?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Several industries such as leather tanning, coal mining, steel and metal processing are responsible for heavy metals contamination in water. Heavy metals contamination in water can have harmful effects on both aquatic and terrestrial animals by entering the food chain. Due to the higher toxicity of heavy metals, it is necessary to remove heavy metal ions from water. There are several physio-chemical methods available, including ion exchange, membrane filtration, chemical oxidation, and electrochemical methods. However, these methods have some disadvantages like expensive and generating harmful byproducts. Biosorption is a cost-effective and eco-friendly method for the removal of heavy metals from contaminated water. Biosorbents are derived from biomasses of plant, bacterial, algal, fungal, agro-waste, etc. The biosorbents have several functional groups on their surface providing them a high binding capacity for heavy metal ions. Mathematical models such as isotherms, thermodynamics, and kinetic studies help explain how heavy metals adsorb on biosorbents. This review provides comprehensive details on the heavy metals heavy metal contaminated in water including the source, toxicity and biosorption of heavy metal ions. This review also provides the mechanism of heavy metal biosorption including mathematical models.
1. Introduction
Heavy metals are a group of elements that exhibit high density and are toxic even at low concentrations (Citation1). They are released into the environment through various anthropogenic activities such as mining, metallurgy, industrial processes, and agricultural practices (Citation2, Citation3). Heavy metal pollution in water sources poses a serious threat to human health and the environment due to their non-biodegradable nature and tendency to accumulate in living organisms (Citation4, Citation5). Heavy metals such as Cr (IV), Cd (II), Ni (II), As (III & V), Pb (II), Cu (II), Zn (II), Sb (II), Hg (II), and Se (II) are highly toxic to living organisms (Citation6). They can accumulate in the body over time, leading to severe health problems (Citation7). Cr (IV) is carcinogenic and can cause lung cancer (Citation8). Cd (II) affects the kidneys and can lead to renal failure (Citation9). Ni (II) is a known carcinogen and can cause lung and nasal cancer (Citation10). As (III & V) are highly toxic and can cause skin lesions, respiratory issues, and cancer (Citation11). Pb (II) affects the nervous system and can lead to developmental delays in children (Citation11). Cu (II) can cause liver and kidney damage. Zn (II) toxicity can lead to gastrointestinal issues (Citation10, Citation11). Sb (II) can cause respiratory issues and liver damage. Hg (II) affects the nervous system and can lead to neurological disorders. Se (II) toxicity can lead to hair loss, nail brittleness, and neurological issues (Citation12).
Conventional methods applied for the elimination of heavy metals from wastewater, such as chemical precipitation, ion exchange, and membrane filtration, have shown effectiveness; however, they are commonly expensive and contribute secondary chemical sludge as extra pollution (Citation13). Furthermore, the implementation of these technologies at a large scale, particularly in industrial settings, entails exorbitant costs (Citation13). Consequently, there exists a pressing need for a suitable and economically viable technique that not only exhibits environmental friendly but also proves to be cost-effective, to effectively address the challenge of eliminating heavy metals from contaminated water (Citation14). Recently, there has been a rising interest in the use of biosorption as an environmentally sustainable and economically viable substitute for heavy metal removal (Citation15, Citation16).
Biosorption, as a biological method that harnesses the potential of inert biomass, emerges as a promising and compelling alternative for the amelioration of heavy metals in wastewater (Citation17). The process of remediating heavy metals from wastewater through biosorption represents a low-cost, uncomplicated, and environmentally sustainable technique for the efficient removal of heavy metal ions (Citation18). By employing dead biomass, biosorption effectively removal heavy metal ions from water, thereby mitigating the negative impact of heavy metal contamination on the environment and human health. This technique offers numerous advantages, including its simplicity, cost-effectiveness, and compatibility with various types of biomass (Citation19). Furthermore, biosorption also minimizes the generation of secondary chemical sludge. Overall, the implementation of biosorption in the removal of heavy metal presents a viable and attractive solution to the persistent problem of heavy metal pollution in wastewater (Citation6, Citation20).
The biosorbent is derived from several biomass of plant, microbe, algae and agro-waste based (Citation21, Citation22). This high level of effectiveness can be attributed to the exceptional properties of heavy metal removal possessed by these biosorbents (Citation23, Citation24). Biosorption is a metabolically independent method and dead biomasses are involved in this process (Citation25). Furthermore, a broad range of biosorbents have been successfully used to remove and eliminate heavy metal pollution, including rice and wheat husk, lignite, agricultural waste, bananas, and citrus peels (Citation26). These biosorbents consist of functional groups such as carboxyl, hydroxyl, and amino groups that demonstrate a strong affinity towards heavy metal ions. The biosorption process is influenced by a variety of factors, including the specific biosorbent utilized, pH levels, temperature, contact duration, and the initial concentration of metal ions in the solution (Citation27–29).
The processes implicated in biosorption entail diverse mechanisms including ion exchange, surface complexation, and precipitation. Ion exchange involves the exchange of heavy metal ions in the solution with other ions present on the surface of the biosorbent (Citation30). Surface complexation involves the formation of chemical bonds between the functional groups on the biosorbent's surface and the heavy metal ions (Citation31). Precipitation arises when heavy metal ions react with the functional groups present on the surface of the biosorbent, resulting in the formation of insoluble complexes (Citation32). Recent developments in biosorption research have resulted in the introduction of novel biosorbent materials and the application of mathematical models to explore biosorption kinetics and thermodynamics (Citation33). Mathematical models like Langmuir, Freundlich, and Temkin isotherms, in addition to pseudo-first-order and pseudo-second-order kinetics, have been employed to elucidate the biosorption process and forecast the performance of biosorbents under varying circumstances (Citation34–36).
This review aims to provide a comprehensive analysis of heavy metal ion pollution in water, focusing on its origins, environmental and health implications, and the application of eco-friendly techniques for its removal. The investigation will explore the various origins of heavy metal contamination, encompassing industrial procedures, agricultural practices, and natural origins, highlighting the varied pathways through which these contaminants infiltrate water sources. It will also address the harmful effects of heavy metal ions on human health and the environment, underscoring the pressing requirement for efficient remedial approaches. Moreover, the review will assess environmentally friendly techniques for the elimination of heavy metal ions, such as biosorption and the utilization of natural adsorbents. It will present an elaborate analysis of the underlying principles of these techniques, their benefits and constraints, and their potential for widespread implementation in water treatment. Moreover, the examination will provide a summarization of critical isotherm, kinetic, and thermodynamic investigations regarding the adsorption of heavy metal ions, offering insights into the factors and variables impacting the adsorption process. Through this comprehensive investigation, the review aims to contribute to the advancement of sustainable solutions for the removal of heavy metal ions from water, thereby ensuring the protection of human health and the environment.
2. Source and toxicity of heavy metal ions
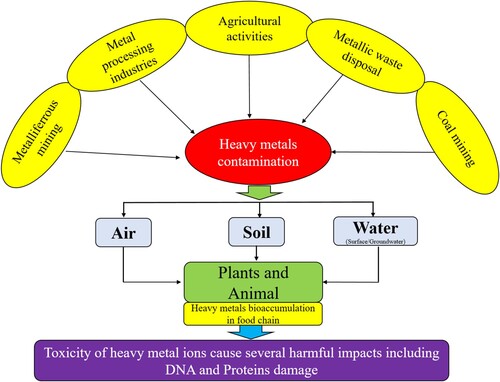
Heavy metal contamination in the water is caused by several natural and anthropogenic sources. Natural processes can eventually result in the entry of heavy metals into water, including volcanic eruptions, erosion, forest fires, etc (Citation37). On the other hand, anthropogenic activities of heavy metal contamination include several industrial processes (Citation38). Some important heavy metal contamination in water is represented in .
A wide and extensive range of industrial processes, encompassing a diverse range of activities such as the treatment and processing of animal hides, the application of a thin layer of metal to various materials, the manufacturing of batteries, the operations within the glass industry, agricultural practices, the proper disposal of household waste, and the intricate industrial processes involved in the production of pharmaceuticals, have all been unequivocally identified as significant contributors to the widespread and all-encompassing dispersion of contaminants consisting of heavy metals throughout the environment (Citation38, Citation39). The toxic heavy metal ions such as Cr (VI), Cd (II), Ni (II), As (III & V), Pb (II), Cu (II), Zn (II), Sb (II) and Se (II) have posed a substantial and noteworthy threat to both the well-being of human beings and the delicate balance of the natural ecosystem. The act of producing Pb (II) has consequently led to the generation of approximately ten million metric tons of this particular chemical element (Citation39). A significant and considerable portion, more specifically 85.10%, has been allocated for usage within the battery industry, where it finds its purpose and usefulness.
Additionally, an additional 5.5% has been employed and utilized for the development and creation of pigments, which serve various artistic and industrial purposes. The remaining 2.1% of these heavy metal contaminants have been designated for miscellaneous industrial procedures (Citation40). Furthermore, it is worth noting that Pb (II) is commonly and extensively utilized in gasoline in the form of tetraethyl and tetramethyl compounds, which are primarily used as anti-knocking agents to improve the performance and efficiency of engines. On the contrary, Cr (VI) finds its application and utility in a wide range of industries and processes, including but not limited to the production of steel, the preservation of wood, the plating of different objects with a protective layer of Cr (VI), the creation and development of various pigments, and the intricate and precise process of electroplating, which is of great significance in numerous industries and applications (Citation41). Some important heavy metal ions, their sources and toxicity are given in .
Table 1. Different heavy metals and their sources with their Toxicity.
Zn (II) is one of the important trace elements which are beneficial for the physiological function of living tissues. But excess zinc leads to many health issues like a stomach cramp, vomiting, nausea, anaemia etc. Sugarcane bagasse and rice straw have the capacity of Zinc adsorption at 0.12 m2/g (Citation54, Citation55). The Cd (II) is used in paints, rubber, cement, paper and other many other applications. Exposure to Cd (II) at lower levels can cause skin irritation and also ulceration but the long-term exposure to cadmium is highly toxic as it can cause damage to certain body organs like the kidney as well as the liver. Cd (II) has been exposed to water or aquatic life by different industries and is hazardous for the people who eat fish. It can cause lung cancer and can also cause different bone diseases like osteomalacia, and osteoporosis in human beings as well as in animals. Cadmium is derived as and by-product of zinc or lead refining. Cd (II) can cause reproductive disorders like Testicular necrosis, estrogen-like effects, affection of steroid hormone synthesis and also cause Itai-Itai disease, stones in the kidney, tubular damage as well as glomerular kidney (Citation51, Citation56).
Hg (II) is present in liquid form at room temperature. Exposure to this metal can cause many diseases like Niigata and minimata disease in japan which was reported. Mercury exposure can also affect the endocrine, reproductive as well as nervous system of humans (Citation53, Citation57). The elemental Hg (II) is non-toxic when exposed orally and highly toxic when it is exposed through inhalation. Inorganic mercury-less toxic in nature (insoluble < soluble), Organic mercury-more toxic in nature. Most of the lead pollution is caused by batteries as well as automobiles (Citation58). Exposure to lead can cause diseases in human beings like reproductive disorders, endocrine disorders, GIT disorders and disorders in the hepatic system. A gastrointestinal sign includes Diarrhea, colic etc. and also causes paralysis and sometimes leads to death of a person (Citation53, Citation59).
Algae are distributed extensively in marine and freshwater environments and play a vital role as biosorbents for removing heavy metals from contaminated water (Citation60). Arsenic is the 20th most abundant metal in the earth’s crust (1.5–3.0 mg/kg) and it is carcinogenic and has highly extensive exposure through drinking water. It can cause bloody diarrhea, dehydration, weakness and hind limb paralysis. According to the Times of India, about 19% of Indians consume water with lethal arsenic levels. WHO reported that the long-term use of arsenic can cause skin, lung, kidney or bladder cancer. According to WHO Arsenic is a toxic metalloid that can cause different serious health problems and can also be described as ‘the greatest mass poisoning in human history’ (Citation61).
