Abstract
Shade, in ecological sense, is not merely a lack of light, but a multi-faceted phenomenon that creates new and complex settings for community and ecosystem dynamics. Tolerating shade therefore affects plants’ ability to cope with other stressors, and also shape its interactions with surrounding organisms. The aim of this broad review was to map our current knowledge about how shade affects plants, plant communities and ecosystems – to gather together knowledge of what we know, but also to point out what we do not yet know. This review covers the following topics: the nature of shade, and ecological and physiological complexities related to growing under a canopy; plants’ capability of tolerating other stress factors while living under a shade – resource trade-offs and polytolerance of abiotic stress; ontogenetic effects of shade tolerance; coexistence patterns under the canopy – how shade determines the forest structure and diversity; shade-induced abiotic dynamics in understorey vegetation, including changing patterns of irradiance, temperature and humidity under the canopy; shade-driven plant–plant and plant–animal interactions – how shade mediates facilitation and stress, and how it creates differentiated environment for different herbivores and pollinators, including the role of volatile organic compounds. We also discuss the ways how vegetation in understorey environments will be affected by climate change, as shade might play a significant role in mitigating negative effects of climate change. Our review shows that living under a shade affects biotic and abiotic stress tolerance of plants, it also influences the outcomes of both symbiotic and competitive plant–plant and plant–animal interactions in a complex and dynamic manner. The current knowledge of shade-related mechanisms is rather ample, however there is much room for progress in integrating different implications of the multifaceted nature of shade into consistent and integral understanding how communities and ecosystems function.
Introduction
Light availability varies beneath plant canopies and between gap and understorey locations, and there is strong variation among plant species in the ability to grow and survive in different strata within the vegetation canopy. Shade tolerance, which is commonly defined as the species-specific minimum light required for survival, is a concept initially developed in forest science (e.g. Gayer Citation1898; Zon and Graves Citation1911; Shirley Citation1943), but it has been increasingly regarded in ecology as a key driver of long-term ecosystem dynamics (Shugart and West Citation1980; Bonan and Shugart Citation1989; Bugmann et al. Citation1996). However, shade is often treated simply as conditions of low light conditions, whereas it actually involves a wide range of environmental factors with various effects on plants (), and there is still much to understand about the complex ecological implications of shade and shade tolerance (; see also reviews on shade tolerance by Valladares and Niinemets Citation2008; Grubb Citation2015).
Table 1. What we know about shade and shade tolerance, and what we do not know yet.
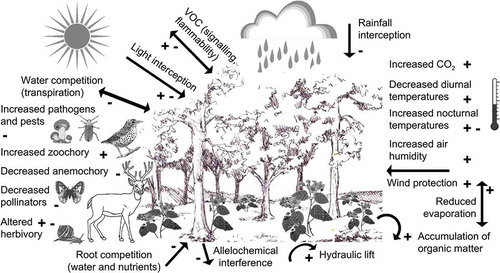
Figure 1. Understorey plant life involves coping with a wide range of environmental changes. While some environmental conditions are clearly beneficial for understorey plants (e.g. increased organic matter in the soil, wind protection, increased seed dispersal by animals) others are disadvantageous (e.g. root competition for water and nutrients, increased phytophagous fungi and pests, decreased number of pollinators) as indicated in the figure by plus and minus symbols, respectively. At least four environmental factors can have either negative or positive effect on recruitment of plant individuals: reduced irradiance (positive under photoinhibitory conditions, negative when light interception by the canopy severely limits understorey photosynthesis), herbivory (positive when the canopy acts as a barrier for herbivores, negative when more herbivores are found in the shade), volatile organic compounds (VOCs, positive when attracting pests’ enemies, negative when increasing flammability), and increased nocturnal temperatures (positive when cold snaps are attenuated, negative when respiration and thus carbon loss is enhanced).
Environmental complexity can cause the light requirement for plant survival and growth to be higher under natural environments than in greenhouse conditions (Baltzer and Thomas Citation2007). Shade can act directly to limit photosynthesis but it can also act indirectly on the potential for carbon gain through morphological and physiological acclimation responses (Niinemets and Valladares Citation2004; Niinemets Citation2007; Laanisto and Niinemets Citation2015). Vegetation dynamics and succession in many communities is primarily driven by interspecific differences in resource uptake and stress tolerance, with light frequently being a key resource (Canham et al. Citation1994; Jucker et al. Citation2015; Zhang et al. Citation2015). Thus, differences in shade tolerance between co-occurring species are central to ecosystem dynamics and community ecology. The importance of shade tolerance has long been recognised, and the mechanisms involved in the tolerance of low light (whether suboptimal irradiance or modified spectral range) and the implications of shade tolerance for regeneration and vegetation dynamics have been incorporated in the current theories of vegetation succession (Bazzaz and Pickett Citation1980; Kobe and Coates Citation1997; Lusk et al. Citation2015; Santo-Silva et al. Citation2015). However, shade tolerance is estimated in a wide variety of ways on many different scales, making comparisons difficult (Kobe and Coates Citation1997; Niinemets and Valladares Citation2006; Baltzer and Thomas Citation2007; Holmgren et al. Citation2012; Miyashita and Tateno Citation2014), which is why shade-related studies hardly form an integral and comprehensive body of research.
Shade is often associated with cooler temperatures during day, warmer temperatures at night, and higher air humidity and soil moisture. It may also be associated with increased herbivory (Baraza et al. Citation2004) and greater competition for below-ground resources because dense above-ground vegetation also exploits below-ground space heavily (Valladares and Pearcy Citation2002). Reducing light intensity, damping temperature variation and ameliorating soil conditions under the canopy are some mechanisms by which established plants facilitate other plants (Valiente-Banuet and Ezcurra Citation1991; Pugnaire et al. Citation2004), but in most cases facilitation effects are less significant as water and nutrient availabilities become lower (Maestre et al. Citation2006). Established plants, typically trees and shrubs, casting shade on others are physical ecosystem engineers that directly or indirectly control the availability of resources to other organisms (Jones et al. Citation1997).
Shade can thus be considered in its literal sense of low light, or in a more general and ecologically meaningful sense that takes into account which suite of factors, including low light, together with altered atmospheric and substrate conditions, and biotic interactions. In this review, we advocate the latter treatment of shade because natural habitats rarely provide shaded locations where light availability alone distinguishes them from open locations. Though the current dynamic vegetation models include a large number of traits to characterise different plant functional types, the predictions of these models are currently rather tentative, making vegetation dynamics one of the largest sources of uncertainty in Earth system models (Cramer et al. Citation2001; Baudena et al. Citation2014; Fischer et al. Citation2016). Decreasing this uncertainty for densely vegetated areas requires both improvements in trait databases, including estimates of tolerance to key environmental drivers, and advances in understanding of the mechanisms associated with competition between species for light (Purves and Pacala Citation2008), and on species’ tolerance of interacting stressors, v (i.e. stress polytolerance, Niinemets and Valladares Citation2006; Hallik et al. Citation2009; Laanisto and Niinemets Citation2015; Kunstler et al. Citation2016).
Since shade, in its ecological sense, involves much more than low light supply, and because tolerance to a given stress is modified by the simultaneous presence of other stresses and ecological interactions, new research questions in this area cannot be adequately addressed with the current simplified conceptual understanding of shade. This review aims to provide a more comprehensive framework in which to consider the ecological consequences for plants of being located in shaded parts of heterogeneous habitats. It is divided into three broad sections that cover some of the most promising aspects of the current shade tolerance research. First, we discuss the nature of shade, the ecological trade-offs associated with living and surviving in the understorey, the effects of other stresses on shade tolerating species, whether polytolerance depends on phylogeny and ontogeny, and whether stress tolerance patterns affect species coexistence in shaded conditions. Second, we elaborate the ecological and physiological complexity of understorey life – how shade affects other abiotic, and biotic stress factors, and the characteristics of competition in the understorey. Finally, we address aspects of global change with respect to shade, in particular how elevated CO2, climate warming, and invasive species affect plant life in the understorey, and whether shade can help to mitigate some of the effects of climate and global change.
Shade tolerance and community dynamics
The challenge of shade for plants
Although the way in which plants cope with shade has been extensively studied, there is still debate about the importance of different traits, patterns of biomass allocation, and developmental and reproductive strategies (; Poorter and Bongers Citation2006; Valladares and Niinemets Citation2008; Niinemets Citation2010). Three main strategies have been identified: tolerance of low light by maximising net carbon gain (Givnish Citation1988); tolerance of low light by enhanced persistence and increased investments in storage and defence (Kitajima Citation1994; Kobe and Coates Citation1997). Avoidance of low light by escaping the understorey – e.g. through allometric adjustments – is a third strategy, followed by certain shade intolerant plants when they germinate in the shade or when neighbours’ growth decreases light availability (Henry and Aarssen Citation1997; Zavala et al. Citation2011; Zhang et al. Citation2015). Whereas the first two strategies allow plants to persist in the understorey for long periods of time, and are thus characteristic of shade tolerant plants, the third strategy can only be successful in coping with shade for a short period of time as plants that invest heavily in greater stem extension are unable to also invest in leaf construction. Unless stem extension results in increased light availability this strategy, which is characteristic of moderately tolerant and intolerant species, rapidly becomes unsustainable (Henry and Aarssen Citation1997). Valladares and Niinemets (Citation2008) suggested that carbon gain and defence/storage strategies in the shade are not mutually exclusive because different suites of traits are responsible for the successful performance of different species in the understorey. However, at least for some groups of species, such as palms, which have monopodial architecture and little architectural plasticity, this hypothesis is unsupported (Ma et al. Citation2015).
