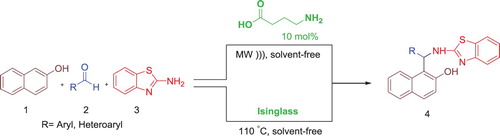
ABSTRACT
γ-aminobutyric acid (GABA) and Isinglass a collagen peptide have been utilized as highly efficient bifunctional biocatalysts for the efficient and convenient synthesis of 2-aminobenzothiazolomethyl-2-naphthols through a one-pot three-component Mannich reaction between diverse aldehydes, 2-naphthol and 2-aminobenzothiazole under solvent-free condition in high yields. Moreover, GABA could be recycled and reused at least four times without noticeable loss of its activity.
GRAPHICAL ABSTRACT
Introduction
Heterocyclic compounds are among the most important targets in organic compounds synthesis due to their countless uses in pharmaceutical applications ( Citation1,Citation2). Multi-component reactions (MCRs) are effective approaches to obtain various complicated organic compounds including pharmacologically heterocyclic precursors in a single step and one-pot reaction ( Citation3–13). Among this variety, the Mannich reaction is an outstanding synthetic method for the synthesis of multifarious biologically active heterocyclic compounds ( Citation14–17). A special case of the Mannich reaction is the Betti reaction which was discovered at the beginning of the twentieth century ( Citation18). Nowadays, the name has grown to refer to any condensation reactions between aldehydes, primary aromatic amines and phenols which produce Betti base ( Citation19). The Betti reaction serves versatile synthetic building blocks with a broad range of application such as essential intermediates for many multistep organic syntheses and preparing a lot of nitrogen-containing pharmacores ( Citation20,Citation21).
During the recent years, environmental concerns have grown and developing non-polluting procedures and utilizing the environmentally benign methods have become more popular such as using recyclable catalysts ( Citation22–24), performing the reactions under solvent-free conditions or replacing harmful and toxic solvents with green ones such as water ( Citation25–28). Furthermore, to have highly atom economy and atom efficiency, other clean energy sources such as microwave irradiation could be employed ( Citation29–33).
In the last decade, an explosive growth in the research and development of organo- and biocatalysts and specially the bifunctional ones have been observed. Hence, many scientists have been attracted to this field ( Citation34–38). One of the invaluable compounds with high efficiency as organocatalysts is amino acids and their analogues. In fact, using these kinds of compounds as bifunctional organocatalysts represent remarkable activity in reactions with nucleophilic and electrophilic substrates with all due attention to their amphoteric nature with both amine and acid functionalities. The morphology of the active sites of the catalyst is extremely important in incorporating effectively with the substrates in the catalytic cycle ( Citation39). Among amino acids, L-proline has been widely used as an organocatalyst in MCRs ( Citation40–43) but according to the fact that it is toxic and has chronic effects on lungs and passes through the placental barrier in humans and after biodegredation the products become more toxic, it has no useful aspect in a green synthetic procedure. Hence, our attention turned towards readily available substances such as γ-aminobutyric acid (GABA) and isinglass (IG) a collagen peptide which could both be used as organo- and biocatalysts. GABA has not ever been used as a bifunctional organocatalyst and the only reported usage of this compound in organic reactions is as base in Knoevenagel condensation reaction for the synthesis of pyridazines from hydrazones ( Citation44–46). IG is a biopolymer obtained from the dried swim bladders of fish. It is a form of collagen which is readily soluble in organic acids and has been used mainly for the clarification or fining of some alcoholic beverages, in the pharmaceutical industry and as ingredients or processing aids in food production ( Citation47,Citation48). IG is a rod-shaped amphoteric molecule with a vast composition of amino acids such as alanine, glycine, valine, leucine, isoleucine, proline, phenylalanine, tyrosine, serine, threonine, methionine, arginine, histidine, lysine, aspartic acid, glutamic acid, hydroxyproline, and hydroxylysine ( Citation49). Founded on our previous report ( Citation50), these two organic compounds could readily act as bifunctional organocatalysts that contain both Lewis base and Lewis acid sites.
