ABSTRACT
Quaternary (last 2.6 million years) botany involves studying plant megafossils (e.g. tree stumps), macrofossils (e.g. seeds, leaves), and microfossils (e.g. pollen, spores) preserved in peat bogs and lake sediments. Although megafossils and macrofossils have been studied since the late eighteenth century, Quaternary botany today is largely dominated by pollen analysis.
Quaternary pollen analysis is just over 100 years old. It started primarily as a geological tool for correlation, relative dating, and climate reconstruction. In 1950 a major advance occurred with the publication by Knut Fægri and Johs Iversen of their Text-book of Modern Pollen Analysis which provided the foundations for pollen analysis as a botanical and ecological tool for studying past dynamics of biota and biotic systems. The development of radiocarbon dating in the 1950s freed pollen analysis from being a tool for relative dating. As a result of these developments, pollen analysis became a valuable implement in long-term ecology and biogeography.
Selected contributions that Quaternary botany has made to ecology and biogeography since 1950 are reviewed. They fall into four general parts: (1) ecological aspects of interglacial and glacial stages such as location and nature of glacial-stage tree refugia and long-term soil development in glaciated and unglaciated areas; (2) biotic responses to Quaternary environmental change (spreading, extinction, persistence, adaptation); (3) ecological topics such as potential niches, the nature of vegetation, and tree and forest dynamics; and (4) its application to ecological topics such as human impact in tropical systems, conservation in a changing world, island palaeoecology, plant–animal interactions, and biodiversity patterns in time.
The future of Quaternary botany is briefly discussed and 10 suggestions are presented to help strengthen it and its links with ecology and biogeography. Quaternary botany has much to contribute to ecology and biogeography when used in conjunction with new approaches such as ancient-DNA, molecular biomarkers, and multi-proxy palaeoecology.
1. Introduction
Quaternary (last 2.6 million years) botany involves the study of plant megafossils (e.g. tree stumps, wood remains), plant macrofossils (e.g. seeds, fruits, leaves), and plant microfossils (e.g. pollen, spores, phytoliths, charcoal, non-pollen palynomorphs) preserved in peat and in lake and ocean sediments. Early studies naturally concentrated on the impressive occurrence of large fossil trunks and stumps of pine trees preserved in peat bogs in north-west Europe (e.g. Tait Citation1794; Dau Citation1829; Steenstrup Citation1841; Vaupell Citation1857; Maxwell Citation1915). Quaternary plant macrofossils began to be investigated systematically in the late nineteenth century (e.g. Nathorst Citation1870, Citation1892; Reid C Citation1899; Andersson G Citation1902, Citation1909). These and related studies (see Birks HJB Citation2008 for a review) resulted in the Blytt-Sernander subdivision of the post-glacial (Holocene) into the Pre-Boreal, Boreal, Atlantic, Sub-Boreal, and Sub-Atlantic periods (Blytt Citation1881; Sernander Citation1890). This scheme became the dominant paradigm for Holocene climate change in the early twentieth century and dominated until the wide-ranging review by von Post (Citation1946) of the prospect for pollen analysis in the study of Earth’s climatic history.
Quaternary pollen analysis as we know it began in July 1916 when the Swedish geologist Lennart von Post presented a lecture on Skogsträdpollen i sydsvenska torvmosselagerfäljder (Forest tree pollen in south Swedish peat deposits) at the 16th Scandinavian Meeting of Natural Scientists in Kristiania (now Oslo) (von Post Citation1916). The use of pollen analysis in Quaternary geology spread rapidly, not only within the Nordic countries but also throughout Europe, the Baltic countries, Russia, New Zealand, and the Americas in the 1920s (Birks HJB and Berglund Citation2018). It was primarily used as a geological tool for correlation, reconstructing forest and climate history, and relative dating of archaeological material, sea-level changes, and peat-stratigraphical changes.
A major turning point in the development of pollen analysis and hence in Quaternary botany was the 1933 Baltic Course on pollen analysis led by von Post. The course participants were primarily young geologists from the Baltic countries with two botanists – Knut Fægri from Norway and Johannes (Johs) Iversen from Denmark. Both had recently defended their doctoral theses in plant ecology. They became life-long friends and planned to write a small monograph about pollen analysis not primarily as a geological dating tool but about their vision of pollen analysis as an ecological tool for studying long-term vegetation dynamics and human impact on vegetation (Birks HJB and Berglund Citation2018). Such early ideas about ecological pollen analysis were not, however, confined to Norway or Denmark, as increasingly more ecological studies involving pollen analysis were being made elsewhere in Europe by, for example, Aario, Bertsch, Firbas, Godwin, Rudolph, Szafer, and Welten (Birks HJB and Berglund Citation2018). Fægri and Iversen published their Text-book of Modern Pollen Analysis in 1950 (Fægri and Iversen Citation1950) and it rapidly became the ‘bible’ for pollen analysts. It represented a paradigm shift in Quaternary pollen analysis. It highlighted the importance of, for example, detailed pollen identifications, of understanding the statistical background to pollen counting, and of the need for sound botanical and ecological knowledge in all Quaternary pollen analysis (Birks HJB and Berglund Citation2018).
The development of radiocarbon dating by WF Libby in the late 1940s and early 1950s provided a means of deriving an absolute chronology for events in the Late Glacial and Holocene. It freed pollen analysis from being a relative chronological tool. Pollen analysis was now able to make contributions to modern ecology and biogeography such as documenting the spatial and temporal patterns in pollen stratigraphies, reconstructing directions and rates of tree spread, identifying pollen assemblages and hence past vegetation types that lack modern analogues today, and studying the timing of human impact on past vegetation not only in temperate but also in tropical areas (Birks HJB et al. Citation2016b; Edwards KJ et al. Citation2017; Birks HJB and Berglund Citation2018).
There are several reviews about Quaternary floristics and vegetational history of different geographical areas (see for examples). There are also some reviews about specific contributions of different aspects of Quaternary botany to modern ecology or biogeography (see for examples). The aim of this paper is to to provide a coherent review of several of these contributions. It falls into four general parts: (1) ecological aspects of interglacial and glacial stages such as tree glacial-stage refugia and long-term soil development; (2) biotic responses to environmental change (spreading, extinction, persistence, adaptation) in the Quaternary; (3) ecological concepts and topics that have developed as a result of Quaternary botanical studies such as potential niches, realised environmental spaces, no-analogue assemblages, the nature of vegetation, and long-term tree and forest dynamics; and (4) the applications of Quaternary botany to ecological or biogeographical topics such as the extent of human impact in tropical systems, conservation in a changing world, island biology, plant–animal interactions, and biodiversity studies. The topics reviewed make no attempt to be exhaustive. Several key ecological topics such as understanding the diversity of tropical forests, the role of fire as a disturbance factor in different geographical areas, and ecosystem resilience are only mentioned briefly because much remains to be understood about them through Quaternary botanical studies. The four main parts, are subdivided into 14 sections and there is inevitably some overlap between some of the sections and subsections of other sections because some of the underlying patterns or processes involved (e.g. species extinction and extermination, tree spreading, no-analogue assemblages, tree and forest dynamics, human impact, historical legacies) are important in several aspects of Quaternary botany and its contributions to ecology and biogeography.
Table 1. Selected reviews on Quaternary floristic and vegetational history of different geographical areas. See Birks HJB and Berglund (Citation2018) for further examples.
Table 2. Selected reviews of contributions of Quaternary botany to modern ecology or biogeography.
Although Quaternary botany began in the nineteenth century with studies of both plant megafossils and plant macrofossils, particularly tree stumps and large seeds preserved in peat bogs, the study of macrofossils fell out of favour in the 1950s with the development of modern pollen analysis and refined pollen identifications (Birks HJB Citation2014; Birks HJB and Berglund Citation2018). A major resurgence of interest in macrofossils began in the mid-1960s as a means of providing unambiguous evidence for local presence and of complementing and validating pollen-analytical results (e.g. Birks HH and Birks Citation2000; Birks HH Citation2008; Birks HJB Citation2008, Citation2014; Jackson and Booth Citation2013; Jackson et al. Citation2014; Birks HJB and Berglund Citation2018). Since the early 1970s there has also been an increasing integration of Quaternary botanical analyses in multi-proxy palaeolimnological studies (e.g. Bradshaw EG et al. Citation2005; Rasmussen Citation2005; Rasmussen and Bradshaw Citation2005; Ammann et al. Citation2013a, Citation2013b). Palaeolimnology rapidly developed in the 1980s in response to concerns about the causes of recent lake acidification and lake eutrophication (Smol Citation2008). Quaternary botany has proved to be important in providing histories of lake catchments relevant to understanding lake dynamics (e.g. Birks HH et al. Citation2000). In this review plant macrofossil and palaeolimnological studies, often involving pollen analysis, are discussed where appropriate. Despite the great breadth of techniques within Quaternary botany (see Berglund Citation1986; Smol et al. Citation2001; Daniau et al. Citation2019), the subject is still dominated by pollen analysis. As Roberts N (Citation2014, p. 33) notes, ‘Palynology is the single most important branch of terrestrial palaeoecology for the late Pleistocene and Holocene’.
Although this review discusses some aspects of tropical systems and islands worldwide, there is an inevitable bias towards studies in Europe and North America. This is because these are the areas where detailed Quaternary botanical data are most abundant and because of my own research experiences. No attempt has been made to cite all relevant original publications for two reasons – length considerations and many of these early publications are cited in other reviews on different aspects of Quaternary botany (e.g. Birks HJB and Birks Citation1980; Birks HJB Citation1981b, Citation1986, Citation1993a, Citation1996, Citation1998, Citation2008, Citation2012a, Citation2014; Birks HJB and Gordon Citation1985; Lang Citation1994; Bennett Citation1997; Birks HH and Birks Citation2000; Birks HJB and Willis Citation2008; Birks HJB et al. Citation2010, Citation2016b, Citation2016c; Mitchell FJG Citation2011; Jackson and Blois Citation2015; Birks HJB and Tinner Citation2016a, Citation2016b; Edwards KJ et al. Citation2017; Birks HJB and Berglund Citation2018).
I avoided using numerous abbreviations. Those that are widely used are: yr (year), yr BP or cal yr BP (calibrated years before present, with present defined as 1950 CE), kyr (thousand years), LGM (last glacial maximum), and MIS (marine isotope stage).
2. Quaternary glacials, interglacials, and tree refugia
2.1. Glacial and interglacial stages
As a result of detailed palaeoceanographic studies since about 1955 involving deep-ocean sediment-cores, stable-isotope measurements, and micropalaeontological studies of faunal assemblages, the Quaternary period is now known to have been a period of very marked and widespread climatic and environmental changes (Imbrie and Imbrie Citation1979; Ruddiman Citation2013a; Bradley Citation2015). Large terrestrial ice-sheets started to develop in the Northern Hemisphere about 2.75 million years ago, resulting in multiple glacial-interglacial oscillations driven by variations in orbital insolation as a result of periodic fluctuations in Earth’s orbit on Milankovitch timescales of 100 (orbital eccentricity), 41 (Earth’s obliquity), and 19–23 (precessional) thousand year intervals (Imbrie and Imbrie Citation1979; Ruddiman Citation1990, Citation2013a; Bradley Citation2015).
Before about 1.25 million years ago, glacial–interglacial oscillations appear to be symmetric with small ice volumes and a periodicity of 41,000 years. Between about 1.25 and 0.7 million years ago, there was a fundamental shift, the so-called Mid-Pleistocene Transition, when the dominant frequency of climate oscillations changed to 100,000 years. The causes of this change that occurred under a broadly similar orbital forcing remain unclear (e.g. Duval et al. Citation2015; Head and Gibbard Citation2015; Maslin and Brierley Citation2015; Chalk et al. Citation2017; Voosen Citation2018). Possible causes include (1) a decline in atmospheric CO2 concentrations triggered by weathering or (2) enhanced ocean uptake and storage or the removal by glacial erosion of thick sediment (regolith) exposing a high-friction crystalline Precambrian Shield bedrock which increased ice stability and an associated change in ice-sheet response to orbital forcing (Chalk et al. Citation2017).
Glacial-stage conditions account for 75–80% of the Quaternary, whereas the remaining 20–25% consists of shorter interglacial stages during which conditions were similar to, or slightly warmer than, the present day (Porter Citation1989; Jackson and Overpeck Citation2000; Birks HJB and Willis Citation2008; Tzedakis et al. Citation2012, Citation2017). During the glacial stages, environmental conditions were very different from the present interglacial (Holocene (= post-glacial) plus the recent ‘Anthropocene’) in which we live today (Porter Citation1989; Jackson and Overpeck Citation2000; Birks HJB and Willis Citation2008). Much of Europe north of 40°N was covered by large terrestrial ice-sheets and widespread permafrost with temperatures possibly 10–25°C lower than present. High aridity and temperatures 2–5°C lower than today were characteristic of more southerly areas (Birks HJB and Willis Citation2008). Global atmospheric CO2 concentrations were as low as 180 ppmv during glacial stages rising to pre-industrial levels of 280 ppmv in interglacial stages (Birks HJB and Willis Citation2008; Bradley Citation2015). Given these extreme conditions in the glacial stages, an obvious question (Bhagwat and Willis Citation2008; Birks HJB and Willis Citation2008; Gavin et al. Citation2014; Birks HJB Citation2015) is where did forest trees survive these long and repeated glacial-stage conditions?
2.2. Glacial-stage tree refugia
Current palaeobotanical evidence suggests that many European trees may have survived the last glacial maximum (LGM) in relatively narrow refugial elevational belts (ca. 500–800 m) in the rugged terrain of mountains in southern Europe (including parts of the Caucasus) and possibly in parts of western Asia (van der Hammen et al. Citation1971; Bennett et al. Citation1991; Lang Citation1994; Bhagwat and Willis Citation2008; Birks HJB Citation2015) and the southern Mediterranean basin. These belts (‘classical’ macrorefugia sensu Birks HJB Citation2015) lay between lowland xeric steppe-like vegetation too dry for tree growth and high-elevation tundra-like vegetation, or permanent snow or ice too cold for tree growth (Birks HJB and Willis Citation2008; Birks HJB Citation2015). Tzedakis et al. (Citation2012) provide palynological evidence for such an LGM macrorefugium in the Pindus Mountains of western Greece. Such mid-elevation bands of mesic trees can be seen today in parts of the Andes, American Rockies (), the Californian Sierra Nevada, the Pamir, parts of the Sino-Himalayan region, and the Tien Shan in Kazakhstan (van der Hammen et al. Citation1971; Birks HJB and Willis Citation2008; Birks HJB Citation2015; Birks HJB and Birks HH pers. obs.). Trees may also have occurred scattered in locally moist sites (e.g. water seepages, ravines), so-called ‘microrefugia’ (Rull Citation2009, Citation2010; Dobrowski Citation2011; Birks HJB Citation2015) (or ‘cryptic’ or ‘hydrologic’ refugia) (Hofreiter and Stewart Citation2009; Stewart JR et al. Citation2010; McLaughlin et al. Citation2017) in Europe and the Middle East during the LGM as they do today on the Tibetan Plateau in Sichuan and Qinghai (), in the Zagros Mountains of Iran, and in parts of eastern Turkey, Tajikistan, Uzbekistan, and Kazakhstan (Birks HJB and Willis Citation2008; Birks HJB Citation2015; Birks HJB and Birks HH pers. obs.). There is increasing evidence from macrofossils and macroscopic charcoal remains that Picea, Pinus and Larix may have grown locally in such microrefugia during the LGM, along with Betula, Salix, and possibly Populus, Alnus, and Ulmus in southern, central, eastern, and north-eastern Europe (e.g. Bhagwat and Willis Citation2008; Birks HJB and Willis Citation2008; Juřičková et al. Citation2014; Monegato et al. Citation2015). Fagus may have grown in western Europe (de Lafontaine et al. Citation2014a, Citation2014b; cf. Huntley Citation2014) and it and Quercus may have grown during the Younger Dryas in the Harz Mountains in Germany, the northernmost low mountain range in central Europe (Robin et al. Citation2016a, Citation2016b; cf. Giesecke Citation2016), as did Pinus in the Allerød interstadial. Carcaillet and Blarquez (Citation2017) report macrofossils and charcoal of Pinus cembra and Larix decidua of presumed LGM age from a sub-alpine lake at 2214 m elevation in the western Alps. They suggest that these trees survived the LGM in microrefugia on high unglaciated nunataks above the glacial limit (Cossart et al. Citation2012) even though there are no LGM radiocarbon determinations for any of the macrofossils or charcoal found (see Finsinger et al. (Citation2019) for a critical analysis of Carcaillet and Blarquez (Citation2017)). The interpretation of tree growth in microrefugia as far north as the north-eastern edge of the great Fennoscandian ice-sheet in Russia at 60°N (Birks HJB and Willis Citation2008) is keenly contested by Tzedakis et al. (Citation2013) who view the available palaeobotanical evidence (mainly macroscopic charcoal) for microrefugia as being controvertible. They propose that no temperate trees grew north of 46°N but that populations of boreal conifers may have occurred in eastern Europe to 49°N and at even higher latitudes east of the Fennoscandian ice-sheet. Much remains to be discovered about tree distributions in Europe (and elsewhere) during the LGM (Firbas Citation1949; van der Hammen et al. Citation1971; Iversen Citation1973; Bennett et al. Citation1991; Lang Citation1994; Holm and Svenning Citation2014; Birks HJB Citation2015; Birks HJB and Tinner Citation2016a, Citation2016b; Lumibao et al. Citation2017; Carcaillet et al. Citation2018; Giesecke and Brewer Citation2018). Körner (Citation2003, Citation2005, Citation2012) review current eco-physiological understanding of the role of low temperatures and aridity in controlling tree limits today.
Figure 1. (a) Mid-elevation belt of Pinus contorta, P. ponderosa, Picea engelmannii, Betula papyrifera, and Populus tremuloides between lowland xeric Artemisia tridentata sagebrush-steppe and high-elevation snow and dry open alpine vegetation, Borah Peak, Idaho, USA. This is a possible modern analogue for a'classical' macrorefugium for trees in the mountains of southern Europe during the last glacial maximum (LGM). Photograph: HJB Birks. (b) Local stands of Picea crassifolia along water seepages at 3600 m on the south-eastern Tibetan Plateau, Sichuan, China. This is a possible modern analogue for a 'microrefugium' for trees in Europe during the LGM. Modern pollen percentages of Picea at this site are less than 3%. Photograph: HJB Birks.

Krebs et al. (Citation2004) present a refugium probability index for individual sites to help detect possible refugia for Castanea sativa, a tree whose native range is heavily obscured by extensive human impact. The index uses information from the full time span recorded at 1471 sites and includes data on site elevation, dating, and pollen occurrences and values. It suggests that the main refugia for Castanea were around the Mediterranean basin, the Transcaucasian region, and north-west Anatolia, and possibly in Syria and Lebanon. This index approach – updated by Krebs et al. (Citation2019) – deserves to be used further, particularly with the extensive data-sets now available in the European Pollen Database (Giesecke et al. Citation2014a; Giesecke et al. Citation2016) and in Neotoma (Grimm EC et al. Citation2018; Williams et al. Citation2018).
An indirect approach to identify possible LGM refugia of trees involves species-distribution modelling (e.g. Birks HJB and Willis Citation2008; Svenning et al. Citation2008b; Ohlemüller et al. Citation2011; Gavin et al. Citation2014; Holm and Svenning Citation2014). This involves modelling present-day tree distributions in relation to contemporary climate using different species–climate modelling algorithms to derive an ensemble of predictive models (e.g. Norberg A et al. Citation2019). Given these modern tree–climate model ensembles and LGM climate-model simulations (e.g. Fordham et al. Citation2017), it is possible to hindcast the potential LGM ranges for trees under LGM climates. Such predictions in Europe (e.g. Birks HJB and Willis Citation2008; Svenning et al. Citation2008b; Ohlemüller et al. Citation2011) suggest that potential LGM tree ranges may have been larger than generally thought, especially for conifers. A large potential range does not, of course, mean that there were widespread forests in the LGM in Europe, only that there was an extensive potential area where small localised populations may have occurred locally in microrefugia. Palaeobotanical data (e.g. Willis and van Andel Citation2004; Bhagwat and Willis Citation2008; Birks HJB and Willis Citation2008; Kuneš et al. Citation2008) generally support these predicted potential ranges but there are many areas in central and eastern Europe where there are no palaeobotanical data to test these predictions. Similar modelling approaches have been used to predict tree distributions during the LGM on the Iberian Peninsula (Garzón et al. Citation2007; Alba-Sánchez et al. Citation2010), in the central Mediterranean (Marta et al. Citation2013), and in the Altai Mountains of Siberia (Hais et al. Citation2015); deciduous tree distributions during the LGM in Europe and south-west Asia (Leroy and Arpe Citation2007); and Araucaria angustifolia distribution during the LGM in Brazil (Bergamin et al. Citation2019). These and other recently published modelling studies (e.g. Waltari et al. Citation2007; Porto et al. Citation2012; Gavin et al. Citation2014; Janská et al. Citation2017; Hao et al. Citation2018) suggest that some species or vegetation types may have had more extensive geographical ranges in the LGM than previously thought. Such modelling studies assume that modern tree distributions are in equilibrium with contemporary climate and hence the modern tree–climate models are realistic and robust and that the LGM climate simulations are equally robust and reliable (Worth et al. Citation2014). Svenning and Sandel (Citation2013) review in detail vegetation disequilibrium with climate today and in the past and highlight the limitations of species-distribution modelling today, in the past, and in the future (see also Ordonez Citation2013; Birks HJB Citation2015). Other modelling approaches free of some of these assumptions of equilibrium involve using present-day topoclimate, climate stability, extreme conditions, and distinct differences from the surrounding matrix to quantify and locate potential microrefugia (e.g. Ashcroft et al. Citation2012; Reside et al. Citation2014; Valencia et al. Citation2016; Michalak et al. Citation2018).
Despite advances in modelling (e.g. Graham CH et al. Citation2010; Ohlemüller et al. Citation2011; Gavin et al. Citation2014; Snell RS et al. Citation2014), there is no substitute for detailed palaeoecological evidence for tree persistence in refugia during the LGM. There is an increasing number of detailed studies in areas that may have been LGM macrorefugia for trees, for example on the Balkan Peninsula (e.g. Tzedakis et al. Citation2012) and the Appenine Peninsula (e.g. Ravazzi et al. Citation2006; Kaltenrieder et al. Citation2009; Samartin et al. Citation2012, Citation2016; de Beaulieu et al. Citation2017). Detailed multi-proxy studies at Lago dello Costa in the Euganean Hills of north-east Italy (Kaltenrieder et al. Citation2009, Citation2010; Samartin et al. Citation2016) indicate that mean July air temperatures (based on chironomid remains that are independent of the pollen record) never fell below 10–13°C during the LGM. These air temperature values are above the range (8–10°C) necessary for tree growth at this latitude, assuming that there was adequate soil moisture (Körner Citation2012). The global mean of root-zone temperature at the tree-line during the growing season is 6.4 ± 0.7°C (Körner and Paulsen Citation2004). Körner (Citation2012) notes that a growing season mean corresponds closely to air temperature. The pollen and macrofossil stratigraphies indicate that Larix and Betula grew locally around the lake, and Acer, Tilia, Ulmus, Quercus (deciduous), Carpinus-type, Fagus, and Corylus were likely to have been present in low amounts through the LGM. Spores of Ophioglossum vulgatum and other pteridophytes suggest moist soil conditions locally. Gubler et al. (Citation2018), using 47 temperature loggers within the Euganean Hills over 11 months, show that areas in these Hills above 200 m elevation may have provided suitable climate conditions for local LGM refugia. The temperature gradients today are broad enough to permit both sub-montane (e.g. Fagus sylvatica) and Mediterranean (e.g. Quercus ilex) taxa to occur in the Hills whereas both are absent from the adjacent low-lying Po Plain.
Few studies have rigorously tested if an area had actually been an LGM refugium as many studies assume that the study site must have been in a refugial area and studies are therefore designed to confirm this assumption. In contrast, Wang Y et al. (Citation2017) rigorously test using pollen, plant macrofossils, and sedimentary ancient DNA the null hypothesis that St. Paul Island, Alaska was not an LGM refugium for Picea, Betula, Alnus, and Salix (see Graham RW et al. (Citation2016) and section 12 and subsection 13.3). They conclude from several lines of evidence that dwarf Salix was the only shrub present during the LGM along with some species of Ericaceae.
The rapid development and increasing application of molecular techniques and phylogenetic studies of extant tree populations on a European scale and the critical integration of available palaeobotanical and modern phylogenetic data (e.g. Hewitt GM Citation1993, Citation1996, Citation1999, Citation2000, Citation2001; Petit et al. Citation2002a, Citation2002b, Citation2008; Cheddadi et al. Citation2006; Magri et al. Citation2006; Hu et al. Citation2009; Médail and Diadema Citation2009; Roberts DR and Hamann Citation2015; Bagnoli et al. Citation2016; Mandák et al. Citation2016; Lumibao et al. Citation2017; Piotti et al. Citation2017) are providing new and challenging insights into where modern populations may have survived the LGM (e.g. Jackson Citation2006b; Magri et al. Citation2006; Provan and Bennett Citation2008). The assumption behind using variability of genetic markers in organelle DNA to infer past refugial areas is that the markers of the colonising population at a site have persisted there through time. The occurrence of a particular marker is unlikely to disappear from the population and may thus help identify past refugial or colonisation areas (Barnard-Kubow et al. Citation2015; Lumibao et al. Citation2017; cf. Sjölund et al. Citation2017; Giesecke and Brewer Citation2018).
Giesecke and Brewer (Citation2018) review the available phylogeographic studies on European trees. They conclude, in contrast to classical ideas of LGM tree refugia, that populations in the central and southern parts of the three southern European peninsulas may not have been involved in the Holocene tree colonisation of central and norther Europe (e.g. Magri et al. Citation2006). Results from an increasing number of phylogeographic studies conflict with the traditional view that European trees primarily survived the LGM in macrorefugia within the Iberian, Appenine, and Balkan peninsulas (e.g. van der Hammen et al. Citation1971; Bennett et al. Citation1991; Lang Citation1994). Instead they support the hypothesis of LGM occurrences in microrefugia in central and eastern Europe (e.g. Fineschi et al. Citation2003; Magri et al. Citation2006; Havrdová et al. Citation2015; Mandák et al. Citation2016). The emerging picture (Bagnoli et al. Citation2016) based on the currently available palaeoecological and phylogeographical data is of tree populations surviving in both ‘classical’ macrorefugia in parts of southern and eastern Europe and in scattered, even locally frequent, microrefugia in central, eastern, and possibly western and north-eastern Europe (Birks HJB Citation2015). Not all the haplotypes present in, for example, extant populations of Fagus sylvatica in Europe today were involved in the Holocene colonisation of Europe by beech (Magri et al. Citation2006), highlighting the complexity of glacial refugial survival and subsequent interglacial colonisation (see also Vendramin et al. (Citation1999) for similar findings for Abies alba). Clearly much remains to be discovered about macrorefugia, microrefugia, and LGM occurrences. Surprises continue to arise. For example, Carpinus betulus pollen has recently been reported from Holocene sequences from Iberia, indicating its former occurrence and recent decline from this peninsula (Abel Schaad et al. Citation2014). Although it has been generally assumed that Carpinus did not grow in Iberia during the LGM (e.g. Huntley and Birks Citation1983), the absence of any LGM evidence does not prove its LGM absence (Giesecke and Brewer Citation2018). Carpinus pollen shows erratic patterns of appearance and disappearance in sequences from Spain and southern France which may reflect the ongoing regional extermination of Carpinus from parts of Iberia (Muñoz Sobrino et al. Citation2018; cf. Grivet and Petit Citation2003).
2.3. Conclusions
The last 2.6 million years of Earth’s history have been characterised by very marked changes in climate, alternating between long glacial stages and short interglacial stages. European trees appear to have survived the LGM in narrow elevational macrorefugial belts in the mountains around the Mediterranean basin and in small, locally moist microrefugia in different areas of Europe. Detecting such microrefugia using pollen analysis and other botanical techniques is very difficult and often contentious. Alternative approaches to identifying LGM refugia involve species-distribution modelling and phylogenetic studies (e.g. Gavin et al. Citation2014). Current available data suggest that trees survived in both macrorefugia in the mountains of southern Europe and in microrefugia in central, eastern, and possibly western and north-eastern Europe. Phylogenetic studies show that not all haplotypes present today within, for example, Fagus sylvatica were involved in its post-LGM colonisation and spread. New discoveries of, for example, Carpinus betulus pollen in Iberia highlight how much remains to be discovered about refugia and LGM phytogeography in Europe.
3. The glacial–interglacial cycle and long-term soil changes
3.1 Iversen’s glacial–interglacial cycle
Pollen-analytical studies of glacial and interglacial deposits in northern and central Europe reveal strikingly similar general palynological patterns from the end of a glacial stage through the succeeding interglacial stage (ca. 13,000–30,000 years duration) and into the following glacial stage. Although the taxa and their relative abundances vary from one interglacial to another, there are such strong general ecological similarities that Iversen (Citation1958) proposes a simple conceptual model or metaphor of the major ecological processes for the observed broad-scale patterns of interglacial pollen stratigraphy in northern and central Europe, the so-called glacial–interglacial cycle consisting of four major phases – cryocratic, protocratic, mesocratic, and telocratic (see ). This model is derived, in part, from von Post’s (Citation1946) tripartite division of the Holocene (see Birks HJB and Berglund Citation2018).
Figure 2. The glacial–interglacial cycle for north-west Europe showing the broad changes in biomass (above-ground productivity), soil status, and temperature that take place during a glacial stage (cryocratic – blue) and its associated interglacial stage (protocratic, mesocratic, oligocratic and telocratic – orange). The largest changes in temperature occur at the glacial–interglacial transitions; that is at the beginning and end of the cryocratic phase, particularly at the cryocratic–protocratic transition. There are also fine-scale climatic changes within an interglacial stage and a glacial stage. Based on Iversen (Citation1958), Andersen ST (Citation1994), and Birks HJB and Birks (Citation2004). In the Holocene (present interglacial), the Homo sapiens phase (Birks HJB Citation1986) replaces the oligocratic and telocratic phases at sites with fertile soils (Birks HJB Citation1986).
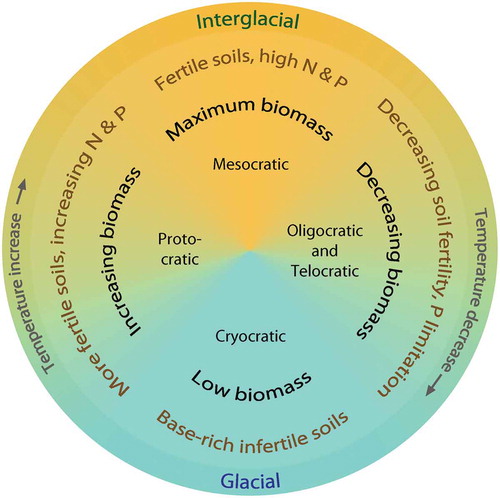
The cryocratic phase, the longest of the phases (ca. 50,000–100,000 years duration), represents the cold, often glacial, and arid stage with sparse assemblages of pioneer, arctic-alpine, steppe, and ruderal herbs growing on skeletal mineral soils, frequently disturbed by freeze-thaw activities. Climate is cold, dry, and continental (van der Hammen et al. Citation1971; Birks HJB Citation1986; Lang Citation1994). At the onset of an interglacial, temperature and moisture rise and the protocratic phase begins. Shade-intolerant and often base-demanding herbs, shrubs, and trees (e.g. Betula, Salix, Populus, Sorbus aucuparia, Pinus) immigrate into formerly glaciated areas and expand to form a mosaic of grassland, shrub, and open woodland growing on unleached, fertile soils with increasing nitrogen and phosphorus content and with a low humus content. Nitrogen-fixing plants (e.g. Hippophaë rhamnoides, Dryas octopetala, legumes such as Anthyllis vulneraria, Astragalus alpinus, and Lotus corniculatus) and aquatic Cyanobacteria (Iversen Citation1967, Citation1973; van Geel et al. Citation1989; Ammann et al. Citation2013a; Pfeiffer et al. Citation2013) are important in this phase, as they are today in many primary successions on glacier forelands (Matthews Citation1994).
The mesocratic phase is characterised by the development of temperate deciduous forests of Tilia, Ulmus, Quercus, Corylus, Fraxinus, Taxus, Carpinus, and Alnus and fertile brown-earth soils (Iversen Citation1967, Citation1973). Shade-intolerant herbs and shrubs are rare as a result of competition and habitat loss, except in naturally open ‘interglacial’ refugia (Birks HJB and Willis Citation2008; Birks HJB Citation2015) such as alpine habitats, cliffs, river gorges, and shallow calcareous soils (Pigott and Walters Citation1954) or in forest openings resulting from fire, wind-throw, and, possibly, grazing mega-fauna (Birks HJB Citation2005; Mitchell FJG Citation2005).
Iversen (Citation1958) terms the last retrogressive phase of the cycle the telocratic phase with open conifer-dominated woodland, ericaceous heaths, and bog vegetation growing on infertile (low available phosphorus; Boyle Citation2007; Peltzer et al. Citation2010) podsols with mor humus and peat. Iversen (Citation1958) suggests that temperature falls at the onset of this phase but Andersen ST (Citation1964, Citation1966, Citation1994) shows that climate deterioration does not occur until well into the phase of soil retrogression, suggesting that soils deteriorate independently of climate change. Andersen calls this phase of impoverished soils prior to climatic deterioration the oligocratic phase. Major climatic changes occur at the onset of the cryocratic phase as forest-cover diminishes, frost action and cryoturbation destroy the leached infertile acid soils, and shade-intolerant herbs expand onto the newly exposed mineral soils.
In addition to these phases identified in the interglacial cycle by Iversen (Citation1958), an additional phase is uniquely identified in the Holocene – the Homo sapiens phase (Birks HJB Citation1986) – with the onset of forest clearance and prehistoric shifting cultivation and livestock farming. The durations of the phases within the interglacial cycle at a given site are not fixed as the duration of the phases may depend on site factors such as soils, elevation, exposure, and vegetation thresholds and inertia (see subsection 14.2; Birks HJB Citation1986).
The general interglacial-cycle model or metaphor proposed by Iversen (Citation1958) is applicable to many other ecological and phytogeographic settings (Birks HJB Citation1986). For example, in the Mediterranean basin, van der Hammen et al. (Citation1971), Tzedakis (Citation2007), Tzedakis et al. (Citation2009), and Birks HJB and Tinner (Citation2016a, Citation2016b) suggest an interglacial cycle in which the major processes determining the observed palynological patterns are changes in humidity, in addition to temperature. After a cryocratic phase of Artemisia–Amaranthaceae (Chenopodiaceae) steppe, temperate taxa (e.g. deciduous Quercus, Ostrya, Carpinus) form open forests in the protocratic phase mixed with evergreen trees such as Quercus ilex and Olea europaea and Mediterranean shrubs (e.g. Pistacia). In the following mesocratic phase, warm-temperate and Mediterranean conifers (e.g. Abies, Pinus) expand into the deciduous and evergreen forest and tree cover increases, probably in response to increasing moisture availability. Towards the end of an interglacial, corresponding broadly to the oligocratic phase, moisture-demanding taxa such as Fagus, Alnus, and Abies gradually replace Mediterranean evergreen broadleaved trees while broadleaved deciduous taxa remain important. Finally, forest cover declines and steppe-like vegetation expands during the climatic deterioration (temperature decrease, reduced moisture) in the telocratic phase near the transition into the next dry and cold cryocratic glacial stage (Tzedakis Citation2007; Tzedakis et al. Citation2009; Birks HJB and Tinner Citation2016a, Citation2016b).
As more and more interglacial pollen stratigraphies are studied in areas such as central and southern Europe and the Mediterranean basin, an apparent order emerges within interglacial palynological patterns when viewed at the broad scale of an entire interglacial of 13,000–30,000 years (Cheddadi et al. Citation2005). However, within each phase of the interglacial (about 4000–8000 years) there is often great variation between interglacials, hence the ability of pollen stratigraphy to differentiate many, but not all (see Tzedakis and Bennett Citation1995) of the different interglacials in Europe (Birks HJB Citation1986; de Beaulieu et al. Citation2001, Citation2006; and ).
Figure 3. Summary pollen diagram of the composite long pollen sequence from the Velay Plateau in the south-eastern part of the French Massif Central. The interglacial stages are shaded in pale brown and the corresponding marine isotope stages (MIS) are shown. Note the change between interglacial stages with dominant temperate trees and cold, dry glacial stages with dominant Poaceae and steppe taxa. The Holocene is MIS 1, the Ribains interglacial is MIS 5e (= Eemian), the Bouchet I interglacial is MIS 7c, the Landos interglacial is MIS 9e, and the Praclaux interglacial is MIS 11e (= Holsteinian). Redrawn and modified from de Beaulieu et al. (Citation2001).
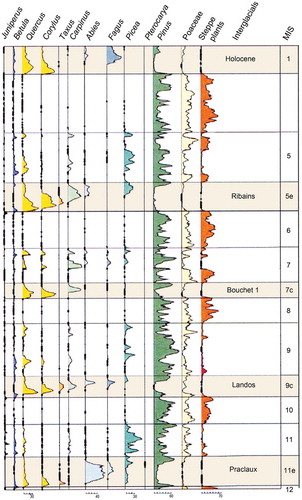
Figure 4. Comparison of interglacial tree pollen stratigraphies in five interglacial stages in the composite long pollen sequence from the Velay Plateau in the south-eastern part of the French Massif Central (). Major tree pollen taxa are coloured identically between interglacials. The approximate age of the onset of each interglacial is also shown along with the correlations with marine isotope stages (MIS). Redrawn and modified from de Beaulieu et al. (Citation2006).
