ABSTRACT
A perspective on incorporating the United Nations Sustainable Development Goals (UNSDG) and Green Chemistry Principles (GCP) into high school chemistry curricula is presented. The framework is based upon the Johnstone-Mahaffy model for chemistry understanding which specifically links student learning to the affective or human-centred domain. Reference is made to the origins of high school chemistry curricula, recommendations from the recent Royal Society of Chemistry survey Green Shoots: A Sustainable Chemistry Curriculum for a Sustainable Planet, student engagement practices, and studies in adolescent mental health. The origins and organization of the UNSDG and GCP are outlined, with the similarities to high school chemistry curricula illustrated. Justification for the implementation of the UNSDG and GCP is given, with a specific example presented in the context of the International Baccalaureate chemistry curriculum, and the GCP are rendered in educator- and student-appropriate language.
GRAPHICAL ABSTRACT
Introduction
During his September 1940 address, commemorating the bicentennial of the University of Pennsylvania, United States President Franklin D. Roosevelt decried the rise of world tyranny and reaffirmed a nation’s role in developing future citizens: ‘We cannot always build the future for our youth, but we can build our youth for the future’ (Citation1). The future – or present – for which our youth must be built includes accelerating climate change (Citation2), record low water levels in many of the Earth’s major river systems (Citation3), increased food insecurity (Citation4), and increasing concern over poly- and perfluoroalkyl substances (Citation5). The youth themselves recognize the future they face and the tools with which we should equip them. A 2021 Royal Society of Chemistry (RSC) survey of how climate change and sustainability are taught in science and chemistry revealed that both educators and students felt ‘sustainability and climate change are not prioritised enough in chemistry lessons or career guidance’ (Citation6). More revealing, the survey established that ‘Young people are worried about climate change and want to take action. Four in five young people feel climate change is an urgent priority to solve and three in four are actively looking for ways to help’ (Citation6). The concerns revealed in the RSC survey are supported by current research in mental health. A recent large-scale study (N = 10,000) of adolescents and young adults (aged 16–25 years) quantified that climate change-related anxiety cuts across cultural and socio-economic lines (Citation7), and a second eight-year longitudinal study (N = 2244) indicated that 62.1% of adolescents experienced increasing, moderate, or high persistent climate change-related worry (Citation8). Worry and anxiety themselves are not problematic: rather, where these emotions situate a student on the continuum between hope and despair correlates with a student acting on those emotions or not (Citation9).
The twenty-year transition of high school chemistry curricula from an extractive to sustainable worldview is illustrated by the Netherlands Ministry of Education, Culture and Science reform. A study commissioned in 2002 found the national pre-university curriculum was largely unchanged from the 1970s, was based upon chemistry from the nineteenth century and the first half of the twentieth century (Citation10) and had its origins in the first chemistry curriculum introduced in 1848 (Citation11). For an overview of further curricular and pedagogical advances the reader is directed to papers by Eilks (Citation12) and Kolopajlo (Citation13). Nevertheless, a 2022 editorial in Nature called for more rapid implementation of green - and sustainability-focused instruction in school and post-secondary chemistry education (Citation14).
