ABSTRACT
This cross-disciplinary course bridging the disciplines of art and chemistry provides an exceptional environment in which first-year students and beyond are engaged and challenged in new ways. This dual lab/studio-based course, titled “Art in the Anthropocene: Greener Art through Greener Chemistry,” enables students to use their imagination, creativity, and innovation to respond to environmental issues and concerns through art. Students are asked to reflect on the nature and implications of the actual physical materials that artists use and to achieve a renewed sense of social and ethical responsibility through the content of their artwork. The curriculum is designed so that teachers guide students on how chemical processes are used to make art materials in an environmentally friendly way. The overall goal is to apply green chemistry principles in the making of artworks that can be crafted with reclaimed, recycled, and naturally available materials, non-toxic solvents and paints, and using sustainable forms of energy while keeping ethical values in mind.
GRAPHICAL ABSTRACT
Introduction and rationale
After analyzing feedback from students who have experienced performing a green chemistry experiment in General Chemistry and in Honors General Chemistry and an art-based experiment in Inorganic Chemistry, the following outcomes emerged:
The communion of art and chemistry concepts covered in hands-on activities was unpredictably attractive to our science students. Likewise, students in creative fields were fascinated with exploring the chemistry of their art materials. All students self-reported that they were motivated to take this class because of the growing awareness of the perilous state earth’s natural world is in.
Students currently majoring in Chemistry or intending to major in a science field requested to perform more of this type of cross-disciplinary hands-on learning activities. Arts-focused students were equally interested in the hands-on technical/materials focus that provides a balance to the conceptual and aesthetic focus of most studio courses.
The cross-listing of this course as an ART/CHE course was essential to its success since input from a professor in the Art Department was necessary for technical/material skills and expertise in contemporary art themes and practices while the chemistry professor brought a deep expertise in chemical/material processes and ensured all course activities were safe and non-toxic.
To remediate the concern about how attractive this course would be to the student population outside of the Chemistry and Art majors and minors, it was decided to also count this course for the Humanities and Fine Arts distribution requirement at Washington College.
It became justified that a stand-alone, four-credit course relying mainly on hands-on activities would fulfill the desire of the students, would contribute to the professional development of the author, and would create a platform to offer a unique distribution course available to a variety of majors. This lab-based course would also provide the opportunity to create an exceptional environment in which first-year students and beyond would be engaged and challenged in new ways as described in the rationale below.
The rationale for this course is four-fold:
Synergistic relationship between chemistry and art
After implementing art-infused chemistry experiments in existing chemistry courses such as General and Inorganic Chemistry, enlightenment occurred. Students realized that there was a logical connection between the two disciplines, which satisfied all types of learners. Students started to explore chemical concepts to make art in a more environmentally friendly way. Creating an artwork using nature as a model and mentor was more rewarding than having the correct written answer on a standard test. Students started to use their imagination to explore ways to explain chemical bonding, chemical properties, and chemical reactivity of all types of matter using forms of art.
This type of exercise requires students to master fundamental chemistry concepts and how to apply green chemistry principles while using their creative nature. Uniting the disciplines of green chemistry and art in one relevant curriculum was the springboard to this cross-disciplinary course.
Unique distribution course
Washington College is a private liberal arts and science institution, which embraces the values of curiosity, leadership, and moral courage. Students are expected to ‘develop habits of analytic thought and clear communication, aesthetic insight, ethical sensitivity, and civic responsibility ( Citation1).’ To achieve these goals, students are required to complete courses in the four following categories: Foreign Language Requirement, Natural Science and Quantitative Requirement, Humanities and Fine Arts Requirement, and Social Sciences Requirement. In the Chemistry Department, students can take either General Chemistry or Chemistry of the Environment to fulfill the Natural Science and Quantitative Requirement. However, General Chemistry is not taught as a distribution course since most of the students majoring in the sciences need this course for their major. Chemistry of the Environment is cross-listed between the Departments of Chemistry and Environmental Sciences and Studies and therefore the amount of spots in this one section course is limited. This new course allows the Chemistry and the Art Departments to offer a unique distribution course while enriching both the chemistry and art curriculum.
