ABSTRACT
1,8-Diazabicyclo[5.4.0]undec-7-ene (DBU) has received a significant importance in organic synthesis during recent years because of its interesting ability to catalyze organic reaction sufficiently at mild conditions. DBU can work as a catalyst under simple conditions inducing the operational simplicity of the experimental procedures by reducing the side products and waste. The notable advantages in these cases include the less expensive catalyst which is commercially readily available, easy to handle, and most importantly recoverable. The DBU as a catalyst has been explored for several practical methodologies and is highly useful for the synthesis of a wide variety of organic materials. The selectivity (chemo-, regio-, and stereo-) of these reactions is highly impressive and applicable in modern chemical transformations. The DBU possesses enhanced basic properties and high mechanical as well as thermal stability. In recent years, a large number of organic transformations have been carried out using DBU as a catalyst. The DBU has successfully been employed to conduct a wide variety of organic reactions including usual chemical modifications, cyclizations, eliminations, esterifications, condensation reactions, multicomponent reactions, etc. In fact, during last few decades, a tremendous interest has sparked in various organic transformations promoted by DBU and a large number of publications have appeared in the literature. Since chemical transformations catalyzed by DBU have recently been proven to be highly important in organic synthesis we review in this article the recently appeared significant protocols using DBU as a catalyst.
GRAPHICAL ABSTRACT
1. Introduction
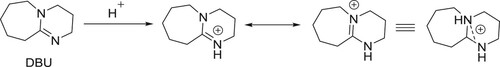
The base plays a critical role in a variety of organic transformations including C–C couplings (Citation1,Citation2), C–hetero atom couplings (Citation2,Citation3), heterocyclic compounds synthesis (Citation3,Citation4), nucleophilic reactions (Citation5), biomass conversions (Citation6), biodiesel productions (Citation2), and biogas upgradations (Citation7). The use of organic bases in the organic synthesis is well reported organic synthesis during current decades (Citation8,Citation9). In this connection, 1,8-diazabicyclo [5.4.0] undec-7-ene (DBU) () is found to be an effective organic base that can be used as a catalyst in a variety of organic transformations including general chemical derivitizations, cyclizations, and multicomponent reactions. DBU is a less expensive and readily available base in most of the synthetic laboratories has low nucleophilicity (Citation10–13).
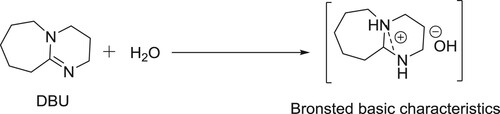
DBU is a sterically hindered base, which exists in liquid form with a boiling point of 261°C. It is one of the strongest organic base containing a pKa value of 12. The presence of adjacent nitrogen atoms is believed to stabilize the protonated species during its basic action ().
DBU is a non-nucleophilic base and due to this reason, it has been found to be useful in many reactions without side reactions due to inherent nucleophilicity of basic nitrogen of DBU (Citation14,Citation15).
Traditionally, DBU has been considered a non-nucleophilic base, but there are few reports where DBU has been shown to function as a better nucleophilic base catalyst than other similar organic bases such as 1,4-diazabicyclo [2.2.2] octane (DABCO) or 4-dimethylaminopyridine (DMAP) (Citation16,Citation17). Organic base catalysts with high reactivity have gained attention as an economically viable alternative to metal catalysts and environmentally green catalysts.
The notable advantages of DBU to be used in many organic reactions as it is less expensive, commercially readily available, homogenous, easy to handle, and most importantly recoverable. The benefit of the DBU catalyst has been explored for the practical methodologies useful for the synthesis of a wide variety of organic materials. In recent years, DBU has been used as a nucleophilic/non-nucleophilic base, catalyst, and complexing ligand and in many organic reactions.
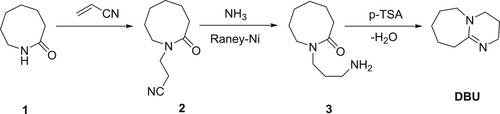
DBU can be synthesized from a lactam, azepan-2-one (1). N-Cyanoethylation of lactam is carried out using acrylonitrile in the presence of a potassium hydroxide as base to produce the nitrite, 2, which is reduced using Raney-nickel in the presence of NH3to give N-(3-aminopropyl)-azepan-2-one (3). The compound 3 further undergoes condensation in the presence of p-toluenesulphonic acid (p-TSA) to yield DBU () (Citation18,Citation19).
There is no comprehensive review on the DBU-catalyzed reactions in recent years. In this regard and the inspiration gained by the effectiveness of DBU in synthetic organic chemistry we review here the significant chemical transformations that are appeared in the literature by the application of DBU as a base and it is highly beneficial to the scientific community working in this area. The high selectivity (chemo-, regio-, and stereo-) of the reactions using DBU is found to be highly impressive and we hope that several methodologies may be developed in the future using DBU.
2. DBU-mediated organic transformations
A brief review of DBU-mediated organic transformations is discussed here.
2.1. Amidation reactions
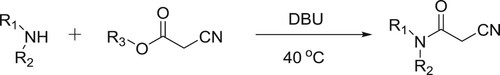
Rajappa et al. have also described the DBU-catalyzed amidations of alkyl cyanoacetates, wherein, DBU acts as a nucleophilic base and replaces the alkoxy moiety and activates the carbonyl functional group attack by the amine () (Citation20).
Sing et al. have been reported a DBU-mediated reaction of 5,6-O-isopropylidene-2,3-bis-O-alkyl ascorbic acid with amines to produce of 3,4-bis-O-alkyl-1-alky1-5-(2-hydroxyethyl)-5-hydroxy-1,5-dihydropyrrol-2-ones in good yields () (Citation21).
Scheme 4. Synthesis of 3,4-bis-O-alkyl-1-alky1-5-(2-hydroxyethyl)-5-hydroxy-1,5 -dihydropyrrol-2-ones.
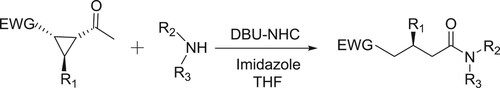
Bode et al. have described the application of DBU-NHC (N-heterocyclic carbene) system in THF for the amidation of α-functionalized aldehydes with amines () (Citation22).
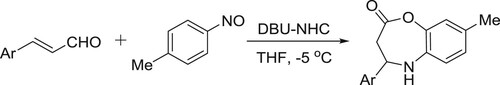
Festa et al. synthesized 1-alkoxypyrazino[1,2-a] indoles from indol-2-carbonitrileson reaction with alcohols under DBU-catalyzed microwave irradiations. This method showed a large scope to access a wide range of indole derivatives () (Citation23).
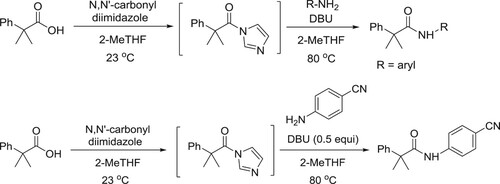
Aboussafy et al. have described a DBU-catalyzed effective amidation process of acyl imidazoles in 2-MeTHF using amines. The rate acceleration is especially evident with traditionally unreactive, electron-deficient anilines () (Citation24).
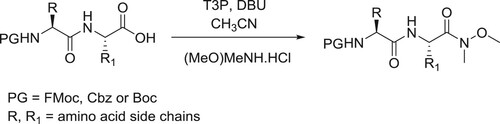
Sharnabai et al. have described the synthesis of N-protected amino weinreb amides and peptidyl weinreb amides in presence of T3P (1-propanephosphonic acid cyclic anhydride) catalyzed by DBU in CH3CN () (Citation25).
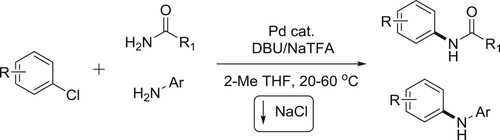
The palladium-catalyzed coupling of aryl and heteroaryl chlorides with primary amides under mild homogeneous reaction conditions has been reported by Gregory et al. Successful C–N coupling is enabled by the use of a unique ‘dual-base’ system consisting of DBU and NaTFA, which serve as proton acceptor and halide scavenger, respectively, using low catalyst loadings (0.5 mol %) with readily available, air-stable palladium precatalysts. The DBU/NaTFA system also enables the room-temperature coupling of primary aryl amines with aryl chlorides and is tolerant of a variety of base-sensitive functional groups () (Citation26).
2.2. Elimination reactions
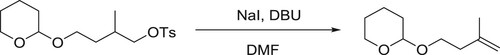
Wolf et al. have been used DBU as effective catalyst for the dehydrohalogenation and elimination of sulphonic acids in one pot conversion of tosylates to alkenes via primary () (Citation27).
Yadav et al. have synthesized a variety of 2-methylbenzofurans from dehydroiodination of 2-iodomethyl-2,3-dihydrobenzofurans catalyzed by DBU () (Citation28).
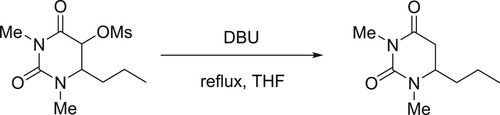
Otter et al. have discussed the DBU-catalyzed efficient elimination of methanesulfonicacid from its ester in the final step during the synthesis of 1,3-dimethyl-6-propyluracil () (Citation29).
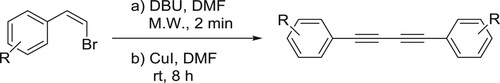
Zhang et al. have efficiently been synthesized symmetrical diynes from (Z)-aryl vinyl bromides catalyzed by DBU in the presence of CuI in DMF. The catalyst DBU carries out the dehydrohalogenation of aryl vinyl bromides resulting in the formation of aryl alkynyls, which is then deprotonated by DBU and in the presence of Cul forms copper aryl alkynylide. Then, the homocoupling of copper aryl alkynylide produces the target product () (Citation30).
2.3. Cyclization reactions
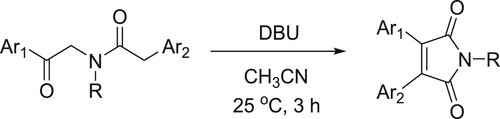
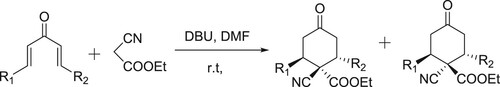
Pal et al. have synthesized symmetrical (or) unsymmetrical 3,4-diaryl maleimides catalyzed by DBU through oxidative cyclization process () (Citation31).
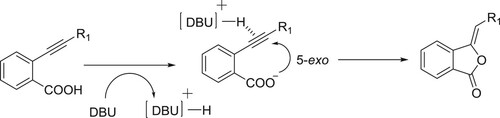
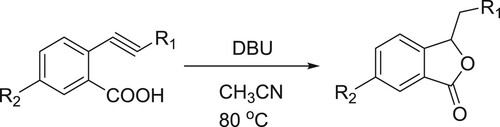
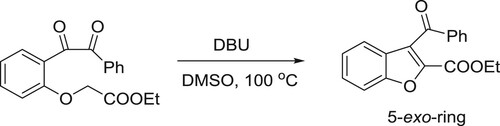
The conjugated acid of DBU assists the intermolecular 5-exo cyclization of the carboxylate to the triple bond (). Kanazawa et al. have efficiently been used DBU as a catalyst for 5-exo intramolecular cyclization reaction of O-alkyl benzonic acids to give corresponding phthalides () (Citation32).
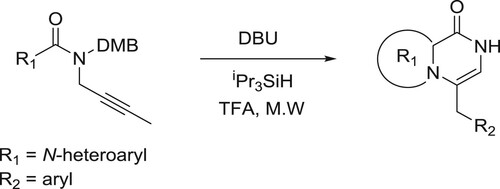
Llauger et al. have efficiently been synthesized the fused heteroaryl pyrazinones from aryl(prop-2-yn-1-y1)-1/-1-pyrro1e-2-carboxamides catalyzed by DBU via a sequential hydro amination/cyclization reaction () (Citation33).
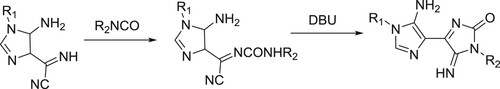
Dias et al. have efficiently synthesized of 4,4’-bi-1H-imidazol-2-ones from 5-amino-α-imino-1H-imidazole-4-acetonitriles and aryl/alkyl isocyanates () (Citation34).
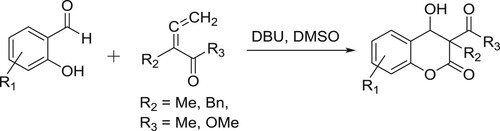
Bensulong et al. have carried out DBU-mediated regioselective intramolecular cyclization/dehydration of ortho-diketophenoxyethers to give corresponding 2,3-substituted γ-benzopyranones () (Citation35).