The United Nations Sustainable Development Goals (UNSDG) and Green Chemistry Principles (GCP) serve as guiding frameworks for governments and corporations to generate wealth, while sustaining planet Earth. These frameworks assume: (a) that science and industry are essential engines of economic and social advancement; and (b) the pace of growth cannot outstrip Earth’s capacity to re-provision itself. The 17 UNSDG were developed out of several UN committees and conferences between 1992 and 2015, culminating in the 2030 Agenda for Sustainable Development. The UNSDG are positioned as
an urgent call for action by all countries – developed and developing – in a global partnership. They recognize that ending poverty and other deprivations must go hand-in-hand with strategies that improve health and education, reduce inequality, and spur economic growth – all while tackling climate change and working to preserve our oceans and forests. (Citation15)
The 12 GCP were codified by Paul Anastas and John Warner, in their 1998 book Green Chemistry: Theory and Practice (Citation16). These industrial chemistry design principles were the consequence of a shift in governmental oversight, enacted by the United States Pollution Prevention Act of 1990 (Citation17), which focused industry on mitigating pollution at its source, rather than through remediation. Since that time, GCP have played a central role in five Nobel Prizes in Chemistry (2001, 2005, 2010, 2021, and 2022) (Citation18–22). In terms of GCP in education, John Warner established the first Green Chemistry Ph.D. program in 1997 at the University of Massachusetts at Boston, and co-founded Beyond Benign, a non-profit organization devoted to supporting K-20 educators. The promise of GCP in chemistry education was best captured by their co-creator Paul Anastas: ‘(c)urricula based on the 12 Principles of Green Chemistry cast the field of chemistry in an entirely different light. Hazard and waste become recognized as design flaws or, more positively, as opportunities for innovation’ (Citation23). Given that chemistry is typically introduced during the final years of publicly mandated education, high school curricula provide the greatest opportunity for infusing the UNSDG and GCP. Considering just the four largest principally English-language educational systems (the United States of America, the United Kingdom, Canada, and Australia), each of these countries mandate education to at least an age of 16 years, or its equivalent (Citation24–27). The central challenge to implementing the UNSDG and GCP in these curricula are the academic, bureaucratic, and technocratic dialects of governments and corporations. For the UNSDG to be successfully connected to the GCP in pursuit of sustainability, this union needs to be directed through publicly mandated education.
This paper presents the UNSDG and GCP in terms accessible to high school chemistry educators and students. It seeks to provide a perspective that shifts the current extractive worldview, where chemical reaction success is measured by yield of product, with a regenerative worldview, where success is achieved by optimization of resources, minimization of waste, and mitigation of hazard. Consequently, a ‘this and’ remedy to address student needs is suggested, rather than a ‘this or’ excision of time-tested chemistry learning objectives. The paper structure is intended to utilize high school chemistry educators’ prior knowledge with curriculum design and implementation to support them in fulfilling the objectives of these two sustainability frameworks. This is done by:
outlining the organization of the UNSDG and GCP,
illustrating similarities between their organization and that of high school curricula,
justifying the UNSDG and GCP in guiding high school chemistry instruction,
rendering the UNSDG and GCP in educator- and student-appropriate language, and
providing an example for integrating the UNSDG and GCP into a high school curriculum, specifically the International Baccalaureate (IB).
Similarities between the UNSDG and high school chemistry curricula
Each of the UNSDG are structured by Goal, Target, and Indicator (). Through data mining, the United Nations compiles relevant statistics sources (e.g. national Education Ministry databases) into a proprietary metadata set which serves to connect related Goals. For example, Goal 12 is linked to Goals 4 and 13 through an identically worded indicator that differs only in number designation. The indicator serves as a measure of success in fulfilling the Goal and Target, simultaneously linking the Goals. The UNSDG are organized similarly to regional, national, and international chemistry curricular documents. Each of the Goals are analogous to an overall learning standard, encountered by high school educators, such as ‘By the end of this course, students will investigate organic compounds and organic chemical reactions, and use various methods to represent the compounds’ (Citation28). An overall standard is broken down into several specific expectations, such as ‘By the end of this course, students will build molecular models for a variety of simple organic compounds’ (Citation28), and finally an indicator of how student learning success will be measured is devised, such as ‘the student can correctly build at least four models provided five simple condensed structural formulas.’ Furthermore, the overall and specific standards connect to standards for related core ideas. For the example provided above this includes structure (applying VSEPR theory to differentiate between trigonal planar and tetrahedral carbon), energetics (bond enthalpy calculations), and physical properties (trends in a homologous series).
Table 1. Comparison of the UNSD Goals 4, 12, and 13 (Citation29).