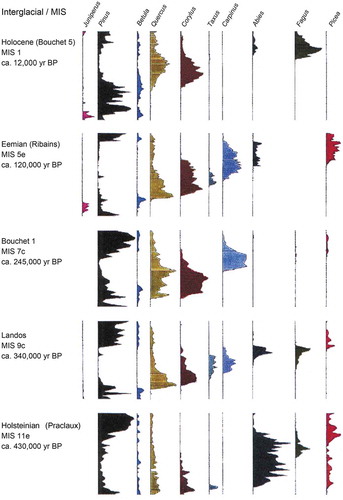
The relative order of arrival and expansion of tree taxa in the early protocratic phase of an interglacial is broadly consistent from one interglacial to another (). In contrast, the order of arrival and expansion of tree taxa in the mesocratic and oligocratic phases are more variable and less predictable ( and ). The reasons for this contrast between arrival and expansion patterns in the protocratic, mesocratic, and oligocratic phases in different interglacials within a specific geographical area are unclear (Birks HJB and Tinner Citation2016a). Locations of preceding glacial-stage refugia (both ‘classical’ macrorefugia and microrefugia; see Kupryjanowicz et al. Citation2018a), propagule dispersal mechanisms, competition from established vegetation, soil conditions, availability of ‘empty niches’, and genetic adaptation may all have been important. The current spread and invasive behaviour of certain ‘exotic’ taxa (e.g. Aesculus hippocastaneum, Rhododendron ponticum, Tsuga spp., Pinus spp., Picea spp.) highlight the complexity of understanding why some taxa have high invasion abilities today whereas other taxa do not (Birks HJB and Tinner Citation2016a). This complexity may have existed in the mesocratic phases of the Holocene and earlier interglacials as taxa such as Fagus, Carpinus, Taxus, Corylus, Picea, Abies, and Pterocarya all have very different histories in different interglacials ( and ; West RG Citation1980; Watts Citation1988; Lang Citation1994).
The general conceptual glacial–interglacial model for ecological processes behind the observed patterns of interglacial pollen stratigraphies in northern and central Europe (Iversen Citation1958) is strongly criticised by Bartlein and Prentice (Citation1989) as being ‘vastly oversimplified’ because it represents ‘the Quaternary as repetitions of a cycle between glacial and interglacial conditions’. They recognise that there are broad similarities between different parts of the palaeoclimatic record (see Jackson and Overpeck Citation2000) with interglacial stages (about 12,000, 120,000, 245,000, 340,000, and 430,000 years ago) all characterised by high summer insolation in the Northern Hemisphere, low global ice-volume, and high atmospheric CO2 concentrations. In detail, each interglacial may have been climatically different (Tzedakis and Bennett Citation1995; Tzedakis et al. Citation2017) leading to differences in their flora and pollen stratigraphy ( and ; Watts Citation1988; Tzedakis and Bennett Citation1995). For example, Bartlein and Prentice (Citation1989) emphasise that in the previous interglacial (Eemian, marine isotope stage (MIS) 5e), the July insolation at 65°N was almost 50% higher than 10,000 years ago in the Holocene (MIS 1) and the CO2 concentrations were higher than in the Holocene. The glacial-stage maxima differ greatly from one another (Bartlein and Prentice Citation1989). For example, the LGM was extreme in its ice volume. Within this stage between 15,000 and 75,000 years ago, there were considerable variations in insolation, ice volume, and CO2, as there were in previous glacial stages. Bartlein and Prentice (Citation1989) conclude that the Holocene and the LGM within the preceding glacial stage are not particularly representative of the Quaternary as whole, as for most of the last 2.6 million years climate has ‘fluctuated around states intermediate between these two extremes’.
Watts (Citation1988) notes that there is much more evidence for the role of climate on interglacial vegetation history than was known at the time of Iversen (Citation1958). For example, there is now evidence based on orbital calculations of enhanced seasonality with warmer summers and colder winters early in interglacials. Watts (Citation1988) suggests that Iversen’s (Citation1958) model of ecological processes needs modifying in light of palaeoclimatic knowledge about the forcing role of increased summer insolation early in interglacials and of changing seasonality through an interglacial. In addition, Watts (Citation1988) proposes that as rapid changes in ice-sheet volume, sea-level, and storm-track positions (Kutzbach and Guetter Citation1986) affect the climatic and vegetational development at the onset of an interglacial, these changes ‘should be incorporated into a new, more comprehensive model’. No such model has yet been developed that combines pollen-stratigraphical patterns, ecological processes, and currently known palaeoclimate forcings. However, Tzedakis et al. (Citation2009, Citation2012, Citation2017) and the Past Interglacials Working Group of PAGES (Citation2016) are making major advances in modelling and understanding the complexity of interglacial climates. A major challenge is to integrate these advances into interpretations of pollen-stratigraphical patterns between different interglacials.
Iversen’s (Citation1958) glacial–interglacial cycle was the first attempt to consider the role of soil changes in long-term vegetation dynamics. It stimulated palynologists to study changes in soil development within interglacials and during the late-glacial and to consider the role of soil changes on vegetation dynamics. The following two subsections consider soil development in unglaciated and glaciated areas in Europe.
3.2. Long-term soil development in unglaciated areas
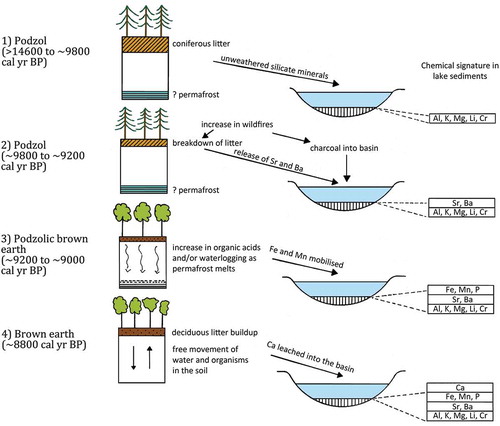
Permafrost was widespread during the last glacial stage in Europe from south of the Fennoscandian ice-sheet to the foothills of the Alps and the northern edge of the Mediterranean basin (Iversen Citation1967, Citation1973; Willis Citation1996). Willis et al. (Citation1995, Citation1997) suggest that in much of unglaciated central and southern Europe, although there was widespread steppe-like herb-dominated vegetation, there were also localised stands of coniferous trees in microrefugia (Birks HJB and Willis Citation2008). The soils would have been cold or even semi-permanently frozen, nutrient-poor, and podsolic, as in the modern boreal forest. In today’s Hungary, the late-glacial vegetation was predominantly coniferous forest (mainly Pinus, with some Picea, Larix, and Juniperus; Willis et al. Citation1995). Early in the Holocene, deciduous woodland of Quercus, Corylus, Tilia, and Ulmus rapidly developed, presumably in response to regional climate change and rising temperature. Willis et al. (Citation1997), using a combination of pollen and charcoal stratigraphy and sediment geochemistry, consider the questions whether climate change results in a change from podsol to brown-earth soils, which in turn causes a change in forest composition, or does the vegetation change first and subsequently alter the soil type. They also consider what mechanisms are involved in the development of a brown-earth soil from a podsol. Their results suggest that the early-Holocene increase in deciduous trees was not consequent on soil type. Deciduous trees increased on podsolic soils or podsolic brown-earths, and this increase was one of the triggers for the development of brown-earth soils (; Willis et al. Citation1997).
Figure 5. Schematic diagram summarising the main stages and associated pedological changes (1–4) occurring in the transition from a podsol supporting coniferous forest in the late-glacial (stages 1 and 2) to a brown-earth soil with deciduous forest in the early-mid Holocene (stage 4) at Kis-Mohos Tó (NE Hungary) via an intermediate phase of a podsolic brown-earth with deciduous trees in the early Holocene (stage 3). The chemical record in the lake sediment at Kis-Mohos Tó is summarised. Redrawn from Willis et al. (Citation1997).
Working at the same site in north-east Hungary, Jeffers et al. (Citation2011a) consider the role of nitrogen cycling in the change from Pinus to Quercus pollen dominance in the early Holocene. They ask if changes in nitrogen cycling lead to vegetation change or does the vegetation change alter the nitrogen dynamics? They studied stable nitrogen isotopes (δ15N) and elemental concentrations of total nitrogen in the lake sediments in relation to the pollen stratigraphies of Pinus and Quercus. Using non-linear population dynamic models, they explore whether the vegetational changes were a response to changes in nitrogen cycling. They show that as Quercus replaced Pinus in the early Holocene () in response to climate change, rates of nitrogen cycling increased. However, the mechanisms by which the trees interacted with nitrogen remained stable across the threshold changes in climate and dominant tree taxa, suggesting that the changes in tree populations over 8000 years were not driven by nitrogen availability. A similar modelling approach in conjunction with pollen, charcoal, and dung-fungal spores (a proxy for herbivore density – see subsection 13.2) stratigraphies, with independently inferred temperatures based on chironomids, and with stable nitrogen isotopes has been used to model the role of climate, herbivory, fires, and nitrogen availability on vegetation dynamics in the late-glacial and early Holocene in north-west Ireland (Jeffers et al. Citation2012) and East Anglia (Jeffers et al. Citation2011b). This type of integrated multi-disciplinary study is resulting in further research on long-term changes in nitrogen and other nutrient cycling during the Holocene (McLauchlan et al. Citation2013a; Citation2013b); on understanding the role of changing disturbance regimes on biogeochemical cycling (McLauchlan et al. Citation2014, Citation2019); and on providing a long-term palaeoecological perspective for the assessment of ecosystem services (e.g. Jeffers et al. Citation2015; see subsection 11.4) and the control of ecosystem structure and function (Jeffers et al. Citation2018).
3.3. Long-term soil development and mycorrhiza in glaciated areas
Ecologists have long been interested in the composition and role of mycorrhizal fungi in soil and ecosystem development (e.g. Dickie et al. Citation2013). Read (Citation1993a, Citation1993b) proposes a predictable sequence of mycorrhizal types during a primary succession and subsequent vegetation and soil development in temperate and boreal forests (see also Lambers et al. Citation2007; Ammann et al. Citation2013a). Read (Citation1993a, Citation1993b) suggests that non-mycorrhizal plants colonise open substrates with high phosphorus content early in a primary succession and that these are replaced by arbuscular mycorrhizal plant species, followed by ectomycorrhizal trees with an arbuscular understorey. These are then followed by ectomycorrhizal trees with an ericoid understorey, and finally by a dominant ericoid mycorrhizal vegetation (Dickie et al. Citation2013). In reality, all mycorrhizal types can occur at almost all stages of ecosystem development and subsequent retrogression (Read Citation1993a; Cázares et al. Citation2005; Lambers et al. Citation2007; Dickie et al. Citation2013), but are there any consistent patterns in mycorrhizal types over the long time-scale of a north-west European interglacial cycle?
Kuneš et al. (Citation2011) study four pollen sequences in southern Scandinavia representing four different interglacials including the Holocene. They derive from the pollen data – transformed to minimise biases due to differential pollen representation – reconstructions of light, soil reaction, and nitrogen (a proxy for above-ground productivity (Hill MO and Carey Citation1997)) through the interglacials using Ellenberg indicator values (Ellenberg et al. Citation1991; Hill MO et al. Citation2004). Above-ground productivity is inferred to be low initially in the protocratic phase, to peak in the mesocratic phase, and to decline slowly in the oligocratic + telocratic phase (except in the Homo sapiens phase of the Holocene). The dominant trees of the oligocratic + telocratic phase mainly have ‘phosphorus-mining’ ectomycorrhiza, whereas the protocratic and mesocratic trees mainly have ‘phosphorus-scavenging’ arbuscular mycorrhiza although some ectomycorrhizal trees (e.g. Quercus, Pinus, Betula) are abundant in the protocratic and mesocratic phases (Kuneš et al. Citation2011). (The assignment of these interglacial trees to different mycorrhizal types follows Harley and Harley (Citation1987).) The long-term shift from ‘P-scavenging’ arbuscular mycorrhiza to ‘P-mining’ ectomycorrhiza during the different interglacials suggests that available phosphorus levels in the soils were becoming depleted from the mesocratic to the oligocratic + telocratic phases (see Boyle Citation2007). A similar depletion occurs in the retrogressive phases of the long chronosequences in different geographical areas studied by Wardle et al. (Citation2004) (see also Birks HJB and Birks Citation2004; Peltzer et al. Citation2010; ).
With the increasing availability of detailed information concerning mycorrhizal types in regional floras (e.g. Harley and Harley Citation1987; Peat and Fitter Citation1993; Hempel et al. Citation2013) and of root-symbiotic nitrogen fixation by plants (Tedersoo et al. Citation2018), there is considerable scope to investigate changes in mycorrhizal types and nitrogen fixation during long-term vegetational changes using pollen-stratigraphical data (e.g. Kuneš et al. Citation2011; Ammann et al. Citation2013a) and ancient DNA (Zobel et al. Citation2018).
A very detailed fine-resolution late-glacial study (ca 8 years per sample) at Gerzensee () on the Swiss Plateau (Ammann et al. Citation2013a, Citation2013b) shows very rapid palynological changes at Termination A (Oldest Dryas/Bølling), about 14,665 calibrated (cal) years before present (yr BP), the onset of the late-glacial (). Using stable oxygen-isotope ratios from bulk carbonate in the lake sediments as an independent climate proxy (primarily temperature), Amman et al. (Citation2013a, Citation2013b) interpret the palynological changes in terms of rapid vegetation responses and ecosystem processes to rapid warming between 14,830 and 14,440 yr BP. Vegetation composition, physiognomy, cover, biomass, and pollen richness (inferred from pollen-accumulation rates), as well as rates of palynological changes and inferred soil types and processes, all changed rapidly within about 200 years. Betula forest and subsequently Betula and Pinus forest developed in the late Bølling and Allerød between 14,445 and 12,710 yr BP, accompanied by a build-up of humus and nitrogen in the soil and phosphorus becomes available through weathering (). This detailed study provides unique insights into ecosystem palaeoecology and highlights the rapid operation and interaction of ecological processes in the past (cf. Laliberté et al. Citation2013).
Figure 6. Gerzensee, a kettle-hole lake on the Swiss Plateau at 603 m elevation (46.83°N, 7.55°E). This site has been the focus of detailed studies on rapid warming and associated biotic changes in the late-glacial and early Holocene (Ammann and Oldfield Citation2000; Ammann et al. Citation2013a, Citation2013b, Citation2013c), for example the study summarised in . The Bernese Alps are in the background. Photograph: AF Lotter.

Figure 7. Vegetation and soil development around Termination A (Oldest Dryas/Bølling transition) about 14,665 calibrated years ago at Gerzensee, Switzerland (see ). Changes in stable oxygen-isotope ratios (δ18O‰) of bulk carbonate in the lake sediments (A) provide a climate proxy independent of the pollen stratigraphy. The physiognomy of the past vegetation (B) and vegetation type (C) are inferred from pollen (D) and plant-macrofossil data (the nitrogen-fixing shrub Hippophaë rhamnoides is shown in orange in B). Pollen-accumulation rates (grains cm–2 yr–1) (D) reflect vegetation cover and biomass, whereas rates of palynological change (per 70 yr) (E) highlight times of marked change shown by a small or big * that are 50% or 75% greater than the mean rate of change, respectively. Palynological richness (α-diversity) (F) is estimated as number of taxa cm–2 yr–1 to allow for the changes in pollen-accumulation rates (D). The inferred soils (G) are summarised in terms of the extent of the active layer, amount of nitrogen-fixation based on the abundance of nitrogen-fixing plants in the pollen and plant-macrofossil stratigraphies, and general soil types. Inferred changes in the potential nitrogen and phosphorus resources during the vegetation development are also shown (H). All the stratigraphical data are smoothed with a running mean over five samples. Redrawn and modified from Ammann et al. (Citation2013b) and Birks HJB et al. (Citation2016b).
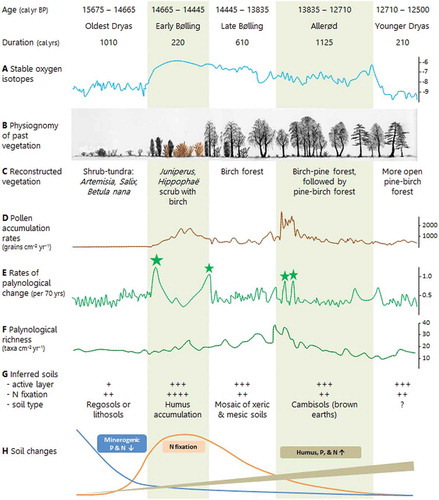
The putative dominant mycorrhizal types in a north-west European glacial–interglacial cycle, along with suggested ordinal levels of above-ground productivity, available phosphorus levels, and nitrogen fixation are summarised in . The hypothetical sequence of Read (Citation1993a, Citation1993b) appears to be only partly applicable to long-term vegetation and soil changes, as arbuscular, ectomycorrhizal, ericoid mycorrhiza, and non-mycorrhizal herbs were probably present in all phases except the oligocratic + telocratic phase where arbuscular mycorrhiza may be largely absent (see Kuneš et al. Citation2011).
Table 3. Possible dominant mycorrhizal types and nitrogen fixers in a north-west European glacial–interglacial cycle and suggested levels of above-ground productivity, available soil phosphorus levels, and amount of nitrogen fixation. This scheme is based on many sources including Harley and Harley (Citation1987), Read (Citation1993a, Citation1993b), Cázares et al. (Citation2005), Kuneš et al. (Citation2011), and Dickie et al. (Citation2013).
3.4. Conclusions
Iversen’s (Citation1958) glacial–interglacial cycle was one of the first attempts to explore the role of edaphic changes in long-term vegetation dynamics (see also Iversen Citation1967, Citation1973). It provides a simple conceptual model that integrates climate and soil within a glacial–interglacial couplet. It has stimulated palynologists to infer changes in soil development within interglacials (e.g. Andersen ST Citation1964, Citation1966, Citation1994; Kuneš et al. Citation2011) and during the late-glacial (e.g. Iversen Citation1954; Berglund and Malmer Citation1971; Willis et al. Citation1997; Ammann et al. Citation2013a, Citation2013b) and to model the role of soil changes on vegetation dynamics (e.g. Jeffers et al. Citation2011a, Citation2011b, Citation2012, Citation2018). As more is discovered about mycorrhizal ecology (e.g. Lambers et al. Citation2007; Dickie et al. Citation2013) and as ancient DNA is used to decipher mutualisms in the past (Zobel et al. Citation2018), the role of soil changes in influencing vegetation history should receive increasing attention.
The next three sections discuss the major biotic responses to long-term environmental change – spreading, extinction, and persistence and adaptation.
4. Holocene tree-spreading and range dynamics
4.1. Introduction and isopollen maps
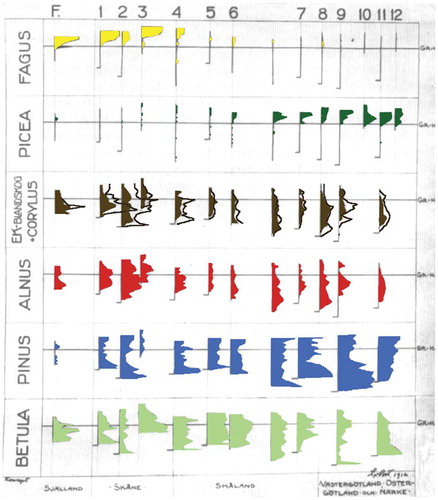
Vegetation varies in space and in time. Fossil pollen assemblages – a proxy for past vegetation – vary in time at points in space and in space at points in time. When von Post (Citation1916) introduced pollen analysis as a tool for reconstructing past vegetation, he illustrated with a large wall-chart how pollen assemblages varied temporally and spatially in a transect of 12 bog sites from Zealand in Denmark, through the Fagus sylvatica region and across the southern limit of Picea abies in southern Sweden into south-central Sweden up to the northern limit of Quercus (). Although never published by von Post, an early draft of it was found later and published by Fries M (Citation1967). It clearly shows spatial and temporal variation in the relative abundances of Fagus and Picea pollen along the transect. a major limitation was the lack of any absolute chronology and von Post was forced to rely on the well-marked peat-stratigraphical change from dark, highly humified to paler, unhumified fresh peat at Weber’s so-called ‘Grenzhorizont’ as his only chronological horizon. This horizon coincides with the Sub-Boreal–Sub-Atlantic transition of about 500 BCE. Throughout his scientific career, von Post’s Swedish motto was ‘Tank horisontellt, handla vertikalt’ (Think horizontally, work vertically) (Edwards KJ et al. Citation2017; Richards K Citation2017; Gaillard et al. Citation2018). As his 1916 wall-chart shows (), von Post was clearly thinking and working in both space and time even at the very beginnings of pollen analysis.
Figure 8. The series of pollen diagrams presented by Lennart von Post in his 1916 lecture in Kristiania along a south-west to north-east transect from Zealand (site F), through Skåne and Småland (sites 1–8) and into Västergötland, Östergötland, and Närke on the borders of the north and central Swedish uplands (sites 9–12). The southern limit of Picea abies lies between sites 3 and 4, and the northern limit of Fagus sylvatica is near site 6. The EK-Blandskog + Corylus curve is the combined values of Quercus, Ulmus, Tilia, and Corylus (‘Quercetum Mixtum’). The colours are those that von Post used in his original lecture-chart. As on all his diagrams, von Post has signed it in the bottom right-hand corner. Based on a diagram in Fries M (Citation1967) but extensively modified. This series of pollen diagrams was subsequently published in very different formats by von Post (Citation1924, Citation1926a).
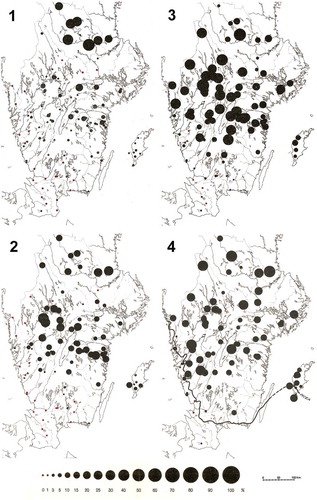
Von Post’s interest in the spatial variation in pollen assemblages in southern Scandinavia continued along with his extensive pollen-stratigraphical studies. In 1924 he published a series of maps of different pollen taxa using symbols of different sizes to represent different percentage ranges for four or six time periods of ‘post-arctic time’: Boreal, Atlantic, Sub-Boreal, early Sub-Atlantic, middle Sub-Atlantic, and recent (von Post Citation1924). These maps (; ‘pollen-analytical maps’ sensu von Post Citation1924) were based on about 250 stratigraphical sequences analysed as part of the peat-resource inventory by the Swedish Geological Survey (von Post Citation1922, Citation1929; Nordlund Citation2014). This mapping approach using different sized symbols continues to be used by palynologists to display basic spatial patterns at a range of spatial scales (e.g. Pigott and Huntley Citation1980; Davis MB et al. Citation1986, Citation1991; Woods and Davis Citation1989; Douda et al. Citation2014; Magri et al. Citation2015; Brewer et al. Citation2017). They are particularly useful in areas with high relief or varied habitat diversity.
Figure 9. Picea pollen percentages (expressed as percentages of total tree pollen) at over 250 sites in southern Sweden in the (1) Sub-Boreal, (2) early Sub-Atlantic, (3) middle Sub-Atlantic, and (4) recent times. The native southern range limit (in 1924) is indicated as a solid line on the map for recent times (4). Modified from von Post (Citation1924).
As pollen-analytical studies increased in number and geographical coverage (Birks HJB and Berglund Citation2018), pollen data were frequently mapped at local (e.g. Firbas Citation1934), regional (e.g. Godwin Citation1934a, Citation1934b, Citation1940; Jessen Citation1949; Ritchie Citation1976), or sub-continental scales (e.g. Rudolph Citation1930; Neustadt Citation1959) using a variety of symbols, pie-charts, or clock-faces to display different relative abundances. Szafer (Citation1935) introduces isopollen maps of isofrequency contours joining geographical localities with the same pollen percentages for a given taxon (e.g. Picea, Fagus) at the time that the map represents. Firbas (Citation1949) adopts this approach and presents isopollen maps to summarise stages in the Holocene spread and expansion of forest trees into central Europe. Sauramo (Citation1940) and Bertsch (Citation1953) provide similar isopollen maps for Finland and central Europe, respectively. Kondratiene and Šeiriene (Citation2003) construct isopollen maps for the major trees in the Butënai (Holsteinian) interglacial in Lithuania and Kupryjanowicz et al. (Citation2018a) present isopollen maps for the last (Eemian) interglacial in Poland based on 187 pollen sequences. Both these studies use regional pollen-assemblage zones as the basis of correlation between sequences. The Polish maps suggest several directions of tree spread that differ from spreading directions in the Holocene (Ralska-Jasiewiczowa et al. Citation2004), illustrating the complexity of tree-spreading patterns between interglacials. An alternative approach to correlation of pollen sequences was pioneered by Auer (Citation1958, Citation1965) in Fuego-Patagonia by using volcanic tephra layers of different appearance and texture as the basis for correlation.
4.2. Chronologies and databases
All these early analyses had to use biostratigraphical pollen zones, peat-stratigraphical features, or tephra layers as the only means of correlation between sites (Firbas Citation1949; Iversen Citation1950) and of establishing a relative time-scale as no independent time-scale was available until the advent of radiocarbon dating in the late 1940s and early 1950s (Libby and Arnold Citation1949; Libby Citation1965). Radiocarbon dating freed Late Quaternary pollen analysis from its being a relative dating tool and thus allowed a fuller and more detailed exploration of spatial and temporal patterns in Holocene fossil pollen assemblages. Isopollen maps using radiocarbon dates for site-to-site correlation have been constructed at regional (e.g. Birks HJB and Saarnisto Citation1975; Birks HJB et al. Citation1975a; Ralska-Jasiewiczowa Citation1983), sub-continental (e.g. Bernabo and Webb Citation1977), or continental scales (e.g. Huntley and Birks Citation1983; Huntley and Webb Citation1989; Williams et al. Citation2004). The synthesis by Huntley and Birks (Citation1983) is based on 423 sites across Europe but in the absence of any database containing the raw data, much of the data were extracted directly from published pollen diagrams. The chronology of the maps was in uncalibrated radiocarbon years before present rather than today’s norm of calibrated calendar years. About one-third of the sites lacked any independent chronology and their use in this pioneering study relied entirely on biostratigraphical correlation (). More spatially detailed isopollen maps based on 190 pollen sequences with limited radiocarbon chronologies were constructed for Poland by Ralska-Jasiewiczowa et al. (Citation2004). Williams et al. (Citation2004) present isopollen maps based on 759 pollen stratigraphies across North America, a large proportion of which has radiocarbon chronologies to provide temporal control (Webb T et al. Citation1983a, Citation1983b; see also Jacobson GL et al. Citation1987 for isopollen maps for smaller areas in North America). The detailed study of the Eemian interglacial in Poland (Kupryjanowicz et al. Citation2018a) is based entirely on pollen-stratigraphical correlations as this interglacial is beyond the range of radiocarbon dating.
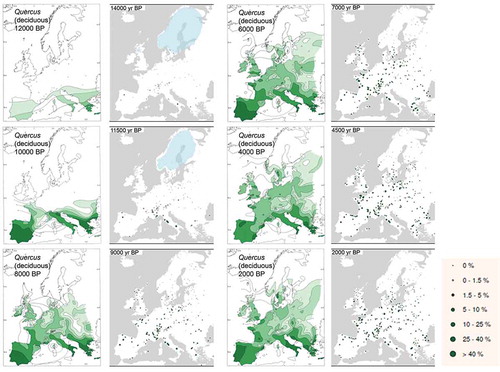
Figure 10. Maps of Quercus (deciduous) pollen percentages across Europe for 12,000, 10,000, 8000, 6000, 4000, and 2000 radiocarbon years before present (BP) drawn as isopollen contours representing different percentage values (modified from Huntley and Birks Citation1983), and for 14,000, 11,500, 9000, 7000, 4500, and 2000 calibrated years BP drawn as different sized solid circles representing different pollen percentage values (modified from Brewer et al. Citation2017). The blue shading on the dot maps for 14,000 and 11,500 yr BP shows the likely extent of glacial ice in Fennoscandia. The two sets of maps have been approximately correlated in time using the IntCal13 radiocarbon calibration curve and CalPal (www.calpal-online.de) as the isopollen maps are in radiocarbon years BP and the dot maps are in calibrated years BP.
As the quantity and quality of pollen-stratigraphical data increased globally, an important development in the 1980s (Birks HJB and Berglund Citation2018) was the creation of open-access databases (Jackson et al. Citation2000a; Grimm EC et al. Citation2013) such as the European Pollen Database (Fyfe et al. Citation2009; Giesecke et al. Citation2014a, Citation2016) and the Neotoma paleoecology database (Grimm EC Citation2008; Brewer et al. Citation2012; Goring et al. Citation2015; Grimm EC et al. Citation2018; Williams et al. Citation2018). Other pollen databases are being developed in Latin America, Africa, parts of Asia, and the Indo-Pacific area (e.g. Grimm EC et al. Citation2013, Citation2018; Flantua et al. Citation2015; Edwards KJ et al. Citation2017 – see Supplementary information in Gillson and Marchant (Citation2014) for details of palaeoecological databases). Many of these are being integrated within Neotoma (Mitchell FJG Citation2011; Edwards KJ et al. Citation2017; Grimm EC et al. Citation2018; Williams et al. Citation2018). The databases currently contain data from 5000+ pollen-stratigraphical sequences and 8000+ modern pollen surface-samples. Considerable effort is being expended to develop robust age-depth models for a very large number of the sequences in these databases (e.g. Blois et al. Citation2011; Giesecke et al. Citation2014a, Citation2016; Flantua et al. Citation2016a).
Brewer et al. (Citation2017) use the European Pollen Database to construct maps for 54 different pollen taxa at 500 calibrated-year intervals for the last 15,000 years based on 828 sites. They plot the pollen percentages in five taxon-specific abundance classes as different sized circles, rather than as isopollen contours (see ). The choice of percentage categories for each taxon is based on empirical thresholds (Lisitsyna et al. Citation2011) derived from comparing modern pollen spectra with contemporary plant-distribution data at a European scale (Brewer et al. Citation2017). These new detailed pollen maps with generally reliable, independent chronologies show few simple spatial patterns of tree or shrub spread or expansion across Europe but highlight considerable variation from one time to another and from one area to another. This variation () contrasts to the rather clearer spatial patterns identifiable in the isopollen maps of Huntley and Birks (Citation1983) based on 423 sites, many of which lacked any reliable independent chronology. Isopollen contours tend to smooth out or even obscure the fine-scale spatial variability that is apparent in the non-contoured, non-interpolated maps of Brewer et al. (Citation2017). Despite the large number of sequences used in the Brewer et al. (Citation2017) maps, there is still an uneven distribution of sites. As more data are contributed to the European Pollen Database and to Neotoma this unevenness may decrease but it will never totally disappear because of the absence of suitable sites for pollen analysis in parts of the Mediterranean area and its islands, in parts of central and eastern Europe, and in Iberia and the Balkans.
4.3. Isochrone maps
An alternative mapping approach is to map the times of particular past pollen-stratigraphical events, for example the first rise in pollen percentages of a particular taxon, resulting in so-called isochrone maps. Such maps present both spatial and temporal information in one map (). The contours or isochrones are lines joining geographical localities at which presumed analogous pollen-stratigraphical events occurred at about the same age. Such maps have been widely constructed in Europe, particularly Fennoscandia, but also in Russia and eastern North America (see ). Variants on the isochrone approach to mapping have been developed by Lang (Citation1994), Giesecke and Bennett (Citation2004), and Giesecke and Brewer (Citation2018). For major European trees, Lang (Citation1994) plots the age at individual sites of the rational-limit for the pollen taxon in question, namely the age at which its pollen begins to rise to sustained high values. Giesecke and Bennett (Citation2004) and Giesecke and Brewer (Citation2018) avoid defining rational or empirical limits in their analysis of the Holocene history of Picea abies in Fennoscandia or of trees in Europe, respectively. In the case of Picea, they simply plot, as interpolated maps, the ages for the beginning of the continuous Picea pollen curve, the times at which values of 1, 3, 5, and 10% are first reached, and the time when its pollen attained maximum values. For the European trees, Giesecke and Brewer (Citation2018) set an initial threshold to the pollen data to reduce the effect of small pollen values creating false presences (Lisitsyna et al. Citation2011). They then use empirical pollen threshold-values (Lisitsyna et al. Citation2011) to represent regional presence; more frequent regional occurrence; common occurrence; and dominance, co-dominance, or highest Holocene pollen value (see Giesecke and Brewer (Citation2018) and Giesecke et al. (Citation2017) for details).
Table 4. Selected examples of Holocene isochrone maps for different geographical areas.
Figure 11. Isochrones (in radiocarbon years BP) for Quercus pollen in Britain and Ireland. Modified from Birks HJB (Citation1989).
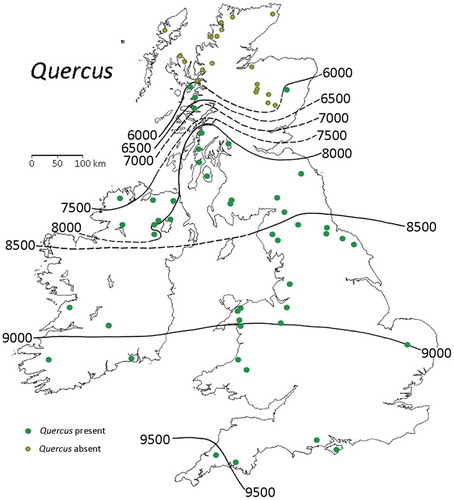
Although popular, isochrone maps have certain critical limitations. They show no information about the pollen-stratigraphical event being mapped and they are thus a poor means of displaying spatial variation in pollen data. They can also be misleading if rigorous definitions are not consistently followed when determining the stratigraphical position and age of the event being mapped and if ages are used from different studies where the definitions of the presumed analogous event are not identical. Tallantire (Citation1972) and Giesecke and Bennett (Citation2004) provide critiques of the problems in constructing isochrones maps. If carefully constructed, they can provide summaries of the direction of spread and the areas of first expansion but they are difficult to interpret in terms of first arrival because of the difficulties in interpreting low pollen values – are such values the result of long-distance pollen transport or do they reflect pollen from local but small nearby populations (e.g. Hesselman Citation1916; Bennett Citation1985, Citation1986, Citation1988a, Citation1988b; Davis MB and Sugita Citation1997; Davis MB Citation2000)? Pollen stratigraphies may seriously underestimate the point in time when a tree taxon actually arrived at a site (e.g. Welten Citation1944; Watts Citation1973; Bennett Citation1983; Citation1985, Citation1988a; Birks HJB Citation1986, Citation1989; Froyd Citation2005; Tinner and Lotter Citation2006). Presumed arrival times and hence spreading rates are usually estimated from particular critical threshold-values or the so-called empirical or rational limits (e.g. Birks HJB Citation1989). Such thresholds or limits may, however, potentially fail to detect the signal of individual trees or even small localised populations within the source area of a pollen or macrofossil site; the not infrequent problem of ‘false absences’ in palaeoecology and vegetational history for which there is currently no solution (see Birks HJB Citation2014; Birks HJB and Birks Citation2016). Estimates of Holocene tree-spreading rates based on isochrone maps (e.g. Birks HJB Citation1989) may be misleading as the maps (and the available data on which the maps are based) may not include information from microrefugia that are, by definition, difficult or impossible to identify unambiguously from pollen-stratigraphical data (Birks HJB and Willis Citation2008; Feurdean et al. Citation2013). As Bennett (Citation1985) concludes in his discussion of the spread of Fagus grandifolia across eastern North America, ‘[t]he spread of F. grandifolia across the continent was achieved at very low population densities. The detection and tracking of such a spread is only marginally possible with current pollen-analytical techniques.’ McLachlan and Clark (Citation2004) reach a similar conclusion for Betula alleghaniensis and F. grandifolia. Low-density tree populations may also be difficult to detect using macrofossils (Lesser and Jackson Citation2011; Birks HJB Citation2014). Such populations are ‘blind spots’ in the Quaternary botanical landscape.
4.4. Tree-spreading and range-expansion processes
The current detailed pollen maps of Brewer et al. (Citation2017) reveal, as did the earlier maps of Huntley and Birks (Citation1983), unexpectedly complex spatial and temporal patterns of tree spread and range expansion, with some tree taxa seemingly spreading at very fast rates from a range of presumed refugial source areas (see Giesecke et al. Citation2017; Giesecke and Brewer Citation2018). These maps raise many questions about the interpretation of pollen-stratigraphical data in terms of local presence, invasion, and range expansion, about how trees spread and expand over large areas so quickly, about what ecological factors may have caused major pollen-stratigraphical changes (e.g. Giesecke Citation2005a; Tinner and Lotter Citation2006), and whether tree pollen assemblages existed in the past that appear to have no modern pollen analogues today (e.g. Jackson and Williams Citation2004; Finsinger et al. Citation2017b; see subsections 7.2.2, 7.2.3, and 11.3.7).
Estimated rates of spread of trees during the Holocene for the European mainland and for Britain and Ireland are summarised in . The European rates based on the pollen maps of Brewer et al. (Citation2017) are rough estimates based on simple visual inspection of the maps at 1000-year intervals and are approximate mean values. Because Betula and Pinus were widespread in the late-glacial and early Holocene, no European rates can meaningfully be estimated for these taxa from these maps. Giesecke and Brewer (Citation2018) estimate spreading rates on the basis of their isochrone maps. The estimates from Feurdean et al. (Citation2013) attempt to take account of known microrefugia by considering known LGM tree presences outside the main southern macrorefugia in Iberia, Italy, and the Balkans.