Simultaneous exposure to the disciplines of chemistry and art
A unique characteristic of this course is the consistent and simultaneous exposure of students to both disciplines. Students realize from the first hands-on session the implications of the interdependence of chemistry and art and their interactions with each other as well as with other systems like society and environment. Connecting the principles of green chemistry and art to students’ lives and what is happening in society is an essential component of this course. This course is infusing the concept of systems thinking in both education and practice as it allows the students to analyze the connections between chemical, ecological, and human systems ( Citation2 , Citation3). An example of placing art and chemistry in society was presented during a moss graffiti experiment. Students had to discuss where the green chemistry principles are present in the making of the moss paint mixture and during the application process but also question the wild collection of moss on a massive scale and the development of techniques for cultivating mosses for a landscaping, horticultural, or artistic use.
This course also provides a platform to attract potential majors to each discipline from an early start. It also acts as a stimulus for students to realize they can double major (or major in one discipline and minor in the other one).
Implementation of ethical values
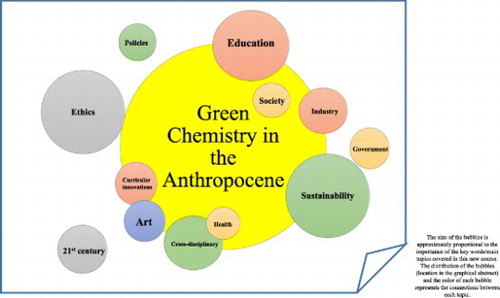
After narrowing down the content, format, and learning outcomes of this cross-disciplinary course, it became evident that ethics was the main principle driving the ideals of sustainable development. Ethics is an inherent component of this course across the proposed units from both a scientist’s and artist’s point of view, as this course addresses how research methods and experimentation in both fields have objective as well as poetic and metaphoric resonance. We, as educators, are responsible to convey the moral and ethical standards of our profession and illuminate how our fields relate to, affect, and are affected by real world concerns. This is why we believe that students should learn about ethics while thinking about sustainable practices in their professional and personal lives. We placed special emphasis throughout our course development on the concept of the Anthropocene and how professional chemists and artists address the fact that human activity is arguably the dominant force now affecting our climate and ecologies worldwide.
Since the relationship man-nature-technology is at the core of sustainable development education, it provides a sense of belonging to the environment. An awareness of the individual of his/her role in society under conditions of finite resources leads to a sense of being useful and contributes to an individual’s social identity.
An awareness of ethics based on the synergy between art and chemistry and in the context of widescale human impact on the environment and sustainable development is relevant for the following reasons:
Most Chemistry Departments don’t systematically include ethics education in their curriculum even though personal and social responsibility is an integral part of the Chemical Professional’s Code of Conduct and the Global Chemists’ Code of Ethics published by the American Chemical Society (Citation4, Citation5). Preparing our students to be active citizens of the twenty-first century does not only rely on understanding the nature of science and the practice of chemistry, but also on being aware of the key ethical issues associated with working in a laboratory such as: data and record keeping, assignment of credit (authorship), peer review, openness (confidentiality, conflict of interest), research misconduct (fabrication and falsification of data and plagiarism), laboratory safety, and collegiality.
As stated by Haack and Hutchison (Citation6) ‘Green chemistry often aligns with students’ personal ethics regarding stewardship of the environment and gives them an opportunity to protect the planet from undesired impacts of chemistry’. To further students’ knowledge about ethics of sustainability, some of the key ethical concepts of sustainability such as ‘environmental ethics’, ‘ecological principles’, and ‘environmental justice’ are incorporated in this 200-level course.
From an art point of view, it is incumbent on any studio program to inform students about the health and environmental dangers associated with many traditional art materials and processes. Unfortunately, and surprisingly, this is not something that is uniformly addressed in undergraduate classes nationwide. This course incorporates this awareness and adopted the college art department’s existing green materials policy in which no toxic materials are used in the studio building. This keeps students and faculty safer while also raising larger pedagogical, ethical, and conceptual issues to find more sustainable ways to live and work.
Beyond fundamental best practices in a studio setting, there are central ethical concerns around materials and processes that are hot topics in contemporary art and society today. These issues touch on aesthetic and conceptual considerations. For one thing, does the world really need more art objects? How might students create an art experience that does not rely on manifesting more objects and toxic trash in a world already overflowing with detritus? What are an artist’s obligation and options to participate in art discourse while also keeping a small environmental footprint? This brings longstanding critiques in the art world over ‘art as a commodity’ to be possessed by the few who can afford it (rather than as meaningful philosophical discourse open to all).