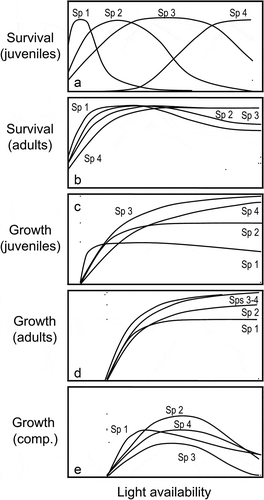
Figure 2. Idealised survival (a–b) and growth (c–e) responses to light availability in four species of contrasting shade tolerances. While species 1 is a typical shade tolerator, species 4 does not tolerate shade, and the other two species represent various intermediate cases. Different responses are observed in juveniles (a and c) and adults (b and d–e) of the very same species, with adults exhibiting less pronounced interspecific differences in their responses to light than juveniles. Competition (e; when growth response is analysed in multi-specific scenarios) affects non-linearly the response to light, tending to decrease growth for a given light availability.
Polytolerance to abiotic stress
Plants in most communities are subjected to multiple stresses, but the extent to which they can tolerate several stresses simultaneously is still poorly understood. Trade-offs between responses to various stresses have been suggested, as contrasting structural and physiological traits are required to tolerate different stresses (Pivovaroff et al. Citation2014; Grubb Citation2015). These trade-offs are generally considered rather universal, and mainly dependent on physico-chemical constraints, whereas biological adaptations are regarded as less important (Sack Citation2004; Laanisto and Niinemets Citation2015). For instance, as tolerance of shade requires a large leaf area, whereas tolerance of drought relies on an extensive root system, it has been suggested that drought tolerance and shade tolerance are inversely correlated (Smith and Huston Citation1989). However, such trade-offs might only hold for certain species and communities (Grubb Citation2015).
Trade-offs with low explanatory power, and even a complete absence of a trade-off between shade tolerance and drought tolerance have been recently found, suggesting that there is at least some capacity to tolerate both of these stresses, especially in species from warmer climates (Markesteijn and Poorter Citation2009). A meta-analysis using data from 806 woody species from the northern hemisphere (Laanisto and Niinemets Citation2015) showed that adaptation to different abiotic stress factors in woody plants is highly complex. Simultaneous tolerance of shade and drought was related to the length of the growing season and dormancy. These are the key factors explaining why woody plants are less able to tolerate both shade and drought in habitats where the growing season is short and the water table is high (Laanisto and Niinemets Citation2015). While this pattern was independent of species region of origin and leaf type, the trade-off patterns were different for angiosperms and gymnosperms. This has also been shown to be the phylogenetic line of division in other stress tolerance studies (Stahl et al. Citation2013; Coyle et al. Citation2014; but see Hallik et al. Citation2009). Dormancy enables angiosperms to respond more successfully to additional stress factors besides shade and drought; however, gymnosperms, which have lower polytolerance, can tolerate shade and drought more easily when other environmental factors are favourable (Pivovaroff et al. Citation2014; Laanisto and Niinemets Citation2015).
The main alternative to traditional theories (e.g. Smith and Huston Citation1989) for explaining the variability in species ecological potentials and trade-offs has been the trait-based approach (Niinemets et al. Citation2009; Ouédraogo et al. Citation2013). The extent of interspecific variability in tolerance towards different types of stress indicates that adaptive evolution and ecological filtering play key roles in polytolerance (Sack Citation2004; Niinemets and Valladares Citation2006; Valladares and Niinemets Citation2008; Hawkins et al. Citation2014; Grubb Citation2015; Laanisto and Niinemets Citation2015). Examination of polytolerance patterns, including trade-off vs. orthogonal responses to drought and light, is vital for understanding species distributions, and the potential for niche differentiation along natural gradients of irradiance and water supply.
Ontogenetic effects: shade tolerance from seedlings to adults
Although all species can benefit from growing in gaps in moist ecosystems (Augspurger Citation1984), many germinate better in understorey shade and some do not germinate in gaps at all. The capacity to germinate in the understorey or in gaps might be linked to water limitations in the prevailing habitat for germination (Augspurger Citation1984), with larger seed size compensating for unpredictability in rainfall conditions, at least in tropical rainforests (O’Brien et al. Citation2013). Overall, a requirement of light for germination cannot distinguish pioneer from non-pioneer species because seed and seedling light requirements are not necessarily the same (Figueroa and Lusk Citation2001). While the initial growth rate of tree seedlings has been shown to have strong correlations with the whole spectrum of functional traits under shady conditions, there are clear divergences between different leaf habit types (Modrzynski et al. Citation2015).
As plants grow, they generally invest less of their total mass in photosynthesising leaves and more in respiring roots, stems and branches. Consequently, their requirement for light increases with their size (Givnish Citation1988; Niinemets Citation2006; Poorter et al. Citation2012). Although ranking of different species’ light requirements remains fairly constant with ontogeny, minimum light requirement can shift during plant ontogeny (Kneeshaw et al. Citation2006; Niinemets Citation2006; Lusk et al. Citation2008). The trade-offs between shade tolerance and tolerance to scarcity of other resources can also be influenced by ontogenetic stage (). As for the trade-off between tolerance of drought and shade, investment in roots increases more rapidly with plant size in shade intolerant than in shade tolerant species (Niinemets Citation1998; Lusk et al. Citation2008). Thus, larger trees can usually be expected to be more tolerant of drought due to their deeper root systems (Cavender-Bares and Bazzaz Citation2000), although it depends on root architecture. This effect is greater in shade intolerant than shade tolerant species. Experimental studies on seed germination together with studies on seedling, juvenile and adult plants reveal important temporal changes in the traits conferring shade tolerance, in the dimensions of the ecological niche and in the nature and strength of plant–plant interactions during the lifetime of a plant (Miriti Citation2006; Quero et al. Citation2008). Recent studies have shown that the influence of shade tolerance on biomass allocation can differ in juvenile and adult trees of the same species (Franceschini and Schneider Citation2014; Grubb Citation2015)
Light partitioning, species coexistence and the niche
There is a consensus that shade tolerance is at the heart of many ecological processes scaling from populations to ecosystems (Smith and Huston Citation1989; Zavala et al. Citation2007; Laanisto and Niinemets Citation2015). In particular, interspecific differences in the ability to compete for light and in shade tolerance are considered key determinants of forest stand structure and dynamics. Microclimatic effects generated by the forest canopy buffer macroclimatic changes, and create “climatic lags” that help to maintain understorey biodiversity (De Frenne et al. Citation2013; Nieto‐Lugilde et al. Citation2014).
Earlier studies have supported the notion that gap colonisers are capable of rapid growth in high light, whereas late-successional species are capable of sustained, though slow growth in the understorey (Bazzaz and Pickett Citation1980). Thus, successional replacement in temperate forests has been typically explained by a trade-off between growth at low and high light, with shade tolerant species growing faster in shade than shade intolerant species and with species interchanging at higher light availabilities (Shugart Citation1984). Incorporation of competition between light-demanding and shade-tolerant species in dynamic vegetation models has led to more realistic predictions of vegetation composition (Smith et al. Citation2001; Hickler et al. Citation2004). Shade tolerance has been shown to be one of four attributes (along cold tolerance, drought tolerance and growth form) required to provide realistic simulations of forest dynamics in central and northern Europe (Bugmann Citation1996; Fischer et al. Citation2016), and it is now recognised to have played a key role in the dynamics of European primeval forests which were darker and denser than traditionally assumed (Birks Citation2005; Xu et al. Citation2015). Despite this recognition, the main mechanisms underlying species coexistence and segregation along light gradients remain under debate in most vegetation types.
The light partitioning hypothesis (LPH) is among the most extended paradigms of the role of light in driving forest dynamics and species coexistence. This hypothesis is based on three main premises: (i) there must be variation in light availability, (ii) species must show a differential distribution with respect to light availability, and (iii) there must be trade-offs in species’ growth that generate their different positions along the light gradient (Goldberg Citation1990; Poorter and Arets Citation2003; Stahl et al. Citation2013). This hypothesis has been validated in a competitive dynamic model scenario (Pacala et al. Citation1996) and experimental evidence has confirmed it in temperate moist forest ecosystems (e.g. Abrams and Mostoller Citation1995) and in tropical forests (e.g. Bazzaz and Pickett Citation1980; Poorter and Arets Citation2003). However, a number of studies suggest that the light partitioning hypothesis might not explain species distribution patterns in species-rich ecosystems, since many species overlap strongly in their light requirements (Wright et al. Citation2003; Getzin et al. Citation2014; Lilles et al. Citation2014). Some of the failure of the LPH is due to the degree of shade considered, with some studies not exploring plant performance at very low light levels (i.e. <2% of sunlight). Plant survival and growth exhibit non-linear responses to light, with exponential decreases at very low light levels that are rare in many temperate ecosystems, particularly in dry ones, but frequent in tropical forests (Poorter Citation1999).