3. Heavy metals removal
3.1. Physiochemical methods
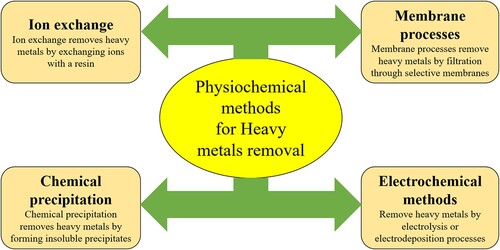
There are several physical and chemical methods are available for the removal of heavy metal ions. Some well-known physiochemical (conventional) methods for heavy metal removal are membrane filtration, precipitation, flotation, precipitation and physical adsorption. A few important physiochemical methods are described in .
These methods have some limitations such as not being effective at very low heavy metal ion concentrations in the water, these methods are expensive and generate large amounts of secondary pollutants (Citation46, Citation62). The physiochemical methods are described below as:
3.1.1. Ion exchange
It is the process in which the capability to exchange cations with that of metals in wastewater is used. The exchange of anions, as well as cations, occurs in aqueous solution. Many types of materials have been used in the ion exchange process like natural (carbon, silicate, and alumina) or synthetic (resins, zeolites) (Citation63). Out of which zeolites are mostly used in this type of method. There are some limitations also to the ion exchange process it is highly sensitive to the pH of the aqueous solution and ion exchange is non-selective in this method (Citation63, Citation64). It was found successfully that the ion-exchange process is useful for the removal of heavy metals like Fe (II), Zn (II), Cd (II), and Pb (II) from mine water with the help of natural zeolites (Citation65). High regeneration of material can take place by ion-exchange process and this process is metal selective in nature but on the other side there are some drawbacks like high cost and large chemical consumption can take place (Citation66). Membrane fouling, pH sensitivity, and non-selectivity of the membrane are some of the major drawbacks of this operation (Citation67).
3.1.2. Chemical precipitation
This methodology proves to be advantageous in the management of wastewater that contains metals by generating a solid substance that cannot dissolve through the introduction of specific chemical substances. This approach is widely employed and straightforward, serving the purpose of retrieving heavy metals from wastewater (Citation63). These techniques are used for wastewater treatment because of ease of handling and operation as well as lower cost. Due to the simplification, convenience and effectiveness in the process treatment of effluent at higher concentrations, limestone as well as lime are mostly used as a chemical precipitant (Citation68). There are some limitations of this technique and the limitation is that it needs a large number of chemicals during treatment and can produce sludge in high amounts and they face problems during disposing of these sludge in the environment. The term chemical precipitation is inexpensive and simple and most of the metals can be removed but on the other part, there are some drawbacks to this method there is a problem with the disposal and a large amount of sludge is produced (Citation66, Citation68).
3.1.3. Membrane process
In most cases, precipitation refers to the formation of insoluble inorganic metal precipitates (Citation69). This technique is used for the removal of different heavy metals in aqueous solution. The removal of suspended solids, oils, organic as well as inorganic materials, and heavy metal recovery is done by this process (Citation70). In this method, solid wastes are produced and chemical consumption is also less and has higher efficiency (>95% for single metal) on the other side there are some drawbacks of this method like higher initial and running costs, low flow rates and the removal percentages can decrease in the presence of other metals (Citation66, Citation70).
3.1.4. Electrochemical method
Under this technique, the redox reaction takes place for the removal of heavy metals from the industrial effluents under the influence of external direct current in the electrolyte solution (Citation64, Citation71). This method is metal-selective and there is no consumption of chemicals taking place. Pure metals can be achieved from this method but on the other hand, there a limitations of this method like high capital cost, and high running cost so it is not much essential method (Citation71).
The physicochemical methods offer effective solutions for the removal of heavy metal ions from water, with each method having its own set of advantages and disadvantages. The selection of the most appropriate method depends on factors such as the specific heavy metal ions present, the concentration of these ions, and the overall requirements of the water treatment process (Citation72). The advantages and disadvantages of physiochemical methods are mentioned in the .
Table 2. Advantages and limitations of physiochemical methods.
3.2. Biosorption
The term biosorption is considered to be an economic, efficient, effective and eco-friendly method for the removal of water contamination from wastewater (Citation73). Biosorption is considered to be one of the most effective processes of advanced wastewater treatments that can reduce the trace hazardous organic as well as inorganic wastes left in effluents after the conventional treatments (Citation74). Biosorption is a technique which is used for removing pollutants from water, mainly those pollutants that are not easily degradable such as dyes and metals (Citation75, Citation76). The phenomenon termed chelation, coordination, complexion, ion-exchange, adsorption as well as micro precipitation are all involved in the overall metal uptake by the process called biosorption (Citation77). The main advantages of the process termed biosorption are the use of inexpensive biological materials as well as its effectiveness in the reduction of concentration of heavy metal ions (Citation77). The term biosorption has advanced in future because of its quick (rapid intrinsic as well as kinetics) and the shuttered dissolved metals out of complex dilute solutions with higher efficiency (Citation78).
Biosorption offers a promising approach for the removal of heavy metal contamination in wastewater by employing natural or modified biological materials to adsorb heavy metal ions. This methodology presents several advantages over traditional physicochemical techniques, such as cost-effectiveness, eco-friendliness, and sustainability in comparison to methods like ion exchange, chemical precipitation, and membrane processes (Citation79). The benefits of biosorption include the utilization of inexpensive and renewable biosorbents, such as agricultural residues or microbial biomass, thus reducing the need for chemical agents and energy-intensive processes (Citation80). Moreover, biosorption demonstrates a remarkable specificity towards specific metal ions, thus reducing the chances of interference from other ions in the wastewater. Nonetheless, biosorption does have its limitations. The adsorption capacity of biosorbents is frequently lower than that of synthetic adsorbents, and the process may be slower, necessitating prolonged contact durations for efficient metal removal (Citation81). Additionally, the performance of biosorption can be influenced by variables like pH, temperature, and the existence of competitive ions, requiring meticulous adjustment of conditions to enhance efficiency (Citation82). compares biosorption with physicochemical methods, highlighting the benefits and challenges of heavy metal removal from wastewater.
Table 3. Physiochemical methods comparison with biosorption methods.
3.2.1. Mechanism of biosorption
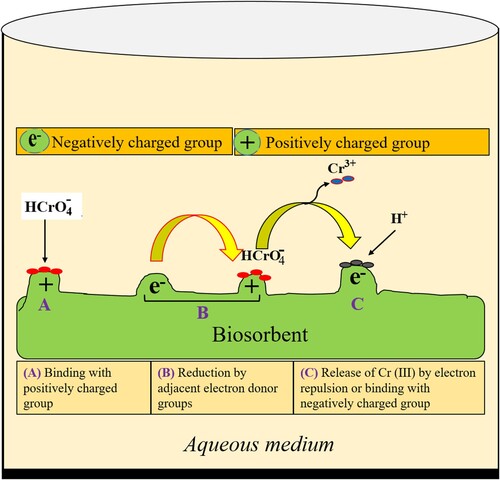
The mechanism underlying biosorption is a complex phenomenon. Anionic hexavalent chromium ions interact with positively charged groups that are present on the surface of the biosorbent. These ions are subsequently transformed into Cr (III) ions through various pathways (Citation91). The process of biosorption of hexavalent chromium ions can be divided into three primary stages, as depicted in .
Initially, the negatively charged hexavalent chromium ions form bonds with the positively charged groups on the biosorbent surface. Furthermore, there is a reduction process in which the hexavalent chromium ions are transformed into trivalent chromium ions by electron donor groups. Finally, in this step, trivalent chromium ions are either released into the aqueous solution due to electronic repulsion with other positive groups or bind with other negative groups present on the biosorbent surface (Citation92).
Biosorption is the sticking together of the molecules of liquid, solid or dissolved solids to the surface of the liquid as well as the solid surface. Because of metal recovery and reuse it has become one of the excellent techniques for wastewater treatment (Citation64). It is a process which can offer the flexibility in the operation as well as in the design. This process can sometimes become reversible which is why the regeneration of biosorbents took place with the help of different desorption processes. It is the physiochemical phenomenon which occurs naturally in some biomasses or the ability of certain biomass to accumulate heavy metals from wastewater which are caused by certain industrial byproducts (Citation93).
3.2.2. Biosorbent materials
The biosorbents are derived from several biomasses such as plant residue, agriculture waste, fruit waste, microbial biomass, algal biomass and green synthesized nanomaterials (Citation94). Plants, algae, bacteria, fungi and yeast have proved to be potential biosorbents for the removal of heavy metal ions (Citation95). Some important biosorbents and their heavy metal removal efficiency are shown in .
Table 4. Heavy metal removal efficiency of some important biosorbent.
The mechanism of biosorption is divided into two types one is metabolism-dependent (dependence on the cell metabolism) and the second type is metabolism metabolism-independent (Citation103, Citation104). The biosorbents have several functional groups such as carbonyl, phosphoryl, hydroxyl, etc., present on their surface and these functional groups play an important role in the biosorption of heavy metal ions (Citation105). The functional present on the biosorbent surface can be analyzed by Fourier-transform infrared spectroscopy (FTIR) instrument. The biosorbent materials are categorized into several categories such as cellulose materials, activated carbon, green synthesized nanomaterials, etc. Cellulose materials-based biosorbents are widely used biosorbent materials. These are widely abundant, cheap and eco-friendly. Many forms of cellulosic materials used as biosorbents are fibers, leaves, barks, shells, stems, husks and many other parts of plants (Citation106).
3.2.3. Preparation of biosorbents
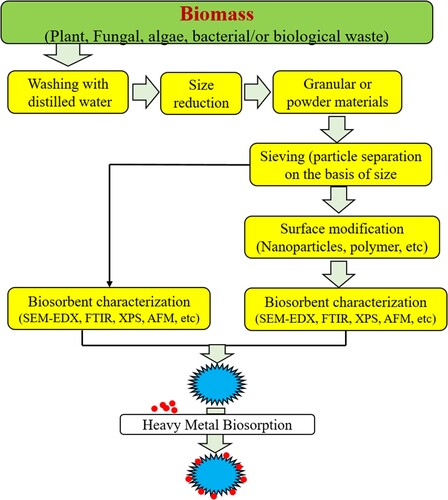
Moreover, the preparation of biosorbents from raw materials is also an easy and simple process (Citation106). In this process, there are a few steps involved including collection of biomass, dry and size reduction. The biosorbent preparation processes are described in .
Activated carbon is the most commonly used biosorbent all over the world. Activated carbon not only removes the toxic metals from the waste water but can also able to remove all the contaminants present in the wastewater. For the production of activated carbon numerous agricultural wastes are used like bagasse, tea waste, coconut shell, apple waste, peanut hull, sawdust, rice husk, banana pith, tree bark, sugarcane bagasse and many more. This type of adsorbent is used in both columns as well as in the batch process (Citation107, Citation108).
The utilization of natural zeolite as a binding agent for the elimination of heavy metal ions is of utmost significance. These naturally occurring materials, known as hydrated alumina silicates, possess notable sorption and ion-exchange properties and fall within the category of Tectosilicates (Citation109, Citation110). Zeolites exhibit a distinct three-dimensional porous structure, which contributes to their versatility in various applications. Numerous investigations have provided empirical evidence supporting their efficacy in the removal of metal cations from wastewater. The employment of natural zeolites in wastewater treatment represents one of the earliest and most promising fields of application. Many natural zeolites worldwide have demonstrated commendable ion-exchange capabilities for cations such as heavy metal ions and ammonium. Additionally, modified zeolites exhibit the highest capacity for biosorption of anions and organic matter (Citation110, Citation111).
3.2.4. Advantages and disadvantages of biosorbents
Biological materials such as plants, algae, bacteria, fungi, and yeast remove heavy metal ions from water, offering several advantages and disadvantages. The advantages and disadvantages of biosorbents are mentioned in .
Table 5. Advantages and disadvantages of biosorbents use for removal of heavy metals.
In general, biosorption represents a promising approach for the removal of heavy metal ions, particularly in scenarios emphasizing cost-effectiveness and ecological sustainability. Nevertheless, a comprehensive assessment of the distinct advantages and disadvantages of various biosorbents is essential for their efficient utilization (Citation123).