The preceding example reveals how high school chemistry educators are ideally suited to support implementation of these UNSDG in terms of their (a) familiarity with national education policies; and (b) expertise in scope and sequence of curriculum delivery and student assessment. The intent of the UN metadata positions the high school educator as a critical actor in implementing the UNSDG. In reference to the common 4.7.1/12.8.1/13.3.1 indicator, ‘(i)t measures what governments intend and not what is implemented in practice in schools and classrooms’ (Citation30).
Justification for GCP in high school chemistry curricula
The 150-year history of science curricular reform in the United States, and chemistry in particular, has been thoroughly reviewed elsewhere (Citation31–33). The reforms can be generalized for the purposes of this chapter by four foci: subject, society, student, and technology, with each focus driven by stakeholders in science education. Among the most influential of these were the American Chemical Society, government and non-government policy organizations, educational psychologists and theorists, and corporations (Citation31–33). Each stakeholder influenced science education for a multitude of reasons: to safeguard the intellectual integrity of science; to foster a pool of incipient post-secondary students and future STEM professionals; to enact reforms and the means for measuring their success; and to enact technological progress within industry and education.
Apart from cyclical changes in focus between the needs of our technological society (e.g. The Sputnik Correction (Citation34), the National Defence Education Act (Citation35)) and those of students (e.g. humanistic science educationFootnote1), chemistry curricula maintain an extractive worldview with a few notable exceptions, among them the IB (Citation37), Massachusetts (Citation38), Australian national (Citation39), Hong Kong (Citation40), North Rhine-Westphalia (Citation41), and Netherlands national (Citation42). Although sustainability themes do appear in the Next Generation Science Standards (Citation43), they are presented in the Earth and Space Science domain, rather than the Physical Science, which contains standards conventionally associated with chemistry. The GCP offer an alternative means for measuring the objectives and outcomes of chemistry education based upon sustainability principles (Citation44). Consequently, through their focus on sustainability, the GCP can serve the objectives of the UNSDG, while ensuring that jurisdictional learning objectives are met.
The language of the UNSDG and GCP
Though the UNSDG and GCP are intended for a wide variety of stakeholders, they were originally written for expert audiences outside of high school chemistry education. Educators who are specifically trained as chemistry subject specialists serve to translate the experts’ body of knowledge to novice learners: the chemistry students. This gap between the subject expert and the novice came to the forefront of chemistry education through the separate work of Herron (Citation45) and Johnstone (Citation46). More recently, Mahaffy (Citation47) introduced humanist pedagogy to Johnstone’s content- and concept-driven model (). In short, Johnstone and Mahaffy’s model for chemistry learning can be broken down into four domains:
‘Atomic and molecular-level’ – molecular.
‘Descriptive and functional-level’ – macroscopic.
‘Representational-level’ – symbolic.
‘Student-centred’ – human element.
Figure 1. Reprinted with permission from Ref. (Citation47). Copyright 2006 American Chemical Society.
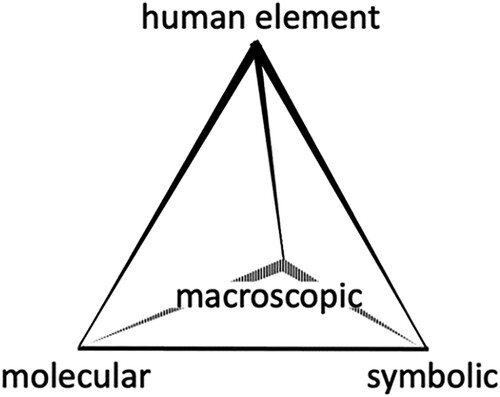
Based upon one author’s experience (K.C.H) in teaching chemistry from middle school through second-year university, the following four axioms transfer across these levels of chemistry education (correlations to the Johnstone-Mahaffy conceptual framework are included):
Chemicals are made of atoms – molecular
Atoms are countable – macroscopic
Chemicals can be dangerous or innocuous – symbolic
Chemicals can be challenging or expensive to obtain – human element
In Canadian curricula (Citation49–51), mole-mass relationships are generally introduced in Grades 10 or 11, although extensive properties (mass, volume) are familiar to elementary school students. Atomic theory is usually introduced in Grades 9 or 10. Hazard systems, such as the United Nations Globally Harmonized System of Classification and Labelling of Chemicals (GHS), the American Hazard Communication Standard (HCS), and the European Union Classification, Labelling and Packaging Regulation (CLP), are generally taught in Grades 8 or 9, and humanist themes, specifically with a sustainability focus, are often introduced earlier. These four axioms were chosen because they are apparent across existing curricula. Furthermore, three of the axioms can be used to group the GCP at a novice-level of understanding, according to Mahaffy’s extension of Johnstone’s model ().