Benzothiazoles and 2-Aminobenzothiazoles are two important groups of heterocyclic compounds with helpful biological activities such as anti-inflammatory ( Citation51), anti-convulsant ( Citation52,Citation53), anti-tumor ( Citation54–57), anti-fungal ( Citation58), anti-bacterial ( Citation59), analgesic ( Citation60), anti-malarial ( Citation61), anti-leishmanial ( Citation62) and anti-HIV ( Citation63) activities. During the recent years, several methods for the synthesis of 2-aminobenzothiazolomethyl-2-naphthols have been developed ( Citation64–77 ) including various catalysts and reagents such as a combination of heteropolyacid (HPA) ( Citation64), LiCl ( Citation65), sodium dodecyl sulfate (SDS) ( Citation66), 1-methyl-2-pyrrolidonium hydrogen sulfate ([Hnmp]HSO4) ( Citation67), 3-methyl-1-(4-sulfonic acid)propyl-imidazolium hydrogen sulfate ([(CH2)3SO3HMIM][HSO4]) ( Citation68), and sodium lauryl sulfate (SLS) ( Citation69). Despite the advantages of these approaches, they suffer from some drawbacks including using toxic catalysts, environmental pollution, expensive catalysts, unavailable substrates, difficult preparation methods, prolonged reaction times, requirement of solvents, and unsatisfactory yields. Thus, development of an effective, efficient, inexpensive and facile method to overcome such problems in the synthesis of 2-aminobenzothiazolomethyl-2-naphthols seems essential.
According to the aforementioned explanations and the undeniable effectiveness of organocatalysts in organic reactions and in continuation of our interest on the synthesis of heterocylic compounds ( Citation64,Citation76), herein we introduce GABA as a recyclable, water soluble, inexpensive and green bifunctional organocatalyst for the first time and IG as an easily available, effective and anti-toxic biocatalyst in the synthesis of 2-aminobenzothiazolomethyl-2-naphthols under solvent-free conditions ().
Results and discussion
We chose 2-naphthol (1), 2-aminobenzothiazole (3) and 4-chlorobenzaldehyde (2b) (1:1:1) as the model substrates to investigate the optimization of the reaction conditions (). Based on the model reaction, several reaction conditions were investigated to gain the optimized reaction conditions. As shown in , since the reaction failed to provide the desired product in the absence of a catalyst (Entry 1), the effect of a variety of readily available catalysts on the reaction progress was studied. Chitosan as a basic catalyst and citric acid as an acidic one were totally inferior (Entries 2 and 3). To our delight, a 96% yield of product 4b was obtained in the presence of 10 mol% 4-aminobutyric acid as a bifunctional organocatalyst (Entry 4). Anthranilic acid and IG also provided 4b in 91% and 64% yields, respectively (entries 5 and 6). The reaction did not complete with fewer than 10 mol% of GABA and an increased amount of catalyst was not effective in achieving higher yields (Entries 7 and 8). The effect of microwave irradiation power on the reaction progress was also studied. The best result in the shortest time was obtained with 900 W (100% power). Decreasing the power lengthened the reaction and lowered the yields (Entries 9 and 10)
Table 1. Optimization of the reaction conditions (Method A).
To prove the effect of microwave irradiation on the completion of the present Mannich reaction, the synthesis of the desired product 4b was carried out in oven at 120°C for 18 h, with grinding for a few times in between, which did not proceed further than the coagulated form. This result revealed the essential role of microwave irradiation in the completion of the reaction in a very short time with high yields and without the requirement of any kinds of solvents, additives or energy consuming heating and cooling procedures became clear.
The recyclability of GABA was examined and the results are summarized in . To check the recyclability of the catalyst, the crude reaction mixture was treated with water and the catalyst was recycled by a simple recrystallization from ethanol to be reused for further catalytic reactions. The yields of the obtained products after four recycles were almost the same without significant loss of catalytic activity.
Table 2. Recyclability of GABA.
With the optimized reaction conditions in hand, a broad range of aldehydes was subjected to this protocol (). Substituted aromatic aldehydes bearing both electron-donating and electron-withdrawing groups, aliphatic and heteroaromatic aldehydes such as furfural and thenaldehyde were all well tolerated and gave the desired products 4a–o in high yields.
Table 3. Aldehyde scope for the synthesis of of 2-aminobenzothiazolomethyl-2-naphthols in the presence of GABA as the catalyst under solvent-free conditions.