In temperate forests, the hypothesis that successional dynamics are driven by trade-offs in growth rates across light gradients has been partly abandoned in favour of a low light survival – high light growth trade-off (Pacala et al. Citation1996), although it has also been suggested that both mechanisms could operate in certain temperate forests (Lin et al. Citation2002). It has also been suggested that other trade-offs associated with tolerances to disturbance and low temperatures (Stott and Loehle Citation1998), or waterlogging and drought (Niinemets and Valladares Citation2006; Laanisto and Niinemets Citation2015), interact with shade tolerance to enable species coexistence in temperate forests. Phylogenetic background also has a significant role in influencing trade-off patterns in woody species through differentiated adaptations (Hawkins et al. Citation2014; Laanisto and Niinemets Citation2015); for example, larger hydraulic conduits in angiosperms in comparison with gymnosperms are thought to be key reason of their successful radiation (Zanne et al. Citation2014).
Trade-offs between survival in shade and fast growth in high light have been reported in Mediterranean forests (Sánchez-Gómez et al. Citation2006), but shade tolerance also exhibits a trade-off with drought and strategies related to disturbance (Zavala et al. Citation2000). Resprouter species that can re-establish vegetatively following disturbance or herbivory tend to be shade tolerant as a consequence of their ability to persist in the understorey without significant growth (Retana et al. Citation1999). In contrast, species that regenerate from seed after disturbance tend to be shade intolerant but more drought tolerant than resprouters (Ojeda Citation1998). It is increasingly evident that shade tolerance is affected by water availability (Sánchez‐Gómez et al. Citation2006) and by many other factors, so that niche partitioning for light cannot by itself explain the coexistence of many species (see, for example, the case of the common European deciduous trees Fagus sylvatica and Quercus pubescens in sub-Mediterranean forests (Kunstler et al. Citation2005)).
In moist tropical ecosystems, species coexistence and forest dynamics are to a great extent driven by functional differences in the capacity to compete for light, and several trade-offs at various ontogenetic stages have been invoked to explain niche differentiation (Kitajima and Poorter Citation2008; Dent et al. Citation2013). Niche specialisation along the light gradient by seedlings has been linked to a trade-off between growth in high light and survival in the shade, rather than by reversals in growth rank between low- and high-light environments (Kitajima Citation1994). A trade-off between growth rate and maximum height of adult plants has been shown to promote coexistence, because shorter species invest fewer resources in support and more in reproduction under shaded conditions (Kohyama et al. Citation2003).
Niche-based hypotheses to explain species coexistence have also been challenged by the neutral theory, which states that local coexistence is driven by broader biogeographical and evolutionary processes rather than by deterministic ecological mechanisms, emphasising the importance of intraspecific variability (Hubbell Citation2001; Valladares et al. Citation2015). This theory is attractive for its simplicity, but provocative for its assumption that individuals of different species in rich communities are ecologically equivalent. However, the existence of clear successional and spatial segregation between tropical species suggests a role for niche partitioning, with functional groups or guilds of species segregating along environmental gradients (Purves and Pacala Citation2008; Getzin et al. Citation2014). However, high within-species variability in leaf trait responses has been shown to be significantly affected by shading also in sub-boreal conifer species with different niche preferences (Lilles et al. Citation2014). Both approaches, niche and neutrality, can be regarded as the opposing ends of a continuum from competitive to stochastic exclusion, with neutrality becoming more pronounced as species richness, niche overlap and dispersal capabilities increase (Gravel et al. Citation2006; Valladares et al. Citation2015), for example towards the Equator, where the prolonged vegetation period allows to successfully overcome tolerating several stress factors at the same time (Laanisto and Niinemets Citation2015).
Understorey life: much more than coping with shade
The understorey can be cooler or warmer, wetter or drier and usually more fertile than the overstorey
The presence of a canopy generates a whole suite of alterations in the abiotic environment () due to comprehensive intricacies from direct effects of light availability to plant individuals to indirect influences on the whole ecosystem level – not just irradiance, temperature and humidity under the shade, but also the processes in soils are affected by the presence of the canopy. We are currently only grasping the implications of these modifications for plant survival and growth in the understorey. During daytime, reduced irradiance makes the understorey environment some degrees cooler than the above-canopy environment, while obstruction of convective and radiative heat loss by the canopy makes the understorey a few degrees warmer at night (Jacobs et al. Citation1994; Niinemets and Valladares Citation2004). Thus, reduced daily oscillations in air temperature are typically found in the understorey. This creates differences in air humidity, with vapour pressure deficit being generally lower in the understorey than in the open (Niinemets and Valladares Citation2004). These differences have important implications for plant carbon gain, as air temperature affects photosynthesis and respiration, and air humidity affects stomatal conductance (Sellin et al. Citation2010). Thus, photosynthetic water use efficiency in the shade is reduced both because of lower carbon gain under limiting light and due to increased stomatal conductance (Niinemets and Valladares Citation2004; Valladares et al. Citation2008), though the latter is compensated by high understorey CO2 concentrations in the tropics (Tomimatsu et al. Citation2014). Apart from the direct effects of higher humidity on stomata, lower air temperature, wind speed and vapour pressure deficit reduce the transpiration rate in the understorey at given stomatal conductance. These environmental modifications are partially counteract the negative effects of low light on water use efficiency. Due to this complex interplay of factors, the issue of whether plants in the shade always have reduced water use efficiency than their counterparts in high light requires further research.
Increased humidity in the shade also affects the performance of other understorey organisms. Fungi in particular can benefit from more humidity, and their increased abundance can have significant negative impacts on plant performance. For instance, the mildew Microsphaera alphitoides reduces the capacity of the European deciduous oak Quercus robur to survive in the shade; survival of seedlings with mildew infestation is lower under reduced light (Jacobs Citation2003). In addition, bacteria, fungi, algae, lichens and epiphytes on leaf surface are more abundant in the humid understories in tropical rainforests, and significantly reduce light interception by leaves (Anthony et al. Citation2002; Lindow and Brandl Citation2003).
Due to canopy interception, 10–30% less precipitation can reach the soil beneath the canopy, especially when it is received in the form of short spells of light rain, fog and dew (Liu Citation1997). In some ecosystems, canopy interception can represent as much as half of the rainfall (Thurow et al. Citation1987). Interception of rainfall, coupled with strong root competition for water by established trees or shrubs, can make the substrate beneath canopies drier than in nearby gaps in tropical and temperate forest ecosystems, particularly during dry years (Abrams and Mostoller Citation1995; Kursar Citation1998). Finally, the presence of a canopy affects the properties of the soil both directly, by physical, chemical and biological root-mediated processes, and indirectly, by altering the abiotic conditions that influence microbial activity and organic matter decomposition. In general, soils beneath a canopy are more fertile and less compact than those in the open (Gómez-Aparicio et al. Citation2005). However, soil fertility itself might not affect the shade tolerance patterns of understorey plants (Sendall et al. Citation2015).
All these examples illustrate radically different abiotic conditions in the understorey compared to open sites for a wide range of factors beyond irradiance. While some of these alterations have a negative effect on plant performance in the understorey, others are beneficial (), and this suite of environmental changes will have different net effects on each plant species, depending mostly on how the plant perceives and modulates its capacity to cope with all environmental changes taking place below the canopy.
Competition for resources or habitat amelioration?
All the abiotic modifications generated directly or indirectly by a canopy are capable of influencing plant-to-plant interactions. Recent analyses of the consequences of shade tolerance for plant communities have produced a shift of interest from considering competition for light as a driver of succession to attenuation of high light stress and excessive transpiration by neighbours as a potential driver of facilitation (Pagès et al. Citation2003; Valladares and Niinemets Citation2008). There is ample support for the importance of “canopy and soil effects” due to “nurse” plants being beneficial for the establishment and growth of understorey plants (Callaway Citation1995; Gómez-Aparicio et al. Citation2005; Brooker et al. Citation2008). However, canopy plants absorb a large proportion of the available irradiance, water and nutrients, so there is always likely to be competition for resources between canopy and understorey plants. The substrate can also be more fertile beneath a canopy, but plants can interfere with each other via allelopathic compounds, making the facilitation-competition balance for a given set of abiotic conditions more negative (Pugnaire et al. Citation2004). In general, negative effects of a canopy on plant fitness are due to an excessive capture of irradiance and rainfall by the canopy, while positive effects are related to increased moisture and nutrient availability and less extreme temperatures beneath the canopy (Tielbörger and Kadmon Citation2000). Whether the net result for the target plant is positive or negative depends on the availability of resources in the environment and on the target plant’s capacity to cope with resource shortages (). The interplay between shade tolerance and phenotypic plasticity of the interacting species is also important for community dynamics, because the extent of both can affect the sign and magnitude of species interactions (Valladares et al. Citation2007; Semchenko et al. Citation2012).