Table 2. Correlation between the Johnstone-Mahaffy conceptual framework and GCP.
Axiom A is best articulated by one of science’s broadest and most successful communicators, Richard Feynman: ‘If we were to name the most powerful assumption of all, which leads one on and on in an attempt to understand life, it is that all things are made of atoms … ’ (Citation52). Furthermore, because axiom A provides an essential conceptual framework that underpins all understanding in chemistry, it is applicable to all GCP. For novice learners the GCP can be summarized into three groups:
account for the quantity of chemicals used and remaining in a process
utilize and synthesize chemicals that present the least harm, and
either recover as much as possible, or waste as little chemicals as possible.
In other words, middle and high school chemistry can be taught through GCP 1, 2, and 3, while capturing the intent of all twelve GCP, for which there is preliminary evidence (Citation44). These novice-level groupings of the GCP align with several Targets of UNSDG 12 (). The rationale behind these alignments is as follows: Targets 12.3 and 12.6 involve measurement, which is introduced early in K-12 education, with data processing; Target 12.4 involves hazard management; and Targets 12.2 and 12.5 involve recovering chemicals or minimizing waste.
Table 3. Alignment between novice GCP groupings and targets of the UNSDG 12.
An example: UNSDG, GCP and the IB Chemistry curriculum
IB Chemistry is one of the curricula, listed earlier, well-suited to the UNSDG, given its student-centred, international, and sustainability focus, including explicit GCP standards. Indicator 4.7.1/12.8.1/13.3.1 specifically targets global citizenship and education for sustainable development, both of which figure prominently in the IB Chemistry curriculum. Apart from organization around accepted content and sequence, shared by most school chemistry curricula, the IB Chemistry curriculum is additionally organized around the Nature of Science (NoS). An established component of contemporary scientific literacy, NoS serves as a humanistic and epistemological framework to help students understand the strengths and limitations of science as knowledge and knowing, and the collaborative nature of the scientific endeavor. As stated by the National Research Council:
Epistemic knowledge is knowledge of the constructs and values that are intrinsic to science. Students need to understand what is meant, for example, by an observation, a hypothesis, an inference, a model, a theory, or a claim and be able to distinguish among them. (Citation53)
The IB program delineates NoS through five strands, which are further delineated through ten Aims, characteristic of a humanistic, or student-centred, curriculum. Specifically, Aim 8 is consistent with UNSDG Goal 12: ‘become critically aware, as global citizens, of the ethical implications of using science and technology’ (Citation34). The most robust connection between the UNSDG and the IB Chemistry curriculum are found between Goal 12, Target 12.8, and Aim 8, as outlined in . The curriculum document further states, in reference to the international nature of scientific endeavor, that IB Chemistry students
need to be aware of the moral responsibility of scientists to ensure that scientific knowledge and data are available to all countries on an equitable basis and that they have the scientific capacity to use this for developing sustainable societies. (Citation37)
Table 4. Alignment between the UNSDG and the IB Chemistry Curriculum.