We have next optimized the reaction conditions between 2-naphthol (1), 2-aminobenzothiazole (3), and benzaldehyde (2a) as the model substrates in the presence of IG as catalyst. IG biocatalyst was obtained based on our previous report ( Citation50) and ball-milling approach was used to prepare nano-IG biocatalyst. As can be seen in , a few methods were examined to achieve the desired product 4a in higher yields. Using ultrasonic irradiation in different solvents was totally insufficient and gave a trace amount of the final product (Entries 1–4). Ball-milling the reaction mixture led to a yield of 70% in 3 h (Entry 5). Performing the reaction under reflux conditions were also monitored using ethanol, toluene or water as solvent (Entries 6–8). Utilizing ethanol as the solvent gave 97% of 4a in a prolonged reaction time of 23 hours (Entry 6). Gratifyingly, using solvent-free conditions at 110°C led to the formation of the desired product in 95% yield in 110 mins (Entry 9). The reaction yield decreased to fewer than 25 mg of IG and increasing the amount of catalyst did not affect the reaction yield (Entries 10 and 11).
Table 4. Optimization of the reaction conditions (Method B).
Once the optimized reaction conditions had been determined, the scope of aldehydes in the present three-component synthesis of 2-aminobenzothiazolomethyl-2-naphthols was investigated. As shown in , aldehydes bearing electron withdrawing groups in ortho- and para-positions gave the highest yields. This observation might be due to the electronic effects of electron withdrawing substituents such as F, Cl, Br, CN, and NO2 that better activate the aldehyde to be more easily attacked by the nucleophile ().
Table 5. Aldehyde scope for the synthesis of of 2-aminobenzothiazolomethyl-2-naphthols in the presence of IG as the catalyst under solvent-free conditions.
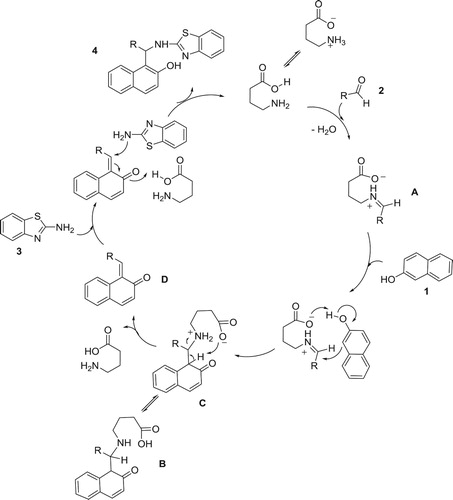
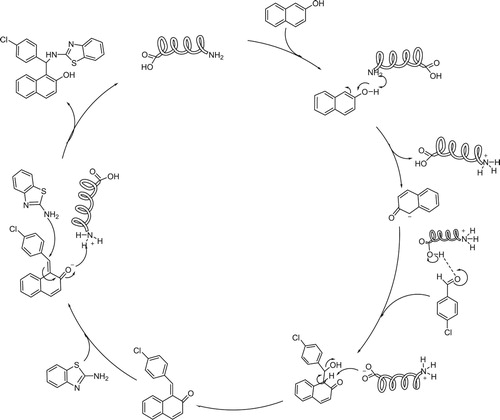
Based on the above results and the previous reports, plausible mechanisms for these two methods are given in and . The reaction mechanism for method A starts with the nucleophilic addition of the amine group of amino acid to the carbonyl group of aldehyde followed by dehydration which leads to the formation of iminium ion A that undergoes reaction with 2-naphtol to give the Mannich base B. Intermediate B is in equilibrium with its zwitterion form C that undergoes amino acid elimination to form the ortho-quinone methide D. Finally, o-QM D readily reacts with 2-aminobenzothiazole to produce the desired product 5 and GABA goes back to the catalytic cycle as an active catalyst (). The proposed mechanism for method B is depicted in .
To show the efficiency of GABA and IG for the Mannich reaction, a comparison of the obtained results in this study with the previous reported methodologies has been summarized in . It is obvious that two superior methodologies in terms of using bifunctional organocatalysts without any post-modifications have been developed which involve advantages such as catalyst recyclability, low cost, high atom economy, non-toxicity, environmentally benign and non-corrosiveness.
Table 6. Comparison of the obtained results in this study with the previous reported methodologies.