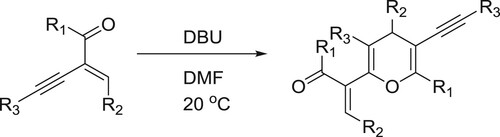
Yu et al. have developed a DBU promoted self-hetero [4 + 2] cycloaddition reaction of 2-(1-alkynyl)-2-alken-1-ones to afforded highly substituted 4H-pyrans in metal-free conditions. In this reaction DBU acts as a conjugated yne-enones as a nucleophilic catalyst () (Citation36).
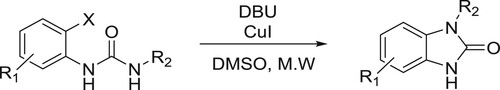
Li et al. have efficiently been utilized DBU-CuI-mediated system to carry out the cyclization of N’-substituted N-(halophenyl) urea’s to give corresponding N-substituted 1,3-dihydrobenzimidazol-2-ones () (Citation37).
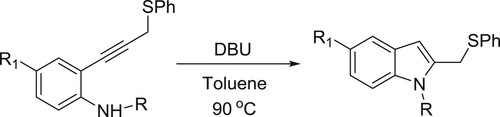
Zhou et al. have been carried out DBU-catalyzed intramolecular cyclization of 2-alkynylanilines which leads to give various analogues of indole () (Citation38).
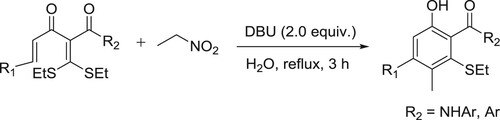
For the first time, an eco-friendly and sustainable tandem [5C + 1C] cycloaromatization of α-alkenoyl ketene dithioacetals and nitroethane in water for the efficient synthesis of ortho-acylphenols was reported by Haifeng Yu et al. The green approach to ortho-acylphenols not only avoided the use of harmful organic solvents, which could result in serious environmental and safety issues, but also exhibited fascinating features such as good substrate scope, excellent yields, simple purification for desired products, ease of scale-up, and reusable aqueous medium () (Citation39).
2.3. Etherification and (thio) esterification
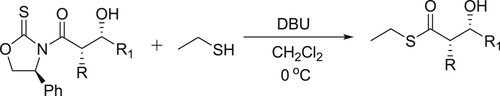
Wu et al. have efficiently been synthesized ethyl thioesters catalyzed by DBU and removal of the 4-phenyloxazolidinethione auxiliary, which results in the conversion of N-acyl-β-hydroxy-oxazolidinethiones () (Citation40).
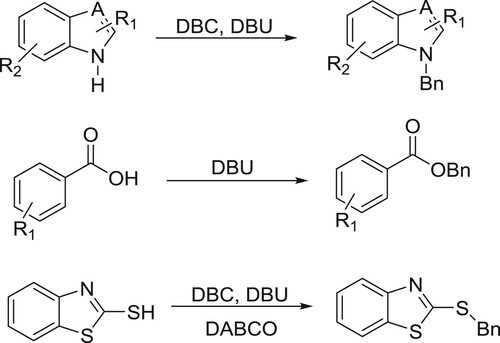
Shieh et al. have successfully been used DBU accelerated green chemistry for N-, O-, and S-benzylation with dibenzyl carbonate (DBC) () (Citation41).
Nishikubo et al. have efficiently been synthesized of poly(p-nitrobenzylmethylacrylate) and poly[4-(4-nitrobenzyloxy)styrene] from polymethacrylic acid and poly(4-hydroxystyrene) respectively underwent reaction with p-nitrobenzylbromide in the presence of DBU catalyst. It has been used as a base for the etherification and esterification of polymers too () (Citation42).
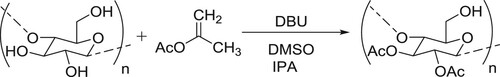
Chen et al. have employed catalytic amount of DBU for the transesterification reaction resulting in the per-O-acetylation of cellulose, by using isopenyl acetate (IPA) as an acetylation reagent in DMSO. In this reaction DBU catalyst acts as acyl transfer agent () (Citation43).
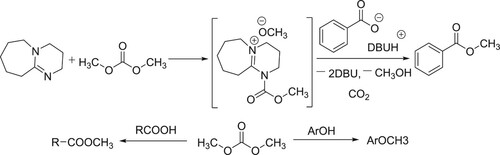
DBU is an effective nucleophilic catalyst for carboxylic acid esterification with dimethyl carbonate (DMC). This reaction pathway of this new class of nucleophilic catalysis has been reported by Shieh et al.. N-acylation of DBU with DMC generates the carbamate intermediate which further reacts with carboxylate anion affords the corresponding methyl esters in excellent yields and this method is particularly valuable for the synthesis of methyl esters that bear acid-sensitive functionality () (Citation17).
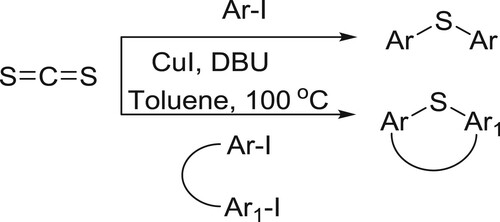
Zhao et al. have efficiently been synthesized diaryl thioethers and S-cycles from carbon disulfide and aryl iodides catalyzed by the CuI in the presence of DBU. This reaction was successfully operated in the construction of sulfur-containing cyclic moieties () (Citation44).
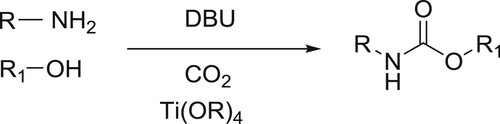
Organic syntheses, such as that of N-substituted carbamic acid esters (CAEs), utilizing low purity and low concentrations of CO2 directly, may help achieve two important goals-reducing CO2 emission, and producing useful core chemicals without the energy-consuming processes of purifying and concentrating CO2. Here, Hiroki Koizumi et al. demonstrated a new synthetic approach for CAEs that uses a combination of two types of CO2 capture mechanisms, which are constituted by combining an amine and 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU), and an alcohol and DBU, to form a protonated DBU carbamate salt and a protonated DBU alkyl carbonate salt, respectively. This new approach of CAE synthesis can be applied even to the simulated exhaust gas from fire-power plants, such as 3 vol % of CO2 in N2 or 15 vol % of CO2 in N2, also containing SO2, NO2, and CO () (Citation45).
2.4. Halogenation reactions
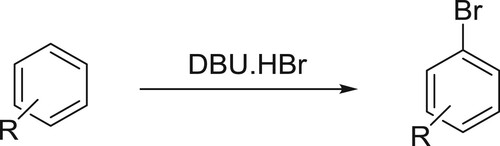
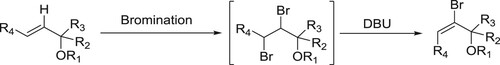
Muathen et al. have successfully employed DBU hydrobromide perbromide (DBU.HBr) as a new mild, stable, and recoverable brominating agent for an array of aromatic compounds. This catalyst shows remarkable stability and remains unchanged for several months whereas compared with Py.HBr which loses 30% of its activity in a period of one month when it exposed to air () (Citation46).
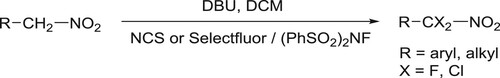
Butler et al. have efficiently been synthesized di α-halogenation (chlorination/fluorination) of nitromethyl group attached to an aryl/alkyl moiety by using NCS/(PhSO2)2NF/Selectfluor and DBU as a base in DCM () (Citation47).
An efficient one-pot method for selectively elimination bromine of allyl alcohol derivatives has been developed by Kutsumura et al. () (Citation48).
2.5. Isomerization reactions
A mixture of substituted pyrrolidin-2-ones was equilibrated using DBU, during the final step of a herbicide synthesis by Moriyaso et al. () (Citation49).
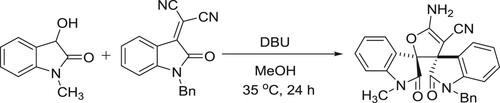
The first DBU-catalyzed Michael/Pinner/isomerization cascade of 3-hydrooxindoles with isatylidene malononitriles was developed by Zhu et al. This protocol also provides an efficient method for the synthesis of α-cyano-γ-butyrolactonebispirooxindoles () (Citation50).
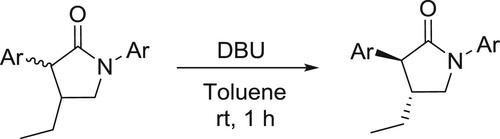
An easy approach to isomerization of δ-thiolactams to the conjugated α, β-unsaturated isomers was developed by Sosnicki et al. () (Citation51,Citation52).
Isoaromatizationof4-a1kylidene-3-oxo-1-cyclohexene-1-carboxylatestothecorrespondingmethyl-4-alky1-3-hydroxybenzoates has been achieved using DBU as the catalyst by Campbell et al. () (Citation53).
Jiahui Duan et al. have described a DBU-mediated cascade strategy of propargylamines with dimethyl 3-oxoglutarate for constructing a functionalized benzo[c]chromen-6-one core has been achieved. This cascade process presumably involves a sequence of 1,4-conjugate addition, followed by lactonization, alkyne–allene isomerization, enol–keto tautomerization, 6π-electrocyclization, and aromatization. A photophysical survey reveals that the benzo[c]chromen-6-one products exhibit fluorescence properties and show potential for exploring fluorescent material applications. This protocol has few advantages such as mild reaction conditions, simple operation, transition metal-free, inert atmosphere-free, rich structural diversity, large-scale synthesis and good functional group tolerance () (Citation54).
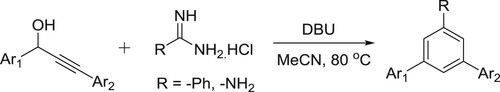
A straightforward, efficient yet effortless approach for the synthesis of structurally important triarylated pyrimidine derivatives has been successfully developed by Rimpa De et al. using secondary propargyl alcohol and commercially available amidines under mild basic conditions. The reaction is believed to proceed via base-mediated redox isomerization of propargyl alcohol into a chalcone and a subsequent N–C–N fragment condensation reaction with the in situ generated chalcone. The procedure may be successfully employed to generate a large array of polysubstituted pyrimidine derivatives from milligram to multigram scale, atom and cost effective, operationally simple () (Citation55).
2.6. Horner-Wadsworth-Emmons reaction
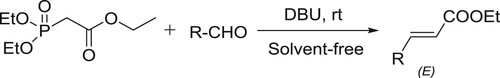
The Horner-Wadsworth-Emmons reaction, a modification of the Wittig reaction has been employed frequently for the synthesis of α,β-unsaturated esters. Ando et al. efficiently developed the solvent-free Horner-Wadsworth-Emmons reaction with a variety of aldehydes in the presence of DBU to give E-α,β-unsaturated esters and ketones in high yields. The E-selectivity of the product was high and the used DBU catalyst was recovered () (Citation56,Citation57).
2.7. Baylis–Hillman reaction
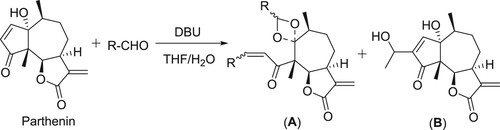
Baylis–Hillman reaction of parthenin involved in the formation of the unexpected 1,3-dioxolanes (A) in the presence of catalytic amount of DBU, in the case of small aromatic and aliphatic aldehydes, whereas in case of higher aliphatic aldehydes resulted a normal Baylis–Hillman product (B) () (Citation58).
A highly regioselective DBU-catalyzed annulation of Morita-Baylis–Hillman carbonates with isothiocyanates was developed. This method allows an efficient and rapid synthesis of spirocyclic oxindole dihydrothiophene products in moderate to high yields with excellent regioselectivities under simple conditions. A plausible reaction mechanism is also proposed by Zhao et al. () (Citation59).
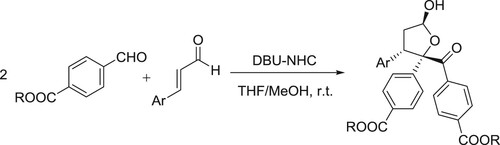
Ze Liang et al. have successfully been developed a cascade assembly between isatin-derived Morita–Baylis–Hillman carbonates and o-hydroxybenzylideneacetones has been developed under the relay catalysis of Pd(PPh3)4 and DBU, affording a spectrum of 1,2,3,4-tetrahydrodibenzo[b,d]furan architectures incorporating a spirooxindole motif in moderate to good yields with excellent diastereoselectivity. The fused indole analogues were similarly furnished by employing the benzylideneacetones having an o-TsNH group () (Citation60).
2.8. Mannich-type reaction
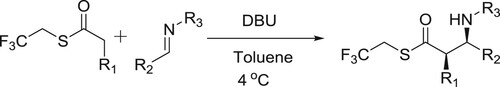
Utsumi et al. have efficiently used DBU in catalytic amount to carry out the Mannich-type reaction of trifluoroethyl thioesters with diverse imines to afford protected β-amino acids () (Citation61).