Two curricular standards from the IB Chemistry curriculum specifically demonstrate how the aims of the UNSDG and GCP can be met through high school chemistry learning. Sub-topic 20.1, ‘Types of organic reactions,’ provides the following NoS guidance: ‘scientists have collaborated to work on investigating the synthesis of new pathways and have considered the ethical and environmental implications of adopting green chemistry’ (Citation37). Sub-option D.6, ‘Environmental impact of some medications,’ provides similar NoS guidance: ‘the scientific community must consider both the side effects of medications on the patient and the side effects of the development, production and use of medications on the environment (i.e. disposal of nuclear waste, solvents and antibiotic waste)’ (Citation37). These two learning objectives are clear, measurable examples of Indicator 4.7.1/12.8.1/13.3.1, thereby fulfilling both the UNSDG and GCP objectives.
Conclusion
A vital consideration for all educators of K-12 students is engagement. Whereas university students are pursuing introductory chemistry as a pre-requisite to a program of study, K-12 chemistry learning is broadly mandated worldwide, including countries in the bottom half of the Human Development Index (Citation54). Considering again the educational systems of the United States of America, the United Kingdom, Canada, and Australia, each of these countries mandate chemistry education for high school students (Citation42, Citation52, Citation50, Citation39). The difference in motivation for learning chemistry between Grade 10 and university students is self-evident to high school teachers. The decline in adolescents’ motivation to learn science is well-documented and multivariate (Citation56, Citation57). Cooper and Stowe (Citation58), in their seminal review of chemical education research (CER), illuminated a gap in our understanding of how high school students learn chemistry:
(b)ecause of the vast number of studies in CER over the years, we have chosen to exclude studies on precollege chemistry education, learning in the laboratory, and studies on the affective domain. (such as identity, motivation, expectations, value, and interest)
Current high school students face significant existential threats, from human action (weapons of mass destruction, nuclear warfare, biological and chemical warfare, catastrophic climate change, and ecological collapse); from natural catastrophe (pandemics, asteroid impact, supervolcanic eruption), and emergent threat unique to their time, specifically artificial intelligence (Citation59). This list of threats, compiled by the Global Challenges Foundation (apart from asteroid impact, supervolcanic eruption, and artificial intelligence) is virtually identical to the list used by Bybee and Mau to survey science educators in 1986 (Citation60), and those existential threats faced by the authors when they were high school students. Choosing the Spanish Influenza pandemic as an arbitrary reference point, students have been learning amidst significant existential threats for over a century, suffering well-documented harm in terms of their mental health (Citation61-Citation63).
Returning to the findings of the RSC survey, Green Shoots: A Sustainable Chemistry Curriculum for a Sustainable Planet: ;Young people are worried about climate change and want to take action. Four in five young people feel climate change is an urgent priority to solve and three in four are actively looking for ways to help’ (Citation6). This paper has set out to demonstrate how education stakeholders can foster student agency and provide students with a pathway to their future.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Notes
1 A curricular movement with its foundations in the 1970s, humanistic science education, is based upon “values, the nature of science, the social aspects of science, the culture of science, and the human nature of science revealed through its sociology, history, and philosophy” (36).
References
- The American Presidency Project. Franklin D. Roosevelt. Address at the University of Pennsylvania. https://www.presidency.ucsb.edu/documents/address-university-pennsylvania (accessed Nov 26, 2022).
- Tollefson, J. Climate Change Is Hitting the Planet Faster Than Scientists Originally Thought. Nature 2022, doi:10.1038/d41586-022-00585-7.
- Howes, L. What Happens When the Water in Our Rivers and Lakes Reaches Record Lows? C&EN 2022, 26–33. doi:10.47287/cen-10038-cover.
- Paslakis, G.; Dimitropoulos, G.; Katzman, D. A Call to Action to Address COVID-19–Induced Global Food Insecurity to Prevent Hunger, Malnutrition, and Eating Pathology. Nutrition Reviews January 2021, 79 (1), 114–116. doi:10.1093/nutrit/nuaa069.
- Erickson, B. Toxic PFAS Found in Freshwater Fish. C&EN Global Enterp 2023, 101 (3), 16–16. doi:10.1021/cen-10103-polcon2.