3.3. Factors affecting heavy metal biosorption
3.3.1. pH
The consideration of the pH at which biosorption takes place is of utmost importance due to its direct impact on the biosorption process and capacity. Both the chemistry of the metal ions employed in the process and the functional groups of the biosorbent are influenced by the pH level. Typically, the biosorption capacity tends to increase with rising pH until it reaches the optimal pH, at which point the maximum biosorption capacity is attained (Citation125). However, it should be noted that higher pH values may lead to the occurrence of metal precipitation, as a result of the presence of metal hydroxides or hydroxide anionic complexes. It is crucial to bear in mind that under low acidic pH conditions, the biosorption of metal ions such as copper, cadmium, nickel, cobalt, and zinc is typically hindered (Citation126). When the acidity of the biosorbent is low, the interaction between the groups and the metal cations is impeded due to the protonation of the groups and the resultant repelling force. However, with increasing acidity levels, functional groups like carboxyl, hydroxyl, and phosphate groups undergo deprotonation, leading to the acquisition of negative charges (while amino and imidazole groups become neutral). The biosorption capacity and rate would both rise as a consequence of this modification, which would improve the binding of metal cations (Citation126). Deprotonation of all acidic groups would be achieved by increasing pH to a higher value. However, this would also cause metal hydroxides to precipitate as free metal cations, which would reduce the process's effectiveness in removing metal (Citation127).
The influence of pH and the functional groups of the biosorbent on the chemistry of metal ions has been previously demonstrated. The pH levels play a crucial role in determining the emergence of different species of metal ions, as they govern the form and concentration of these ions in an aqueous solution (Citation128). The dominance of each form of Cr (III) is influenced by the pH level. The biosorption process may change due to the varying affinities of different species for the functional groups present in the biosorbent. Moreover, variations in the solubility and mobility of metal ion species could also potentially impact the biosorption process (Citation127, Citation129).
3.3.2. Temperature
The biosorption capacity and surface activity of the biosorbent are subject to the influence of temperature. The impact of temperature on the biosorption process is contingent upon the specific procedure employed. In contrast to endothermic biosorption processes, whereby an elevation in temperature is observed, exothermic biosorption processes would experience a decrease in the removal of metal ions (Citation130). Conversely, in the case of an exothermic biosorption process, a reduction in temperature would result in a heightened removal of metal ions. Conversely, for an endothermic biosorption process, the opposite effect would be observed. Temperature fluctuations ranging from 20°C to 35°C do not exert any influence on the biosorption process (Citation131). As the temperature increased, the ability of peanut shells to biosorb lead (II) ions diminished, thereby indicating that the process was exothermic (Citation131). Olukanni et al. (Citation132) conducted a study on the biosorption of heavy metals, including cadmium, zinc, and silicate, using Pseudomonas aeruginosa. The authors found that heavy metal biosorption increased with temperature but experienced a significant reduction after reaching the optimum temperature (Citation132).
3.3.3. Contact time
Contact time is generally defined as a designated time frame for the biosorption process. Although it does not directly impact the biosorption capacity, the duration in which the sorbate and biosorbent are in contact can act as a potential constraint. By prolonging the contact period in an experimental setup, the biosorbent material can fully demonstrate its maximum potential for biosorption. Once the maximum biosorption capacity of the biosorbent is attained under the specific conditions, wherein the binding sites of the biosorbent have become utterly saturated, any attempt to prolong the contact time between the biosorbent and the target substance would prove to be futile and ineffectual (Citation119). Kanu et al. (Citation133) investigated the biosorption of Pb(II) using Rooibos shoot powder (RSP). They observed that as the duration of biosorption increased, the rate of Pb(II) adsorption onto RSP also increased. It took nearly 60 min to achieve complete saturation of the active sites. The maximum biosorption capacity was noted at this point. The rapid sorption rate with increasing time was attributed to the attraction of active functional groups to Pb(II), resulting in stronger surface binding. As the surface sites of the RSP became saturated, the rate of Pb(II) uptake was controlled by the transport of Pb(II) ions from the exterior to the interior sites. Therefore, 60 min was deemed sufficient for biosorption to reach equilibrium (Citation133).
3.3.4. Biosorbent dosage
When multiple metal ions are present, increasing the biomass dose would decrease the competition between them to bind to the active groups. Nevertheless, the implementation of a small biomass dose in complexly contaminated water will restrict the capacity of the biosorbent to absorb biosorption and intensify the competition for the binding site. When multiple metal ions are present, the competition between them to bind to the operative groups would be decreased by elevating the biomass dose. The biomass utilized in the majority of documented investigations varied from 0.5 to 6.0 g/L (Citation134, Citation135). Singh et al. (Citation136) studied the adsorption of Cr(VI) using chitosan-coated MnO2 nanoparticles. They examined the effect of adsorbent doses ranging from 0.5 to 2 g/L and found that the maximum removal of Cr(VI) was achieved at a dose of 2 g/L (Citation136).
3.3.5. Effect of initial heavy metal ion concentration
The examination of the efficiency of banana peel as a biosorbent for heavy metals was the subject of a research study, wherein it was observed that the biosorption of metals experiences an increase with a rise in the initial metal concentration (Citation137). The impact of increasing the initial metal concentration is similar to that of extending the contact time. However, the utilization of a low metal concentration does not accurately reflect the actual maximum biosorption capacity of the biosorbent, as there may be multiple unoccupied metal binding sites. To fully exploit the potential of metal removal, it is imperative to elevate the metal concentration until the binding sites of the biosorbent reach complete saturation. Once the biosorbent attains saturation, further increasing the initial metal concentration proves to be ineffective. The majority of published research studies have implemented experimental conditions wherein the initial metal concentration ranges from 5 to 200 mg/L (Citation138, Citation139). Bamisaye et al. (Citation140) examined Cu(II) biosorption using a Chitosan-Walnut shell (WNS-CH) composite. They studied the effect of initial Cu(II) ion concentrations ranging from 20 to 200 mg/L and found that maximum Cu(II) removal occurred at 20 mg/L (Citation140).
4. Isotherms, kinetics and thermodynamics of heavy metal biosorption
4.1. Isotherm study
The attainment of equilibrium in the biosorption process is achieved through the utilization of a sorption isotherm. This isotherm serves the purpose of evaluating the connection between the equilibrium concentration of metal ions and the quantity of metal ions bound by the unit mass of the biosorbent (Citation141). The equilibrium between the solid and liquid phases is predominantly established with the assistance of the Langmuir Isotherm. The study of the complex relationship between a material's adsorption capacity and the residual concentration of heavy metal ions present in it, all while maintaining a constant temperature, is the focus of isotherms, which are essential tools in the area of adsorption studies. Numerous isotherm models, each with specific advantages and disadvantages in predicting adsorption behavior, have been proposed and used extensively in the field of adsorption. These models encompass a variety of isotherm models including Freundlich, Langmuir, Temkin, Halsey, Harkin-Jura (H-J), D-R, Redlich-Peterson, and Jovanovic isotherm models (Citation142). The examples of some isotherm model and their application in heavy metal adsorption are showed in .
Table 6. Examples of isotherm models for heavy metal biosorption.
The maximum achievable biosorption of Spirulina platensis is 97.1%, and the adsorption capacities of the adsorbent for zinc ions were examined using both Langmuir and Freundlich isotherms. It was concluded that the Langmuir isotherm showed a stronger correlation, suggesting a superior fit (Citation151). In the studies done on peanut husk as a biosorbent, it was found that the better-fitted experimental data are in the Langmuir isotherm model (Citation152). A few important isotherms are explained here which are frequently used for heavy metal biosorption.
4.1.1. Langmuir isotherm
This model is derived considering monolayer heavy metals biosorption on the homogenous surface of biosorbent. This model can incorporate the thermodynamic consideration of the sorption process. This is accomplished by neglecting any surface interaction between two absorbed molecules (Citation153). The mathematical expression is shown in Equation 1.
(1)
(1) Where,
(mg/g) is maximum uptake capacity and b (L/mg) is the isotherm constant which can be calculated from the slope and intercept of the plot between
vs Ce.
4.1.2. Freundlich isotherm
It is entirely an empirical model and the biosorption of heavy metal ions onto heterogeneous biosorbent surfaces can occur without the saturation of the heavy metals binding site. The utilization of this particular model allows for the description of the biosorption process, wherein multiple layers of molecules are deposited onto the surface of the adsorbent. This is accomplished by taking into consideration the heterogeneity of the surface and the interactions between the adsorbing molecules and the adsorbent material (Citation19).
The linear form of Freundlich isotherm is given in Equation 2.
(2)
(2) Where kf (mg/g) and n are the isotherm constants and calculated from the intercept and slope of the plot between log
vs. log
.
4.1.3. Temkin isotherm
The Temkin isotherm model offers prognostications about the equally distributed binding energies tied to surface biosorption, thereby enhancing our comprehension of the process. This model is underpinned by the assumption that the decrease in heat connected to biosorption would progress linearly with an increase in coverage of the biosorbent. Consequently, it implies that the biosorption process is predominantly influenced by the harmonious allocation of binding energies among all molecules in the layer, until a specific threshold is reached (Citation154). Equation 3 illustrates the manifestation of the Temkin isotherm.
(3)
(3) Where R is the gas constant that is universally accepted and has a value of 8.341 J mol−1 K−1. The variable T denotes the temperature, measured in Kelvin. Additionally, bT and AT are the constants associated with the Temkin isotherm. AT is connected to heat adsorption, quantified in L/g, while bT corresponds to the maximum binding energy, expressed in kJ/mol. The determination of AT and bT involves the calculation of the intercept and slope from the qe vs ln Ce plots.
4.1.4. Halsey isotherm
The multilayer biosorption of heavy metal ions on the surface of the biosorbent at a greater distance from the adsorbent surface is described by the isotherm model mentioned in this study (Citation155). The Halsey isotherm is presented in Equation 4.
(4)
(4) Where, the Halsey isotherm constants, denoted as
and
, can be acquired by analyzing the slope and intercept of the plot between
and
.
4.2. Kinetics of heavy metal biosorption
The two most frequently employed models for the study of the kinetics of heavy metal biosorption are the pseudo first order kinetic model and the pseudo second order kinetic model, as stated by reference (Citation156). To determine the optimal contact time required to achieve sorption equilibrium, the analysis of the kinetics of the biosorption process is carried out. Furthermore, this analysis can offer valuable insights into the effects of process parameters such as temperature and pH, which are crucial for a comprehensive understanding of the biosorption properties exhibited by the given biosorbents, as emphasized by reference (Citation157). The remarkable effectiveness of Spirullina biomass as a biosorbent for heavy metal ions, particularly rhenium, in both industrial effluents and batch solutions has been demonstrated. The biosorption of rhenium utilizing Spirullina biomass demonstrates a closer alignment with the pseudo second-order kinetic model, as indicated by experimental evidence. Overall, these findings support the utilization of Spirullina biomass as an efficient and reliable biosorbent for the removal of heavy metal ions, such as rhenium, from contaminated water sources (Citation158).
4.2.1. Pseudo-first order kinetics
The pseudo-first-order model considers the reversible attachment of ligands with an adsorbent surface (Citation159). The pseudo-first-order kinetic model assumes that the uptake rate of heavy metals with time is directly proportional to the amount of available active sites on the adsorbent surface (Citation160). The linear expression of pseudo-first order is shown in Equation 5.
(5)
(5) The equilibrium rate constant ks can be determined by calculating the slope of
the plot of the adsorption capacity (qt) versus time (t). The adsorption capacities at time t and equilibrium, denoted as qt and qe respectively, are measured in milligrams per gram (mg/g). Zhang et al. (Citation160) studied the biosorption of Fe(II) and Mn(II) ions from aqueous solutions using rice husk ash. They reported that the biosorption data fitted well with the pseudo-first-order kinetic model, with R2 values of 0.98 for Fe(II) and 0.97 for Mn(II) (Citation160).
4.2.2. Pseudo-second order kinetics
The rate-limiting step in the biosorption process is assumed to be surface sorption, which includes chemisorption. This assumption is based on previous research (Citation161). The mathematical representation of the pseudo-second-order model can be seen in Equations 6 and 7.
