japan PCT Int. Appl. WO 03, 47, 564, 2002; Noriyuki, K.; Hitoshi, M.; Chem. Abstr., 2003, 139, 36532c.
- Jani, M.K.; Shah, B.R.; Undavia, N.K.; Trivedi, P.B. Chem. Abstr., 1994, 121, 35513p.
- Safonova, T.V.; Keremov, A.F.; Ershova, Y.A. Khim. Farm. Zn., 1998, 32, 11 (Eng); Safonova, T.V.; Keremov, A.F.; Ershova, Y.A. Chem. Abstr., 1999, 131, 18975e.
- Jean-Damien, C.; David, B.; Ronald, K.; Julian, G.; Pan, Li; Robert, D. Vertex Pharmaceuticals Incorporated, USA; PCT Int. Appl. WO 02 22, 608, 2002; Jean-Damien, C.; David, B.; Ronald, K.; Julian, G.; Pan, Li; Robert, D. Chem. Abstr., 2002, 136, 247584x.
- Nakaguti, O.; Shimazaki, N.; Shimazaki, M.; Nakatuka, M. Eur. Pat. Appl., 1986 , 168, 005; Nakaguti, O.; Shimazaki, N.; Shimazaki, M.; Nakatuka, M. Chem. Abstr., 1986, 105, 191118p.
- Papesh, V.; Schroeder, E.F. US. Pat 2714559, 1956; Papesh, V.; Schroeder, E.F. Chem. Abstr., 1956, 50, 11370.
- Tokutake, N. Brit. Pat.146836B, 1977; Tokutake, N. Chem. Abstr., 1977, 87, 102370.
- Kurono, M. JP, 62, 267, 272, 1987; Kurono, M. Chem. Abstr., 1988, 109, 37382t.
- Pershin, N.G.; Sherbakova, L.I.; Sakolova, V.N. Pharmacol. Taksiko., 1972, 35, 466.
- Metolcsy, G. World Rev. Pest Contr., 1971, 10, 50.
- Regnier, G.L.; Canevar, R.J.; Douarec, L.; Halstop, S.; Daussy, J.; J. Med. Chem., 1972, 15, 295. doi: 10.1021/jm00273a600
- (a) Zapol’kii, V.A.; Namyslo, J.C.; Altug, C.; Gjikaj, M.; Kaufmann, D.E. Synthesis, 2008, 304. (b) Kaname, T.; Masaaki, T.; Yukitoshi, M.; Kuniyoshi, O.; Katsuyuki, I.; Hikari, M.; Tomoji, A. J. Heterocycl. Chem., 1987, 24, 1003. (c) Masaaki, T.; Yukitoshi, M.; Hikari, M.; Kaname, T. Chem. Pharm. Bull., 1985, 33, 2129. (d) Khanzhin, N. U.S. Patent 7, 066, 612, 2007; Khanzhin, N. Chem. Abstr., 2007, 146, 337905; (e) Davey, D.D. EP Patent 1, 429,149, 1991; Davey, D.D. Chem. Abstr., 1991, 115, 183357.
- (a) Hu, M.; Wu, J.; Zhang, Y.; Qiu, F.; Yu, Y. Tetrahedron, 2011, 67, 2676. (b) Hu, B.; DiMagno, S. Org. Biomol. Chem., 2015, 13, 3844. doi: 10.1016/j.tet.2011.01.062
- (a) Shao, Z.; Pan, Q.; Chen, J.; Yu, Y.; Zhang, G. Tetrahedron, 2012, 68, 6565. (b) Ni, H.; Zhang, G.; Yu, Y. Current Org. Chem., 2015, 19, 776. doi: 10.1016/j.tet.2012.05.057
- (a) Guo, S.; Chen, B.; Zhao, D.; Chen, W.; Zhang, G. Adv. Synth. Catal., 2016, 358, 3010. (b) Zhang, G.; Chen, B.; Guo, X.; Guo, S.; Yu, Y. Adv. Synth. Catal., 2015, 357, 1065. (c) Chen, B.; Guo, S.; Guo, X.; Zhang, G.; Yu, Y. Org. Lett., 2015, 17, 4698. (d) Guo, S.; Chen, B.; Guo, X.; Zhang, G.; Yu, Y. Tetrahedron, 2015, 71, 9371–9375. (e) Chen, B.; Ni, H.; Guo, X.; Zhang, G.; Yu, Y. RSC Adv., 2014, 4, 44462. (f) Zhang, G.; Ni, H.; Chen, W.; Shao, J.; Liu, H.; Chen, B.; Yu, Y. Org. Lett., 2013, 15, 5967. (g) Zhang, Y.; Liu, S.; Yu, Hu, M. Zhang, G.; Yu, Y. Tetrahedron, 2013, 69, 2070. doi: 10.1002/adsc.201600423
- (a) Riadi, Y.; Geesi, M. Chem. Pap., 2017, 1. doi:10.1007/s11696-017-0325-2. (b) Riadi, Y.; Massip, S.; Leger, J. M.; Jarry, C.; Lazar, S.; Guillaumet, G. Tetrahedron, 2012, 68, 5018. (c) Dehbi, O.; Tikad, A.; Bourg, S.; Bonnet, P.; Lozach, O.; Meijer, L.; Aadil, M.; Akssira, M.; Guillaumet, G.; Routier, S. Eur. J. Med. Chem. 2014, 80, 352. (c) Routier, S.; Guillaumet, G.; Tikad, A.; Dehbi, O. Preparation of Pyrido[3,2-d]pyrimidine Derivatives as Inhibitors of CDK1, DYRK1A, CDK5 and/or GSK3 kinases, WO 2011135259, Chem. Abstract 155:637809, 2011.
- (a) Riadi, Y.; Mamouni, R.; Azzalou, R.; El Haddad, M.; Routier, S.; Guillaumet, G.; Lazar, S. Tetrahedron Lett., 2011, 52, 3492. (b) Riadi, Y.; Mamouni, R.; Routier, S.; Guillaumet, G.; Lazar, S. Environ. Chem. Lett., 2014, 12, 523. (c) Riadi, Y.; Geesi, M.; Dehbi, O.; Bakht, M.A.; Alshammari, M.; Viaud-Massuarde, M.-C. Green Chem. Lett. Rev., 2017, 10, 101. doi: 10.1016/j.tetlet.2011.04.121
- (a) Knittel, D.; Hemetsberger, H.; Weidmann, H. Monatsh. Chem., 1970,101, 157. (b) Gilchrist, T. L.; Mendonca, R. ARKIVOC, 2000, 769. (c) Patonay, T.; Jekȍ, J.; Rimàn, E. Synth. Commun., 2002, 32, 2403. doi: 10.1007/BF00907535