Table 5. Estimated rates of spread (m yr–1) of trees during the Holocene in Britain and Ireland (Birks Citation1989) and on the European mainland based on Huntley and Birks (Citation1983), Feurdean et al. (Citation2013), Brewer et al. (2017), and Giesecke and Brewer (Citation2018).Citation2017
The estimated spreading rates when northern microrefugia are taken into account (Feurdean et al. Citation2013) are naturally lower (60–260 m yr –1) than estimates based on spreading from southern macrorefugia only (115–540 m yr –1) (; Feurdean et al. Citation2013; see also Cheddadi et al. Citation2014). The crude estimates based on the basic but detailed pollen maps of Brewer et al. (Citation2017) range from 200 to 1000 m yr –1, with Corylus and Ulmus having rates as high as 1000 m yr –1. These taxa are not considered by Feurdean et al. (Citation2013). When they are excluded and Betula and Pinus are excluded because no spreading rates can be meaningfully estimated from the maps of Brewer et al. (Citation2017) for these taxa, the ranges of tree-spreading rates in mainland Europe () are 250–1000 m yr–1 (based on Giesecke and Brewer Citation2018), 200–500 m yr–1 (Brewer et al. Citation2017), 70–170 m yr–1 (Feurdean et al. Citation2013), and 40–2000 m yr–1 (Huntley and Birks Citation1983). It is likely that the earlier rates (Huntley and Birks Citation1983; Huntley and Webb Citation1989; Huntley Citation1991) based on the then available but limited data and without any consideration of microrefugia are over-estimates of tree-spreading rates. However, these early estimates have been used in several species-climate distribution models to predict the possible magnitude of trees in response to future climate change. When tree occurrences in microrefugia are considered (Feurdean et al. Citation2013), it is clear that spreading rates based on pollen data that ignore microrefugia are over-estimates of the rates at which trees have (and could) spread. McLachlan et al. (Citation2005) reached similar conclusions using molecular techniques for Fagus grandifolia and Acer saccharum in eastern North America. If many Holocene tree-spreading rates are over-estimates, this affects predictions about how species may respond to future climate change (see also Svenning and Skov Citation2004; Snell RC and Cowling Citation2015). The hypothesis that the estimated Holocene tree-spreading rates (e.g. Huntley and Birks Citation1983) may greatly exceed likely tree-spreading rates in response to future climate change has stimulated debates about the effects of dispersal and forest fragmentation on tree-spreading rates (Roberts L Citation1989; Malanson and Cairns Citation1997), ‘assisted migration’ (Roberts L Citation1989; Neilson et al. Citation2005; Pearson RG Citation2006; McLachlan et al. Citation2007; Hoegh-Goldberg et al. Citation2008; Hällfors et al. Citation2016, Citation2017; Miller KM and McGill Citation2018), ‘assisted colonisation’ (Hunter Citation2007; Hewitt N et al. Citation2011; Pykälä Citation2017), ‘managed relocation’ (Richardson et al. Citation2009; Stone Citation2010; Schwartz et al. Citation2012), and ‘translocation’ (Hobbs RJ et al. Citation2018) in conservation biology. If actual Holocene tree-spreading rates are closer to the Feurdean et al. (Citation2013) estimates based on known microrefugia (), then the ‘assisted migration’ debate needs to be re-opened and critically re-assessed in light of the ever-increasing evidence for possible microrefugia (e.g. de Lafontaine et al. Citation2014a; Birks HJB Citation2015; Robin et al. Citation2016a; Carcaillet and Blarquez Citation2017 – but see Tzedakis et al. Citation2013; Huntley Citation2014; Giesecke Citation2016; Finsinger et al. Citation2019), revised Holocene tree-spreading rates (e.g. Feurdean et al. Citation2013; Cheddadi et al. Citation2014; Giesecke et al. Citation2017; Giesecke and Brewer Citation2018), and the detailed pollen maps of Brewer et al. (Citation2017) and Giesecke and Brewer (Citation2018). Blois (Citation2013) discusses other approaches to estimating tree-spreading rates using dendrochronology and molecular techniques in Pinus ponderosa in northern Wyoming (Lesser and Jackson Citation2012, Citation2013). She estimates rates of 125–280 m yr–1 for one population and between 350 and 3300 m yr–1 for three other populations but with most rates of spread less than 500 m yr–1 and one fast outlier.
Despite the key role that tree spreading plays in interglacial vegetation history, we are largely ignorant of the ecological mechanisms that enable trees to move at such surprisingly fast rates (e.g. Pigott and Huntley Citation1980; Walker Citation1982a; Birks HJB Citation1986, Citation1989; Webb T Citation1986; Huntley Citation1996; Kolstrup Citation2007) and of the processes that allow new tree species to invade and expand into existing forest vegetation (e.g. Watts Citation1973; Walker Citation1982a; Birks HJB Citation1986, Citation1989; With Citation2002; Martin PH et al. Citation2009; Birks HJB and Tinner Citation2016a). Here, I discuss the observed rates of spread and the possible processes operative in broad-scale changes of range limits. I consider the possible processes operative in tree invasions in subsection 9.6.
Clement Reid (Citation1899, p.25) was perhaps the first to consider rates of spread of trees when he wrote ‘[t]hough the Post-glacial period counts its thousands of years, it was not indefinitely long, and few plants that merely scatter their seed could advance more than a yard [~1 m] in a year, for though the seed might be thrown further, it would be several seasons before an oak for instance would be sufficiently grown to form a fresh starting point. The oak, to gain its most northerly position in North Britain after being driven out by the cold, probably had to travel fully six hundred miles [965 km] and this without external aid would take something like a million years’. Skellam (Citation1951) used a simple diffusion model of random dispersal of acorns to estimate that oak would have only travelled about 135 km (85 miles) within the 11,500 years of the Holocene (i.e. at a rate of spread of 13.5 m yr–1; see ). The observed rates (Table 5) of 50–500 m yr–1 falsify the hypothesis that the Holocene spread of oak conforms to a simple random diffusion model of acorn dispersal (Birks HJB Citation1989) and suggest that birds such as jays, rooks, and wood pigeons and small mammals such as squirrels may have played an important role in the spread of oak (Birks HJB Citation1989). As Clark et al. (Citation1998) note, the rates suggested from pollen data are too high to have been produced by traditional dispersal mechanisms in tree dispersal biology and demography. They call this dilemma of rapid tree spread ‘Reid’s Paradox’ (see also Powell and Zimmermann Citation2004; Phillips et al. Citation2008).
Isochrone () and isopollen () maps give the impression that trees spread and advance as a continuously spreading or moving front across the landscape and invade and occupy all suitable sites (.1). This hypothesis seems unlikely for many, if not all, mesocratic trees (e.g. Watts Citation1973; Walker Citation1982a; Davis MB Citation1987; Giesecke Citation2005a). An alternative hypothesis (.2–12.4; e.g. Rudolph Citation1930; Fægri Citation1949; Firbas Citation1949; Smith AG Citation1965; Godwin Citation1966, Citation1975; Watts Citation1973; Walker Citation1982a; Bennett Citation1985, Citation1988b; Birks HJB Citation1986; Giesecke Citation2013; Giesecke and Brewer Citation2018) proposes that taxa spread by long-distance chance dispersal of propagules (.2) from cryocratic and protocratic macrorefugial and scattered microrefugial populations into locally favourable habitats or into forest openings created by windthrow, death, fire, or disease beyond the main range of the taxon to form small outliers. These mature, and in turn, act as seed parents for local expansion of these outliers and further establishment in new gaps if conditions (e.g. climate) are suitable (.3, 12.4). These eventually expand, coalesce with the main population, and produce propagules, some of which may be dispersed beyond the range limits into new gaps. The result is a discontinuous expansion of the range-limit that may appear as a continuous range-expansion at the broad spatial scale of isopollen or isochrone maps (Birks HJB Citation1989). This hypothesis first presented by Rudolph (Citation1930) explicitly decouples tree spread from tree-population expansion (Giesecke and Brewer Citation2018). Pollen-stratigraphical data often record population expansion unambiguously but are very poor at identifying population arrival or local geographical spread (Watts Citation1973; Bennett Citation1983, Citation1985, Citation1988a; Giesecke and Brewer Citation2018).
Figure 12. Possible scenarios for tree spreading across a large area in an interglacial stage. 1. The moving-front or continuous wave hypothesis where trees 'march' across the landscape. 2. Rare far-distance dispersal events form small outlying populations. 3. Populations expand from the outlying populations into locally favourable sites or enclaves. 4. Merging of large and small populations. Small scattered populations expand (as in 3) and are a source for dispersal events, as are the large populations. Scenarios 2, 3, and 4 combined are likely to be most important. Based, in part, on Davis MB (Citation1987) and Giesecke (Citation2013).
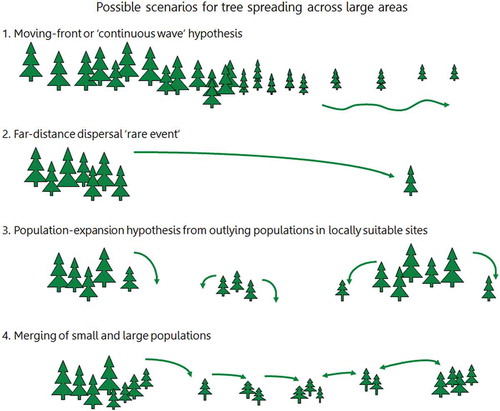
Can the hypotheses of a moving-front or small outlying populations as the process for tree spread be tested? Population models that incorporate life-history constraints of limited seed production and survival and that allow for the leptokurtic (‘fat-tailed’) shape of seed shadows and stochasticity in population growth and spread do not rule out rapid spread through extremely rare long-distance events. They predict that spreading potential is likely to be limited and that dispersal and life-history traits strongly affect the capacity for rapid spread (Clark Citation1998; Clark et al. Citation2001a; Citation2003; McLachlan et al. Citation2005).
If these dispersal-model results are applied to the early-Holocene spread of Quercus, it can be assumed that a single acorn could be deposited some kilometres away from the parent oak tree. If this seed germinates and grows into a tree which in turn disperses its seeds primarily close to itself, this single tree becomes a ‘founder’ of a new outlying population (.2). Support for this type of process comes from the distribution of DNA haplotypes of Quercus in western France (Petit et al. Citation1997, Citation2001; Giesecke Citation2013). The distribution of chloroplast DNA markers shows a mosaic with different areas dominated by distinct haplotypes. Chloroplasts and their haplotypes are maternally inherited and are thus retained in the acorn. The DNA markers suggest that the oak population in each area may have originated from one single founder tree through the maternal line (Giesecke Citation2013).
There is ever-increasing genetic and palaeobotanical evidence for tree populations in microrefugia further north than presumed from pollen data (e.g. see above and McLachlan et al. Citation2005; Birks HJB and Willis Citation2008; Peterson and Graves Citation2016). These populations, some of which may have grown as close as 500 km south or east of the ice margin, may have consisted of trees in locally favourable habitats and the populations were too small to be detected by pollen analysis. These northern populations, as well as the larger populations in macrorefugia further south, expanded in the early Holocene. Without knowledge of these northern microrefugia, their expansion could be easily interpreted as a rapid spread of more southerly populations, resulting in high estimated rates of spreading, which may never actually have occurred (Giesecke Citation2013).
Increases of pollen-accumulation rates (PAR; grains cm–2 yr–1) can be used as a proxy for the population increase of the tree taxon in question (Watts Citation1973; Tsukada Citation1981, Citation1982a, Citation1982b, Citation1982c, Citation1983; Bennett Citation1983, Citation1986, Citation1988a, Citation1988b). There is usually an exponential increase in PAR that is most parsimoniously interpreted as reflecting the expansion of small populations that were present near the site even before its pollen can be detected in standard pollen counts. Considered together, results from theoretical models (Clark et al. Citation1998), the regional distributions of haplotypes (Petit et al. Citation1997), and the exponential increases of PAR estimates (Giesecke Citation2005a) are not explicable in terms of the simple moving-front hypothesis (.1) but are all consistent with the outlying population hypothesis, which, in turn, depends on long-distance dispersal from refugia (.2-12.4; Giesecke Citation2013). The moving-front model (.1) may not be applicable to continental- or regional-scale tree spreading, but it may be relevant at a more local-scale (Giesecke Citation2013).
Detailed studies of tree- and shrub-spreading rates and subsequent expansion in the xeric areas of Wyoming, Utah, and Montana (Lyford et al. Citation2003) demonstrate the complexity of the ecological processes involved (Jackson et al. Citation2009b). Juniperus osteosperma spread into Wyoming and southern Montana as a series of long-distance dispersal events over 30–135 km (.2) which were facilitated to some degree by climate variability between 7500 and 8400 years ago and by the geographical distribution and connectivity of suitable habitats within the landscape. Further expansion of these initial outlying populations and the colonisation of sites to the south of the outliers did not occur during a widespread wet period in the Rockies from 5400 to 2800 years ago. The onset of dry conditions 2800 years ago resulted in the rapid colonisation of J. osteosperma into sites throughout its current range (.3, 12.4). This detailed study suggests that regional climate change was an important extrinsic driver interacting with landscape structure in determining the spatial and temporal patterns of colonisation and expansion of J. osteosperma (Lyford et al. Citation2003). As Lyford et al. (Citation2003) conclude ‘past and future migrations may follow neither a front-like advance nor a process of long-distance colonization, expansion, and backfilling that is partly predictable from the environmental mosaics’. A similar set of complex processes and interactions with climate and/or fire appear to have been operative in the range development of Pinus edulis in north-eastern Utah (Gray et al. Citation2006), of P. ponderosa in northern Wyoming and elsewhere in the Rockies (Lesser and Jackson Citation2012, Citation2013; Blois Citation2013; Norris et al. Citation2016), and of P. monophylla and J. osteosperma in south-central Idaho (Weppner et al. Citation2013).
Thanks to an increasing number of detailed studies involving pollen and plant-macrofossil assemblages, radiocarbon chronologies, and independent palaeoclimatic reconstructions from, for example, tree-rings, peat stratigraphy and bog hydrology based on testate amoebae, or chironomid-based air-temperature inferences, a picture is emerging of populations of some trees being locally present in low numbers, presumably as a result of long-distance dispersal of propagules (.2), for several centuries prior to population expansion. Exogenous or extrinsic factors (sensu Williams et al. Citation2011a) such as subtle changes in one or more ecologically critical climatic variables or interactions between variables (Jackson et al. Citation2009b), and changes in habitat conditions, habitat availability (e.g. Bush and Hall Citation1987; Bennett and Birks Citation1990), or land-use (e.g. Bradshaw RHW and Lindbladh Citation2005) or a combination of changes in climate, fire, and human impact (e.g. Valsecchi et al. Citation2008; Edwards ME et al. Citation2015) may have initiated population expansion and regional spread (.3, 12.4; see subsections 9.2, 9.3, and 9.6). Endogenous factors, such as factors influencing local propagule deposition and probability of establishment within the surrounding forest, can, over several generations, lead to population expansions and coalescence with the main population (.4). Population growth may then flatten off or even decrease due to interspecific competition, density-dependent self-thinning, and intraspecific competition and to the apparent inability of several temperate forest trees to regenerate under themselves (Birks HJB Citation1986). A new forest composition is thus assembled over several generations as a result of these exogenous and endogenous factors and their interactions, as well as other factors (Jackson et al. Citation2009b). Green (Citation1982) shows that invasion and expansion into existing forests in Nova Scotia may coincide with rare, extensive natural disturbances, suggesting that availability of large openings may be an important prerequisite for invasion and expansion for some trees. Almost all temperate mesocratic trees require openings for establishment and regeneration, possibly because of nutrient release, increased irradiance, relaxed root competition, or reduced host-specific insect predation (see subsection 9.6). Gaps are thus probably essential for invasion and expansion, as existing vegetation provides ‘inertia’ (Pearsall Citation1959; Smith AG Citation1965) or ‘resistance’ to vegetational change. Invasion and range expansion can occur, in theory, without climate change (Watts Citation1973). However, it appears to be most commonly favoured by climate change. For example, extensive gaps providing conditions of low competition for invaders may be commoner in vegetation following climate change across a critical threshold that restricts seed production or regeneration of the residents (e.g. Walker Citation1982a; Jackson et al. Citation2009b). Storms, pathogen outbreaks, fire, or other disturbances may create more frequent and more extensive gaps in forests affected by, for example, prolonged drought, senescence, nutrient shortage, or pests and pathogens than stands growing under optimal conditions. Liang et al. (Citation2018) present a physiologically based landscape model to study how climate change, dispersal, disturbance, and competition interact to influence tree-range shifts and conclude that interspecific competition and disturbance are important in understanding range shifts.
Given that climate change and its variability occur at all ecologically relevant time and spatial scales (Jackson and Overpeck Citation2000; Jackson et al. Citation2009b), it is not surprising that range expansions or contractions may, when examined in detail, appear to show a ‘stop’ or a ‘go’ behaviour (‘ratchet’ sensu Jackson et al. Citation2009b) – see for selected examples.
Table 6. Selected examples of range expansions or contractions of taxa that appear to show a ‘stop’ or ‘go’ behaviour during the Holocene.
Bennett and Birks (Citation1990) suggest that within British and Irish trees there is a continuum in their expansion behaviour in the Holocene. At one extreme (.1) trees spread rapidly across widespread suitable habitat with a predictable reproductive means and rapid rates of population expansion (e.g. Corylus, Populus, Salix, Ulmus). At the other extreme (.3) are taxa confined to local ‘island-like’ habitats and with infrequent opportunities for reproduction (e.g. Alnus). Between these extremes (.2) are taxa such as Betula and Pinus that although they have widespread suitable habitats and frequent reproduction, they appear to have experienced some ‘dispersal limitation’. Taxa such as Carpinus and Fagus (and Picea alba in Scandinavia) that although they too occupy widespread suitable habitats and frequently reproduce today, appear to have experienced ‘expansion limitation’ and may have been present in low numbers for hundreds or even thousands of years prior to population expansion (Firbas Citation1949; Iversen Citation1950). Many factors may contribute to such expansion limitation – unsuitable regional climate, low habitat availability, human activity, disturbance regime, and competition and resistance or inertia from pre-existing vegetation. Although palaeoecologists have long discussed ‘migration lags’ (e.g. Davis MB Citation1978, Citation1986a; Birks HJB Citation1981b, Citation1986; Bennett Citation1983; Prentice Citation1983, Citation1986b, Citation1988a, Citation1992; Webb T Citation1986; Bennett and Willis Citation1995), it is important to distinguish between ‘dispersal limitation’ and ‘expansion limitation’, both of which can result in delayed expansion which has often been interpreted as a ‘migration lag’ and part of what ecologists call ‘immigration credit’ (Jackson and Sax Citation2010). Dispersal limitation in a long-term perspective is difficult to demonstrate as it requires not only detailed pollen-analytical and plant-macrofossil data but also an independent palaeoclimate reconstruction. Such a reconstruction is required to establish if the regional climate was suitable for the taxon in question. In the early Holocene at Kråkenes in western Norway, Birks HJB and Birks (Citation2008) demonstrate in a fine-resolution study that tree Betula exhibited a dispersal lag of about 700 years. Chironomid-inferred air temperatures suggest that mean July temperatures were at least 10°C 670–720 years before the first macrofossils of tree Betula were found (.2). It is likely that other trees (e.g. Fraxinus, Quercus, Tilia) show dispersal limitation or expansion limitation or both but there are currently no detailed data to test these hypotheses. Judging by the long discontinuous tail of low pollen values prior to the main expansion and rise in pollen values shown by Picea abies in Fennoscandia (e.g. Huttunen and Tolonen Citation1972; Miller U and Robertson Citation1979; Almquist-Jacobson Citation1994; Giesecke Citation2005b) it is likely that it commonly experienced extended expansion limitation (.3). Pinus in Scotland may also have been subject to expansion limitation, as shown by the presence of its stomata, evidencing local presence, 600–1600 years prior to the rise of pine pollen (Froyd Citation2005).
Figure 13. The changing relative abundance expressed as a percentage of the maximum attained values of tree taxa through time in Britain and Ireland. The top panel (1) shows how relative abundance changes through time in the absence of any detectable dispersal limitation or expansion limitation, as shown by the Holocene behaviour of Corylus, Populus, Salix, or Ulmus. The middle panel (2) shows relative abundance changes for taxa that experience dispersal limitation but little or no expansion limitation (e.g. Betula, Pinus). The bottom panel (3) shows relative abundance changes for taxa that may experience not only dispersal limitation but also prolonged expansion limitation (e.g. Alnus, Carpinus, Fagus) that may extend for 2000–3000 years. Of these, Alnus is the most extreme in its expansion limitation due to the rarity of its 'island-like' wet habitats. There are not enough detailed data to assess the long-term behaviour of Fraxinus, Quercus, or Tilia.
DL = Dispersal limitation; EL = Expansion limitation; EP = Expansion phase.
Seeley et al. (Citation2019) assess environmental and dispersal controls on the distribution of Fagus grandifolia in the Great Lakes region, USA. They conclude that both environmental (climatic) factors and dispersal limitations appear to govern the western range limit of F. grandifolia and show different species–environment relationships between populations at the range-edge and in the core of the range. This study highlights the complexity of understanding a species’ range and the potential importance of dispersal limitation.
Given the current hypothesis (.2–12.4; Giesecke Citation2005a, Citation2013; Giesecke and Brewer Citation2018) about how trees spread and expanded in the Holocene, chance colonisation as a result of long-distance dispersal (sensu Garcia et al. Citation2017; Jordano Citation2017; .2) must have been as important or even more important than local propagule dispersal and seedling establishment (Smith JMB Citation1978; Clark Citation1998; Clark et al. Citation1998, Citation2001a; Higgins and Richardson Citation1999). The difficulty about invoking chance events such as long-distance dispersal (‘jump-dispersal’ Davis MB and Sugita Citation1997; Davis MB Citation2001b) in palaeoecology and historical biogeography is, as Smith JMB (Citation1978, p.218) emphasises, ‘distinguishing the impossible from the highly improbable, especially in the largely unknown conditions of the past’ (see also Godwin Citation1923; Palmgren Citation1929; Talling Citation1951; Jeffries Citation1989). However, it is important to recall Simpson’s (Citation1952) discussion of probabilities of dispersal in geologic time where low-probability events may become nearly certain over long time spans. There is an increasing number of convincing cases for chance long-distance dispersal (over 100 km or more) in Holocene tree palaeoecology. For example, Webb SL (Citation1986, Citation1987) elegantly shows that the spread of Fagus grandifolia from lower Michigan to eastern Wisconsin across either Lake Michigan or parts of the Illinois Prairie Peninsula in the south may have been facilitated by the now extinct (since 1914 CE) passenger pigeon (Ectopistes migratorius). Other carefully argued cases for the role of long-distance jump dispersal of propagules in Holocene tree biogeography include the spread of Picea glauca in the Western Interior of Canada (Ritchie and MacDonald Citation1986), the colonisation of Pinus ponderosa in northern Wyoming (Lesser and Jackson Citation2013), the Holocene spread of fagaceous trees (Castanea, Fagus, Quercus) by blue jays (Cyanocitta cristata) in eastern North America (Johnson WC and Webb Citation1989), the spread of Fagus grandifolia and Tsuga canadensis in the Great Lakes Region (Davis MB et al. Citation1986; Davis MB Citation1989a, Citation2001a), and the Holocene spread of Quercus in Britain and Ireland (Lowe et al. Citation2006). Wilkinson (Citation1997) suggests that broad-scale dispersal of trees with wind-dispersed seeds may, in reality, be dependent on animals and that wind dispersal is effective only for local dispersal over distances of a few canopy diameters (see also Nathan Citation2006). Nathan et al. (Citation2001, Citation2002, Citation2008) and Schupp et al. (Citation2010) as they provide detailed examples of, and reviews on, wind dispersal of propagules.
Palaeoecological studies of range expansions have naturally concentrated on trees as their pollen are well represented in lake or mire sediments. An obvious question is how did forest shrubs and herbs expand their ranges (Bennett Citation1988c)? It has long been assumed that herbs spread rapidly as a result of their generally short generation times (Bennett Citation1988c). Detailed studies on forest herbs demonstrate low and very local propagule dispersal (e.g. Matlack Citation1994; Cain et al. Citation1998; Citation2000; Vellend et al. Citation2003, Citation2006). However, several of these studies highlight the importance of rare long-distance dispersal events through ingestion and subsequent defaecation by animals such as deer, bears, small mammals (e.g. squirrels), and birds (e.g. jays) (e.g. Pakeman Citation2001; Vellend et al. Citation2003, Citation2006). Peterson and Graves (Citation2016) suggest, on the basis of genome analysis, that the shrub Dirca palustris, a plant of mesic Fagus-Acer forests in eastern North America, grew within 500 km of the Laurentide ice-sheet at the LGM with temperate trees such as F. grandifolia (McLachlan et al. Citation2005). In contrast, Willner et al. (Citation2009) propose that many species closely associated with European beech forests are limited by Holocene dispersal lags rather than by specific environmental requirements. Much remains to be elucidated about forest shrubs and herbs, their history, and their dispersal limitations (see also Primack and Miao Citation1991; Honnay et al. Citation2002; Clark et al. Citation2003; Higgins et al. Citation2003; Tackenberg Citation2003; Tackenberg et al. Citation2003; van der Veken et al. Citation2007; Svenning et al. Citation2008a; Felde et al. Citation2018).
Pollen maps for trees in Europe and eastern North America show that taxon abundances and ranges have been very dynamic over time-spans of thousands of years and spatial scales of 105–109 km2. Such maps provide unique insights into long-term dynamics of range boundaries and relative abundances. Spatial pollen data-sets have been used to estimate mean square dispersion rates (= diffusivity or diffusion constants (km2 yr–1) based on the combined diffusion and growth models (Skellam Citation1951) commonly used in the quantitative analysis of biological invasions (Birks HJB Citation1989; Giesecke et al. Citation2017; Giesecke and Brewer Citation2018). The long-term trends in the spatial range of a taxon such as Fagus sylvatica in Europe during the Late Quaternary can be modelled as a simple logistic model (Magri Citation2008; cf. Saltré et al. Citation2013). The model suggests that the range expanded exponentially for over 10,000 years until about 3500 BP. In the last three millennia, beech’s range continued to expand but at a slower rate, tending towards an equilibrium value, possibly reaching carrying capacity. Giesecke et al. (Citation2017) fit similar logistic functions to tree-range increases in Europe north of 47°N. They interpret the inflection point, the point at which the exponential increase slows, as a convenient parameter to compare the differences in time required by different tree taxa to fill their ranges. The inflection points are 11,200 yr BP (Ulmus), 11,100 yr BP (Corylus), 10,300 yr BP (Tilia), 10,100 yr BP (Quercus), 9500 yr BP (Alnus), 5900 yr BP (Abies), 4900 yr BP (Fagus), and 4800 yr BP (Carpinus). Interestingly, Picea shows no obvious inflection point or exponential increase.
4.5. Phylogeography
In the last 25 years with the development of DNA analysis of extant plant populations, extensive studies have been made of the genetic variation across Europe of populations of, for example, Fagus sylvatica (Magri et al. Citation2006), Quercus (‘white oaks’) (Petit et al. Citation2002a), Quercus cerris (Bagnoli et al. Citation2016), Pinus sylvestris (Dering et al. Citation2017), Abies alba (Liepelt et al. Citation2009; Cheddadi et al. Citation2014), Picea abies (Collignon and Favre Citation2000; Tollefsrud et al. Citation2008, Citation2009; Tsuda et al. Citation2016), and Alnus (Havrdová et al. Citation2015; Mandák et al. Citation2016). Important links are established between phylogeography and palaeoecology (e.g. Petit et al. Citation2001, Citation2002b, Citation2004, Citation2005; Magri et al. Citation2006; Jackson Citation2006b; Hu et al. Citation2009; Giesecke and Brewer Citation2018) through the bringing together of spatial DNA of extant populations and spatial pollen data from fossil assemblages.
Studies on chloroplast DNA variation in 2613 extant populations of eight species of Quercus in Europe (Q. canariensis, Q. faginea, Q. frainetto, Q. macranthera, Q. petraea, Q. pubescens, Q. pyrenaica, Q. robur) identify 32 chloroplast DNA variants (Petit et al. Citation2002a). Using the then available fossil pollen data, Petit et al. (Citation2002b) infer major LGM refugia on the Iberian and Appenine peninsulas and in the eastern Balkans and reconstruct two major phases of colonisation, one in the late-glacial and one in the early or mid Holocene (see also Brewer et al. Citation2002; Citation2006).
Magri et al. (Citation2006) closely integrate data about chloroplast DNA and nuclear genetic markers (isozymes) from nearly 600 extant populations of Fagus sylvatica across Europe with all the then available pollen and plant-macrofossil data (408 and 80 radiocarbon-dated sites, respectively). They show that there are 20 chloroplast DNA and microsatellite haplotypes in European populations today but only three comprise over 80% of populations – one is centred on Iberia, one in the southern Balkans, and one is widespread. Of the nuclear genetic markers identified, there is only one widespread group, with smaller groups in Italy, southern Balkans, and Iberia.
By combining Quaternary pollen and extant genetic data and by assuming that if a population today at locality X belongs to nuclear-marker group A, then the first population to reach or expand at locality X at, say, 4000 years ago also belonged to nuclear-marker group A, Magri et al. (Citation2006) show that of the 20 chloroplast haplotypes only one had several LGM refugia and one had refugia in Italy, whereas the other haplotypes were mainly centred in the Balkans. The isozyme types had refugia primarily in Italy, the Balkans, and Iberia but only one type had several refugia. The overall conclusion is that there were multiple LGM refugia as far north as 45°N () but that only a very few of the chloroplast haplotypes or isozyme groups contributed to the Holocene spread and expansion of Fagus across Europe. The majority of the genetic types persisted in different areas around the Mediterranean basin and contributed little to the Fagus sylvatica population range in Europe today.
Figure 14. Suggested location of refugial areas for Fagus sylvatica during the last glacial maximum (blue circles) and the major spread into Europe during the Holocene (blue arrows). The green shaded areas are the present native range of F. sylvatica. Modified from Magri et al. (Citation2006).
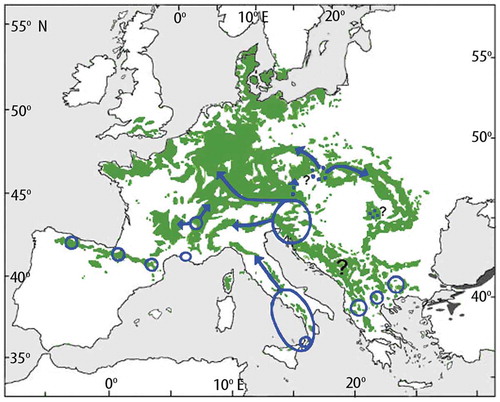
These detailed integrated pollen-DNA studies of extant Quercus and Fagus in Europe highlight the complex genetic structure of present-day populations, the many possible LGM refugia not only in the Mediterranean basin but also in central Europe, and the complex colonisation and expansion patterns in the Holocene. There is no convincing evidence for advancing fronts but much evidence for complex events that may well have included long-distance dispersal events to create outlying populations and subsequent expansion and coalescence in response to a multitude of environmental and biotic changes (Hu et al. Citation2009). Lowe et al. (Citation2006) present a detailed integrated analysis of phylogeography, palaeoecology, and spatial modelling of the Holocene spread of Quercus in Britain and Ireland that illustrates the potential of such integration in understanding the origin and development of species distributions and the genetic variation of populations within a taxon’s range (see also Sjölund et al. (Citation2017) for a detailed phylogeographic study of Fagus sylvatica in Britain). Despite the many investigations on the history, phylogeography, and ecology of F. sylvatica (e.g. Giesecke et al. Citation2007; Bradshaw RHW et al. Citation2010), the ecological controls on its distribution and abundance remain poorly understood. As Giesecke et al. (Citation2007) conclude ‘[o]ur analysis … shows that no single driving force or climatic parameter can adequately account for the observed changes in its abundance and distributional limits … [and] climatic control cannot be rejected as an explanation for Holocene changes in distribution and abundance. … On the other hand, non-climatic factors help explain and the pattern of population expansion in the lowlands of central Europe, which is largely consistent with observations that disturbance and anthropogenic clearance accelerated the growth of F. sylvatica populations’.
4.6. Conclusions
Basic pollen maps display pollen values at different sites and at different times. They can be interpreted, with varying degrees of confidence, in terms of taxon presence, colonisation, expansion, and contraction locally and regionally (e.g. Douda et al. Citation2014) and in terms of latitudinal richness gradients of woody plants at the genus (Silvertown Citation1985) or family (Haskell Citation2001; Vázquez-Rivera and Currie Citation2015) levels and their development through time. Pollen maps can also be combined with broad-scale genetic studies of extant populations of a particular taxon to provide insights into the LGM survival and Holocene spread of the taxon (e.g. Magri et al. Citation2006). By summarising broad-scale patterns of past changes in response to past climate change, pollen maps can help to predict the biotic impacts of future climate change (Tinner et al. Citation2013). Maps can also be used to test process-based vegetation models by hindcasting vegetation distribution in the past and comparing these hindcasts with mapped pollen syntheses for particular time intervals (e.g. Miller PA et al. Citation2008; Henne et al. Citation2011; Saltré et al. Citation2013; Tinner et al. Citation2013; Giesecke et al. Citation2017), thereby providing some insights into the predictive skill of such models beyond the present-day. Maps of individual taxa can be combined to reconstruct past biomes or broad vegetation types (e.g. Prentice et al. Citation1996; Williams et al. Citation2004; Dyke Citation2005), to assess whether pollen assemblages occurred in the past that have no modern pollen counterparts or analogues today (e.g. Williams et al. Citation2001; Jackson and Williams Citation2004; Williams and Jackson Citation2007; Veloz et al. Citation2012; Finsinger et al. Citation2017b; see section 7.2), and to provide a factual basis for vegetation reconstruction and landscape planning for a future with climate change (e.g. Henne et al. Citation2015).
Despite the inevitable generalisations that spatial display and analysis can introduce and the chronological uncertainties in all palaeoecological data, displaying complex palynological data spatially permits the detection of patterns and trends in both time and space. The scales represented are primarily at the biogeographical, regional, and landscape scales. Spatial displays of palynological data can thus open up many research directions in ecological biogeography as well as phylogeography and palaeoecology (Prentice Citation1983), and allow us to ‘think horizontally, work vertically’ (Edwards KJ et al. Citation2017; Richards K Citation2017; Gaillard et al. Citation2018). The challenge, however, is to interpret these spatial patterns in terms of underlying processes in rigorous ways. Invasion biologists (Coutts et al. Citation2018) in discussing ‘invasion lags’ recognise the problems in identifying such lags as ‘[t]he stories we tell ourselves and our inability to infer process from pattern’. The same inabilities easily arise in interpreting spatial and temporal patterns of palynological and other palaeobotanical data.
5. Extinctions
5.1. Introduction
The term ‘extinction’ is increasingly used in titles of publications in ecology, biogeography, conservation biology, and nature management (see Box S1 for examples). Despite this wide usage, the various kinds of extinction relevant to the Quaternary and the present-day are rarely distinguished. Leopold (Citation1967) identifies three main extinction types – blanket, partial, and regional. Blanket and partial extinctions are considered to be ‘true extinctions’ (sensu Guilday Citation1967). Blanket extinction is the total loss of a phyletic line such as a genus, family, or higher category, whereas partial extinction is the loss of a member (e.g. species) of a group incidental to the evolution of the phyletic line. True extinctions have occurred throughout the history of life on Earth (Bennett Citation1997). Regional extinction is the extermination of a taxon in one area although it continues to exist in another area. Such regional extinctions are termed ‘extirpations’ by Reid EM (Citation1920) with reference to Pliocene exterminations of plants in Britain that did not return in the Early Pleistocene and by Martin PS (Citation1958) to refer to changes in the geographical range and loss of taxa from an area previously occupied. Regional extinctions have also been frequent throughout Earth’s history of life (Bennett Citation1997).
For ease of understanding, here I refer to blanket extinction as total extinction, partial extinction as species extinction, and regional extinction as extermination, as partial extinction (sensu Leopold Citation1967) is an ambiguous term.
Bennett (Citation1997) and Nogués-Bravo et al. (Citation2018) review the three major types of biotic response to environmental change – distributional range shifts (dispersal and range expansion or contraction), adaptation and evolution, and extinction. Given the rapid and frequent marked alternations in climate during the last 2.6 million years of the Quaternary between short interglacial-stage conditions and long glacial-stage conditions, many examples of species extinctions in extinct genera (total extinctions), in living genera (species extinctions), and broad-scale range shifts (exterminations) could be expected. Range shifts are discussed in subsection 4.4 and adaptation is briefly considered in subsection 6.3.
5.2. True extinctions
5.2.1. Pliocene and Early and Middle Pleistocene
True extinctions of plants in the Quaternary, Neogene, or Palaeogene are very difficult to detect from fossils. Pollen and spores generally do not provide enough morphological characters to distinguish between extinct and extant members of a genus. Macrofossils, particularly seeds and fruits and, in some cases, leaves, allow more precise differentiation, but there are relatively few diagnostic plant morphological features comparable to those in dental morphology that permit vertebrate palaeontologists to infer evolutionary rates (Leopold Citation1967).There are also problems relating to different taxonomic treatments. Some palaeobotanists assign Tertiary fossils to extinct fossil taxa and yet assume that all Quaternary fossils belong to extant taxa. In contrast, European palaeobotanists such as Chandler, Clement and Eleanor Reid, Szafer, and Jentys-Szaferowa meticuously assigned Late Tertiary fossils to living taxa only if they considered the relationship to be sure. If they were uncertain of the relationship, they related the fossil to the nearest living form by cf. or aff. They only described and named a new species (or genus) if the fossil material in comparison with extensive modern reference collections was compellingly different (Leopold Citation1967). The European Late Tertiary floras are largely based on seeds and fruits that form a firmer basis than leaves for assignment to extinct taxa, whereas the American Late Tertiary floras are mainly based on fossil leaves. The result is that most Late Cenozoic records of extinct versus extant taxa come from Europe (Leopold Citation1967). For example, based on seed morphology, nine successive species of Stratioides have been described between the Eocene and the Pleistocene by Chandler (Citation1923), but only one species, S. aloides, is extant.
If total extinctions due to human activity in the late Holocene are excluded, the youngest extinct woody genus is the Late Palaeogene (Oligocene) Fagopsis (Fagaceae) (MacGintie Citation1953; Leopold Citation1967) but some extinct herbaceous genera are younger, for example Hemitrapa (Lythraceae) (Miki Citation1959) and Stipidium (Poaceae) (Elias MK Citation1935); both appear to have gone extinct in the Late Neogene (Pliocene). There are, however, close morphological similarities between the extinct Hemitrapa and the extant Trapa (Miki Citation1959; Doweld Citation2016), suggesting that the extinction of Hemitrapa may not qualify as a strict ‘total extinction’. Aracites interglacialis (?Araceae) suffered total extinction in the Middle Pleistocene (see below).