(6)
(6) Where,
is the equilibrium constant
(7)
(7) Here,
and h are constants that can be calculated from the plot between t/qt vs t. Singh et al. (Citation136) studied the adsorption of Cr(VI) by chitosan-coated MnO2 nanoparticles from contaminated water. They reported that the pseudo-second-order model best fitted the adsorption data, indicating the chemosorption of Cr(VI) onto chitosan-coated MnO2 nanoparticles (Citation136).
4.3. Thermodynamics
The analysis of the thermodynamic properties, which includes the alterations in enthalpy (ΔH°), Gibbs free energy (ΔG°), and entropy (ΔS °), can be performed at different temperatures. If an increase in ΔH° is observed, it indicates that the process of adsorption requires an input of heat and can be further enhanced by raising the temperature. On the contrary, a negative result or value of ΔG° could imply that the adsorption process occurs spontaneously and its magnitude increases proportionally as the temperature increases (Citation11). The thermodynamic parameters were ascertained by employing Equations 8 and 9.
(8)
(8)
(9)
(9) The equilibrium concentration (Cae) is represented in mg/L, while the equilibrium metal ion concentration in the bulk solution (Ce) is also expressed in mg/L. The reaction temperature (T) is measured in Kelvin (K), and the universal constant (R) is equal to 8.314 mol−1 K−1. The values of ΔS° and ΔH° were determined by analyzing the intercept and slope of the plot between the natural logarithm of kc and the reciprocal of the reaction temperature (1/T).
Few studies have explored the relationship between the two thermodynamic parameters (ΔH° and ΔS°) and temperature. Most literature assumes that these parameters are relatively independent of temperature and can be considered constants with reliable accuracy. Ortiz-Oliveros et al. (Citation162) evaluated the removal of Pb ions using six different adsorbents from five literature sources, examining the temperature dependence of enthalpy and entropy. The study suggests that ΔH° and ΔS° parameters exhibit slight temperature dependence. This observation led to the proposal of an intriguing thermodynamic relationship between these two parameters due to their strong mutual causal correlation (Citation162). Ciobanu et al. (Citation163) studied the thermodynamic parameters for Cu(II) ions biosorption on algae biomass and derived biochars at various temperatures. They found that the calculated thermodynamic parameters for each case indicated a spontaneous and feasible biosorption process, regardless of the nature of the biosorbent (Citation163).
5. Longevity and environmental impacts of biosorbents used for heavy metal removal
Biosorbents offer a promising solution for heavy metal removal from wastewater due to their efficacy, cost-effectiveness, and environmentally friendly nature (Citation80, Citation164, Citation165). However, it is essential to carefully evaluate the durability and ecological consequences of biosorbents to ensure their sustainable application. The longevity of biosorbents is impacted by various factors including the type of biosorbent, the concentration of heavy metals in the wastewater, and the operational parameters (Citation166, Citation167). Generally, biosorbents can be utilized multiple times until they become saturated, at which point regeneration or replacement is necessary. The methods for regeneration vary depending on the biosorbent and may entail chemical or thermal processes (Citation121). Sufficient regeneration measures can extend the longevity of biosorbents and reduce overall costs. In addition, the fabrication of biosorbents can result in environmental effects (Citation168). The process of production or harvesting biomass for biosorbent creation may necessitate the use of land, water, and energy, leading to potential environmental harm. As a result, it is crucial to evaluate the environmental impact of biosorbent production and explore sustainable procurement approaches (Citation169, Citation170).
6. Technology challenges and future prospects
Both the prevalence of heavy metal pollution in aquatic ecosystems and the availability of environmentally benign techniques for removing the metals from polluted water have grown. Microbial detoxification is a very promising technique as a prospective heavy metal removal approach since it is both environmentally safe and very effective at removing heavy metals, alt-hough some areas still require improvement when using these methods at the industrial level (Citation171). Upon performing an in-depth literature analysis, it seems necessary to conduct more studies in the following areas:
A comprehensive economic assessment of biosorption methods (encompassing expenses related to adsorbent production, energy consumption, labor, etc.) should be conducted. It is infrequent for studies to provide a direct cost assessment of the materials utilized in the remediation of heavy metals.
Conducting a detailed analysis of biosorption methodologies is crucial for their effective implementation on a large scale, particularly in water treatment facilities.
Conducting a thorough life cycle analysis and techno-economic assessment is imperative for the successful integration of these methodologies at an industrial scale.
7. Conclusion
The discharge of metallic waste into soil, air, and water from industrial processes can harm humans and other animals. Heavy metal pollution poses significant health risks, including carcinogenic effects and severe diseases at high exposure levels. Physiochemical methods for heavy metal removal are available but often expensive and produce toxic sludge. Biosorption, however, offers a cost-effective, environmentally friendly alternative. Biosorption is effective even at low levels of heavy metals in wastewater. Isotherms, kinetics, and thermodynamic studies provide detailed insights into the behavior of heavy metal biosorption onto biosorbents. Embracing biosorption can mitigate the harmful impacts of heavy metal pollution, contributing to a cleaner and safer environment.
Author contributions
Concept, validation, formal analysis, writing-original draft preparation: Shijie Xie. Author has read and agreed to the published version of the manuscript.
Institutional review board statement
Not applicable.
Informed consent statement
Not applicable.
Acknowledgements
The author expresses gratitude to the Institute of International Rivers and Eco-Security at Yunnan University for offering essential assistance in conducting this research endeavor.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data mentioned in this manuscript.
Additional information
Funding
References
- Elwakeel, K.Z.; Ahmed, M.M.; Akhdhar, A.; Sulaiman, M.G.M.; Khan, Z.A. Recent Advances in Alginate-Based Adsorbents for Heavy Metal Retention from Water: A Review. Desalin. Water Treat. 2022, 272, 50–74. doi:10.5004/dwt.2022.28834.
- Elgarahy, A.M.; Eloffy, M.G.; Guibal, E.; Alghamdi, H.M.; Elwakeel, K.Z. Use of Biopolymers in Wastewater Treatment: A Brief Review of Current Trends and Prospects. Chin. J. Chem. Eng. 2023, 64, 292–320. doi:10.1016/j.cjche.2023.05.018.
- Mishra, S. Biomedical Impact of Heavy Metal Ions on Human Health. Int. J. Biochem. Physiol. 2023, 8 (1). doi:10.23880/ijbp-16000215.
- Adnan, M.; Xiao, B.; Xiao, P.; Zhao, P.; Li, R.; Bibi, S. Research Progress on Heavy Metals Pollution in the Soil of Smelting Sites in China. Toxics 2022, 10 (5), 231. doi:10.3390/toxics10050231.
- Mashabi, R.A.; Khan, Z.A.; Elwakeel, K.Z. Chitosan- or Glycidyl Methacrylate-based Adsorbents for the Removal of Dyes from Aqueous Solutions: A Review. Mater. Adv. 2022, 3 (14), 5645–5671. doi:10.1039/D2MA00320A.
- Elgarahy, A.M.; Elwakeel, K.Z.; Mohammad, S.H.; Elshoubaky, G.A. A Critical Review of Biosorption of Dyes, Heavy Metals and Metalloids from Wastewater as an Efficient and Green Process. Cleaner Eng. Technol. 2021, 4, 100209. doi:10.1016/j.clet.2021.100209.
- Zurutuza, I.; Isasti, N.; Detemple, E.; Schwinn, V.; Mohrbacher, H.; Uranga, P. Effect of Nb and Mo Additions in the Microstructure/Tensile Property Relationship in High Strength Quenched and Quenched and Tempered Boron Steels. Metals. (Basel) 2020, 11 (1), 29. doi:10.3390/met11010029.
- Elwakeel, K.Z.; Elgarahy, A.M.; Khan, Z.A.; Almughamisi, M.S.; Al-Bogami, A.S. Perspectives Regarding Metal/Mineral-Incorporating Materials for Water Purification: With Special Focus on Cr(vi) Removal. Mater. Adv. 2020, 1 (6), 1546–1574. doi:10.1039/D0MA00153H.
- Elwakeel, K.Z.; Ahmed, M.M.; Akhdhar, A.; Alghamdi, H.M.; Sulaiman, M.G.M.; Hamza, M.F.; Khan, Z.A. Effect of the Magnetic Core in Alginate/Gum Composite on Adsorption of Divalent Copper,: Cadmium, and Lead Ions in the Aqueous System. Int. J. Biol. Macromol. 2023, 253, 126884. doi:10.1016/j.ijbiomac.2023.126884.
- Guo, H.; Liu, H.; Wu, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Nickel Carcinogenesis Mechanism: DNA Damage. Int. J. Mol. Sci. 2019, 20 (19), 4690. doi:10.3390/ijms20194690.
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, Mechanism and Health Effects of Some Heavy Metals. Interdiscip. Toxicol. 2014, 7 (2), 60–72. doi:10.2478/intox-2014-0009.
- Naja, G.; Volesky, B. Multi-Metal Biosorption in a Fixed-Bed Flow-through Column. Colloids Surf., A 2006, 281 (1–3), 194–201. doi:10.1016/j.colsurfa.2006.02.040.
- Elgarahy, A.M.; Elwakeel, K.Z.; Elshoubaky, G.A.; Mohammad, S.H. Untapped Sepia Shell–based Composite for the Sorption of Cationic and Anionic Dyes. Water Air Soil Pollut. 2019, 230 (9). doi:10.1007/s11270-019-4247-1.
- Elbasiouny, H.; Darwesh, M.; Elbeltagy, H.; Abo-alhamd, F.G.; Amer, A.A.; Elsegaiy, M.A.; Khattab, I.A.; Elsharawy, E.A.; Ebehiry, F.; El-Ramady, H.; Brevik, E.C. Ecofriendly Remediation Technologies for Wastewater Contaminated with Heavy Metals with Special Focus on Using Water Hyacinth and Black Tea Wastes: A Review. Environ. Monit. Assess. 2021, 193 (7). doi:10.1007/s10661-021-09236-2.
- El Batouti, M.; Al-Harby, N.F.; Elewa, M.M. A Review on Promising Membrane Technology Approaches for Heavy Metal Removal from Water and Wastewater to Solve Water Crisis. Water. (Basel) 2021, 13 (22), 3241. doi:10.3390/w13223241.
- Sathya, K.; Nagarajan, K.; Carlin Geor Malar, G.; Rajalakshmi, S.; Raja Lakshmi, P. A Comprehensive Review on Comparison among Effluent Treatment Methods and Modern Methods of Treatment of Industrial Wastewater Effluent from Different Sources. Appl. Water. Sci. 2022, 12 (4). doi:10.1007/s13201-022-01594-7.
- Hopkins, D.; Hawboldt, K. Biochar for the Removal of Metals from Solution: A Review of Lignocellulosic and Novel Marine Feedstocks. J. Environ. Chem. Eng. 2020, 8 (4), 103975. doi:10.1016/j.jece.2020.103975.
- Capodaglio, A.G. Biorefinery of Sewage Sludge: Overview of Possible Value-Added Products and Applicable Process Technologies. Water. (Basel) 2023, 15 (6), 1195. doi:10.3390/w15061195.
- Kapahi, M.; Sachdeva, S. Bioremediation Options for Heavy Metal Pollution. J. Health Pollut. 2019, 9 (24). doi:10.5696/2156-9614-9.24.191203.
- Abd Elnabi, M.K.; Elkaliny, N.E.; Elyazied, M.M.; Azab, S.H.; Elkhalifa, S.A.; Elmasry, S.; Mouhamed, M.S.; Shalamesh, E.M.; Alhorieny, N.A.; Abd Elaty, A.E.; Elgendy, I.M.; Etman, A.E.; Saad, K.E.; Tsigkou, K.; Ali, S.S.; Kornaros, M.; Mahmoud, Y.A.-G. Toxicity of Heavy Metals and Recent Advances in their Removal: A Review. Toxics 2023, 11 (7), 580. doi:10.3390/toxics11070580.
