?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Background: Plant communities are usually characterised by species composition and abundance, but also underlie a multitude of complex interactions that we have only recently started unveiling. Yet, we are still far from understanding ecological and evolutionary processes shaping the network-level organisation of plant diversity, and to what extent these processes are specific to certain spatial scales or environments.
Aims: Understanding the systemic mechanisms of plant–plant network assembly and their consequences for diversity patterns.
Methods: We review recent methods and results of plant–plant networks.
Results: We synthetize how plant–plant networks can help us to: (a) assess how competition and facilitation may balance each other through the network; (b) analyse the role of plant–plant interactions beyond pairwise competition in structuring plant communities, and (c) forecast the ecological implications of complex species dependencies. We discuss pros and cons, assumptions and limitations of different approaches used for inferring plant–plant networks.
Conclusions: We propose novel opportunities for advancing plant ecology by using ecological networks that encompass different ecological levels and spatio-temporal scales, and incorporate more biological information. Embracing networks of interactions among plants can shed new light on mechanisms driving evolution and ecosystem functioning, helping us to mitigate diversity loss.
Introduction
‘No plant is an island’
Traditionally, it has been proposed that environmental heterogeneity, competitive ability and niche differences between species are the main drivers of biodiversity in ecological communities (Grubb Citation1977; Chesson Citation2000; HilleRisLambers et al. Citation2012). As highlighted by Grubb (Citation1977), niche differentiation includes diversifying life forms and functional traits, phenology and particularly regeneration strategies through space and time. These processes also contribute to and result from differences in species interactions in ecological and evolutionary time scales, respectively. In addition, plant communities harbour a very large variety of complex interactions that we have only recently started to unveil (Callaway Citation2007; Klein et al. Citation2016; Mescher and Pearse Citation2016; Levine et al. Citation2017). Diversity coupled with the complexity of biotic interactions challenge our current understanding of plant diversity and the organisation of plant communities. Recent applications of network theory to the study of biotic interactions among plant species is helping us to tackle such complexity, revealing hitherto hidden aspects of organisation of plant community diversity. However, network-level mechanisms driving local scale diversity and the role of plant interaction networks for shaping the diversity of plant communities remain unclear, overlooked aspects. A better understanding of plant–plant interaction networks by means of a unified framework is therefore key to improving our knowledge of plant diversity.
But, why should one care about plant networks? Plant diversity is more than a list of species, a collection of herbarium specimens, a summary index, a phylogenetic tree or a multidimensional trait space. Rather, biotic interactions are foundational to plant diversity at the local, community scale (Grace and Tilman Citation1990; Callaway Citation2007; Levine et al. Citation2017). That is, the type, intensity, and diversity of interactions embedded in a network-like structure are all contributing mechanisms to the diversity and stability of plant communities.
We define plant networks as systems of plants (such as individuals, species or meta-communities) linked by ecological relationships (such as competition, facilitation or concurrence). Plant networks can therefore encapsulate emergent properties of plant communities and provide further information beyond species richness or phylogenetic similarity about the potential mechanisms of community assembly, community dynamics and the resistance of plant diversity to environmental change (Grilli et al. Citation2017; Losapio and Schöb Citation2017; Saiz et al. Citation2017). In this sense, plant networks extend ‘classic’ knowledge of systematic and functional botany to explain ecological patterns of plant diversity and improve our understanding of evolutionary processes shaping plant communities (Verdú and Valiente-Banuet Citation2008; Kefi et al. Citation2012). This improved knowledge of plant community ecology can have important implications to advance our forecasting ability and can benefit practices for conserving single populations and entire ecosystems simultaneously.
We reviewed the literature on plant–plant networks and identified three main challenges to be tackled for a better understanding of how networks of plant–plant interactions can influence biodiversity. First, along with competition, simultaneously considering other widespread interactions among plants within this trophic level that shape the structure and dynamics of ecological communities, such as facilitation, commensalism, and parasitism (Callaway Citation2007; Burns and Zotz Citation2010). For example, facilitation and competition can potentially balance each other, resulting in a broad range of possible context-dependent outcomes (Callaway and Walker Citation1997; Levine Citation1999; Choler et al. Citation2001; Schöb et al. Citation2014a).
Second, one major challenge is to describe and quantify, in addition to pairwise interactions, direct and indirect interaction effects among multiple species. Although research to date in plant and theoretical ecology has mainly focused on interactions between pairs of species (Chesson Citation2000), species pairs rarely interact in isolation from the rest of other species and their interactions in the community (Bascompte and Jordano Citation2014; Levine et al. Citation2017). Pairwise interactions can be affected by other species interactions (Mayfield and Stouffer Citation2017), as interactions between two species can change depending on the presence of other community members and the interactions among them (Losapio et al. Citation2019).
Third, plant interactions can have important implications for ecological processes at different levels of organisation as well as at different temporal and spatial scales. These include effects on plant eco-physiology (Schöb et al. Citation2014a; Körner Citation2018), demography (Verdú and Valiente-Banuet Citation2008), structure and diversity of communities (Butterfield et al. Citation2013; Kikvidze et al. Citation2015), and potentially even contributing to evolutionary diversity at the biome scale (Valiente-Banuet et al. Citation2006). An adequate characterisation of plant networks in each of the three cases will inform us about the implications of biotic interactions in different ecological processes. Notably, characterising plant–plant interactions is the main challenge in most cases, a non-trivial, fundamental aspect for constructing reliable plant networks which we will discuss below.
The development of ecological network theory (Bersier Citation2007; Fortuna and Bascompte Citation2008) has provided valuable new analytical methods for studying ecological communities that can be translated to plant community ecology. Originally, network analysis was applied to study predation and then mutualistic interactions (McCann Citation2011; Bascompte and Jordano Citation2014). The study of food webs and mutualistic networks has substantially helped to understand the contribution of trophic interactions to ecological and evolutionary processes such as the factors driving biodiversity. Despite the historical interest in biotic interactions in plant ecology (Grace and Tilman Citation1990; Callaway Citation2007; Levine et al. Citation2017), particularly of competition, plant networks have rarely been considered. Only with the development of network analysis based on both graph theory and statistical mechanics (Jordán and Scheuring Citation2004), along with advances in user-friendly, open-source software (e.g. Csardi and Nepusz Citation2006; Dormann et al. Citation2008), plant ecologists have begun to use ‘modern’ network theory. Indeed, plant networks are now receiving increasing attention as models and case studies are rapidly growing in number.
In this review, we synthesise the recent contribution of the network approach to address species interactions and diversity patterns in plant communities. In particular, we highlight how studying the organisation and dynamics of plant–plant interactions from the network perspective can help to (a) assess the balance between facilitation and competition that may result in a wide range of potential outcomes; (b) model the role of plant–plant interactions beyond pairwise competition in structuring plant communities, and (c) forecast the broader ecological implications of plant–plant interactions.
First, we present the different types of plant networks. Second, we revise indirect interactions among plant species (i.e. interactions between two species mediated by a third species) and propose network framework to address them. Third, we discuss the assumptions, advantages and weaknesses of different methods and models, and their implications for our understanding of ecological processes. We conclude with highlighting main gaps and unsolved problems, and we propose avenues for future research.
Plant networks
Ecological networks are composed of ‘nodes’ (e.g. species) connected by ‘links’ (e.g. biotic interactions) (). Nodes and links are then projected into a matrix, where nodes form rows and columns, and links form matrix entries (). Two types of ecological networks have been mainly used: unipartite and bipartite (). In unipartite networks, there is one set of nodes and links can connect any node within the network (e.g. predation in food webs). In bipartite networks, there are two distinct sets of nodes, and links can only connect nodes between sets (e.g. pollinators by plants in pollination networks). A third type of network is the multi-layer network, in which nodes are connected across different networks (i.e. layers) which can represent multiple types of interactions, time or space ().