Perhaps most importantly from an art perspective is to raise student awareness of truth in materials. In other words, the materials and processes themselves are a core aspect of any art work’s meaning. While every contemporary artist understands the ethical dimensions involved, it is not something the average undergraduate student tends to be aware of. Making itself, manipulating materials, has long been a central philosophical and practical consideration for the professional artists and scientists alike. To experiment with materials – a phenomenological and embodied experience – is to understand more deeply the implications of materiality and the limits imposed by natural science laws. It is a profound, not a sideline, aspect of being a thoughtful and informed artist or scientist today.
Course design and objectives
This course is currently offered as a Special Topics course at the 200-level and fulfills the distribution requirements for either the Humanities and Fine Arts or the Natural Sciences and Quantitative requirements. It is also available to all majors and minors in Art and Chemistry who can take this course as an elective in their own discipline.
This course is co-taught by Professor Anne Marteel-Parrish (Chemistry Department) and Associate Professor Heather Harvey (Department of Art and Art History) for a duration of two hours and 45 min twice a week. Both instructors were present for the duration of each lecture. Students spend time in the John Toll Science Center for all experimental work and in the Larrabee Art Studio at Washington College for all art studio assignments.
Six student-learning outcomes are emphasized in this cross-disciplinary course. The core emphasis of all learning outcomes was to explore significant synergies and areas of overlap between art and chemistry, and particularly how a growing awareness of massive human impact on the environment (i.e. the Anthropocene) is transforming both fields.
Outcome 1: greener art through greener chemistry in practice
Students should be able to implement green chemistry principles to create benign-by-design art materials (implementation of green chemistry guidelines for all art and science projects).
Outcome 2: imagination and creativity put to work
Students will use environmentally benign tools to create a series of unique artworks made with eco-friendly art materials they either fabricate or harvest themselves.
Outcome 3: exposure of science students to art, and art students to science
Students will develop creativity and research rigor through the juxtaposition of disciplines.
Outcome 4: sustainability
Students will ensure that sustainability is explored and incorporated into any larger meanings of their art projects through form and/or content of art.
Outcome 5: safety
Students will develop a proper concern for safety while using art materials and creating artworks.
Outcome 6: ethics from a scientist’s point of view
Students will understand the responsibilities associated with serving the public interest and safety while further advancing the knowledge of science.
Outcome 7: ethics from an artist’s point of view
Students will understand the inherent conceptual links between the materials and processes used to create a work of art and the ultimate meanings conveyed. Additionally, students will be exposed to the intellectual rigor and material experimentation required in sound artistic research.
Overall course structure: To meet the learning outcomes of the course, four units were introduced, each focused on particular art media from both a chemical and studio perspective. Materials covered included paint, clay, plaster, found objects, metals, and metal rust. The intent was to cover a broad range of elements present in the periodic table, to give examples of the different states of matter, to introduce chemical and physical properties, and to discuss the different types of bonding such as ionic, covalent, and metallic as well as secondary bonding present in art materials and projects. Students were asked to fill out a questionnaire on the first day of classes to share their expectations. They also had the opportunity to fill out a final questionnaire specific to the content and format of the course as well as suggest improvements for future iterations of the course. For each unit we first introduced nuts and bolts foundational concepts from both a chemist’s and artist’s point of view, then engaged in a range of hands-on explorations of materials. The culminating assignment for every unit was an independently devised and developed studio project using the materials procured and concepts explored over the course of the unit. Following that culminating assignment students then submitted a final unit self-assessment and reflection.
Assessment of studio and creative assignments focused primarily on students’ developing a voice and point of view, as well as on demonstrating an increased ability to engage in independent artistic research and to discuss art in a verbally articulate manner. Emphasis was placed on meaning and mood, and specifically how materials and processes used in each unit added to an artwork’s content. Technical artistic skills were not a major part of students’ grades for this course. The reasoning for this is two-fold: first, it would be unrealistic and unfair to expect non-art students to master advanced studio skills in one semester; second, and more philosophically, an over-reliance on technical skill has rightly been heavily critiqued in the arts for well over a century, although the reasons for this are too complex to fully address here.
Unit 0 or introductory unit: introduction to the cross-disciplinary dimension of green chemistry and art
The students were introduced to the thinking processes of scientists and artists, to chemical hazards and product safety in the laboratory as well as to ethics in the laboratory and studio and the code of conduct as a scientist. We also explored the surprising commonalities between what professional artists and their professional scientist counterparts do.