- Elgarahy, A.M.; Mostafa, H.Y.; Zaki, E.G.; ElSaeed, S.M.; Elwakeel, K.Z.; Akhdhar, A.; Guibal, E. Methylene Blue Removal from Aqueous Solutions Using a Biochar/Gellan Gum Hydrogel Composite: Effect of Agitation Mode on Sorption Kinetics. Int. J. Biol. Macromol. 2023, 232, 123355. doi:10.1016/j.ijbiomac.2023.123355.
- Elgarahy, A.M.; Elwakeel, K.Z.; Elshoubaky, G.A.; Mohammad, S.H. Microwave-Accelerated Sorption of Cationic Dyes onto Green Marine Algal Biomass. Environ. Sci. Pollut. Res. 2019, 26 (22), 22704–22722. doi:10.1007/s11356-019-05417-2.
- Bădescu, I.S.; Bulgariu, D.; Ahmad, I.; Bulgariu, L. Valorisation Possibilities of Exhausted Biosorbents Loaded with Metal Ions – A Review. J. Environ. Manag. 2018, 224, 288–297. doi:10.1016/j.jenvman.2018.07.066.
- Lima e Silva, A.A.d.; Carvalho, M.A.R.d.; Souza, S.A.L.d.; Dias, P.M.T.; Silva Filho, R.G.d.; Saramago, C.S.d.M.; Bento, C.A.d.M.; Hofer, E. Heavy Metal Tolerance (Cr, Ag and Hg) in Bacteria Isolated from Sewage. Braz. J. Microbiol. 2012, 43 (4), 1620–1631. doi:10.1590/S1517-83822012000400047.
- Elgarahy, A.M.; Maged, A.; Elwakeel, K.Z.; El-Gohary, F.; El-Qelish, M. Tuning Cationic/Anionic Dyes Sorption from Aqueous Solution onto Green Algal Biomass for Biohydrogen Production. Environ. Res. 2023, 216, 114522. doi:10.1016/j.envres.2022.114522.
- Ungureanu, E.L.; Mocanu, A.L.; Stroe, C.A.; Panciu, C.M.; Berca, L.; Sionel, R.M.; Mustatea, G. Agricultural Byproducts Used as Low-cost Adsorbents for Removal of Potentially Toxic Elements from Wastewater: A Comprehensive Review. Sustainability 2023, 15 (7), 5999. doi:10.3390/su15075999.
- Salama, A.; Rashad, E.; Elgarahy, A.; Elwakeel, K. Effect of Green Synthesized Iron Oxide Nanoparticles on Bacterial Microbiome for Clean up the Crude Oil. Aswan Univ. J. Environ. Stud. 2023, 4 (1), 49–81. doi:10.21608/aujes.2023.178805.1110.
- Monachese, M.; Burton, J.P.; Reid, G. Bioremediation and Tolerance of Humans to Heavy Metals through Microbial Processes: A Potential Role for Probiotics? Appl. Environ. Microbiol. 2012, 78 (18), 6397–6404. doi:10.1128/AEM.01665-12.
- Pratush, A.; Kumar, A.; Hu, Z. Adverse Effect of Heavy Metals (As,: Pb. Hg, and Cr) on Health and Their Bioremediation Strategies: A Review. Int. Microbiol. 2018, 21 (3), 97–106. doi:10.1007/s10123-018-0012-3.
- Elwakeel, K.Z.; Al-Bogami, A.S.; Elgarahy, A.M. Efficient Retention of Chromate from Industrial Wastewater onto a Green Magnetic Polymer Based on Shrimp Peels. J. Polym. Environ. 2017, 26 (5), 2018–2029. doi:10.1007/s10924-017-1096-0.
- Elwakeel, K.Z.; Elgarahy, A.; Mohammad, M.; H, S. Use of Beach Bivalve Shells Located at Port Said Coast (Egypt) as a Green Approach for Methylene Blue Removal. J. Environ. Chem. Eng. 2017, 5 (1), 578–587. doi:10.1016/j.jece.2016.12.032.
- Elwakeel, K.Z.; Elgarahy, A.M.; Mohammad, S.H. Magnetic Schiff’s Base Sorbent Based on Shrimp Peels Wastes for Consummate Sorption of Chromate. Water Sci. Technol. 2017, 76 (1), 35–48. doi:10.2166/wst.2017.184.
- Buhani; Dewi, J.S.; Fajriyah, N.S.; Rilyanti, M.; Suharso; Sumadi; Elwakeel, K.Z. Modification of Non-Activated Carbon from Rubber Fruit Shells with 3-(Aminopropyl)-Triethoxysilane and its Adsorption Study on Coomassie Brilliant Blue and Methylene Blue in Solution. Water Air Soil Pollut. 2023, 234 (9). doi:10.1007/s11270-023-06506-2.
- Benettayeb, A.; Morsli, A.; Elwakeel, K.Z.; Hamza, M.F.; Guibal, E. Recovery of Heavy Metal Ions Using Magnetic Glycine-Modified Chitosan—Application to Aqueous Solutions and Tailing Leachate. Appl. Sci. 2021, 11 (18), 8377. doi:10.3390/app11188377.
- Elgarahy, A.M.; Al-Mur, B.A.; Akhdhar, A.; El-Sadik, H.A.; El-Liethy, M.A.; Elwakeel, K.Z.; Salama, A.M. Biosorption Kinetics of Cerium(III) and Cobalt(II) from Liquid Wastes Using Individual Bacterial Species Isolated from Low-level Liquid Radioactive Wastes. Environ. Sci. Pollut. Res. 2022, 30 (6), 15198–15216. doi:10.1007/s11356-022-23241-z.
- Elwakeel, K.Z.; El-Bindary, A.A.; Ismail, A.; Morshidy, A.M. Magnetic Chitosan Grafted with Polymerized Thiourea for Remazol Brilliant Blue R Recovery: Effects of Uptake Conditions. J. Dispersion Sci. Technol. 2016, 38 (7), 943–952. doi:10.1080/01932691.2016.1216436.
- Hegazy, G.E.; Soliman, N.A.; Ossman, M.E.; Abdel-Fattah, Y.R.; Moawad, M.N. Isotherm and Kinetic Studies of Cadmium Biosorption and its Adsorption Behaviour in Multi-metals Solution Using Dead and Immobilized Archaeal Cells. Sci. Rep. 2023, 13 (1). doi:10.1038/s41598-023-29456-5.
- Singh, V.; Singh, N.; Rai, S.N.; Kumar, A.; Singh, A.K.; Singh, M.P.; Sahoo, A.; Shekhar, S.; Vamanu, E.; Mishra, V. Heavy Metal Contamination in the Aquatic Ecosystem: Toxicity and Its Remediation Using Eco-Friendly Approaches. Toxics 2023, 11 (2), 147. doi:10.3390/toxics11020147.
- Luhar, I.; Luhar, S.; Abdullah, M.M.A.B.; Razak, R.A.; Vizureanu, P.; Sandu, A.V.; Matasaru, P.-D. A State-of-the-Art Review on Innovative Geopolymer Composites Designed for Water and Wastewater Treatment. Materials. (Basel) 2021, 14 (23), 7456. doi:10.3390/ma14237456.
- Nunes, N.; Ragonezi, C.; Gouveia, C.S.S.; Pinheiro de Carvalho, MÂA. Review of Sewage Sludge as a Soil Amendment in Relation to Current International Guidelines: A Heavy Metal Perspective. Sustainability 2021, 13 (4), 2317. doi:10.3390/su13042317.
- Wuana, R.A.; Okieimen, F.E. Heavy Metals in Contaminated Soils: A Review of Sources,: Chemistry, Risks and Best Available Strategies for Remediation. ISRN Ecol. 2011, 2011, 1–20. doi:10.5402/2011/402647.
- Sharma, P.; Singh, S.P.; Parakh, S.K.; Tong, Y.W. Health Hazards of Hexavalent Chromium (Cr (VI)) and its Microbial Reduction. Bioengineered 2022, 13 (3), 4923–4938. doi:10.1080/21655979.2022.2037273.
- Kubier, A.; Wilkin, R.T.; Pichler, T. Cadmium in Soils and Groundwater: A Review. Appl. Geochem. 2019, 108, 104388. doi:10.1016/j.apgeochem.2019.104388.
- Huff, J.; Lunn, R.M.; Waalkes, M.P.; Tomatis, L.; Infante, P.F. Cadmium-induced Cancers in Animals and in Humans. Int. J. Occup. Environ. Health 2007, 13 (2), 202–212. doi:10.1179/oeh.2007.13.2.202.
- Genchi, G.; Carocci, A.; Lauria, G.; Sinicropi, M.S.; Catalano, A. Nickel: Human Health and Environmental Toxicology. Int. J. Environ. Res. Public Health 2020, 17 (3), 679. doi:10.3390/ijerph17030679.
- Mustapha, M.U.; Halimoon, N. Microorganisms and Biosorption of Heavy Metals in the Environment: A Review Paper. J. Microb. Biochem. Technol. 2015, 7 (5). doi:10.4172/1948-5948.1000219.
- Sharma, S.; Tiwari, S.; Hasan, A.; Saxena, V.; Pandey, L.M. Recent Advances in Conventional and Contemporary Methods for Remediation of Heavy Metal-Contaminated Soils. 3. Biotech. 2018, 8 (4). doi:10.1007/s13205-018-1237-8.
- Bilal, M.; Rasheed, T.; Sosa-Hernández, J.; Raza, A.; Nabeel, F.; Iqbal, H. Biosorption: An Interplay between Marine Algae and Potentially Toxic Elements—A Review. Mar. Drugs. 2018, 16 (2), 65. doi:10.3390/md16020065.
- Sumiahadi, A.; Acar, R. A Review of Phytoremediation Technology: Heavy Metals Uptake by Plants. IOP Conf. Ser. Earth Environ. Sci. 2018, 142, 012023. doi:10.1088/1755-1315/142/1/012023.
- K, K.; Sardar, R.; Bhargavi, U.R.; Devi, E.; Bhunia, I.; Tiwari, B.; N, O. Advances in Exopolysaccharides Based Bioremediation of Heavy Metals in Soil and Water: A Critical Review. Carbohydr. Polym. 2018, 199, 353–364. doi:10.1016/j.carbpol.2018.07.037.
- Shafiq, M.; Alazba, A.A.; Amin, M.T. Removal of Heavy Metals from Wastewater Using Date Palm as a Biosorbent: A Comparative Review. Sains Malaysiana 2018, 47 (1), 35–49. doi:10.17576/jsm-2018-4701-05.
- Jamshed, Z.; VAmit, P. Review on Heavy Metal Pollution in Major Lakes of India: Remediation through Plants. Afr. J. Environ. Sci. Technol. 2017, 11 (6), 255–265. doi:10.5897/AJEST2017.2299.
- Sulaymon, A.H.; Mohammed, A.A.; Al-Musawi, T.J. Multicomponent Biosorption of Heavy Metals Using Fluidized Bed of Algal Biomass. J. Eng. 2023, 19 (4), 469–484. doi:10.31026/j.eng.2013.04.05.
- Marshall, W.E.; Champagne, E.T. Agricultural Byproducts as Adsorbents for Metal Ions in Laboratory Prepared Solutions and in Manufacturing Wastewater. J. Environ. Sci. Health A Environ. Sci. Eng. Toxicol. 1995, 30 (2), 241–261. doi:10.1080/10934529509376198.
- Zinc in the Soil and its Importance for the Plants and Human Health. An Integratedreview. Middle East J. Appl. Sci. 2021. doi:10.36632/mejas/2021.11.2.43.
- Wang, S.; Wang, N.; Yao, K.; Fan, Y.; Li, W.; Han, W.; Yin, X.; Chen, D. Characterization and Interpretation of Cd (II) Adsorption by Different Modified Rice Straws under Contrasting Conditions. Sci. Rep. 2019, 9 (1). doi:10.1038/s41598-019-54337-1.
- Park, J.-D.; Zheng, W. Human Exposure and Health Effects of Inorganic and Elemental Mercury. J. Prev. Med. Public Health 2012, 45 (6), 344–352. doi:10.3961/jpmph.2012.45.6.344.