Conclusion
In conclusion, we have developed inexpensive, efficient, facile and green organocatalyzed methods to generate 2-aminobenzothiazolomethyl-2-naphthols by coupling 2-naphthol, 2-aminobenzothiazole and various aldehydes by using GABA and IG as a novel recyclable bifunctional biocatalyst under microwave irradiation and solvent-free conditions, respectively, in high yields. These protocols have the advantages of low cost, solvent-free reaction conditions, environmentally benign, easy and clean work-up procedure, and no requirement of column chromatography, excellent yields, and recyclability of the catalyst.
Supplemental_Material.docx
Download MS Word (3.6 MB)Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
Maryam Fardpour is Ph.D. in Organic Chemistry from University of Tehran (UT). She got her M.S. in Organic Chemistry from Iran University of Science and Technology (IUST) and her B.S. in Applied Chemistry from Khaje Nasir Toosi University of Technology (KNTU). Her research inte rest lie in Green Chemistry, Multi-Component reactions, C-H activation and functionalization reactions and synthesis of heterocyclic compounds.
Ali Safari was born in Tehran, Iran in 1990. He received his BS degrees in applied chemistry in 2012 from the Islamic Azad University Central Tehran Branch and his MS degrees in Organic Chemistry in 2015 from Iran University of Science and Technology.
Shahrzad Javanshir was born in Tehran, Iran in 1960. She received her BS, MS, and and M.Phil. degrees in Chemistry and Organic Chemistry in 1983, 1984, and 1985, respectively, from the University of Claude Bernard Lyon I, France, and her PhD degree in Organic Chemistry, in 2007, from Alzahra University, Tehran, Iran. She is currently Associate Professor of Organic Chemistry at Iran University of Science and Technology, Tehran, Iran. Her research interests include organic synthesis (heterocyclic and medicinal chemistry), heterogeneous catalysis, and green chemistry.
ORCID
Shahrzad Javanshir http://orcid.org/0000-0002-3161-0456
References
- Gribble, G.W. Perkin Trans, J. Chem. Soc., 2000, 1, 1045. doi: 10.1039/a909834h
- Nobuyoshi, A.; Akihiko, O.; Chikara, M.; Tatsuya, T.; Masami, O.; Hiromitsu, S. J. Med. Chem., 1999, 42, 2946. doi: 10.1021/jm990094r
- Tanaka, K.; Toda, F. Chem. Rev., 2000, 100, 1025. doi: 10.1021/cr940089p
- Rothenberg, G.; Downie, A.P.; Raston, C.L.; Scott, J.L. J. Am. Chem. Soc., 2001, 123, 8701. doi: 10.1021/ja0034388
- Martins, M.A.P.; Frizzo, C.P.; Moreira, D.N.; Buriol, L.; Machado, P. Chem. Rev., 2009, 109, 4140. doi: 10.1021/cr9001098
- Cave, G.W.V.; Raston, C.L.; Scott, J.L. Chem. Commun., 2001, 2159. doi: 10.1039/b106677n
- Kaupp, G. CrystEngComm, 2003, 5, 117. doi: 10.1039/b303432a
- Tanaka, K. Solvent-free Organic Synthesis; Wiley-VCH: Weinheim, 2003.
- Schneider, F.; Szuppa, T.; Stolle, A.; Ondruschka, B.; Hopf, H. Green Chem., 2009, 11, 1894. doi: 10.1039/b915744c
- Choudhary, G.; Peddinti, R.K. Green Chem., 2011, 13, 276. doi: 10.1039/C0GC00830C
- Cheng, C.; Jiang, B.; Tu, S.J.; Li, G. Green Chem., 2011, 13, 2107. doi: 10.1039/c1gc15183e
- Shaabani, A.; Maleki, A.; Rezayan, A.H.; Sarvary, A. J. Mol. Divers., 2011, 15, 41. doi: 10.1007/s11030-010-9258-1
- Altug, C.; Burnett, A.K.; Caner, E.; Durust, Y.; Elliott, M.C.; Glanville, R.P.J.; Gu, C.; Westwell, A.D. Tetrahedron, 2011, 67, 9522. doi: 10.1016/j.tet.2011.10.005
- Verkade, J.M.M.; van Hemert, L.J.C.; Quaedflieg, P.J.L.M.; Rutjes, F.P.J.T. Chem. Soc. Rev., 2008, 37, 29. doi: 10.1039/B713885G
- Li, K.; He, T.; Li, C.; Feng, X.; Wang, N.; Yu, X. Green Chem., 2009, 11, 777. doi: 10.1039/b817524a
- Kumar, A.; Gupta, M.K.; Kumar, M. Green Chem., 2012, 14, 290. doi: 10.1039/C1GC16297G
- Baudoux, J.; Lefebvre, P.; Legay, R.; Lasne, M.; Rouden, J. Green Chem., 2010, 12, 252. doi: 10.1039/B915681J
- Betti, M. Gazz. Chim. Ital. 1900, 30, 301.
