ABSTRACT
Recent oil spills causing pollution in oceans and other bodies of water require sorbents that are both cost-effective and eco-friendly. Novel magnetic silica sorbent materials (MSS) have been synthesized using ash from rice husks or sugarcane bagasse and red dust, a waste generated in the steel industry. The designed MSS exhibited a definite response to external magnetic fields. The MSS, based on rice husk ash and bagasse ash, showed oil loading capacities of 1.93 and 2.09 times their weight, respectively, and also exhibited recyclability. The MSS were also functionalized to make them oleophilic and hydrophobic using sodium oleate. These functionalized rice husk ash and bagasse ash-based MSS showed oil loading capacities of 3.22 and 2.69 times their weight, respectively. Magnetic separation provided a convenient method for the removal of magnetizable particles by applying an appropriate external magnetic field. The MSS at the end of their utilization cycles were successfully incorporated into geopolymeric material, enabling the process to be zero waste and to achieve the overall goal of finding a green approach to use these wastes after oil spillage cleanup.
GRAPHICAL ABSTRACT
Introduction
Oil pollution has become an environmental concern all over the world. Oil spillage into the ocean can be caused by oil leaking from oil drilling operations and from oil tankers spilling oil during its transportation (Citation1). Oil can be released during production, distribution, and usage. The history of global oil spills has been summarized by Idris et al. Citation2013 (Citation2). The oceans are becoming more polluted every day from oil spills due to routine shipping, draining, and dumping activities (Citation3), with about 400,000 Mg (metric tonnes) of oil spillage taking place per year around the world through various avenues (Citation4). Between 1970 and 2010, about 5.71 million Mg (metric tonnes) of oil spillage took place solely due to tanker incidents (Citation5, Citation6). In the Gulf of Mexico oil spill in 2010, about 78.0 m3 (206 million gallons) of petroleum products were released, and it is known as one of the worst environmental disasters in human history (Citation7). Recently, 21,000 Mg (metric tonnes) of oil spilled into the Daldykan river and its surroundings in Russia on May 29, 2020, and it is expected to take 5–10 years for a complete clean-up (Citation8). Oil spillage into bodies of water leads to health hazards to marine or aquatic life and can adversely impact the marine ecology for years. In disaster management, oil spill remediation has been reported to be the most expensive kind, at about $4230 to $5283 per m3 in the U.S.A., thus having substantial economic consequences (Citation9).
It has been reported in the literature that seven approaches are available for recovering oil from water, which includes the use of (a) booms, (b) skimmers, (c) dispersants, (d) in-situ burnings, (e) bioremediation, (f) sorbents, (g) membranes, and (h) magnetic nanocomposites (Citation10–12). Booms are mechanical floating barriers that only prohibit the further spreading of oil spillage, whereas skimmers are mechanical types of equipment useful for physical removal of oil spillage floating on the water surface. The dispersants, basically various detergents, are conventionally used to dissolve the oil by dispersing oil in seawater. However, chemicals such as 2-butoxyethanol are toxic in nature and have been found to be harmful to coral reefs and fish. Dissolved oil, including toxic components, can be dispersed great distances, and thus can have harmful effects for large volumes and areas of water bodies.
In the in-situ burning technique, a thick oil layer is burned by flamethrowers hung under helicopters and it was found to be successful in oil spill operations in Northern Canada in 1958, Norway in 1988, Alaska, U.S.A. (Exxon Valdez) in 1989 and Newfoundland, Canada in 1993 (Citation12). The major drawback of this approach is the release of toxic gases and particulates in the ocean and above it, affecting marine and avian life. The technique of bioremediation can be used as an ex-situ as well as an in-situ approach, and it is environment friendly and relatively economical (Citation12). The major disadvantages of in-situ burning and the use of bioremediation are that both these approaches lead to the loss of spilled oil that could otherwise be recovered by innovative approaches recently developed.