- Green Shoots: A Sustainable Chemistry Curriculum for a Sustainable Planet; Royal Society of Chemistry: Cambridge, 2021. https://www.rsc.org/globalassets/22-new-perspectives/sustainability/sustainability-curriculum/green-shoots-a-sustainable-chemistry-curriculum-for-a-sustainable-planet.pdf (accessed Nov 26, 2022).
- Hickman, C.; Marks, E.; Pihkala, P.; Clayton, S.; Lewandowski, R.E.; Mayall, E.E.; Wray, B.; Mellor, C.; van Susteren, L. Climate Anxiety in Children and Young People and Their Beliefs About Government Responses to Climate Change: A Global Survey. Lancet Planet. Health 2021, 5 (12), e863–e873. doi:10.1016/S2542-5196(21)00278-3.
- Sciberras, E.; Fernando, J. Climate Change-Related Worry Among Australian Adolescents: An Eight-Year Longitudinal Study. Child Adoles. Ment. Health 2022, 27, 22–29. doi:10.1111/camh.12521.
- Stevenson, K.; Peterson, N. Motivating Action Through Fostering Climate Change Hope and Concern and Avoiding Despair Among Adolescents. Sustainability 2016, 8, 6. doi:10.3390/su8010006.
- Koten, G.; van; Kruijff, B.; de; Driessen, H.; Kerkstra, A.; Meinema, H. Bouwen aan Scheikunde; Enschede: SLO, 2002. https://elbd.sites.uu.nl/wpcontent/uploads/sites/108/2017/04/1810_26_bouwenaanscheikunde.pdf (accessed Nov 26, 2022).
- Apotheker, J. The Development of a New Chemistry Curriculum in the Netherlands: Introducing Concept-Context Based Education. Afr. J. Chem. Educ 2014, 4 (2), 44–63. https://www.ajol.info/index.php/ajce/issue/view/11103.
- Linkwitz, M.; Eilks, I. An Action Research Teacher’s Journey While Integrating Green Chemistry into the High School Chemistry Curriculum. Sustainability 2022, 14 (17), 10621. doi:10.3390/su141710621.
- Kolopajlo, L. Green Chemistry Pedagogy. Phys. Sci. Rev 2017, 2 (2), doi:10.1515/psr-2016-0076.
- Chemistry Education Needs a Green Reset. Nature 2022, 604, 598. doi:10.1038/d41586-022-01109-z.
- United Nations Department of Economic and Social Affairs, Sustainable Development. https://sdgs.un.org/goals (accessed Nov 26, 2022).
- Anastas, P.; Warner, J. Green Chemistry: Theory and Practice; Oxford University Press: New York, 1998; p 30
- Pollution Prevention Act of 1990. https://www.epa.gov/p2/pollution-prevention-act-1990 (accessed Nov 26, 2022).
- The Nobel Prize in Chemistry. 2001. https://www.nobelprize.org/prizes/chemistry/2001/summary/ (accessed Nov 26, 2022).
- The Nobel Prize in Chemistry. 2005. https://www.nobelprize.org/prizes/chemistry/2005/summary/ (accessed Nov 26, 2022).
- The Nobel Prize in Chemistry 2010. https://www.nobelprize.org/prizes/chemistry/2010/summary/ (accessed Nov 26, 2022).
- The Nobel Prize in Chemistry. 2021. https://www.nobelprize.org/prizes/chemistry/2021/summary/ (accessed Nov 26, 2022).
- The Nobel Prize in Chemistry. 2022. https://www.nobelprize.org/prizes/chemistry/2022/summary/ (accessed Nov 26, 2022).
- Anastas, P.; Beach, E. Changing the Course of Chemistry. In Green Chemistry Education: Changing the Course of Chemistry: Anastas, P., Levy, I., Parent, K., Eds.; ACS Symposium Series 1011; American Chemical Society: Washington, DC, 2009. p 2. https://pubs.acs.org/doi/pdf/10.1021bk-2009-1011.ch001
- United States Compulsory School Attendance Laws, Minimum and Maximum Age Limits for Required Free Education. https://nces.ed.gov/programs/statereform/tab5_1.asp (accessed Nov 26, 2022).