Figure 1. Elements of plant networks. Plant networks are composed by nodes (e.g. species, individuals, genotypes, phenotypes, functional groups, vegetation patches) connected by links (e.g. competition, facilitation, commensalism, parasitism, co-occurrence). Nodes and links are represented in matrices and therefore network objects. A matrix can be either square for unipartite networks or rectangular for bipartite networks. Several matrices can be further combined in plant multilayer networks.
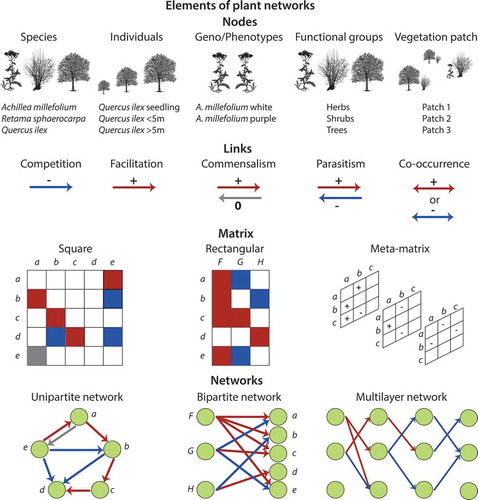
Plant–plant interactions maybe considered as unipartite, bipartite or multi-layer networks. In unipartite networks, plants belong to a single set, while in bipartite networks there are two different sets. In the former case, plants are ecologically equivalent, while in the latter case, structural and functional differences between plants are identified a priori. For instance, two different sets of plants can be arranged on the basis of growth form (e.g. tree and liana), functional role (e.g. competitor and subordinate species) or life-history stage (e.g. seedling and adult). These groups represent the two distinct sets of nodes in a bipartite plant–plant network. This type of bipartite network model assumes no direct interactions among members of each set of nodes (e.g. among trees in tree–liana networks).
Links in plant networks can be established either by directly measuring a selected type of interaction or by measuring species associations and then inferring interactions on the basis of patterns of spatial distribution. In the first case, the strength and sign of plant interactions is determined by any proxy for fitness (i.e. survival, reproductive success or growth rate) and quantified in the presence and absence of other plant species (Connell Citation1961; Verdú and Valiente-Banuet Citation2008, Citation2011; Alcantara and Rey Citation2012; Delalandre and Montesinos-Navarro Citation2018). Experimental manipulation of plant neighbours has been used to estimate the strength of competition or facilitation to quantify links in plant networks. Common practices have included the removal of single individual/species, manipulation of neighbour identity/density around a target plant or sowing at different density (Grace and Tilman Citation1990; Callaway Citation2007).
When looking at spatial patterns of species distribution within communities, positive or negative species associations are based on spatial co-occurrence data that are used as a proxy for inferring positive or negative interactions among species, respectively (Burns and Zotz Citation2010; Saiz and Alados Citation2011a; Montesinos-Navarro et al. Citation2018; Losapio et al. Citation2018a). Contrary to plant networks based on fitness measurements, networks based on co-occurrence are representative of the spatial organisation of communities and can be expressed with different spatial resolution. It is important to keep in mind that the spatial distribution of species is potentially affected by biotic interactions together with other ecological processes such as colonisation, dispersion, habitat filtering and species turnover (Connell Citation1961; Kikvidze et al. Citation2015), so the information that can be derived from these spatial networks is different from that derived from manipulative experiments. Therefore, the interpretation of networks constructed from spatial patterns is not as straightforward as direct interactions since species distribution may result from different factors, in addition to interactions. Using statistical tools (e.g. Bayesian Networks) at community scale, including covariates that bear additional ecological information (such as abiotic conditions) and focusing on fine spatial resolution (i.e. centimetres) can help building informative plant interaction networks (Saiz et al. Citation2017; Staniczenko et al. Citation2017; Montesinos-Navarro et al. Citation2018; Losapio et al. Citation2018a).
Links in plant networks usually represent interspecific interactions, in terms of qualitative or quantitative net effects of biotic interactions on the fitness or spatial distribution of species (Kefi et al. Citation2012). Links are often depicted as categorical variables including ‘-1ʹ or ‘-‘ for negative effects and ‘1ʹ or ‘+’ for positive effects, which can represent the outcome of interactions as well as reciprocal effects between species ().
Advantages and disadvantages of the network approach
The application of network approaches to characterise plant communities presents several features that make this methodology convenient for addressing classic questions in plant ecology. Networks can be created to explore any system of interacting components at different levels, from individuals to species, or groups of species, or the community as a whole, across multiple spatial and temporal scales (Olesen et al. Citation2010; Baskerville et al. Citation2011). In addition, ecological networks can unveil patterns that methods traditionally used in community ecology cannot, such as indirect biotic interactions or emergent properties of ecological communities (e.g. extinction cascades, Bascompte and Jordano Citation2014). However, the use of networks is not exempt of problems, particularly because measuring or inferring plant interactions is not always an easy task.
Measuring biotic interactions at the community level can be challenging as the potential number of interactions increases exponentially with species richness (Scutari and Denis Citation2014), so that the quantification of these interactions in the field or using experimental designs is often unfeasible. Fine-scale spatial distribution patterns can help to overcome this limitation in combination with measures of environmental heterogeneity plus independent proxies for growth rate and fitness. However, spatial patterns alone may sometimes be insufficient to estimate biotic interactions even at the appropriate spatial scale (Connell Citation1961; Delalandre and Montesinos-Navarro Citation2018; Freilich et al. Citation2018). In addition, studies on ecological networks have demonstrated that ecological drivers behind network structure can be obscured by neutral factors such as abundance distribution or the number of interactions observed (Dormann Citation2007).
In the use of interaction networks in plant ecology it is implicitly assumed that the network reasonably reflects the ecological process. Building networks using experiments where biotic interactions are explicitly measured can establish a clear connection between network links and biotic interactions. However, the challenge of building realistic networks is dealing with context dependence, as it is not reasonable to assume that competitive effects inferred from pairwise experiments would hold the same in a diverse community (Levine et al. Citation2017). For this reason, pairwise interactions do not necessarily scale in multispecific context as interactions between two species change in the presence of other species (Losapio et al. Citation2019). On the other hand, limitations associated with co-occurrence networks can be partially overcome by accounting for environmental heterogeneity, plant dispersal patterns and survey scale (Saiz et al. Citation2018; Losapio et al. Citation2018a). This can be done by modelling plant distribution first as a function of environmental factors and second in response to neighbours. Null models to estimate the significance of observed patterns must be included in any analysis (Bascompte and Jordano Citation2014), and the use of networks should be encouraged to study emergent properties that cannot be addressed otherwise. This way, networks will become a promising tool to better understand the role of direct and indirect biotic interactions in plant communities.
Plant–plant interactions in ecological networks
Competitive networks
Competitive networks represent competition among plants (). Competitive interactions are often represented as direct links, constructed of the net outcome of negative interactions. The context dependence of interactions may be further investigated by simulations. For example, Laird and Schamp (Citation2006) have proposed a theoretical model of competitive networks based on species dominance, in which species are hierarchically ranked according to their direct net effects on other species, and organised in intransitive ways, like in a ‘rock-paper-scissors game’ where species A outcompetes B, B outcompetes C and C outcompetes A. These competitive outcomes are then organised in square matrices with varying levels of species richness. Results have indicated that intransitivity promoted species coexistence.
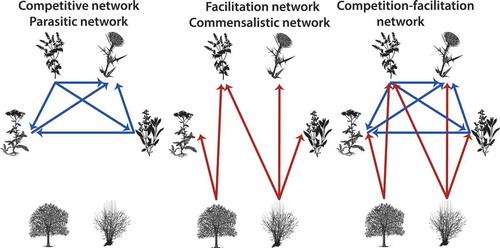
Figure 2. Types of plant networks. Competition-facilitation networks are composed by both competitive (blue) and facilitative (red) interactions. Competitive or parasitic networks are composed only by negative interactions, while facilitation and commensalistic networks are composed only by positive interactions.