Sorbent materials used for recovering oil are capable of adsorbing and/or absorbing oil spillage from water surfaces. Among the different techniques used for oil recovery, adsorption is found to be simple and relatively cost-effective (Citation10). About 200 different kinds of sorbent materials are available commercially and can be categorized into three major groups: (a) organic and agriculturally based materials, (b) synthetic materials, and (c) inorganically based sorbents (Citation10). The agro-based materials can be amorphous silica, lignin, activated carbons, cotton fibers, rice husk, corn stalks, corn cobs, and powdered corn cobs; these are environment friendly, non-toxic, low cost, and biodegradable in nature (Citation2, Citation10, Citation13). However, they have limited sorption capacity and are hydrophilic. Since they are economically feasible, they nevertheless are typically chosen for use in oil recovery. Synthetic materials (e.g. polyethylene, polypropylene, polyurethane, and butyl rubber) are hydrophobic and thus oleophilic in nature, but are non-biodegradable, thus posing environmental concerns (Citation14–16). Inorganic materials, such as perlite, graphite, vermiculite, clays, and zeolites, have high adsorption capacities up to 3–4 times their weight and are also recyclable, but are prone to fouling and aging due to accumulating absorbed water in their porous matrices (Citation10). Recently, to overcome the limitation of these conventional sorbents, advanced materials have been introduced. Generally possessing large specific surface areas and oleophilic natures, such materials include multiwall carbon nanotubes, magnetic carbon nanotubes sponges (Citation10), magnetic iron oxide nanoparticles coated with sulfonated asphaltenes (Citation17), chitosan-coated magnetic nanoparticles (Citation18), magnetic/sawdust composites (Citation19), amine functionalized magnetic nanoparticles (Citation20), magnetic iron oxide nanoparticles (Citation6), coconut oil-based magnetic nanofluids (Citation3), magnetic scaffolds (Citation9), and palm fatty acid functionalized magnetic nanoparticles (Citation21).
Apart from the use of nanomaterials for oil spillage, their applications for enhancing oil recovery can be extended innovatively. The utility of nanomaterials for reducing oil viscosity and thereby enhancing its mobility and altering oil reservoir permeability has not been investigated in depth (Citation22). The iron oxides magnetite, their nanoparticles, and hematite are ferromagnetic, super paramagnetic (size <15 mm), and weakly ferromagnetic, respectively (Citation23). Thus, hematite and particularly magnetite could be used innovatively to recover oil from oil spill sites, as well as from oil production operations, because they can absorb oil and can also adhere to the surface of electromagnetic magnets in the form of magnetic adsorbent materials.
Nevertheless, the synthesis of such advanced nanomaterials is quite cumbersome and energy-intensive. The synthesis requires costly, high-purity synthetic chemical compounds and involves sophisticated instrumentation. Making such compounds has been limited to laboratory-based studies to understand the basic physiochemical characteristics in-depth, since upscaling for commercial viability has been unrealized so far.
The use of porous amorphous silica has been increasing globally in the areas of medicine, catalysis, sensing, and absorption due to their very high specific surface area, low density, and interconnected pore structure. Due to its amorphous, porous structure, rice husk ash has been reported to be capable of oil sorption (Citation1). Combining this ash with magnetic particles could produce a new separation material. When used with a magnetic separation technique, such magnetic silica particles could provide a unique approach to recover oil by developing appropriate mechanical equipment (Citation24).
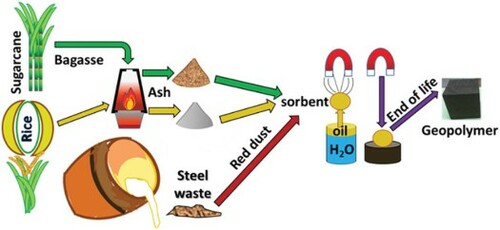
In spite of voluminous research work and the availability of adsorbent, there is a need to develop a techno-economically feasible approach to address oil recovery from oil spillage and from oil-contaminated water from oil extraction and production operations. In particular, a method is should be found that does not require disposal of the materials used (Citation25). Magnetic and porous sorbent materials are needed, particularly those originating from agricultural and industrial wastes that are abundantly available, easily accessible, eco-friendly, recyclable, and inexpensive. In the present study, novel magnetic silica sorbent materials (MSS) were developed using red dust, a waste from the steel industry, and silica from agricultural waste (i.e. rice husk and bagasse). Rice husks and sugarcane bagasse were chosen because they are secondary agricultural residues removed at central processing facilities that are not useful as animal feed. Electricity can be generated when they are combusted to form ash (Citation26). To improve its hydrophobicity and oleophilic characteristics for recovery of spilled oil, MSS have been functionalized using sodium oleate as a surfactant.