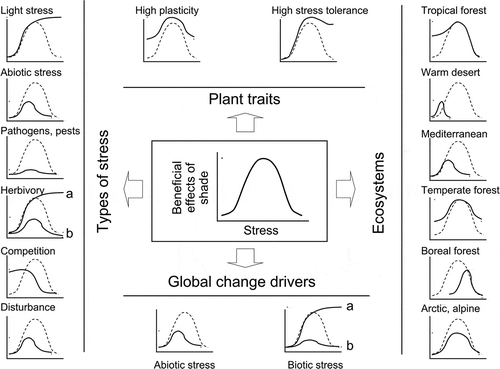
Figure 3. Understorey life (shade sensu lato) can be advantageous for plants when compared to life in the open. We suggest that how these beneficial effects of shade vary with stress can be represented by a Gaussian bell, with an intermediate range of stress intensity for which understorey life is clearly advantageous. In general, the advantages of living in the shade would be reduced at either low or high levels of stress because environmental conditions cannot be ameliorated by a canopy at the ends of the stress gradient, either because there is no condition to be enhanced with respect to the open when stress is low or because stress is exacerbated by limiting light when the former is very intense. But, the net balance of advantages and disadvantages, and thus the shape of this beneficial effect of the shade vs. stress intensity curve, varies depending on the type of stress, the traits of the target plant and the overall conditions of the ecosystem (compare continuous lines with dashed lines representing the general model in each graph). While phenotypic plasticity and stress tolerance of the target plant tend to increase the potential benefits of living in the shade, most stresses tend to decrease them. However, high light stress makes the shade increasingly beneficial at increasing stress till benefits reach a species-specific plateau, and competition-induced stress makes the shade beneficial only at low levels of stress. Shade becomes relatively more beneficial at low levels of stress in dry and hot ecosystems, but more beneficial at high levels of stress in boreal forests due to the nature of the predominant stress in each ecosystem, i.e. drought vs. freezing temperatures respectively. Stresses induced by the many drivers of global change also affect the beneficial effects of the shade vs. stress intensity curve. Shade can be increasingly beneficial with increasing herbivory if the canopy is effective in keeping away the herbivores (a, central graph at the left hand side column of graphs) or it can be disadvantageous if herbivores are more active or in greater number in the shade (b). Analogously, the stress induced by invasive species can make the shade increasingly beneficial if invasive species prefer high light habitats (a, graph at the right in the lower row of graphs) or it can make it disadvantageous over most of the stress range if invasive species prefer understorey habitats (b).
In semi-arid ecosystems, positive effects of plant cover on both resource availability and plant performance beneath are well documented. Plants can improve soil mycorrhizal networks and increase infiltration of water, so recruitment for many species is greater in shady microsites than in open sites (Verdú and Valiente-Banuet. Citation2008). In Mediterranean ecosystems, where summer drought is a key abiotic species filter, the interplay between shade and water availability is subtler and more complex, so net positive effects of carbon gain can alter along environmental gradients (Maestre et al. Citation2006; Pivovaroff et al. Citation2014). In Mediterranean evergreen oak savannas, tree cover has a positive effect on soil water supply and the costs associated with higher transpiration can be overcome by the benefits of increased infiltration (Joffre and Rambal Citation1993). In sub-humid Mediterranean forests, canopy cover can also exert a positive effect on seedling recruitment, although through very different mechanisms. In Mediterranean oak forest ecosystems soil waterlogging hampers seedling emergence and survival, resulting in sheltered microsites, where canopy interception decreases the risk of waterlogging (Urbieta et al. Citation2008). Positive effects of partial canopy cover on seedling recruitment have been detected in other Mediterranean forests, but the facilitative effects seem to disappear towards the driest end of the gradient (Maestre et al. Citation2006). The suggestion that the importance of facilitation increases with the severity of abiotic stress, i.e. the stress gradient hypothesis is currently under intense debate (Lortie and Callaway Citation2006; Maestre et al. Citation2006). Recent community studies on plant–plant interactions that account for the evolutionary history of the interacting species have indicated that certain ancient species lineages can cope with the current climates because of the nurse effects provided by more recently-evolved taxa (the wet Tertiary-dry Quaternary transition that affected the flora of Mediterranean-type ecosystems Valiente-Banuet et al. Citation2006). These studies also show that facilitation turns into competition when interacting species become more closely related (Valiente-Banuet and Verdu Citation2008). The overall role of facilitation in plant community dynamics has been studied intensively (Valiente-Banuet and Verdu Citation2013), and we argue that a better understanding of the ecology of understorey life is crucial for making further progress in this field ().
Plant–animal interactions are different in the understorey and in gaps
Both biotic and abiotic stresses operate in plant communities to shape neighbourhood plant responses, and these stresses change along gap-understorey gradients. Shrub cover can have a positive influence on tree recruitment by protecting tree seedlings from herbivores, and this positive effect operates in conjunction with abiotic stress amelioration in vegetated sites ( and ; Smit et al. Citation2007). Interestingly, the relative importance of biotic and abiotic effects changes along stress gradients, and also depends on functional traits of the interacting species (Gómez-Aparicio et al. Citation2008). Thus, for example, mortality due to resource limitation in the understorey can be balanced by mortality due to herbivory in gaps. In other cases, herbivores are more abundant, or exert greater feeding pressure in the understorey, so that the overall impact of herbivory can be greater in shade than in the open (Baraza et al. Citation2004). Ultraviolet radiation, which varies in intensity with total irradiance along the gap-understorey gradient, has a significant impact on the interactions between plants and animals. The intensity of insect herbivory increases when the ultraviolet band of solar radiation is attenuated (Mazza et al. Citation2002). Thus, the net outcome of plant–herbivore interactions depends not only on herbivory pressure and the light environment, but also on the identity of both the canopy species and the understorey species (Smit et al. Citation2007). Results from different studies exploring plant–plant interactions in multifactor scenarios (e.g. Gómez-Aparicio et al. Citation2005; Quero et al. Citation2008; Valladares et al. Citation2008) indicate that shade tolerance can influence the outcome of these interactions in a complex manner. Interestingly, plants under stress emit volatile organic compounds (VOCs), which can attract the enemies of herbivores, leading to reduced herbivory pressure under a canopy of VOC-emitting plants (Baldwin et al. Citation2006; Copolovici et al. Citation2014). This topic deserves further attention to fully assess the net outcome of plant–plant interactions mediated by their influence on animals. Plant responses to herbivory under different levels of resource availability is an active topic of research in which the proponents of competing theories are seeking empirical support (Wise and Abrahamson Citation2007; Blande et al. Citation2014).
Pollination and dispersal by animals also alter along the gap-understorey gradient. The light environment significantly affects the pollinator assemblages of plant species, affecting their reproduction and fitness (Herrera Citation1997). Although many animal-dispersed seeds are brought to understorey locations (e.g. Gómez et al. Citation2004), seed predation, particularly by small rodents, is also more intense in the understorey. Thus, plant cover can have detrimental effects on seedling recruitment (Pérez-Ramos et al. Citation2008) despite the many beneficial effects of a plant cover for seedling recruitment (see a revision in Callaway Citation2007).
Global change and shade localities
Understanding future Earth responses to global change is one of the most pressing issues for humanity. Global change alters many environmental drivers and modifies numerous biotic and abiotic factors that collectively have profound effects on plant performance in the shade. Preliminary reviews suggest that many of the uncertainties associated with multi-species interactions and dynamics may be increased by global climate change (Parmesan Citation2006). Rather than providing a detailed review of all aspects of global climate change related to understorey plant life, we concentrate on key issues that can modify vegetation performance in the understorey, with emphasising new combinations of factors and environmental conditions.
Elevated CO2
Increased CO2 concentrations enhance light-saturated photosynthetic rate and growth under shade (DeLucia and Thomas Citation2000; Kim et al. Citation2015), and also result in higher soluble carbohydrate concentrations within plants (e.g. Tjoelker et al. Citation1998). This could imply that under increased CO2 concentrations seedlings of a given age could be larger, and have greater carbon reserves. Both of these modifications would be expected to increase survival in shaded conditions. Although growth enhancement depends strongly on nutrient availability, and declines as plants increase in size, there is evidence that slower-growing species, including many shade-tolerant species, can respond more strongly to elevated CO2 than species of open habitats (Tjoelker et al. Citation1998; Hattenschwiler Citation2001).
Although the effects of elevated CO2 on maximum photosynthesis may be short-lived or limited under nutrient-limited conditions, elevated CO2 also causes reductions in stomatal conductance and improved water use efficiency (e.g. Saxe et al. Citation1998), and accordingly plants can support larger leaf areas in drier forests. Furthermore, elevated CO2 increases the low-light quantum yield of photosynthesis (i.e. the amount of CO2 fixed per unit light intercepted) (e.g. DeLucia and Thomas Citation2000), implying that plants maintain a positive carbon balance under lower light, compared with their performance under ambient CO2 concentration. Thus, enhanced quantum yield results in an overall increase in leaf area (Pritchard et al. Citation1999). Forest understories may therefore be darker than in the future, benefitting more shade tolerant species (Birks Citation2005; Xu et al. Citation2015). Differences in species’ growth responsiveness and leaf area production might therefore alter vegetation dynamics significantly in atmospheres with elevated CO2. Information on the responses of many species to elevated CO2 is lacking, however, complicating inclusion of the effects of CO2 concentration on shade tolerance into global models (Kim et al. Citation2015).
Warming understorey
Effects of global warming are further complicated by contrasting regional scenarios. While moderate warming is predicted for the southern hemisphere, temperatures in the northern hemisphere are predicted to rise by ca. 5 K by the end of 2100. Whereas winter warming is predicted for northern boreal and temperate forests, summer warming is predicted for Mediterranean and tropical environments (Meehl et al. Citation2007). Warm temperatures can increase respiration through dynamic thermal acclimation (Atkin and Tjoelker Citation2003), potentially accelerating losses of carbon, which are hard to compensate for when photosynthetic rates in the shade are low. Additionally, understorey vegetation dynamics in the Mediterranean are predicted to change significantly due to increasing frequency of wildfires and extreme climatic events, including heat waves and prolonged droughts (Barros et al. Citation2015). Acclimation of respiration to increased temperatures has been shown to be crucial for understorey plants of low productivity ecosystems (Zaragoza‐Castells et al. Citation2008). Warm episodes in winter can deplete carbohydrate reserves and seriously impact winter survival, though only in colder climates (Ögren et al. Citation1997). For evergreen conifers, in addition to carbon depletion, episodes with high light and high air temperatures when the substrate is still frozen can be particularly damaging due to both photoinhibition and desiccation stresses, especially when foliar temperatures of conifer shoots can exceed ambient air temperatures under these conditions by 5–10°C (Germino and Smith Citation1999).