The competitive network model of Allesina and Levine (Citation2011) has considered different competitive abilities of species in relation to limiting environmental factors. This model assumed that species competed in numerous patches, each of which was limited by a combination of up to five factors. Results showed that coexistence via intransitive competition was a stabilising niche mechanism, and that heterogeneous environmental conditions together with network structure may favour diversity. Since then, accumulated evidence has shown that intransitivity might play an important role in maintaining biodiversity. Along the same line, the theoretical model proposed by Grilli et al. (2017) has built on the role of intransitive competition for gap colonisation in forest ecosystems. Using random matrix theory, the authors showed that intransitive competition may maintain plant diversity by increasing the stability of the community owing to lowering competitive exclusion.
In a pairwise competition experiment, Godoy et al. (Citation2017) have tested the contribution of intransitive competition to explaining stability in annual plant communities. They found that intransitive competition was not common, and coexistence was driven by pairwise niche differences when species richness was relatively low. These empirical results seem to contradict the foregoing theoretical expectation. Similarly, Nakagawa et al. (Citation2015) have explored whether intraspecific competition was evenly distributed in the community. Building a competitive network between trees in a fir (Abies sachalinensis) plantation, where links were the effect of neighbours on tree size, they showed that competition was stronger between trees of similar age and that this effect might have driven the spatial distribution of age classes.
Conversely, using co-occurrence data, Soliveres et al. (Citation2015) have found a positive correlation between the degree of intransitivity of competitive networks and species richness, suggesting a positive effect of intransitive competition on plant diversity. In summary, theoretical models point toward a major role of network-level intransitive competition for supporting species coexistence, whereas empirical evidence is still sparse to deduce a conclusion. Notably, whether plant intransitive networks are common or infrequent in nature and their importance for species diversity across real-world ecosystems remain unknown.
Facilitation networks
The pioneering study of Verdú and Valiente-Banuet (Citation2008) has described facilitation patterns using seedling recruitment, seed-set and co-occurrence patterns of plants associated with foundation plant species, which are plants with important structural and functional roles in the ecosystem (Ellison Citation2019). Their results showed that facilitation networks across desert ecosystems exhibited a nested structure which was similar to other mutualistic networks (Bascompte and Jordano Citation2014). This nested pattern implies that the establishment and survival of facilitated specialist species was enhanced by generalist foundation species that interact with a broad range of other species. The arrangement of facilitative interactions in a nested way resulted in plant communities less vulnerable to loss of species (Verdú and Valiente-Banuet Citation2008).
Several subsequent studies have explored factors that could explain nestedness in plant facilitation networks. Nestedness has been explained by a combination of species abundance and their phylogenetic relationships (Verdú and Valiente-Banuet Citation2011; Marcilio-Silva et al. Citation2015). This pattern was consistent across different habitats such as drylands and forest–grassland ecotones. Phylogenetic relationships have been used as a proxy for functional dissimilarity, as closely related species are assumed to be more ecologically similar (Verdú and Valiente-Banuet Citation2011). Verdú et al. (Citation2010) have found different phylogenetic patterns when considering facilitation networks based on seedling or adult facilitated plants, suggesting that an ontogenetic shift from facilitation to competition across species occurred and highlighting potential consequences for the organisation and evolution of the whole community.
Ecological networks can also help to understand dynamic properties of plant communities, such as the process of ecological succession. Recruitment networks have been used for this purpose, in which plant–plant interactions (i.e. who recruits beneath whom) were modelled as a flow of resources (Alcantara and Rey Citation2012). In food webs, resources are nutrients that flow across species. In recruitment networks, available space and gaps within vegetation can be modelled as resources. In these gaps, plant species recruit and facilitate the establishment of other species, and these facilitated species can eventually replace the facilitators over time. This approach allows identifying species associated in connected sets (i.e. tight groups of species that facilitate each other), where species are likely to persist over time, or other network subsets in which species are more likely to disappear (Alcantara and Rey Citation2012). In addition, it has been shown that recruitment networks can project realistic ecological succession using forest communities as a case of study (Alcantara et al. Citation2015).
Finally, plant networks can explain variation in facilitation patterns across successional gradients. Losapio et al. (Citation2018b) have considered a multi-layer network with facilitation by foundation plant species of different ages and associated species in seed and adult stages. Their results showed that life history stages and ontogeny drive the organisation of plant networks, suggesting that different processes are operating within the same community across the succession. For example, seed bank development was mainly due to random events while the establishment of adult plants increased with the successional age of plants. Understanding plant networks over the course of the lifetime of an organism is therefore necessary for predicting the dynamics of communities across spatial and temporal scales.
Combined competition-facilitation networks
Competition and facilitation usually co-occur within the same plant community (Callaway and Walker Citation1997; Brooker et al. Citation2008), although they have often been treated separately (Maestre et al. Citation2009). Thus, competitive networks neglect facilitative effects such as recruitment and reduced physiological stress, while facilitation networks overlook competitive interactions among subordinate species (Schöb et al. Citation2013) or negative feedbacks on foundation species (Schöb et al. Citation2014a; Citation2014b). Combined competition-facilitation networks have the advantage of considering both competitive and facilitative interactions within the same community or ecosystem ()).
The first reference to what can be considered as a plant network in literature (De Vries et al. Citation1954) included both putative competition and facilitation. Based on rank correlation between species abundances, it depicted a network – so called ‘constellation of plants’ – of both positive and negative co-occurrence links among 45 grassland species. Further studies have used transplant experiments (Turkington and Harper Citation1979) and applied community matrix theory (Roxburg and Wilson Citation2000; Dormann Citation2007) – a matrix that includes the direct effects between species in a dynamic population model. These studies have substantially contributed to understanding the consequences of species interactions, mainly competition, for the diversity and stability of plant communities. Nevertheless, ecological networks that comprise both competition and facilitation are still rare and poorly understood.
Recent work on inferring plant–plant interactions, based on spatial patterns, has considered both competition and facilitation simultaneously. Here, positive spatial association has been taken to indicate putative facilitation, while negative association to indicate putative competition (Callaway Citation2007). Using this approach, several authors have explored different factors that affect the spatial structure of plant communities. For instance, Fuller et al. (Citation2008) have shown that tree size was related to the spatial organization of tropical-forest networks, and Losapio et al. (Citation2018a) have found that a small number of stress-tolerant species were important for the structure an alpine-tundra network. However, the consideration of both positive and negative links simultaneously remains an overlooked aspect of plant networks which can be important for species coexistence given that a balance between positive and negative associations can have stabilising effects for the network structure (Saiz et al. Citation2017).
Analytical proposals to combine facilitation and competition within a given network have resulted in the seminal theoretical framework of Kefi et al. (Citation2012), in which the authors organised interactions among functional types and provided pathways to incorporate non-trophic interactions into ecological networks. They considered both direct and indirect effects of foundation species on the population density of associated species, showing that including facilitation in the network can increase species persistence and consequently diversity. Overall, including both competition and facilitation within the same plant network is a more realistic approach that naturally has consequences for modelling plant communities ().
Parasitic and commensalistic networks
Apart from competition and facilitation, interactions among plants can also be obligate parasitic and commensalistic (i.e. when species need their partners to grow and reproduce). However, parasitism and commensalism at plant community level have been largely overlooked. Parasitic and commensalistic plant networks describe host–parasite interactions and host–guest interactions between plants. Given the asymmetrical nature of these interactions and the clear functional differences between the two distinct sets of partners, both parasitic and commensalistic interactions are better represented by bipartite networks. Studies have mainly focused on epiphyte–tree interactions in tropical forests and often measured interactions by integrating the ecological knowledge of the species and spatial patterns, but less often using fitness components.