- United Kingdom School Leaving Age. https://www.gov.uk/know-when-you-can-leave-school (accessed Nov 26, 2022).
- Province of Alberta Education Act. https://www.alberta.ca/education-guide-education-act.aspx (accessed Nov 26, 2022)
- State of Victoria School Leaving Age. https://www.vic.gov.au/if-your-child-wants-leave-school-early (accessed Nov 26, 2022)
- The Province of Ontario Curriculum, Grades 11 and 12: Science, 2008.https://www.edu.gov.on.ca/eng/curriculum/secondary/2009science11_12.pdf (accessed Nov 26, 2022).
- Sustainable Development Goal Indicators: Metadata Repository. https://unstats.un.org/sdgs/metadata (accessed Nov 26, 2022).
- Sustainable Development Goal Indicator Metadata. https://unstats.un.org/sdgs/metadata/files/Metadata-04-07-01.pdf (accessed Nov 26, 2022).
- DeBoer, G.E. A History of Ideas in Science Education: Implications for Practice; Teachers College Press: New York, 1991.
- DeBoer, G. E. The History of Science Curriculum Reform in the United States. In Handbook of Research on Science Education, Lederman, H., Abell, S., Eds.; Routledge, New York, 2014; Vol 2: pp 559–578.
- Pea, R.; Collins, A. Learning How to Do Science Education: Four Waves of Reform. In Designing Coherent Science Education: Implications for Curriculum, Instruction, and Policy: Yael, K., Linn, M., Roseman, J., Eds.; Teachers College: New York, 2008; pp 3–12.
- Sputnik at 50: Looking Back at the Space Race. https://www.npr.org/2007/09/30/14829195/sputnik-left-legacy-for-u-s-science-education (accessed Nov 26, 2022).
- United States National Defence Education Act of 1958. https://www.govinfo.gov/content/pkg/STATUTE-72/pdf/STATUTE-72-Pg1580.pdf. (accessed Nov 26, 2022).
- Aikenhead, G. Science Education for Everyday Life: Evidence-Based Practice; Teachers College Press: New York, 2006; p 2.
- The International Baccalaureate Diploma Programme; Chemistry Guide: Cardiff, 2016.
- Massachusetts Department of Elementary and Secondary Education. https://www.doe.mass.edu/frameworks/scitech/2016-04.pdf. (accessed Nov 26, 2022).
- Australian Curriculum, Assessment and Reporting Authority. https://www.australiancurriculum.edu.au/senior-secondary-curriculum/science/chemistry/?unit = Unit+4 (accessed Nov 26, 2022).
- Chemistry Curriculum and Assessment Guide (Secondary 4-6); Govt; Hong Kong: Logistics Dept., 2007, https://www.edb.gov.hk/attachment/en/curriculum-development/kla/science-edu/Chem_C_and_A_Guide_updated_Eng_22082018.pdf (accessed Jan 12, 2023).
- Ministerium für Schule und Bildung des Landes Nordrhein-Westfalen, Kernlehrplan für die Sekundarstufe II - Gymnasium/Gesamtschule in Nordrhein-Westfalen Chemie, 1. Aufl.; Düsseldorf, DE, 2022. https://www.schulentwicklung.nrw.de/lehrplaene/lehrplan/314/klp_gost_ch_2022_06_07.pdf (accessed Jan 12, 2023).
- Ottevanger, W., Oorschot, F., Spek, W., Boerwinkel, DJ, Eijkelhof, H., Vries, M. de, Hoeven, M. van der, & Kuiper, W. Kennisbasis natuurwetenschappen en technologie voor de onderbouw vo: een richtinggevend leerplankader. SLO: Enschede, 2014. https://www.slo.nl/publicaties/@4177/kennisbasis/ (accessed Jan 25, 2023).
- 2013. Next Generation Science Standards: For States, by States; NGSS Lead States, Ed.; National Academies Press: Washington, D.C., 2013.