In warmer climates, mid-day heat during summer can exceed the heat stress limit of photosynthesis and this, in combination with elevated evaporative demands, can have a serious impact on plant survival and growth. Although mid-day thermal maxima are buffered in the understorey, warming can counteract elevated CO2 effects on foliage area, resulting in more open understories with amplified levels of abiotic stress (Valladares et al. Citation2008).
Changing water availability in the understorey
The combination of low light availability and dry substrates is becoming increasingly common in many Mediterranean (Valladares et al. Citation2008) and tropical (Kursar Citation1998) ecosystems. In contrast, waterlogged understories are becoming more frequent in temperate and boreal ecosystems (Meehl et al. Citation2007; Barros et al. Citation2015). Waterlogged conditions during winter are increasingly frequent in many temperate forests, but winter-dormant trees are essentially insensitive to waterlogging. Given that warmer winters in boreal ecosystems can break dormancy earlier, waterlogging is likely to become a more severe stress. Given the trade-offs between waterlogging and shade tolerance (Niinemets and Valladares Citation2006), a higher incidence of waterlogging events in flat areas may more than counterbalance the effects of elevated CO2 on foliage area, leading to more open northern forests in the future.
Tree seedling regeneration under severe drought frequently requires facilitation by shrubs, but limiting herb competition alters the precise role of facilitation in the colonisation process, at least in temperate sub-mediterranean ecosystems (Kunstler et al. Citation2006). Thus, it is not only the individual plant performance that is challenged by simultaneous changes in shade and soil water but also the balance of plant–plant interactions.
Exotic invasive species in the understorey
Invasive non-native species are recognised as one of the main global change drivers of biodiversity change; they can alter ecosystem function, producing, in turn, feedbacks that drive further changes in community composition (Gómez-Aparicio and Canham Citation2008). Earlier studies described invasive plants as occurring primarily in forest gaps and open environments. Thus, intact, closed-canopy forests were believed to be resistant to exotic plant invasions. But intact forests only weakly resisted invasion by Acer platanoides in North America (Martin and Marks Citation2006). Once established, Acer platanoides exerted a profound change in understorey light quantity, becoming an important driver of suppression of native species and conspecific success in invaded riparian communities (Reinhart et al. Citation2006). There is an increasing awareness of understorey colonisation by invasive plants, many of which exhibit high phenotypic plasticity in response to light (Niinemets et al. Citation2003). In fact, a large ecological niche breadth involving the capacity to perform well both in high and low light conditions is believed to contribute to invasive success (Sultan Citation2001; Traveset et al. Citation2008).
The expansion of invasive species in the understorey is affected by other global change drivers such as elevated CO2, altered precipitation and increased soil nutrients. The increasing abundance of the non-indigenous evergreen Prunus laurocerasus in the understorey of Swiss temperate forests has been related to increasing atmospheric CO2 (Hättenschwiler and Körner Citation2003). The magnitude of the impact of invasive species increases with increasing soil fertility in north-eastern United States (Gómez-Aparicio and Canham Citation2008), and increased climate variability is expected to favour invasion by exotic species in the understorey of South American ecosystems through altered herbivory pressure, as extreme climate events such as El Niño trigger significant and complex changes in herbivore populations (Manrique et al. Citation2007).
Conclusions
Ecologists should not treat shade merely as a lack of light. Canopy shade influences understorey plant life in multifaceted ways, creating new and complex environmental settings for community and ecosystem dynamics (, ). Therefore, the ability of a species to tolerate shade has numerous aspects that also affect its ability to cope with other stressors, and also shape its interactions with surrounding species. Recent studies have shown that shade tolerance is not straightforwardly related to other stress factors, as depicted by traditional theories which assume that strict physico-chemical constraints determine abiotic stress tolerance trade-offs. Recognition of this complexity opens up new research perspectives. In this review we have identified some promising areas for future research, but there are still major gaps in understanding the full ecological implications of shade tolerance.
Detailed documentation of shade-related polytolerance patterns, and identification of the mechanisms they involve have yet to be carried out. Such documentation would provide new understanding of the causes of species distribution patterns along abiotic stress gradients and the potential for species niche differentiation in different environments characterised simultaneously by shade and other stresses. An important and still understudied aspect of polytolerance is the effect of ontogeny – how understory plants in different stages of life cope with other stress factors, and how much intraspecific trait variability is involved. The main mechanisms underlying species coexistence and segregation along light gradients also remain poorly understood in most vegetation types. Filling these gaps in our knowledge would provide the basis for better dynamic vegetation models. Similarly, the way in which vegetation canopies affect, both directly and indirectly, the properties of soil, and ecosystem cycles, need better models incorporating biogeographic differences in shade-related trade-offs.
Both biotic and abiotic stresses operate in plant communities to shape plant neighbourhood responses and these stresses alter profoundly along gap-understorey gradients. Recent results indicate that shade tolerance can influence the outcome of plant–plant interactions in a complex manner. However, globe-wide similarities in species (and functional type) assemblage patterns along environmental and disturbance gradients, suggest the existence of unifying mechanisms. Finally, the ways in which vegetation in understorey environments will be affected by climate change deserve more scientific attention, as shade might play a significant role in mitigating negative effects of climate change.
Acknowledgments
Thanks are due to Christian Messier, Martin Lechowicz, Niels Anten, Frank Sterck, Fernando Maestre and the members of the Spanish network GLOBIMED (www.globimed.net) for stimulating discussions. We also thank Michael J. Hutchings, the editors and anonymous reviewers for comments that helped to improve early versions of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
Notes on contributors
Fernando Valladares
Fernando Valladares is Research Professor at Spanish Council for Scientific Research (CSIC) and Associate Professor at University Rey Juan Carlos (Madrid). His research is focused on phenotypic, population and community responses to global environmental changes.
Lauri Laanisto
Lauri Laanisto is Senior Researcher at the Estonian University of Life Sciences. His research interests include macroecology and macrophysiology, and his current research focuses mainly on global patterns of plant stress.
Ülo Niinemets
Ülo Niinemets is Professor of plant physiology at the Estonian University of Life Sciences and Member of the Estonian Academy of Sciences. His research interests cover a variety of topics including plant stress dynamics and acclimation, volatile organic compound emissions, carbon gain and trace gas exchange models.
Miguel A. Zavala
Miguel A. Zavala holds an Engineering degree from Polytechnic University of Madrid (1993) and a PhD from Princeton University (2000). He is currently the Head of the Forest and Restoration Ecology group at Universidad de Alcala (Madrid). His research interest includes the development of models of vulnerability of Mediterranean ecosystems and adaptation under global change.
References
- Abrams MD, Mostoller SA. 1995. Gas exchange, leaf structure and nitrogen in contrasting successional tree species growing in open and understory sites during a drought. Tree Physiology 15:361–370.
- Anthony PA, Holtum JAM, Jackes BR. 2002. Shade acclimation of rainforest leaves to colonization by lichens. Functional Ecology 16:808–816.
- Atkin OK, Tjoelker MG. 2003. Thermal acclimation and the dynamic response of plant respiration to temperature. Trends in Plant Science 8:343–351.
- Augspurger CK. 1984. Light requirements of neotropical tree seedlings: a comparative study of growth and survival. The Journal of Ecology 72:777–795.
- Baldwin IT, Halitschke R, Paschold A, Von Dahl CC, Preston CA. 2006. Volatile signaling in plant-plant interactions: “talking trees” in the genomics era. Science 311:812–815.
- Baltzer JL, Thomas SC. 2007. Physiological and morphological correlates of whole-plant light compensation point in temperate deciduous tree seedlings. Oecologia 153:209–223.
- Baraza E, Gómez JM, Hódar JA, Zamora R. 2004. Herbivory has a greater impact in shade than in sun: response of Quercus pyrenaica seedlings to multifactorial environmental variation. Canadian Journal of Botany 82:357–364.
- Barros VR, Field CB, Dokke DJ, Mastrandrea MD, Mach KJ, Bilir TE, Chatterjee M, Ebi KL, Estrada YO, Genova RC, Girma B. 2015. Climate change 2014: impacts, adaptation, and vulnerability. Part B: regional aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge (UK): Cambridge University Press.
- Baudena M, Dekker SC, van Bodegom PM, Cuesta B, Higgins SI, Lehsten V, Reick CH, Rietkerk M, Scheiter S, Yin Z, Zavala MA. 2014. Forests, savannas and grasslands: bridging the knowledge gap between ecology and dynamic global vegetation models. Biogeosciences Discussions 11:9471–9510.
- Bazzaz FA, Pickett STA. 1980. Physiological ecology of tropical succession: a comparative review. Annual Review of Ecology and Systematics 11:287–310.
- Birks HJB. 2005. Mind the gap: how open were European primeval forests? Trends in Ecology & Evolution 20:154–156.
- Blande JD, Holopainen JK, Niinemets Ü. 2014. Plant volatiles in polluted atmospheres: stress responses and signal degradation. Plant, Cell & Environment 37:1892–1904.
- Bonan GB, Shugart HH. 1989. Environmental factors and ecological processes in boreal forests. Annual Review of Ecology and Systematics 20:1–28.