The pioneering study by Burns (Citation2007) in New Zealand forests has found that interactions between epiphytes and host trees were organised in a nested way, in which generalist epiphytes were the first to colonise host trees while specialist epiphytes grew only in a subset of trees. A similar result was obtained for forest networks in Amazonian (Sfair et al. Citation2010) and Chile (Taylor et al. Citation2016). Nestedness in commensalistic networks suggested that facilitation by soil accumulation on host trees may be the mechanism responsible for epiphyte succession (Burns Citation2007). According to this model, early-generalist colonists ameliorate environmental conditions within host trees for later recruiting species that are more specialised and less stress tolerant. The distribution of epiphytes was clustered at both fine and large spatial scales (Burns and Zotz Citation2010), indicating that positive feedbacks may operate to drive the establishment of epiphyte communities over trees.
Strikingly, all these plant–epiphyte networks were structurally similar to those of facilitation networks in desert ecosystems discussed above (Verdú and Valiente-Banuet Citation2008) and to pollination networks (Bascompte and Jordano Citation2014), as they all showed a nested structure of species interactions. These patterns suggest that common processes may underlie the formation of ecological networks regardless of study systems, whether plant commensalism in forests or facilitation in deserts. Indeed, both facilitation and commensalistic networks tend to show a nested pattern, so that specialist species tend to avoid interacting with small sets of specialist partners, in this way maximising the interaction overlap between generalist and specialist species (Bascompte and Jordano Citation2014). A unified network framework can therefore help unveiling common underlying organising principles across ecological systems.
Nestedness in commensalistic plant networks has further been explored using phylogeny and traits tools. It appeared to be independent of the phylogeny of epiphytes and trees (Silva et al. Citation2010) in a tropical forest, possibly owing to the blurring of the signal of phylogenetic filtering in the vast taxonomic and phylogenetic diversity present. On the other hand, phenotypic traits have shown stronger predictive power. Analysing bromeliad communities in tropical forests, Sáyago et al. (Citation2013) have found that tree size, wood density and bark texture were important factors contributing to the nested assembly of commensalistic networks.
Overall, commensalistic networks change with forest age as the distribution of commensalistic interactions was more nested in old than young forests (Piazzon et al. Citation2011). The importance of mature forests as well as of tree abundance, tree size and bark texture was further confirmed by a later study in montane forests. As for previously mentioned plant facilitation networks (Losapio et al. Citation2018b), these results suggest that ecological networks can change over the lifetime of organisms, related to ontogenetic stage and plant size.
In a similar way to plant commensalistic networks, there has been little consideration of parasitic interactions in plant–plant networks. Plant parasitic networks seem to be composed by species-specific interactions organised in a modular way. For instance, generalist mistletoes do not share host trees but colonise distinct groups of specific hosts (Genini et al. Citation2012). Studies on the structure of interactions between epiphytes, mistletoes and lianas with their host trees have found that coevolutionary dynamics can shape the spatial distribution of parasitic interactions (Blick and Burns Citation2009). These authors found evidence for competition among lianas for access to host trees, while host specificity limited species associations (Blick and Burns Citation2011). A functional perspective has been also considered in plant parasitic networks, however, no clear pattern has emerged so far.
Blick et al. (Citation2012) have related the structure of plant parasitic networks to plant traits. Their results showed that phenotypic similarity between mistletoes and host trees did not result in a particular network structure. This result contradicts results in plant–insect mutualistic networks, where interaction intimacy – i.e. the degree of symbiotic relationship between two partners – leads to differences in patterns of specialisation and might affect network organisation (Bascompte and Jordano Citation2014). However, the intensity of interaction intimacy in plant communities can substantially vary among individuals and communities, depending on life history traits and interaction types. We propose that in mistletoe–tree parasitic networks as well as in plant facilitation networks interaction intimacy can be an important factor of coevolution, which, in turn, can further drive interaction intimacy. On the other hand, in transient or unstable plant competition networks in disturbed environments we predict lower interaction intimacy than in other plant networks.
Beyond the perspective of direct and pair-wise plant interactions
Individual plants in nature are confronted with a variety of neighbours of the same and of various numbers of different species. Nevertheless, interspecific biotic interactions are often described at the pairwise level, considering direct net effects between species pairs (e.g. Turkington and Harper Citation1979; Grace and Tilman Citation1990; Roxburg and Wilson Citation2000; Godoy et al. Citation2017). However, it is increasingly recognised that interactions between two species change depending on the presence of other species in the community (Levine et al. Citation2017; Mayfield and Stouffer Citation2017; Losapio et al. Citation2019; Aschehoug and Callaway Citation2015). In other words, a third species can change the effects of one species on another one. Yet, it remains largely unexplored how pairwise interactions scale at the community level.
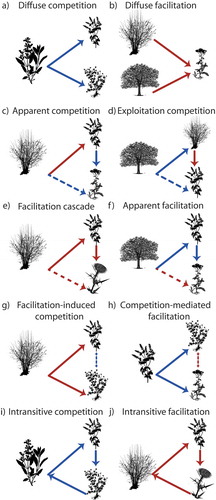
In the following paragraphs, we review the main classes of non-pairwise effects among plants: diffuse interactions, indirect interactions and intransitive interactions (). During the last few years, excellent reviews on indirect interactions have been published (Sotomayor and Lortie Citation2015; Levine et al. Citation2017; Godoy et al. Citation2018). Thus, our next paragraph focuses on how plant networks have helped to assess the relevance of the different non-pairwise and indirect effects among plants for plant diversity.
Diffuse interactions
Diffuse interactions take place when several species interact – compete with or facilitate more than one species within the community (Pianka Citation1974; Moen Citation1989; Vandermeer Citation1990; Callaway and Pennings Citation2000) (). Theory proposes that competitive inhibition by many species in diffuse competition can be equivalent to strong competitive inhibition by fewer competing species (Pianka Citation1974). Diffuse competition among three plant species has been reported in field experiments in salt marshes (Callaway and Pennings Citation2000), indicating that diffuse interactions can cancel direct competitive effects between plant species. There is also evidence that a single species may facilitate many other subordinate species at the same time, leading to diffuse facilitation (Chacòn-Labella et al. Citation2016; Losapio et al. Citation2018a). The outcome of diffuse interactions at the community level can therefore be modelled as a single network to highlight the net effects of biotic interactions on plant diversity.
Indirect interactions
Indirect interactions occur when multiple interaction pathways can take place between the same two species, so that the effects of one plant on another can be mediated by a third species (Grace and Tilman Citation1990; Callaway Citation2007) (). While the intermediary species can be other organism than plants, such as pollinators, mycorrhizal fungi or herbivores, here we focus only on interactions among plants, avoiding for instance insect-mediated indirect interactions which deserves a separate discussion (Sauve et al. Citation2014).
Indirect interactions can buffer net effects, reversing the outcome of direct interactions (Levine Citation1999), and have the potential to impact community dynamics (Levine Citation1976). Indirect interactions can occur through interaction chains and higher-order interactions (Wootton Citation1994). Interaction chains are sequences of direct species interactions resulting in indirect effects that are mediated by changes in population size, density or growth rate of intermediary species (Sotomayor and Lortie Citation2015; Levine et al. Citation2017). On the other hand, for higher-order interactions the effects of interactions between two species on a third species are mediated by changes in phenotypes or functional traits (Vandermeer Citation1990; Ohgushi and Hambäk Citation2015; Levine et al. Citation2017).
Examples of indirect interactions are depicted in . One species can indirectly decrease the abundance of a second species by directly increasing the abundance of its competitor ()), a case of apparent competition. Negative indirect effects can also arise when a species suppresses a facilitator of another species, a case of exploitation competition ()). For instance, LLambi et al. (Citation2018) reported that an invasive plant suppressed a native plant by outcompeting its facilitator. A species can also indirectly increase the abundance of another species by facilitating its facilitator, as in the case of facilitation cascade (Altieri et al. Citation2007) ()). Conversely, one species can indirectly increase the abundance of a second species by suppressing its competitor, as in apparent facilitation (Levine Citation1999) ()).