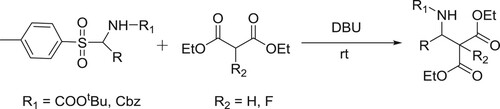
DBU has been successfully employed as a catalyst to carry out Mannich-type of reaction of α-amido-p-tolysulfones treated with diethyl malonates/diethyl flouro malonates to produce β-amino esters/α–fluoro-β-amino esters () (Citation62).
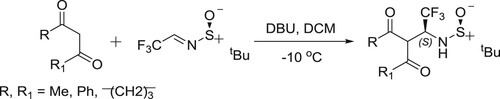
Mazzeo et al. have successfully been used DBU-mediated Mannich type addition of 1,3-dicarbonyl compounds to (S)-N-tert-butanesulfinylfluoroacetaldimine carried out under dramatically different conditions () (Citation63).
2.9. Coupling reactions
Traditionally, mineral bases are used in Suzuki–Miyaura cross-coupling reactions. The use of DBU as an alternative base for Suzuki–Miyaura cross-coupling protocols was discussed by Chanthavong et al. () (Citation64).
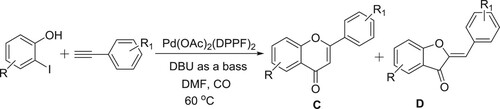
Ciattini et al. have successfully been used DBU-Pd(OAc)2(DPPF)2 system, wherein DBU acts as a base, for carrying out the carbonylative coupling of ethynyl arenes and 2-hydroxylaryl iodides leads to resulting in the mixtures of flavones (C) and aurones (D) in different yields () (Citation65).
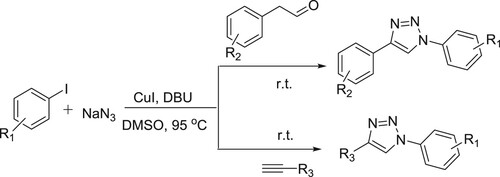
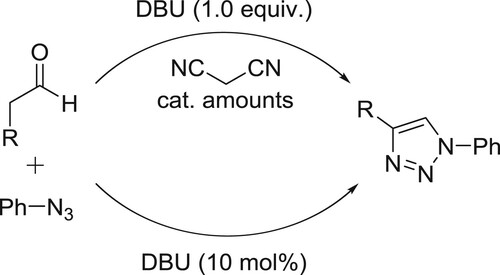
A convenient CuI/DBU-catalyzed one-pot method has been developed for the synthesis of 1,4-disubstituted 1,2,3-triazoles through the coupling of aryl iodides with sodium azide, followed by the intermolecular cyclization between the generated aryl azides and phenyl acetaldehyde derivatives or alkynes derivatives in DMSO producing the desired products in excellent yields () (Citation66).
2.10. Nef reaction
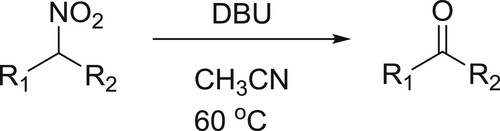
Selective Nef reaction of different secondary nitro compounds has been converted into the corresponding ketones under basic conditions using DBU in acetonitrile. In this reaction, DBU acts a base () (Citation67).
2.11. Strecker-type reaction
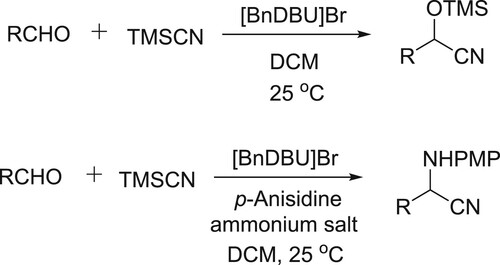
Raj et al. have efficiently been used DBU based basic ammonium salt ([BnDBU]Br) for synthesis of α-aminonitriles and cyanohydrins utilizing TMSCN as the source of cyanide ion. In this reaction catalyst helps in activating the silyl group of TMSCN, thus releasing the cyanide ion () (Citation68).
2.12. Alkylation reactions
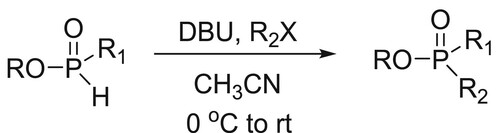
Gavara et al. have efficiently carried out the alkylation of various alkyl phosphinates and some H-phosphonate diesters were promoted by the base DBU. This method provides an easy access to import H-phosphinate building blocks and synthesis of methyl-H-phosphinate esters in excellent yields () (Citation69).
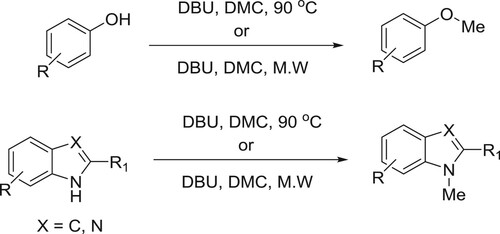
Shieh et al. have efficiently been used catalytic amount of DBU along with dimethyl carbonate (DMC) to affect the methylation of bezimidazoles, phenols, and indoles () (Citation70).
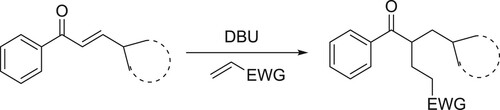
An efficient and atom-economic transformation of enones with electron-deficient alkenes in the presence of DBU under mild conditions has been developed by Tian et al. This protocol offers a direct access to 1,5-dicarbonyl compounds in synthetically useful yields using vinylogous strategy via dienolate intermediates () (Citation71).
2.13. Acylation reactions
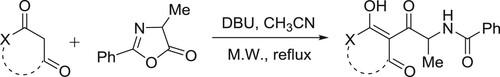
A new method for the synthesis of diversely functionalized oxazoles has been developed by Cordaro et al. Catalytic amount of DBU has been employed to afford the reaction of oxazolone with enolizable cyclic 1,3-dicarbonyls resulting in the formation of C-acylated derivatives () (Citation72).
Singh et al. have efficiently been synthesized 1,3-diketones/α,β-unsaturated-1,3-diketones from N-heterocyclic carbene (NHC) intramolecular acylation of haloketones with aldehydes/α,β-unsaturated aldehydes () (Citation73).
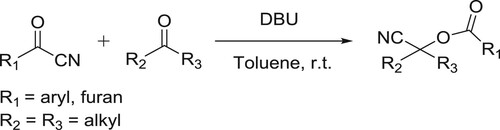
Zhang et al. have successfully been synthesized the corresponding O-acyl cyanohydrin adducts in higher yields from a variety of substituted acetone or pentan-3-one, cyclohexanones, cyclopentanone, and various acyl cyanides catalyzed by DBU () (Citation74).
2.14. Benzoin condensation
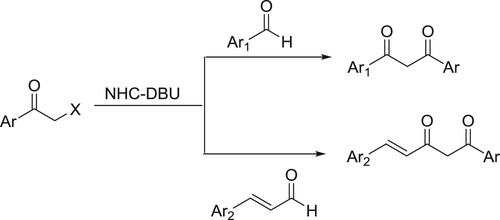
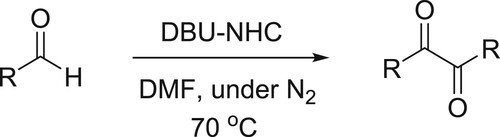
An efficient and simple one-pot procedure for the synthesis of α-diketones from a variety of aldehydes via benzoin condensation under the influence of a catalytic amount of DBU-NHC has been developed by Shimakawa et al. () (Citation75).
2.15. Synthesis of azalactones and cyclic hemiacetals
The N-heterocyclic carbene (NHC)-catalyzed annulation of enals with nitroso compounds in the presence of DBU in THF has described by Yang et al. Unexpected seven membered 4-azalactone was formed according to a process involving a 1,2-Bamberger-type rearrangement () (Citation76).
DBU-NHC catalyzed transformation of α,β-unsaturated aldehydes and 4-formylbenzoates to cyclic hemiacetals was described by Yoshida et al. Various substrates were converted to the corresponding cyclic hemiacetals in a stereoselective manner () (Citation77).
2.16. Multicomponent reactions
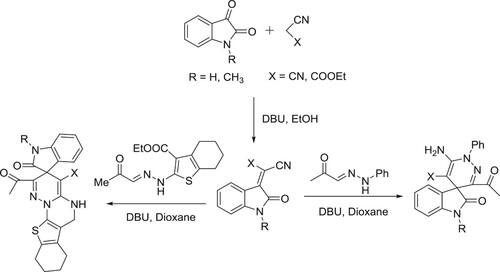
The catalyst DBU has been used as a catalyst for a variety of multicomponent reactions in organic chemistry. DBU-catalyzed synthesis of 2-amino-4-aryl-6,7,8,9-tetrahydro-5H-benzo[7]annu-lene-1,3-dicarbonitriles () (Citation78) and synthesis of pyrrolo[2,1-a]isoquinolines have been reported by Subhend et al. () (Citation79).
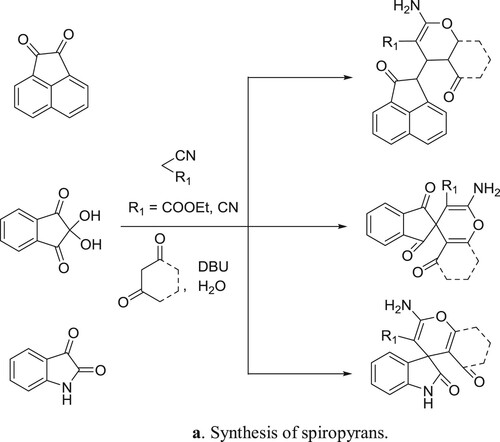
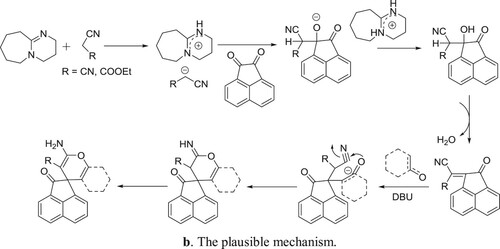
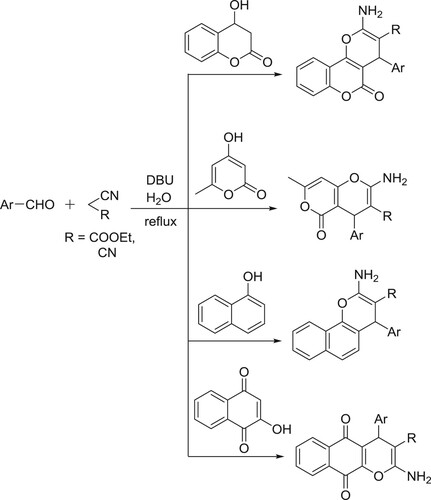
Khurana et al. have efficiently been synthesized spiropyrans by the reaction of 1,3-dicarbonyl compounds treated with acenaphthoquinone/isatin, malononitrile/ninhydrin/ethylcyanoacetate in one pot manner () (Citation80). The plausible mechanism is depicted in .
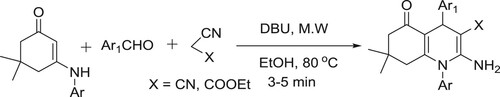
N-Aryl quinoline analogues have efficiently been synthesized by DBU catalyzed in one pot reaction of 3-arylamino-5,5-dimethylcyclohex-2-enone, aromatic aldehydes, and ethyl cyano acetate/malononitrile under microwave irradiation by Singh co-workers () (Citation81).
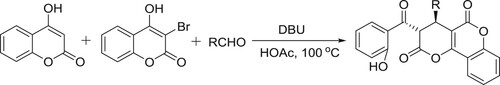
DBU/HOAc catalyzed system has been employed for a diastereoselective synthesis of pyrano fused coumarins via three-component reaction of aldehydes, 3-bromo-4-hydroxycoumarin and 4-hydroxy coumarin by Ahadi et al. () (Citation82).
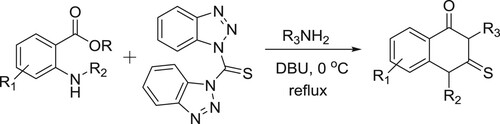
Tiwari et al. have efficiently been reported the synthesis of 2-thioxo-2,3-dihydroquinazoline-4(1H)-ones via one-pot, simple and convenient DBU-mediated reaction of primary amines, anthranilic esters, and bis(benzotriazolyl)methanethione () (Citation83). The same research group have also been employed the DBU-mediated one-pot synthesis of dithiocarbamates via reaction of amines, mercaptans, and bis(benzotriazolyl)methanethione (Citation84).
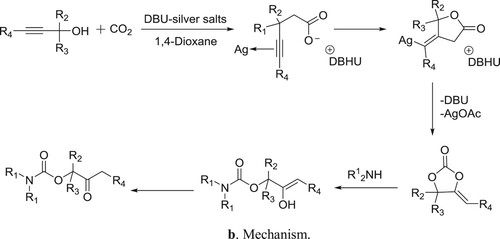
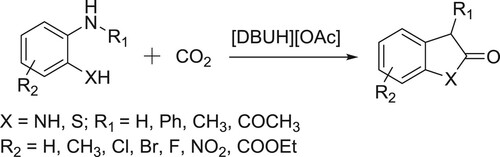
DBU-silver salts catalyzed system has been utilized for the synthesis of β-oxoalkyl carbamates using three-component reaction of secondary amines, propargylic alcohols, and carbon dioxide by Qi et al. () (Citation85). The proposed mechanism for the reaction is depicted in .