- Brooker RW, Maestre FT, Callaway RM, Lortie CL, Cavieres LA, Kunstler G, Liancourt P, Tielbörger K, Travis JM, Anthelme F, Armas C. 2008. Facilitation in plant communities: the past, the present, and the future. Journal of Ecology 96:18–34.
- Bugmann H. 1996. Functional types of trees in temperate and boreal forests: classification and testing. Journal of Vegetation Science 7:359–370.
- Bugmann HK, Yan X, Sykes MT, Martin P, Lindner M, Desanker PV, Cumming SG. 1996. A comparison of forest gap models: model structure and behaviour. Climatic Change 34:289–313.
- Callaway RM. 1995. Positive interactions among plants. The Botanical Review 61:306–349.
- Callaway RM. 2007. Positive interactions and interdependence in plant communities. New York (NY): Springer-Verlag.
- Canham CD, Finzi AC, Pacala SW, Burbank DH. 1994. Causes and consequences of resource heterogeneity in forests: interspecific variation in light transmission by canopy trees. Canadian Journal of Forest Research 24:337–349.
- Cavender-Bares J, Bazzaz FA. 2000. Changes in drought response strategies with ontogeny in Quercus rubra: implications for scaling from seedlings to mature trees. Oecologia 124:8–18.
- Copolovici L, Kännaste A, Remmel T, Niinemets Ü. 2014. Volatile organic compound emissions from Alnus glutinosa under interacting drought and herbivory stresses. Environmental and Experimental Botany 100:55–63.
- Coyle JR, Halliday FW, Lopez BE, Palmquist KA, Wilfahrt PA, Hurlbert AH. 2014. Using trait and phylogenetic diversity to evaluate the generality of the stress‐dominance hypothesis in eastern North American tree communities. Ecography 37:814–826.
- Cramer W, Bondeau A, Woodward FI, Prentice IC, Betts RA, Brovkin V, Cox PM, Fisher V, Foley JA, Friend AD, Kucharik C. 2001. Global response of terrestrial ecosystem structure and function to CO2 and climate change: results from six dynamic global vegetation models. Global Change Biology 7:357–373.
- De Frenne P, Rodríguez-Sánchez F, Coomes DA, Baeten L, Verstraeten G, Vellend M, Bernhardt-Römermann M, Brown CD, Brunet J, Cornelis J, Decocq GM. 2013. Microclimate moderates plant responses to macroclimate warming. Proceedings of the National Academy of Sciences 110:18561–18565.
- DeLucia EH, Thomas RB. 2000. Photosynthetic responses to CO2 enrichment of four hardwood species in a forest understory. Oecologia 122:11–19.
- Dent DH, DeWalt SJ, Denslow JS. 2013. Secondary forests of central Panama increase in similarity to old‐growth forest over time in shade tolerance but not species composition. Journal of Vegetation Science 24:530–542.
- Figueroa JA, Lusk CH. 2001. Germination requirements and seedling shade tolerance are not correlated in a Chilean temperate rain forest. New Phytologist 152:483–489.
- Fischer R, Bohn F, de Paula MD, Dislich C, Groeneveld J, Gutiérrez AG, Kazmierczak M, Knapp N, Lehmann S, Paulick S, Pütz S. 2016. Lessons learned from applying a forest gap model to understand ecosystem and carbon dynamics of complex tropical forests. Ecological Modelling 326:124–133.
- Franceschini T, Schneider R. 2014. Influence of shade tolerance and development stage on the allometry of ten temperate tree species. Oecologia 176:739–749.
- Gayer K. 1898. Der Waldbau. vierte, verbesserte Auflage edition. Berlin (Germany): Verlagsbuchhandlung Paul Parey.
- Germino MJ, Smith WK. 1999. Sky exposure, crown architecture, and low‐temperature photoinhibition in conifer seedlings at alpine treeline. Plant, Cell & Environment 22:407–415.
- Getzin S, Wiegand T, Hubbell SP. 2014. Stochastically driven adult–recruit associations of tree species on Barro Colorado Island. Proceedings of the Royal Society of London B: Biological Sciences 281:20140922.
- Givnish TJ. 1988. Adaptation to sun and shade: a whole-plant perspective. Functional Plant Biology 15:63–92.
- Goldberg DE. 1990. Components of resource competition in plant communities. In: Grace JB, Tilman D, editors. Perspectives on plant competition. San Diego (CA): Academic Press. p. 27–49.
- Gómez JM, Valladares F, Puerta‐Piñero C. 2004. Differences between structural and functional environmental heterogeneity caused by seed dispersal. Functional Ecology 18:787–792.
- Gómez-Aparicio L, Canham CD. 2008. Neighborhood models of the effects of invasive tree species on ecosystem processes. Ecological Monographs 78:69–86.
- Gómez-Aparicio L, Gómez JM, Zamora R, Boettinger JL. 2005. Canopy vs. soil effects of shrubs facilitating tree seedlings in Mediterranean montane ecosystems. Journal of Vegetation Science 16:191–198.
- Gómez‐Aparicio L, Zamora R, Castro J, Hódar JA. 2008. Facilitation of tree saplings by nurse plants: microhabitat amelioration or protection against herbivores? Journal of Vegetation Science 19:161–172.
- Gravel D, Canham CD, Beaudet M, Messier C. 2006. Reconciling niche and neutrality: the continuum hypothesis. Ecology Letters 9:399–409.
- Grubb PJ. 2015. Trade-offs in interspecific comparisons in plant ecology and how plants overcome proposed constraints. Plant Ecology & Diversity 9:3–33.
- Hallik L, Niinemets Ü, Wright IJ. 2009. Are species shade and drought tolerance reflected in leaf‐level structural and functional differentiation in Northern Hemisphere temperate woody flora? New Phytologist 184:257–274.
- Hättenschwiler S. 2001. Tree seedling growth in natural deep shade: functional traits related to interspecific variation in response to elevated CO2. Oecologia 129:31–42.
- Hättenschwiler S, Körner C. 2003. Does elevated CO2 facilitate naturalization of the non‐indigenous Prunus laurocerasus in Swiss temperate forests? Functional Ecology 17:778–785.
- Hawkins BA, Rueda M, Rangel TF, Field R, Diniz‐Filho JAF. 2014. Community phylogenetics at the biogeographical scale: cold tolerance, niche conservatism and the structure of North American forests. Journal of Biogeography 41:23–38.
- Henry HA, Aarssen LW. 1997. On the relationship between shade tolerance and shade avoidance strategies in woodland plants. Oikos 80:575–582.
- Herrera CM. 1997. Thermal biology and foraging responses of insect pollinators to the forest floor irradiance mosaic. Oikos 78:601–611.
- Hickler T, Smith B, Sykes MT, Davis MB, Sugita S, Walker K. 2004. Using a generalized vegetation model to simulate vegetation dynamics in northeastern USA. Ecology 85:519–530.
- Holmgren M, Gómez-Aparicio L, Quero JL, Valladares F. 2012. Non-linear effects of drought under shade: reconciling physiological and ecological models in plant communities. Oecologia 169:293–305.
- Hubbell SP. 2001. The unified neutral theory of biodiversity and biogeography (MPB-32). Vol. 32. Princeton (NJ): Princeton University Press.
- Jacobs AFG, Van Boxel JH, El-Kilani RMM. 1994. Nighttime free convection characteristics within a plant canopy. Boundary-Layer Meteorology 71:375–391.
- Jacobs P. 2003. Mildew (Microsphaera alphitoides) as possible important factor in limiting height growth of oak (Quercus robur) [MSc thesis]. Wageningen: Forest and Nature Conservation, Wageningen University.
- Joffre R, Rambal S. 1993. How tree cover influences the water balance of Mediterranean rangelands. Ecology 74:570–582.
- Jones CG, Lawton JH, Shachak M. 1997. Positive and negative effects of organisms as physical ecosystem engineers. Ecology 78:1946–1957.
- Jucker T, Bouriaud O, Coomes DA. 2015. Crown plasticity enables trees to optimize canopy packing in mixed‐species forests. Functional Ecology 29:1078–1086.
- Kim D, Oren R, Qian SS. 2015. Response to CO2 enrichment of understory vegetation in the shade of forests. Global Change Biology. doi:10.1111/gcb.13126
- Kitajima K. 1994. Relative importance of photosynthetic traits and allocation patterns as correlates of seedling shade tolerance of 13 tropical trees. Oecologia 98:419–428.
- Kitajima K, Poorter L. 2008. Functional basis for resource niche partitioning by tropical trees. In: Carson WP, Schnitzer SA, editors. Tropical forest community ecology. London: Blackwell. p. 172–188.
- Kneeshaw DD, Kobe RK, Coates KD, Messier C. 2006. Sapling size influences shade tolerance ranking among southern boreal tree species. Journal of Ecology 94:471–480.
- Kobe RK, Coates KD. 1997. Models of sapling mortality as a function of growth to characterize interspecific variation in shade tolerance of eight tree species of northwestern British Columbia. Canadian Journal of Forest Research 27:227–236.
- Kohyama T, Suzuki E, Partomihardjo T, Yamada T, Kubo T. 2003. Tree species differentiation in growth, recruitment and allometry in relation to maximum height in a Bornean mixed dipterocarp forest. Journal of Ecology 91:797–806.