- Betti, M. Org. Synth. 1929, 9, 60. doi: 10.15227/orgsyn.009.0060
- Gyemant, N.; Engi, H.; Schelz, Z.; Szatmari, I.; Toth, D.; Fulop, F.; Molnar, J.; de Witte, P. J. Cancer, 2010, 103, 178. doi: 10.1038/sj.bjc.6605716
- Gandhi, M.; Olyaei, A.; Raoufmoghaddam, S. Synth. Commun., 2008, 38, 4125. doi: 10.1080/00397910802279860
- Miura, T.; Imai, K.; Ina, M.; Tada, N.; Imai, N.; Itoh, A. Org. Lett., 2010, 12, 1620. doi: 10.1021/ol1003719
- Lu, A.; Liu, T.; Wu, R.; Wang, Y.; Wu, G.; Zhou, Z.; Fang, J.; Tang, C. J. Org. Chem., 2011, 76, 3872. doi: 10.1021/jo2002819
- Stacey, J.M.; Dicks, A.P.; Goodwin, A.A.; Rush, B.M.; Nigam, M. J. Chem. Educ., 2013, 90, 1067. doi: 10.1021/ed300819r
- Singh, M.S.; Chowdhury, S. RSC Adv., 2012, 2, 4547. doi: 10.1039/c2ra01056a
- Mondal, J.; Modak, A.; Nandi, M.; Uyama, H.; Bhaumik, A. RSC Adv., 2012, 2, 11306. doi: 10.1039/c2ra22291d
- Gong, K.; Wang, H.; Ren, X.; Wang, Y.; Chen, J. Green Chem., 2015, 17, 3141. doi: 10.1039/C5GC00384A
- Bienayme, H.; Hulme, C.; Oddon, G.; Schmitt, P. Chemistry, 2000, 6, 3321. doi: 10.1002/1521-3765(20000915)6:18<3321::AID-CHEM3321>3.0.CO;2-A
- Dastan, A.; Kulkarni, A.; Torok, B. Green Chem., 2012, 14, 17. doi: 10.1039/C1GC15837F
- Battini, N.; Battula, S.; Kumar, R.R.; Ahmed, Q.N. Org. Lett., 2015, 17, 2992. doi: 10.1021/acs.orglett.5b01271
- Murugavel, G.; Punniyamurthy, T. J. Org. Chem., 2015, 80, 6291. doi: 10.1021/acs.joc.5b00738
- Zhang, M.; Liu, Y.H.; Shang, Z.R.; Hu, H.C.; Zhang, Z.H. Catal. Commun., 2017, 88, 39. doi: 10.1016/j.catcom.2016.09.028
- Díaz-Ortiz, Á; Prieto, P.; de la Hoz, A. Chem. Rec. 2018, Jul 23.