Materials and methods
Materials
Red dust powder generated at the Arcelor Mittal steel manufacturing facility (Burns Harbor, IN, U.S.A.) was supplied by Phoenix Services (Portage, IN, U.S.A.). Rice husks were collected from Falcon Rice Mill located in Crowley, LA, U.S.A. Sugar cane bagasse was obtained from Lula Westfield (Paincourtville, LA, U.S.A.). Characteristics of rice husk ash and sugarcane bagasse ash are detailed in Table S1 (Online Resource 1). Oleic acid and denatured ethanol (90.5%) were purchased from Loudwolf (Dublin, CA, U.S.A.) and Duda Energy (Decatur, AL, U.S.A.), respectively. Sodium hydroxide in pellet form was purchased from Fisher Scientific (Waltham, MA, U.S.A.). ACS grade acetone was purchased from Aldon Corporation (Avon, NY, U.S.A.). Sodium silicate (∼45% w/w solution) was purchased from Occidental Chemical Corp. (Dallas, TX, U.S.A.). Class F fly ash was received from Martin Lake Power Plant (MLP) (Tatum, TX, U.S.A.). The chemical composition of fly ash is shown in Table S1 (Online Resource 1). Fly ash is a byproduct from the combustion of coal to produce electricity.
Methods
Preparation of ash from bagasse and rice
The rice husks and sugar cane bagasse were placed in a forced-air oven separately at 260°C for 4 h to burn the biomass down to black ash. The black ash was then combusted at 575°C for 24 h in a muffle furnace to obtain rice husk ash (RHA) and sugar cane bagasse ash (SCBA). The RHA and SCBA contained 94.3% and 71.4% silicon dioxide, respectively (Citation27). The chemical composition from X-ray fluorescence (XRF) of both the ashes is shown in Table S1 (Online Resource 1). XRF was performed using a model Quant’X EDXRF Analyzer C10020XRF from Thermo Fisher Scientific (Madison, WI, U.S.A.). Particle size distribution was conducted on rice hull ash and bagasse ash by suspending them in glycerol using a laser light scattering technique with a ‘Microtrac’ particle size analyzer. The mean particle size for rice hull ash and bagasse ash was found to be 6.17 and 6.18 μm, respectively (Citation27).
Preparation of magnetic silica sorbent material (MSS)
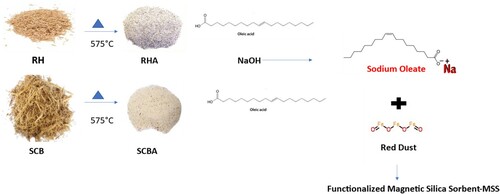
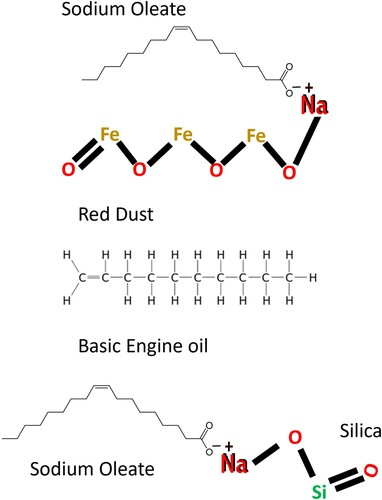
Sodium oleate was prepared by adding 1.42 g of sodium hydroxide to 11.12 mL of oleic acid dissolved in 100 mL of ethanol, and then stirred at room temperature for 24 h. shows the overall scheme of the preparation of the MSS.