- Kunstler G, Curt T, Bouchaud M, Lepart J. 2005. Growth, mortality, and morphological response of European beech and downy oak along a light gradient in sub-Mediterranean forest. Canadian Journal of Forest Research 35:1657–1668.
- Kunstler G, Curt T, Bouchaud M, Lepart J. 2006. Indirect facilitation and competition in tree species colonization of sub‐Mediterranean grasslands. Journal of Vegetation Science 17:379–388.
- Kunstler G, Falster D, Coomes DA, Hui F, Kooyman RM, Laughlin DC, Poorter L, Vanderwel M, Vieilledent G, Wright SJ, et al. 2016. Plant functional traits have globally consistent effects on competition. Nature 529:204–207.
- Kursar TA. 1998. Relating tree physiology to past and future changes in tropical rainforest tree communities. In: Markham A, editor. Potential impacts of climate change on tropical forest ecosystems. Dordrecht: Springer. p. 223–239.
- Laanisto L, Niinemets Ü. 2015. Polytolerance to abiotic stresses: how universal is the shade–drought tolerance trade‐off in woody species? Global Ecology and Biogeography 24:571–580.
- Lilles EB, Astrup R, Lefrançois ML, Coates KD. 2014. Sapling leaf trait responses to light, tree height and soil nutrients for three conifer species of contrasting shade tolerance. Tree Physiology tpu092 34:1334–1347.
- Lin J, Harcombe PA, Fulton MR, Hall RW. 2002. Sapling growth and survivorship as a function of light in a mesic forest of southeast Texas, USA. Oecologia 132:428–435.
- Lindow SE, Brandl MT. 2003. Microbiology of the phyllosphere. Applied and Environmental Microbiology 69:1875–1883.
- Liu S. 1997. A new model for the prediction of rainfall interception in forest canopies. Ecological Modelling 99:151–159.
- Lortie CJ, Callaway RM. 2006. Re‐analysis of meta‐analysis: support for the stress‐gradient hypothesis. Journal of Ecology 94:7–16.
- Lusk CH, Falster DS, Jara‐Vergara CK, Jimenez‐Castillo M, Saldaña‐Mendoza A. 2008. Ontogenetic variation in light requirements of juvenile rainforest evergreens. Functional Ecology 22:454–459.
- Lusk CH, Jorgensen MA, Bellingham PJ. 2015. A conifer–angiosperm divergence in the growth vs. shade tolerance trade‐off underlies the dynamics of a New Zealand warm‐temperate rain forest. Journal of Ecology 103:479–488.
- Ma RY, Zhang JL, Cavaleri MA, Sterck F, Strijk JS, Cao KF. 2015. Convergent evolution towards high net carbon gain efficiency contributes to the shade tolerance of palms (Arecaceae). PloS one 10:e0140384.
- Maestre FT, Valladares F, Reynolds JF. 2006. The stress‐gradient hypothesis does not fit all relationships between plant–plant interactions and abiotic stress: further insights from arid environments. Journal of Ecology 94:17–22.
- Manrique R, Gutiérrez JR, Holmgren M, Squeo FA. 2007. Reduced herbivory during simulated ENSO rainy events increases native herbaceous plants in semiarid Chile. Plant Ecology 191:21–31.
- Markesteijn L, Poorter L. 2009. Seedling root morphology and biomass allocation of 62 tropical tree species in relation to drought‐and shade‐tolerance. Journal of Ecology 97:311–325.
- Martin PH, Marks PL. 2006. Intact forests provide only weak resistance to a shade‐tolerant invasive Norway maple (Acer platanoides L.). Journal of Ecology 94:1070–1079.
- Mazza CA, Izaguirre MM, Zavala J, Scopel AL, Ballaré CL. 2002. Insect perception of ambient ultraviolet‐B radiation. Ecology Letters 5:722–726.
- Meehl GA, Stocker TF, Collins WD, Friedlingstein P, Gaye AT, Gregory JM, Kitoh A, Knutti R, Murphy JM, Noda A, Raper SC. 2007. Global climate projections. Climate change, 283. In: Solomon S, et al. editors. Climate change 2007: the physical science basis. Cambridge (UK): Cambridge University Press; p. 747–847.
- Miriti MN. 2006. Ontogenetic shift from facilitation to competition in a desert shrub. Journal of Ecology 94:973–979.
- Miyashita A, Tateno M. 2014. A novel index of leaf RGR predicts tree shade tolerance. Functional Ecology 28:1321–1329.
- Modrzynski J, Chmura DJ, Tjoelker MG. 2015. Seedling growth and biomass allocation in relation to leaf habit and shade tolerance among 10 temperate tree species. Tree Physiology tpv053 35:879–893.
- Nieto‐Lugilde D, Lenoir J, Abdulhak S, Aeschimann D, Dullinger S, Gégout JC, Guisan A, Pauli H, Renaud J, Theurillat JP, Thuiller W. 2014. Tree cover at fine and coarse spatial grains interacts with shade tolerance to shape plant species distributions across the Alps. Ecography 38:578–589.
- Niinemets U. 2006. The controversy over traits conferring shade‐tolerance in trees: ontogenetic changes revisited. Journal of Ecology 94:464–470.
- Niinemets U. 2007. Photosynthesis and resource distribution through plant canopies. Plant, Cell & Environment 30:1052–1071.
- Niinemets Ü. 1998. Growth of young trees of Acer platanoides and Quercus robur along a gap-understory continuum: interrelationships between allometry, biomass partitioning, nitrogen, and shade tolerance. International Journal of Plant Sciences 159:318–330.
- Niinemets Ü. 2010. A review of light interception in plant stands from leaf to canopy in different plant functional types and in species with varying shade tolerance. Ecological Research 25:693–714.
- Niinemets Ü, Valladares F. 2004. Photosynthetic acclimation to simultaneous and interacting environmental stresses along natural light gradients: optimality and constraints. Plant Biology 6:254–268.
- Niinemets Ü, Valladares F. 2006. Tolerance to shade, drought, and waterlogging of temperate Northern Hemisphere trees and shrubs. Ecological Monographs 76:521–547.
- Niinemets Ü, Valladares F, Ceulemans R. 2003. Leaf‐level phenotypic variability and plasticity of invasive Rhododendron ponticum and non‐invasive Ilex aquifolium co‐occurring at two contrasting European sites. Plant, Cell & Environment 26:941–956.
- Niinemets Ü, Wright IJ, Evans JR. 2009. Leaf mesophyll diffusion conductance in 35 Australian sclerophylls covering a broad range of foliage structural and physiological variation. Journal of Experimental Botany 60:2433–2449.
- O’Brien MJ, Philipson CD, Tay J, Hector A. 2013. The influence of variable rainfall frequency on germination and early growth of shade-tolerant dipterocarp seedlings in Borneo. PloS one 8:e70287.
- Ögren E, Nilsson T, Sundblad LG. 1997. Relationship between respiratory depletion of sugars and loss of cold hardiness in coniferous seedlings over‐wintering at raised temperatures: indications of different sensitivities of spruce and pine. Plant, Cell & Environment 20:247–253.
- Ojeda F. 1998. Biogeography of seeder and resprouter Erica species in the Cape Floristic Region—Where are the resprouters? Biological Journal of the Linnean Society 63:331–347.
- Ouédraogo DY, Mortier F, Gourlet‐Fleury S, Freycon V, Picard N. 2013. Slow‐growing species cope best with drought: evidence from long‐term measurements in a tropical semi‐deciduous moist forest of Central Africa. Journal of Ecology 101:1459–1470.
- Pacala SW, Canham CD, Saponara J, Silander Jr JA, Kobe RK, Ribbens E. 1996. Forest models defined by field measurements: estimation, error analysis and dynamics. Ecological Monographs 66:1–43.
- Pagès JP, Pache G, Joud D, Magnan N, Michalet R. 2003. Direct and indirect effects of shade on four forest tree seedlings in the French Alps. Ecology 84:2741–2750.
- Parmesan C. 2006. Ecological and evolutionary responses to recent climate change. Annual Review of Ecology, Evolution, and Systematics 37:637–669.
- Pérez‐Ramos IM, Urbieta IR, Maranón T, Zavala MA, Kobe RK. 2008. Seed removal in two coexisting oak species: ecological consequences of seed size, plant cover and seed‐drop timing. Oikos 117:1386–1396.
- Pivovaroff A, Sharifi R, Scoffoni C, Sack L, Rundel P. 2014. Making the best of the worst of times: traits underlying combined shade and drought tolerance of Ruscus aculeatus and Ruscus microglossum (Asparagaceae). Functional Plant Biology 41:11–24.
- Poorter H, Niklas KJ, Reich PB, Oleksyn J, Poot P, Mommer L. 2012. Biomass allocation to leaves, stems and roots: meta‐analyses of interspecific variation and environmental control. New Phytologist 193:30–50.
- Poorter L. 1999. Growth responses of 15 rain‐forest tree species to a light gradient: the relative importance of morphological and physiological traits. Functional Ecology 13:396–410.
- Poorter L, Arets EJ. 2003. Light environment and tree strategies in a Bolivian tropical moist forest: an evaluation of the light partitioning hypothesis. Plant Ecology 166:295–306.
- Poorter L, Bongers F. 2006. Leaf traits are good predictors of plant performance across 53 rain forest species. Ecology 87:1733–1743.
- Pritchard S, Rogers H, Prior SA, Peterson C. 1999. Elevated CO2 and plant structure: a review. Global Change Biology 5:807–837.