- Stacey, J.M.; Dicks, A.P.; Goodwin, A.A.; Rush, B.M.; Nigam, M. J. Chem. Educ., 2013, 90, 1067. doi: 10.1021/ed300819r
- Pratt, R.C.; Lohmeijer, B.G.G.; Long, D.A.; Waymouth, R.M.; Hedrick, J.L. J. Am. Chem. Soc., 2006, 128, 4556. doi: 10.1021/ja060662+
- Tsutsumi, Y.; Yamakawa, K.; Yoshida, M.; Ema, T.; Sakai, T. Org. Lett., 2010, 12, 5728. doi: 10.1021/ol102539x
- Mondal, J.; Modak, A.; Nandi, M.; Uyama, H.; Bhaumik, A. RSC Adv., 2012, 2, 11306. doi: 10.1039/c2ra22291d
- Feu, K.S.; de la Torre, A.F.; Silva, S.; de Moraes Junior, M.A.F.; Corrêa, A.G.; Paixão, M.W. Green Chem., 2014, 16, 3169. doi: 10.1039/C4GC00098F
- Venugopala, K.N.; Krishnappa, M.; Nayak, S.K.; Subrahmanya, B.K.; Vaderapura, J.P.; Chalannavar, R.K.; Gleiser, R.M.; Odhav, B. Eur. J. Med. Chem. 2013, 65, 295. doi: 10.1016/j.ejmech.2013.04.061
- Fu, L.; Lin, W.; Hu, M.H.; Liu, X.C.; Huang, Z.B.; Shi, D.Q. ACS Comb. Sci., 2014, 16, 238. doi: 10.1021/co4001524
- Kumaragurubaran, N.; Juhl, K.; Zhuang, W.; Bogevig, A.; Jorgensen, K.A. J. Am. Chem. Soc., 2002, 124, 6254. doi: 10.1021/ja026412k
- Rao, S.N.; Mohan, D.C.; Adimurthy, S. Org. Lett., 2013, 15, 1496. doi: 10.1021/ol4002625
- Li, Y.; Chen, H.; Shi, C.; Shi, D.; Ji, S. J. Comb. Chem., 2010, 12, 231. doi: 10.1021/cc9001185
- Ballatore, C.; Crowe, A.; Piscitelli, F.; James, M.; Lou, K.; Rossidivito, G.; Yao, Y.; Trojanowski, J.Q.; Lee, V.M.Y.; Brunden, K.R.; Smith, A.B. Bioorg. Med. Chem., 2012, 20, 4451. doi: 10.1016/j.bmc.2012.05.027
- Ferguson, G.N.; Valant, C.; Horne, J.; Figler, H.; Flynn, B.L.; Linden, J.; Chalmers, D.K.; Sexton, P.M.; Christopoulos, A.; Scammells, P.J. J. Med. Chem., 2008, 51, 6165. doi: 10.1021/jm800557d
- Ballatore, C.; Brunden, K.R.; Piscitelli, F.; James, M.J.; Crowe, A.; Yao, Y.; Hyde, E.; Trojanowski, J.Q.; Lee, V.M.Y.; Smith, A.B. J. Med. Chem., 2010, 53, 3739. doi: 10.1021/jm100138f
- Hickman, D.; Sims, T.J.; Miles, C.A.; Bailey, A.J.; de Mari, M.; Koopmans, M.J. Biotechnol. J., 2000, 79, 245. doi: 10.1016/S0168-1656(00)00241-8
- Leach, A.A. J. Inst. Brew., 1967, 73, 8. doi: 10.1002/j.2050-0416.1967.tb03011.x
- Eastoe, J.E. Biochem. J. 1957, 65, 363. doi: 10.1042/bj0650363
- Javanshir, S.; Pourshiri, N.S.; Dolatkhah, Z.; Farhadnia, M. Monatsh. Chem. 2017, 148, 703. doi: 10.1007/s00706-016-1779-6
- el-Shorbagi, A.N.; Sakai, S.; el-Gendy, M.A.; Omar, N.; Farag, H.H. Chem. Pharm. Bull. 1989, 37, 2971. doi: 10.1248/cpb.37.2971
- Chopade, R.S.; Bahekar, R.H.; Khedekar, P.B.; Bhusari, K.P.; Ram Rao, A.R. Archiv der Pharmazie, 2002, 335, 381. doi: 10.1002/1521-4184(200211)335:8<381::AID-ARDP381>3.0.CO;2-S
- Amnerkar, N.D.; Bhusari, K.P. Eur. J. Med. Chem., 2010, 45, 149. doi: 10.1016/j.ejmech.2009.09.037
- Shi, D.F.; Bradshaw, T.D.; Wrigley, S.; McCall, C.J.; Lelieveld, P.; Fichtner, I.; Stevens, M.F.G. J. Med. Chem., 1996, 39, 3375. doi: 10.1021/jm9600959