- Kalyani, P.; Hemalatha, K.P.J. Biosorption of Heavy Metals in the Environment-A Review Paper. Int. J. Curr. Res. Acad. Rev. 2016, 4 (11), 66–74. doi:10.20546/ijcrar.2016.411.011.
- Charkiewicz, A.E.; Backstrand, J.R. Lead Toxicity and Pollution in Poland. Int. J. Environ. Res. Public Health 2020, 17 (12), 4385. doi:10.3390/ijerph17124385.
- Dadwal, A.; Mishra, V. Review on Biosorption of Arsenic From Contaminated Water. CLEAN Soil Air, Water 2017, 45 (7). doi:10.1002/clen.201600364.
- Ghosh, P.K.; Maiti, T.K.; Pramanik, K.; Ghosh, S.K.; Mitra, S.; De, T.K. The Role of Arsenic Resistant Bacillus Aryabhattai MCC3374 in Promotion of Rice Seedlings Growth and Alleviation of Arsenic Phytotoxicity. Chemosphere 2018, 211, 407–419. doi:10.1016/j.chemosphere.2018.07.148.
- Sheel, A.; Pant, D. Recovery of Gold from Electronic Waste Using Chemical Assisted Microbial Biosorption (Hybrid) Technique. Bioresour. Technol. 2018, 247, 1189–1192. doi:10.1016/j.biortech.2017.08.212.
- Qasem, N.A.A.; Mohammed, R.H.; Lawal, D.U. Removal of Heavy Metal Ions from Wastewater: A Comprehensive and Critical Review. NPJ Clean Water 2021, 4 (1). doi:10.1038/s41545-021-00127-0.
- Malik, D.S.; Jain, C.K.; Yadav, A.K. Removal of Heavy Metals from Emerging Cellulosic Low-cost Adsorbents: A Review. Appl. Water. Sci. 2016, 7 (5), 2113–2136. doi:10.1007/s13201-016-0401-8.
- Giripunje, M.D.; Fulke, A.B.; Meshram, P.U. Remediation Techniques for Heavy-Metals Contamination in Lakes: A Mini-review. CLEAN Soil Air Water 2015, 43 (9), 1350–1354. doi:10.1002/clen.201400419.
- Acharya, J.; Kumar, U.; Rafi, P.M. Removal of Heavy Metal Ions from Wastewater by Chemically Modified Agricultural Waste Material as Potential Adsorbent-A Review. Int. J. Curr. Eng. Technol. 2018. doi:10.14741/ijcet/v.8.3.6.
- Jabłońska, B.; Siedlecka, E. Removing Heavy Metals from Wastewaters with Use of Shales Accompanying the Coal Beds. J. Environ. Manag. 2015, 155, 58–66. doi:10.1016/j.jenvman.2015.02.015.
- Pohl, A. Removal of Heavy Metal Ions from Water and Wastewaters by Sulfur-Containing Precipitation Agents. Water Air Soil Pollut. 2020, 231 (10). doi:10.1007/s11270-020-04863-w.
- Gavrilescu, M. Removal of Heavy Metals from the Environment by Biosorption. Eng. Life Sci. 2004, 4 (3), 219–232. doi:10.1002/elsc.200420026.
- Barakat, M.A. New Trends in Removing Heavy Metals from Industrial Wastewater. Arabian J. Chem. 2011, 4 (4), 361–377. doi:10.1016/j.arabjc.2010.07.019.
- Liu, C.; Wu, T.; Hsu, P.-C.; Xie, J.; Zhao, J.; Liu, K.; Sun, J.; Xu, J.; Tang, J.; Ye, Z.; Lin, D.; Cui, Y. Direct/Alternating Current Electrochemical Method for Removing and Recovering Heavy Metal from Water Using Graphene Oxide Electrode. ACS Nano 2019, 13 (6), 6431–6437. doi:10.1021/acsnano.8b09301.
- Yadav, M.; Singh, G.; Jadeja, R.N. Physical and Chemical Methods for Heavy Metal Removal. Pollut. Water Manage. 2021, 377–397. doi:10.1002/9781119693635.ch15.
- Gupta, V.K.; Nayak, A. Cadmium Removal and Recovery from Aqueous Solutions by Novel Adsorbents Prepared from Orange Peel and Fe2O3 Nanoparticles. Chem. Eng. J. 2012, 180, 81–90. doi:10.1016/j.cej.2011.11.006.
- Volesky, B.; Holan, Z.R. Biosorption of Heavy Metals. Biotechnol. Prog. 1995, 11 (3), 235–250. doi:10.1021/bp00033a001.
- Jeenathunisa, N.; Jeyabharathi, S. Biosorption of Heavy Metal from Textile and Dye Industrial Effluent. Acta Sci. Pharm. Sci. 2021, 5 (11), 98–104. doi:10.31080/asps.2021.05.0735.
- Kapoor, A. Fungal Biosorption – an Alternative Treatment Option for Heavy Metal Bearing Wastewaters: A Review. Bioresour. Technol. 1995, 53 (3), 195–206.
- Najmanova, J.; Mackova, M.; Macek, T.; Kotrba, P. Preparation of Transgenic Flax with Enhanced Metal Tolerance. J. Biotechnol. 2007, 131 (2), S38–S39. doi:10.1016/j.jbiotec.2007.07.065.
- Demirbas, A. Heavy Metal Adsorption onto Agro-Based Waste Materials: A Review. J. Hazard. Mater. 2008, 157 (2–3), 220–229. doi:10.1016/j.jhazmat.2008.01.024.
- Yaashikaa, P.R.; Kumar, P.S.; Saravanan, A.; Vo, D.-V.N. Advances in Biosorbents for Removal of Environmental Pollutants: A Review on Pretreatment,: Removal Mechanism and Future Outlook. J. Hazard. Mater. 2021, 420, 126596. doi:10.1016/j.jhazmat.2021.126596.
- Karić, N.; Maia, A.S.; Teodorović, A.; Atanasova, N.; Langergraber, G.; Crini, G.; Ribeiro, A.R.L.; Đolić, M. Bio-Waste Valorisation: Agricultural Wastes as Biosorbents for Removal of (in)Organic Pollutants in Wastewater Treatment. Chem. Eng. J. Adv. 2022, 9, 100239. doi:10.1016/j.ceja.2021.100239.
- Ordóñez, J.I.; Cortés, S.; Maluenda, P.; Soto, I. Biosorption of Heavy Metals with Algae: Critical Review of Its Application in Real Effluents. Sustainability 2023, 15 (6), 5521. doi:10.3390/su15065521.
- El-Naggar, N.E.-A.; Hamouda, R.A.; Mousa, I.E.; Abdel-Hamid, M.S.; Rabei, N.H. Biosorption Optimization, Characterization, Immobilization and Application of Gelidium Amansii Biomass for Complete Pb2+ Removal from Aqueous Solutions. Sci. Rep. 2018, 8 (1). doi:10.1038/s41598-018-31660-7.
- Djedidi, Z.; Bouda, M.; Souissi, M.A.; Cheikh, R.B.; Mercier, G.; Tyagi, R.D.; Blais, J.-F. Metals Removal from Soil,: Fly Ash and Sewage Sludge Leachates by Precipitation and Dewatering Properties of the Generated Sludge. J. Hazard. Mater. 2009, 172 (2–3), 1372–1382. doi:10.1016/j.jhazmat.2009.07.144.
- Ariffin, N.; Abdullah, M.M.A.B.; Mohd Arif Zainol, M.R.R.; Murshed, M.F.; Hariz-Zain; Faris, M.A.; Bayuaji, R. Review on Adsorption of Heavy Metal in Wastewater by Using Geopolymer. MATEC Web Conf. 2017, 97, 01023. doi:10.1051/matecconf/20179701023.
- Perumal, S.; Atchudan, R.; Thirukumaran, P.; Yoon, D.H.; Lee, Y.R.; Cheong, I.W. Simultaneous Removal of Heavy Metal Ions Using Carbon Dots-Doped Hydrogel Particles. Chemosphere 2022, 286, 131760. doi:10.1016/j.chemosphere.2021.131760.
- Blöcher, C.; Dorda, J.; Mavrov, V.; Chmiel, H.; Lazaridis, N.K.; Matis, K.A. Hybrid Flotation—Membrane Filtration Process for the Removal of Heavy Metal Ions from Wastewater. Water Res. 2003, 37 (16), 4018–4026. doi:10.1016/S0043-1354(03)00314-2.
- Al-Enezi, G.; Hamoda, M.F.; Fawzi, N. Ion Exchange Extraction of Heavy Metals from Wastewater Sludges. J. Environ. Sci. Health A 2004, 39 (2), 455–464. doi:10.1081/ESE-120027536.
- Benettayeb, A.; Haddou, B. New Biosorbents Based on the Seeds,: Leaves and Husks Powder of Moringa Oleifera for the Effective Removal of Various Toxic Pollutants. Int. J. Environ. Anal. Chem. 2021, 103 (18), 6859–6884. doi:10.1080/03067319.2021.1963714.
- Osman, A.I.; El-Monaem, E.M.A.; Elgarahy, A.M.; Aniagor, C.O.; Hosny, M.; Farghali, M.; Rashad, E.; Ejimofor, M.I.; López-Maldonado, E.A.; Ihara, I.; Yap, P.-S.; Rooney, D.W.; Eltaweil, A.S. Methods to Prepare Biosorbents and Magnetic Sorbents for Water Treatment: A Review. Environ. Chem. Lett. 2023, 21 (4), 2337–2398. doi:10.1007/s10311-023-01603-4.
- Benettayeb, A.; Usman, M.; Tinashe, C.C.; Adam, T.; Haddou, B. A Critical Review with Emphasis on Recent Pieces of Evidence of Moringa Oleifera Biosorption in Water and Wastewater Treatment. Environ. Sci. Pollut. Res. 2022, 29 (32), 48185–48209. doi:10.1007/s11356-022-19938-w.
- Song, Y.; Lei, S.; Zhou, J.; Tian, Y. Removal of Heavy Metals and Cyanide from Gold Mine Waste-water by Adsorption and Electric Adsorption. J. Chem. Technol. Biotechnol. 2015, 91 (9), 2539–2544. doi:10.1002/jctb.4859.
- Ren, B.; Jin, Y.; zhao, L.; Cui, C.; Song, X. Enhanced Cr(VI) Adsorption Using Chemically Modified Dormant Aspergillus Niger Spores: Process and Mechanisms. J. Environ. Chem. Eng. 2022, 10 (1), 106955. doi:10.1016/j.jece.2021.106955.
- Wang, T.; Zheng, X.; Wang, X.; Lu, X.; Shen, Y. Different Biosorption Mechanisms of Uranium(VI) by Live and Heat-Killed Saccharomyces Cerevisiae under Environmentally Relevant Conditions. J. Environ. Radioact. 2017, 167, 92–99. doi:10.1016/j.jenvrad.2016.11.018.
- Buhani; Suharso; Rilyanti, M.; Antika, F.D.R.; Lestari, L.P.; Sumadi; Ansori, M.; Elwakeel, K.Z. Functionalization of Carbon from Rubber Fruit Shells (Hevea Brasiliensis) with Silane Agents and its Application to the Adsorption of Bi-Component Mixtures of Methylene Blue and Crystal Violet. Environmental Science and Pollution Research 2023. doi:10.1007/s11356-023-28031-9.
- Oyewole, O.A.; Zobeashia, S.S.L.-T.; Oladoja, E.O.; Raji, R.O.; Odiniya, E.E.; Musa, A.M. Biosorption of Heavy Metal Polluted Soil Using Bacteria and Fungi Isolated from Soil. SN Appl. Sci. 2019, 1 (8). doi:10.1007/s42452-019-0879-4.
- Asif, Z.; Chen, Z. Removal of Arsenic from Drinking Water Using Rice Husk. Appl. Water. Sci. 2015, 7 (3), 1449–1458. doi:10.1007/s13201-015-0323-x.