Detailed recent macrofossil studies from Late Pliocene deposits in south-eastern Belarus (Velichkevich and Zastawniak Citation2003) provide a wealth of information on extant and extinct plant taxa. The deposits yielded macrofossils of 170 taxa in 82 genera and 43 families. Most (137) are identified to species level. Of these 137, 78 (48%) are species extinctions, although one reappears in the Middle Pleistocene where it suffered total extinction (see below). The most striking feature of the 78 species extinctions is the preponderance of extinct taxa in aquatic families such as Potamogetonaceae (14 extinct species out of 16 taxa), Cyperaceae (11 out of 24), Salviniaceae (4 out of 4), Najadaceae (3 out of 3), Cabombaceae (3 out of 3), Haloragaceae (3 out of 4), Nymphaceae (3 out of 4), and Lythraceae (2 out of 2). In contrast, there are only three extinct tree or shrub species (Myrica, Betula (two taxa)). The phytogeographical affinities of the overall assemblage are East Asian (with 11 exterminated taxa) and North America (13 exterminated taxa), along with many Eurasian, Holarctic, or cosmopolitan taxa.
Stachowicz-Rybka (Citation2011, Citation2015a, Citation2015b, Citation2015c) present similarly detailed macrofossil data from a series of Early and Middle Pleistocene interglacials in Poland that are correlated with Marine Isotope Stages (MIS) 21, 20, 17, and 15–13 (Cromerian I, Glacial AI, Cromerian III, and Cromerian IV, respectively). The rich macrofossil assemblages contain several extinct species as well as some exterminated taxa not native in Poland today such as Azolla filiculoides, Euryale ferox, and Dulichium arundinaceum. The numbers of extinct species are almost the same in each of these interglacials () and belong almost exclusively to genera that today grow in open water or telmatic conditions. An exception to the dominance of aquatic species extinctions is the Early and Middle Pleistocene is the species extinction of the shrub Eurya stigmosa in the Early Pleistocene on Madeira (Góis-Marques et al. Citation2019), a taxon previously only known from the European Tertiary (see subsection 12.3).
Table 7. Numbers of total and species partial extinctions and exterminations of taxa in recently studied Late Pliocene sequences in Belarus and Early or Middle Pleistocene interglacials in Poland along with their assumed correlations with Marine Isotope Stages (MIS) and interglacial stages in western Europe (Lindner et al. Citation2013). (Based on Stachowicz-Rybka Citation2015a and data from Mamakowa and Velichkevich Citation1993a; Velichkevich and Granoszewski Citation1996; Velichkevich and Lesiak Citation1996; Velichkevich and Mamakowa Citation2003; Velichkevich and Zastawniak Citation2003; Velichkevich et al. Citation2004; Stachowicz-Rybka Citation2011, Citation2015a, Citation2015b, Citation2015c; Drzymulska Citation2018).
The rich macrofossil floras of the Middle Pleistocene Mazovian interglacial stage (equivalent to the European Holsteinian and the British Hoxnian interglacial stages, and MIS 11; 370–425 kyr) have been critically re-examined (Velichkevich and Mamakowa Citation1999, Citation2003; Velichkevich et al. Citation2004). Re-determinations of these macrofossils show five extinct species – Aldrovanda doktoruvskyi, Carex paucifloroides, Caulinia (Najas) goretskyi, Nymphaea cinera, and Brasenia borysthenica var. heterosperma – out of nearly 200 taxa (3% of the total fossil flora: Table 7); one regionally extinct taxon Scirpus torreyi (also present in the last interglacial in eastern Europe and extant in North America today); and one totally extinct taxon Aracites interglacialis. (Mamakowa and Velichkevich Citation1993a). As in the Early Pleistocene (), species extinctions in these stages are largely confined to aquatic taxa. The Eemian interglacial in Poland (Mamakowa Citation1989; Granoszewski Citation2003; Kupryjanowicz Citation2008; Kupryjanowicz et al. Citation2018b, Citation2018c) supported one extinct species (Potamogeton sukaczevii) and several exterminated terrestrial and aquatic taxa (e.g. Acer tataricum, Aldrovanda vesiculosa, Brasenia schreberi, Buxus sempervirens, Carex aquatilis, Ilex aquifolium, Najas flexilis, Osmunda cinnamonea, Potamogeton vaginatus, Salvinia natans, Tilia tomentosa). A careful re-examination of the macrofossil assemblages from 10 Vistulian (= Weichselian) sites in Poland (Velichkevich and Mamakowa Citation1999) identifies only three extinct species – Potamogeton panormitanoides, P. dorofeevi, P. cf. oxyphyllus – all aquatics. As in many full-glacial assemblages there are several taxa in these assemblages that were exterminated but which occur today in, for example, Fennoscandia (e.g. Salix polaris, Silene uralensis).
At present, there appears to be only one example of total extinction of a plant in the entire Quaternary at a time with no hints of human intervention. This is Aracites interglacialis. (Mamakowa and Velichkevich Citation1993a) whose distinctive seeds disappear near the end of the Holsteinian interglacial stage in Poland, Belarus, Russia, the Ukraine (Mamakowa and Velichkevich Citation1993a), Finnish Lapland (Aalto et al. Citation1992), and, most surprisingly, the English Midlands (Field et al. Citation2017). There are no known records from younger deposits (Katz et al. Citation1965; Donner Citation1995). Morphologically similar seeds have been found in Pliocene or Miocene deposits in the Netherlands, Poland, Russia, Siberia, northern Canada, and northern Greenland (Bennike Citation1990). The Tertiary finds have received various names (e.g. Hippuris globosa, Carpolithes johnstrupii, Aracispermum orientalis, A. jugutum, Caricoidea globosa, Myrica johnstrupii, Aracites johnstrupii, A. interglacialicus, A. interglacialis), reflecting their uncertain taxonomic affinities. They have been assigned to the Araceae, Myricaceae, Scheuchzeriaceae, Cyperaceae, Cucurbitaceae, and Hippuridaceae (Bennike Citation1990; Mamakowa and Velichkevich Citation1993b). Bennike (Citation1990) proposes that the Tertiary and Middle Pleistocene fossils are morphologically similar and calls them all Aracites globosa. However, Aalto et al. (Citation1992) suggest that the Tertiary and Middle Pleistocene remains can be separated and determined and named fossils from the Middle Pleistocene deposits at Naakenavaara in Finnish Lapland (which Donner (Citation1995) correlates with the Holsteinian stage, MIS 11) as Aracites interglacialis. This extinct fossil is formally described as Aracites interglacialis by Velichkevich (Citation1977, Citation1982). At present there is a general consensus (Nikitin Citation1979) that fossils from the Palaeogene and older Neogene are assigned to the genus Aracispermum and fossils from the Late Pliocene and Pleistocene are assigned to Aracites (Mamakowa and Velichkevich Citation1993a). Aracites is generally thought to belong to the Araceae (Velichkevich and Zastawniak Citation2006).
Little is known about the ecological preferences of A. interglacialis. Judging by its associated fossils of Eriophorum vaginatum, Calluna vulgaris, Sphagnum spp. (e.g. Nikitin Citation1957; Aalto et al. Citation1992; Field et al. Citation2017), Aracites was a plant of oligotrophic mires rather than of open water. Indirect evidence for it not being an open-water aquatic is that Field et al. (Citation2017) find no remains of the aquatic Azolla filiculoides, which is regularly found in British Holsteinian (= Hoxnian) assemblages. These assemblages mainly come from lacustrine situations in kettle-holes rather than mire conditions (Field et al. Citation2017). The preference of Aracites for mires rather than open-water conditions may explain its apparent absence from Early Pleistocene interglacial assemblages in eastern Europe that mainly derive from fluviatile or limnic settings.
Aracites interglacialis clearly had a wide geographical range during the Holsteinian interglacial in Europe, ranging from England to the Ukraine and Russia. Its macrofossils are likely to be found elsewhere in Europe. It is unclear what caused its total extinction in the Middle Pleistocene, It is, as far as is known, the only total extinction of a vascular plant genus until the Late Quaternary and associated human activity.
Field et al. (Citation2000) discuss macrofossils of Caulina goretskyi (Najas goretskyi) and Potamogeton occidentalis from Middle Pleistocene interglacial deposits in Brittany. Both taxa are ‘critical’ in that their macrofossils are very similar morphologically to the extant N. flexilis and P. maackianus, respectively. The two fossil taxa represent two species extinctions in western Europe in addition to the total extinction of Aracites interglacialis.
There is a major difference in the number of extinct species and exterminated taxa between the Late Pliocene and the Early Pleistocene (Cromerian I; ), possibly because of marked climate change at the Pliocene–Pleistocene boundary (Mosbrugger et al. Citation2005; Teodoridis et al. Citation2017; Martinetto et al. Citation2018). The number of extinct species and exterminated taxa remains relatively constant until the Mazovian (= Holsteinian, MIS 11; ). It is unclear why these extinctions or exterminations documented by detailed macrofossil analyses are almost entirely of aquatic taxa. It does not appear to be a taphonomic artefact as most of the assemblages are from limnic or fluviatile environments and the total number of taxa (terrestrial and aquatic) is about the same for each stage. Seeds and fruits of aquatic plants today show a similar variation in morphology, size, surface patterns, and other features to macrofossils of terrestrial taxa, so there is no reason to suppose that carpological analysts are more likely to notice subtle morphological differences in macrofossils of aquatic taxa compared to macrofossils from terrestrial taxa from different interglacial stages. A possible hypothesis is that Late Pliocene, Early Pleistocene, and Middle Pleistocene aquatic and telmatic floras were simply more species rich than today. As a result of a series of environmental bottlenecks associated with rapid environmental changes due to climatic shifts and habitat changes caused by the alternating glacial and interglacial stages and rapid interstadial climate changes (cf. Willis et al. Citation2007c) and associated ‘cryptic biotic transitions’ (Cooper et al. Citation2015), species extinctions were frequent, leaving a depauperate temperate aquatic flora with species that today only have broad ecological tolerances and widespread geographical ranges. Interestingly, macrofossils of many of the aquatic taxa with widespread geographical ranges today are also present in Late Pliocene or Early Pleistocene assemblages. The long-term history of the aquatic flora may thus be similar to that of European Late Cenozoic trees (van der Hammen et al. Citation1971; Lang Citation1994) with the progressive species extinction of many taxa, leaving an impoverished aquatic flora with a small number of taxa with wide ecological tolerances and good dispersal abilities, and hence widespread ranges. Although not studied in comparable detail to the Polish sequences, macrofossils and pollen from Middle or Late Pleistocene interglacials in western Europe show the species extinctions of Potamogeton and Caulina (Najas) taxa and the exterminations of predominantly aquatic or wetland taxa such as Azolla filiculoides, Brasenia schreberi, Chamaecyparis thyoides, Dulichium arundinaceum, Euryale ferox, Najas minor, Nymphoides aquatica, Proserpinaca cf. palustris, Salvinia natans, and Urtica kiovensis (e.g. Tralau Citation1959, Citation1963a; Huckerby and Oldfield Citation1976; Oldfield and Huckerby Citation1979; Watts Citation1988; Lang Citation1994; Field et al. Citation2000; Field and Lewis 2019Citation2018).
5.2.2. Late Pleistocene and Holocene
In the Late Quaternary (last 125,000 years) there appears to have been only one instance of total extinction of a flowering plant (Easter Island Palm) but this is almost certainly due to human activity and only one well-documented natural species extinction (Picea critchfieldii). In contrast, there are very many examples of exterminations.
The apparent total extinction of the Easter Island Palm Paschalococus disperta (Dransfield et al. Citation1984) is discussed in subsection 12.3. It is the only documented total extinction of a plant genus in the Late Quaternary and its extinction was almost certainly a direct or indirect result of human activity (Bennett Citation1997; Marchant et al. Citation2009). The only well documented example of a species extinction in the Late Quaternary is Picea critchfieldii (Jackson and Weng Citation1999) in the south-eastern United States. During the last glacial stage, Picea pollen occurred as far south as Louisiana (Jackson and Givens Citation1994) and the Florida panhandle (e.g. Watts et al. Citation1992) not with its usual contemporary boreal associates such as Larix laricina or Abies balsamea but with temperate deciduous trees. Whitehead (Citation1965) notes an unusually long-coned form of Picea in the south-east states. Jackson and Weng (Citation1999), in a detailed morphometric analysis of these cones, propose that this form is an extinct species, P. critchfieldii. During the Late Pleistocene it ranged from Louisiana to Georgia and as far north as Tennessee (Jackson and Weng Citation1999). In the Tunica Hills of Louisiana its pollen and macrofossils occur with remains of temperate deciduous trees such as Quercus, Fagus, Fraxinus, Carya, Juglans nigra, Acer, and Ulmus. It went extinct there about 15,000 years ago, at about the time that temperate deciduous associates were beginning to spread northward. Extant Picea species in eastern North America are boreal (P. glauca, P. mariana) or montane (P. rubens) and are associated with cool climates today. Judging by its Late Pleistocene associates, P. critchfieldii had a warmer tolerance than the extant species. However, its remains at a site in north-western Georgia occur with Pinus banksiana, P. resinosa, P. strobus, and Picea glauca between 22,000 and 15,000 years ago, suggesting that the climate response of P. critchfieldii may have overlapped in the past with P. glauca and Pinus banksiana (Jackson and Weng Citation1999). Mander et al. (Citation2014) show using apotome fluorescence microscopy that P. critchfieldii pollen is morphologically distinct from pollen of P. mariana, P. glauca, and P. rubens, so more information about its palaeoecology may soon become available.
Miller DM et al. (Citation2014) report macrofossils of Picea from last interglacial and early last-glacial sediments at Ziegler Reservoir in Colorado that cannot currently be assigned to any extant species. The taxon resembles extant P. pungens from Colorado in some features but differs in other characteristics. They suggest these fossils may represent ancestral P. pungens, an extinct variant of P. pungens, or an extinct sister species.
Watts (Citation1988) discusses the problems of documenting extinction in the European Quaternary flora with reference to Azolla tegeliensis (*), Eucommia ulmoides, E. europaea (*), Pterocarya fraxinifolia, P. ‘limburgensis’(*), P. hupehensis, and Picea omorikoides (*) and P. omorica. Species marked with an asterisk were originally described as extinct fossil taxa, but are now thought, on the basis of representative and extensive modern reference material and critical examinations, to be almost certainly minor morphological variations of extant species. Re-determination by Watts (Citation1988) of fossils of taxa previously thought to represent extinct taxa has shown that the seeds of the taxon Erica scoparia var. macrosperma at the Gort interglacial (≡ Holsteinian stage) in western Ireland (Jessen et al. Citation1959) are from Bruckenthalia spiculifolia (Menke Citation1970; but see Nelson EC Citation2009), a dwarf-shrub heath confined today to montane areas in the Balkans. Its seeds and its pollen have now been found widely in north-west Europe in interglacial and early Weichselian (last glacial stage) interstadials (e.g. Birks HJB and Peglar Citation1979; Whittington Citation1994; Granoszewski Citation2003). It represents a spectacular regional extermination.
Watts (Citation1971) shows that fossil seeds of ‘Micromenyanthes microsperma n. sp.’ from Gort (Jessen et al. Citation1959) are from an unrecognised species of Nymphoides, closely similar to the North American N. cordata but different from European N. peltata. They were subsequently redetermined by Watts (Citation1988) to be N. aquatica, a species today confined to the south-eastern US. As Watts (Citation1988, p.184) emphasises ‘the sheer difficulty of obtaining adequate reference material for pollen and macrofossils for the whole north temperate region of the world will be appreciated’. Such reference material is essential not only to identify fossil material but also to evaluate critically whether the fossils fall outside the range of modern reference material and hence may represent extinct taxa. For example, Tralau (Citation1962) illustrates the practical problems of distinguishing seeds of Late Pliocene Najas lanceolata (considered to be an extinct taxon) from extant N. tenuissima.
5.3. Regional exterminations
In contrast to the very few known instances of true extinctions of plants in the Quaternary, there are a vast number of exterminations (regional extinctions) or extirpations through the Quaternary as a result of changes in geographical ranges. Extermination is a concept that covers a very wide range of geographical scales ranging from continents, biogeographical regions, or even individual catchments of a lake studied palaeoecologically. It should also be emphasised that exterminations based on pollen identifications at the generic level may conceal a large number of species-level exterminations (Bennett Citation1997). Exterminations represent range shifts.
Europe lost many temperate tree genera that today are only found native in the warm-temperate/sub-tropical evergreen forests of south-eastern China or eastern North America (Box S2; e.g. van der Hammen et al. Citation1971; Tallis Citation1991; Latham and Ricklefs Citation1993; Lang Citation1994; Mai 1995; Martinetto Citation1999; Willis and McElwain Citation2014; Combourieu-Nebout et al. Citation2015; Magri et al. Citation2017; Martinetto et al. Citation2017) as a result of the many relatively cold stages in the Late Pliocene and Early Quaternary and of the west–east mountain chains (e.g. Alps, Pyrenees, Carpathians, Caucasus Mountains) and the Mediterranean Sea providing barriers to the southward retreat of the Arcto-Tertiary geoflora. Svenning (Citation2003) presents a detailed analysis and suggests that these Late Pliocene and Early Quaternary tree exterminations were largely driven by climatic factors.
The 73 tree genera present in the European Pliocene (according to van der Hammen et al. (Citation1971), Mai (Citation1995), and (Svenning Citation2003)) can be grouped into three – exterminated, relict and rare, and widespread (Box S2). The modern climatic tolerances of these groups are summarised in (based on Svenning Citation2003). Taxa widespread in Europe today tolerate a mean annual temperature <0.0°C whereas taxa with a tolerance >2.5°C are either regionally extinct in Europe or have southern relictual distributions (, Box S2). The relictual genera are concentrated in areas with mean annual precipitation < 270 mm yr–1 where they often occupy locally damp sites in gorges or along streams and rivers. Although the relictual genera are nearly as thermophilic as the genera now extinct in Europe, they are generally more drought tolerant than the exterminated genera (; see also Huang et al. Citation2015). Several relictual taxa favour unstable scree slopes and have a strong ability to sprout, in contrast to the widespread evergreen broad-leaved trees that grow on stable slopes and produce a dense canopy (Tang and Ohsawa Citation2002). Interestingly some of the exterminated tree taxa are spreading today after re-introduction into parts of Europe (e.g. Aesculus, Ailanthus, Cedrus, Robinia, Tsuga (Kleinbauer et al. Citation2010); Rhododendron ponticum (Birks HJB and Tinner Citation2016a)). Eiserhardt et al. (Citation2015) suggest that the exterminations of taxa in Europe (Box S2) during the Pliocene or Early Pleistocene may have resulted in significant and substantially larger than random losses of phylogenetic diversity in relation to cold tolerance. Taxa surviving in areas where there was strong extermination are phylogenetically more clustered, suggesting that non-random losses of phylogenetic diversity may be of increasing concern with increasing severity of extermination. These conclusions provide important warnings of potential further phylogenetic depauperation if future anthropogenic climate change affects taxa as it did in the Pliocene and Early Pleistocene (Eiserhardt et al. Citation2015).
Table 8. Comparison of modern climatic requirements of cool-temperate tree genera that grew in Europe in the Neogene but had been exterminated by the early Pliocene, that were present in Europe in the Neogene but have relictual disjunct distributions today, and that were present in the Neogene and are widespread in Europe today (based on Svenning Citation2003). Minimum growing degree days and the moisture index (actual evapotranspiration/potential evapotranspiration) are only available for genera in North America.
Besides trees, several genera of shrubs, wetland shrubs or herbs, and aquatics, as well as Proserpinaca cf. palustris were also exterminated from north-west Europe during the Neogene or Pleistocene (see ). See above for a discussion of species extinctions of aquatic plants in the Late Pliocene and Early and Middle Pleistocene.
Table 9. Selected genera or species other than trees that were exterminated from north-west Europe during the Neogene or Pleistocene. See Tralau (Citation1959, Citation1963a), van der Hammen et al. (Citation1971), Huckerby and Oldfield (Citation1976), Watts (Citation1988), Lang (Citation1994), Mai (Citation1995), Drzymulska (Citation2018), and Góis-Marques et al. (Citation2019) for details.
Exterminations of plants in the Quaternary were by no means confined to the Early or Middle Pleistocene or to Europe. A large number of exterminations of arctic and alpine plants occurred at the end of the last glaciation in eastern North America (e.g. Baker RG Citation1965; Leopold Citation1967; Watts Citation1967; Birks HJB Citation1976, Citation1981a), lowland mainland Europe (e.g. Tralau Citation1963b; Lang Citation1994; Birks HH Citation2008; Birks HJB and Willis Citation2008), and Britain and Ireland (e.g. Mitchell GF Citation1954; Godwin Citation1975; Birks HH Citation2008). Several plants that do not occur today in the central European mountain ranges formerly occurred in northern, central, or eastern Europe (Birks HJB and Willis Citation2008) including Cassiope tetragona, Diapensia lapponica, Koenigia islandica (Lang Citation1994), Papaver radicatum, Pedicularis hirsuta, P. lanata, Ranunculus hyperboreus, Salix polaris, Saxifraga cespitosa, S. rivularis, Silene uralensis, and S. furcata (Tralau Citation1963b; Godwin Citation1975; Birks HH Citation2008). There are several possible reasons for their absences in the central European mountains today. The most likely explanation is climate (Dahl Citation1998) as these species today generally have northern distributions in Scandinavia (including Svalbard) and/or are confined to high elevations, suggesting they have an intolerance of warm summer or winter conditions and/or a need for long day-lengths. The extermination of Larix sp. from Kotelny Island, northern Siberia after about 38,000 yr BP (van Geel et al. Citation2017) may be a result of a shift to long snowy winters and hence the development of moist tundra vegetation.
Exterminations of trees have also occurred in the Southern Hemisphere (Kershaw and McGlone Citation1996). For example, the tree flora of eastern Australia included Dacrydium, Phyllocladus, and the southern beech of the Nothofagus brassii-type group (Kershaw Citation1984; Bennett Citation1997). In New Zealand several genera have been exterminated since the Late Cretaceous including Gondwanan relicts such as the podocarps Microcachrys and Microstrobus and trees that reached New Zealand well after it became isolated but which have not persisted there to the present-day (e.g. Acacia, Casuarina, Eucalyptus) (Mildenhall Citation1980; Bennett Citation1997). The explanations for these exterminations are unclear as there is no obvious temporal patterns to the exterminations (Bennett Citation1997).
Holocene exterminations include Carpinus betulus from Iberia (Abel Schaad et al. Citation2014; Muñoz Sobrino et al. Citation2018), Larix sibirica from the Swedish Scandes Mountains (Kullman Citation1998, Citation2017), the aquatics Najas marina from Ireland (Watts Citation1978), N. minor from Denmark (Bennike et al. Citation2001), N. tenuissima from northern and central Finland (Tralau Citation1962), and Trapa natans from Britain (Flenley et al. Citation1975; Schofield and Bunting Citation2005), and Pinus sylvestris in the late Holocene of Ireland (Watts Citation1984; but see McGeever and Mitchell Citation2016), the Isle of Skye (Birks HJB and Williams Citation1983), the Outer Hebrides (Wilkins Citation1984), and elsewhere in Britain (Bennett Citation1984). As Bennett (Citation1995) discusses, several trees and shrubs have been exterminated during the Holocene on many islands of Britain and Ireland. These are discussed in subsection 12.3. Possible explanations for these Holocene exterminations are diverse and include climate change (L. sibirica, P. sylvestris, T. natans), human activity (P. sylvestris, T. natans), and habitat loss (C. betulus, N. marina, N. minor, N. tenuissima) (see Vargot et al. Citation2016). Marchant et al. (Citation2009) discuss in detail plant extinctions and exterminations in the Holocene and Turvey (Citation2009b) presents an overview of all Holocene extinctions, both plant and animal, many of which have a strong anthropogenic cause.
5.4. Conclusions
The most striking feature of plant extinctions (other than exterminations at a range of spatial scales) in the Quaternary (Leopold Citation1967; Bennett Citation1997; Botkin et al. Citation2007) is how relatively few non-anthropogenic species extinctions there have been – about 70 taxa, nearly all aquatic or telmatic species, in the Early and Middle Pleistocene and Picea critchfieldii as the only example in the Late Quaternary (Jackson and Weng Citation1999). There is only one known non-anthropogenic total plant extinction in the Quaternary – Aracites interglacialis in the Holsteinian stage (Field et al. Citation2017). Each total or species extinction and extermination will have its own taxon-specific explanation. One possible cause for the species extinction of P. critchfieldii and the regional extermination of many tree genera during the Late Neogene and through the Pleistocene (van der Hammen et al. Citation1971), and of many arctic and alpine taxa at the end of the last glacial stage (Birks HH Citation2008; Birks HJB and Willis Citation2008) is rapid climate change associated with abrupt transitions between a glacial stage and an interglacial stage (or vice versa). A key question is whether exterminations of taxa present in Europe during the Neogene and Early–Middle Pleistocene occurred during glacial or interglacial stages (Petit et al. Citation2008; Magri Citation2010). If exterminations had mainly occurred during interglacial stages, this would support pessimistic views about the impacts of future global warming on population and taxon survival (Petit et al. Citation2008). However, as Svenning (Citation2003) shows (see also ), extant Asian and American cogeners of extinct European tree taxa are less cold-tolerant than currently widespread European tree taxa. This suggests therefore that exterminations of these taxa may have occurred during glacial rather than interglacial stages (Petit et al. Citation2008). At present the available palaeoecological data are not sufficiently detailed stratigraphically to evaluate these contrasting scenarios. Another potentially important factor is increased climatic variability (Petit et al. Citation2008) during, for example, the Late Pliocene and Early Pleistocene (Willis et al. Citation2007c), the Mid-Pleistocene Transition (ca. 1.25–0.75 Ma) (Tzedakis et al. Citation2006; Postigo-Mijarra et al. Citation2010; Magri and Palombo Citation2013; Duval et al. Citation2015; Maslin and Brierley Citation2015; Chalk et al. Citation2017), and rapid interstadial climate changes such as short warming events (Huntley et al. Citation2013) and associated ‘cryptic biotic transitions’ (Cooper et al. Citation2015). However, in a detailed analysis of the disappearance of 12 tree taxa from southern Europe during the Quaternary, Magri et al. (Citation2017) find few temporal or spatial patterns in these exterminations.
Ecological forecasts using species distribution models predict that future global warming may cause widespread regional exterminations and even species or total extinctions (e.g. Thomas CD et al. Citation2004; Thuiller et al. Citation2005; Botkin et al. Citation2007). Wiens JJ (Citation2016) proposes on the basis of recent re-sampling studies that climate-related local exterminations are already widespread among plant (59%) and animal (50%) species. ‘Local’ extermination refers here to individuals of a given species being totally absent from a locality (e.g. a quadrat) in which the species occurred earlier. He notes that it can, however, be difficult to distinguish between ‘local’ extermination and a substantial decline in abundance that results in the species being undetected at the locality in a subsequent survey (Tingley and Beissinger Citation2009). The fossil plant record indicates that only one species underwent total extinction and about 70 aquatic and one tree species suffered species extinctions during the entire Quaternary despite evidence for major climatic changes during the last 2.6 million years. This discrepancy between such ecological forecasts and the fossil record is the so-called ‘Quaternary conundrum’ (Botkin et al. Citation2007; Mander et al. Citation2014). Understanding this conundrum (e.g. Bennett Citation1997; Citation2004; Jansson and Dynesius Citation2002; Willis and Niklas Citation2004) is critically important in developing realistic forecasts of the effects of future climate change on plant biodiversity (Botkin et al. Citation2007).
In eastern North America isopollen maps (e.g. Williams et al. Citation2004) show that tree taxa such as Abies balsamea, Larix laricina, Picea spp., and Pinus banksiana shifted their ranges northwards to the Great Lakes region and beyond in response to climate change after about 15,000 years ago (Ordonez and Williams Citation2013b), resulting in their extermination in southern and south-eastern states (e.g. Georgia) where they had grown during the LGM (e.g. Watts Citation1970). In Europe, trees similarly spread northwards from their macrorefugia in southern Europe at about this time but in contrast to North America, some populations remained in or near their refugial areas (Huntley and Birks Citation1983; Bennett et al. Citation1991; Hampe and Jump Citation2011). Exterminations of arctic, alpine, arctic-alpine, and boreal taxa in Europe and also in North America primarily involved range shifts and contraction of range, particularly at the southern edges of their range. Species-distribution modelling results predict that range-edge populations, not surprisingly, may be more susceptible to future climate change than central ‘core’ populations (Honnay et al. Citation2002). Such range-edge populations are often of important conservation value as they may be unique genetically or even represent endemic sub-species or varieties (e.g. Hampe and Arroya Citation2002; Hampe and Petit Citation2005; Willis and Birks Citation2006; Martín et al. Citation2008; Hampe and Jump Citation2011; Lepais et al. Citation2013; Lumibao et al. Citation2017; Erichsen et al. Citation2018).
Although there have been very few true extinctions of plants in the Late Quaternary, there have been many exterminations. Much remains to be understood about when and why and under what conditions these extinctions and exterminations occurred and about the ‘Quaternary conundrum’. Climate relicts or marginal populations that are isolated due to climate-driven range shifts preserve ecological and evolutionary histories that can span millennia. They can thus be viewed as ‘natural laboratories’ for understanding how long-term environmental change has impacted species and populations using modern genetic approaches (Woolbright et al. Citation2014). Such understanding is important if the Quaternary record is to be used as a source of information about the effects of future global warming on plants and on biodiversity. McElwain and Punyasena (Citation2007) comment in their discussion of mass extinction events and the plant record in Deep-time that ‘[m]odel projections of future biodiversity response to global climate change, based on modern plant distributions alone, are limited by the difficulty of scaling from individual species responses as independent and non-interacting entities to species responses within the ecological network of communities. In this respect, palaeobiological research has a strong advantage, providing empirical evidence of how whole communities were shaped by natural global warming events of the past. Perhaps the past could be a key to our future?’ Much information about extinctions and exterminations remains to be exploited from fossils in the Quaternary and Neogene (e.g. Saupe et al. Citation2015; Magri et al. Citation2017) and from models that incorporate adaptations, range-shifts, fragmentation, speciation, dispersal, and extinction in response to Late Quaternary climate change (Rangel et al. Citation2018).
6. Persistence, adaptation, and plasticity
6.1. Introduction
As discussed above, Quaternary pollen-analytical and plant-macrofossil records provide abundant evidence for plant spread and range expansion (‘migration’ Birks HJB Citation1989; Huntley and Webb Citation1989; Huntley Citation1991; Hobbs RJ et al. Citation2018) over large geographical areas (see subsections 4.4 and 4.5). These records also provide a small amount of evidence for true extinctions (subsection 5.2) and much evidence for exterminations and range shifts (subsection 5.3). The other major biotic response to environmental change at Quaternary time-scales is persistence (‘toleration’), possibly with a shift in habitat (Bennett Citation1997; Dawson TP et al. Citation2011; Hobbs RJ et al. Citation2018; Nogués-Bravo et al. Citation2018). As many modern plant populations display adaptive differentiation along latitudinal or elevational gradients (Davis MB and Shaw Citation2001), it is likely that past populations may have been similarly differentiated as they spread from their LGM macro- and microrefugia to their present ranges (Davis MB and Shaw Citation2001). There is increasing interest amongst global-change biologists, ecological geneticists, biogeographers, and palaeoecologists in the role of persistence, of evolutionary adaptation, and of phenotypic plasticity in influencing biodiversity patterns today and in relation to future environmental change (see for relevant references). Is there any Quaternary botanical evidence for persistence, adaptation, or plasticity in plants in relation to environmental changes during the Quaternary?
Table 10. Selected examples of publications on the role of persistence, evolutionary adaptation, and of phenotypic plasticity in influencing biodiversity patterns today and in the future.
6.2. Persistence
Some trees (e.g. Fitzroya cupressoides, Juniperus occidentalis, J. scopulorum, Pinus longeava, P. aristata, P. albicaulis. P. flexilis, P. heldreichii, Sequoiadendron giganteum, Taxodium distichum, Thuja occidentalis) have survived in situ for centuries or even millennia (all are over 1500 years old) and presumably have tolerated a wide range of environmental changes, including climate change at decadal, centennial, or even millennial scales (e.g. Molisch Citation1938; Schulman Citation1954; Lanner Citation2007; Sussman Citation2010; Piovesan et al. Citation2018). Several clonal alpine ‘cushion’ or ‘mat’ plants have also grown in situ for over 1000–2000 years (Körner Citation2003; Nagy and Grabherr Citation2009; de Witte and Stöcklin Citation2010). Some taxa have persisted over at least the last 21,000 years within or near their LGM refugia (e.g. Bennett et al. Citation1991; Nowak et al. Citation1994; Jackson et al. Citation2005; Magri et al. Citation2006; Birks HJB and Willis Citation2008) or at or near their mid-Holocene range-limit where they persist vegetatively (e.g. Pigott and Huntley Citation1978, Citation1980, Citation1981; Pigott Citation1981a, Citation1989, Citation1991, Citation2012). Several of the habitats where some long-lived taxa grow today are ‘extreme’, or ‘azonal’ habitats such as very dry shallow soils often on limestone, steep ravines and vertical rock-faces, rock outcrops, or very wet sites. It is possible that many of these taxa may have undergone habitat shifts over short distances (1-5m – 10 km; Scherrer and Körner Citation2010, Citation2011) to sites of different aspect, slope, topography, or elevation in response to environmental change (e.g. Dawson TP et al. Citation2011; Magyari et al. Citation2018; Orbán et al. Citation2018), as is occurring today in response to current environmental change (e.g. Visser and Both Citation2005; Lenoir et al. Citation2008, Citation2010; Bertrand et al. Citation2011; Lenoir and Svenning Citation2013, Citation2015; Wasof et al. Citation2013; Steinbauer et al. Citation2018).
6.3. Adaptation and plasticity
Adaptation as described by RA Fisher is the shift of a population towards a phenotype that best fits the current environment (Orr Citation2005; Brown SK and Blois Citation2016). Davis MB et al. (Citation2005) propose that such adaptive evolution may aid long-term persistence in response to environmental change and may permit the exploitation of new niches. At present, there is, as far as I know, no evidence to support this hypothesis for plants in the context of Quaternary or even Holocene time-scales. In contrast, there is strong evidence for functional trait evolution in ‘Deep-time’ (McElwain Citation2018). In ecological time-scales of 5–25 years there are examples of rapid adaptive response to environmental change (e.g. Leimu and Fischer Citation2008; Franks et al. Citation2014; Merilä and Hendry Citation2014), such as the impacts of drought on the flowering time of the annual Brassica rapa over a seven-year period (Franks et al. Citation2007). Other examples of rapid adaptation include Arabidopsis lyrata (Vergeer and Kunin Citation2013), A. thaliana (Ågren and Schemske Citation2012; Lasky et al. Citation2012; Brachi et al. Citation2013; Lau et al. Citation2014), and Pinus contorta (Rehfeldt et al. Citation1999).
Given the inherent taxonomic and morphological limitations of pollen-analytical and macrofossil records, it is currently not possible to identify, let alone distinguish, between adaptive evolutionary shifts and phenotypic plasticity as being important for in situ persistence, or for spreading and expansion in response to environmental change, or to discern the spatial and temporal scales at which these processes may play a role. It is likely, but as yet unproven, that they have been key processes in population persistence over long periods of climate change (Bradshaw WE and Holzapfel Citation2006; Merilä and Hendry Citation2014; Nogués-Bravo et al. Citation2018). A meta-analysis of extant plant populations (Merilä and Hendry Citation2014) suggests, however, that most responses to climate change have been mediated by phenotypic plasticity (e.g. Rehfeldt et al. Citation1999; Gienapp et al. Citation2008; Matesanz et al. Citation2010; Nicotra et al. Citation2010; Franks et al. Citation2014) rather than by adaptation.
Basic Quaternary botanical data from detailed macrofossil analyses can provide possible insights into allopatric speciation within the Middle or Late Quaternary. Kienast et al. (Citation2018) report a seed of Stellaria jacutica, endemic to Yakutia and the Russian Far East Magadan Oblast, in permafrost deposits 125 kyr old. S. jacutica is the only large-seeded Stellaria in north-east Siberia today but it is closely related to southern Siberian large-seeded Stellaria taxa centred on the Altai-Sayan mountains. Kienast et al. (Citation2018) propose that the ancestor of S. jacutica spread north during cold dry stages, became isolated, and allopatric speciation occurred prior to the last interglacial.
6.4. Conclusions
Clearly there are many unresolved and challenging questions relating to persistence and tolerance, adaptive evolution, and phenotypic plasticity in the context of Quaternary biology. Is adaptive evolution influenced by spread (see Wilson JRU et al. Citation2009; Clobert et al. Citation2012)? Conversely, is spread influenced by adaptive evolution or plasticity? Do tolerance, adaptive evolution, and spread interact in specific situations to reduce or increase the risk of local or regional extermination, or total extinction? These questions may one day be answerable thanks to the very rapid advances currently being made in high throughput next-generation sequencing of ancient DNA, in palaeogenetics, and in palaeogenomics (e.g. Fortes et al. Citation2013; Hofreiter et al. Citation2014; Parducci and Bennett Citation2017; Parducci et al. Citation2017; Schmid et al. Citation2017; Nogués-Bravo et al. Citation2018; Olajos et al. Citation2018; Taberlet et al. Citation2018) and applied, for example, to the ‘resurrection’ approach of Project Baseline (Franks et al. Citation2007; Shaw RG and Etterson Citation2012; Etterson et al. Citation2016) involving ancestral seeds of known age (e.g. Franks et al. Citation2007). This approach has been very effective with, for example, Daphnia spp. ephippia preserved in dated lake sediments (e.g. Hairston et al. Citation1999, Citation2005; Kerfoot et al. Citation1999; Limburg and Weider Citation2002; Kerfoot and Weider Citation2004; Brede et al. Citation2009; Orsini et al. Citation2012, Citation2013a, Citation2013b, Citation2016). All these new developments and approaches should, whenever possible, be linked to detailed multi-proxy palaeoecological studies so as to maximise the use of ‘[a]ncient DNA to understand evolutionary and ecological processes’ (Orlando and Cooper Citation2014). Ideas on the role and rate of evolutionary processes in ecological systems are also changing (see Lallensack Citation2018) – 'Seeing such rapid evolution in action has changed ecologists’ picture of what they thought was a predictable and fundamental ecological process. … Everything about ecology has to be re-examined in light of the fact that evolution is more important than we thought,’ [Stephen Ellner].