- Wells, G.; Bradshaw, T.D.; Diana, P.; Seaton, A.; Shi, D.F.; Westwell, A.D.; Stevens, M.F.G. Med. Chem. Lett. 2000, 10, 513. doi: 10.1016/S0960-894X(00)00027-5
- Hutchinson, I.; Chua, M.S.; Browne, H.L.; Trapani, V.; Bradshaw, T.D.; Westwell, A.D.; Stevens, M.F.G. J. Med. Chem., 2001, 44, 1446. doi: 10.1021/jm001104n
- Racané, L.; Kralj, M.; Šuman, L.; Stojković, R.; Tralić-Kulenović, V.; Karminski-Zamola, G. Bioorg. Med. Chem., 2010, 18, 1038. doi: 10.1016/j.bmc.2009.12.054
- Singh, T.; Srivastava, V.K.; Saxena, K.K.; Goel, S.L.; Kumar, A. Archiv der Pharmazie 2006, 339, 466. doi: 10.1002/ardp.200500265
- Palkar, M.; Noolvi, M.; Sankangoud, R.; Maddi, V.; Gadad, A.; Nargund, L.V.G. Archiv der Pharmazie 2010, 343, 353. doi: 10.1002/ardp.200900260
- Westaway, S.M.; Thompson, M.; Rami, H.K.; Stemp, G.; Trouw, L.S.; Mitchell, D.J.; Seal, J.T.; Medhurst, S.J.; Lappin, S.C.; Biggs, J.; Wright, J.; Arpino, S.; Jerman, J.C.; Cryan, J.E.; Holland, V.; Winborn, K.Y.; Coleman, T.; Stevens, A.J.; Davis, J.B.; Gunthorpe, M.J. Bioorg. Med. Chem. Lett. 2008, 18, 5609. doi: 10.1016/j.bmcl.2008.08.105
- Takasu, K.; Inoue, H.; Kim, H.S.; Suzuki, M.; Shishido, T.; Wataya, Y.; Ihara, M. J. Med. Chem. 2002, 45, 995. doi: 10.1021/jm0155704
- Delmas, F.; Giorgio, C.D.; Robin, M.; Azas, N.; Gasquet, M.; Detang, C.; Costa, M.; Timon-David, P.; Galy, J.P. Antimicrob. Agents Chemother 2002, 46, 2588. doi: 10.1128/AAC.46.8.2588-2594.2002
- Racane, L.; Tralic-Kulenovic, V.; Fiser-Jakic, L.; Boykin, D.W.; Karminski Zamola, G. Heterocycles 2001, 55, 2085. doi: 10.3987/COM-01-9305
- Javanshir, S.; Ohanian, A.; Heravi, M.M.; Naimi-Jamal, M.R.; Bamoharram, F. J. Saudi Chem. Soc 2014, 18, 502. doi: 10.1016/j.jscs.2011.10.013
- Kumar, A.; Rao, M.S.; Rao, V.K. Aust. J. Chem. 2010, 63, 1538. doi: 10.1071/CH10209
- Shaabani, A.; Rahmati, A.; Farhangi, E. Tetrahedron Lett. 2007, 48, 7291. doi: 10.1016/j.tetlet.2007.08.042
- Yi, Y.; Hongyunv, G. Chin. J. Org. Chem. 2011, 31, 96.
- Shaterian, H.R.; Hosseinian, A. Res. Chem. Intermed. 2015, 41, 793. doi: 10.1007/s11164-013-1230-0
- Sahu, P.K.; Sahu, P.K.; Agarwal, D.D. RSC Adv. 2014, 4, 40414. doi: 10.1039/C4RA03847A
- Yang, L. E-J. Chem. 2012, 9, 2424. doi: 10.1155/2012/707324
- Shaterian, H.R.; Mohammadnia, M. S. Afr. J. Chem. 2013, 66, 60.
- Hosseinian, A.; Shaterian, H.R. Phosphorus, Sulfur Silicon Relat. Elem. 2012, 187, 1056. doi: 10.1080/10426507.2012.664221
- Maghsoodlou, M.T.; Karima, M.; Lashkari, M.; Adrom, B.; Aboonajmi, J. J. Iran. Chem. Soc. 2017, 14, 329. doi: 10.1007/s13738-016-0981-0
- Goli-Jolodar, O.; Shirini, F. J. Iran. Chem. Soc. 2016, 13, 457. doi: 10.1007/s13738-015-0754-1
- Goli-Jolodar, O.; Shirini, F.; Seddighi, M. RSC Adv. 2016, 6, 44794. doi: 10.1039/C6RA08486A
- Javanshir, S.; Saghiran Pourshiri, N.; Dolatkhah, Z.; Farhadnia, M. Monatsh. Chem 2017, 148, 703. doi: 10.1007/s00706-016-1779-6
- Kamali, F.; Shirini, F. Appl. Organomet. Chem. 2018, 32, e3972. doi: 10.1002/aoc.3972