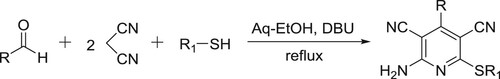
Mamgain et al. have efficiently been synthesized highly functionalized pyridine derivatives in the aqueous ethanol by the reaction of malononitrile, aldehydes, and thiophenols in the presence of DBU () (Citation86).
Khurana et al. have employed a highly efficient DBU system for one-pot synthesis of substituted 2-amino-4H-benzo[h]chromenes, 2-amino-4H-benzo[g]chromenes, dihydropyrano[4,3-b]pyranes, and 3,4-dihydropyrano[3,2-c]chromenes in the aqueous medium () (Citation87).
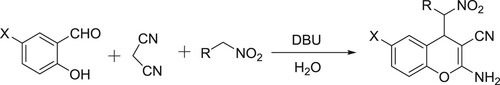
DBU-catalyzed one-pot synthesis of 2-amino-4-(nitroalkyl)-4H-chromene-3-carbonitriles has been reported by Zonouzi et al. () (Citation88).
Chauhan et al. have discussed a one-pot procedure for the synthesis of a novel series of biologically important spiropyrazolone derivatives via sequential organo cascade Michael/Michael 1,2-addition reactions between β-nitrostyrene, β-ketoester, and pyrazolone () (Citation89).
An efficient, practical, simple, and scalable approach for the synthesis of 4H-pyrimido[2,1-b]benzothiazoles using three-component cyclo-condensation of β-ketoesters, substituted aromatic aldehydes, and 2-aminobenzothiazole catalyzed by DBU-CPB system has been reported by Singh et al. () (Citation90).
2.17. Carbonylation reactions
DBU acts both as a catalyst and a trapping agent in these types of reactions.
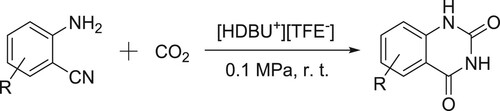
A protic DBU-based ionic liquid [HDBU+][TFE-] catalyzed CO2 conversion at room temperature and atmospheric pressure to give quinazoline-2,4-(1H,3H)-diones reported by Zhao et al. () (Citation91).
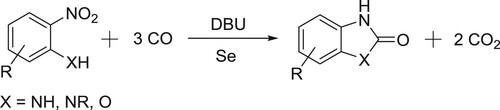
Wang et al. have efficiently been carried out selenium-catalyzed one-pot reductive carbonylation of 2-nitroaniline/2-nitrophenols to give 2-benzoxazolones () (Citation92).
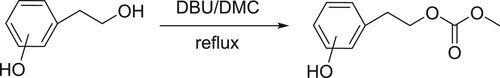
Bernini et al. have been reported chemoselective and efficient carbomethoxylation of the alcoholic groups of phenols with dimethyl carbonate (DMC) catalyzed by DBU () (Citation93).
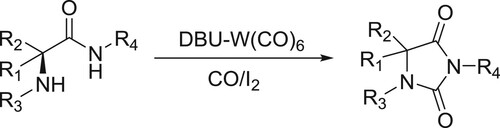
Oxidative catalytic carbonylation of α-amino amides using DBU-W(CO)6 as a catalyst, CO as the carbonyl source and I2 as the oxidant has been reported by Dumbris et al. () (Citation94).
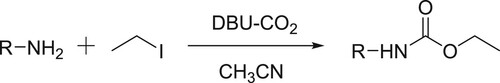
Perez et al. have employed a DBU-CO2 carbamic complex system to carry out selective trans-carboxylation of several amines followed by O-alkylation to give N-alkyl carbamates () (Citation95).
DBU-based ionic liquid was used for the carbonylation of o-phenylenediamines with CO2 to 2-benzimidazolones under solvent-free conditions. In this reaction, the ionic liquid performs dual function with the cation activating CO2 and the anion activating o-phenylenediamines () (Citation96).
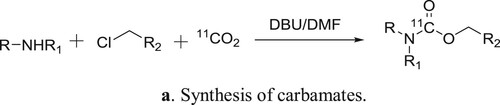
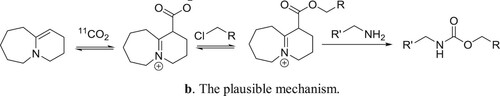
Hooker et al. have used DBU as a catalyst for the one-pot direct incorporation of [11C]CO2 into carbamates in a convenient way () (Citation97). The plausible mechanism is depicted in .
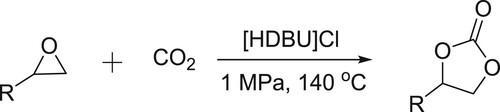
Yang et al. have efficiently been synthesized cyclic carbonates by the reaction of epoxides and carbon dioxide in the presence of DBU-based ionic liquid () (Citation98).
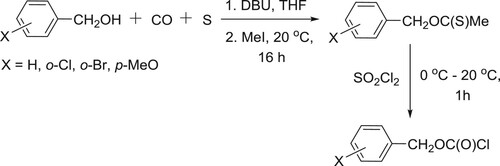
Mizuno et al. have efficiently synthesized S-methyl-O-benzyl carbonothioates by the reaction of carbonylation of benzyl alcohols with carbon monoxide and sulfur (or carbonyl sulfide) in the presence of DBU, followed by the esterification using methyl iodide and chlorination of S-methyl-O-benzyl carbonothioates using sulfuryl chloride () (Citation99). The synthesis of S-alkyl carbonothioates has been reported by the same research group () (Citation100).
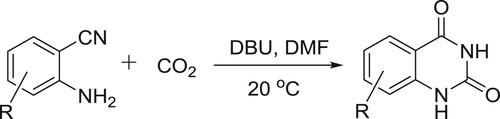
Xiang et al. have efficiently synthesized quinazolinones from carbon dioxide and 2-aminobenzonitriles in the presence of DBU-coupled ionic liquid under mild conditions. This protocol could also be conducted on a gram scale may have promising and practical applications in the synthesis of quinazolinones () (Citation101).
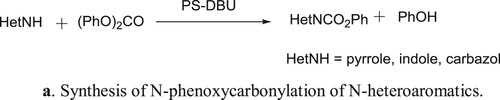
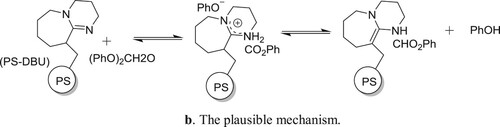
Polymer-bound DBU (PS-DBU, DBU = 1,8-diazabicyclo[5.4.0]undec-7-ene) is an effective and selective catalyst for solvent less N-phenoxycarbonylation of N-heteroaromatics (pyrrole (1), indole (2), and carbazole (3)) with diphenyl carbonate (DPC), used as an eco-friendly active carbonyl species in place of phosgene-derivatives. The immobilized catalyst is less active than unsupported DBU but can be recovered easily at the end of catalytic run and recycled effectively. This method has been reported by Eugenio et al. () (Citation102). The proposed mechanism for the reaction is depicted in .
2.18. Condensation/Cycloaddition/addition reactions
DBU has been employed for a variety of condensation, cycloaddition, and addition reactions.
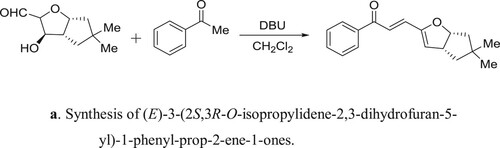
Gangwar et al. have efficiently been synthesized of (E)-3-(2S,3R-O-isopropylidene-2,3-dihydrofuran-5-yl)-1-phenyl-prop-2-ene-1-ones catalyzed by DBU a reaction of 1,2-O-isopropylidene-α-D-xylofuran-1,5-dialdoses with different acetophenones () (Citation103). The proposed mechanism for the reaction is depicted in .
Scheme 85a. Synthesis of (E)-3-(2S,3R-O-isopropylidene-2,3-dihydrofuran-5-yl)-1-phenyl-prop-2-ene-1-ones.
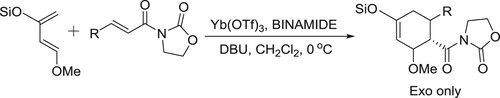
Harada et al. described DBU/Yb(OTf)3/chiral bis-urea complex (BINAMIDE) mediated asymmetric highly enantioselective Diels–Alder reaction of Danishefsky-Type diene and electron-deficient of olefines () (Citation104).
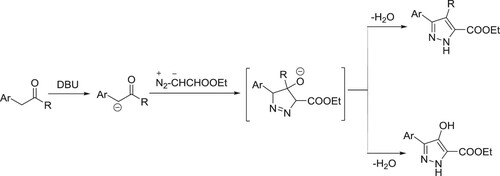
One-pot synthesis of pyrazole-5-carboxylates by 1,3-dipole cycloadditions of ethyl diazoacetate with α-methylene carbonyl compounds catalyzed by DBU has been reported by Gioiello et al. () (Citation105).
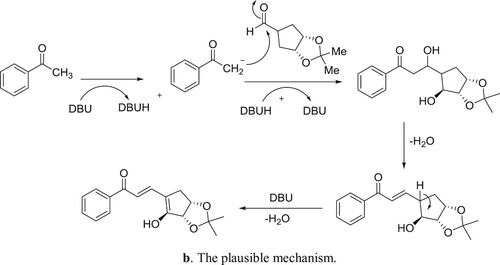
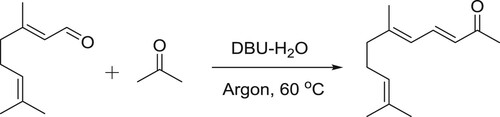
DBU-H2O is an effective catalytic system for aldol condensation reactions. DBU alone is inactive for these types of reactions due to its a Lewis basic characteristic but along with equimolar amount of water, it is transformed into a structure with Bronsted basic characteristics () and thus, catalyzes the reaction () (Citation106).
The Aldol type of reaction of α-keto esters with cyclopent-2-enone catalyzed by DBU has been reported by Shi et al. () (Citation107).
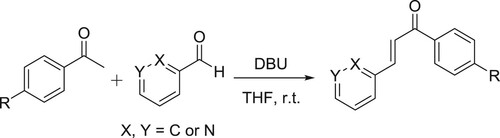
Downs et al. have carried out the DBU-catalyzed Aldol-type condensation reaction to synthesize a new series of 2/3-azachalcones from pyridinecarboxaldehydes () (Citation108).
Shen et al. have described DBU-catalyzed Mukaiyama-Aldol () and Mukaiyama-Michael () reactions of ketene silyl acetals under solvent and metal-free conditions (Citation109).
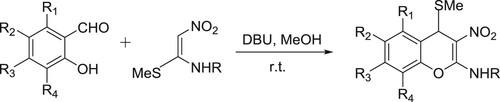
Rao et al. have efficiently synthesized 2-alkylamino-3-nitro-4-alkylsulfanyl-4H-chromenes from the reaction of 2-hydroxybenzaldehydes and nitroketene N, S-acetals catalyzed by DBU () (Citation110).
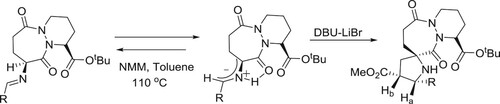
DBU-LiBr catalyzed regio-and stereo-specific cycloaddition of imines of (1S,9S)-t-butyl-9-amino-octahydro-6,10-dioxo-6H-pyridazino[1,2-a][1,2]diazepine-1-carboxylate with a variety of chiral 1,3-dipolarophiles to afford enantiopure spiro-cycloadducts in excellent yields was reported by Dondas et al. () (Citation111).
Mizuno et al. have employed DBU as a catalyst for the reaction of 2-aminobenzonitriles and carbon dioxide to produce 1H-quinazoline-2,4-diones () (Citation112).
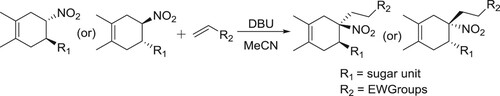
The stereoselective Michael addition reaction between chiral 5-glyco-4-nitrocyclohex-1-enes and several electron-deficient alkenes proceeded in the presence of catalytic amount of DBU was reported by Areces et al. () (Citation113).
Scheme 97. The stereoselective Michael addition reaction between chiral 5-glyco-4-nitrocyclohex-1-enes.
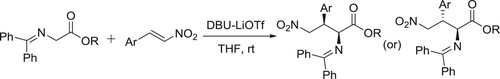
Li et al. have used the DBU-LiOTf catalyzed system for Michael addition of glycine amines to aromatic nitroalkenes. In this reaction, LiOTf acts as an additive () (Citation114).
DBU-catalyzed, a facile and an efficient method for the synthesis of spirocyclic-2-oxindole derivatives with incorporated 6-amino-4H-pyridazines and their fused derivatives was reported by Abdelhamid et al. () (Citation115).