As a starting point, the definition of green chemistry, the principles of green chemistry, and the concept of sustainability were introduced followed by a discussion of the main chemistry areas of emphasis such as:
Description of the periodic table (nature of elements and different groups)
Review of types of elements leading to the formation of different types of compounds such as ionic and inorganic (Unit 1), minerals (Unit 2), and metallic (Unit 3)
Review on different types of bonding (covalent, ionic, and metallic; intramolecular and intermolecular forces)
Review on chemical and physical properties and changes
On the first day, students were asked to think about how the green chemistry principles can be related to art methods and techniques based on the 12 principles of Green Chemistry and the article by Walker ( Citation7) on ‘Art, Science, and Reality’. They also needed to address what Walker’s essay suggests about overlap in the aims and processes of science vs. art.
To illustrate the point about using nature as a model, a moss graffiti experiment ( Citation8) was included for students to familiarize themselves with the use of a lab notebook, laboratory regulations, and the lab environment; to use their critical thinking skills to compare the outcome of three different methods of paint making and the impact of using three different painting supports; to gather information about where chemistry is present while making the moss mixture and applying the moss mixture to the three painting supports; and to analyze how the 12 principles of green chemistry are applied in this activity. Students had similar expectations regarding learning standards of research and recording from an artist’s perspective. They were required to keep a sketchbook throughout the semester, for example, to record studio insights, develop creative/conceptual ideas, and track material investigations. During the painting unit, for example, students were expected to maintain ongoing swatches of every variation of paint color and paint binders made over the course of several classes. Alongside their swatches, students kept detailed notes regarding how the sample was made should they want to recreate it. Sketchbook entries interwove chemical/material/procedural information alongside developing aesthetic and thematic ideas for upcoming studio projects. Through keeping simultaneous lab and art notebooks, students experienced firsthand the commonalities between how scientists and artists think, including how they track progress and learn from experimentation and research.
A Sharpie pen experiment ( Citation9) was also introduced early on to explain the concept of solubility. As with every assignment throughout the semester, we explored both the chemical and aesthetic ramifications of the materials used and exposed students to contemporary artists working in similar veins. Physical changes were exemplified by mixing carbon powder, sulfur powder, and copper sulfate and examining how color relativity affects perception of an individual color ( Citation10). We used this same initial physical changes lesson to introduce students to basic color theory and color mixing techniques which we then revisited more fully in the weeks to follow.
After giving students the basic chemistry and art background and information needed through the semester, the first unit was started.
Unit 1: coloring the world with environmentally friendly paints
The first unit focused on the exploration and preparation of environmentally friendly paints using natural resources. For the culminating studio assignment students used paints they personally produced in a final independently devised painting. Students were introduced to three types of paints: oil, water, and acrylic from a historical and contemporary point of view after which they learned about the composition of paint and paints as solutions. The physical properties of solutions and changes associated with paints, an introduction to media such as watercolor, egg tempera paint, oil, acrylic and the advantages and disadvantages of each medium were presented.
Following this lecture-type introduction students transitioned to the preparation of natural pigments. Students harvested soil samples and used pigments present in common fruit, vegetables, coffee, tea, and spices. They also prepared natural binders (casein-, egg tempera-, gouache- and gelatin-based) and observed the impact of green chemistry on the creation of paints in a variety of assignments.
Assignments ranged from a quiz related to the chemistry of paints, the creation of paint swatches using commercially available non-toxic powdered pigments as well as environmentally friendly paints, a color wheel, idea generation exercises to develop their final painting, and in-class critiques, as well as a painting study and a reflection on the painting study. Besides creating a final painting, students were asked to write a project summary as well as a studio project self-assessment for this final project as well as for Units 2 and 3.
Unit 2: exploring our soil and discovering the world of natural clay and plaster
The second unit relied on the chemistry related to the composition of clay and plaster such as the formation of natural clay and clay minerals and plaster, the basic structures and identification of clay and plaster minerals, and the type of binding involved in natural clay and plaster as well as the chemical and physical properties of the different types of clay and plaster in general. We also explored historical and contemporary examples of artists working with these materials. The additional components of this unit were related to the hand building techniques of clay such as the pinch pot (press and squeeze), coil method (roll, press, and smooth), slab method (roll and cut), press and form, all involving an understanding of the chemistry, as well as a brief presentation of the evolution of the hand building techniques and coverage of additive and subtractive construction methods. Similar basic tools and techniques used by artists working with plaster were also introduced. This unit concluded by looking at the chemistry related to the firing process. Students did not use an oven or a kiln but let their final clay-based piece dry naturally.