Having discussed the three major types of biotic response to environmental change (Bennett Citation1997; Nogués-Bravo et al. Citation2018) – distributional range shifts, extinction, and persistence and adaptation – I now consider Quaternary botany’s contributions to ideas on ecological niche theory, no-analogue assemblages, the nature of vegetation, and tree and forest dynamics.
7. Realised environmental space, potential niches, and no-analogue pollen assemblages
7.1. Niche theory
Jackson and Overpeck (Citation2000) and Jackson (Citation2000) (see also Ackerly Citation2003, Citation2015) add to conventional ecological niche theory the concept of realised environmental space and hence of the potential niche. The concept is derived from Quaternary botanical and palaeoclimatological data and is an important addition to basic niche theory. This section draws extensively on Jackson and Overpeck (Citation2000), Jackson (Citation2000), and Jackson and Williams (Citation2004).
Hutchinson (Citation1957, Citation1978) conceptualises the niche of a species as a multidimensional space or hypervolume whose n dimensions are defined by the environmental variables that influence the fitness of individuals of the species (Jackson and Overpeck Citation2000; Colwell and Rangel Citation2009). The variables that form the dimensions may include resources consumed or used by individuals (‘Eltonian niche’; Elton Citation1958), or non-resource variables that directly or indirectly affect individuals. Jackson and Overpeck (Citation2000) focus on non-resource variables and thus define a species niche as being similar to Leibold’s (Citation1996) requirement niche, Whittaker et al.’s (Citation1973) habitat hyperspace, Hutchinson’s (Citation1978) scenopoetic niche, Grinnell’s (Citation1917, Citation1924) niche (James et al. Citation1984; Soberón Citation2007; Wake et al. Citation2009), and Austin et al.’s (Citation1990, Citation1997) environmental niche. Most niche theory assumes that sites exist in the natural world that cover all possible combinations of environmental niche variables (cf. Austin Citation1990, Citation1992, Citation1999), although Hutchinson (Citation1957) comments on possible exceptions. Griesemer (Citation1992) and Holt (Citation2009) emphasise that the Hutchinsonian niche concept is static and does not consider temporal changes in the environment and in population responses. Environmental changes can, however, lead to the emergence and disappearance of combinations of environmental niche variables (Jackson and Overpeck Citation2000). Blonder (Citation2016, Citation2017) and Blonder et al. (Citation2014, Citation2017a) revisit Hutchinson’s n-dimensional hyperspace and develop new approaches for delineating the boundaries and probability densities within such hypervolumes, thereby making the hyperspace concept potentially more operational in a range of questions. Blonder (Citation2017) discusses the many applications of the hypervolume concept including how climate change can limit the overall climate space and realised niches of species over time, as explored by Jackson and Overpeck (Citation2000) and Jackson (Citation2000).
Conventional niche theory distinguishes the fundamental niche of a species as a subset of the environmental space defined by the n dimensions of the hypervolume and comprises all the combinations of variables that permit survival and reproduction of individuals (Hutchinson Citation1978). The regeneration niche of Grubb (Citation1977) is the fundamental niche for successful regeneration, establishment, reproduction, and persistence (Blonder Citation2017) and is particularly relevant when considering dynamics and range limits of species as well as the adult-survival niche (see Jackson et al. (Citation2009b) for the role of the regeneration niche in considering species responses to climate change). In conventional niche theory, the actual niche space occupied by a species is a subset of the fundamental niche – the realised niche – constrained by biotic factors that may prevent individuals of a species from occurring in parts of its fundamental niche (Jackson and Overpeck Citation2000; Blonder Citation2016).
Jackson and Overpeck (Citation2000) and Jackson (Citation2000) emphasise that large parts of environmental space in the Hutchinsonian niche concept may be empty and not represented in the real world at any given time. Empty environmental space can arise for several reasons (Jackson and Overpeck Citation2000; Blonder Citation2016). First, spatial heterogeneity in geology, soils, topography, etc. results in not all combinations of variables being able to occur (Jackson and Overpeck Citation2000). Second, many niche variables co-vary. Winter and summer mean temperatures are highly correlated, whereas summer temperature and annual precipitation do not occur in all possible combinations. Some combinations, for example extremely low temperature and high precipitation, are physically impossible in the current Earth system (Jackson Citation2000; Jackson and Overpeck Citation2000). Third, the frequency and intensity of environmental change in, for example, the Quaternary inevitably leads to parts of the environmental space drifting between being empty or full, resulting in so-called ‘nomadic niches’ (Jackson and Overpeck Citation2000).
The absence of certain environmental combinations constrains niche shape and size (Jackson and Overpeck Citation2000). A finite set of combinations of the n variables critical for a species will exist at any given time. Jackson and Overpeck (Citation2000) and Jackson (Citation2000) term the particular realisation of environmental conditions that occur in nature at a certain time the realised environmental space (RES) and the intersection of the species’ fundamental niche with the RES as the potential niche for the species at that time (.1). Species differ in their fundamental niches and different species respond to different environmental variables. As a result, particular species combinations may develop or disappear as the environment changes (.2; Jackson and Overpeck Citation2000).
Figure 15. (1) Fundamental, potential, and realised niches of a species in response to two environmental variables. The realised niche is where populations of the species actually occur and is a subset of the potential niche constrained by biotic, abiotic, and other factors. The potential niche is where the fundamental niche intersects with the realised environmental space at a particular time. (2) A schematic representation of how changes in the realised environmental space between time 1 and time 2 can affect species co-occurrences. At time 1, the potential niches of species 1 and species 2 overlap and the species can potentially co-occur at sites within the intersection. At time 2, the potential niches of the two species do not overlap and hence they will not co-occur in the realised world. (3) The four modes of population response of five species (a-e) to environmental change. Species a mode 1: persistence. Species b modes 1 and 2: shift within local habitat conditions. Species c modes 1, 2, and 3: spread to distant newly-suitable sites and disappear from some former sites. Species d modes 1 and 4: widespread extirpation without colonisation of new areas, thereby changing from being a widespread species to a local or rare species. Species e is the inverse of species d where a formerly rare species colonises extensive new areas. Redrawn from Jackson and Overpeck (Citation2000).
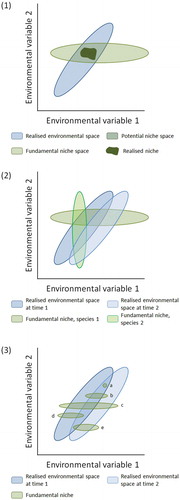
Advances in palaeoclimatology (e.g. Ruddiman Citation2013a; Bradley Citation2015) show that climate changes continually at all time-scales and that climate is always changing. Jackson and Overpeck (Citation2000) provocatively suggest that the term ‘climate change’ is redundant as climate is always changing in space and time. A particular realisation of climate viewed as a multivariate entity at a given time and place is rarely, if ever, repeated. Each point in time represents a unique combination of climate variables and gradients, along with changing concentrations of atmospheric CO2 and of variables such as other trace gases and volcanic and dust aerosols that can all influence climate (Jackson and Overpeck Citation2000).
Jackson and Overpeck (Citation2000) and Jackson (Citation2000) use their model of RES and potential niches to make several predictions. The potential niche of a species will change in size, shape, and position as the RES changes. Individuals of different species can only co-occur when their potential niches overlap (.2). Species that have relatively slow evolutionary response times may track environmental change by persistence (‘tolerance’ sensu Jackson and Overpeck (Citation2000); see subsection 6.2), migration (see subsection 4.4), or extinction (see section 5; Bennett Citation1997; Nogués-Bravo et al. Citation2018). In contrast, species with relatively rapid evolutionary response times may track environmental changes by adjustments in their fundamental niche. There is, however, little evidence for this in terrestrial plants during the Quaternary (see subsection 6.3). Particular species combinations (‘associations’ sensu Jackson and Overpeck Citation2000) may develop under some environmental conditions and then disappear as the environment changes (.2). Pairs or groups of species with similar fundamental niches and rapid evolutionary response times may track environmental change by parallel adjustments in their fundamental niches (Jackson and Overpeck Citation2000). There is, however, no evidence for this in terrestrial plants within the Quaternary.
This model suggests that there are four major modes of biotic response to environmental change in the context of the Quaternary (.3; Jackson and Overpeck Citation2000; Dawson TP et al. Citation2011).
Populations stay in place (persistence or toleration) (‘stayers’ Hobbs RJ et al. Citation2018)
Populations shift along local habitat gradients (e.g. elevation, soil moisture)
Populations spread to, colonise, and expand in suitable areas and may decrease in or even disappear (extirpation) from formerly occupied areas, resulting in range shifts (‘movers’ Hobbs RJ et al. Citation2018)
Populations undergo local extermination (extirpation) due to mortality and lack of regeneration and fail to colonise new territory, resulting in range contractions or even extinction not only locally, but also regionally.
There may be lags in all these responses (Ordonez Citation2013)
Pollen-analytical and other palaeobotanical data provide examples of all four modes in the context of the Holocene and other interglacials and of modes 1, 2, and 4 for late-glacial ecological dynamics (see Jackson and Overpeck (Citation2000) and subsections 4.4, 5.3, 6.2, 6.3, and 9.6 for examples). summarises the likely frequency of these four modes of response in species populations and species combinations (‘associations’) within Europe and North America during the Late Quaternary. Of course, as shown in .3, the four modes are not necessarily independent.
Table 11. The frequency of the four modes of response in species populations and species combinations (‘associations’) within Europe and North America during the late-Quaternary.
Ackerly (Citation2015) discusses from a modern ecologist’s viewpoint the importance of Jackson and Overpeck’s (Citation2000) ideas of realised environmental space and potential niches. For Ackerly, the key idea in Jackson and Overpeck (Citation2000) is that not all environments exist at a given point in time at regional or global scales. It is thus essential to consider broad-scale climate patterns in climate space, the analogue to Hutchinson’s niche-space concept (Citation1957, Citation1978). The fact that not all conditions or combinations exist has, as Ackerly (Citation2015) emphasises, profound implications for understanding biotic distributions. Hutchinson (Citation1957, Citation1978) clarifies the distinction between the fundamental and the realised niche and thus the roles of abiotic and biotic factors as determinants of niche breadth and hence geographical distributions. Obviously, field observations of where a taxon occurs only tell us about the dimensions and breadth of its realised niche. Jackson and Overpeck’s (Citation2000) demonstration that not all conditions exist means that it is not possible to know the past or future climatic limits of the realised or fundamental niche in certain parts of modern climate space. This creates a major limitation in extrapolating from modern distributions into potential distributions under past or future climates (Ackerly Citation2003, Citation2015), using, for example, species distribution modelling.
7.2. No-analogue pollen assemblages
7.2.1. Identification and interpretation
No-analogue assemblages are fossil pollen assemblages that do not resemble modern pollen assemblages and hence lack modern analogues. In the last 50–60 years, analogue analysis has been a major approach to the interpretation of fossil assemblages in terms of past vegetation and/or environment (e.g. Birks HJB Citation1973; Birks HJB and Birks Citation1980; Overpeck et al. Citation1985; Jackson and Williams Citation2004). Such an analysis consists of two parts: (1) a comparative part where modern and fossil pollen assemblages are compared qualitatively or quantitatively to identify matching assemblages and to assess their degree of similarity, and (2) an interpretative part where the assumption is made that the relationships between modern pollen assemblages and the vegetation they are derived from (and more indirectly the environment in which the modern vegetation grows) are the same as between fossil pollen and past vegetation (and environment) (Jackson and Williams Citation2004). There are several ways of conducting analogue analyses. These range from visual comparisons to multivariate numerical matching procedures (e.g. Overpeck et al. Citation1985, Citation1992; Gavin et al. Citation2003; Jackson and Williams Citation2004; Lytle and Wahl Citation2005; Williams and Shuman Citation2008; Simpson GL Citation2012). The basic requirement for all these methods is collections of modern pollen assemblages sampled from the same sedimentary environment (e.g. lakes) that the fossil assemblages of interest are derived from (e.g. Whitmore et al. Citation2005; Gonzales et al. Citation2009a; Davis BAS et al. Citation2013; Williams et al. Citation2018).
Iversen’s (Citation1954; see also Iversen Citation1967, Citation1973) classic reconstruction based on very detailed pollen analyses of the Danish late-glacial suggests that the late-glacial flora, vegetation, landscape, and environment had no widespread modern analogues. This idea of the unique floristic, vegetational, and environmental nature of the late-glacial and the lack of any widespread modern analogues is supported by results from later studies on late-glacial and LGM sediments elsewhere in Europe, in North America, and South America (e.g. Cushing Citation1963, Citation1965; Birks HJB Citation1976, Citation1981a; Graham RW and Grimm Citation1990; Jackson and Williams Citation2004; Gonzales et al. Citation2009b; Correa-Metrio et al. Citation2012a; Birks HJB and Berglund Citation2018).
Jackson and Williams (Citation2004) and Simpson GL (Citation2012) discuss the problems in convincingly recognising no-analogue fossil assemblages. Despite developments in the numerical quantification of the dissimilarity between fossil and modern assemblages, such distance metrics do not indicate if two assemblages are dissimilar enough to be considered non-equivalent or non-analogous (Jackson and Williams Citation2004). The critical question is what level of dissimilarity is appropriate to conclude the assemblages are not analogous. Various approaches have been developed to establish critical dissimilarity values between modern and fossil assemblages and hence to identify possible no-analogue fossil assemblages and, at the same time, for the critical value to minimise the number of false negative and false positive matches (Wahl Citation2004). These approaches include examining the numerical distribution of the dissimilarities, Monte Carlo resampling, detecting ‘jumps’ or breaks in the ordered dissimilarities, receiver operating characteristic (ROC) curves, and logistic regression modelling (Jackson and Williams Citation2004; see Overpeck et al. Citation1985; Waelbroeck et al. Citation1998; Gavin et al. Citation2003; Oswald et al. Citation2003; Williams Citation2003; Sawada et al. Citation2004; Wahl Citation2004; Lytle and Wahl Citation2005; Simpson GL Citation2012 for technical details).
Jackson and Williams (Citation2004) also discuss the problems of false-negative matches that suggest that no modern analogous pollen assemblage exists when, in reality, an analogue exists and of false-positive matches that suggest that a modern analogue exists when, in reality, no analogue exists. In the case of false-negatives, they propose in light of HH Birks’ (Citation2003) critique of the interpretation of late-glacial pollen assemblages in Scotland, western Norway, and Minnesota as being non-analogous, that there are three alternative hypotheses that might explain no-analogue pollen assemblages (Jackson and Williams Citation2004).
A ‘deep no-analog’ hypothesis where the pollen assemblage reliably reflects groups of plant taxa growing together in the same habitat that do not occur together today (e.g. Ostrya, Picea, and Ulmus in the Minnesotan late-glacial (e.g. Cushing Citation1963); Armeria maritima, Astragalus alpinus, Helianthemum, and Polemonium caeruleum in the north-west European late-glacial (e.g. Godwin Citation1975)).
A ‘shallow no-analog’ hypothesis where the fossil pollen assemblages reflect anomalous landscapes or pollen-source areas in which individual vegetation types resemble modern vegetation types in composition, but with a spatial arrangement of vegetation types (density, size, mosaic structure) unlike any today (e.g. the Danish late-glacial as envisaged by Iversen (Citation1954, Citation1967, Citation1973) or the central Minnesotan late-glacial as reconstructed by Birks HJB (Citation1976)).
A ‘no no-analog’ hypothesis where the fossil pollen assemblages reflect anomalously steep gradients in regional vegetation and climate, resulting in the close proximity of different vegetation types that today are widely separated, leading to long-distance pollen dispersal and resulting in the occurrence of, for example, pollen of temperate deciduous trees and prairie herbs in Picea-dominated pollen assemblages in the central Minnesotan late-glacial (e.g. Cushing Citation1963; Birks HJB Citation1976).
When late-glacial pollen and plant-macrofossil assemblages are studied together in detail, the pollen assemblages often appear to have no convincing modern analogues whereas the macrofossil assemblages usually closely resemble the composition of modern vegetation types (e.g. Baker RG Citation1965; Birks HJB Citation1976; Birks HH and Mathewes Citation1978; Birks HH Citation1984, Citation2003; Jackson et al. Citation2000b; Mortensen et al. Citation2011, Citation2014). This supports the ‘no no-analog’ hypothesis of Jackson and Williams (Citation2004). If this is correct, far-distant transported extra-regional pollen may well represent a larger component of late-glacial pollen assemblages than is generally envisaged (Fægri Citation1981).
Jackson and Williams (Citation2004) also discuss false-positive matches between fossil and modern pollen assemblages. These arise when the fossil pollen assemblages resemble modern assemblages but other evidence such as plant macrofossils suggest that past vegetation as a whole lacks modern analogues despite there being positive fossil and modern pollen matches. Examples of such false-positives are frequent in unglaciated eastern North America during the LGM (20,000–23,000 yr BP) (Jackson et al. Citation2000b; Jackson and Williams Citation2004). LGM pollen assemblages there are dominated by Picea, Pinus, and Quercus, along with herbs and low amounts of pollen of Carya and other deciduous trees (Jackson et al. Citation2000b). In contrast, contemporaneous macrofossils from Georgia, Louisiana, Mississippi, and Tennessee include Betula papyrifera, Carpinus caroliniana, Carya spp., Fagus grandifolia, Juglans nigra, and Ulmus americana, and the extinct Picea critchfieldii (Jackson et al. Citation2000b; Jackson and Williams Citation2004). Jackson and Williams (Citation2004) present several hypotheses to explain these false-positive matches – the ‘deep no-analog’, the ‘shallow no analog’, and low pollen production of deciduous trees relative to Pinus and Picea in response to the lowered CO2 atmospheric concentrations during the LGM (180–200 ppmv). These lowered concentrations may have imposed a severe limitation on the carbon budgets of plants, resulting in shifts in resource allocation and reproductive strategies (Jackson et al. Citation2000b; Jackson and Williams Citation2004). See Dippery et al. (Citation1995) and Ehleringer et al. (Citation1997) for examples of the impacts of lowered and elevated CO2 values on C3 and C4 photosynthetic pathways, and Cowling (Citation1999), Cowling and Sykes (Citation1999), and Harrison and Prentice (Citation2003) for discussions of the role of lowered CO2 concentrations on LGM vegetation and plant–climate interactions.
It is surprising that available floristic information provided by plant macrofossils is often ignored in deciding that pollen assemblages lack modern pollen analogues and by inference lack modern vegetational analogues. For example, Finsinger et al. (Citation2017b) suggest, on the basis of pollen assemblages alone, that Allerød late-glacial and early-Holocene assemblages in much of Europe have high similarity (i.e. are analogous) with pollen assemblages from pre-industrial cultural landscapes. They suggest that this might be a result of low pollen-taxonomic resolution within many herbaceous taxa (e.g. Asteraceae, Cyperaceae, Poaceae), thereby reducing the ability to distinguish pollen assemblages produced by different species assemblages. Alternatively, they propose that this apparent analogousness may be a result of many late-glacial pollen assemblages containing pollen of steppe taxa and thus these assemblages resemble late-Holocene pollen assemblages because steppe elements increased again following forest clearance and with agriculture. However, available plant-macrofossil data clearly show that many of the herbaceous taxa of the late-glacial (e.g. Bistorta vivipara, Saxifraga spp., Silene acaulis, Thalictrum alpinum; Iversen Citation1973; Godwin Citation1975; Lang Citation1994; Birks HH Citation2008) are very different from the ruderal flora of Europe today.
As discussed by Jackson and Williams (Citation2004), analogue analysis consists of two parts: (1) a comparative part where modern and fossil pollen assemblages are compared, and (2) an interpretative part where an analogous fossil pollen assemblage is interpreted to reflect the modern vegetation that produced the matching modern assemblage (what Jackson and Williams (Citation2004) term ‘singularity’). The situation can arise of convergence (sensu Jackson and Williams Citation2004) when several different vegetation types may produce similar modern pollen assemblages or of divergence (sensu Jackson and Williams Citation2004) when one vegetation type may produce different modern pollen assemblages. Clearly convergence and divergence can create problems in the interpretative part where fossil pollen assemblages are interpreted, via modern analogues, in terms of past vegetation. Alternatively, a non-analogue fossil assemblage is assumed to reflect past vegetation that does not resemble a modern vegetation type. As discussed above, false-negative no-analogues and false-positive analogues create major problems in interpreting the pollen assemblages in terms of past vegetation because of ‘deep no-analog’, ‘shallow no-analog’, and ‘no no-analog’ situations. As Jackson and Williams (Citation2004) note, ‘[a]n important goal for paleoecologists should be to devise studies capable of discriminating among these three hypotheses’.
7.2.2. How do no-analogue pollen assemblages arise?
No-analogue fossil pollen assemblages and by assumption past vegetation have long been interpreted as a response to unusual no-analogue ecological and biogeographic factors (Jackson and Williams Citation2004). These factors include lags in spreading or population expansion (Watts Citation1973; Davis MB Citation1976, Citation1981a, Citation1986a; Birks HJB Citation1986, Citation1989), soil development, climate (Webb T Citation1987; Minckley et al. Citation2019), geographical barriers (Watts Citation1980), dispersal limitation (Bennett Citation1988b, Citation1997), megafauna (Watts Citation1977), the location of LGM macro- and microrefugia (West RG Citation1964, Citation1980), and a combination of factors (Cushing Citation1963; Wright Citation1984).
Jackson and Williams (Citation2004) present an alternative and more general hypothesis for the occurrence of no-analogue pollen assemblages and vegetation types based on the existence of unique environmental conditions and gradients and on the theory of realised environment space (RES) and potential niches ( and above) developed by Jackson (Citation2000) and Jackson and Overpeck (Citation2000) (see also Webb T Citation1987; Williams et al. Citation2001, Citation2004). Individual taxa have unique responses to the multivariate environmental and biotic factors that define the n dimensions of the hypervolume that contains the realised and potential niches of the taxa. Taxa can occur together if their fundamental niches overlap and if this overlap falls within the RES at a particular time (.2). If the RES changes, the potential niches of taxa may no longer overlap with the RES, and the composition of the vegetation assemblage may change (.3). Past pollen and hence vegetation assemblages with modern analogues may occur in areas and at times when the RES matches environmental conditions somewhere on Earth today (Jackson and Williams Citation2004). Past pollen and vegetation assemblages lacking modern analogues would have occurred in areas and at times when the RES had no modern counterpart. Taxa with potential niches that overlap with the past RES would form no-analogue vegetation types and potentially no-analogue pollen assemblages.
The realised environmental space and hence the potential niche of Jackson (Citation2000) and Jackson and Overpeck (Citation2000) can be generalised along the lines of the realised niche of a species to include biotic variables that may prevent individuals of a taxon from occurring in parts of its fundamental niche and limit its potential niche. As discussed in subsection 13.2, late-glacial no-analogue pollen assemblages in Indiana and Ohio (Gill et al. Citation2009, Citation2012) may have resulted not only from regional climate change but also from the loss of keystone megaherbivores releasing palatable hardwood trees such as Ostrya/Carpinus and Fraxinus nigra-type from herbivore pressure and intensified fire regime (see subsection 13.2). At Cupola Pond in Missouri (Jones RA et al. Citation2017) there are markedly no-analogue pollen assemblages between 17,000 and 11,000 yr BP but there are almost no dung-fungus spores such as Sporormiella-type and very low charcoal values, suggesting that the no-analogue pollen assemblages there did not result from megaherbivore pressure, grazing, or disturbance, or fire. They probably resulted from no-analogue climatic conditions in the RES due to different solar insolation values and increased seasonality in the late-glacial (see Williams et al. Citation2001; Williams and Jackson Citation2007). The rarity of Sporormiella-type spores, an indicator of megaherbivores, is also matched at late-glacial sites in Kentucky and Tennessee where no-analogue pollen assemblages occur (Liu et al. Citation2013).
gives suggested drivers within the RES that might have induced no-analogue pollen assemblages and possibly no-analogue vegetation types during different stages in the Late Quaternary of north-west Europe and eastern North America. As RA Jones et al. (Citation2017) conclude ‘[b]etter-constrained pollen chronologies … as well as independent paleoenvironmental proxies (charcoal, Sporormiella, paleotemperature, lake-level) should help reveal the biogeographic and ecological mechanisms underlying the late-glacial transition’ with its ‘complexity of the late-glacial no-analog communities’.
Table 12. Suggested drivers within the realised environment space (RES) resulting in no-analogue pollen assemblages and possible no-analogue vegetation types during different stages in the late-Quaternary in north-west Europe and eastern North America.
The existence of no-analogue pollen assemblages and, by inference, no-analogue vegetation assemblages in the past has important ecological, evolutionary, and conservation implications that are now being considered in ecological theory (Ackerly Citation2003; Jackson and Blois Citation2015), dynamic modelling (Veloz et al. Citation2012; Svenning and Sandel Citation2013; Ordonez and Williams Citation2013a; Reu et al. Citation2014), and conservation science (Williams et al. Citation2013a, Citation2013b; Ordonez et al. Citation2016) (see also subsection 11.3.7).
7.2.3. Future no-analogue ecosystems
The demonstration of no-analogue pollen assemblages and the careful evaluation of probable no-analogue past floras and vegetation types in response to unique changes in the realised environmental space is stimulating ecologists and conservation biologists to consider the idea of ‘no-analogue ecosystems’ developing in the near future in response to the rapid and on-going changes in climate, land-use, nitrogen deposition, biological invasions, biotic interactions, and disturbance regimes (e.g. Flessa and Jackson Citation2005a; Jackson Citation2007, Citation2013b; Williams and Jackson Citation2007; Williams et al. Citation2007; Hobbs RJ et al. Citation2009, Citation2013; Bridgewater et al. Citation2011; Buckley Citation2013; Radeloff et al. Citation2015; Mahony et al. Citation2017).
The recognition of such ecosystems today is (and will be in the near future) much easier than the recognition of no-analogue or novel vegetation types in the past, as present systems and their constituent species can be directly observed. However, despite this ease of recognition, the existence of novel ecosystems today and their development in the future create new challenges in, for example, restoration ecology (e.g. Higgs ES et al. Citation2014; Higgs EC and Jackson Citation2017); management (e.g. Ordonez et al. Citation2014; Morelli et al. Citation2016); species, ecosystem, and landscape conservation (e.g. Hobbs RJ et al. Citation2014b; Armsworth et al. Citation2015; Jackson Citation2016; Backstrom et al. Citation2018); predictive ecology and biogeography (e.g. Ohlemüller et al. Citation2006; Beckage et al. Citation2011; Buckley Citation2013); ecological theory (e.g. Radeloff et al. Citation2015); species modelling (e.g. Veloz et al. Citation2012; Williams et al. Citation2013a; Catullo et al. Citation2015); and ecological dynamics (e.g. Jackson and Williams Citation2004; Hobbs RJ et al. Citation2018) (see subsection 11.3).
7.3. Conclusions
The concepts of realised environmental space and potential niches as presented by Jackson and Overpeck (Citation2000) are proving to be important tools in understanding no-analogue pollen and macrofossil assemblages and the determinants of vegetation composition at broad spatial and temporal scales and are an essential addition to contemporary niche theory in ecology and biogeography (Ackerly Citation2003, Citation2015; Feeley and Silman Citation2010). The understanding of how no-analogue assemblages arose in the past and hence how they may arise in the future is an important contribution of Quaternary botanical studies to ecology and biogeography.
8. The nature of vegetation, the community concept, and Quaternary botany
In this section, I consider the contributions of Quaternary botany to our understanding of the nature of vegetation, the community concept, and the organisation of community composition, and discuss whether pollen assemblages reflect ‘communities’ in the sense of modern communities as recognised by ecologists. This section draws, in part, on Jackson (Citation2000) and Jackson and Overpeck (Citation2000).
8.1. Clements, Gleason, and palynological views on the nature of vegetation
Early ideas on the post-glacial history and movement of plants (e.g. Clements Citation1904; Cowles Citation1911; Gleason Citation1923) considered these as zonal processes with latitudinal, longitudinal, and elevational shifts of an entire and identical vegetation type or plant association that can be recognised today (Jackson and Overpeck Citation2000). Clements (Citation1916 – see Eliot Citation2007) formalised this idea in his clisere concept – a temporal series of associations corresponding to those found along a spatial gradient today. In the same year, von Post (Citation1916) presented pollen analysis of peat as a means of reconstructing temporal changes in pollen assemblages but often in a spatial context (e.g. Fries M Citation1967; Birks HJB and Berglund Citation2018; see ). Von Post, like all his colleagues at the time, assumed that pollen and the vegetation that produced the pollen are a reflection of regional climate and that changes in pollen stratigraphy are therefore a response to climate change (von Post Citation1946; Birks HJB Citation2008). Pollen analysts in the first 50 years of their subject were clearly Clementsian in their ideas about the nature of vegetation, as were many modern ecologists until the early 1960s. Clementsian ideas continue in global ecological research involving global or continental biome models based on plant functional type, physiology, and dominance, soil properties, and climate (e.g. Prentice et al. Citation1992; Bradshaw RHW and Sykes Citation2014).
Many plant sociologists until the 1960s considered vegetation to consist of tightly co-evolved associations (Kohler Citation2008) that would move and behave as an integrated unit. The ideas of Gleason (Citation1917, Citation1926, Citation1927, Citation1939; see Matthews Citation1996; Nicolson and McIntosh Citation2002; Eliot Citation2007) of individualistic behaviour of species primarily in response to broad-scale environmental variables, with some stochastic factors of dispersal, colonisation, and establishment operative at fine scales in space and time (Jackson and Overpeck Citation2000), were largely ignored (see McIntosh Citation1975; Nicolson Citation1990; Matthews Citation1996) until the work of Curtis (Citation1959) and his ‘Wisconsin school’ with its continuum concept of vegetation (see McIntosh Citation1993; Nicolson Citation2001) and, most particularly, of Whittaker RH (Citation1951, Citation1953, Citation1956, Citation1960, Citation1967; see Westman and Peet Citation1982) and his development of direct gradient analysis.
RH Whittaker’s (Citation1956, Citation1960) demonstration that species do not turn over together along environmental gradients in the Great Smoky Mountains or the Siskiyou Mountains refuted Clements’ (Citation1916) clisere concept. It initiated a paradigm shift in how many modern ecologists viewed species occurrences in vegetation by proposing a new community concept as a ‘loosely ordered complexity’ (Whittaker RH Citation1967), in contrast to Clementsian ideas of communities as distinct integrated units in time and space. This loosely ordered complexity resulted in the continuum concept of vegetation being more widely adopted (McIntosh Citation1967; see also Dansereau et al. Citation1968 for responses). Palynologists such as Fægri (Citation1947, Citation1963), Iversen (Citation1954, Citation1960), Andersen ST (Citation1961), and Cushing (Citation1963, Citation1965) proposed that as plants react individualistically to their environment (and to other biota) today and not as collective units such as integrated communities, approaches to interpreting pollen-analytical data should focus on individual taxa, their ecological tolerances, and the extent to which they are in equilibrium with their present environment (Cushing Citation1963, Citation1965).
By the late 1970s and 1980s many Quaternary palaeoecologists generally agreed that taxa respond individualistically to the major environmental changes of the Late Quaternary (Jackson Citation2000). Taxa that occur today appear to have had different centres of distribution during the LGM and apparently spread and expanded at different rates and in different directions during the Holocene (e.g. Firbas Citation1949; Iversen Citation1950; Davis MB Citation1976, Citation1981a; Huntley and Birks Citation1983; Birks HJB Citation1989). Many modern vegetation types have no long history (3000–6000 years maximum) (e.g. Webb T Citation1987, Citation1988a; Huntley Citation1990a, Citation1990b; Overpeck et al. Citation1992; Jackson Citation2006a; see subsections 9.8, 11.2, and 11.3) and vegetation composition and patterns along elevational gradients changed markedly and in an individualistic way during the Holocene (e.g. Jackson and Whitehead Citation1991; Spear et al. Citation1994; Jackson et al. Citation2005; Hobbs RJ et al. Citation2018). No-analogue pollen and macrofossil assemblages are widely documented in the early Holocene, the late-glacial, and earlier interglacials (e.g. Cushing Citation1963, Citation1965; West RG Citation1964; Birks HJB Citation1976, Citation1981a; Jacobson GL et al. Citation1987; Overpeck et al. Citation1992; see subsection 7.2). The interpretations of how these patterns and dynamics had been generated were less widely understood or agreed upon (Jackson Citation2000). The individualistic patterns interpreted from pollen-analytical data were viewed as stochastic manifestations arising from factors such as geographical barriers, random dispersal and colonisation processes, and refugial locations (e.g. Davis MB Citation1976, Citation1981a, Citation1983a; Huntley and Birks Citation1983; Birks HJB Citation1989; see also Jackson Citation2000) . This stochastic view was, in many ways, inspired by Gleason’s (Citation1926) hypothetical examples in which random colonisation events resulted in multiple stable vegetation types (see Nicolson and McIntosh Citation2002) but in an identical environment (Jackson Citation2000). At the same time, plant and animal ecologists were paying increasing attention to stochasticity and non-equilibrium conditions in community structure (e.g. Sousa Citation1979; McIntosh Citation1980, Citation1987; Simberloff Citation1980; Wiens JA Citation1984; Giller and Gee Citation1987; see also Jackson Citation2000).
An alternative environmentally deterministic interpretation of Quaternary pollen-stratigraphical data was developed at about the same time (e.g. Webb T Citation1981, Citation1986; Prentice Citation1983, Citation1986b; Webb T et al. Citation1983a) largely associated with the Cooperative Holocene Mapping Project (COHMAP; Wright et al. Citation1993). COHMAP showed the importance of broad-scale climate change in long-term ecological dynamics. It was a major turning point not only in Holocene palaeoclimatology but also in Holocene palaeoecology (Birks HJB Citation2008) and resulted in several contributions relevant to our understanding of drivers of vegetation change and community history (e.g. Webb T Citation1986, Citation1987, Citation1988a; Huntley Citation1988, Citation1990a, Citation1990b; Williams et al. Citation1998), particularly extrinsic deterministic factors (Williams et al. Citation2011a).
8.2. The community concept in modern ecology and palaeoecology
The term community has generated an immense amount of debate, controversy, and terminological confusion in modern ecology, particularly plant ecology (e.g. Fauth et al. Citation1996; Vellend Citation2010, Citation2016; Stroud et al. Citation2015; Wilson JB et al. Citation2019), as well as in Deep-time palaeoecology (e.g. Miller W Citation1993; DiMichele Citation1994; DiMichele and Phillips Citation1996; Bonuso et al. Citation2002). The debates have centred on what is a community, what is community ecology, and how are communities structured and organised (e.g. Wilson JB Citation1991; Palmer MW and White Citation1994; Lawton Citation1999; Gotelli Citation2004; Lortie et al. Citation2004; Simberloff Citation2004; Ricklefs Citation2008, Citation2009; Brooker et al. Citation2009; Roughgarden Citation2009; Vellend Citation2010, Citation2016; D’Amen et al. Citation2017; Wilson JB et al. Citation2019). In ecology, community refers to the total living biotic component of an ecosystem including plants, animals, fungi, and microbes (Callow Citation1998). It implies interactions between individuals and species in the form of interference, exploitative and apparent competition, facilitation, predation, mutualism, and commensalism (Callow Citation1998). Plant ecologists commonly use community to refer to the botanical component of an ecosystem. The term assemblage is more general and refers to a collection of plants and/or animals and other biota in an area or the fossil remains of these organisms in a geological layer or sequence. Assemblage does not imply interactions between the living organisms. In the case of fossil assemblages (e.g. pollen assemblages), many factors such as pollen production, dispersal, sedimentation, and preservation play important roles in the formation of a fossil assemblage (Birks HJB and Birks Citation1980). The composition of a fossil assemblage can provide information about the past biota and indirectly the past environment in which it formed and hence the past biotic and abiotic history of the site at which it is preserved (Birks HJB and Birks Citation1980; Callow Citation1998).
Community ecology is the study of communities, in particular, the interactions between populations, and between populations and their abiotic environment in a specified area and the role that these interactions have on the composition and structure of that community. A community should be viewed not simply as the sum of its constituent species and their populations but also in terms of its emergent properties such as composition, structure, diversity, biogeochemistry, and physiognomy that are not features of the component populations. The terms community and community ecology encompass a very wide range of spatial scales. Moreover, one or more communities may occur and be described and studied within other communities, for example in forests (Callow Citation1998; Vellend Citation2010, Citation2016).
How communities are organised centres on three main hypotheses (see subsections 9.2 and 9.3 and Jackson and Blois (Citation2015)). Clements (Citation1905) developed his holistic or ‘organismal’ concept of the community and viewed it as a super-organism or discrete unit with sharp boundaries in space and time (McIntosh Citation1998). Gleason (Citation1917, Citation1926, Citation1927, Citation1939) proposed his individualistic concept of the community (also known as the open or continuum concept), with the abundance of populations of species varying gradually along complex environmental gradients but varying individually and not necessarily in the same way as other species’ populations. In this concept, it is possible that the individualistic distributions of different species can give rise to seemingly discrete units as well as to a continuum. As Webb DA (Citation1954) notes ‘the pattern of variation shown by the distribution of species among quadrats of the Earth’s surface chosen at random hovers in a tantalizing manner between the continuous and the discontinuous’. More recently, an alternative concept – the neutral theory of the community or meta-community – has been proposed by Hubbell (Citation2001). In this, all species are functionally equivalent, and the abundance of a species’ population changes by stochastic demographic processes such as random births and deaths. Each population has the same adaptive value (competitive and dispersal abilities) and local and regional composition represents a balance between speciation or dispersal that increases diversity and random extinctions that decrease diversity (Jackson and Blois Citation2015).
In the Quaternary botanical world, Fægri (Citation1947, Citation1963), Iversen (Citation1954, Citation1960), Andersen ST (Citation1961), and Cushing (Citation1963, Citation1965) all propose that pollen-stratigraphical data indicate that plants react individualistically to their environment such as climate, soils, other biota, topography, and other factors and not as collective units such as communities or floristic elements (Cushing Citation1963, Citation1965). Pollen-analytical and macrofossil data thus support the individualistic concept of Gleason (Citation1917, Citation1926, Citation1927, Citation1939) and Whittaker’s (Citation1967) concept of loosely ordered complexity of present-day plant communities in contrast to the Clementsian concept of communities as distinct integrated units in space and time.