A separate case is facilitation-induced competition ()), in which one species is facilitating two other species that are therefore indirectly competing against each other. This can particularly be the case in facilitation-driven systems (e.g. arid environments), where it has been found that foundation species (Retama sphaerocarpa) had positive effects on annual plant species that thereby competed with each other (Schöb et al. Citation2013). Conversely, two species can indirectly facilitate each other by outcompeting a common competitor in competition-mediated facilitation ()). Evidence for this type of interaction has been found among animals rather than among plants, where several species of parrotfish (genera Sparisoma and Scarus) and corals (Montastraea) are mutually facilitating each other by competitively excluding macroalgae (Dictyota) in coral reefs (Bozec et al. Citation2013). We hypothesise that indirect interactions with positive outcomes would increase network nestedness as a result of lower competition among facilitated species that occur only within subsets of abundant, generalist facilitators, ultimately leading to higher stability of the community.
Both diffuse and indirect interactions may be explored using Bayesian network inference. By examining all potential direct and indirect relationships among species in a community to assess the conditional dependencies among species abundances, it might be possible to identify interactions that significantly affect a given focal species (Scutari and Denis Citation2014; Staniczenko et al. Citation2017). This technique overcomes the challenge of estimating an unfeasible number of parameters by applying a heuristic search for optimal solutions. A network is proposed by different algorithms and sequentially compared to co-occurrence relationships observed through goodness-of-fit statistics. Then, the network is modified and the process iterated until maximum fitness is reached, and the network that best matches the data can be selected. This tool has been widely used to study interaction patterns in disciplines such as molecular biology and medical bioinformatics (Chai et al. Citation2014), but its application to study ecological interactions has been much less explored (but see Staniczenko et al. Citation2017; Delalandre and Montesinos-Navarro Citation2018; Montesinos-Navarro et al. Citation2018).
Intransitive interactions
When interactions between species are non-hierarchical and net direct effects cannot be linearly ranked, intransitive loops can emerge leading to even competitive dominance (). Despite plant communities being often envisioned as characterised by hierarchical, competitive dominance (Grace and Tilman Citation1990; Keddy Citation2017), the list of studies reporting intransitivity has rapidly grown recently as we discussed above, although not to unanimous acceptance (Levine et al. Citation2017). The swap from interaction hierarchies to intransitive loops is likely to emerge in spatially or temporally heterogeneous systems when there is more than one limiting resource or where the growth rates of species and their resource acquisition are highly context-dependent (Allesina and Levine Citation2011). Intransitivity may also occur in facilitative interactions ()), due to indirect reciprocity (Boyd et al. Citation2003), but its frequency and relevance is yet to be identified in natural communities. Although intransitive facilitation is a well-established concept in evolutionary biology, the ecological implications of this type of interaction network are still unexplored also under a theoretical point of view.
Theory has proposed that intransitive competition in complex ecological networks can enhance community persistence (Laird and Schamp Citation2006; Allesina and Levine Citation2011, Grilli et al. 2017). Network properties such as connectance (Alcantara et al. Citation2015; Saiz et al. Citation2018), relationships between the subnetworks within the network (Alcantara and Rey Citation2012) and the competitive ranking among species (Laird and Schamp Citation2006) can significantly influence the persistence of species within communities. Moreover, compartmentalisation and a high number of species participating in these intransitive loops can mitigate species loss (Alcantara and Rey Citation2012). Despite these modelling studies indicating how intransitive interactions can support plant diversity by enhancing community stability, the empirical evidence for the importance of intransitive interactions is controversial as intransitive competition alone might not be strong enough to determine species persistence within a community (Godoy et al. Citation2017).
Taken together, these studies suggest that non-pairwise interactions occur at the whole-network level and thus can be relevant for community dynamics. These diffuse, indirect and intransitive interactions ‘spread’ across the networks and have the potential to reverse negative effects of direct pairwise interactions, with fundamental consequences for species persistence and species diversity maintenance. However, we still do not know enough to draw causal relationships between these interactions and the diversity of species in plant communities that we observe in nature. Indirect interactions in plant networks may be hard to identify, however, it appears to be an important step in order to better understand the stability and functioning of ecological systems.
Ecological implications of plant interactions across organisational levels
A combination of the accumulated knowledge on network theory and plant ecology is tackling the three challenges mentioned above. Network theory improves models of plant community structure and dynamics (Kefi et al. Citation2012; Grilli et al. 2017), particularly by combining different interaction types within and across trophic levels (Sauve et al. Citation2014; Losapio et al. Citation2019). Ecological networks have been used to answer relevant questions in plant ecology at different scales, from populations to ecosystems ().
Box 1. Effects of livestock grazing on a spatial plant network.
Figure B1. Plant spatial networks for the natural (left) and overgrazed (right) communities in Mediterranean ecosystems (South East Spain). Nodes (dots) represent plant species, blue links represent positive links (aggregation) and red links represent negative links (segregation).
Overgrazing had a strong impact on the structure of plant networks (, ). Overgrazing substantially simplified the community and caused the loss of many positive associations between species, mainly as a result of reduced richness, as can be seen from the increase in the values of NC and contemporary decrease in D and R (). In particular, overgrazing breaks the patchy structure of the vegetation, precluding the persistence of species that require the presence of plant patches to survive. Interestingly, both communities show a modular organization, indicating the resistance of plant networks to overgrazing. However, the modular network structure relies only on one single species in overgrazed communities, the unpalatable species at the center of the network (), thus making the network more vulnerable. These results shed new light on the profound consequences of overgrazing on the structure and functioning of plant communities.
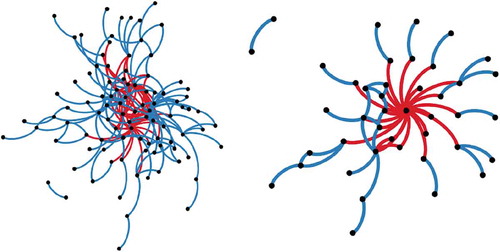
Table B1. Structure of spatial networks in two communities with different livestock grazing intensity. NC is the proportion of non-connected species; D is the link density; R is the link ratio; M is the modularity. ** and *** indicate that modularity is significantly higher than expected by 1000 random networks.
Plant network structure has been linked to community robustness and stability. Some properties of plant facilitation networks such as nestedness or scale-free degree distributions can maximise the number of species occurring in the community (Verdú and Valiente-Banuet Citation2008; Saiz and Alados Citation2011a; Kefi et al. Citation2012). On the other hand, recruitment networks have been shown to be structured in discrete subsets (Alcantara and Rey Citation2012). Saiz and Alados (Citation2014) have found that grazing simplifies the structure of the plant community, with livestock disturbance reducing the number of species and interactions within the community. Meanwhile, in the same environment, the tussock grass Stipa tenacissima increased the spatial aggregation of subordinate species (Saiz and Alados Citation2011a).
Functional traits, spatio-temporal dynamics and conservation
Plant networks can contribute to conservation by gaining a better understanding of how network structure can be altered by extrinsic factors (e.g. environmental change) and can buffer local population extinction. These areas constitute a potential framework to assess how plant interactions contribute to species persistence and community robustness against species loss, with important implication for conservation strategies. Using a modelling approach, Losapio and Schöb (Citation2017) have proposed a framework for assessing the response of plant networks to environmental change. Their model integrated facilitation networks with functional traits as criteria of species sensitivities to environmental change, showing that the resistance of plant communities and inherent diversity was supported by stress-tolerant foundation species.
Other applications of plant networks have been oriented to studies of spatio-temporal dynamics. In particular, processes involved in ecological successions have been examined by both focusing on foundation-species effects and other components such as replacement and turnover. Overall, the role of foundation species in plant communities is comparable to that of keystone species in food webs (Jordán Citation2009; Ellison Citation2019). Foundation species play a key role for structuring plant facilitation networks, especially in stressful environments such as arid (Verdú and Valiente-Banuet Citation2008, Citation2011; Saiz and Alados Citation2011b; Losapio et al. Citation2018b) and alpine ecosystems (Losapio et al. Citation2018a). These plant networks show a scale-free degree distribution (Jordán and Scheuring Citation2004), meaning that the majority of plant species is loosely associated to other species while only few species have a large number of neighbours (Saiz and Alados Citation2011b).