Assignments consisted of a quiz and several hands on studio experiments including: a ‘speed sculpture’ incorporating locally harvested clay as well as found objects collected by students, an in-class collaborative plaster sculpture, and a final individual sculpture combining different types of clay, plaster, and at least one found object.
Unit 3: introduction to metals and rust as an artistic medium
The third unit focused on the chemistry of metals, the process of rusting, and the multitude of ways artists today work with these materials. Students were introduced to metal recycling and the concept of electroplating. Students were exposed to an activity testing criteria for rust promotion and inhibition, as well as using rust as paint pigment, and the combined effect of metals rusting. The final project consisted of a final project summary and self-assessment related to the final self-directed studio art project for which students had to incorporate all types of materials and techniques covered this semester (environmentally friendly paints, clay, a found metallic object, and a found object).
The list of units, topics covered, key assignments related to green chemistry for each unit, and overall performance are summarized in .
Table 1. Summary of topics, key green chemistry principles addressed for each unit, and overall performance.
Course assessment and evaluations
Since this was the first iteration of this course, most of the assessment data are qualitative except for the ones presented below. As highlighted in and , these units and assignments were designed to closely align with our desired student learning outcomes, which were covered consistently in each unit.
Table 2. Examples of green-chemistry based assignments and performance for each unit.
Table 3. Assessment of student learning objectives incorporating green chemistry and art based assignments.
The average of the art/studio assignments counted for 45%. The same percentage was attributed to the chemistry components. The remaining 10% was given for attendance and participation.
showed a superior performance for Units 0 and 1 compared to Units 2 and 3. This was expected since most students in the class had some experience with paints. The final project incorporating all classes of materials covered in this course was a challenge for some students as reflected in the lowest score of the semester.
The average performance regarding the assessment of our student learning objectives presented in showed a superior performance dealing with the imagination and creativity put to work but a much lower aptitude to keep up with pre-lab assignments based on finding data from the literature and assessing the safety and toxicity associated with chemicals.
Rubrics, frequent in-class discussion and critique, as well as individual meetings were used to provide comprehensive feedback. The use of an online course management program, such as Canvas, was used to post and collect assignments and record grades. Oral and written communication skills were emphasized throughout the course.
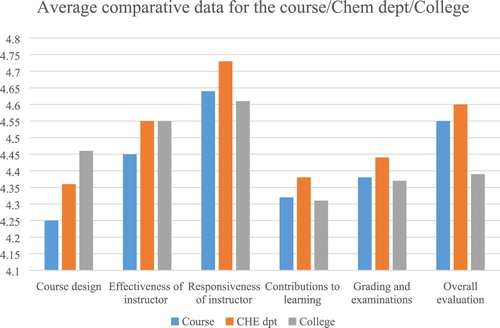
Both anonymous end-of-the-semester surveys as well as standard student course evaluations were analyzed to provide qualitative as well as quantitative feedback. Students at Washington College are asked to fill out a standard survey for each of their courses at the end of each semester. This survey asked students to rank their satisfaction on a scale of 0 (lowest) to 5 (highest). Average data representing the level of satisfaction of students for different areas of evaluation in this class were compared to the average data obtained for the Chemistry Department and the college as a whole. These data are summarized in and demonstrate high student satisfaction for all areas with the mean ranging between 4.25 and 4.64. The response rate was 15 students in Spring 2018.
Students enjoyed integrating chemistry into art in ways that they had not previously thought of.
They were able to ‘harness how using sustainable materials made an impact’. The hands-on approach made the class go by faster despite the length of the class twice a week.
Students enjoyed the ‘very interactive learning on both the chemistry and art aspects’ and ‘the dual nature of the course, switching between chemistry and art’ as well as ‘how there was a balance of what was taught in class’. Students appreciated the level of preparedness of the professors and ‘that both professors were available as needed, and taught the course together rather than going back and forth.’