Jackson and Overpeck (Citation2000) ask the stimulating question ‘is all the world Gleasonian?’ They define Gleasonian as being close to Gleason’s (Citation1926) own environmentally deterministic view of plant associations at broad spatial and temporal scales, where Gleason emphasised physiological tolerances as constraints to community structure and composition (see also Gleason Citation1927; Nicolson and McIntosh Citation2002; Eliot Citation2007). Jackson (Citation2000) and Jackson and Overpeck (Citation2000) propose that the observed patterns of terrestrial palynological (and hence vegetational) changes are largely explicable as biotic responses to the multivariate environmental changes of the realised environmental spaces (.2, 15.3). If we assume that fundamental niches vary among species – a basic tenet of ecological niche theory (Hutchinson Citation1978) – in terms of their size, shape, orientation, and location within environmental niche space and in other niche spaces (e.g. regeneration niche: Grubb Citation1977), temporal changes in RES will result in predictably individualistic responses of species, including disintegration of species associations and creation of new associations (Jackson and Overpeck Citation2000). As the magnitude of environmental change increases, species population responses will change from tolerance and persistence (mode 1, .3) to habitat shift (mode 2, .3), and finally to spread to new areas or local extinction (modes 3, 4, .3).
The hypothesis that tree taxa spread independently (e.g. Davis MB Citation1976, Citation1981a; Birks HJB Citation1989) does not exclude the idea that associated taxa such as forest herbs and shrubs spread and expanded together with the trees (Walker Citation1982a; Jackson and Overpeck Citation2000). The fundamental niche of many species that co-exist today are probably broadly similar on several environmental dimensions. Environmental change may thus stimulate the spread and expansion of species in the same general direction of the tree concerned (Jackson and Overpeck Citation2000). Some niche variables may be linked to vegetation physiognomy and many forest herbs may require a cool, moist, and shaded microclimate. It is not surprising therefore to see in pollen stratigraphies forest pollen or spores of shrubs, herbs, and ferns expand in the past at the same time or very soon after the expansion of the tree(s) that they grow under (e.g. Birks HH and Mathewes Citation1978). Aquatic and lake-shore assemblages often show comparable composition and structure along the terrestrial–telmatic–open-water gradient but under contrasting climatic regimes (Jackson and Overpeck Citation2000). The RES for terrestrial species and vegetation and for aquatic assemblages are probably not the same. The RES in aquatic habitats may consist of local environmental factors such as lake-water depth and chemistry and exposure as well as regional variables such as climate, whereas the RES for terrestrial habitats include many aspects of regional and local climate.
In conclusion, at the broad temporal and spatial scales that pollen analysts generally operate within, the Late Quaternary world is quasi-deterministic (Jackson Citation2000) with strong Gleasonian aspects and some Clementsian ideas (e.g. Watt Citation1964; Wilson JB et al. Citation2019). Stochastic neutral processes such as propagule dispersal, establishment, colonisation, disturbance, and mortality, may play important roles at particular spatial and temporal scales, for example in primary and secondary succession, patch dynamics, and landscape mosaics (see Prentice Citation1983, Citation1992; Chapin and Shaver Citation1985; Webb T Citation1986 for examples and discussion; Jackson Citation2000). As Jackson (Citation2000) discusses, in some rare situations such stochastic processes may be important at broad scales (regional to continental, centuries to millennia) by constraining the size of the realised niche compared to the size of the potential niche. Moreover, there is increasing evidence for the role of long-term neutral processes such as historical legacies or contingencies in vegetation history (e.g. Davis MB Citation1976, Citation1981a; Tzedakis and Bennett Citation1995; Jackson and Blois Citation2015; Svenning et al. Citation2015; Herzschuh et al. Citation2016; see subsections 9.2.2 and 11.3.4).
Despite the major advances in the ecological interpretation of pollen-stratigraphical data, there is still a tendency to equate fossil pollen assemblages with plant ‘communities’ and to apply approaches and quantitative models for investigating and analysing modern community data (e.g. Ferrier et al. Citation2002, Citation2007; D’Amen et al. Citation2017; Blonder et al. Citation2017a, Citation2017b; Gaüzère et al. Citation2018; Jeffers et al. Citation2018; see subsections 9.5.2 and 9.5.3) directly to fossil pollen-assemblage data (e.g. Delcourt PA and Delcourt Citation1987; He and Orlóci Citation1998; Rull Citation2012; Blois et al. Citation2013a, Citation2013b, Citation2014; Maguire KC et al. Citation2015, Citation2016; Nogués-Bravo et al. Citation2016; Stivrins et al. Citation2016; Dietl Citation2016a; Lyons et al. Citation2016b; Eroglu et al. Citation2018). Such applications naturally raise the question, do pollen assemblages reflect plant communities in the sense that modern ecologists use the term community?
8.3. Do pollen assemblages reflect plant communities?
In the early days of pollen analysis, pollen assemblages from bogs and lakes were interpreted as reflecting broad-scale forest types (e.g. mixed deciduous forest, pine forest, beech forest) (e.g. Jessen Citation1920; Auer Citation1921). As pollen analysis became more sophisticated, thanks to major advances in microscopy and pollen morphology and hence in the identification of many more pollen and spore types (e.g. Erdtman Citation1943; Fægri and Iversen Citation1950; Erdtman et al. Citation1961), and a shift from a primarily geological and chronological approach to a more botanical and ecological approach, the interpretation of pollen assemblages shifted more towards the reconstruction of vegetation types forming a vegetational mosaic within the pollen-source area of the study site (e.g. Iversen Citation1954; Andersen ST Citation1961; Cushing Citation1963; Birks HJB Citation1973, Citation1976, Citation1981a). The development of pollen-representation factors (R-values sensu Davis MB Citation1963), Prel- and Rrel-values and ‘general-purpose correction factors’ (sensu Andersen ST Citation1970), and the ‘extended R-value’ models (Parsons and Prentice Citation1981; Prentice and Parsons Citation1983; Prentice Citation1986a) allows the quantitative estimation of plant abundances from fossil pollen assemblages in the pollen-source area of the site (Birks HJB and Birks Citation1980). These developments led to Prentice’s (Citation1985, Citation1988a) model of pollen deposition that attempts to quantify the pollen-source area and pollen production and dispersal biases and to predict the effect of site size on the relative pollen representation of different taxa (see also Jackson Citation1994; Jackson and Lyford Citation1999). Results from such models (e.g. Prentice Citation1985, Citation1988b; Sugita Citation1993, Citation1994; Theuerkauf et al. Citation2016) suggest that in small- to medium-sized lakes or mires (5–100 ha), the majority of pollen comes from within a 50-km distance (Jacobson GL Citation1988; Davis MB Citation2000). Consequently, pollen assemblages can be used to reconstruct vegetation composition in these regional-scale settings with, for example, the REVEALS model (Sugita Citation2007a; Theuerkauf et al. Citation2016) or quantitative approaches within a Bayesian framework (e.g. Paciorek and McLachlan Citation2009; Dawson A et al. Citation2016). In the REVEALS model the source area defines its resolution, as vegetation patches smaller than the pollen-source area cannot be distinguished (Theuerkauf and Couwenberg Citation2017).
Pollen assemblages from smaller basins represent smaller source areas (Prentice Citation1985, Citation1988a; Jacobson GL Citation1988), but in small lakes (<5 ha) the shore vegetation is over-represented in the pollen assemblage to an unknown extent, and in small mires, pollen from local or extra-local trees can disturb the pollen signal (Matthias and Giesecke Citation2014; Theuerkauf and Couwenberg Citation2017). Pollen assemblages from very small hollows (20–50 m diameter) (e.g. Bradshaw RHW Citation1988, Citation2013) can provide high spatial resolution of the past vegetation at the stand-scale if quantitative reconstruction methods such as the LOVE (Sugita Citation2007b) or MARCO POLO (Mrotzek et al. Citation2016) models are used. However, these models only reconstruct the composition of the vegetation close to the site (Jacobson GL Citation1988), not the vegetation patterns or mosaic structure of the surrounding landscape (Theuerkauf and Couwenberg Citation2017).
As Theuerkauf and Couwenberg (Citation2017) discuss, there are currently two quantitative approaches to increase the spatial resolution of vegetation reconstructions based on pollen assemblages from small- to medium-sized lakes. These are the multiple scenario approach (MSA) of Bunting MJ and Middleton (Citation2009) and the extended downscaling approach (EDA) of Theuerkauf and Joosten (Citation2009, Citation2012), Theuerkauf et al. (Citation2014), and Theuerkauf and Couwenberg (Citation2017). The MSA involves two stages: (1) it defines multiple scenarios in which predefined vegetation types are assigned to landscape units defined by elevation, soil type, moisture, and other factors and (2) it uses a pollen-dispersal model to estimate pollen deposition based on the various scenarios. The modelled pollen data are then compared with the observed pollen assemblage and the best matching scenarios are assumed to be the appropriate past vegetation composition and mosaic pattern. It is an approach with much potential if, for example, the matching procedure is made within a Bayesian modelling framework with an emphasis on model selection (e.g. Hilborn and Mangel Citation1997; Mangel Citation2006; Clark Citation2007; Stephens et al. Citation2007). The EDA also compares modelled with observed pollen deposition but in contrast to the MSA, it uses many sites, thereby allowing forward modelling to determine the vegetation cover associated with the landscape units. Like the MSA, the EDA defines its landscape units based on abiotic features such as soil type and topography. Both the MSA and EDA intuitively build on the concept of the landform-vegetation zone or unit introduced into palynology by Ritchie and Lichti-Federovich (Citation1963) and Lichti-Federovich and Ritchie (Citation1968), defined as an area characterised by a broadly uniform geomorphology, bedrock geology, soil, and vegetation. Although vegetation is rarely constant at a fine scale, the range of vegetation types is often relatively consistent within a landform-vegetation zone because of the recurring topographical and ecological patterns in the area. The landform-vegetation zone appears to be the functional spatial unit recorded by pollen assemblages from small- or medium-sized basins (Janssen Citation1970, Citation1981; Birks HJB et al. Citation1975a; Birks HJB et al. Citation1975b; Birks HJB Citation1993a; Felde et al. Citation2014a). Within such a unit, pollen assemblages – particularly of the dominant taxa – are broadly similar, whereas between such zones the assemblages are often different (Janssen Citation1970; Felde et al. Citation2014a). The spatial scale of landform-vegetation zones in a region defines, in part, the scale of spatial resolution of fossil pollen assemblages in that region (Janssen and Törnqvist Citation1991). Tests of the MSA and EDA are producing promising results (e.g. Theuerkauf and Joosten Citation2009, Citation2012; Bunting MJ et al. Citation2018; Pratt Citation2018). Hopefully both approaches will be used by palynologists to reconstruct quantitatively not only past vegetation composition using the REVEALS or related models but also to reconstruct past vegetation and landscape patterns and mosaic structure. Such reconstructions would form a firm basis for considering past communities rather than simply equating a fossil pollen assemblage with a past plant community.
In a recent study, Calder et al. (Citation2019) use pollen and charcoal stratigraphies from six small lakes within the sub-alpine zone of northern Colorado to demonstrate that changes in regional climate and fire regimes (Calder et al. Citation2015) in the last 2000 years result not only in vegetation-state change (Calder and Shuman Citation2017) but also in novel landscape patterns. This study illustrates how detailed Quaternary botanical studies can link to landscape ecology (see below).
8.4. Conclusions
Fossil pollen assemblages are a rich source of information about floristic and vegetation composition and dynamics (Birks HJB Citation1993a; Davis MB Citation2000; Seppä and Bennett Citation2003). However many of the concepts of conventional community ecology and modelling are inadequate to extract and interpret fully this information. There is a conflict in the spatial scales of modern community ecology and the vegetation-sensing properties of fossil pollen assemblages (Delcourt HR et al. Citation1983; Birks HJB Citation1986; Delcourt HR and Delcourt Citation1991; Jackson Citation1994). Although often ignored by palynologists, the rapidly developing theories, models, metaphors, and methodologies of landscape ecology (e.g. Forman and Godron Citation1986; Turner MG and Gardner Citation1991; Wagner HH and Fortin Citation2005; Wiens JA and Moss Citation2005; Jones HG and Vaughan Citation2010; Ewers et al. Citation2013) provide new and exciting possibilities for linking ecological theory and pollen-assemblage data at the relevant spatial scales (Birks HJB Citation1986; Jackson Citation1994; Birks HJB et al. Citation2016c; for examples see Meltsov et al. Citation2013; Matthias et al. Citation2015) . Pollen assemblages reflect the vegetation composition of the pollen-source area of the site under study (Davis MB Citation2000). This source area will commonly support several vegetation types or communities (Rackham Citation1992). In a simulation study, Bunting MJ et al. (Citation2004) show that the pollen-source area of a site is primarily an expression of the mosaic structure of the different vegetation types within the landscape. Pollen assemblages thus do not reflect a community but a landscape mosaic of different communities or vegetation types (Cushing Citation1963). The use of quantitative models developed for modern community data to analyse fossil pollen-assemblage data (see above) runs the danger of producing misleading or even erroneous results and interpretations.
9. Tree and forest dynamics
9.1. Introduction
Pollen analysis has provided much information about forest history and structure, broad-scale patterns of tree expansion and contraction, and fine-scale forest dynamics. This section is primarily concerned with the dynamics and history of temperate forest systems in north-west Europe and eastern North America because of personal familiarity and the wealth of available information from these areas.
This large section considers causal hypotheses for changes in interglacial pollen stratigraphies in terms of environmental forcing, biotic interactions, neutral processes, and, in the case of the Holocene, human impact. It then discusses the reconstruction of forest structure in temperate areas in light of current ideas of prehistoric pasture woodlands. The use of pollen-stratigraphical data in ecological models at a range of scales is reviewed. The key processes in forest dynamics of tree invasions and forest-stand dynamics are outlined. The concepts of stability and change are discussed in the context of Quaternary botany. The antiquity of contemporary woodland types is considered for Britain, and finally the likely extent of forest cover and landscape openness in the Holocene are reviewed.
9.2. Causal hypotheses for change in interglacial pollen stratigraphies
In the early days of pollen analysis, von Post (Citation1916, Citation1918, Citation1924) interpreted pollen-stratigraphical changes as a reflection of long-term vegetation changes in response to regional climate change (Birks HJB Citation2008; Edwards KJ et al. Citation2017; Birks HJB and Berglund Citation2018; Giesecke and Brewer Citation2018). For von Post’s interpretation to be valid, it assumes that trees spread rapidly into the study area or that the relevant trees were already present in the area (Giesecke and Brewer Citation2018). Von Post (Citation1946) justified his interpretation that pollen-stratigraphical changes are a response to regional climate change through the concepts of ‘revertence’ and ‘regional parallelism’ (Birks HJB Citation2008). Revertence refers to early-Holocene pollen assemblages reappearing or expanding towards the present-day in response to late-Holocene cooling that resulted in the late-Holocene decline of mid-Holocene thermophilous trees. Regional parallelism refers to the occurrence of ecologically analogous changes in pollen assemblages in different areas with different taxa responding in parallel to regional climate changes in the different areas. Von Post (Citation1946) regarded factors such as soil development and time required to spread from LGM refugia to be of secondary importance compared to regional climate change as drivers of the observed palynological patterns. He implicitly adopted part of the hypothesis presented by Rudolph (Citation1930) that proposes that tree dispersal and establishment and tree-population expansion are different processes that may have occurred at very different times leading to possible expansion lags (see subsection 4.4). Relevant to von Post’s climate interpretation is that Rudolph (Citation1930) regarded tree-population expansion as a response to regional climate change (Giesecke and Brewer Citation2018). Rudolph (Citation1930) proposed that there were outlying small populations of many taxa growing in local pockets prior to expansion when conditions (e.g. regional climate) became more favourable (see also Smith AG Citation1965; Watts Citation1973; Godwin Citation1975; Bennett Citation1988b; see subsection 4.4; .2-12.4)
An alternative hypothesis to explain observed pollen-stratigraphical changes was proposed by Bertsch (Citation1940) and Fægri (Citation1940, Citation1949). It suggests that changes in tree-pollen dominance reflects differences in tree-spreading rates and in the location of LGM refugia (see also Davis MB Citation1976, Citation1981a). A related non-climatic hypothesis was presented by Iversen (Citation1960, Citation1967, Citation1973). It proposes that the Holocene tree-pollen changes prior to prehistoric human impact are primarily driven by different shade tolerances, soil preferences, and other endogenous population properties such as longevity of the major forest trees.
These early palaeoecological hypotheses can be expressed in current ecological terminology (Jackson and Blois Citation2015) as follows:
Pollen-stratigraphical changes are a response to external, i.e. exogenous, abiotic environmental factors such as climate change (von Post Citation1946). The composition of pollen assemblages are structured primarily by whether potential members’ fundamental or Grinnelian niches overlap with the prevailing local environmental conditions (Jackson and Blois Citation2015) and hence by the physiological and demographic responses of taxa to external abiotic environmental factors (Jackson and Blois Citation2015). These external factors can result in the two types of change distinguished by Williams et al. (Citation2011a) – extrinsic change externally driven by abrupt climate change and intrinsic change resulting from internal thresholds, tipping points, and other non-linear biotic responses to gradual climate change. This hypothesis is what Jackson and Blois (Citation2015) term ‘environment assembly’ and in it is called ‘environmental forcing’ (H1). It assumes that plant distributions, abundances, vegetation, and hence pollen assemblages, are in equilibrium over the spatial and temporal scales of interest (Howe and Webb Citation1983; Prentice Citation1983).
Pollen-stratigraphical changes are a response to internal, i.e. endogenous, biotic factors such as growth rates, longevity, and competition, ecological properties such as shade tolerance, and responses to disturbances, for example fire or storms (Iversen Citation1960, Citation1967, Citation1973). This hypothesis is termed ‘interaction assembly’ by Jackson and Blois (Citation2015) and ‘biotic interactions’ (H2 in ). It is based on the Eltonian niche (Hutchinson Citation1957; Soberón Citation2007) and considers communities to be structured primarily by interactions between taxa (Post Citation2013).
Pollen-stratigraphical changes are structured by random processes, particularly propagule dispersal, recruitment, and mortality, and proximity of LGM refugia (Bertsch Citation1940; Fægri Citation1940, Citation1949; Davis MB Citation1976, Citation1981a). Such ‘neutral assembly’ (Jackson and Blois Citation2015) or ‘neutral processes’ (H3 in ) are non-equilibrial and may be influenced by historical legacies of, for example, past survival, demographic, or dispersal events. Such assemblages may display ‘ecological drift’ as a result of singular events (Chase and Myers Citation2011; Jackson and Blois Citation2015).
Figure 16. The three major hypotheses (H1–H3) and processes for changes in pollen-assemblage composition and abundance: environmental forcing (H1), biotic interactions (H2), and neutral processes (H3) that can cause ecological drift (Jackson and Blois Citation2015). These processes may interact in the real world. Exogenous environmental change (e.g. climate change) may be a major driver of assemblage properties both directly and indirectly by influencing interactions between taxa and initiating neutral processes. Climate varies continuously and may influence processes within all three hypotheses to modify assemblage properties (Jackson and Blois Citation2015). The three underlying hypotheses H1, H2, and H3 correspond to the hypotheses discussed in the text. Modified from Jackson and Blois (Citation2015).
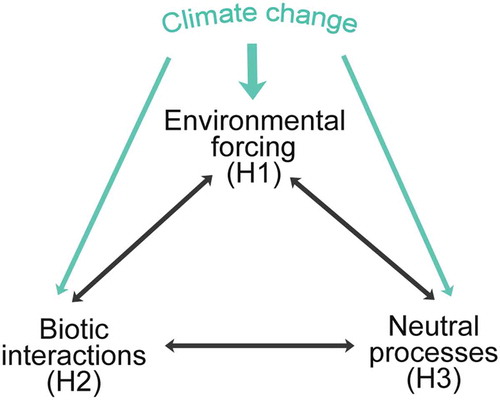
These three hypotheses about drivers of long-term (104–105 years), broad-scale forest succession and development correspond closely to current ideas about underlying processes in short-term (101–102 years), local ecological succession (e.g. Måren et al. Citation2018). Local community species assembly during a succession can be driven by deterministic, stochastic, or neutral processes (e.g. Chu et al. Citation2007; Kreyling et al. Citation2011; Bhaskar et al. Citation2014; Zhou et al. Citation2014), or by a combination of all three (e.g. Gravel et al. Citation2006; Adler et al. Citation2007). Despite over a century of research on ecological succession, the relative importance and temporal dynamics of these different processes within a succession and in different systems and what drives this vegetation change (e.g. Chase Citation2010; Chase and Myers Citation2011; Zhou et al. Citation2014; Måren et al. Citation2018; Wilson JB et al. Citation2019) remain unclear; what Måren et al. (Citation2018) term a ‘conundrum’. It has its roots in the early work on succession by Clements (Citation1916) who interpreted succession as a primarily deterministic process with predictable species, niches, and vegetation replacements (Clementsian model – Måren et al. Citation2018) and the contrasting views of Gleason (Citation1926) who interpreted succession as an outcome of stochastic processes and individualistic responses of taxa to their environment (Eliot Citation2007) (Gleasonian model – Måren et al. Citation2018). A recent addition to the conundrum comes from neutral theory (e.g. Hubbell Citation2001; Missa Citation2005; Chu et al. Citation2007; Zhou et al. Citation2014). This adds to the stochastic perspective of succession by highlighting how many patterns observable in nature today, including succession, may develop not only under high stochasticity but also in the absence of any differences among taxa in their responses to the environment or to each other (demographic equivalence). A neutral system is one with species equivalence such that species’ responses are driven by colonisation-extinction dynamics and interactions rather than niche differences (Hubbellian model – Måren et al. Citation2018). A detailed replicated study of post-fire succession over 12 years in grazed and ungrazed west Norwegian heaths (Måren et al. Citation2018) shows that stochastic individualistic species responses early in the succession were replaced later by more niche-driven dynamics and that sheep grazing reduced predictability in both the successional trends and the species-level dynamics, especially in plant functional groups such as bryophytes that are disturbance-intolerant. Chase and Myers (Citation2011) provide a framework for assessing how niches structure composition in the face of stochastic neutral processes.
Do available pollen-analytical data permit the testing of these hypotheses over long-term periods? There is much support for the environment-forcing hypotheses (H1 in ). The strong spatio-temporal broad-scale coherence of pollen assemblages over broad areas and the close correspondence with independent palaeoclimate reconstructions and with palaeoclimate simulations from general circulation models establish that climate is the strongest factor to explain such broad-scale coherence (e.g. Birks HJB Citation2008; Jackson and Blois Citation2015). Despite the overall importance of exogenous climate factors to explain the spatial and temporal patterns in regional-scale pollen-assemblage data, at a finer scale of individual lakes or hollows within forest stands (e.g. Iversen Citation1973; Andersen ST Citation1978; Jacobson GL and Bradshaw Citation1981; Birks HJB Citation1986; Bradshaw RHW Citation2013), the observed assemblage changes may represent the results of endogenous interactions between individuals and the impacts of disturbance (H2 in ; Jackson and Blois Citation2015). These factors may themselves be influenced by changing climate. As Jackson and Blois (Citation2015) conclude ‘[a]lthough these interactions play out locally and often briefly, even the broadest geographic patterns are spatial aggregations of local interactions, and all temporal changes, regardless of scale, are ultimate outcomes of population processes and interactions. Paleoecological dynamics … cannot be understood fully without considering population processes and species interactions’. The neutral assembly hypothesis (H3 in ) is based on Hubbell’s (Citation2001) neutral theory of biodiversity and biogeography. It assumes that taxon-niche properties are not important in assemblage structure and composition, which instead result from ecological drift. Processes behind such drift are largely random in relation to niche properties and result in assemblages that cannot be predicted from exogenous environmental factors or endogenous taxon interactions (Jackson and Blois Citation2015). The model proposed above by Jackson and Blois (Citation2015) contrasts with the model of Wiens JJ (Citation2011) at the biogeographical scale where most patterns are created by niche differences. Abiotic factors may be important whereas biotic interactions are considered to play a minor role.
Current interpretations of available pollen-stratigraphical data highlight that exogenous environmental change is a strong driver of pollen-assemblage change, particularly at broad spatial and temporal scales. Endogenous taxon interactions largely determine the outcomes of environmental change on pollen assemblages, often in subtle ways. Interactions of neutral processes with environmental change and taxon interactions can result in indeterminancies as well as historical legacies (Jackson and Blois Citation2015). Jackson and Blois (Citation2015) rightly emphasise that endogenous interactions and neutral processes should be considered more explicitly in the interpretation of palynological data.
I now review the evidence for the three major hypotheses influencing changes in interglacial pollen stratigraphies and a fourth major hypothesis (human impact) incluencing palynological changes in the Holocene interglacial. Because so much more is known about Holocene pollen stratigraphies and environmental history than for previous interglacials, this review is primarily confined to Holocene interglacial pollen stratigraphy.
9.2.1. Environmental forcing hypothesis
In testing this hypothesis (H1 in ) it is essential to have environmental records (e.g. climate reconstructions) that are independent of the pollen record so as to avoid problems of the frequent circular argument of reconstructing climate from the pollen record and then using this pollen-based reconstruction to interpret the observed pollen record. Much work in palaeoclimatology (see Birks HJB Citation2008; Ruddiman Citation2013a; Bradley Citation2015) using general circulation models and biomisation procedures (COHMAP Members Citation1988; Wright et al. Citation1993; Bartlein et al. Citation1998; Prentice et al. Citation1998; Webb T et al. Citation1998; Webb T and Kutzbach Citation1998; Williams et al. Citation1998) shows, at a broad scale, good correspondence between simulated climate and mapped pollen-stratigraphical data, especially near the transition from the Late Pleistocene to the Holocene and thus the role of extrinsic exogenous climate change in initiating the spread and expansion of trees and the development of forest over much of the temperate areas of the Northern Hemisphere. In addition, there have been great advances in palaeolimnology (e.g. studies of lake-level changes), peat stratigraphy (e.g. surface bog-wetness reconstructions), and a multitude of other palaeoclimate (e.g. geochemical, isotopic records) proxies (see Oldfield Citation2005; Birks HJB Citation2008; Verschuren and Charman Citation2008; Bradley Citation2015). As Flessa and Jackson (Citation2005a, p.99) conclude ‘Paleoclimate inference is no longer heavily dependent on biological proxies, but can now draw on a greater variety of both biological and non-biological data. Thus, paleontological data on past distribution and abundance of organisms can now be used as response variables in studies in which climate change has been independently inferred’ (e.g. Battarbee Citation2000; Shuman et al. Citation2002; Webb T et al. Citation2003; Shuman et al. Citation2004; Oldfield Citation2005; Birks HJB and Birks Citation2008; Shuman et al. Citation2009; Grimm EC et al. Citation2011; Booth et al. Citation2012; Shuman and Serravezza Citation2017).
Whilst the role of extrinsic exogenous climate change in driving vegetation change and thus pollen assemblages in the late-glacial/Holocene transition at about 11,700 yr BP is very clear and undisputed (see below), the role of climate change in driving pollen-stratigraphical changes within the Holocene is less clear, especially in Europe. In a numerical analysis of 59 well-dated Holocene pollen sequences in Europe, Giesecke et al. (Citation2011) identify statistically significant palynological changes clustered at about 10,600, 9500, 8200, 4800, 3700, and 1200 yr BP and suggest that European pollen assemblages often changed synchronously in the last 11,500 years, supporting the earlier conclusion of Tinner and Lotter (Citation2001, Citation2006) that a climate event like the so-called ‘8.2 ka’ event (Alley et al. Citation1997) triggered the expansion of Fagus sylvatica in different parts of Europe. The only driver that could result in pollen-assemblage compositional change at a subcontinental scale is climate change, either as an abrupt step-like change (extrinsic – sensu Williams et al. Citation2011a) or as a series of progressive climate changes that acted together rather like a large broad-scale disturbance event (Tinner and Lotter Citation2001, Citation2006; Giesecke et al. Citation2011), similar to the concept of intrinsic climate change of Williams et al. (Citation2011a) (see also Jackson et al. Citation2009b).
Giesecke et al. (Citation2011) contrast the Holocene expansion of Corylus avellana and Alnus glutinosa pollen in Europe as special cases of synchronous expansions. For both taxa, their populations must have increased from palynologically undetectable occurrences, despite being prolific pollen producers. Such populations were probably present but with very low pollen abundances prior to their expansion in response to the climatic triggers that initiated population expansion over a wide area. The striking expansion of Corylus at the same time across an area from Scotland to the European Alps and in more continental areas farther east suggests a critical climate threshold for hazel was reached at the same time (ca. 10,600 yr BP or earlier) over a large part of Europe. There is a striking similarity in the pattern of Corylus pollen curves when plotted together on an absolute age scale from 5°W to 20°E across Europe (Giesecke et al. Citation2011).
The dynamics of Alnus is unusual in that it shows a large expansion at about 9500 yr BP only around the Baltic Sea region but site-specific erratic intrinsic behaviour in western Europe, especially the British Isles (Bennett and Birks Citation1990). Giesecke et al. (Citation2011) suggest ‘[w]hat is true for these two species may also play a role for many other trees that spread early without leaving evidence of their presence and only expanded when the climate became suitable (Rudolph Citation1930; Godwin Citation1975)’ (see also Giesecke and Brewer Citation2018).
In a related study, Seddon et al. (Citation2015) estimate rates of change in pollen-assemblage composition at 54 sites across northern Europe to identify times of abrupt assemblage change. They find high rates of turnover within the late-glacial and around the onset of the Holocene (12,750–11,500 yr BP) and between 11,000–10,750, 10,250–10,000, 7750–7250, 3250–3000, and 1750–250 yr BP. In contrast to Giesecke et al. (Citation2011), they interpret these Holocene compositional patterns as generally intrinsic site-specific events rather than as responses to broad-scale extrinsic processes. They propose that during the early–mid Holocene (9000–6000 yr BP), palynological and hence vegetation responses were non-linear and highly variable within and between geographical areas. At the late-glacial/Holocene transition, the rate and magnitude of climate change were large enough to override any site-specific thresholds whereas during the Holocene, responses were mediated by intrinsic site-specific factors resulting in varying turnover rates within and between areas. The analysis by Seddon et al. (Citation2015) suggests that the underlying dynamics of palynological turnover are different at different times, with extrinsic linear responses in the late-glacial and at the onset of the Holocene to broad-scale climate forcing, changing to intrinsic non-linear site-specific responses throughout much of the Holocene.
The two synthetic studies discussed above suggest that climate change has clearly been important in driving vegetation and hence pollen-stratigraphical changes at the transition from the late-glacial to the Holocene and possibly some changes within the Holocene. However, many Holocene pollen-stratigraphical changes are not unambiguously explicable in terms of our current understanding of past climate change and of interactions between climate variables during the Holocene, at least in Europe.
Although exogenous environmental forcing appears to be necessary to explain the long-term dynamics seen at the scale of glacial–interglacial couplets, are other factors also important? Jackson and Blois (Citation2015) suggest that ‘species and populations are not billiard balls knocked around the landscape by changing climate; climate change drives community change via processes that involve individuals, populations, and communities’. Many ecologists today agree with Diamond’s (Citation1975) summary ‘communities are assembled through selection of colonists, adjustment of their abundances, and compression of their niches, in part so as to match the resource curve of the colonists to the resource production curve’.
In summary, some of the major Holocene pollen-stratigraphical changes in Europe appear to be responses to exogenous extrinsic climate change, for example at the onset of the Holocene, at 10,600 yr BP, and at the ‘8.2 ka’ event. There are, however, several other palynological changes that are not parsimoniously interpretable as being responses to extrinsic climate change. The following three subsections consider alternative hypotheses.
9.2.2. Biotic interaction hypothesis
The biotic interaction hypothesis (H2 in ) involves a variety of internal endogenous biotic factors such as growth rates, longevity, flowering age, dispersal mechanisms, shade tolerance, and competition as potential drivers of the progressive spread and expansion of trees under conditions of suitable climate (e.g. Iversen Citation1960, Citation1967, Citation1973; Huntley and Birks Citation1983; Birks HJB Citation1986, Citation1989). Spread and possibly expansion were probably initiated by rapid climate change at the onset of the Holocene (H1 environmental forcing in ) and each tree taxon may have spread in different directions over thousands of kilometres from different LGM refugia at rates of 500–1000 m yr–1 (Huntley and Birks Citation1983; Giesecke and Brewer Citation2018) until it reached its climate limits, attained equilibrium with climate, and increased its abundance (see subsection 4.4). Some taxa such as Betula, Corylus, Pinus, and Ulmus apparently reached their climate limits and attained equilibrium with climate early in the Holocene, whereas other taxa may not have reached such limits even after 11,000 years since deglaciation (e.g. Fagus, Picea). Some distributions seemingly in climate equilibrium today may not have been in equilibrium in the past. Differences in, for example, location of LGM refugia (both micro- and macro-refugia), routes and inherent rates of dispersal, threshold responses, and a range of ecological and competitive tolerances and traits (see table 1.1 in Birks HJB Citation1986), and the presence of barriers to spread such as mountain ranges, existing vegetation, unsuitable soils, and unfavourable climate, may account for the diverse patterns of tree pollen abundances observed in time and space (e.g. Huntley and Birks Citation1983; Giesecke and Bennett Citation2004; Giesecke et al. Citation2007, Citation2017; Williams et al. Citation2011a; Brewer et al. Citation2017; Giesecke and Brewer Citation2018).
In the Holocene of north-west Europe, many mesocratic trees did not expand in an order predictable from their climatic tolerances as judged by modern distributions (e.g. Ellenberg Citation1988; Ellenberg et al. Citation1991; Prentice and Helmisaari Citation1991; Hill MO et al. Citation2004; Zimmermann et al. Citation2009) or in the order they expanded in previous interglacials (Firbas Citation1949; Iversen Citation1960, Citation1967, Citation1973; see ). For example, in the Holocene, Hedera and Viscum were present in Denmark before Alnus and Tilia even though the latter two grow farther north today and are less sensitive to low temperatures, as shown by detailed autecological studies (e.g. Iversen Citation1944; McVean Citation1955; Pigott and Huntley Citation1980; Pigott Citation1981a). In terms of any simple model of climate control influencing arrival and/or expansion order, one would expect a priori, Alnus and Tilia to have expanded before Hedera and Viscum. Iversen (Citation1960, Citation1967, Citation1973) argues that the sequence of arrivals and expansion cannot be a direct reflection of climate change and proposes differential spreading rates and lags in expansion over at least 1000 years. Walker (Citation1978), Jackson et al. (Citation2009b), and Williams et al. (Citation2011a) discuss the possible role of intrinsic climate ‘syndromes’, namely combinations of interacting climate variables that may be compensatory under different boundary conditions in influencing plant distribution, growth, abundance, and reproduction (see Iversen (Citation1944), Hintikka (Citation1963), and Neilson and Wullstein (Citation1983) for examples of such plant–climate syndromes). Little is known, however, about climate syndromes and their relevance as drivers of long-term vegetation change (Jackson et al. Citation2009b; Williams et al. Citation2011a).
Biotic interactions of potential relevance include facilitation, mutualism, trophic relationships (predation, parasitism, herbivory), and, probably most important, competition for resources (e.g. light, water, nutrients, space) (Diamond Citation1975; Weiher and Keddy Citation1999; Jackson and Blois Citation2015). Communities structured by interactions have limited membership that is set by equilibrium population processes resulting from interspecific competition (Jackson and Blois Citation2015).
Following from Iversen’s (Citation1958) glacial–interglacial cycle, Iversen (Citation1960, Citation1967, Citation1973) presents an ecological interpretation of early- and mid-Holocene forest development in Denmark in terms of endogenous biotic interaction factors. This interpretation is based on the known different shade tolerances, longevities, reproductive behaviour, and soil preferences of the major forest trees and built on the pioneering work by Vaupell (Citation1863) on the importance of light in woodland succession and dynamics such that light-demanding species will be replaced by shade-tolerant trees in the long run, provided that the conditions for growth are favourable for the latter (Iversen Citation1960; see also Jensen Citation1910, Citation1949; Körner Citation2005). Iversen (Citation1960) provides a simple null hypothesis (Birks HJB Citation1986, Citation1993) against which alternative hypotheses to explain the observed pollen-stratigraphical trends can be tested (e.g. Prentice Citation1983, Citation1988a; Birks HJB Citation1986; Bennett Citation1988b; Bennett and Willis Citation1995; Shuman et al. Citation2004; Williams et al. Citation2011a; Jackson and Blois Citation2015).
What data can test Iversen’s (Citation1960) null hypothesis of biotic interactions? Odgaard (Citation1994) analysed numerically three detailed Holocene pollen sequences from western Jutland, Denmark. After partialling out the effect of age as a covariable (= conditional variable), the ordering of pollen-taxon scores on the first partial or conditional correspondence analysis (pCA) (capturing 36% of the residual variation) reflects a light-demanding to shade-tolerant gradient of taxa. Taxa such as Juniperus and Salix have high positive scores whereas Alnus, Corylus, Fraxinus, Tilia, and Ulmus have high negative scores. Betula, Pinus, and Populus have intermediate scores. These results suggest that although the pollen stratigraphies are different at the three sites, the dominant underlying patterns (‘latent structure’) are identical, reflecting near synchronous responses at each site. Similar results of a dominant light–shade gradient as represented by the first axis of partial CAs of Holocene pollen sequences in Norway, western Scotland, and central England (HJB Birks, unpublished) suggest the importance of shade-tolerance and competition for light in long-term vegetation dynamics in the early and mid Holocene (Birks HJB Citation1986; Bennett and Lamb Citation1988). Bennett (Citation1988b, p.715) concludes that ‘historical data suggest that the composition of plant communities is determined, at least in part, by competition’.