- Babazad, Z.; Kaveh, F.; Ebadi, M.; Mehrabian, R.Z.; Juibari, M.H. Efficient Removal of Lead and Arsenic Using Macromolecule-Carbonized Rice Husks. Heliyon 2021, 7 (3), e06631. doi:10.1016/j.heliyon.2021.e06631.
- Pillai, P.; Kakadiya, N.; Timaniya, Z.; Dharaskar, S.; Sillanpaa, M. Removal of Arsenic Using Iron Oxide Amended with Rice Husk Nanoparticles from Aqueous Solution. Mater. Today: Proc. 2020, 28, 830–835. doi:10.1016/j.matpr.2019.12.307.
- Zhao, S.; Sun, K.; Xie, P.; Zhang, S.; Zhang, J.; Zhu, Y.; Sun, Z. Mercury Removal from Coal Combustion Flue Gas by Mechanochemically Sulfur Modified Straw Coke and its Mercury Stability. Fuel 2024, 355, 129498. doi:10.1016/j.fuel.2023.129498.
- Badessa, T.S.; Wakuma, E.; Yimer, A.M. Bio-Sorption for Effective Removal of Chromium(VI) from Wastewater Using Moringa Stenopetala Seed Powder (MSSP) and Banana Peel Powder (BPP). BMC Chem. 2020, 14 (1). doi:10.1186/s13065-020-00724-z.
- Joshi, H.K.; Vishwakarma, M.C.; Kumar, R.; Sharma, H.; Joshi, S.K.; Bhandari, N.S. Adsorption of Cd2+ from Synthetic Wastewater by Modified Leaves of Eupatorium Adenophorum and Acer Oblongum: Thermodynamics, Kinetics and Equilibrium Studies. Discover Water 2022, 2 (1). doi:10.1007/s43832-022-00018-6.
- González-Tavares, C.; Salazar-Hernández, M.; Talavera-López, A.; Salgado-Román, J.M.; Hernández-Soto, R.; Hernández, J.A. Removal of Ni(II) and Cu(II) in Aqueous Solutions Using Treated Water Hyacinth (Eichhornia Crassipes) as Bioadsorbent. Separations 2023, 10 (5), 289. doi:10.3390/separations10050289.
- Davis, T.A.; Volesky, B.; Mucci, A. A Review of the Biochemistry of Heavy Metal Biosorption by Brown Algae. Water Res. 2003, 37 (18), 4311–4330. doi:10.1016/S0043-1354(03)00293-8.
- Asnaoui, H.; Laaziri, A.; Khalis, M. Study of the Kinetics and the Adsorption Isotherm of Cadmium(II) from Aqueous Solution Using Green Algae (Ulva Lactuca) Biomass. Water Sci. Technol. 2015, 72 (9), 1505–1515. doi:10.2166/wst.2015.359.
- Blaga, A.C.; Zaharia, C.; Suteu, D. Polysaccharides as Support for Microbial Biomass-Based Adsorbents with Applications in Removal of Heavy Metals and Dyes. Polymers. (Basel) 2021, 13 (17), 2893. doi:10.3390/polym13172893.
- Abdelhamid, H.N.; Mathew, A.P. Cellulose-based Materials for Water Remediation: Adsorption. Catal. Antifouling. Front. Chem. Eng. 2021, 3. doi:10.3389/fceng.2021.790314.
- Sharma, G.; Sharma, S.; Kumar, A.; Lai, C.W.; Naushad, M.; Shehnaz; Iqbal, J.; Stadler, F.J. Activated Carbon as Superadsorbent and Sustainable Material for Diverse Applications. Adsorpt. Sci. Technol. 2022, 2022, 1–21. doi:10.1155/2022/4184809.
- De Gisi, S.; Lofrano, G.; Grassi, M.; Notarnicola, M. Characteristics and Adsorption Capacities of Low-Cost Sorbents for Wastewater Treatment: A Review. Sustainable Mater. Technol. 2016, 9, 10–40. doi:10.1016/j.susmat.2016.06.002.
- Erdem, E.; Karapinar, N.; Donat, R. The Removal of Heavy Metal Cations by Natural Zeolites. J. Colloid Interface Sci. 2004, 280 (2), 309–314. doi:10.1016/j.jcis.2004.08.028.
- Margeta, K.; Vojnovic, B.; Zabukovec Logar, N. Development of Natural Zeolites for their Use in Water-treatment Systems. Recent Pat. Nanotechnol. 2011, 5 (2), 89–99. doi:10.2174/187221011795909170.
- Abdelwahab, O.; Thabet, W.M. Natural Zeolites and Zeolite Composites for Heavy Metal Removal from Contaminated Water and Their Applications in Aquaculture Systems: A Review. Egypt. J. Aquat. Res. 2023, 49 (4), 431–443. doi:10.1016/j.ejar.2023.11.004.
- Buhani; Istikomah; Suharso; Sumadi; Sutarto; Alghamdi, H.M.; Elwakeel, K.Z. Cationic Surfactant-Modified Tetraselmis Sp. for the Removal of Organic Dyes from Aqueous Solution. Molecules 2023, 28 (23), 7839. doi:10.3390/molecules28237839.
- Elwakeel, K.Z.; Elgarahy, A.M.; Al-Bogami, A.S.; Hamza, M.F.; Guibal, E. 2-Mercaptobenzimidazole-Functionalized Chitosan for Enhanced Removal of Methylene Blue: Batch and Column Studies. J. Environ. Chem. Eng. 2021, 9 (4), 105609. doi:10.1016/j.jece.2021.105609.
- Hamza, M.F.; Fouda, A.; Elwakeel, K.Z.; Wei, Y.; Guibal, E.; Hamad, N.A. Phosphorylation of Guar Gum/Magnetite/Chitosan Nanocomposites for Uranium (VI) Sorption and Antibacterial Applications. Molecules 2021, 26 (7), 1920. doi:10.3390/molecules26071920.
- do Carmo, S.N.; Merib, J.; Dias, A.N.; Stolberg, J.; Budziak, D.; Carasek, E. A Low-cost Biosorbent-Based Coating for the Highly Sensitive Determination of Organochlorine Pesticides by Solid-Phase Microextraction and Gas Chromatography-Electron Capture Detection. J. Chromatogr., A 2017, 1525, 23–31. doi:10.1016/j.chroma.2017.10.018.
- Wei, Y.; Salih, K.A.M.; Rabie, K.; Elwakeel, K.Z.; Zayed, Y.E.; Hamza, M.F.; Guibal, E. Development of Phosphoryl-Functionalized Algal-PEI Beads for the Sorption of Nd(III) and Mo(VI) from Aqueous Solutions – Application for Rare Earth Recovery from Acid Leachates. Chem. Eng. J. 2021, 412, 127399. doi:10.1016/j.cej.2020.127399.
- Priya, A.K.; Gnanasekaran, L.; Dutta, K.; Rajendran, S.; Balakrishnan, D.; Soto-Moscoso, M. Biosorption of Heavy Metals by Microorganisms: Evaluation of Different Underlying Mechanisms. Chemosphere 2022, 307, 135957. doi:10.1016/j.chemosphere.2022.135957.
- Elwakeel, K.Z.; Elgarahy, A.M.; Elshoubaky, G.A.; Mohammad, S.H. Microwave Assist Sorption of Crystal Violet and Congo Red Dyes onto Amphoteric Sorbent Based on Upcycled Sepia Shells. J. Environ. Health Sci. Eng. 2020, 18 (1), 35–50. doi:10.1007/s40201-019-00435-1.
- Adewuyi, A. Chemically Modified Biosorbents and Their Role in the Removal of Emerging Pharmaceutical Waste in the Water System. Water. (Basel) 2020, 12 (6), 1551. doi:10.3390/w12061551.
- Elwakeel, K.Z.; Aly, M.H.; El-Howety, M.A.; El-Fadaly, E.; Al-Said, A. Synthesis of Chitosan@activated Carbon Beads with Abundant Amino Groups for Capture of Cu(II) and Cd(II) from Aqueous Solutions. J. Polym. Environ. 2018, 26 (9), 3590–3602. doi:10.1007/s10924-018-1243-2.
- Huang, D.; Li, B.; Ou, J.; Xue, W.; Li, J.; Li, Z.; Li, T.; Chen, S.; Deng, R.; Guo, X. Megamerger of Biosorbents and Catalytic Technologies for the Removal of Heavy Metals from Wastewater: Preparation,: Final Disposal, Mechanism and Influencing Factors. J. Environ. Manag. 2020, 261, 109879. doi:10.1016/j.jenvman.2019.109879.
- Devanesan, S.; AlSalhi, M.S. Effective Removal of Cd2+, Zn2 + by Immobilizing the Non-Absorbent Active Catalyst by Packed Bed Column Reactor for Industrial Wastewater Treatment. Chemosphere 2021, 277, 130230. doi:10.1016/j.chemosphere.2021.130230.
- Ghosh, S.; Selvakumar, G.; Kennedy Ajilda, A.A.; Webster, T.J. Microbial Biosorbents for Heavy Metal Removal. New Trends Removal Heavy Met. Ind. Wastewater 2021, 213–262. doi:10.1016/B978-0-12-822965-1.00010-6.
- Rana, V.; Ghosh, D.; Maiti, S.K. Removal of Heavy Metals from Coke-plant Effluents by Using Wetlands. New Trends Removal Heavy Met. Ind. Wastewater 2021, 263–299. doi:10.1016/B978-0-12-822965-1.00011-8.
- Wei, W.; Wang, Q.; Li, A.; Yang, J.; Ma, F.; Pi, S.; Wu, D. Biosorption of Pb (II) from Aqueous Solution by Extracellular Polymeric Substances Extracted from Klebsiella Sp. J1: Adsorption Behavior and Mechanism Assessment. Sci. Rep. 2016, 6 (1). doi:10.1038/srep31575.
- Wang, J.; Chen, C. Chitosan-Based Biosorbents: Modification and Application for Biosorption of Heavy Metals and Radionuclides. Bioresour. Technol. 2014, 160, 129–141. doi:10.1016/j.biortech.2013.12.110.
- Gadd, G.M. Biosorption: Critical Review of Scientific Rationale,: Environmental Importance and Significance for Pollution Treatment. J. Chem. Technol. Biotechnol. 2008, 84 (1), 13–28. doi:10.1002/jctb.1999.
- Blázquez, G.; Hernáinz, F.; Calero, M.; Martín-Lara, M.A.; Tenorio, G. The Effect of pH on the Biosorption of Cr (III) and Cr (VI) with Olive Stone. Chem. Eng. J. 2009, 148 (2–3), 473–479. doi:10.1016/j.cej.2008.09.026.
- Surisetty, V.R.; Kozinski, J.; Rao Nageswara, L. Biosorption of Lead Ions from Aqueous Solution UsingFicus benghalensisL. J. Eng. 2013, 2013, 1–8. doi:10.1155/2013/167518.
- Taşar, Ş; Kaya, F.; Özer, A. Biosorption of Lead(II) Ions from Aqueous Solution by Peanut Shells: Equilibrium. J. Environ. Chem. Eng. 2014, 2 (2), 1018–1026. doi:10.1016/j.jece.2014.03.015.
- International Proceedings of Economics Development and Research. doi:10.7763/ipedr.
- Olukanni, D.O.; Agunwamba, J.C.; Ugwu, E.I. Biosorption of Heavy Metals in Industrial Wastewater Using Microorganisms (Pseudomonas Aeruginosa). Am. J. Sci. Ind. Res. 2014, 5 (2), 81–87. doi:10.5251/ajsir.2014.5.2.81.87.
- Kanu, S.A.; Moyo, M.; Zvinowanda, C.M.; Okonkwo, J.O. Biosorption of Pb(II) from Aqueous Solution Using Rooibos Shoot Powder (RSP). Desalin. Water Treat. 2015, 57 (12), 5614–5622. doi:10.1080/19443994.2015.1004116.
- Mehrotra, T.; Dev, S.; Banerjee, A.; Chatterjee, A.; Singh, R.; Aggarwal, S. Use of Immobilized Bacteria for Environmental Bioremediation: A Review. J. Environ. Chem. Eng. 2021, 9 (5), 105920. doi:10.1016/j.jece.2021.105920.