The obtained sodium oleate was stored at room temperature until it was used as a functionalizing material for the MSS.
Synthesis of functionalized MSS
To improve the hydrophobic and oleophilic nature of the ashes, they were functionalized with sodium oleate, as shown in . First, 2 g of sodium oleate was dissolved in 100 mL of water, and then 10 g of each ash was dispersed in this solution separately. The mixture was stirred for 30 min at room temperature. After 30 min, the excess sodium oleate was decanted and the functionalized material was dried at 60°C for 24 h. The decanted solution was further reused for the functionalization of fresh RHA and SCBA. The functionalized RHA and SCBA were separately mixed with red dust using a mortar and pestle to achieve a homogeneous mixture of the two MSS. Red dust was used as received. Red dust (10%) was mixed with functionalized RHA, while 20% of red dust was mixed with functionalized SCBA. Red dust was mixed with the ashes to make the silica sorbent material sufficiently magnetic so that the oil loaded MSS could be removed using a permanent magnet from the water surface. As seen in Table S1 (Online Resource 1), the silica content of SCBA is only 71.4% in compared to 94.2% of RHA. SCBA additionally contains 11.2% Al2O3, compared to 0.8% Al2O3 in RHA. Due to their differing compositions, it was necessary to have 20% red dust to achieve comparable magnetic characteristics in the SCBA based magnetic sorbent.
Oil recovery from water surfaces
Non-functionalized materials were magnetic, but had not been treated with sodium oleate. The functionalized materials were magnetic and had been modified with sodium oleate. Motor oil was poured into pure water in a beaker to simulate an oil spill. The functionalized or non-functionalized powdered sorbent material was adhered to a permanent magnet ( and ).
Then the permanent magnet was brought in contact with the contaminated water surface and the MSS was allowed to saturate with oil. We carried out various experiments to optimize the maximum time needed for absorption of oil using sorbent materials and found that a period of 10 min was optimum and sufficient to complete removal of the oil from the water surface. After that, the oil laden sorbent material was removed from the permanent magnet and used for further study.
Extraction of oil from MSS
Oil loaded sorbent material (10 g) was mixed with 50 mL of acetone. The sorbent material was then separated and washed with acetone until the oil was completely removed. The oil-free MSS material was then dried and used again. For the used MSS, no significant changes were observed in the loading capacity for repeated cycles.
Geopolymerization of recycled MSS material after oil removal from water surface
To evaluate the compressive strength for geopolymerized samples, three 5.08 cm cube samples of each were prepared. Various constituents were weighed and mixed to homogenize them for 5 min using a Kitchen Aid 600TM mixer. Next, a 16 mol L−1 sodium hydroxide solution was added to this homogenized mix and stirred in for another five min time duration. After this, sodium silicate (∼45% w/w) solution was added and stirred in for two more min. The control batch of geopolymer paste was comprised of 75.47 g of NaOH solution, 113.20 g of sodium silicate solution, and 706 g of fly ash. To investigate the geopolymerization potential of recycled MSS material after oil removal from the water surface, 54 g of fly ash of geopolymer composition was replaced with recycled MSS material. The resulting material was poured into 5.08 cm cube molds and was vibrated for 5 min by placement on a vibrating table to ensure uniformity throughout the sample. All the specimens were then cured in the oven at 70˚C for 24 h. The specimens were demolded the next day and kept at room temperature in sealed plastic bags for 2 more days. The geopolymer samples were subjected to a compression test as per ASTM C109 (Citation28). A 0.35 MPa/s loading rate was used until the failure of each specimen occurred.
Results and discussion
Red dust
The chemical composition of red dust is shown in Table S1 (Online Resource 1). The red dust was found to be magnetic in nature. It contained iron in the form of hematite, magnetite, and wüstite. The magnetite is a ferromagnetic phase with high magnetization (∼92 Am2 kg−1) and low coercivity (10–40 mT) whereas hematite is anti-ferromagnetic with low magnetization [∼0.4 Am2 kg−1] and high coercivity (100–400 mT). Coercivity is the resistance of a ferromagnetic material to becoming demagnetized. The magnetization of different phases are linearly additive in non-interacting mixtures (Citation29). Sodium oleate has a high affinity for the surface of iron oxide particles (Citation30) in red dust. Mixing of functionalized RHA/SCBA with red dust resulting in the MSS becoming responsive towards the external magnetic field and selectively adsorbing the oil on the water surface.