The importance of competition for resources, particularly light and water, in long-term vegetation succession is supported by the ability of generalised dynamic vegetation models such as LPJ-GUESS to simulate Holocene vegetation change remarkably well (e.g. Miller PA et al. Citation2008; Bradshaw RHW and Sykes Citation2014). Sensitivity analyses show that competition, natural disturbance, and the magnitude of inter-annual variability are all important in determining the establishment, biomass, and even the presence of species near their bioclimatic limits. Current modelling attempts with LPJ-GUESS are, however, unable to simulate satisfactorily the Holocene behaviour of Fagus sylvatica or Picea abies in Fennoscandia (Bradshaw RHW et al. Citation2000; Cowling et al. Citation2001; Giesecke et al. Citation2007; Miller PA et al. Citation2008; Saltré et al. Citation2013; Bradshaw RHW and Sykes Citation2014). There are indications for an overall broad-scale climatic control, but large areas are modelled as being suitable for these taxa during the early Holocene whereas the pollen records suggest only small scattered populations that do not contribute to any significant expansion of range until the late Holocene (Bradshaw RHW and Sykes Citation2014). One hypothesis about what might have prevented these small populations from expanding early in the Holocene is changes in inter-annual climate variability rather than changes in climate mean values (Giesecke et al. Citation2008, Citation2010). A major challenge is thus how to detect biotic responses to inter-annual variability and how to distinguish them from responses due to differences in climate means. Despite major advances in pollen-analytical studies in terms of geographical coverage, chronological control, methodology, and data handling and display, and in linking such studies with dynamic vegetation modelling, Giesecke and Brewer (Citation2018) conclude that ‘in the 100 years since his [von Post] observations we have still not been able to document the presumed climatic reasons for the late expansion of Fagus and Picea in Sweden or the early Holocene dynamics of Corylus and Alnus’.
In answering the question of what processes are sufficient to explain the dynamics observed in pollen-stratigraphical data, Jackson and Blois (Citation2015) conclude that ecological interactions matter, and ultimately changes observed in pollen stratigraphies reflect the outcomes of interactions among populations of different species, as influenced, in part, by a changing environment. Although these interactions occur locally and often briefly, broad geographic patterns are probably spatial aggregations of such interactions. All temporal changes, regardless of scale, are outcomes of population processes and species interactions. Pollen-stratigraphical changes cannot be understood without considering such processes and interactions (Jackson and Blois Citation2015).
9.2.3. Neutral processes
Neutral assembly assumes that taxon-niche properties are not relevant to community composition or structure as taxa are ecologically equivalent (Hubbell Citation2001) and thus that composition and structure result entirely from neutral ecological drift (e.g. Whitfield Citation2002; Missa Citation2005; Alonso et al. Citation2006; Hunter Citation2007; Vellend et al. Citation2014; Jackson and Blois Citation2015). Properties of such communities include virtually unlimited membership; no equilibrium; influences of historical legacies of past dispersal and demographic events; and tendencies to drift as a result of a singular event (Hubbell Citation2001; Clark and McLachlan Citation2003; Jackson and Blois Citation2015). Such ecological drift processes are effectively random with respect to taxon-niche properties (Grinnellian and/or Eltonian) resulting in community composition and structure that is unpredictable from either taxon interactions or the environment (Jackson and Blois Citation2015).
As discussed in subsection 11.3.4, the potential impact of historical legacies or contingencies (‘ecological memory’ – Ogle et al. Citation2015) is being increasingly recognised by ecologists, conservation biologists, and biogeographers (see Flessa and Jackson Citation2005a). Such legacy effects are apparent over a range of time scales (100–1000 yrs; see Box S4). Legacy effects over Holocene time-scales such as thousands of years are also attracting attention from palaeoecologists (e.g. Davis Citation1976, Citation1981a; Lindbladh Citation1999; Svenning and Skov Citation2007a; Jackson et al. Citation2009b; Booth et al. Citation2012; Pederson et al. Citation2014; Svenning et al. Citation2015). For example, Davis (Citation1976, Citation1981a) interprets spatial and temporal patterns of Holocene forest development in eastern North America as largely resulting from random processes of propagule dispersal and location of LGM refugia. Over longer Quaternary interglacial–glacial time-scales, Tzedakis and Bennett (Citation1995) propose that the abundance and distribution of tree populations during a cold and dry glacial stage may be critical in determining the tree composition of the following interglacial, as recorded in the pollen stratigraphy of a long continuous sequence of several interglacial and glacial stages in Greece. Mild, moist, cold stages may lead to similarities between successive interglacials by preserving part of the characteristic features of the preceding interglacial that may then influence the composition of the following interglacial. Herzschuh et al. (Citation2016) present evidence for such legacies in interglacials derived from the preceding glacial stage during the Plio-Pleistocene transition in the Russian Far East (see subsection 11.3.4). Such historical legacies represent neutral processes and can arise by interactions across scales (Jackson and Blois Citation2015), involving, for example, climate variation and biotic responses at different temporal scales due to the strong impact of historical processes such as dispersal, recruitment, mortality, and survival (Jackson et al. Citation2009b). Although these processes involve aspects of Eltonian and/or Grinnellian niche concepts, the processes are basically neutral because future ecological outcomes cannot be predicted from niche properties and the contemporary environment alone (Jackson and Blois Citation2015).
Despite the interest in neutral assembly among ecologists and biogeographers (e.g. Abrams Citation2001; Silander Citation2002; Whitfield Citation2002; McGill Citation2003; Missa Citation2005), there have been few attempts to assess the role of neutral processes other than as historical legacies in determining pollen stratigraphies. One approach involves ‘random walks’ to simulate pollen stratigraphies and to assess whether environmental forcing is necessary to explain such stratigraphies (Blaauw et al. Citation2010). The simulated profiles resemble observed stratigraphies with the successive dominance of different taxa; changing combinations, abundances, and turnover of taxa; abrupt events; long-term trends; quasi-cyclic behaviour; extinctions; and immigrations. This pioneering study (Blaauw et al. Citation2010) shows that realistic, temporal stratigraphical structure can arise in the absence of a coherent forcing mechanism such as environmental change (Jackson and Blois Citation2015) and highlights the need for replicated, multi-proxy data for reliable reconstructions of past vegetation, climate, and other environmental changes (Blaauw et al. Citation2010).
Correa-Metrio et al. (Citation2014) analyse numerically an 86,000-year pollen sequence from Guatemala to test whether the observed palynological changes are attributable to neutral random drift or to environmental change as reflected by changes in sediment magnetic susceptibility and charcoal concentrations. They use change in variance ratios (Schluter Citation1984; Fischer et al. Citation2001) as an explicit test for neutrality and regression analysis of individual taxa as an explicit test for environmental forcing. Taxon interchangeability emerges as an inadequate mechanism to explain the pollen stratigraphy at millennial scales (see also Kelly CK et al. Citation2008). There is no evidence for local taxon loss despite considerable climate variability (Hodell et al. Citation2008; Correa-Metrio et al. Citation2012a, Citation2012b). Random walks are unlikely therefore to have played a major role in influencing the pollen stratigraphy. Taxon presence and abundance were similarly not influenced by moisture availability or fire frequency, arguing for the possible role of compensatory mechanisms (sensu Connell et al. Citation1984), disjunct populations (Bush Citation2002; Sublette Mosblech et al. Citation2011), phenological and phenotypic plasticity (Toledo Citation1982; Coleman et al. Citation1994), and suppressed individuals in playing major roles in maintaining diversity (Correa-Metrio et al. Citation2014). Although Correa-Metrio et al. (Citation2014) conclude that environmental change explains most of the observed stratigraphic patterns, neutrality may have played a critical role in maintaining long-term tropical biodiversity at times of extreme environmental stress over the last 86,000 years. Moreover, neither environmental forcing nor biotic interactions responding to environmental changes completely explain taxon co-existence at this site. Taxon abundances depend on their abundance in the previous pollen sample, supporting the idea that a portion of the temporal variation in such stratigraphical data may be a result of neutrality and random processes, mostly expressed as historical drift (Adler et al. Citation2007; Correa-Metrio et al. Citation2014).
Turning to the shorter time span of the Holocene and a temperate area of relatively low biodiversity, Clark and McLachlan (Citation2003) use pollen data from eight sites in Ontario to test neutral model predictions using trends in mean and variance of individual pollen data. The results obtained are not consistent with predictions from neutral drift as the relative abundances of all the major taxa reached relatively constant values and their variances and coefficients of variation among the sites tended to stabilise or decline. If community dynamics are characterised by neutral drift, variances among sites are predicted to increase with time due to the accumulation of random changes in abundances. Clark and McLachlan (Citation2003) conclude that although the equalising processes implicit in the neutral model may not be absent or unimportant in nature, they must operate together with strong stabilising forces associated with biotic interactions and resource competition. These conclusions were strongly challenged by Volkov et al. (Citation2004) but these criticisms were vigorously answered by Clark and McLachlan (Citation2004).
9.2.4. Human impact and European tree and forest dynamics
The fourth main driver of change in Holocene tree and forest dynamics is human impact in the so-called Homo sapiens phase (Birks HJB Citation1986), especially in Europe but also elsewhere (see subsections 10.2 and 14.2 and sections 11 and 12). No attempt is made here to discuss in detail the role of humans in influencing rates and directions of important causative processes and observed palynological patterns (for reviews see, for example, Iversen Citation1967, Citation1973; Birks HJB Citation1986; Berglund Citation2003; Birks HJB and Tinner Citation2016a, Citation2016b; Birks HJB and Berglund Citation2018).
Human activity resulted in local and regional forest destruction and the creation of clearings, cultivated areas, heaths and pastures, and other open habitats. The natural rates and directions of change that occurred in response to environmental forcing, biotic interactions, and neutral processes in the first 4000–5000 years of the Holocene in Europe may have been disrupted and changed by the appearance and ever-increasing importance of Homo sapiens and agriculture. It is extremely difficult to identify and distinguish between natural and human-induced changes as climate changed, biota interacted, and neutral processes continued. The problem is to isolate unambiguously the effects of human activity from the other potential drivers of change (e.g. Berglund Citation2003; Finsinger et al. Citation2010; Henne et al. Citation2013).
In north-west Europe, there was a widespread sharp fall in Ulmus pollen values about 6000 years ago, probably as a result of interactions between prehistoric human activities and a tree pathogen with Ulmus pollen values halving within five years (Peglar and Birks Citation1993). Similarly, 5000–6000 years ago, Abies alba disappeared from the Mediterranean and sub-Mediterranean lowlands of the Italian Peninsula, probably in response to excessive Neolithic disturbance by fire and browsing (Tinner et al. Citation2013; Birks HJB and Tinner Citation2016a). The Abies collapses were rapid, with its pollen values halving within 13 and 22 years in Italy (Colombaroli et al. Citation2007) and southern Switzerland (Tinner et al. Citation1999), respectively.
In some areas of central and north-west Europe, forest clearance and subsequent derelictions of clearings may have facilitated local colonisation and expansion of new immigrants such as Fagus sylvatica, Picea abies, and possibly Carpinus betulus (Huntley and Birks Citation1983; Birks HJB Citation1986; Birks HJB and Tinner Citation2016a). While the establishment of F. sylvatica during Mesolithic times followed climate change (cooling and moisture increase) in southern and south-central Europe (Tinner and Lotter Citation2001), it is possible that the expansion of F. sylvatica across central Europe in the last 4000–5000 years may have been greatly facilitated by the creation of abundant large clearings within Tilia- or Quercus-dominated forests on well-drained soils (Birks HJB and Tinner Citation2016a). In some areas, depending on soil conditions, mixed Fagus–Ilex–Quercus forests developed whereas in other areas there was a rapid change from Tilia- or Quercus-dominance to Fagus-dominance (Andersen ST Citation1978; Birks HJB Citation1986). These changes commonly occurred after an extensive phase of human activity involving clearance and grazing followed by the abandonment of cleared and/or cultivated areas (Birks HJB and Tinner Citation2016a). This abandonment may have occurred as a result of local human population collapse following, for example, climate change, emigration, or over-exploitation of resources (Bradshaw RHW and Lindbladh Citation2005). Other types of secondary woodland developed in areas beyond the natural range of Fagus, for example woods of pure Betula spp., Fraxinus excelsior, Ilex aquifolium, Quercus spp., or Taxus baccata became established on particular soil types following abandonment of cleared or cultivated areas, relaxation in grazing pressure, reduction in fire frequency, or changes in disturbance regimes (Birks HJB Citation1986, Citation1989).
The westward, northward, and southward expansion of Picea abies through the Baltic countries, Finland, Sweden, and Norway over the last 6000–7000 years (Huntley and Birks Citation1983; Giesecke and Bennett Citation2004; Giesecke and Brewer Citation2018) may have been a contemporaneous response to subtle step-wise climate changes; a delayed spread and subsequent expansion unrelated to simple climate change; a response to forest disturbance creating gaps for colonisation; or, most likely, a combination of all these factors (Giesecke Citation2004). Whatever its causes, the invasion of Picea into northern and central Fennoscandia over the last 6000–7000 years resulted in major changes in forest composition and structure, soil conditions, and natural fire regime within the boreal forest (Seppä et al. Citation2009b; Ohlson et al. Citation2011).
In general, disturbance-sensitive taxa such as Abies, Acer, Fraxinus, Tilia, and Ulmus declined while disturbance-resistant taxa such as Corylus avellana, Fagus (resprouters), Ostrya carpinifolia, Picea (non-palatable), Pinus (non-palatable resprouter), and Quercus, expanded (Tinner and Ammann Citation2005). Fagus, Picea, and Quercus were also favoured by humans for their valuable acorns or timber, often forming monospecific stands (Tinner et al. Citation2013; Schwörer et al. Citation2015). Continued forest clearances and agriculture, interspersed by periods of abandonment and secondary regeneration, occurred due to the development and expansion of more permanent land-use practices (e.g. animal husbandry, ploughing, crop cultivation, woodland management) during the late Neolithic, Bronze Age, Iron Age, and Roman, Viking, medieval, and recent times. Forests initially became more open and pasture-woodland, scrub-pasture, and hazel coppice expanded. However, increased human interference including regular burning (Tinner et al. Citation2005) led ultimately to the widespread deforestation of much of Europe and the development of extensive pastures and of ‘commons’, fields, heaths, maquis, and settlements (Fyfe et al. Citation2015). These processes were particularly intense in the lowlands of Mediterranean Europe where practically no unplanted forests survive (e.g. Colombaroli et al. Citation2007; Tinner et al. Citation2009; Pini et al. Citation2017). Almost all extensive and naturally forested areas surviving today in Europe have been managed by selective silviculture over many centuries (e.g. Birks HJB Citation1993a; Rey et al. Citation2013; Bradshaw RHW et al. Citation2015; Birks HJB and Tinner Citation2016a).
9.3. Multiple causal hypotheses
With palaeoecology increasingly involving many different sedimentary proxies (e.g. Flessa and Jackson Citation2005a; Birks HH and Birks Citation2006; Shuman and Marsicek Citation2016; Birks HJB and Berglund Citation2018), it is now possible to reconstruct past environment, especially climate, independently of the pollen record. As a result, there is increasing evidence for interactions between the main natural drivers of change () plus human impact (e.g. Tinner et al. Citation2003; Vescovi et al. Citation2007; Valsecchi et al. Citation2008; Rey et al. Citation2019).
Neutral processes can interact with environmental forcing (Jackson et al. Citation2009b) as can biotic interactions (). As Jackson and Blois (Citation2015) note, a small change in temperature or moisture may shift the competitive balance in favour of one taxon, leading to its increase at the expense of other taxa (e.g. Booth et al. Citation2012; Clifford and Booth Citation2015). Interactive effects can be profound and complex, with environmental drivers being contingent on biotic interactions or vice versa. For example, Jackson and Blois (Citation2015) suggest that ‘the late-glacial megaherbivore decline may have been a proximal driver of vegetation changes that ultimately were a response to climatic changes that occurred centuries or more before (Gill et al. Citation2012)’.
The abrupt mid-Holocene decline of Tsuga canadensis in eastern North America was originally interpreted by Davis MB (Citation1981b, Citation1989c) as a result of a widespread insect- or pathogen-mediated attack (see also Allison et al. Citation1986; Bhiry and Filion Citation1996). Detailed climate reconstructions based on lake-level change and other proxies independent of the pollen record suggest that climate change may have been a major driver of this abrupt decline (e.g. Calcote Citation2003; Shuman et al. Citation2004; Foster et al. Citation2006; Marsicek et al. Citation2013). There is the possibility that pests or pathogens may have affected Tsuga trees in response to their stressed condition resulting from an abrupt climate change (Shuman et al. Citation2001; Calcote Citation2003; Foster et al. Citation2006), suggesting a complex interaction between climate, tree growth, and herbivory.
An obvious question to ask is whether a given vegetation type is a result of biotic interactions, neutral processes, environmental drivers, or human impact. Jackson and Blois (Citation2015) consider this to be a ‘futile’ question, as communities are a result of many interacting factors (Levins and Lewontin Citation1980). Any ‘explanation’ may, as Jackson and Blois (Citation2015) emphasise, be more a matter of causal ‘thickets’ than ‘chains’ (sensu Wimsatt Citation2007). Depending on the spatial and temporal scales, environmental change, biotic interactions including human impact, and neutral processes can all be important drivers of tree and forest dynamics.
9.4. Forest structure in temperate areas
Forests in lowland Europe and eastern North America during the early and mid Holocene (ca. 9000–6500 years ago) have long been assumed to be ‘climax forest’ (Iversen Citation1960), ‘primeval forest’ (Iversen Citation1967, Citation1973), ‘Urwald’ (Firbas Citation1949), ‘virgin forest’ (Jones EW Citation1945), ‘closed forest’ (Svenning Citation2002), or ‘wildwood’ (Rackham Citation2006). These inferred reconstructions of old tall trees forming a closed canopy, clumps of saplings and young trees in gaps created by natural disturbance such as windthrow, fire, or tree death, and a sparse field and ground flora are based on many ecological studies on the remaining widespread stands of presumed ‘primeval’ or ‘primary’ forest (Sabatini et al. Citation2018) in, for example, parts of the Balkan peninsula, remote areas in the foothills of the southern Alps, and lowland areas in eastern Europe (e.g. Iwaschkewitsch Citation1929; Fröhlich Citation1930; Markgraf F and Dengler Citation1931; Mauve Citation1931; Treguboov Citation1941; Jones EW Citation1945; Pigott Citation1975; Peterken Citation1996; Bradshaw RHW et al. Citation2011).
This long-standing and widely accepted hypothetical reconstruction of forest structure was challenged by Vera (Citation1997, Citation2000) who proposes that large herbivores within the forest maintained a much more open landscape and prevented the dominance of closed canopy ‘high forest’. Vera (Citation2000) proposes as a null hypothesis that (1) Corylus avellana, Quercus petraea, and Q. robur survive in a closed forest and regenerate in gaps in the canopy (Watt’s (Citation1947) ‘gap phase’ model of regeneration), and (2) large herbivores such as aurochs, bison, deer, elk, and wild horse followed the early-Holocene vegetation development but did not influence the course of the process of forest development or regeneration. Vera (Citation2000) tests his null hypothesis using selected published material including pollen-stratigraphical data. Although it is virtually impossible to reconstruct population densities of large herbivores in prehistoric times (Bradshaw RHW and Mitchell Citation1999), Vera (Citation2000) argues that their impact on forest structure can be inferred from the pollen record. Vera (Citation2000) emphasises that modern observations suggest that Corylus and Quercus require canopy gaps for regeneration (cf. Watt Citation1919; Rackham Citation2003, Citation2006) and as central European pollen records are dominated by these taxa in the mid Holocene, prehistoric forest must have been more open than today to allow the regeneration of Corylus and Quercus. Having rejected his null hypothesis, Vera (Citation2000, p.370) proposes an alternative hypothesis: ‘The natural vegetation consists of a mosaic of large and small grasslands, scrub, solitary trees, and groups of trees, in which the indigenous fauna of large herbivores is essential for the regeneration of the characteristic trees and shrubs of Europe. The wood-pasture can be seen as the closest modern analogy for this landscape.’ As Farjon (Citation2017a) notes ‘wood pasture’ is an incorrect translation of Domesday’s silva pastilis; it should strictly be pasture woodland. Vera’s (Citation2000) ideas have attracted much attention among forest conservationists and ecologists (e.g. Kirby Citation2003, Citation2004a; Kirby and Branson Citation2009; Fisher M Citation2012; Rotherham Citation2013; King Citation2017; Alexander K et al. Citation2018), particularly those favouring rewilding, wilding (see subsection 11.2.2) or, perhaps more contentiously, ecological restoration (see Fisher M Citation2013). Both the terms rewilding and ecological restoration imply knowledge of a baseline or reference conditions (see subsection 11.2.2).
The ideal test of Vera’s (Citation2000) pasture-woodland hypothesis would be a long-term grazing exclusion experiment conducted 6500–9000 years ago so that one can compare forest composition, or at least its proxy, fossil pollen assemblages, in areas with and without large herbivores. Such a ‘natural experiment’ is provided by insular Ireland that only supported wild boar and possibly some red deer during the early and mid Holocene (Mitchell FJG Citation2005). Mitchell FJG (Citation2005) compares pollen-analytical values for Corylus and Quercus for the period 6500–9500 years ago from England and lowland Europe with values from Ireland for the same time period. He shows that there are no statistically significant differences between Corylus and Quercus pollen values from Ireland and England and mainland Europe. These results suggest that the presence or absence of large herbivores had little or no impact on the relative abundances of Corylus and Quercus pollen in these areas and large herbivores were therefore not essential for the maintenance of Corylus and Quercus in early- or mid-Holocene forests. The pollen data that Mitchell FJG (Citation2005) uses in this analysis are from lakes and bogs with a pollen-source area of several square kilometres (regional pollen-source area). As different vegetation types and mosaics can occur within such an area, it is difficult to reconstruct the degree of landscape openness from such regional-scale pollen data. More local-scale pollen data are clearly required.
Pollen assemblages from small (20–30 m diameter) hollows derive most of their pollen from a much more restricted source areas with a radius of 500–100 m (‘stand-scale’ palynology, Bradshaw RHW Citation2013; Birks HJB and Berglund Citation2018). Mitchell FJG (Citation2005) assembles modern pollen data from small hollows throughout lowland Europe and calibrates the tree pollen values to the tree cover within the pollen-source area, He then applies this calibration to fossil assemblages from small hollows in southern Sweden, Denmark, and Ireland and shows that there are no indications of open-forest canopies (tree pollen values <50%) or pasture woodland at a local scale until about 3000 years ago, coincident with extensive human impact. There is no indication of open-forest canopies before human impact irrespective of the presence or absence of large herbivores. Pollen data reflecting two different spatial scales (regional and local) provide no support for Vera’s pasture-woodland hypothesis in lowland temperate Europe (Bradshaw RHW et al. Citation2003; Bradshaw RHW and Hannon Citation2004; Birks HJB Citation2005; Mitchell FJG Citation2005).
Other types of palaeoecological data such as fossil beetle assemblages (Whitehouse and Smith Citation2010) can be used to test the pasture-woodland or closed-forest hypotheses. Whitehouse and Smith (Citation2010) analysed 36 early- and mid-Holocene beetle assemblages from Britain. The assemblages when considered as tree, open-ground, or dung taxa show how variable the British prehistoric landscape was in terms of closed forest and open ground (e.g. Fyfe Citation2007). Open areas were clearly of local significance and were an important feature of the Holocene landscape in providing interglacial microrefugia for open-ground taxa (see Pigott and Walters Citation1954; Iversen Citation1967, Citation1973; Svenning Citation2002; Birks HJB and Willis Citation2008; Thomas CD Citation2009). The beetle assemblages suggest, however, that the role of herbivores in creating open areas was minimal until the onset of human impact in the Neolithic (Whitehouse and Smith Citation2010; see also Sandom et al. Citation2014b).
Recently, palynological results that apparently challenge the closed canopy forest hypothesis have been obtained for lowland Europe (e.g. Fyfe et al. Citation2013; Marquer et al. Citation2014, Citation2017; Theuerkauf et al. Citation2014). These studies derive quantitative estimates of past vegetation composition using the Regional Estimates of Vegetation Abundance from Large Sites (REVEALS) model of Sugita (Citation2007a) (see also Hellman et al. Citation2008). This model is designed to minimise representation biases in pollen data that result from differences in pollen production and dispersal of different taxa. In its reconstructions, REVEALS uses modern pollen-productivity estimates (PPEs) to derive quantitative estimates of taxa in the past. As many of the PPEs (e.g. Poaceae) used are derived from modern pollen-vegetation calibrations in cultural landscapes, there is the important problem that such PPEs may differ from PPEs derived from more natural old-growth forests such as Białowieża National Park on the border between Poland and Belarus (Baker AG et al. Citation2016). Poaceae pollen is six times more under-represented in old-growth forest than in south Swedish cultural landscapes (Baker AG et al. Citation2016). The apparent openness of early- and mid-Holocene vegetation as suggested by REVEALS (e.g. Fyfe et al. Citation2013; Marquer et al. Citation2014, Citation2017; Theuerkauf et al. Citation2014) should therefore be treated with much caution as the apparent openness of the landscape may simply be an artefact of applying modern PPEs for Poaceae from open cultural landscapes to early- and mid-Holocene tree-dominated pollen assemblages. Fyfe et al. (Citation2013) emphasise that their vegetation estimates suggesting more openness in British regional vegetation than is suggested by untransformed pollen percentage data ‘cannot at this stage be used to test the Vera hypothesis. The results describe first-order approximations of landscape/woodland openness at the regional scale, not the openness of woodland at the local scale: REVEALS results do not describe vegetation structure’. Farjon (Citation2017a) emphasises that ‘pollen diagrams do not reconstruct past vegetation structure … and no palynologist has ever made such a claim. All they show is the relative abundance of taxa (mainly tree genera but also shrubs and herbs) as indicated by deposited pollen.’
Overall, there is no convincing pollen-analytical or other palaeoecological evidence that supports Vera’s hypothesis, especially in Britain and Ireland. Bradshaw RHW et al. (Citation2003) emphasise that the impacts of past herbivore–vegetation interactions ‘will not be fully resolved until more is known about past ungulate population sizes’ (see also Svenning Citation2002; Smith D et al. Citation2014). Well-designed and detailed local-scale pollen studies are needed where pollen and insect remains are analysed together (Smith D et al. Citation2010) and spores of dung fungi such as Sporormiella-type spores are systematically counted (Svenning Citation2002). Such spores today show a monotonic relationship between accumulation rates and local mammal biomass density (Baker AG et al. Citation2017; see subsection 13.2).
A workshop was held at Knepp Estate in West Sussex (Tree Citation2018) in June 2017 to celebrate the 20th anniversary of Vera’s (Citation1997) doctoral thesis (subsequently translated, expanded, and updated as Vera Citation2000). The workshop’s title was ‘Freeing the Landscape: Grazing animals as ecosystem engineers’ (King Citation2017). All the talks can be viewed at https://knepp.co.uk/vera-conference-1. Many misleading statements were made at the workshop about palynology – ‘grass pollen disappears in the wind’ leading it to be under-represented; and ‘there are no pollen data from the chalk’. According to ecologist MJ Crawley, palynology is ‘not a science but a religion … a bizarre belief system’ based on assumptions such as that keystone species are only wind-pollinated, and that pollen of key species always finds its way into peat bogs (King Citation2017). Farjon (Citation2017a) suggests ‘The Frans Vera acolytes seem to misrepresent palynology’. Moreover, there is a major disconnect in time scales between the ‘acolytes’ and palynologists. The former highlight compelling evidence from lichens, molluscs, and beetles that herbivores play a key role in maintaining open pasture woodland today (e.g. Tree Citation2018). On the other hand, palynologists are discussing the relevance of Vera’s pasture-woodland hypothesis to the structure and composition of early- and mid-Holocene woodlands about 6500–9000 years ago (Mitchell FJG Citation2005). Despite this time gap of several thousand years and detailed stand-scale palynological results, the Vera hypothesis remains surprisingly controversial (e.g. Hodder et al. Citation2009; Fisher M Citation2012; King Citation2017; Farjon Citation2017a; Alexander K et al. Citation2018; Alexander K and Butler Citation2018; Gould Citation2018; Tree Citation2018). King (Citation2017) concludes ‘we are trying to complete the “what was the natural landscape of pre-human Europe” jigsaw, but we do not have the picture on the box, and many of the pieces are missing’. Rackham (Citation2006, p.99) proposes that the Vera model, ‘cannot be simply dismissed, there are ragged robins and devil’s-bits to be explained, as well as a place to be found for the beasts and Mesolithic people who lived on them. On Vera’s model it is easier to find room for ancient trees and their specific invertebrate animals and lichens. Ancient trees fare better in savanna where they escape the competition of younger neighbours and can more easily resist windthrow’. The Vera hypothesis tantalisingly remains ‘non-proven’ (see Rackham Citation2003, Citation2006; Kirby and Baker Citation2013; Shaw H and Whyte Citation2013; Sandom et al. Citation2014b; Kuneš and Abraham Citation2017; Farjon Citation2017b; Fyfe Citation2018).
9.5. Tree-population, ecosystem, and biogeographical models
9.5.1. Tree-population models
Detailed tree pollen-stratigraphical data when expressed as pollen accumulation rates (PAR; grains cm–2 yr–1) from sites of simple morphometry and shape and of small size can be viewed as long time-series of tree populations (at least of the haploid generation) at the scale of the pollen-source area of the site that may exhibit expansions, stability, oscillations, declines, and even local loss over long periods of time (Watts Citation1973). In a seminal paper, Watts (Citation1973) proposes that specific population-growth models such as the exponential and logistic models could be fitted to detailed PAR for the time interval (generally 250–2000 years) of tree arrival (if detectable) and expansion to asymptotic PAR values and assumed population stability. Such models require detailed PAR data with closely-spaced counts and a detailed age scale. Welten (Citation1944) pioneered the estimation of PAR using laminated sediments at Faulenseemoos in the Bernese Oberland and attempted to estimate the duration of population expansions (Watts Citation1973). PAR are not perfect records of past population sizes but they are adequate for the type of population modelling discussed here.
Various attempts have been made to estimate rates of population expansion and population decline as ‘doubling times’ or ‘halving times’. Such estimates are a convenient means of comparing rates of expansion or decline where doubling (or halving) time is simply loge(2/r) where r is the unrestricted rate of increase (or decrease) per individual and is the gradient of a plot of logeN against t where N is the number (PAR) present at time t (see Bennett Citation1983, Citation1986, Citation1988b). Estimates of tree doubling times and tree halving times are summarised in and , respectively, from a large number of studies in different parts of the world. In addition, doubling times of PAR of forest trees in southern Ontario after the marked decline of Tsuga canadensis pollen about 5400 years ago are listed in .
Table 13. Estimates of tree-population doubling times (td = logn(2/r); rounded to nearest 5 years) derived from pollen-stratigraphical data (PAR) from North America (Tsukada and Sugita Citation1982; Tsukada Citation1982c, Citation1982d; Bennett Citation1986, Citation1988a; MacDonald and Cwynar Citation1991; MacDonald Citation1993a; Fuller Citation1998; Edwards ME et al. Citation2015), England (Bennett Citation1983, Citation1988b), Central Europe (Giesecke et al. Citation2007; Bradshaw RHW et al. Citation2010), Italy (Magri Citation1989), north-east Australia (Walker and Chen Citation1987; Chen Citation1988), and Japan (Tsukada Citation1981, Citation1982a, Citation1982b, Citation1982c; Tsukada and Sugita Citation1982; Kito and Takimoto Citation1999). Modified from MacDonald (Citation1993a).
Table 14. Estimates of tree-population halving times (th = loge(2/r); rounded to nearest 5 years) derived from pollen-stratigraphical data (PAR) from North America (Tsukada and Sugita Citation1982), England (Peglar Citation1993a; Peglar and Birks Citation1993), and Japan (Tsukada Citation1983).
Table 15. Estimates of tree-population doubling times (td = loge(2/r); rounded to nearest 5 years) derived from pollen-stratigraphical data (PAR) from two sites in southern Ontario after the marked decline of Tsuga canadensis pollen at about 5400 years ago (calculated from data in Fuller Citation1998).
Bennett (Citation1986) compiles doubling times for trees based on observational data (). They range from 10 to 350 years, with a median of 35 years. The estimates from fossil PAR (range 30–1390 years, median 180 years) are for population increases over large areas (possibly up to 10 km2) and over long time periods, in contrast to the modern data which come from much smaller observational plots and over short time periods (Bennett Citation1986). Comparison of pollen-based doubling-times () highlights inter- and intra-taxon variability. Intra-taxon variability suggests that local environmental and biotic factors may have influenced fecundity and survivorship and hence the population growth of the tree taxon during its population increase (MacDonald Citation1993a). MacDonald and Cwynar’s (Citation1991) data suggest that there is much regional variability in the population growth rates (doubling times 80–1100 years) as Pinus contorta ssp. latifolia spread across western Canada. These data caution against the assumption that population doubling times of a geographically expanding taxon will be similar in all regions (Birks HJB Citation1989; MacDonald Citation1993a).
Table 16. Estimates of tree-population doubling times (td = loge(2/r); rounded to nearest 5 years) calculated from the eigenvalue (λ) of the transition matrix where logeλ = r (see Piñero et al. Citation1984). Data from various sources compiled and documented by Bennett (Citation1986).
In almost all cases examined, increases in PAR are best modelled by an exponential growth model with an abrupt plateau rather than a logistic curve with an asymptote (MacDonald Citation1993a). This predominance of exponential growth suggests that intraspecific competition was not a major influence on population growth and changes in population size were a direct response to changes in environmental conditions (MacDonald Citation1993a). If exponential growth with a sharp plateau is an accurate reflection of population expansion, it raises critical questions about controls on population growth and the appropriate model to use in predicting temporal patterns of plant invasions (MacDonald Citation1993a).
The estimated tree-population doubling times for trees in the north-eastern Australian tropics () show interesting differences between gymnosperm and angiosperm canopy trees and, not surprisingly, between canopy trees and early secondary forest taxa such as Trema. The doubling times from Valle di Castiglione near Rome (Magri Citation1989; ) are higher than elsewhere which are all Holocene in age whereas the Italian estimates are from the last 250,000 years including the last interglacial and the last glacial stages.
There are very few estimates of tree-population halving times (). These range from 5 to 35 years with the exception of the decline of Picea in the early or late Holocene in Japan, which was almost certainly a response to regional climate change. The short halving times for Ulmus in England (Peglar and Birks Citation1993; Peglar Citation1993a) and for Castanea dentata and Tsuga canadensis in eastern North America (Tsukada Citation1983) contrast with the high halving times of Picea. These dramatically low halving times reflect catastrophic events such as pathogenic attacks, possibly interacting with exogenous factors such as human activity or climate change.
The tree-population doubling times in southern Ontario after the decline of Tsuga canadensis in the mid Holocene (Fuller Citation1998) are surprisingly high, with values between 230 and 695 years, and, not surprisingly, 760 and 1000 years for T. canadensis (). Generally there are few estimates for doubling times prior to the Tsuga decline for eastern North America except for Fagus grandifolia (doubling time 240–445 years; Bennett Citation1988a) as almost all the North American values in are from the Pacific Northwest (e.g. Tsukada and Sugita Citation1982; Tsukada Citation1982a). Clearly more work is needed to estimate doubling and halving times for tree populations using the high quality pollen and chronological data within the European Pollen Database (Giesecke et al. Citation2016), the Neotoma database (Grimm EC Citation2008; Grimm EC et al. Citation2018; Williams et al. Citation2018), and the Latin American Pollen database (Flantua et al. Citation2015, Citation2016a).
Attempts have been made to combine data on geographic spread and changes in population abundances (e.g. Delcourt PA and Delcourt Citation1987) with limited success (see MacDonald Citation1993a). In a more detailed approach, Dexter et al. (Citation1987) analyse the geographic spread and population size changes of Fagus grandifolia in terms of a transport model typically used in biological diffusion studies. They propose that 94% of the observed variation in the Holocene pollen percentages of Fagus in eastern North America can be explained by (i) Fagus spread from areas of high population abundance; (ii) a northward drift in distribution; and (iii) local and regional changes in abundance. They conclude that changes in the distribution and abundance of Fagus reflect environmental changes. As Bennett (Citation1988d) argues, determining what these environmental factors are is problematic (cf. Webb T Citation1988b). Bennett (Citation1988a) proposes that variations in population growth rates unrelated to exogenous environmental variables such as endogenous biotic and abiotic factors may explain part of the variation in abundance generated by the Dexter et al. (Citation1987) model.
Bennett (Citation1990) expands the use of exponential and logistic population models to PAR data to include two kinetic models of population growth. The basic idea is that a change in a pollen assemblage may be triggered by taxa increasing by a model different from the basic exponential or logistic population models (e.g. rapid decline in response to a pathogenic attack or to intensive human activity). Models that accommodate such situations are used in reaction kinetics within physical chemistry. Such kinetic equations are, along with the mathematics of population growth, special cases of Lotka’s (Citation1907) general model of ‘the mode of growth of material aggregates’ (Bennett Citation1990). If PAR time-series are examined by reaction kinetic models, viewing PAR population change as a series of reactions between competing taxa, it may be possible to detect some of the underlying mechanisms behind taxon-by-taxon replacement. Using suitable null models it may be possible to test the hypothesis of an observed change being an immediate response to an extrinsic rapid climate shift (sensu Williams et al. Citation2011a) or being a continuing long-term (>1000 years) shift in response to one or more past climatic changes (Davis MB Citation1986b – intrinsic change sensu Williams et al. Citation2011a). Bennett (Citation1990) suggests that 'thinking about long-term vegetation change in population terms (the factors controlling rates of increase and decrease) and in terms of reaction kinetics can lead to insights into the development of plant communities’.
9.5.2. Ecosystem models
In multiproxy palaeoecological studies (e.g. Shuman et al. Citation2004; Flessa and Jackson Citation2005a; Birks HH and Birks Citation2006; Birks HJB and Birks Citation2008), one or more variable-type is studied to provide an independent palaeoenvironmental proxy (e.g. stable isotopes). Responses in the other proxies (e.g. pollen, plant macrofossils) to the inferred palaeoenvironmental changes can be explored and questions of response time, lags, rates of change, turnover, and ecosystem drivers can be quantified (e.g. Lotter and Birks Citation2003b; Birks HJB and Birks Citation2008). The end result is a temporal series of biological assemblages (e.g. pollen) and an independent temporal series of past environmental change (e.g. regional climate). Millennial-scale ecological dynamics, as reconstructed from palaeoecological records, often show non-linear shifts in the trajectory of the drivers of biotic change such that discrete disturbance, stress, and climate regimes can be observed on either side of a breakpoint or threshold in the temporal-series data (e.g. Willis et al. Citation2010a; Jeffers et al. Citation2011b). Such thresholds can initiate cascading changes through ecosystems including shifts between alternative stable states in population and community dynamics (Scheffer and Carpenter Citation2003) but they are very often difficult to predict (Scheffer et al. Citation2009). Can palaeoecological temporal-series be used to assess how interactions between biota respond to abrupt environmental change – do they show gradual intrinsic responses or sharp extrinsic shifts between alternative states (Jeffers et al. Citation2011b)?