Students emphasized that the principles of green chemistry gave new inspiration to artists who are looking to create more sustainable and less wasteful art media. With new knowledge of how harmful some art materials can be, there is room for chemists and artists to work together to find new ways to still express culture and ideas without the negative impact on society and our planet. Green chemistry allows artists to be aware of the possibilities of transforming their art practices into more environmentally friendly techniques. They also made a point that using materials from their own environment (such as soil samples from their childhood beach or berries from their own garden) can create personal connections that are far more meaningful than store bought materials.
However students did not enjoy the chemistry-based lectures at the beginning of each unit as well as the quizzes at the end of each unit (it probably reminded them too much of a traditional chemistry course, which this course is not). Additionally a few students had a difficult time to connect the hands-on activities to the goal of the final project. Some students would have liked to spend less time on the first unit to be able to study metals more in depth as well as an introduction to photography.
Suggestions for improvements are offered in the next section. Pictures of artwork are presented in Appendix 1.
Possibilities for modification and potential challenges
This new course was offered for the first time at Washington College and generated tremendous enthusiasm from the students. As it is the case for any new course, suggestions include:
Implementing shorter quizzes and more frequent hands-on activities directly related to the final project.
Having one combined writing assignment instead of a separate chemistry project summary and a studio project self-assessment.
Spending less time on the lecture component of the different units and instead add a unit on photography originally planned in the syllabus. Ideas for this proposed unit include a cyanotypes experiment and possibly an overview of digital photography allowing students to compare the process of traditional photograph printing to the digital process as well as experiment with the digital recording of a chemical reaction involving heat production during night time.
Including more self-guided hands-on activities based on the comparison of traditional vs. greener chemistry. For example in Unit 1 students could perform a laboratory activity on comparing the toxicity of commercial paints vs. environmentally friendly ones and derive their own conclusions.
Incorporating the notion of making art with found objects as the core material (‘Art with trash’) as an additional or possibly final project. Students found the exercise of getting out in the community and including found objects in their studio art piece very rewarding. Students also requested more field trips like going to a municipal dump, which suggests that focusing on found objects as the primary aesthetic and conceptual material in a project would be appealing and eye-opening in the next edition of this course.
Designing more quantitative assignments evaluating how students improved their ethical awareness as well as their understanding of the application of green chemistry principles after taking this course. Metrics will be developed to add to the qualitative assessment of this new course.
It is worth mentioning that the number of slots for this course was maximized at 16 and several students were unfortunately not able to take the course. Even if these students did not need the course for their major, minor, or for distribution, and some of them will have the opportunity to take the course in the future, the small class size led to the successful implementation of this course due to frequent individualized mentoring, in-class critiques for each art project, and focus on improvement of hands-on and communication skills needed for student growth.
It is believed that with a larger class, the constraint on available chemistry and studio art space for a class larger than 16 as well as the wider distribution of knowledge in chemistry and in art would be a challenge. In different institutional settings, where the co-teaching of such a course is not feasible or the financial impact is too cumbersome, it should be possible to incorporate the principles of green chemistry in at least one unit, especially the unit dedicated to paints. Instead of teaching a full course dedicated to the implementation of green chemistry in art, a first-year seminar may be offered to expose first-year students to the cross-disciplinary nature of green chemistry with art but also business, political science, sociology, ecology, food, and many other systems.
Conclusions
The course design for a skills- and concepts-based course emphasizing the synergistic relationship between green chemistry and art was presented. This course is intended for Chemistry majors and minors, Art majors and minors, or for any student wishing to fulfill either the Humanities and Fine Arts or the Natural Sciences and Quantitative requirements. Assessment data for this course as implemented at Washington College show a very positive student response to this format, with particular appreciation for the rigor of the course, the way instructors treated students and their opinions with respect and the responsiveness and availability of the instructors in and outside of class.
Comparisons of student evaluation data show a comparable overall evaluation to the Chemistry Department and a superior performance compared to the college norm. It is the hope that this course will inspire students to take additional cross-disciplinary courses and will challenge them to live a more sustainable life.