Oil removal from water surfaces using non-functionalized and functionalized MSS material
To investigate the oil loading capacity of MSS, the motor oil was poured into pure water to replicate an oil spill. Then, MSS was adhered to a permanent magnet and was used to recover oil from the surface of the contaminated water. The oil loading capacity of the RHA and the SCBA MSS were found to be 1.93 ± 0.03 g/g and 2.09 ± 0.06 g/g, respectively. As a model compound, motor oil simulated the kind of oil likely to be spilled, which in reality would have many aliphatic and polycyclic aromatic hydrocarbons (Citation31).
The major drawback associated with agriculture-based sorbent materials is that they are hydrophilic and thus possess low sorption capacity (Citation32). Their water absorption capability has resulted in reduced oil loading and sorbent buoyancy. Both of these drawbacks were eliminated by their functionalization with sodium oleate to make them hydrophobic and oleophilic in nature, and magnetic by combining them with red dust.
To investigate the hydrophobicity, oliophilicity, and buoyancy effect of the functionalized MSS using sodium oleate, the experiment is shown in was carried out. This laboratory-scale experiment simulated oil recovery from a water surface. In the experiment, 0.25 g of functionalized MSS was placed at a single point on the oil surface to see if it would absorb water and if it would sink.
Figure 4. (A) Motor oil on the water surface (B) Placed functionalized MSS on the oil surface (C) Oil saturated MSS cluster (D) Collecting oil-saturated MSS cluster.

As soon as the functionalized MSS came in contact with the layer of oil contaminant, it selectively sorbed the oil. It was observed that the functionalized MSS did not adsorb water, due to its hydrophobic and oleophilic nature. The functionalized MSS saturated with oil was then separated and collected from the water surface using a permanent magnet. The oil loading capacity of functionalized rice husk and bagasse ash-based MSS materials was found to be 3.22 ± 0.19 and 2.69 ± 0.12 g/g, respectively. To replicate the oil spillage in sea water, a synthetic 3.5% NaCl water solution was prepared and used for oil absorption capacity of the MSS material and similar oil loading capacity was observed.
Material scientists are increasingly focused on the application of a magnetic field as an alternative to intensify oil adsorption processes, as it overcomes the limitations associated with conventional biomass-based sorbent material characteristics (Citation24). This is due to the fact that magnetic fields are easy to regulate, are generally safe, and are environmental friendly, along with being inexpensive and easy to implement (Citation33). Electromagnets have been used in adsorption processes and the magnetic field has aided in decreasing the equilibrium time, thus increasing the efficiency of the process (Citation9). The magnetic field can intensify the adsorption, depending on the morphology of the adsorbents. Using an electromagnet is also suitable for demagnetization since its reversibility can instantly allow the removal of adsorbent loaded with oil (Citation33).
During oil recovery operations the oil from the spillage area can become miscible, while water is not absorbed on the surface of the MSS, which is strongly adhered to the electromagnet bar. This phenomenon is based on the concept of recovery of oil from water using magnetic oil sorption methods (Citation34). The magnetic component of MSS is responsive to the applied magnetic field, and the use of sodium oleate aids in making MSS quite hydrophobic and oleophilic, so that this method can enhance oil recovery (Citation30).
Recyclability of MSS
The sorbed motor oil was extracted from the magnetic modified rice husk/bagasse ash sorbent material using acetone. The MSS material was then dried in an air oven until it was constant weight and found that the oil recovery efficiency was as high as 95–99.5%. No significant change in the sorption or recyclability efficiency of the MSS was found when the MSS was used up to 5 times. Such recyclability might be expected since MSS is basically a composite of inorganic magnetic red dust and inorganic ash materials. The MSS proved to be highly stable and, therefore reusable without degradation. These magnetically modified silica sorbent materials, if combined with a mechanical skimmer and a magnetic drum could be a straightforward method to achieve oil recovery from water contaminated with oil.