- Ali Redha, A. Removal of Heavy Metals from Aqueous Media by Biosorption. Arab J. Basic and Appl. Sci. 2020, 27 (1), 183–193. doi:10.1080/25765299.2020.1756177.
- Singh, V.; Singh, J.; Mishra, V. Sorption Kinetics of an Eco-Friendly and Sustainable Cr (VI) Ion Scavenger in a Batch Reactor. J. Environ. Chem. Eng. 2021, 9 (2), 105125. doi:10.1016/j.jece.2021.105125.
- Elijah Leizou, K.; Ashraf, M.A. Adsorption of CD II and CR VI Ions on Unripe Banana (Musa Sapientum) Peel Biomass. Sustainable Environ. Benign Mater. Eng. Herit. J. 2022, 6 (2), 45–50. doi:10.26480/gwk.02.2022.45.50.
- Asymmetric Allylic Amination of Allylic Esters Catalyzed by Palladium. Synfacts 2014, 10 (7), 0714–0714. doi:10.1055/s-0034-1378262.
- Farhan, S.N.; Khadom, A.A. Biosorption of Heavy Metals from Aqueous Solutions by Saccharomyces Cerevisiae. Int. J. Ind. Chem. 2015, 6 (2), 119–130. doi:10.1007/s40090-015-0038-8.
- Bamisaye, A.; Adesina, M.O.; Alfred, M.O.; Ige, A.R.; Idowu, M.A.; Adegoke, K.A. Kinetic and Mechanistic Study of the Biosorption of Copper(II) Ion from Wastewater Using Porous Chitosan-Walnut Shell Composite. Chem. Data Collect. 2023, 45, 101024. doi:10.1016/j.cdc.2023.101024.
- Lucaci, A.-R.; Bulgariu, L. Biosorption of Technologically Valuable Metal Ions on Algae Wastes: Laboratory Studies and Applicability. Water. (Basel) 2024, 16 (4), 512. doi:10.3390/w16040512.
- Okpara-Elom, I.A.; Onochie, C.C.; Elom, M.O.; Ezaka, E.; Elom, O. Bioremediation of Heavy Metal Polluted Soil Using Plant Growth Promoting Bacteria: An Assessment of Response. Biorem. J. 2022, 1–20. doi:10.1080/10889868.2022.2143473.
- Rozumová, L.; Životský, O.; Seidlerová, J.; Motyka, O.; Šafařík, I.; Šafaříková, M. Magnetically Modified Peanut Husks as an Effective Sorbent of Heavy Metals. J. Environ. Chem. Eng. 2016, 4 (1), 549–555. doi:10.1016/j.jece.2015.10.039.
- Yu, K.L.; Lee, X.J.; Ong, H.C.; Chen, W.-H.; Chang, J.-S.; Lin, C.-S.; Show, P.L.; Ling, T.C. Adsorptive Removal of Cationic Methylene Blue and Anionic Congo Red Dyes Using Wet-Torrefied Microalgal Biochar: Equilibrium,: Kinetic and Mechanism Modeling. Environ. Pollut. 2021, 272, 115986. doi:10.1016/j.envpol.2020.115986.
- Shafiq, M.; Alazba, A.A.; Amin, M.T. Kinetic and Isotherm Studies of Ni2+ and Pb2+ Adsorption from Synthetic Wastewater Using Eucalyptus Camdulensis—Derived Biochar. Sustainability 2021, 13 (7), 3785. doi:10.3390/su13073785.
- Mohamed, M.S.; Hozayen, W.G.; Alharbi, R.M.; Ibraheem, I.B.M. Adsorptive Recovery of Arsenic (III) Ions from Aqueous Solutions Using Dried Chlamydomonas Sp. Heliyon 2022, 8 (12), e12398. doi:10.1016/j.heliyon.2022.e12398.
- Kumari, S.; Agrawal, N.K.; Agarwal, A.; Kumar, A.; Malik, N.; Goyal, D.; Rajput, V.D.; Minkina, T.; Sharma, P.; Garg, M.C. A Prominent Streptomyces Sp. Biomass-Based Biosorption of Zinc (II) and Lead (II) from Aqueous Solutions: Isotherm and Kinetic. Separations 2023, 10 (7), 393. doi:10.3390/separations10070393.
- Li, X.; Deng, R.; Tang, Z.; Zhou, S.; Zeng, X.; Wang, J.; Hursthouse, A. A Study of the Adsorption and Removal of Sb(III) from Aqueous Solution by Fe(III) Modified Proteus Cibarius with Mechanistic Insights Using Response Surface Methodology. Processes 2021, 9 (6), 933. doi:10.3390/pr9060933.
- Bulgariu, L.; Ferţu, D.I.; Cara, I.G.; Gavrilescu, M. Efficacy of Alkaline-Treated Soy Waste Biomass for the Removal of Heavy-Metal Ions and Opportunities for Their Recovery. Materials. (Basel) 2021, 14 (23), 7413. doi:10.3390/ma14237413.
- Bulgariu, D.; Nemeş, L.(N).; Ahmad, I.; Bulgariu, L. Isotherm and Kinetic Study of Metal Ions Sorption on Mustard Waste Biomass Functionalized with Polymeric Thiocarbamate. Polymers. (Basel) 2023, 15 (10), 2301. doi:10.3390/polym15102301.
- Lima, É; Pinto, D.; Schadeck Netto, M.; Dos Reis, G.; Silva, L.; Dotto, G. Biosorption of Neodymium (Nd) from Aqueous Solutions Using Spirulina Platensis Sp. Strains. Polymers. (Basel) 2022, 14 (21), 4585. doi:10.3390/polym14214585.
- Abdelfattah, I.; Ismail, A.A.; Sayed, F.A.; Almedolab, A.; Aboelghait, K.M. Biosorption of Heavy Metals Ions in Real Industrial Wastewater Using Peanut Husk as Efficient and Cost Effective Adsorbent. Environ. Nanotechnol. Monit. Manage. 2016, 6, 176–183. doi.org/10.1016/j.enmm.2016.10.007.
- Alves, A.R.A.; Yin, Q.; Oliveira, R.S.; Silva, E.F.; Novo, L.A.B. Plant Growth-Promoting Bacteria in Phytoremediation of Metal-Polluted Soils: Current Knowledge and Future Directions. Sci. Total Environ. 2022, 838, 156435. doi:10.1016/j.scitotenv.2022.156435.
- Liu, Y.; Feng, J.; Pan, H.; Zhang, X.; Zhang, Y. Genetically Engineered Bacterium: Principles,: Practices, and Prospects. Front. Microbiol. 2022, 13. doi:10.3389/fmicb.2022.997587.
- Aquino, E.; Barbieri, C.; Oller Nascimento, C.A. Engineering Bacteria for Bioremediation. Prog. Mol. Environ. Bioeng. Anal. Model. Technol. Appl. 2011. doi:10.5772/19546.
- Tálos, K.; Pernyeszi, T.; Majdik, C.; Hegedűsova, A.; Páger, C. Cadmium Biosorption by Baker’s Yeast in Aqueous Suspension. J. Serb. Chem. Soc. 2012, 77 (4), 549–561. doi:10.2298/JSC110520181T.
- Michalak, I.; Chojnacka, K.; Witek-Krowiak, A. State of the Art for the Biosorption Process—a Review. Appl. Biochem. Biotechnol. 2013, 170 (6), 1389–1416. doi:10.1007/s12010-013-0269-0.
- Zinicovscaia, I.; Safonov, A.; Troshkina, I.; Demina, L.; German, K. Biosorption of Re(VII) from Batch Solutions and Industrial Effluents by Cyanobacteria Spirulina Platensis. CLEAN Soil Air Water 2018, 46 (7). doi:10.1002/clen.201700576.
- Liu, S.; Zhang, F.; Chen, J.; Sun, G. Arsenic Removal from Contaminated Soil via Biovolatilization by Genetically Engineered Bacteria under Laboratory Conditions. J. Environ. Sci. 2011, 23 (9), 1544–1550. doi:10.1016/S1001-0742(10)60570-0.
- Zhang, Y.; Zhao, J.; Jiang, Z.; Shan, D.; Lu, Y. Biosorption of Fe(II) and Mn(II) Ions from Aqueous Solution by Rice Husk Ash. BioMed Res. Int. 2014, 2014, 1–10. doi:10.1155/2014/973095.
- Bondarenko, O.; Rõlova, T.; Kahru, A.; Ivask, A. Bioavailability of Cd, Zn and Hg in Soil to Nine Recombinant Luminescent Metal Sensor Bacteria. Sensors 2008, 8 (11), 6899–6923. doi:10.3390/s8116899.
- Ortiz-Oliveros, H.B.; Ouerfelli, N.; Cruz-Gonzalez, D.; Avila-Pérez, P.; Bulgariu, L.; Flaifel, M.H.; Abouzeid, F.M. Modeling of the Relationship between the Thermodynamic Parameters ΔH° and ΔS° with Temperature in the Removal of Pb Ions in Aqueous Medium: Case Study. Chem. Phys. Lett. 2023, 814, 140329. doi:10.1016/j.cplett.2023.140329.
- Ciobanu, A.A.; Bulgariu, D.; Ionescu, I.A.; Puiu, D.M.; Vasile, G.G.; Bulgariu, L. Evaluation of Thermodynamic Parameters for Cu(II) Ions Biosorption on Algae Biomass and Derived Biochars. Symmetry. (Basel) 2023, 15 (8), 1500. doi:10.3390/sym15081500.
- Abdulkhaleq Alalwan, H.; Alminshid, A.; Mustafa Mohammed, M.; Mohammed, M.; Hatem Shadhar, M. Reviewing of using Nanomaterials for Wastewater Treatment. Pollution 2022, 8 (3), 995–1013. doi:10.22059/poll.2022.337436.1329.
- Ali, M.; Alalwan, N.S.; Alminshid, H.A.; & Mohammed, A.H.; M, M. Synthesis and Characterization of Fe3O4- SiO2 Nanoparticles as Adsorbent Material for Methyl Blue Dye Removal from Aqueous Solutions. Pollution 2022, 8 (1), 295–302. doi:10.22059/poll.2021.328697.1157.
- Alalwan, H.A.; Mohammed Ali, N.S.; Mohammed, M.M.; Mohammed, M.F.; Alminshid, A.H. A Comparison Study of Methyl Green Removal by Peroxi-Coagulation and Peroxi-Electrocoagulation Processes. Cleaner Eng. Technol. 2023, 13, 100623. doi:10.1016/j.clet.2023.100623.
- Elwakeel, K.Z.; Elgarahy, A.M.; Guibal, E. A Biogenic Tunable Sorbent Produced from Upcycling of Aquatic Biota-Based Materials Functionalized with Methylene Blue Dye for the Removal of Chromium(VI) Ions. J. Environ. Chem. Eng. 2021, 9 (2), 104767. doi:10.1016/j.jece.2020.104767.
- Qin, H.; Hu, T.; Zhai, Y.; Lu, N.; Aliyeva, J. The Improved Methods of Heavy Metals Removal by Biosorbents: A Review. Environ. Pollut. 2020, 258, 113777. doi:10.1016/j.envpol.2019.113777.
- Gautam, R.K.; Mudhoo, A.; Lofrano, G.; Chattopadhyaya, M.C. Biomass-Derived Biosorbents for Metal Ions Sequestration: Adsorbent Modification and Activation Methods and Adsorbent Regeneration. J. Environ. Chem. Eng. 2014, 2 (1), 239–259. doi:10.1016/j.jece.2013.12.019.
- Bhattacharjee, C.; Dutta, S.; Saxena, V.K. A Review on Biosorptive Removal of Dyes and Heavy Metals from Wastewater Using Watermelon Rind as Biosorbent. Environ. Adv. 2020, 2, 100007. doi:10.1016/j.envadv.2020.100007.
- Sodhi, K.K.; Mishra, L.C.; Singh, C.K.; Kumar, M. Perspective on the Heavy Metal Pollution and Recent Remediation Strategies. Curr. Res. Microb. Sci. 2022, 3, 100166. doi:10.1016/j.crmicr.2022.100166.