Nestedness of plant facilitation networks has been proposed to be also responsible for driving ecological succession as networks become more nested with increasing successional age (Losapio et al. Citation2018a). This suggests that the stability of ecological networks against species loss increases throughout ecological successions, as also evidenced by plant–insect network dynamics (Losapio et al. Citation2015). Results from tropical forests (Marcilio-Silva et al. Citation2015) also highlight the importance of species turnover over the influence of some species traits related to dispersal and canopy size.
The concept of strongly connected components can be useful for predicting dynamical properties of networks based on network topology (Alcantara and Rey Citation2012). These network subsets are groups of nodes where ‘resources’ flow either directly or indirectly, from one to the other and in both directions, and they can represent successional dynamics among species that can persistent over time. Future studies aimed at forecasting interaction-mediated community response to environmental change will need to include different layers of plant fitness information as well as addressing direct and indirect antagonistic and mutualistic interactions (Sauve et al. Citation2016). In sum, plant networks seem a promising tool to improve management strategies by considering how the whole organisation of plant communities can be affected by changes driven by both extrinsic and intrinsic factors.
Ecological network theory has been also succinctly used to model species distribution at different spatial scales, but its application to study plant interactions and plant communities has been much less extended. From a biogeographical perspective, species distribution models have usually considered macroecological abiotic factors to predict species distribution. However, interest in including plant interaction networks into species distribution models has increased recently (Staniczenko et al. Citation2017). Results of Bayesian Networks of annual plant communities showed that considering plant interactions substantially altered assessments of species range changes under future environmental change (Staniczenko et al. Citation2017). Indeed, Bayesian Network inference improves the prediction of plant species distribution models and provides a feasible way of including information about the co-occurrence patterns among all the species in the community (Montesinos-Navarro et al. Citation2018).
At the local scale, it has been also tested whether this technique can precisely infer plant–plant interactions, showing that networks could reflect different processes at different spatial scales, and its sensitivity to the sampling spatial scale requires caution in the interpretation of the inferred links (Delalandre and Montesinos-Navarro Citation2018). Potential future applications include integrating models of individual-based networks (Olesen et al. Citation2010) with species distribution models, then testing these predictions in the field. Particularly relevant to future directions is understanding how current network structure may drive local adaptation to microhabitat conditions as well as to novel interacting partners and extinct partners.
Conclusions
The application of network theory to plant ecology has provided new avenues to study the role of biotic interactions for the structure and dynamics of plant communities. Measuring plant–plant interactions and scaling them at the community level to build meaningful ecological networks are not straightforward and easy tasks. Caution should be taken when interpreting networks resulting from co-occurrence only or pairwise experiments as species interactions are context dependent and vary in the presence of other members of the communities. Statistical tools, simulations and experimental manipulation in the field can help to overcome the problems associated with inferring plant interactions at the network level.
Research along this theoretical line has indicated how the hierarchy of plant competition can influence species persistence and community stability. By highlighting how species are connected, what makes some species more connected than others, and how they influence each other’s persistence through the community network resulted in a deeper understanding of the effects of plant–plant interactions on the maintenance of plant species diversity at the community level. Overall, plant networks reveal properties that are consistent over many different communities and environments, from tropical forests to alpine tundra, and are also common to other classes of ecological and complex networks. Yet, our understanding of processes shaping plant networks as well as their mechanisms underlying the maintenance of plant species diversity is limited. Questions such as how robust are specialised and generalised plant interactions, the combined effects of facilitation and competitive networks on community stability, or the role of interaction diversity for biodiversity maintenance are still largely unanswered under both theoretical and empirical points of view.
Future directions where research on plant networks may move forward can include multi-layer plant networks that encompass different interaction types and trophic levels responsible for plant fitness, such as mutualistic and antagonistic organisms (Sauve et al. Citation2016). Plant network modelling in a context of ecological succession and environmental gradients can reveal mechanisms linking biotic interactions, community dynamics and ecosystem processes. Finally, we still have a very limited knowledge of the role of plant interactions and their networks for eco-evolutionary dynamics (Schöb et al. Citation2018), how plant networks evolve and the consequences of plant network structure for ecosystem functioning. In these areas, relevant questions such as how plant network structure contributes to micro-evolutionary processes, local adaptation or productivity of plant communities are still unanswered. To do so, it seems important moving from the description of local plant networks to formalise mathematical models associated with experimental assessment as well as including other biotic components of ecosystems. Plant network studies that embrace the peculiarity of the plant kingdom across different organisation levels and processes have a great potential for advancing plant ecology and biodiversity science.
Acknowledgements
We thank Christian Schöb for stimulating discussions and for inviting the authors to write this review. We also thank the Editor-in-Chief Laszlo Nagy and four anonymous reviewers for their constructive comments. We thank Concepción L. Alados for sharing data from CEBCE project (CGL2008-0065) that we used for building the networks in . GL is supported by the Swiss National Science Foundation (IZSEZ0_180195) and by the ETH Biocommunication group. AMN is supported by a Juan de la Cierva-Incorporación fellowship from the Spanish Ministry of Economy and Competitiveness (IJCI-2015-23498). H.S. is supported by the European Research Council (ERC Grant agreement 647038 [BIODESERT]) and by a Juan de la Cierva-Formación fellowship from the Spanish Ministry of Economy and Competitiveness (FJCI-2015-26782).
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
Notes on contributors
Gianalberto Losapio
Gianalberto Losapio is a post-doctoral researcher in ecology. His research interests include biodiversity and ecological networks.
Alicia Montesinos-Navarro
Alicia Montesinos-Navarro, a post-doctoral researcher, is interested in how biotic interactions shape the structure of plant communities.
Hugo Saiz
Hugo Saiz is a post-doctoral researcher is interested in biotic interactions for the persistence and functioning of plant communities.
References
- Alcantara JM, Rey PJ, Manzaneda AJ. 2015. A model of plant community dynamics based on replacement networks. J Veg Sci. 26:524–537.
- Alcantara JM, Rey PT. 2012. Linking topological structure and dynamics in ecological networks. Am Nat. 180:186–199.
- Allesina S, Levine JM. 2011. A competitive network theory of species diversity. Proc National Acad Sci USA. 108:5638–5642.
- Altieri AH, Silliman BR, Bertness MD. 2007. Hierarchical organization via a facilitation cascade in intertidal cordgrass bed communities. Am Nat. 169:195–206.
- Aschehoug ET, Callaway RM. 2015. Diversity increases indirect interactions, attenuates the intensity of competition, and promotes coexistence. Am Nat. 186:452–459.
- Bascompte J, Jordano P. 2014. Mutualistic networks. USA: Princeton University Press.
- Baskerville EB, Dobson AP, Bedford T, Allesina S, Anderson TM, Pascual M. 2011. Spatial guilds in the serengeti food web revealed by a Bayesian group model. PLoS Comput Biol. 7:e1002321.
- Bersier LF. 2007. A history of the study of ecological networks. In: Kepes, editor. Biological networks. World Scientific. 365–421.
- Blick R, Burns KC. 2009. Network properties of arboreal plants: are epiphytes, mistletoes and lianas structured similarly? Perspect Plant Ecol Evol Syst. 11:41–52.
- Blick RAJ, Burns KC. 2011. Liana co‐occurrence patterns in a temperate rainforest. J Veg Sci. 22:868–877.
- Blick RAJ, Burns KC, Moles AT. 2012. Predicting network topology of mistletoe–host interactions: do mistletoes really mimic their hosts? Oikos. 121:761–771.