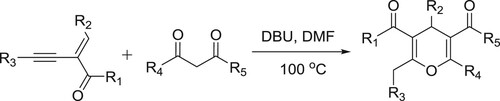
Xiao et al. have reported the catalyst-controlled regio-divergent tandem Michael addition cyclization reaction of 2-(1-alkynyl)-2-alken-1-ones with 1.3-dicarbonyl compounds to afford 4H-pyrans () (Citation116).
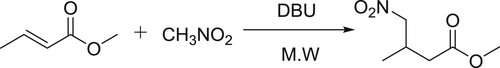
Escalanate et al. have described the synthesis of γ-nitro aliphatic methyl esters using Michael addition reaction of α,β-unsaturated methyl esters with nitro alkanes in the presence of DBU () (Citation117).
DBU has been employed for the Aza-Michael addition reaction of different nitrogen nucleophiles to α,β-unsaturated carbonyl compounds () (Citation118). Aza-Michael addition of aromatic or aliphatic amines to α,β-unsaturated compounds catalyzed by DBU-based ionic liquid under solvent-free conditions have been reported by Ying et al. () (Citation119,Citation120).
Zhang et al. have described DBU-mediated direct stereoselective synthesis of 1-amino-2,5-diarylcyclohexanecarboxylic acid derivatives based on [5 + 1] annulation of divinyl ketones and isocyanoacetate () (Citation121).
Wu et al. have described N-heterocyclic carbene (NHC) catalyzed transformation of 3-halopropenals into equivalent β-acyl-vinyl anionic synthons for the synthesis of cyclic α,β-unsaturated butenolides and pyrazolones in the presence of catalytic amount of DBU () (Citation122).
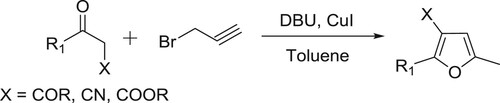
Arcadi et al. have been used a DBU-CuI catalytic system to achieve the reaction of 1,3-dicarbonyl compounds with propargyl bromide to yield 2,3,5-trisubstituted furans via sequential alkylation, cyclization, and isomerization reactions () (Citation123).
DBU has been employed for the reaction of salicylic aldehydes with 3-benzylpenta-3,4-dien-2-one/3-methylpenta-3,4-dien-2-one/ethyl-2-methylbuta-2,3-dienoate to produce corresponding functionalized 2H-1-benzopyrans with good diastereoselectivity () (Citation124).
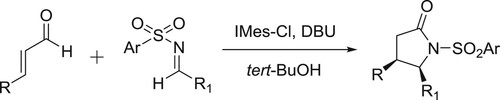
The synthesis of γ-lactams through the direct annulations of enals and N-sulfonyl amines catalyzed by 1,3-bis(2,4,6-trimethylphenyl)-2-chloroimdazolium chloride (IMes-Cl) and in the presence of DBU () (Citation125).
The DBU-catalyzed synthesis of 2-aryl-2H-indazoles via the reaction of 2-nitrobenzyl triphenylphosphonium bromide and aryl isocyanates has been reported by Taher et al. () (Citation126).
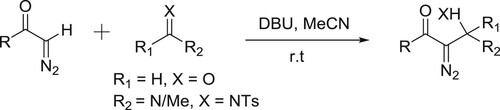
DBU promoted synthesis of β-amino-α-diazocarbonyls/β-hydroxy-α-diazo carbonyls by the reaction of aldehydes/imines and diazomethanes has been reported by Jiang et al. () (Citation127).
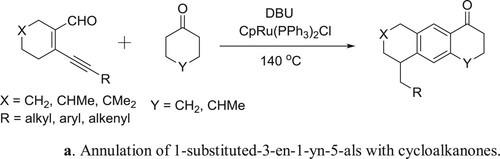
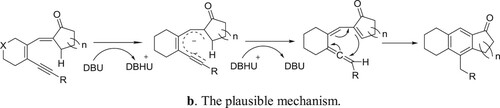
Yang et al. have been employed a DBU-CpRu(PPh3)2Cl system for the one-pot Aldol-condensation aromatization of 3-en-1-yn-5-als with cycloalkanones () (Citation128). The plausible mechanism for the reaction is depicted in .
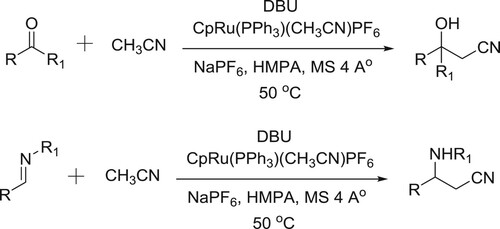
Kumagai et al. have been used DBU-Ru-complex system for the direct incorporation of acetonitrile to imines/aldehydes/activated ketones () (Citation129,Citation130).
Singh et al. have described a highly efficient, recyclable, and eco-friendly protocols for the 1,3-dipolar cycloaddition reaction of different aryl azides with active methylene compounds in the presence of DBU-water system under conventional heating, micro-wave irradiation, and ultrasonic () (Citation131).
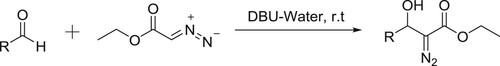
Xiao et al. have efficiently been synthesized β-hydroxy-α-aryl acrylates by DBU-catalyzed condensation of ethyl diazoacetate (EDA) with various aldehydes in water () (Citation132).
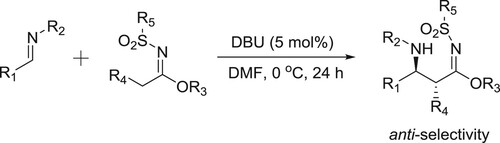
Matsubara et al. have used DBU for the reaction of sulfonylimides treated with various types of N-protected imines to afford β-aminosulfonylimidates in high yields and anti-selectivity () (Citation133).
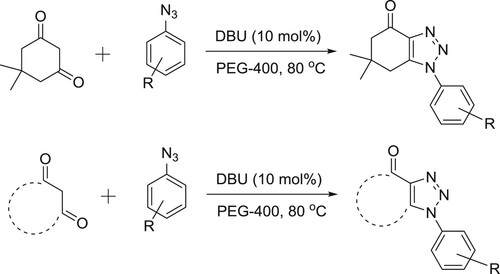
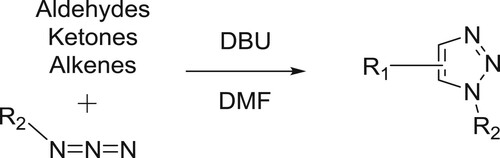
DBU-catalyzed synthesis of fused 1,2,3-triazoles by [3 + 2] cycloaddition of arylazides with activated cyclic C–H acids such as dimedone, 2-hydroxy-naphthalene-1,4-dione, 5-methylcyclohexane-1,3-dione and cyclohexane-1,3-dione in PEG-400 has been reported under heating () (Citation134).
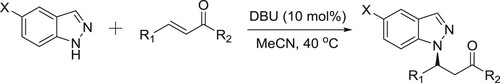
Yang et al. have described a highly efficient DBU-catalyzed aza-Michael reaction of a variety of aromatic and aliphatic enones are well tolerated and afford the corresponding N1-substituted 1H-indazoles in excellent yields with exclusive N1-regioselectivity () (Citation135).
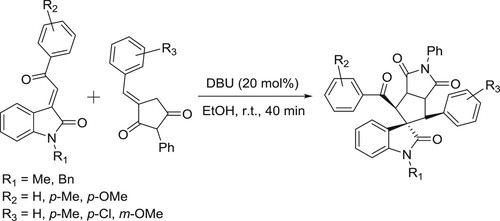
The DBU-catalyzed protocol has been developed by Piyush et al. for the regioselective [3 + 2] cycloaddition reactions of 3-benzylidene succinimides with 3-ylidene oxindoles to furnish spirooxindole derivatives () (Citation136).
Mohammad et al. have described Density functional theory (DFT) methods used to investigate the mechanism of diethylamine-catalyzed cycloaddition reaction of phenyl azide and pentanal. The computational results indicate that the catalyzed cycloaddition reaction is carried out via two one-step transition states that lead to 1,4- and 1,5-regioisomers, and favors the generation of the 1,4-regioisomer. In the absence of a catalyst, the 32CA reactions of azide and alkyne lack poor regioselectivity. Finally, distortion/interaction analysis along the reaction pathways was used to explain the 1,5-regioselectivity () (Citation137).
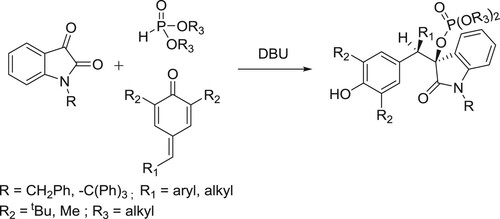
Amjad ali et al. have reported the addition of α-ketoamide to p-quinone methide initiated by dialkylphosphite in the presence of organic base 1,8-diazabicyclo(5.4.0)undec-7-ene (DBU) is explored. Coupling of dialkylphosphites to α-ketoamides in the presence of a base follows [1,2]-phospha-Brook rearrangement, generating corresponding α-phosphonyloxy enolates that are subsequently seized by p-quinone methides (p-QMs). The two-step one-pot 1,6-conjugate addition provides effective access to a series of isatin-incorporated phosphate-bearing 1,6-adducts having two vicinal tertiary carbons with up to 90% yield and >20:1 dr. () (Citation138).
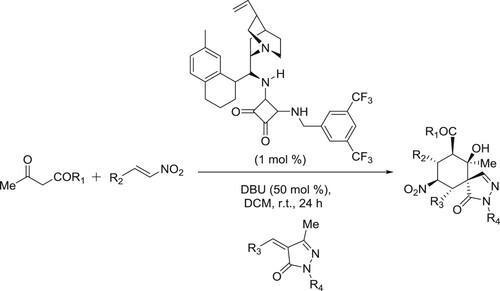
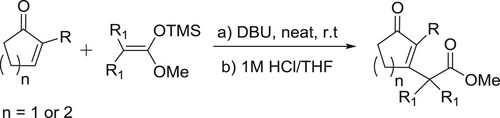
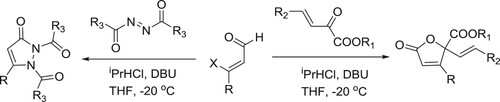
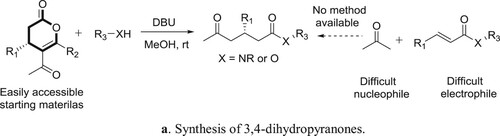
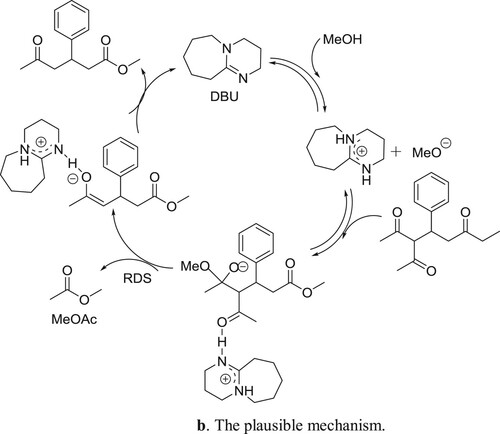
Anton et al. have described a general protocol for the formal Michael addition of acetone to α,β-unsaturated esters and amides, a transformation difficult to perform using current methods. The protocol comprises of an amidine catalyzed relay ring-opening and fragmentation of 3,4-dihydropyranones. The reaction proceeds under mild conditions has a broad substrate scope and the products can be isolated in good to excellent yields. The method can be applied to homochiral substrates with total preservation of chiral information, generating products in high optical purity. Kinetic experiments supported by quantum chemical modeling indicate a mechanism in which the catalyst takes a bifunctional role, acting both as a Brønsted base and as a hydrogen-bond donor () (Citation139). The plausible mechanism is depicted in .
Attilio et al. have succefully reported a fully detailed mechanistic study involving an organocatalyzed 1,3-dipolar cycloaddition via enolate or stabilized vinylogous carbanion intermediates and azide for the synthesis of 1,2,3-triazoles. A detailed investigation of the elementary steps, intermediates, and transition states of the two organocatalyzed metal-free click reactions is supported by DFT calculations and 1H NMR monitoring experiments, providing detailed profiles for both reaction mechanisms. Distortion–interaction activation-strain (DIAS) analysis was also employed to further elucidate the regioselectivity in both reactions () (Citation140).
2.19. Synthesis of carbamic acids and urea
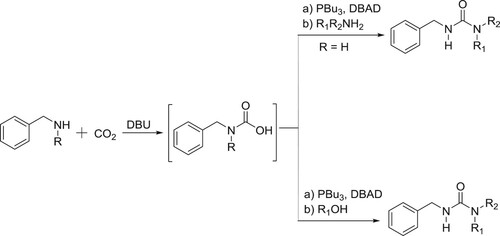
Peterson et al. have employed DBU as the catalyst for the synthesis of carbamates/ureas, by the reaction of in situ generated carbamic acid with amines or alcohols in the presence of Mitsunobu reagent under mild conditions () (Citation141).