Acknowledgments
The implementation of this new course would not have been possible without the support of the Spring 2018 students who dared to take this new course.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes on contributors
Anne Marteel-Parrish grew up in the North of France and got her Engineering Degree in Materials Science from the Ecole Polytechnique de Lille in France in 1999. She received a Ph.D. in Chemistry with concentration in Materials Science from the University of Toledo, Ohio in May 2003. Shortly after she was hired as Assistant Professor in Chemistry at Washington College in Chestertown, Maryland. Anne received tenure and was promoted to the rank of Associate Professor in 2009. She achieved full professorship in 2016. She was the Chair of the Chemistry Department at Washington College from 2010 to 2016, a deputy chair in 2016–2017 and the co-chair in 2017–2018. In 2011 she was invested as the Inaugural Holder of the Frank J. Creegan Chair in Green Chemistry. Anne is the recipient of several national awards such as a Leadership Development Award by the Younger Chemists Committee (YCC) of the American Chemical Society in December 2007, the Washington College Alumni Association Award for Distinguished Teaching in May 2010, the American Chemical Society-Committee on Environmental Improvement (ACS-CEI) Award for Incorporating Sustainability into Chemistry Education in March 2011, and the 2015 Centennial Award for Excellence in Undergraduate Teaching from Iota Sigma Pi, the National Honor Society for Women in Chemistry. Anne was the recipient of the University of Toledo College of Natural Sciences and Mathematics Outstanding Alumna Award for the Academic Year 2016–2017. Anne’s scholarly activities include 13 peer-reviewed teaching- and research-based publications since being hired at Washington College as well as a textbook titled “Green Chemistry and Engineering: A Pathway to Sustainability” with Dr. Martin Abraham from Youngstown University.
Heather Harvey is currently Associate Professor and Chair of Art + Art History at Washington College in Chestertown, MD as well as the Studio Art Program Coordinator. She holds an MFA from Virginia Commonwealth University in Richmond, VA. Her work has been exhibited around the country including: The Painting Center, New York; Delaware Center for Contemporary Arts; the Anderson Gallery, Richmond; Denise Bibro Gallery, New York; the McLean Project for the Arts; Vanderbilt University; McKinney Avenue Contemporary; Dallas; PLAYsPACE, San Francisco; and the Claremont Graduate University Gallery in LA; Second Street in Charlottesville; Maryland Art Place in Baltimore and Salisbury University in Salisbury, MD. Recent awards include the Maryland State Arts Council’s Individual Artist Award for Sculpture in 2017 and 2014, the Virginia Museum of Fine Arts Professional Artist Fellowship, a Sustainable Arts Foundation Grant, and fellowship residencies at the Vermont Studio Center and the Virginia Center for Creative Arts. Her art criticism has been published in Art Papers, Sculpture Magazine, and NYArts. Her essay “Outliers, Fringes, Speculation, and Complicity: On making and teaching complex, contradictory art” appeared in Creative Collaboration in Art Practice, Research, and Pedagogy, published by Cambridge Scholars Publishing in 2018.
Additional information
Funding
References
- Washington College Course Catalog 2018–2019. https://www.washcoll.edu/live/files/8108-2018-2019-catalog (accessed Jul 21, 2018).
- Holme, T.A.; Hutchison, J.E. J. Chem. Educ. 2018, 95 (4), 499–501. doi: 10.1021/acs.jchemed.8b00174
- Mahaffy, P.; Krief, A. Systems Thinking to ReImagine Chemistry. https://www.acs.org/content/dam/acsorg/events/popular-chemsitry/Slides/2016-09-08-systems-thinking.pdf (accessed Jun 21, 2018).
- The Chemical Professional’s Code of Conduct-American Chemical Society. https://www.acs.org/contenVacs/enlcareers/career-services/ethics/the-chemical-professionals-code-of-conduct.html (accessed Jun 21, 2018).
- The Global Chemists’ Code of Ethics. https://www.acs.org/content/dam/acsorg/global/international/scifreedom/global-chemists-code-of-ethics-fi-2016.pdf (accessed Jun 21, 2018).
- Haack, J.; Hutchison, J. ACS Sust. Chem. Eng. 2016, 4, 5889–5896. doi: 10.1021/acssuschemeng.6b02069
- Walker, G.R. Bulletin of the Atomic Scientists Sept. 1964, 20, 9–13. doi: 10.1080/00963402.1964.11454685
- Moss Plants and More. http://mossplants.fieldofscience.com/2015/04/debunking-moss-graffiti.html (accessed Jun 21, 2018).
- Steve Spangler Science. https://www.stevespanglerscience.com/lab/experiments/sharpie-pen-science/ (accessed Jun 21, 2018).
- Greenberg, B.; Patterson, D. In Art in Chemistry: Chemistry in Art, 2nd ed.; Library Unlimited/Teacher Ideas Press, Westport, CT, 2008; p 71.