- Boyd R, Gintis H, Bowles S, Richerson PJ. 2003. The evolution of altruistic punishment. Proc National Acad Sci USA. 100:3531–3535.
- Bozec YM, Yakob L, Bejarano S, Mumby P. 2013. Reciprocal facilitation and non-linearity maintain habitat engineering on coral reefs. Oikos. 122:428–440.
- Brooker RW, Maestre FT, Callaway RM, Lortie CL, Cavieres LA, Kunstler G, Liancourt P, Tielbörger K, Travis JMJ, Anthelme F. 2008. Facilitation in plant communities: the past, the present, and the future. J Ecol. 96:18–34.
- Burns KC. 2007. Network properties of an epiphyte metacommunity. J Ecol. 95:1142–1151.
- Burns KC, Zotz G. 2010. A hierarchical framework for investigating epiphyte assemblages: networks, meta-communities, and scale. Ecology. 91:377–385.
- Butterfield BJ, Cavieres LA, Callaway RM, Cook BJ, Kikvidze Z, Lortie CJ, Michalet R, Pugnaire FI, Schöb C, Xiao S, et al. 2013. Alpine cushion plants inhibit the loss of phylogenetic diversity in severe environments. Ecol Lett. 16:478–486.
- Callaway RM. 2007. Positive interactions and interdependence in plant communities. USA: Springer.
- Callaway RM, Pennings SC. 2000. Facilitation may buffer competitive effects: indirect and diffuse interactions among salt marsh plants. Am Nat. 156:416–424.
- Callaway RM, Walker LR. 1997. Competition and facilitation: a synthetic approach to interactions in plant communities. Ecology. 78:1958–1965.
- Chacòn-Labella J, de la Cruz M, Escudero A. 2016. Beyond the classical nurse species effect: diversity assembly in a Mediterranean semi‐arid dwarf shrubland. J Veg Sci. 27:80–88.
- Chai LE, Loh SK, Low ST, Mohamad MS, Deris S, Zakaria Z. 2014. A review on the computational approaches for gene regulatory network construction. Comput Biol Med. 48:55–65.
- Chesson P. 2000. Mechanisms of maintenance of species diversity. Annu Rev Ecol Syst. 31:343–366.
- Choler P, Michalet R, Callaway RM. 2001. Facilitation and competition on gradients in alpine plant communities. Ecology. 82:3295–3308.
- Connell JP. 1961. The influence of interspecific competition and other factors on the distribution of the barnacle Chthamalus stellatus. Ecology. 42:710–723.
- Csardi G, Nepusz T. 2006. The igraph software package for complex network research. InterJ Complex Syst. 1695:1–9.
- De Vries DM, Baretta JP, Hamming G. 1954. Constellation of frequent herbage plants, based on their correlation in occurrence. Vegetatio. 6:105–111.
- Delalandre L, Montesinos-Navarro A. 2018. Can co-occurrence networks predict plant-plant interactions in a semi-arid gypsum community. Perspect Plant Ecol Evol Syst. 31:36–43.
- Dormann CF. 2007. On community matrix theory in experimental plant ecology. Web Ecol. 8:108–115.
- Dormann CF, Gruber B, Fruend J. 2008. Introducing the bipartite package: analysing ecological networks. R News. 8:8–11.
- Ellison AM. 2019. Foundation species, non-trophic interactions, and the value of being common. iScience. 13:254–268.
- Fortuna MA, Bascompte J. 2008. The network approach in ecology. In: Valladares C, Elosegi E, Senar G, editors. Unity in diversity: reflections on ecology after the legacy of Ramon Margalef. Fundacion BBVA. 371–392.
- Freilich MA, Wieters E, Broitman BR, Marquet PA, Navarrete SA. 2018. Species co-occurrence networks: can they reveal trophic and non-trophic interactions in ecological communities? Ecology. 99:690–699.
- Fuller MM, Wagner A, Enquist BJ. 2008. Using network analysis to characterize forest structure. Nat Resourse Modell. 21:225–247.
- Genini J, Cortes MC, Guimaraes PRJ, Galetti M. 2012. Mistletoes play different roles in a modular host–parasite network. Biotropica. 44:171–178.
- Godoy O, Bartomeus I, Rohr R, Saavedra S. 2018. Towards integrating niche and network theories. Trends Ecol Evol. 33:287–300.
- Godoy O, Stouffer DB, Kraft NJ, Levine JM. 2017. Intransitivity is infrequent and fails to promote annual plant coexistence without pairwise niche differences. Ecology. 98:1193–1200.
- Grace JB, Tilman D. 1990. Perspectives on plant competition. USA: Academic Press.
- Grilli J, Barabás G, Michalska-Smith MJ, Allesina S. 2017. Higher-order interactions stabilize dynamics in competitive network models. Nature. 548:210–213.
- Grubb PJ. 1977. The maintenance of species-richness in plant communities: the importance of the regeneration niche. Biological Reviews. 52:107–145.
- HilleRisLambers J, Adler PB, Harpole WS, Levine JM, Mayfield MM. 2012. Rethinking community assembly through the lens of coexistence theory. Annu Rev Ecol Evol Syst. 43:227–248.
- Jordán F. 2009. Keystone species and food webs. Philos Trans R Soc B. 364:1733–1741.
- Jordán F, Scheuring I. 2004. Network ecology: topological constraints on ecosystems dynamics. Phys Life Rev. 3:139–172.
- Keddy PA. 2017. Plant ecology. UK: Cambridge University Press.
- Kefi S, Berlow EL, Wieters EA, Navarrete SA, Petchey OL, Wood SA, Boit A, Joppa LN, Lafferty KD, Williams RJ, et al. 2012. More than a meal integrating non-feeding interactions into food webs. Ecol Lett. 15:291–300.
- Kikvidze Z, Brooker RW, Butterfield BJ, Callaway RM, Cavieres LA, Cook BJ, Lortie CJ, Michalet R, Pugnaire FI, Xiao S, et al. 2015. The effects of foundation species on community assembly: a global study on alpine cushion plant communities. Ecology. 96:2064–2069.
- Klein T, Siegwolf RTW, Körner C. 2016. Belowground carbon trade among tall trees in a temperate forest. Science. 352:342–344.
- Körner C. 2018. Concepts in empirical plant ecology. Plant Ecol Divers. 11:405–428.
- Laird RA, Schamp BS. 2006. Competitive intransitivity promotes species coexistence. Am Nat. 168:182–193.
- Levine JM. 1999. Indirect facilitation: evidence and predictions from a riparian community. Ecology. 80:1762–1769.
- Levine JM, Bascompte J, Adler PB, Allesina S. 2017. Beyond pairwise mechanisms of species coexistence in complex communities. Nature. 546:56–64.
- Levine SH. 1976. Competitive interactions in ecosystems. Am. Nat. 110:903–910.
- LLambi LD, Hupp N, Saez A, Callaway RM. 2018. Reciprocal interactions between a facilitator, natives, and exotics in tropical alpine plant communities. Perspect Plant Ecol Evol Syst. 30:82–88.
- Losapio G, de la Cruz M, Escudero A, Schmid B, Schöb C. 2018a. The assembly of a plant network in alpine vegetation. J Veg Sci. 29:999–1006.
- Losapio G, Fortuna M, Bascompte J, Schmid B, Michalet R, Neumeyer R, Castro L, Cerretti P, Germann C, Haenni J-P, et al. 2019. Plant interactions shape pollination networks via nonadditive effects. Ecology. 100:e02619.
- Losapio G, Jordán F, Caccianiga M, Gobbi M. 2015. Structure-dynamic relationship of plant–insect networks along a primary succession gradient on a glacier foreland. Ecol Modell. 314:73–79.
- Losapio G, Pugnaire FI, O’Brien MJ, Schöb C. 2018b. Plant life history stage and nurse age change the development of ecological networks in arid ecosystems. Oikos. 127:1390–1397.