2.20. Protected functional group
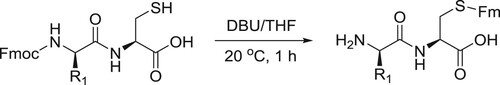
Katritzky et al. have reported DBU-catalyzed transprotection of N-Fmoc (Fmoc: 9-Fluorenylmethyloxycarbonyl) protected cysteine peptides possess free SH groups to afford S-Fm (Fm: 9-Fluorenylmethyl) protected peptides with free amino groups in a convenient way () (Citation142).
2.21. Rearrangement reactions
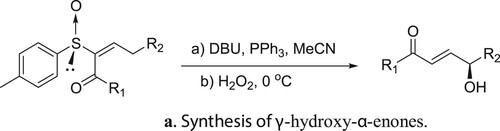
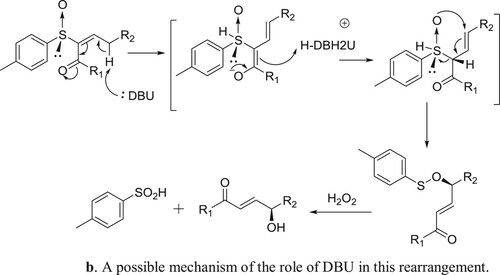
Miura et al. have described DBU catalyzed and triphenyl phosphine-mediated stereoselective asymmetric rearrangement of chiral α-sulfinyl enones and subsequent treatment with aqueous hydrogen peroxide (H2O2) to afford γ-hydroxy-α-enones () (Citation143). A possible mechanism depicting the catalyst role of DBU in this rearrangement reaction is shown in .
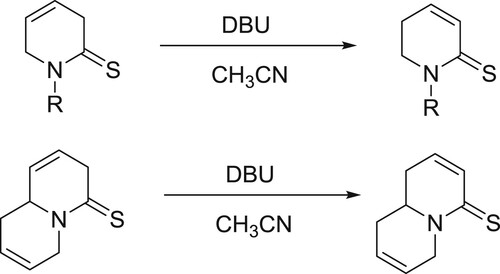
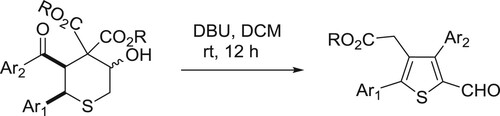
Sathishkannan et al. have been reported DBU-catalyzed system for the rearrangement of tetrahydrothiopyranols for the synthesis of tetrasubstituted thiophenes () (Citation144).
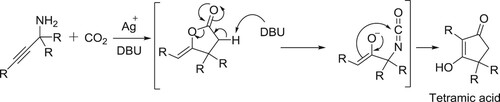
Ishida et al. have reported a new synthetic route for the synthesis of novel tetramic acid derivatives via silver salt catalyzed carbon dioxide insertion into propargylic amines followed by mediated intramolecular rearrangement, in the presence of catalytic amount of DBU () (Citation145).
2.22. Miscellaneous reactions
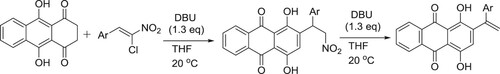
Daniel et al. have efficiently been synthesized a novel series of anthracene-9,10-dione derivatives via a DBU-catalyzed reaction sequence () (Citation146).
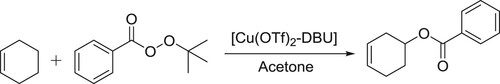
[Cu(OTf)2]-DBU/DBN complex as an efficient catalyst was reported by Sekar et al. for allylic oxidation of olefins with tert-butyl perbenzoate to afford allylic benzoates () (Citation147).
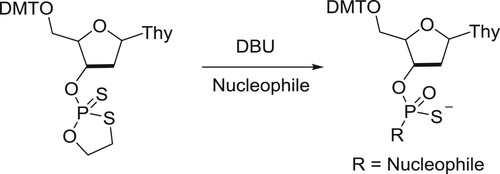
Misiura et al. have described DBU-catalyzed 1,3,2-oxathiaphospholane ring opening condensation reaction with selected C-, O-, S-, and N-nucleophiles () (Citation148).
Scheme 130. 1,3,2-oxathiaphospholane ring opening condensation reaction with selected C-, O-, S-, and N- nucleophiles.
Dehydration of aldoximes to the corresponding nitriles catalyzed by DBU under microwave irradiation has been reported by Sabitha et al. () (Citation149) and decarboxylation of natural 4-hydroxycinnamic acids under microwave irradiation to afford 4-vinylphenols has been reported by Bernini co-workers () (Citation150).
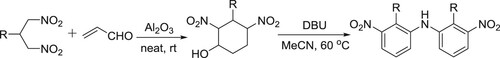
Ballini et al. have efficiently synthesized symmetrical diarylamines from 2-alkyl-1,3-dinitropropanes via two step-synthesis, by involving DBU in the second step, wherein it carries out aromatization () (Citation151).
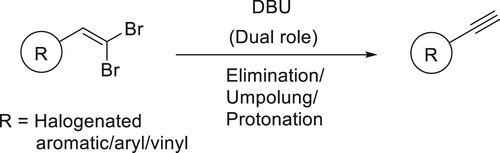
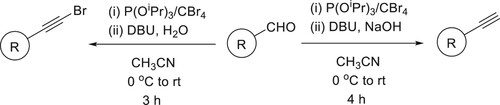
The dual role of the bicyclic amidine base DBU was demonstrated in a synthesis of terminal aryl- and styryl-acetylenes from germinal dibromoalkenes, a tandem process involving elimination/umpolung/protonation occurs in a single step has been reported by Ashok Kumar et al. and Shiva Krishna et al. () (Citation152,Citation153).
Yadagiri et al. have reported a two well-known synthetic reactions Ramirez olefination and Corey-Fuchs reactions are integrated in a one-pot sequential manner for the synthesis of arylacetylenes and 1,3-enynes starting from aldehydes. DBU along with an additive NaOH not only exclusively afforded the terminal alkynes directly from the aldehydes, but also enhanced the rate of reaction. The dynamic nature of DBU also facilitated the isolation of 1-bromoalkynes intermediate products () (Citation154,Citation155).
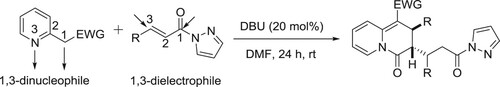
The reaction of 2-pyridylacetates and α,β-unsaturated pyrazolamides with DBU as the catalyst has been developed by Yao et al. A range of unexplored multisubstituted 2,3-dihydro-4H-quinolizin-4-ones are obtained with satisfactory yields (up to 94%) and excellent diastereoselectivities (all cases > 20: 1 dr) via a dearomative [3 + 3] annulation process. This practical method also has few advantages such as transition metal-free, mild reaction conditions, wide functional group tolerance, easy scale-up synthesis, and versatile further derivitizations () (Citation156).
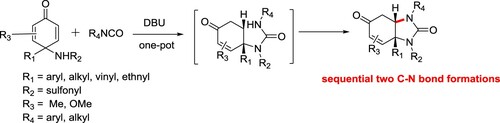
A DBU-catalyzed desymmetrization strategy between cyclohexdienones and isocynates was discovered, affording a series of vicinal diamine-containing heterocycle derivatives excellent diastereoselectivity under mild conditions has been reported by Hongxing et al. This method has advantages such as mild reaction conditions, good yields, excellent diastereoselectivity, most reactions within 30 min, broad substrate scope, this reaction could be performed on a 10 g scale using 1.0 mol% of catalyst loading () (Citation157).
An efficient and facile DBU catalyzed synthesis of highly significant motif 5,7-disubstituted-1,2,4-triazolo[1,5-a]pyrimidines under solvent-free condition has been reported by Yogesh et al. () (Citation158).
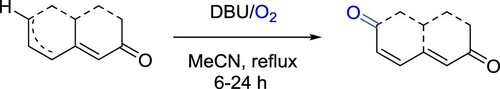
Paresh et al. have described a DBU/O2-promoted novel method for oxidation of dienones to 2,6-dione derivatives. The reaction involves treatment of a dienone with DBU in acetonitrile employing molecular oxygen as the oxidant. This transformation proceeds through a peroxide intermediate that upon Kornblum–DeLaMare rearrangement produces 2,6-diones. The method was successfully utilized for the synthesis of (±)-pleodendione with improved yields versus those of the traditional PDC-TBHP method. Metal-free conditions, an eco-friendly reagent, operationally simplicity are the striking features of this protocol () (Citation159).
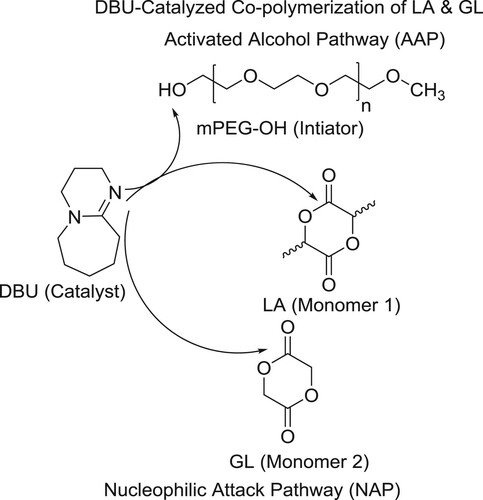
The cyclic organic amidine catalyst, 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU), is gaining popularity for its use in the synthesis of biodegradable aliphatic polyesters, such as poly(lactic-co-glycolic acid) (PLGA). PLGA is one of the most successful polymeric drug delivery materials in the pharmaceutical industry. Currently, commercial PLGA materials are produced via ring-opening copolymerization of lactide and glycolide under the influence of metal catalysts such as tin octoate, and this chemistry has been extensively studied and has been reported by Samruddhi et al. However, not much is known yet about the details of the newer, DBU-catalyzed PLGA polymerization reactions. For this investigation, a full-scale kinetic population balance model was developed that takes into account all possible reactions of the copolymerization, including initiation via activated alcohol and nucleophilic attack pathways, self- and cross-propagation, combination via inter- and intrachain acylation, and DBU deactivation () (Citation160).
3. Conclusions
This review focuses on the catalytic activity of the organic bicyclic amidine base, 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU). DBU catalyst has received a significant importance in recent years due to its interesting ability to conduct organic transformations conveniently and efficiently. It is available commercially, cheap, and a homogenous catalyst. It has been used widely in a variety of reactions, namely amidation, halogenation, condensation, cyclization, esterification, elimination, multicomponent reactions, etc. The benefit of the DBU catalyst has been explored for the practical methodologies useful for the synthesis of a wide variety of organic materials. The selectivity (chemo-, regio-, and stereo-) of these catalysts is highly impressive and applicable in modern chemical transformations. Since chemical transformations catalyzed by DBU have recently been proven to be highly important in organic synthesis.
Acknowledgements
The authors would like to thank the University of Nizwa (UoN) for the generous support of this project.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Ajitha, M.J.; Pary, F.; Nelson, T.L.; Musaev, D.G. ACS Catal. 2018, 8 (6), 4829–4837.
- Venkateswarlu, K. Environ. Chem. Lett. 2021, 19 (5), 3887–3950.
- Zhu, C.; Yue, H.; Jia, J.; Rueping, M. Angew. Chemie Int. Ed. 2021, 60 (33), 17810–17831.
- Liu, H.-W.; Fang, Y.; Wang, S.-Y.; Ji, S.-J. J. Org. Chem. 2020, 85 (5), 3508–3516.
- Maripally, N.; Reddy, V.R.; Donthi, R.; Mutyala, R.; Chandra, R. Tetrahedron Lett. 2020, 61 (9), 151554.
- Marella, R.K.; Madduluri, V.R.; Yu, T.; Venkateswarlu, K.; Kumar, J.V.S.; Sreenivasan, M.; Lakkaboyana, S.K. Mol. Catal. 2021, 507, 111561.
- Sahota, S.; Shah, G.; Ghosh, P.; Kapoor, R.; Sengupta, S.; Singh, P.; Vijay, V.; Sahay, A.; Vijay, V.K.; Thakur, I.S. Bioresour. Technol. Reports. 2018, 1, 79–88.
- Jangid, K.D. Current Green Chem. 2020, 7 (2), 146–162.
- Puleo, T.R.; Sujansky, S.J.; Wright, S.E.; Bandar, J.S. Chem. A Eur. J. 2021, 27 (13), 4216–4229.
- Tewari, N.; Mishra, R.C.; Tiwari, V.K.; Tripathi, R.P. Synlett. 2002, 2002 (11), 1779–1782.
- Reddy, N.S.; Ravinder, K.; Krishnaiah, P.; Venkateswarlu, Y. Synlett. 2001, 2001 (5), 625–626.
- Soloshonok, V.A.; Cai, C.; Hruby, V.J.; Meervelt, L.V.; Yamazaki, T. J. Org. Chem. 2000, 65 (20), 6688–6696.