Zero waste concept
Zero waste emphasizes resource management rather than waste management. The existence of waste shows a lack of innovation strategies since waste does not exist in nature, where everything is a resource: in nature today’s wastes are tomorrow’s precious resource materials. The real solution to pollution lies in the total utilization of raw materials, along with a significant shift towards renewable resources, especially biomass. In the present research work, red dust, an industrial waste from steel manufacturing, along with silica from the agricultural wastes rice husk and sugar cane bagasse, has been utilized simultaneously and synergistically to prepare MSS. These composite materials were found to be useful in recovering oil from oil-contaminated water and in enhancing its recovery. Red dust, rice husk ash (Citation35), sugarcane bagasse ash (Citation36), and crude oil (Citation37) have been successfully used in geopolymeric materials. Thus, the MSS used for oil absorption also has potential for use in geopolymeric material. At the end of its useful life for oil recovery, MSS could achieve the objective of zero waste in reality by its incorporation into geopolymers for use in structures. The geopolymer made using recycled MSS showed a compressive strength of 78.60 MPa for rice husk ash-based MSS, and 75.84 MPa for bagasse ash-based MSS in comparison to 88.47 MPa for control geopolymer composition. This end-use could lead to a new sustainable approach for the relevant industries, generating employment, enhancing the overall economy, and safeguarding the environment (Citation37).
Compared to present technologies, the prepared MSS is likely more ‘green,’ economically viable, and technically applicable with a minimum level of complexity in terms of its use, preparation, recyclability, storage, and disposal. Thus, this material offers a novel eco-friendly method for recovering oil from oil spills and water–oil mixtures generated during oil production operations.
Conclusions
A novel MSS for oil spillage cleanup has been prepared using red dust and ash derived from rice husk or sugarcane bagasse, which are industrial and agricultural wastes, respectively. The use of wastes in the developed process make it more likely to be economically feasible for large scale operation.
The inherent magnetic property of red dust, a waste from the steel industry, can aid in the technical feasibility of the developed novel MSS material in magnetic field operations.
MSS was functionalized using sodium oleate to improve its hydrophobicity and oleophilic nature. Functionalized MSS had a higher oil loading capacity compared to non-functionalized MSS.
The geopolymerization potential for recycled MSS substrate at its end-of-life could allow the process to achieve a zero-waste life cycle.
Further investigation with a more realistic oil mixture is required to determine the feasibility of the developed process.
Supplemental Material
Download MS Word (15.3 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Kudaibergenov, K.; Ongarbayev, Y.; Zulkhair, M.; Tulepov, M.; Tileuberdi, Y. Rice Husk ash for Oil Spill Cleanup. Appl. Mech. Mater. 2014, 446-447, 1508–1511.
- Idris, J.; Eyu, G.; Ahmad, Z.; Chukwuekezie, C. Oil Spills and Sustainable Cleanup Approach. Aust. J. Basic Appl. Sci. 2013, 7 (14), 272–280.
- Nabeel Rashin, M.; Kutty, R.G.; Hemalatha, J. Novel Coconut Oil Based Magnetite Nanofluid as an Ecofriendly Oil Spill Remover. Ind. Eng. Chem. Res. 2014, 53 (40), 15725–15730.
- Varela, A.; Oliveira, G.; Souza Jr, F.; Rodrigues, C.; Costa, M. New Petroleum Absorbers Based on Cardanol-Furfuraldehyde Magnetic Nanocomposites. Polym. Eng. Sci. 2013, 53 (1), 44–51.
- Annunciado, T.; Sydenstricker, T.; Amico, S. Experimental Investigation of Various Vegetable Fibers as Sorbent Materials for Oil Spills. Mar. Pollut. Bull. 2005, 50 (11), 1340–1346.