- Losapio G, Schöb C. 2017. Resistance of plant–plant networks to biodiversity loss and secondary extinctions following simulated environmental changes. Funct Ecol. 31:1145–1152.
- Maestre FT, Callaway RM, Valladares F, Lortie CJ. 2009. Refining the stress-gradient hypothesis for competition and facilitation in plant communities. J Ecol. 97:199–205.
- Marcilio-Silva V, Cavalin PO, Varrasin IG, Oliveira RAC, deSouza JMT, Muschner VC, Marques MCM. 2015. Nurse abundance determines plant facilitation networks of subtropical forest–grassland ecotone. Austral Ecol. 40:898–908.
- Mayfield MM, Stouffer DB. 2017. Higher-order interactions capture unexplained complexity in diverse communities. Nat Ecol Evol. 3:62.1–62.7.
- McCann KS. 2011. Food webs. USA: Princenton University Press.
- Mescher MC, Pearse IS. 2016. Communicative interactions involving plants: information, evolution, and ecology. Curr Opin Plant Biol. 32:69–76.
- Moen J. 1989. Diffuse competition – a diffuse concept. Oikos. 54:260–263.
- Montesinos-Navarro A, Estrada A, Font X, Matias MG, Meireles C, Mendoza M, Honrado JP, Prasad HD, Vicente JR, Early R. 2018. Community structure informs species geographic distribution. PLoS One. 13:e0200556.
- Nakagawa Y, Yokozawa M, Hara T. 2015. Competition among plants can lead to an increase in aggregation of smaller plants around larger one. Ecol Modell. 301:41–53.
- Ohgushi T, Hambäk PA. 2015. Toward a spatial perspective of plant-based indirect interaction webs: scaling up trait-mediated indirect interactions. Perspect Plant Ecol Evol Syst. 17:500–509.
- Olesen JM, Dupont YL, O’Gorman E, Ings TC, Layer K, Meliàn C., Trøjelsgaard C, Pichler DE, Rasmussen C, Woodward G. 2010. From Broadstone to Zackenberg. Space, time and hierarchies in ecological networks. Adv Ecol Res. 42:1–69.
- Olff H, Ritchie ME. 1998. Effects of herbivores on grassland plant diversity. Trends Ecol Evol. 13:261–265.
- Pianka ER. 1974. Niche overlaps and diffuse competition. Proc National Acad Sci USA. 71:2141–2145.
- Piazzon M, Larrinaga AR, Santamaria L. 2011. Are nested networks more robust to disturbance? A test using epiphyte-tree, commensalistic networks. PLoS One. 6:e19637.
- Roxburg SH, Wilson JB. 2000. Stability and coexistence in a lawn community: mathematical prediction of stability using a community matrix with parameters derived from competition experiments. Oikos. 88:395–408.
- Saiz H, Alados CL. 2011a. Structure and spatial self-organization of semi-arid communities through plant–plant co-occurrence networks. Ecol Complexity. 8:184–191.
- Saiz H, Alados CL. 2011b. Effect of Stipa tenacissima L. on the structure of plant co-occurrence networks in a semi-arid community. Ecol Res. 27:595–603.
- Saiz H, Alados CL. 2012. Changes in semi-arid plant species associations along a livestock grazing gradient. PLoS One. 7:e40551.
- Saiz H, Alados CL. 2014. Effect of livestock grazing in the partitions of a semiarid plant–plant spatial signed network. Acta Oecologica. 59:18–25.
- Saiz H, Gómez-Gardeñes J, Nuche P, Girón A, Pueyo Y, Alados CL. 2017. Evidence of structural balance in spatial ecological networks. Ecography. 40:733–741.
- Saiz H, Le Bagousse-Pinguet Y, Gross N, Maestre FT. 2018. Intransitivity increases plant functional diversity by limiting dominance in drylands worldwide. J Ecol. 107:240–252.
- Sauve AM, Fontaine C, Thébault E. 2014. Structure–stability relationships in networks combining mutualistic and antagonistic interactions. Oikos. 123:378–384.
- Sauve AM, Thébault E, Pocock MJ, Fontaine C. 2016. How plants connect pollination and herbivory networks and their contribution to community stability. Ecology. 97:908–917.
- Sáyago R, Lopezaraiza-Mikel M, Quesada M, Álvarez-Añorve MY, Cascante-Marín A, Bastida JM. 2013. Evaluating factors that predict the structure of a commensalistic epiphyte–phorophyte network. Proc R Soc B. 280:20122821.
- Schöb C, Armas C, Pugnaire FI. 2013. Direct and indirect interactions co-determine species composition in nurse plant systems. Oikos. 122:1371–1379.
- Schöb C, Brooker RW, Zuppinger-Dingley D. 2018. Evolution of facilitation requires diverse communities. Nat Ecol Evol. 2:1381.
- Schöb C, Michalet R, Cavieres LA, Pugnaire FI, Brooker RW, Butterfield BJ, Cook BJ, Kikvidze Z, Lortie CJ, Xiao S, et al. 2014b. A global analysis of bidirectional interactions in alpine plant communities shows facilitators experiencing strong reciprocal fitness costs. New Phytol. 202:95–105.
- Schöb C, Prieto I, Armas C, Pugnaire FI. 2014a. Consequences of facilitation: one plant’s benefit is another plant’s cost. Funct Ecol. 28:500–508.
- Scutari M, Denis J-B. 2014. Bayesian networks: with examples in R. USA: CRC Press.
- Sfair JC, Casarin Rochelle AL, Rezende AA, van Melis J, de Lara Weiser V, Martins FR. 2010. Nested liana-tree network in three distinct neotropical vegetation formations. Perspect Plant Ecol Evol Syst. 12:277–281.
- Silva IA, Coelho Ferreira AW, Silveira Lima MA, Soares JJ. 2010. Networks of epiphytic orchids and host trees in Brazilian gallery forests. J Trop Ecol. 26:127–137.
- Soliveres S, Maestre FT, Ulrich W, Manning P, Boch S, Bowker MA, Prati D, Delgado-Baquerizo M, Quero JL, Schöning I, et al. 2015. Intransitive competition is widespread in plant communities and maintains their species richness. Ecol Lett. 18:790–798.
- Sotomayor DA, Lortie CJ. 2015. Indirect interactions in terrestrial plant communities: emerging patterns and research gaps. Ecosphere. 6:1–23.
- Staniczenko PPA, Sivasubramaniam P, Suttle KB, Pearson RG. 2017. Linking macroecology and community ecology: refining predictions of species distributions using biotic interaction networks. Ecol Lett. 20:693–707.
- Taylor A, Saldana A, Zotz G, Kirby C, Diaz I, Burns K. 2016. Composition patterns and network structure of epiphyte–host interactions in Chilean and New Zealand temperate forests. N Z J Bot. 54:204–222.
- Turkington R, Harper JL. 1979. The growth, distribution and neighbour relationships of Trifolium repens in a permanent pasture. II. Inter- and intra-specific contact. J Ecol. 67:219–230.
- Valiente-Banuet A, Rumebe AV, Verdú M, Callaway RM. 2006. Modern quaternary plant lineages promote diversity through facilitation of ancient tertiary lineages. Proc National Acad Sci USA. 103:16812–16817.
- Vandermeer JH. 1990. Indirect and diffuse interactions: complicated cycles in a population embedded in a large community. J Theor Biol. 142:429–442.
- Verdú M, Jordano P, Valiente-Banuet A. 2010. The phylogenetic structure of plant facilitation networks changes with competition. J Ecol. 98:1454–1461.
- Verdú M, Valiente-Banuet A. 2008. The nested assembly of plant facilitation networks prevents species extinctions. Am Nat. 172:751–760.
- Verdú M, Valiente-Banuet A. 2011. The relative contribution of abundance and phylogeny to the structure of plant facilitation networks. Oikos. 120:1351–1356.
- Wootton JT. 1994. The nature and consequences of indirect effects in ecological communities. Annu Rev Ecol Syst. 25:443–466.