- Pugnaire FI, Armas C, Valladares F. 2004. Soil as a mediator in plant-plant interactions in a semi-arid community. Journal of Vegetation Science 15:85–92.
- Purves D, Pacala S. 2008. Predictive models of forest dynamics. Science 320:1452–1453.
- Quero JL, Gómez-Aparicio L, Zamora R, Maestre FT. 2008. Shifts in the regeneration niche of an endangered tree (Acer opalus ssp. granatense) during ontogeny: using an ecological concept for application. Basic and Applied Ecology 9:635–644.
- Reinhart KO, Gurnee J, Tirado R, Callaway RM. 2006. Invasion through quantitative effects: intense shade drives native decline and invasive success. Ecological Applications 16:1821–1831.
- Retana J, Espelta JM, Gracia M, Riba M. 1999. Seedling recruitment. In: Rodà F, Retana J, Gracia CA, Bellot J, editors. Ecology of Mediterranean evergreen oak forests. Berlin: Springer; p. 89–103.
- Sack L. 2004. Responses of temperate woody seedlings to shade and drought: do trade‐offs limit potential niche differentiation? Oikos 107:110–127.
- Sánchez‐Gómez D, Valladares F, Zavala MA. 2006. Performance of seedlings of Mediterranean woody species under experimental gradients of irradiance and water availability: trade‐offs and evidence for niche differentiation. New Phytologist 170:795–806.
- Santo-Silva EE, Withey KD, Almeida WR, Mendes G, Lopes AV, Tabarelli M. 2015. Seedling assemblages and the alternative successional pathways experienced by Atlantic forest fragments. Plant Ecology & Diversity 8:483–492.
- Saxe H, Ellsworth DS, Heath J. 1998. Tree and forest functioning in an enriched CO2 atmosphere. New Phytologist 139:395–436.
- Sellin A, Õunapuu E, Karusion A. 2010. Experimental evidence supporting the concept of light-mediated modulation of stem hydraulic conductance. Tree Physiology 30:1528–1535.
- Semchenko M, Lepik M, Götzenberger L, Zobel K. 2012. Positive effect of shade on plant growth: amelioration of stress or active regulation of growth rate? Journal of Ecology 100:459–466.
- Sendall KM, Lusk CH, Reich PB. 2015. Trade‐offs in juvenile growth potential vs. shade tolerance among subtropical rain forest trees on soils of contrasting fertility. Functional Ecology 30:845–855.
- Shirley HL. 1943. Is tolerance the capacity to endure shade? Journal of Forestry 41:339–345.
- Shugart HH. 1984. A theory of forest dynamics: the ecological implications of forest succession models. Berlin: Springer Verlag.
- Shugart HH, West DC. 1980. Forest succession models. BioScience 30:308–313.
- Smit C, Vandenberghe C, Den Ouden J, Müller-Schärer H. 2007. Nurse plants, tree saplings and grazing pressure: changes in facilitation along a biotic environmental gradient. Oecologia 152:265–273.
- Smith B, Prentice IC, Sykes MT. 2001. Representation of vegetation dynamics in the modelling of terrestrial ecosystems: comparing two contrasting approaches within European climate space. Global Ecology & Biogeography 10:621–637.
- Smith T, Huston M. 1989. A theory of the spatial and temporal dynamics of plant communities. Vegetatio 83:49–69.
- Stahl U, Kattge J, Reu B, Voigt W, Ogle K, Dickie J, Wirth C. 2013. Whole-plant trait spectra of North American woody plant species reflect fundamental ecological strategies. Ecosphere 4:art128.
- Stott P, Loehle C. 1998. Height growth rate tradeoffs determine northern and southern range limits for trees. Journal of Biogeography 25:735–742.
- Sultan SE. 2001. Phenotypic plasticity for fitness components in Polygonum species of contrasting ecological breadth. Ecology 82:328–343.
- Thurow TL, Blackburn WH, Warren SD, Taylor Jr CA. 1987. Rainfall interception by midgrass, shortgrass, and live oak mottes. Journal of Range Management 40:455–460.
- Tielbörger K, Kadmon R. 2000. Temporal environmental variation tips the balance between facilitation and interference in desert plants. Ecology 81:1544–1553.
- Tjoelker MG, Oleksyn J, Reich PB. 1998. Seedlings of five boreal tree species differ in acclimation of net photosynthesis to elevated CO2 and temperature. Tree Physiology 18:715–726.
- Tomimatsu H, Iio A, Adachi M, Saw LG, Fletcher C, Tang Y. 2014. High CO2 concentration increases relative leaf carbon gain under dynamic light in Dipterocarpus sublamellatus seedlings in a tropical rain forest, Malaysia. Tree physiology tpu066 34:944–954.
- Traveset A, Moragues E, Valladares F. 2008. Spreading of the invasive Carpobrotus aff. acinaciformis in Mediterranean ecosystems: the advantage of performing in different light environments. Applied Vegetation Science 11:45–54.
- Urbieta IR, Perez-Ramos IM, Zavala MA, Marañón T, Kobe RK. 2008. Soil water content and emergence time control seedling establishment in three co-occurring Mediterranean oak species. Canadian Journal of Forest Research 38:2382–2393.
- Valiente-Banuet A, Ezcurra E. 1991. Shade as a cause of the association between the cactus Neobuxbaumia tetetzo and the nurse plant Mimosa luisana in the Tehuacan Valley, Mexico. The Journal of Ecology 79:961–971.
- Valiente-Banuet A, Rumebe AV, Verdú M, Callaway RM. 2006. Modern Quaternary plant lineages promote diversity through facilitation of ancient Tertiary lineages. Proceedings of the National Academy of Sciences 103:16812–16817.
- Valiente-Banuet A, Verdú M. 2013. Plant facilitation and phylogenetics. Annual Review of Ecology, Evolution, and Systematics 44:347–366.
- Valiente‐Banuet A, Verdú M. 2008. Temporal shifts from facilitation to competition occur between closely related taxa. Journal of Ecology 96:489–494.
- Valladares F, Bastias CC, Godoy O, Granda E, Escudero A. 2015. Species coexistence in a changing world. Frontiers in Plant Science 6:866.
- Valladares F, Gianoli E, Gómez JM. 2007. Ecological limits to plant phenotypic plasticity. New Phytologist 176:749–763.
- Valladares F, Niinemets Ü. 2008. Shade tolerance, a key plant feature of complex nature and consequences. Annual Review of Ecology, Evolution, and Systematics 39:237–257.
- Valladares F, Pearcy RW. 2002. Drought can be more critical in the shade than in the sun: a field study of carbon gain and photo‐inhibition in a Californian shrub during a dry El Niño year. Plant, Cell & Environment 25:749–759.
- Valladares F, Zaragoza-Castells J, Sánchez-Gómez D, Matesanz S, Alonso B, Portsmuth A, Delgado A, Atkin OK. 2008. Is shade beneficial for Mediterranean shrubs experiencing periods of extreme drought and late-winter frosts? Annals of Botany 102:923–933.
- Verdú M, Valiente‐Banuet A. 2008. The nested assembly of plant facilitation networks prevents species extinctions. The American Naturalist 172:751–760.
- Wise MJ, Abrahamson WG. 2007. Effects of resource availability on tolerance of herbivory: a review and assessment of three opposing models. The American Naturalist 169:443–454.
- Wright SJ, Muller-Landau HC, Condit R, Hubbell SP. 2003. Gap-dependent recruitment, realized vital rates, and size distributions of tropical trees. Ecology 84:3174–3185.
- Xu C, Vergnon R, Cornelissen JHC, Hantson S, Holmgren M, van Nes EH, Scheffer M. 2015. Temperate forest and open landscapes are distinct alternative states as reflected in canopy height and tree cover. Trends in Ecology & Evolution 20:1–3.
- Zanne AE, Tank DC, Cornwell WK, Eastman JM, Smith SA, FitzJohn RG, McGlinn DJ, O’Meara BC, Moles AT, Reich PB, Royer DL. 2014. Three keys to the radiation of angiosperms into freezing environments. Nature 506:89–92.
- Zaragoza‐Castells J, Sánchez‐Gómez D, Hartley IP, Matesanz S, Valladares F, Lloyd J, Atkin OK. 2008. Climate‐dependent variations in leaf respiration in a dry‐land, low productivity Mediterranean forest: the importance of acclimation in both high‐light and shaded habitats. Functional Ecology 22:172–184.
- Zavala MA, Angulo Ó, de la Parra RB, López-Marcos JC. 2007. An analytical model of stand dynamics as a function of tree growth, mortality and recruitment: the shade tolerance-stand structure hypothesis revisited. Journal of Theoretical Biology 244:440–450.
- Zavala MA, Espelta JM, Caspersen J, Retana J. 2011. Interspecific differences in sapling performance with respect to light and aridity gradients in Mediterranean pine–oak forests: implications for species coexistence. Canadian Journal of Forest Research 41:1432–1444.
- Zavala MA, Espelta JM, Retana J. 2000. Constraints and trade-offs in Mediterranean plant communities: the case of holm oak-Aleppo pine forests. The Botanical Review 66:119–149.
- Zhang Q, Zhang TJ, Chow WS, Xie X, Chen YJ, Peng CL. 2015. Photosynthetic characteristics and light energy conversions under different light environments in five tree species occupying dominant status at different stages of subtropical forest succession. Functional Plant Biology 42:609.
- Zon R, Graves HS. 1911. Light in relation to tree growth (No. 92). Washington (DC): US Department of Agriculture, Forest Service.