- Sarcletti, M.; Vivod, D.; Luchs, T.; Rejek, T.; Portilla, L.; Müller, L.; Dietrich, H.; Hirsch, A.; Zahn, D.; Halik, M. Superoleophilic Magnetic Iron Oxide Nanoparticles for Effective Hydrocarbon Removal from Water. Adv. Funct. Mater. 2019, 29 (15), 1805742.
- Atlas, R.M.; Hazen, T.C. Oil Biodegradation and Bioremediation: A Tale of the Two Worst Spills in US History. Environ. Sci. Technol. 2011, 45 (16), 6709–6715.
- A Catastrophic Oil Spill Turns a Russian River Red. https://www.popularmechanics.com/science/environment/a32769626/bloody-russian-river-oil-spill/ (accessed June 10)
- Singh, B.; Kumar, S.; Kishore, B.; Narayanan, T.N. Magnetic Scaffolds in Oil Spill Applications. Environ. Sci. Water Res. Technol. 2020, 6 (3), 436–463.
- Al-Jammal, N.; Juzsakova, T. Review on the Effectiveness of Adsorbent Materials in Oil Spills Clean Up. Sea 2017, 25, 36.
- Li, B.; Zhang, X. Superhydrophobic Nylon Cloth Coated with Modified Silica Used for Oil–Water Separation. Environ. Progr. Sustain. Energy 2019, 38, 3.
- Hoang, A.T.; Pham, X.D. An Investigation of Remediation and Recovery of Oil Spill and Toxic Heavy Metal from Maritime Pollution by a New Absorbent Material. J. Marine Eng. Technol. 2018, 20,159–169.
- Choi, H.-J. Agricultural Bio-Waste for Adsorptive Removal of Crude Oil in Aqueous Solution. J. Mater. Cycles Waste Manag. 2019, 21 (2), 356–364.
- Choi, H.M.; Cloud, R.M. Natural Sorbents in Oil Spill Cleanup. Environ. Sci. Technol. 1992, 26 (4), 772–776.
- Duong, H.T.; Burford, R.P. Effect of Foam Density, Oil Viscosity, and Temperature on Oil Sorption Behavior of Polyurethane. J. Appl. Polym. Sci. 2006, 99 (1), 360–367.
- Tu, W.; Lin, Y.-P.; Bai, R. Enhanced Performance in Phenol Removal from Aqueous Solutions by a Buoyant Composite Photocatalyst Prepared with a Two-Layered Configuration on Polypropylene Substrate. J. Environ. Chem. Eng. 2016, 4 (1), 230–239.
- Abdullah, M.M.; Al-Lohedan, H.A.; Atta, A.M. Novel Magnetic Iron Oxide Nanoparticles Coated with Sulfonated Asphaltene as Crude Oil Spill Collectors. RSC Adv. 2016, 6 (64), 59242–59249.
- Zhang, S.; Lü, T.; Qi, D.; Cao, Z.; Zhang, D.; Zhao, H. Synthesis of Quaternized Chitosan-Coated Magnetic Nanoparticles for Oil-Water Separation. Mater. Lett. 2017, 191, 128–131.
- Di, X.; Zhang, W.; Jiang, Z.; Zhang, M.; Wang, Y.; Liu, F.; Ho, S.-H.; Wang, C. Facile and Rapid Separation of Oil from Emulsions by Hydrophobic and Lipophilic Fe3O4/Sawdust Composites. Chem. Eng. Res. Des. 2018, 129, 102–110.
- Ko, S.; Kim, E.S.; Park, S.; Daigle, H.; Milner, T.E.; Huh, C.; Bennetzen, M.V.; Geremia, G.A. Amine Functionalized Magnetic Nanoparticles for Removal of Oil Droplets from Produced Water and Accelerated Magnetic Separation. J. Nanoparticle Res. 2017, 19 (4), 132.
- Rozi, S.K.M.; Shahabuddin, S.; Manan, N.S.A.; Mohamad, S.; Kamal, S.A.A.; Rahman, S.A. Palm Fatty Acid Functionalized Fe3O4 Nanoparticles as Highly Selective Oil Adsorption Material. J. Nanosci. Nanotechnol. 2018, 18 (5), 3248–3256.