- Jeyaraj, D.A.; Kapoor, K.K.; Yadav, V.K.; Gauniyal, H.M.; Parvez, M. J. Org. Chem. 1998, 63 (2), 287–294.
- Sutherland, K. J. Chem. Commun. 1997, 0 (3), 325–326.
- Im, Y.J.; Gong, J.H.; Kim, H.J.; Kim, J.N. Bull. Korean Chem. Soc. 2001, 22 (9), 1053–1055.
- Aggarwal, K.; Mereu, A. Chem. Commun. 1999, 22, 2311–2312.
- Shieh, W.-C.; Dell, S.; Repič, O. J. Org. Chem. 2002, 67 (7), 2188–2191.
- Moller, F. German Patent 1545855. Faebenfabriken Bayer AG, 1970.
- Reppe, W.; Mitarbeitern. Justus Liebigs Ann. Chem. 1955, 596 (1), 1–4.
- Price, K.E.; Larrivée-Aboussafy, C.; Lillie, B.M.; McLaughlin, R.W.; Mustakis, J.; Hettenbach, K.W.; Hawkins, J.M.; Vaidyanathan, R. Org. Lett. 2009, 11 (9), 2003–2006.
- Singh, B.K.; Verma, S.S.; Dwivedi, N.; Tripathi, R.P. Tetrahedron Lett. 2006, 47 (13), 2219–2222.
- Bode, J.W.; Sohn, S.S. J. Am. Chem. Soc. 2007, 129 (45), 13798–13799.
- Festa, A.A.; Zalte, R.R.; Golantsov, N.E.; Varlamov, A.V.; Van der Eycken, E.V.; Voskressensky, L.G. J. Org. Chem. 2018, 83 (16), 9305–9311.
- Larrivée-Aboussafy, C.; Jones, B.P.; Price, K.E.; Hardink, M.A.; McLaughlin, R.W.; Lillie, B.M.; Hawkins, J.M.; Vaidyanathan, R. Org. Lett. 2010, 12 (2), 324–327.
- Sharnabai, K.M.; Nagendra, G.; Vishwanatha, T.M.; Sureshbabu, V.V. Tetrahedron Lett. 2013, 54 (6), 478–482.
- Gregory, L.B.; John, R.C.; Rebecca, A.G.; Bahar, I.; Dong, L.; Jun, Q.; Frederick, R.; Eric, M.S.; Steven, R.W. Org. Process. Res. Dev. 2019, 23 (8), 1529–1537.
- Wolff, S.; Huecas, M.E.; Agosta, W.C. J. Org. Chem. 1982, 47 (22), 4358–4359.
- Yadav, A.K.; Singh, B.K.; Singh, N.; Tripathi, R.P. Tetrahedron Lett. 2007, 48 (38), 6628–6632.
- Otter, B.A.; Taube, A.; Fox, J.J. J. Org. Chem. 1971, 36 (9), 1251–1255.
- Zhang, W.-S.; Xu, W.-J.; Zhang, F.; Qu, G.-R. Chinese Chem. Lett. 2013, 24 (5), 407–410.
- Pal, M.; Swamy, N.K.; Hameed, P.S.; Padakanti, S.; Yeleswarapu, K.R. Tetrahedron 2004, 60 (18), 3987–3997.
- Kanazawa, C.; Terada, M. Tetrahedron Lett. 2007, 48 (6), 933–935.
- Llauger, L.; Bergami, C.; Kinzel, O.D.; Lillini, S.; Pescatore, G.; Torrisi, C.; Jones, P. Tetrahedron Lett. 2009, 50 (2), 172–177.
- Dias, A.M.; Cabral, I.; Proença, M.F.; Booth, B.L. J. Org. Chem. 2002, 67 (16), 5546–5552.
- Bensulong, S.; Boonsombat, J.; Ruchirawat, S. Tetrahedron 2013, 69 (44), 9335–9348.
- Yu, X.; Cao, Z.; Zhang, J. Org. Biomol. Chem. 2010, 8 (22), 5059–5061.
- Li, Z.; Sun, H.; Jiang, H.; Liu, H. Org. Lett. 2008, 10 (15), 3263–3266.
- Zhou, H.; Zhu, D.; Xing, Y.; Huang, H. Adv. Synth. Catal. 2010, 352 (13), 2127–2131.
- Yu, H.; Zhang, Z.; Zhang, X.; Xu, Y.; Huo, D.; Zhang, L.; Wang, W. Comp. Theory. Chem. 2022, 1209, 113593.
- Wu, Y.; Hu, Q.; Sun, Y.-P.; Yang, Y.-Q. Tetrahedron Lett. 2004, 45 (41), 7715–7717.
- Shieh, W.-C.; Lozanov, M.; Loo, M.; Repič, O.; Blacklock, T.J. Tetrahedron Lett. 2003, 44 (24), 4563–4565.
- Nishikubo, T.; Iizawa, T.; Takahashi, A.; Shimokawa, T. J. Polym. Sci. Part A Polym. Chem. 1990, 28 (1), 105–117.
- Chen, C.-Y.; Chen, M.-J.; Zhang, X.-Q.; Liu, C.-F.; Sun, R.-C. J. Agric. Food Chem. 2014, 62 (15), 3446–3452.
- Zhao, P.; Yin, H.; Gao, H.; Xi, C. J. Org. Chem. 2013, 78 (10), 5001–5006.
- Hioki, K.; Katsuhiko, T.; Kazuhiro, M.; Norihisa, F.; Kazuhiko, S.; Masahito, U.; Seiji, M.; Satoshi, H.; Jun-Chul, C. ACS Sustain. Chem. Eng. 2022, 10 (17), 5507–5516.
- Muathen, H.A. J. Org. Chem. 1992, 57 (9), 2740–2741.
- Butler, P.; Golding, B.T.; Laval, G.; Loghmani-Khouzani, H.; Ranjbar-Karimi, R.; Sadeghi, M.M. Tetrahedron 2007, 63 (45), 11160–11166.
- Kutsumura, N.; Niwa, K.; Saito, T. Org. Lett. 2010, 12 (15), 3316–3319.
- R. R. Chauvette. U. S. A Pat. 1990, 4906769.
- Zhu, Y.-S.; Wang, W.-B.; Yuan, B.-B.; Li, Y.-N.; Wang, Q.-L.; Bu, Z.-W. Org. Biomol. Chem. 2017, 15 (4), 984–990.
- Sośnicki, J.G. Tetrahedron Lett. 2009, 50 (2), 178–181.
- Sośnicki, J.G. Tetrahedron 2007, 63 (48), 11862–11877.
- Campbell, E.; Martin, J.J.; Bordner, J.; Kleinman, E.F. J. Org. Chem. 1996, 61 (14), 4806–4809.
- Jiahui, D.; Xinwei, H.; Pui, Y.C.; Qi, W.; Mengqing, X.; Ruxue, L.; KeKe, X.; Yongjia, S.; Fuk, Y.K. Org. Lett. 2021, 23 (16), 6455–6460.
- Rimpa, D.; Utsav, S.; Antony, S.; Souvik, M.; Jayanta, N.; Mrinal, K.B. New. J. Chem. 2022, 46, 10603–10610.
- Ando, K.; Yamada, K. Tetrahedron Lett. 2010, 51 (25), 3297–3299.
- Ando, K.; Yamada, K. Green Chem. 2011, 13 (5), 1143–1146.
- Shah, B.A.; Taneja, S.C.; Sethi, V.K.; Gupta, P.; Andotra, S.S.; Chimni, S.S.; Qazi, G.N. Tetrahedron Lett. 2007, 48 (6), 955–960.
- Zhao, Y.-Y.; Zhao, S.; Xie, J.-K.; Hu, X.-Q.; Xu, P.-F. J. Org. Chem. 2016, 81 (21), 10532–10537.
- Ze-liang, H.; Peng, C.; Zhi-Chao, C.; Wei, D.; Ying-Chun, C. Org. Lett. 2022, 24 (1), 100–104.
- Utsumi, N.; Kitagaki, S.; Barbas Carlos, F.I.I.I. Org. Lett. 2008, 10 (16), 3405–3408.
- Kadam, S.T.; Kim, S.S. Tetrahedron 2010, 66 (30), 5647–5652.
- Mazzeo, G.; Longhi, G.; Abbate, S.; Mangiavacchi, F.; Santi, C.; Han, J.; Soloshonok, V.A.; Melensi, L.; Ruzziconi, R. Org. Biomol. Chem. 2018, 16 (45), 8742–8750.
- Chanthavong, F.; Leadbeater, N.E. Tetrahedron Lett. 2006, 47 (12), 1909–1912.
- Ciattini, P.G.; Morera, E.; Ortar, G.; Rossi, S.S. Tetrahedron 1991, 47 (32), 6449–6456.
- Jiang, Y.; Li, X.; Zhao, Y.; Jia, S.; Li, M.; Zhao, Z.; Zhang, R.; Li, W.; Zhang, W. RSC Adv. 2016, 6 (111), 110102–110107.
- Ballini, R.; Bosica, G.; Fiorini, D.; Petrini, M. Tetrahedron Lett. 2002, 43 (30), 5233–5235.
- Raj, I.V.P.; Suryavanshi, G.; Sudalai, A. Tetrahedron Lett. 2007, 48 (40), 7211–7214.
- Gavara, L.; Petit, C.; Montchamp, J.-L. Tetrahedron Lett. 2012, 53 (37), 5000–5003.
- Shieh, W.-C.; Dell, S.; Repič, O. Org. Lett. 2001, 3 (26), 4279–4281.
- Tian, Y.; Shang, Y.; Su, W. Asian J. Org. Chem. 2021, 10 (7), 1718–1721.
- Cordaro, M.; Grassi, G.; Risitano, F.; Scala, A. Synlett. 2009, 5 (1), 103–105.
- Singh, S.; Singh, P.; Rai, V.K.; Kapoor, R.; Yadav, L.D.S. Tetrahedron Lett. 2011, 52 (1), 125–128.
- Zhang, W.; Shi, M. Org. Biomol. Chem. 2006, 4 (9), 1671–1674.
- Shimakawa, Y.; Morikawa, T.; Sakaguchi, S. Tetrahedron Lett. 2010, 51 (13), 1786–1789.
- Yang, L.; Tan, B.; Wang, F.; Zhong, G. J. Org. Chem. 2009, 74 (4), 1744–1746.
- Yoshida, M.; Terai, N.; Shishido, K. Tetrahedron 2010, 66 (46), 8922–8927.
- Rong, L.; Tao, S.; Xia, S.; Liu, L.; Yin, S.; Shi, Y. Res. Chem. Intermed. 2012, 38 (7), 1647–1654.
- Naskar, S.; Banerjee, M.; Hazra, A.; Mondal, S.; Maity, A.; Paira, R.; Sahu, K.B.; Saha, P.; Banerjee, S.; Mondal, N.B. Tetrahedron Lett. 2011, 52 (13), 1527–1531.
- Khurana, J.M.; Nand, B.; Saluja, P. J. Heterocycl. Chem. 2014, 51 (3), 618–624.
- Singh, S.K.; Singh, K.N. Monatshefte für Chemie - Chem. Mon. 2012, 143 (5), 805–808.
- Ahadi, S.; Zolghadr, M.; Khavasi, H.R.; Bazgir, A. Org. Biomol. Chem. 2013, 11 (2), 279–286.
- Tiwari, V.K.; Singh, D.D.; Hussain, H.A.; Mishra, B.B.; Singh, A. Monatshefte für Chemie - Chem. Mon. 2008, 139 (1), 43–48.
- Tiwari, V.K.; Singh, A.; Hussain, H.A.; Mishra, B.B.; Tripathi, V. Monatshefte für Chemie - Chem. Mon. 2007, 138 (7), 653–658.
- Qi, C.; Huang, L.; Jiang, H. Synthesis (Stuttg). 2010, 2010 (9), 1433–1440. doi:10.1055/s-0029-1218675.
- Mamgain, R.; Singh, R.; R, D.S. J. Heterocycl. Chem. 2009, 46, 69–73.
- Khurana, J.M.; Nand, B.; Saluja, P. Tetrahedron 2010, 66 (30), 5637–5641.
- Zonouzi, A.; Mirzazadeh, R.; Safavi, M.; Kabudanian Ardestani, S.; Emami, S.; Foroumadi, A. . Iran. J. Pharm. Res. (IJPR) 2013, 12 (4), 679–685.
- Chauhan, P.; Mahajan, S.; Loh, C.C.J.; Raabe, G.; Enders, D. Org. Lett. 2014, 16 (11), 2954–2957.
- Singh, S.; Lal, J. SN Appl. Sci. 2020, 2 (7), 1271.
- Zhao, Y.; Yu, B.; Yang, Z.; Zhang, H.; Hao, L.; Gao, X.; Liu, Z. Angew. Chemie Int. Ed. 2014, 53 (23), 5922–5925.
- Wang, X.; Ling, G.; Xue, Y.; Lu, S. European J. Org. Chem. 2005, 2005 (8), 1675–1679.