- Hoffman, K.C.; Dicks, A.P. Shifting the Paradigm of Chemistry Education by Greening the High School Laboratory. Sustain. Chem. Pharm 2020, 16, 100242. doi:10.1016/j.scp.2020.100242.
- Herron, J.D. Piaget for Chemists. Explaining What “Good” Students Cannot Understand. J. Chem. Educ 1975, 52 (3), 146–150. doi:10.1021/ed052p146.
- Johnstone, A.H. Why Is Science Difficult to Learn? Things Are Seldom What They Seem. J. Comp. Assist. Learn 1991, 7 (2), 75–83. doi:10.1111/j.1365-2729.1991.tb00230.x.
- Mahaffy, P. Moving Chemistry Education into 3D: A Tetrahedral Metaphor for Understanding Chemistry. Union Carbide Award for Chemical Education. J. Chem. Educ 2006, 83 (1), 49–55. doi:10.1021/ed083p49.
- Christenson, S.; Wylie, C.; Reschly, A.L. Handbook of Research on Student Engagement; Springer: New York, 2012.
- Province of Alberta Science Grades 7-8-9 Program of Studies. https://education.alberta.ca/media/3069389/pos_science_7_9.pdf (accessed Nov 26, 2022).
- Province of Alberta Science 10 Program of Studies. https://education.alberta.ca/media/3069384/pos_science_10.pdf (accessed Nov 26, 2022).
- Province of Alberta Chemistry 20-30 Program of Studies. https://education.alberta.ca/media/3069388/pos_chem_20_30.pdf (accessed Nov 26, 2022).
- The Relation of Physics to Other Sciences. https://www.feynmanlectures.caltech.edu/I_03.html (accessed Nov 26, 2022).
- A Framework for K-12 Science Education: Practices, Crosscutting Concepts, and Core Ideas; National Research Council (U.S.), Ed.; The National Academies Press: Washington, D.C, 2012; p. 79
- Teaching Chemistry Around the World; Risch, B., Ed.; Waxmann Verlag GmbH: Münster, 2010; pp 15–43.
- Science Programmes of Study: Key Stage 3. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/335174/SECONDARY_national_curriculum_-_Science_220714.pdf (accessed Nov 26, 2022).
- Potvin, P.; Hasni, A. Analysis of the Decline in Interest Towards School Science and Technology from Grades 5 Through 11. J. Sci. Educ. Technol 2014, 23 (6), 784–802. doi:10.1007/s10956-014-9512-x.
- Fortus, D.; Touitou, I. Changes to Students’ Motivation to Learn Science. Discip. Interdscip. Sci. Educ. Res 2021, 3 (1). doi:10.1186/s43031-020-00029-0.
- Cooper, M.; Stowe, R. Chemistry Education Research from Personal Empiricism to Evidence, Theory, and Informed Practice. Chem. Rev 2018, 118 (12), 6053–6087. doi:10.1021/acs.chemrev.8b00020.
- Westin, U., Ed. Global Catastrophic Risks 2020; Global Challenges Foundation Annual Report: Global Challenges Foundation; Stockholm, 2020.
- Bybee, R.W.; Mau, T. Science and Technology Related Global Problems: An International Survey of Science Educators. J. Res. Sci. Teach 1986, 23 (7), 599–618. doi:10.1002/tea.3660230704.
- Beardslee, W. Children’s and Adolescents’ Perceptions of the Threat of Nuclear War: Implications of Recent Studies. In The Medical Implications of Nuclear War: Solomon, F., Marston, R., Eds.; National Academy Press: Washington, DC, 1986; pp 413–434.
- Kiraly, S.J. Psychological Effects of the Threat of Nuclear War. Can. Fam. Physician 1986, 32, 170–174.
- Christie, D.J.; Hanley, C.P. Some Psychological Effects of Nuclear War Education on Adolescents During Cold War II. Polit. Psychol 1994, 15 (2), 177–199. doi:10.2307/3791737.