- Negin, C.; Ali, S.; Xie, Q. Application of Nanotechnology for Enhancing Oil Recovery – A Review. Petroleum 2016, 2 (4), 324–333.
- Wu, W.; He, Q.; Jiang, C. Magnetic Iron Oxide Nanoparticles: Synthesis and Surface Functionalization Strategies. Nanoscale Res. Lett. 2008, 3 (11), 397.
- Yu, L.; Hao, G.; Liang, Q.; Jiang, W. Fabrication of Magnetic Porous Silica Submicroparticles for Oil Removal from Water. Ind. Eng. Chem. Res. 2015, 54 (38), 9440–9449.
- Smieja, J.M.; Babcock, K.E. The Intersection of Green Chemistry and Steelcase’s Path to Circular Economy. Green Chem. Lett. Rev. 2017, 10 (4), 331–335.
- Henry, C.S.; Lynam, J.G. Embodied Energy of Rice Husk Ash for Sustainable Cement Production. Case Studies in Chemical and Environmental Engineering 2020.
- Garrett, T.D.; Cardenas, H.S.; Lynam, J.G. Sugarcane Bagasse and Rice Husk Ash Pozzolans: Cement Strength and Corrosion Effects When Using Saltwater. Curr. Res. Green Sustain. Chem. 2020, 1-2, 7–13.
- ASTM, A. Standard Test Method for Compressive Strength of Hydraulic Cement Mortars (Using 2-in. or [50-mm] Cube Specimens). Annu. Book ASTM Stand. 2013, 4 (1), 1–9.
- Ahmadzadeh, M.; Romero, C.; McCloy, J. Magnetic Analysis of Commercial Hematite, Magnetite, and Their Mixtures. Aip Adv. 2018, 8 (5), 056807.
- Shekhawat, D. S.; Aggarwal, A.; Agarwal, S.; Imtiaz, M. Magnetic Recovery-Injecting Newly Designed Magnetic Fracturing Fluid with Applied Magnetic Field for EOR, SPE Asia Pacific Hydraulic Fracturing Conference. Society of Petroleum Engineers, 2016
- Kriipsalu, M.; Marques, M.; Maastik, A. Characterization of Oily Sludge from a Wastewater Treatment Plant Flocculation-Flotation Unit in a Petroleum Refinery and its Treatment Implications. J. Mater. Cycles Waste Manag. 2008, 10 (1), 79–86.
- Fuller, A.; Maier, J.R.; Scheffknecht, G.N.; Karampinis, E.; Grammelis, P.; Kakaras, E.; Kalivodova, J. Fly Ash Formation and Characteristics from (Co-)Combustion of an Herbaceous Biomass and a Greek Lignite (Low-Rank Coal) in a Pulverized Fuel Pilot-Scale Test Facility. Energies 2018, 11, 6.
- Virgen, M.D.R.M.; Vázquez, O.F.G.; Montoya, V.H.; Gómez, R.T. Removal of Heavy Metals Using Adsorption Processes Subject to an External Magnetic Field. Heavy Met. 2018, 15, 253–280.
- Kaiser, R., Miskolczy, G. The Recovery of Oil from Water With Magnetic Liouids, Proceedings, Am. Pet. Inst., 1971, 415
- Arnold, M.C.; de Vargas, A.S.; Bianchini, L. Study of Electric-Arc Furnace Dust (EAFD) in Fly Ash and Rice Husk Ash-Based Geopolymers. Adv. Powder Technol. 2017, 28 (9), 2023–2034.
- Pratiwi, K. I. Effect NaOH Concentration on Bagasse Ash Based Geopolymerization, MATEC Web of Conferences, EDP Sciences, 2016, 01025.
- Abousnina, R.; Manalo, A.; Lokuge, W.; Zhang, Z. Effects of Light Crude Oil Contamination on the Physical and Mechanical Properties of Geopolymer Cement Mortar. Cem. Concr. Compos. 2018, 90, 136–149.