- Bernini, R.; Mincione, E.; Crisante, F.; Barontini, M.; Fabrizi, G.; Gentili, P. Tetrahedron Lett. 2007, 48 (39), 7000–7003.
- Dumbris, S.M.; Díaz, D.J.; McElwee-White, L. J. Org. Chem. 2009, 74 (22), 8862–8865.
- Pérez, E.R.; da Silva, M.O.; Costa, V.C.; Rodrigues-Filho, U.P.; Franco, D.W. Tetrahedron Lett. 2002, 43 (22), 4091–4093.
- Yu, B.; Zhang, H.; Zhao, Y.; Chen, S.; Xu, J.; Hao, L.; Liu, Z. ACS Catal. 2013, 3 (9), 2076–2082.
- Hooker, J.M.; Reibel, A.T.; Hill, S.M.; Schueller, M.J.; Fowler, J.S. Angew. Chemie Int. Ed. 2009, 48 (19), 3482–3485.
- Yang, Z.-Z.; He, L.-N.; Miao, C.-X.; Chanfreau, S. Adv. Synth. Catal. 2010, 352 (13), 2233–2240.
- Mizuno, T.; Takahashi, J.; Ogawa, A. Tetrahedron 2002, 58 (50), 10011–10015.
- Mizuno, T.; Nishiguchi, I.; Hirashima, T.; Ogawa, A.; Kambe, N.; Sonoda, N. Tetrahedron Lett. 1988, 29 (37), 4767–4768.
- Gao, X.; Liu, J.; Liu, Z.; Zhang, L.; Zuo, X.; Chen, L.; Bai, X.; Bai, Q.; Wang, X.; Zhou, A. RSC Adv. 2020, 10 (20), 12047–12052.
- Eugenio, Q.; Antonella, A.; Marianna, C.; Angela, D.; Valentina, M. ACS Catal. 2014, 4 (1), 195–202.
- Gangwar, P.; Sharma, A.; Tripathi, R.P. Trends Carbohydrr. Res. 2010, 2, 42–55.
- Harada, S.; Toudou, N.; Hiraoka, S.; Nishida, A. Tetrahedron Lett. 2009, 50 (40), 5652–5655.
- Gioiello, A.; Khamidullina, A.; Fulco, M.C.; Venturoni, F.; Zlotsky, S.; Pellicciari, R. Tetrahedron Lett. 2009, 50 (44), 5978–5980.
- Cota, I.; Chimentao, R.; Sueiras, J.; Medina, F. Catal. Commun. 2008, 9 (11), 2090–2094.
- Shi, M.; Zhang, W. . Tetrahedron 2005, 61 (50), 11887–11894.
- Downs, L.E.; Wolfe, D.M.; Schreiner, P.R. Adv. Synth. Catal. 2005, 347 (2–3), 235–238.
- Shen, Z.-L.; Ji, S.-J.; Loh, T.-P. Tetrahedron Lett. 2005, 46 (3), 507–508.
- Rao, H.S.P.; Geetha, K. Tetrahedron Lett. 2009, 50 (27), 3836–3839.
- Dondas, H.A.; Grigg, R.; Kilner, C. . Tetrahedron 2003, 59 (43), 8481–8487.
- Mizuno, T.; Ishino, Y. . Tetrahedron 2002, 58 (16), 3155–3158.
- Areces, P.; Gil, M.V.; Higes, F.J.; Román, E.; Serrano, J.A. Tetrahedron Lett. 1998, 39 (46), 8557–8560.
- Li, W.; Liu, H.; Du, D.M. Synlett. 2009, 2009 (6), 925–928.
- Abdelhamid, I.A.; Mohamed, M.H.; Abdelmoniem, A.M.; Ghozlan, S.A.S. . Tetrahedron 2009, 65 (48), 10069–10073.
- Xiao, Y.; Zhang, J. Chem. Commun. 2009, 2009 (24), 3594–3596.
- Escalante, J.; Díaz-Coutiño, F.D. Synthesis of γ-Nitro Aliphatic Methyl Esters Via Michael Additions Promoted by Microwave Irradiation. Molecules 2009, 14 (4), 1595–1604.
- Yeom, C.-E.; Kim, M.J.; Kim, B.M. Tetrahedron 2007, 63 (4), 904–909.
- Ying, A.-G.; Liu, L.; Wu, G.-F.; Chen, G.; Chen, X.-Z.; Ye, W.-D. Tetrahedron Lett. 2009, 50 (14), 1653–1657.
- Ying, A.G.; Wang, L.-M.; Deng, H.-X.; Chen, J.-H.; Chen, X.-Z.; Ye, W.-D. Arkivoc 2009, XI, 288–298.
- Zhang, D.; Xu, X.; Tan, J.; Liu, Q. Synlett. 2010, 2010 (6), 917–920.
- Wu, Y.; Yao, W.; Pan, L.; Zhang, Y.; Ma, C. Org. Lett. 2010, 12 (3), 640–643.
- Arcadi, A.; Cerichelli, G.; Chiarini, M.; Di Giuseppe, S.; Marinelli, F. Tetrahedron Lett. 2000, 41 (47), 9195–9198.
- Zhao, G.-L.; Shi, Y.-L.; Shi, M. Org. Lett. 2005, 7 (20), 4527–4530.
- He, M.; Bode, J.W. Org. Lett. 2005, 7 (14), 3131–3134.
- Taher, A.; Ladwa, S.; Thirumalai Rajan, S.; Weaver, G.W. Tetrahedron Lett. 2000, 41 (50), 9893–9897.
- Jiang, N.; Wang, J. Tetrahedron Lett. 2002, 43 (7), 1285–1287.
- Yang, C.-W.; Liu, R.-S. Tetrahedron Lett. 2007, 48 (33), 5887–5889.
- Kumagai, N.; Matsunaga, S.; Shibasaki, M. Tetrahedron 2007, 63 (35), 8598–8608.
- Kumagai, N.; Matsunaga, S.; Shibasaki, M. Chem. Commun. 2005, 2005 (28), 3600–3602.
- Singh, H.; Sindhu, J.; Khurana, J.M. RSC Adv. 2013, 3 (44), 22360–22366.
- Xiao, F.; Liu, Y.; Wang, J. Tetrahedron Lett. 2007, 48 (7), 1147–1149.
- Matsubara, R.; Kobayashi, S. Synthesis (Stuttg) 2008, 2008 (18), 3009–3011.
- Singh, H.; Khanna, G.; Khurana, J.M. Tetrahedron Lett. 2016, 57 (29), 3075–3080.
- Yang, J.; Bao, Y.; Zhou, H.; Li, T.; Li, N.; Li, Z. Synthesis. (Mass) 2016, 48 (8), 1139–1146.
- Tehri, P.; Peddinti, R.K. Org. Biomol. Chem. 2019, 17 (16), 3964–3970.
- Al-Hakim Badawi, M.A. Comp. Theory. Chem. 2022, 1209, 113593.
- Amjad, A.; Raveena, J.; Harish, K.H.; Ravi, P.S. J. Org. Chem. 2022, 87 (8), 5228–5213.
- Anton, A.; Emmelie, H.; Martin, R.; Henrik, S. Eur. J. Org. Chem. 2020, 7, 5436–5444.
- Attilio, C.N.; Kelly, C.S.; Maiara da, S.S.; Tulio, J.A.; Antonio, G.F.; Guilherme, A.M.J.; Claudio, F.T.; Marcio, W.P.; Marco, A.B.F. Org. Biomol. Chem. 2022, 20, 6019–6026.
- Peterson, S.L.; Stucka, S.M.; Dinsmore, C.J. Org. Lett. 2010, 12 (6), 1340–1343.
- Katritzky, A.R.; Abo-Dya, N.E.; Abdelmajeid, A.; Tala, S.R.; Amine, M.S.; El-Feky, S.A. Org. Biomol. Chem. 2011, 9 (2), 596–599.
- Miura, M.; Toriyama, M.; Kawakubo, T.; Yasukawa, K.; Takido, T.; Motohashi, S. Org. Lett. 2010, 12 (17), 3882–3885.
- Sathishkannan, G.; Srinivasan, K. Chem. Commun. 2014, 50 (31), 4062–4064.
- Ishida, T.; Kobayashi, R.; Yamada, T. Org. Lett. 2014, 16 (9), 2430–2433.
- Dauzonne, D.; Fouris, S. Tetrahedron 1993, 49 (39), 8865–8876.
- Sekar, G.; DattaGupta, A.; Singh, V.K. Tetrahedron Lett. 1996, 37 (46), 8435–8436.
- Misiura, K.; Szymanowicz, D.; Olesiak, M.; Stec, W.J. Tetrahedron Lett. 2004, 45 (22), 4301–4305.
- Sabitha, G.; Syamala, M. Synth. Commun. 1998, 28 (24), 4577–4580.
- Bernini, R.; Mincione, E.; Barontini, M.; Provenzano, G.; Setti, L. Tetrahedron 2007, 63 (39), 9663–9667.
- Ballini, R.; Barboni, L.; Femoni, C.; Giarlo, G.; Palmieri, A. Tetrahedron Lett. 2006, 47 (14), 2295–2297.
- Morri, A.K.; Thummala, Y.; Doddi, V.R. Org. Lett. 2015, 17 (18), 4640–4643.
- Krishna Moodapelly, S.; Sharma, G.V.M.; Ramana Doddi, V. Adv. Synth. Catal. 2017, 359 (9), 1535–1540.
- Thummala, Y.; Karunakar, G.V.; Doddi, V.R. Adv. Synth. Catal. 2019, 361 (3), 611–616.
- Thummala, Y.; Morri, A.K.; Karunakar, G.V.; Doddi, V.R. European J. Org. Chem. 2018, 2018 (45), 6280–6285.
- Yao, B.S.; Jian, Q.Z.; Zhen, H.W.; Yong, Y.; Ming, Q.Z.; Wei, C.Y. Org. Chem. Frontiers. 2022, 9, 88–84.
- Hongxing, J.; Changhang, D.; You, H. Org. Lett. 2018, 20 (16), 5006–5009.
- Yogesh, K.P.; Anu, M.; Pratibha, R.; Jaya, S.; Jagdamba, S.; Ramendra, K.S. Curr. Org. Synt. 2020, 17 (1), 73–80.
- Paresh, R.A.; Hanuman, P.K.; Reddy, D.S. J. Org. Chem. 2021, 86 (13), 9200–9205.
- Samruddhi, P.; Jin, Y.; You-Yeon, W. Ind. Engg. Chem. Res. 2021, 60 (41), 14685–14700.

![Scheme 6. Synthesis of 1-alkoxypyrazino [1,2-a] indoles.](/cms/asset/03561ac3-5b9f-4cc0-80a4-9cc7c6dfb408/tgcl_a_2132836_f0007_ob.jpg)

![Scheme 26. Synthesis of poly(p-nitrobenzylmethylacrylate) and poly[4-(4-nitrobenzyloxy)styrene].](/cms/asset/84689dc2-8a23-4a13-8d15-81c0b8664c18/tgcl_a_2132836_f0027_ob.jpg)

![Scheme 38. Synthesis of functionalized benzo[c]chromen-6-one core.](/cms/asset/97ab4ed0-2e4f-43dc-bfb8-785e3852d95f/tgcl_a_2132836_f0039_ob.jpg)
![Scheme 43. Synthesis of 1,2,3,4-tetrahydrodibenzo[b,d]furan motifs.](/cms/asset/28e314a6-f330-4068-bd1d-1ac3518f6c60/tgcl_a_2132836_f0044_ob.jpg)

![Scheme 61. Synthesis of 2-amino-4-aryl-6,7,8,9-tetrahydro-5H-benzo[7]annu-lene-1,3-dicarbonitriles.](/cms/asset/806fcfce-5924-4784-baea-84c3b8146fa5/tgcl_a_2132836_f0062_ob.jpg)
![Scheme 62. Synthesis of pyrrolo[2,1-a]isoquinolines.](/cms/asset/f9569254-0f8a-4cad-bfbd-beedba96e646/tgcl_a_2132836_f0063_ob.jpg)
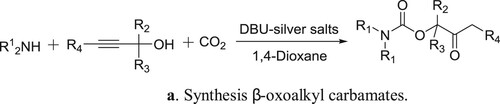
![Scheme 72. Synthesis of 4H-pyrimido[2,1-b]benzothiazoles.](/cms/asset/c51737fd-ca2a-4b8a-9b35-33ef60fadd44/tgcl_a_2132836_f0073_ob.jpg)





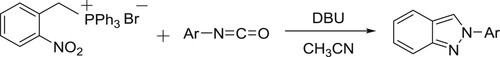

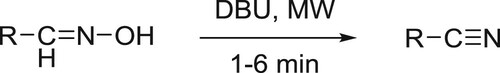

![Scheme 138. Synthesis of 5,7-disubstituted-1,2,4-triazolo[1,5-a]pyrimidines.](/cms/asset/49c329b6-c5ae-4bfe-87ac-2729f9ebc6a8/tgcl_a_2132836_f0139_ob.jpg)