Jeffers et al. (Citation2011a) use ecosystem model-fitting and model-selection analyses of a series of non-linear dynamic models to generate predicted changes in taxon interactions and abundances over time and across abrupt changes in climate, fire, ungulate herbivore density, and nitrogen availability. Relatively simple and transparent mechanistic models of ecological dynamics are used and Akaike Information Criteria (AIC) weights are used to assess the relative amount of support for each mechanistic model. Jeffers et al. (Citation2011a) show that as Quercus replaced Pinus in the early Holocene in response to climate change, rates of nitrogen cycling increased. The tree–nitrogen interaction was unchanged across the climate threshold and the changes in tree type, suggesting that the tree-population shifts were not driven by nitrogen availability. Using the same approach at five sites in Ireland and Britain, Jeffers et al. (Citation2018) assess the extent to which megaherbivores influenced vegetation composition and nutrient cycling at the late-glacial/Holocene transition. Climate change, plant–soil and plant–plant interactions, and reduced burning contributed to the expansion of woody plants and declining nitrogen availability. Shrub biomass, here mainly Corylus pollen accumulation rates, was consistently one of the strongest predictors of ecosystem change, equalling or exceeding the effects of other biotic and abiotic factors. Megaherbivores had little impact on regional vegetation composition or nitrogen availability. This approach (Jeffers et al. Citation2011b, Citation2012, Citation2015, Citation2018) has considerable potential in palaeoecology as it may allow the testing of multiple working hypotheses, distinguishing between chaos or non-linear deterministic dynamics from noise or random effects in pollen-stratigraphical data, and testing the ideas of Deevey (Citation1984) about stress, strain, and stability in past ecological systems.
Davis MB and Botkin (Citation1985) apply a forest-growth simulation model (JABOWA) to explore the sensitivity of cool-temperate forests in eastern North America to rapid climate change and show that response delays of 100–200 years limit the sensitivity of the pollen record to detect rapid temperature changes. Related forest succession models (e.g. FORCLIM; Heiri et al. Citation2006) can simulate Holocene treeline dynamics and have been used to test hypotheses about possible causal factors for the inferred changes in treeline based on pollen and plant macrofossils in the central Swiss Alps (see also Schwörer et al. (Citation2014) for an extension of this approach using a dynamic landscape vegetation model (LANDCLIM) to simulate past and future drivers of Holocene sub-alpine forest dynamics). Henne et al. (Citation2011, Citation2013) and Tinner et al. (Citation2013) illustrate the use of these types of models in predicting future changes and validating causal factors.
Connor et al. (Citation2018a) analyse multi-proxy data (pollen and spores, non-pollen palynomorphs, charcoal, diatoms, chrysophyte cysts, midges, mites, testate amoebae) from a mire in south-western Georgia (Caucasus) with a suite of numerical techniques and population models to assess the major drivers of change in assemblage diversity and population stability. They demonstrate that within the mire ecosystem, the main drivers are both extrinsic and intrinsic but at different stages in the mire development. The model results accord with theoretical predictions that population increases lead to greater stability and declines result in instability at the population level. Dynamics that differ from predictions are explained in terms of random variation and interspecific competition. Population change and diversity are positively correlated in all taxonomic groups, suggesting that population-level instability is greater in more diverse systems, even though such systems are themselves more stable. This study demonstrates the potential of testing population theory with long-term data from detailed palaeoecological studies to assess the predictive power of existing theoretical frameworks.
As computing power increases and algorithms become more refined and efficient, ecological modelling techniques can now fit ever more complex models (Lavine Citation2010). There is an increasing tension between accurate representations of palaeoecological patterns and underlying processes and the need for accessible models that provide novel inferences and robust and valid predictions (LaDeau Citation2010; Lele Citation2010) to bridge the gap between ecological theory and associated modelling and the quality, robustness, and limitations of palaeoecological data (Waller LA Citation2010). Palaeoecologists must be aware of the limitations of their data and the assumptions of statistical and mathematical models in ecology and thus in palaeoecology so as to avoid ‘living dangerously with big fancy models’ (Lavine Citation2010). Nearly 30 years ago Bennett (Citation1990) warned in discussing the use of pollen-stratigraphical data in ecological models that ‘[t]he most severe problem is the paucity of data that are of high enough quality, especially with respect to temporal resolution of samples (Birks HJB and Gordon Citation1985). Future advances in the interpretation of pollen sequences as records of population change will depend on the willingness of palaeoecologists to analyse enough pollen samples … Models are available when there are data with which to test them’. The situation has not changed greatly in the intervening years (Birks HJB and Berglund Citation2018).
9.5.3. Biogeographical models
Although these are not directly relevant to tree and forest dynamics, these models are currently making extensive use of pollen-stratigraphical data. They are discussed here briefly because they relate, in part, to ecosystem models and, in part, to broad-scale models of forest development.
In the last 20–30 years, biogeography has rapidly developed from being primarily descriptive to becoming increasingly quantitative and analytical. Approaches such as co-occurrence analysis (e.g. Sanderson and Pimm Citation2015), null models (e.g. Gotelli and Graves Citation1996), and correlative species distribution models (SDMs; e.g. Elith and Leathwick Citation2009; Franklin J Citation2010; Svenning et al. Citation2011; Norberg A et al. Citation2019) are now extensively used to evaluate patterns of taxon occurrences and co-occurrences, to model occurrences in relation to environmental predictors, particularly climate, to assess present-day biogeographical patterns across space and time, and to predict or hindcast such patterns in the future or in the past. These approaches were developed for the analysis of biogeographical data that consist of presence/absence data of taxon occurrences in different localities (e.g. point, islands, grid squares, regions). Surprisingly, given the complex (closed) quantitative nature of stratigraphic pollen-percentage data (Fagerlind Citation1952; Birks HJB and Gordon Citation1985), taxon co-occurrence analysis has been used with pollen data transformed to presences or absences to evaluate the role of climate, dispersal limitation, and biotic associations in influencing community patterns over broad spatial and temporal scales (e.g. Blois et al. Citation2014; Lyons et al. Citation2016b; cf. Bertelsmeier and Ollier Citation2016; Telford et al. Citation2016). Taxon co-occurrence analysis of pollen presences and absences over broad geographical areas such as eastern North America can result in statistically significant segregated taxon pairs such as the boreal larch Larix laricina and the subtropical mangrove Rhizophora (extended data provided by Lyons et al. Citation2016b) or L. laricina and the southern Taxodium distichum (Supplementary material of Blois et al. Citation2014). Such pairs clearly reflect broad-scale biogeographical patterns and not local community patterns or assembly rules. There is a major confusion of spatial scales between the data and the ecological model implicit in taxon co-occurrence analysis.
Spatial modelling methods that simultaneously consider multiple co-occurrences of taxa to model patterns of taxon assemblages in space and time have been developed as an alternative to SDMs (e.g. Ferrier and Guisan Citation2006; Ferrier et al. Citation2007; Fitzpatrick et al. Citation2013; Nieto-Lugilde et al. Citation2018), so-called community-level models (CLMs). Such models have a greater ecological realism than early attempts to model community assemblages in space and time such as the ‘assemble-then-predict’ or ‘predict-then-assemble’ strategies (Ferrier and Guisan Citation2006). CLMs attempt ‘assemble-and-predict-together’ within a single process (Ferrier and Guisan Citation2006). There are various CLM algorithms (Nieto-Lugilde et al. Citation2018). Generalised dissimilarity modelling (GDM; Ferrier et al. Citation2007) relates dissimilarities in taxon composition between pairs of sites as a biological distance to sites that differ in environmental conditions (environmental distance) and how isolated they are from each other (geographic distance). GDM predictions permit detection of spatial variation in assemblage composition (Nieto-Lugilde et al. Citation2018). GDMs are used by Blois et al. (Citation2013a, Citation2013b) to model drivers of spatial patterns in pollen-assemblage composition from eastern North America. Blois et al. (Citation2013a) model the relationships between climate, geography, and compositional dissimilarity at 1000 year periods using GDM and determine the strongest predictors of compositional dissimilarity. The best set of predictors changes across time with summer temperature and geographical distance being the strongest predictors for most time periods. Climate predicts biotic patterns well in the Holocene but less well in the late-glacial when factors such as dispersal limitation, colonisation, and biotic factors were probably important. Blois et al. (Citation2013b) use GDMs to show that at the broad spatial and temporal scales of eastern North America and 21,000 years, respectively, the ‘space-for-time substitution’ approach provides predictions about 72% as accurate as ‘time-for-time’ predictions in the late-glacial but its performance drops to 14% in the Holocene where temporal variation in climate is small relative to spatial variation. In these applications of GDM with palynological data, the pollen data are analysed as percentages relative to the upland pollen sum that excludes pollen taxa only identified to family level (e.g. Amaranthaceae, Asteraceae, Poaceae). Tree pollen values are therefore inflated in assemblages from prairie sites and give a distorted record of tree values in the mid-Holocene ‘prairie periods’. A different approach is used by Maguire KC et al. (Citation2016) where relative pollen values are converted to a presence/absence matrix after applying a threshold scaled to 5% of the maximum value of the taxon in question.
Svenning et al. (Citation2011), Maguire KC et al. (Citation2015), and Nieto-Lugilde et al. (Citation2018) review the rapidly developing fields of species and community-level distribution modelling within palaeoecology. As Nieto-Lugilde et al. (Citation2018) comment ‘[a] major shortcoming of CLMs is their reliance on presence-absence community composition data’. Howard et al. (Citation2014) (see also Ashcroft et al. Citation2017) show how SDM performance is enhanced by using abundance data. It is likely that SDMs and CLMs may also be improved further with abundance data, as discussed by Blois (Citation2012a) and pioneered by Huntley et al. (Citation2010) and Blois et al. (Citation2013a, Citation2013b).
9.6. Tree invasion and forest-stand dynamics
Pollen-stratigraphical sequences from temperate interglacial stages including the Holocene consistently show a series of tree population expansions (, ), often followed by a compositional equilibrium or stability phase (see ). As discussed in subsection 4.4, we have a poor understanding of how trees seemingly spread at rates of up to 205–1000 m yr–1. The preferred hypothesis (Rudolph Citation1930; Fægri Citation1949; Firbas Citation1949; Smith AG Citation1965; Watts Citation1973; Godwin Citation1975; Walker Citation1982a; Giesecke Citation2013; Giesecke and Brewer Citation2018) is that taxa are spread by long-distance chance dispersal of propagules into locally favourable areas such as openings caused by wind-throw, death, fire, or disease (Bradshaw RHW and Hannon Citation2004) beyond the main range of the taxon in question to form small outliers (see .2). These eventually mature and in turn act as seed parents for local expansion of these outliers and further establishment in newly created gaps (see .3). We similarly have a poor understanding of the processes of how tree invasion, stand development, and forest dynamics may occur as most palynological data lack the spatial and temporal resolution required to infer the underlying processes (see MacDonald Citation1993a; Gillson et al. Citation2008; cf. Rejmánek Citation1999). The account below is, by necessity, largely based on ecological ideas and observations (e.g. Crawley Citation1987; Rejmánek Citation1989; Burke and Grime Citation1996) rather than on direct palynological evidence because relevant fine-scale pollen data are very sparse.
Figure 17. Generalised pollen-stratigraphical patterns and inferred population processes suggested by Watts (Citation1973) in the establishment and expansion of a tree in the mesocratic phase within an interglacial stage. The very approximate durations of the establishment and expansion intervals are also shown. Modified from Birks HJB and Tinner (Citation2016a).
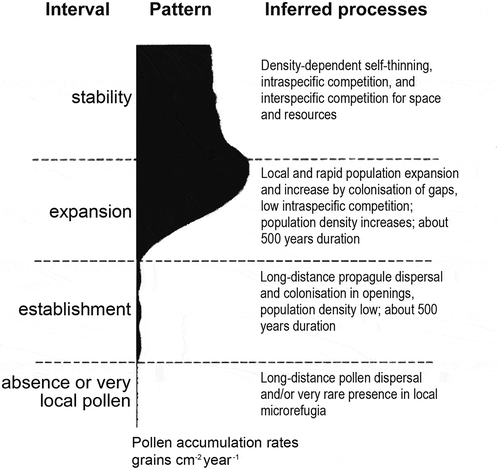
In contrast to the initial slow phase of outlying seedling establishment with low population densities, the subsequent expansion phase may, for some taxa, occur rapidly (.3) because local propagule deposition within the nearby vegetation increases, probability of establishment rises, and population densities grow (Birks HJB Citation1986). Intraspecific competition may remain low at this stage (Watts Citation1973). Over several generations, these populations may expand and coalesce with the main population (.4). Population growth may then flatten off or even decrease owing to competition and the inability of many forest trees to regenerate under their own canopies (; Birks HJB Citation1986). Watts (Citation1973) suggests that during invasion, populations stabilise after a period when the less common ‘invader’ has expanded vigorously at the expense of the common ‘resident’ (Harper Citation1967). Any invading seedlings would initially have a greater probability of establishment and achieving dominance in a gap than seedlings of the ‘residents’. As invasion progresses and the invader’s population increases, intraspecific competition may become more important, and eventually a relative balance between seedling establishment of resident and invading taxa may occur (), thereby enabling co-existence in a quasi-equilibrium until the next invasion occurs, possibly triggered by subtle climate change (Harper Citation1967; Watts Citation1973; Forcier Citation1975; Fox JF Citation1977; Davis MB Citation2001b). Watts (Citation1973) proposes that the major determinants of invasion and population expansion are thus the time required for propagule dispersal and colonisation of gaps in the existing vegetation and for seedlings to mature into seed parents. Interference in seedling establishment, survival, and maturation may be important. Green (Citation1982) shows that tree invasion and expansion into existing forests in Nova Scotia coincided with rare, extensive natural disturbances, suggesting that availability of large openings may be an important determinant of invasion and subsequent expansion. Almost all mesocratic trees require openings for establishment and regeneration, possibly because of nutrient release, increased irradiance, relaxed root competition, or reduced host-specific insect predation (Jones EW Citation1945; Pigott Citation1975).
Invasion and expansion may also occur without climate change (Smith AG Citation1965; Watts Citation1973; Edwards ME et al. Citation2015), although they can be favoured by climate change (e.g. Davis MB Citation2001b; Jackson et al. Citation2009b; Anderson RS et al. Citation2017). Frequent gaps provide conditions of low competition for invaders and may be relatively common in vegetation following climatic change across a critical ecological threshold that restricts, for example, seed production and regeneration of the residents (Walker Citation1982a). Storms, widespread fire, or other catastrophic disturbances may create infrequent but more extensive disturbances in vegetation affected by, for example, prolonged drought, senescence, nutrient shortage, or widespread pathogen attacks (e.g. Foster et al. Citation1998; Romme et al. Citation1998; Turner MG et al. Citation1998; Turner MG and Dale Citation1998) than in vegetation growing under more optimal conditions. Rates of taxon replacement will clearly vary from taxon to taxon according to demographic factors, longevity, gregariousness, competition, and environmental conditions, and may extend over many generations (Walker Citation1982a; Edwards ME et al. Citation2015).
There is considerable interest in modern invasion biology (e.g. Crawley Citation1987, Citation1989; Hengeveld Citation1989; Williamson Citation1996; Williamson and Fitter Citation1996; Davis MA Citation2009; Prins and Gordon Citation2014) and there are potentially interesting parallels between the landscape and stand-scale processes involved in tree invasions in interglacial stages and modern invasions (MacDonald Citation1993a; Gillson et al. Citation2008). Two detailed studies from North America of palaeo-invasions in the Holocene (Davis MB Citation1987; Davis MB et al. Citation1998; Lyford et al. Citation2003) demonstrate the complexity of the processes involved. The study by Lyford et al. (Citation2003) concerns the invasion of Juniperus osteosperma into xeric steppe areas in Wyoming, Montana, and Utah and is discussed in subsection 4.4. The study by Davis MB et al. (Citation1998) focusses on the Holocene invasion of Tsuga canadensis into mixed mesic deciduous-coniferous forests of northern Michigan. It involves pollen analyses of small forest hollows (‘stand-scale’ palynology – Bradshaw RHW Citation2013) which record forest composition at the scale of 1–3 ha (Davis MB et al. Citation1998). Four stands of Tsuga today were formed by an 800-year long invasion by hemlock into patches of Pinus strobus about 3000 years ago. There is no evidence for disturbance during these invasions. Tsuga co-existed with P. strobus for several thousand years but eventually became dominant at different times at three of the stands. The underlying exogenous drivers of these changes may have been climatic changes over the last 4000 years. The history of four nearby Acer saccharum stands is more variable. Three sugar maple stands were originally dominated by Quercus. Two were not invaded by Tsuga and the third was invaded for a few centuries by low numbers of Tsuga only. Clearly invasion by Tsuga depended on the composition of the resident forest. Acer saccharum and Tilia americana increased and by 2000 years ago they had formed mixed mesic maple forest. The fourth Acer stand was invaded by Tsuga but reverted to an Acer stand 500–1000 years ago. The presence of a wood layer in the forest-pond sediments suggests a catastrophic windstorm about 500–1000 years ago that may have played a key part in these complex forest dynamics (Davis MB et al. Citation1998). Further details and additional examples of the complex dynamics of invasion in the past and of local stand dynamics are given by Davis MB (Citation1987, Citation1989c, Citation1994, Citation2001b), Davis MB et al. (Citation1992), MacDonald (Citation1993a), Parshall (Citation2002), and Bradshaw RHW and Lindbladh (Citation2005). These and other detailed studies illustrate how difficult it is to generalise about the underlying processes and to identify the critical drivers of tree invasion and stand development and dynamics in the past (Bennett Citation1988b, Citation1993; Bennett and Willis Citation1995; Fuller Citation1997). The apparent complexity of past tree invasions and stand dynamics warns against assuming simple models for invasions today by introduced taxa. Gillson et al. (Citation2008) and Gillson (Citation2009) propose that palaeoecology and invasion ecology can act in synergy to help understand the ‘landscape ecology of invasive spread’ (With Citation2002). Invasions in the past may be potentially relevant to modern invasions because the processes of establishment and spread (‘invasion’) of taxa can be comparable despite the temporal differences (Rejmánek Citation1999; Richardson et al. Citation2000; Gillson Citation2009; Hobbs RJ et al. Citation2018).
9.7. Stability and change
9.7.1. Stability
Pollen stratigraphies typically consist of two major types of patterns – intervals where the composition and values of the different taxa are relatively constant (); and intervals where the composition and values of some or all taxa change (; e.g. Ritchie Citation1985). Changes may be abrupt (about 20 – 100 years) or gradual (about 200 – 500 years) (Williams et al. Citation2011a). Walker (Citation1966, Citation1972, Citation1989) and Watts (Citation1973) suggest that for some purposes, particularly in interpreting the observed patterns in terms of underlying ecological processes and dynamics, it is important to distinguish between these ‘constant’ and ‘change’ intervals and between abrupt and gradual changes. Nearly all numerical procedures used to partition pollen-stratigraphical data have as their underlying numerical model the delimitation of homogeneous units, so-called pollen-assemblage zones (e.g. Birks HJB and Gordon Citation1985; Birks HJB Citation2012b). Such procedures can provide useful partitions for stratigraphies with abrupt changes but are less effective with stratigraphies with gradual changes. Birks HJB and Gordon (Citation1985) present a variable-barriers approach to identify specifically gradual transitions that may occur over relatively long intervals rather than simply placing partitions in the middle of such gradual transition intervals. The approach also identifies intervals of abrupt change. They appear as late additions to the cluster groups of ‘constant’-type spectra in the agglomerative analysis. This approach is particularly useful in partitioning late-glacial pollen stratigraphies where there can be complex mixtures of ‘stable’ intervals and gradual and abrupt changes (e.g. Birks HH and Mathewes Citation1978; Birks HJB Citation1981a). A 10,000–11,000 year Holocene pollen-stratigraphy from north-west Europe typically consists of about 80–90% of relatively constant ‘stable’ pollen values and 10–20% of changing values as shown by the variable-barriers method (Birks HJB and Gordon Citation1985: Birks HJB unpublished). What do these long intervals of constant pollen values reflect, even when the inherent binomial and multinomial counting errors (Birks HJB and Gordon Citation1985) are considered?
Constancy in pollen composition and values over long time periods implies constancy in the vegetation within the pollen-source area of the site and, by inference, environmental constancy (Fægri and Iversen Citation1950). In their chapter on ‘The interpretation of pollen diagrams’, (Fægri and Iversen Citation1950) present hypothetical pollen-stratigraphical reflections of the same broad-scale climatic oscillation in different vegetational zones on a north–south transect (). For localities far from a vegetational ecotone (localities D and F in ), no major pollen-stratigraphical changes are observed as the vegetation type remains the same. Constancy in pollen composition and values over long periods may thus reflect environmental constancy (‘stability’) or the lack of ecological sensitivity at some localities far from ecotones.
Figure 18. Hypothetical pollen-stratigraphical reflections of the same climate oscillation in different regions along an imaginary transect from south to north. In the far north, locality A, the climatic oscillation results in a retreat of tundra and expansion of ice. Locality B records the classical Allerød–Younger Dryas–early Holocene pattern of Betula woodland–tundra–Betula woodland. Farther south, the climatic oscillation is recorded by other vegetational and hence pollen-stratigraphical changes. Note that at localities D and F no major pollen-stratigraphical changes are observed as no ecotones are crossed. Based on Fægri and Iversen (Citation1950).
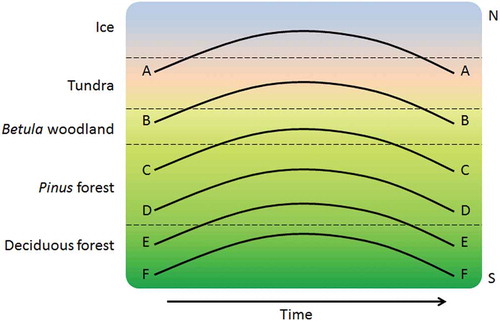
The term stability in ecology and other disciplines can reflect many different concepts; the analysis by Grimm V and Wissel (Citation1997) considers 163 definitions of 70 different stability concepts. They propose that the general term ‘stability’ is so ambiguous as to be useless and it should be replaced by stability properties. These are (1) staying essentially unchanged (constancy), (2) staying essentially unchanged despite the presence of potentially disturbing external influences (resistance), (3) returning to the reference state (or dynamic) after a temporary disturbance (resilience), and (4) persistence through time of an ecological system (persistence). They also propose that the relevant ecological situation where the term ‘stability’ is used should be defined in terms of six features – variable of interest; level of description; reference state; disturbance; spatial scale; and temporal scale. Hansson and Helgesson (Citation2003) also conclude that there are three basic concepts of stability – constancy; robustness (= resistance); and resilience. Within a formal philosophical framework, they show that robustness is a limiting case of resilience, but neither constancy nor resilience can be defined in terms of the other. There are thus two or three basic concepts of stability – constancy, resilience, and persistence.
Pollen-analytical investigations suggest that a given vegetation type or landscape mosaic generally do not persist for more than 10,000–15,000 years due to major secular changes in Earth’s climate system (Jackson Citation2006a). Persistence is thus a stability concept relevant to pollen analysis only at particular time scales and in certain geographical areas (). Pollen analysts and other palaeoecologists (e.g. Smith AG Citation1965; Cole KL Citation1985, Citation1986) imported from plant ecology the concept of biological inertia presented by (Gorham Citation1957) and Pearsall (Citation1959) into long-term vegetation dynamics. Gorham (Citation1957) proposes that ‘once having colonized an area, some plants continue to exist there long after the conditions suitable for their establishment have disappeared’. Inertia is thus a component of extinction debt (Jackson and Sax Citation2010) and of persistence and implies that the species or system of interest can tolerate external fluctuations. The other component of persistence is resistance – a measure of how little the species or assemblage changes in response to external pressures. Inertia and resistance thus determine biotic responses to a perturbation. Smith AG (Citation1965) presents a detailed discussion of the problems and importance of inertia and thresholds in understanding late-glacial and Holocene vegetation history and lays the foundation for several important conceptual developments on the interface between pollen analysis and modern ecology (Birks HJB Citation1993b). Smith AG (Citation1965) postulates that ‘the effect of a climatic change will always depend on whether or not the changing local climate or microclimate could cross the threshold for a vegetational change’ (see ). Ecologists are increasingly recognising the influence of landscape configuration on temporal variability, biotic responses, and ecosystem properties (e.g. Kratz et al. Citation1991; Johnstone et al. Citation2010; Elmendorf et al. Citation2012; Schwörer et al. Citation2017). Davies et al. (Citation2017) show the importance of topography and microclimate in Holocene vegetation dynamics, persistence, and composition at the local scale at five sites in the Scottish Highlands.
In introducing inertia, Smith AG (Citation1965) uses the example from Pearsall (Citation1959) about a closed forest offering no scope for invaders and that such a vegetation and its associated fauna possess an enormous inertia (see also von Holle et al. Citation2003; Martin PH et al. Citation2009). Smith AG (Citation1965) recognises that threshold and inertia are related. He discusses that when a climate change in one area may produce conditions favourable for an additional species, the same climate change in another area may produce conditions only just above the critical physiological threshold for the existence of that species, perhaps only in specialised microhabitats. Thus the mere presence of the existing vegetation and its high production of propagules might act against any rapid vegetation change. If the change was such as to make conditions unfavourable for the existing species, the effects of inertia would be restricted to the life-span of the existing species (see Gorham Citation1957). The concepts of biological inertia and thresholds are used by, for example, Walker (Citation1982b), Grimm EC (Citation1983), Cole KL (Citation1985, Citation1986), Carrión et al. (Citation2001a), and Davies et al. (Citation2017) to interpret observed patterns in pollen or plant-macrofossil stratigraphies. However, Markgraf V (Citation1986) and Bradshaw RHW (Citation1993b) question the importance of biological inertia in long-term vegetation dynamics.
The patterns detectable in pollen-stratigraphical data are scale-dependent both in time and space, as are many ecological data. Different scales of resolution can result in different patterns being detected (e.g. Delcourt HR et al. Citation1983; Green Citation1983; Prentice Citation1983; Birks HJB and Gordon Citation1985; Birks HJB Citation1986; Delcourt HR and Delcourt Citation1988, Citation1991; Roy et al. Citation1996; Jackson Citation2006a; Gillingham et al. Citation2012). Different processes are likely to be important determinants of the patterns observed at different scales. The importance of scale in the interpretation of pollen-stratigraphical data requires fine-resolution temporal data from several sites. Gajewski (Citation1987) presents results from such a study designed to explore the potential importance of external drivers at the temporal scales of centuries and millennia. The study uses annually laminated sediment covering the last 2000 years from seven lakes on a transect from Minnesota to Maine. The temporal resolution has intervals of about 40 years between samples with each pollen sample containing pollen that have accumulated for about ten years. The seven pollen stratigraphies do not exhibit any abrupt changes and only two stratigraphies show slight gradual trends. All seven stratigraphies appear, on visual inspection, to be relatively unchanging and constant.
Gajewski (Citation1987) uses principal components analysis (PCA) to summarise the temporal patterns in each pollen stratigraphy and to identify the hidden ‘latent’ structure within each data-set. PCA identifies comparable temporal scales of variation within each sequence – long-term changes over thousands of years (PCA axis 1: 23–51% of the total variation); medium-frequency oscillations over many centuries (PCA axis 2: 12–28%); and high-frequency fluctuations during many decades (PCA axis 3: 10–15%). Not all palynological changes are replicated at each of the sites. Gajewski (Citation1987) interprets the stratigraphies and underlying trends in terms of the susceptibility of the vegetation of the catchments to change based on proximity to ecotones, soil moisture, landscape topography, and recent disturbance history (Delcourt HR and Delcourt Citation1991). Gajewski (Citation1987) identifies broad-scale climate trends tied to increased winter frequency of the Arctic airmass in the Great Lakes region being reflected by PCA axis 1. PCA axis 2 reflects an intermediate level of medium-frequency cycles over many centuries including the Little Ice Age with cooling of 1–2°C in mean annual temperature from 1450 CE to 1950 CE resulting in changes in the frequency of natural disturbances. Higher frequency fluctuations captured by PCA axis 3 may also reflect disturbance events over many decades (Delcourt HR and Delcourt Citation1991). Gajewski’s (Citation1987) study using several sites with high-resolution stratigraphical data shows that the pollen stratigraphy and, by inference, the vegetation at each site have been influenced by a variety of physical drivers superimposed but occurring at different frequencies (Delcourt HR and Delcourt Citation1991). Thus the current structure and composition of the vegetation along Gajewski’s (Citation1987) transect are a result of physical drivers operative at different frequencies. It also shows the important link between climate and natural disturbance regime in influencing the stratigraphical and vegetational patterns in time and space. It provides an important warning that pollen stratigraphies which show little or no change (‘stable’ or constant composition and values) do not necessarily imply no changes in the vegetation or the environment within the source area of the sites in question. Numerical techniques such as PCA or other ordination or dimension-reduction methods (Legendre and Birks Citation2012), stratigraphically constrained partitioning approaches (Birks HJB Citation2012b), multivariate regression trees (Simpson GL and Birks Citation2012), and recently developed techniques for ‘change-point’ analysis (e.g. Muggeo Citation2003; Hothorn et al. Citation2006; Erdman and Emerson Citation2007) can easily test for ‘constancy’ or ‘change’ within pollen-stratigraphical data. Change over a wide range of temporal and spatial scales is likely to be the rule rather than the exception (Delcourt HR et al. Citation1983; Prentice Citation1983, Citation1986b; Webb T Citation1986; Delcourt HR and Delcourt Citation1991; Jackson et al. Citation2009b). Such changes raise the question of ‘stable states’ that are currently a topic of intense interest in ecological dynamics (e.g. Fukami and Nakajima Citation2011).
9.7.2. Change
Ecological thresholds, where a pollen stratigraphy and, by inference, vegetation type or ecosystem switches from one ‘stable state’ to another, usually within a relatively short time interval, are well documented in many terrestrial ecosystems and have long been recognised in pollen-stratigraphical records (e.g. Smith AG Citation1965; Watts Citation1973, Citation1982; Walker Citation1982b; Cole KL Citation1985; Ritchie Citation1985; Birks HJB Citation1986; Willis et al. Citation2010a). Past and present human impact is well known to be a driver of such switches with evidence to suggest that the likelihood of ecological thresholds may increase when humans reduce resilience (Folke et al. Citation2004; Willis et al. Citation2010a). There are, of course, many thresholds that occur in the absence of humans and a key question is what combination of climatic and non-climatic variables result in a switch from one ‘stable state’ to another – so-called regime shift (see Jeppesen et al. (Citation2018) for a detailed definition and clear descriptions of different types of regime shifts)? Such thresholds can include climate change (e.g. Jackson et al. Citation2009a); natural disturbances such as fire, pathogens, or storms (e.g. Foster et al. Citation1998; Dale et al. Citation2001; Thom and Seidl Citation2016; Morris et al. Citation2018); introduction of non-native biota (e.g. Williamson Citation1999; Simberloff Citation2000); demographic factors (e.g. Carter RN and Prince Citation1981); and interactions between some or all of these drivers (e.g. Pulsford et al. Citation2016; Thom et al. Citation2017; Fyfe et al. Citation2018a). For example, Carrión et al. (Citation2001a) show in central Spain that over the last 9000 years, several threshold changes have occurred, shifting from one ‘stable’ pollen assemblage to another (Pinus to Quercus deciduous) and then a second shift from Quercus evergreen to Pinus. The triggers appear to have been a combination of at least two abiotic drivers. In the first shift, an interval of higher precipitation combined with less evaporation, and in the second, increased aridity combined with increased fire frequency. Such ‘double trigger’ thresholds are not uncommon (e.g. Prentice Citation1983; Jackson et al. Citation2009a, Citation2009b; Willis et al. Citation2010a).
Williams et al. (Citation2011a) discuss abrupt ecological changes from the perspective of Late Quaternary vegetation history. They distinguish between abrupt change driven by extrinsic drivers and abrupt changes driven by intrinsic drivers (). Abrupt changes include biotic responses to abrupt millennial-scale climate changes during the late-glacial (see ; e.g. Birks HH and Ammann Citation2000; Williams et al. Citation2002; Ammann et al. Citation2013a, Citation2013b) or early Holocene (e.g. Tinner and Lotter Citation2001), rapid changes in tree density at the forest–prairie ecotone in Minnesota during the Holocene (e.g. Grimm EC Citation1983; Williams et al. Citation2009), and the spectacular crash in Ulmus pollen abundances in southern England about 6000 years ago (Peglar and Birks Citation1993; Peglar Citation1993a) or of Alnus pollen in Finland and Poland about 600–800 CE (Stivrins et al. Citation2017; Latałowa et al. Citation2019). Often, abrupt ecological changes may have been driven by similarly abrupt climate changes which are common during the Quaternary at all time-scales (Lockwood JG Citation2001; Williams et al. Citation2011a; Bradley Citation2015). Williams et al. (Citation2011a) emphasise that not all abrupt ecological changes necessarily result from abrupt climate change. There is increasing recognition that ecosystems can respond abruptly and non-linearly to an accumulation of multiple drivers. Despite the plethora of terms such as tipping points, ecological surprises, thresholds, regime shifts, critical transitions, bifurcations, and other related terms, the underlying concern is the same – ecosystems are complex and are influenced by a mixture of external drivers, positive and negative loops, and fast and slow linear and non-linear processes (Williams et al. Citation2011a). Ecosystems can thus display apparent stasis (or resilience or resistance) when exposed to an external driver such as climate change until an internal threshold is passed and the system undergoes a large and abrupt regime shift, perhaps to a new ‘stable state’ ().
Figure 19. Contrasts between an abrupt extrinsically forced ecological change (left panels) with an abrupt intrinsically forced change (right panels). In the extrinsically forced change, a large widespread change in temperature (a), e.g. the Younger Dryas–Holocene transition, causes abrupt ecological changes that are synchronous with or slightly lag behind the climate change at sites 1, 2, and 3 (c), resulting in a strong temporal coherence of change (e). With intrinsically forced change, a long-term progressive trend such as droughts and megadroughts (b) are mediated by site-specific (1–6) thresholds (d). These site-specific thresholds cause abrupt changes across the drought period (f). Clusters of site-level abrupt changes may result from extreme events which may impact many sites simultaneously (f). Modified from Williams et al. (Citation2011a).
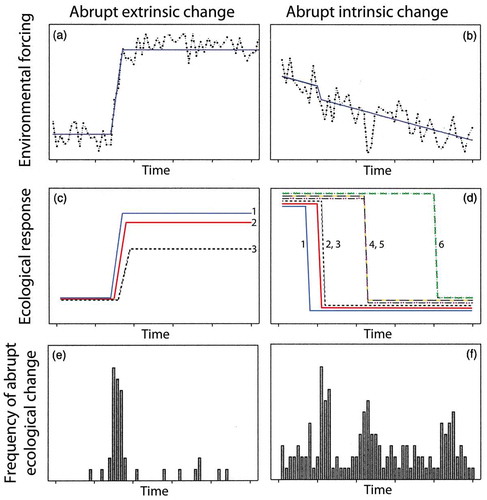
Distinguishing between extrinsic and intrinsic changes can, in part, be done by compiling and analysing a regional network of pollen-analytical records and looking for temporal patterns of change (). Extrinsically driven changes can be expected to show strong temporal coherence whereas intrinsically driven changes may show some but not strong temporal coherence. Examples of extrinsic abrupt changes occur commonly during the late-glacial and early Holocene in the Northern Hemisphere (Williams et al. Citation2002, Citation2011a). In intrinsic abrupt changes, the timing and rate of ecological response to climate change may be influenced by local biotic and abiotic processes and by stochastic processes such as disturbance or local climatic extremes. The result is ‘temporal mosaics’ of abrupt ecological change in which the timings and rates of change vary among species within sites and among sites (Williams et al. Citation2011a). Examples of intrinsic abrupt changes include the mid-Holocene aridification of North Africa (e.g. Ritchie et al. Citation1985; Ritchie and Haynes Citation1987; Haynes et al. Citation1989; Ritchie Citation1994; Claussen Citation1998; deMenocal et al. Citation2000; Kuper and Kröpelin Citation2006; Holmes Citation2008; Kröpelin et al. Citation2008), the early-Holocene aridification of central North America (e.g. Grimm EC Citation1983, Citation2001; Clark et al. Citation2001b, Citation2002; Brown KJ et al. Citation2005; Nelson DM and Hu Citation2008; Williams et al. Citation2009, Citation2010; Grimm EC et al. Citation2011; Shuman Citation2012), and the occurrences of megadroughts in western North America (e.g. Cook et al. Citation2010a, Citation2010b).
One of the first demonstrations by pollen analysis of multiple stable states and abrupt intrinsic shifts is from the Big Woods area of south-central Minnesota that was dominated in pre-settlement times by Acer saccharum, Ostrya virginiana, Tilia americana, Ulmus americana, and U. rubra (Grimm EC Citation1983, Citation1984). Near the transition between prairie and deciduous forest, pollen stratigraphies do not show gradual changes but exhibit abrupt changes in pollen assemblages. It appears that as a result of topographic breaks between gently rolling and hilly terrains and riverine margins, a landscape patch may have been occupied by one vegetation type for centuries or thousands of years and then, with the crossing of a critical ecological threshold, there is an abrupt change to another vegetation type (; Grimm EC Citation1983; Delcourt HR and Delcourt Citation1991). By examining the early records of the land surveyors between 1847 and 1856 CE, Grimm EC (Citation1984) shows that given comparable site conditions of soil drainage and texture, the site’s history of wildfire events dictates which vegetation type may persist as a seemingly ‘stable’ unit over centuries or millennia, i.e. the vegetation in the Big Woods area consists of multiple stable states () that established after a disturbance event such as fire. In the area, the spatial array of fire breaks is a function of the configuration of streams, wetlands, and lakes on the landscape and by the boundaries between gently rolling outwash plains and hilly moraines. The fire-probability pattern (Grimm EC Citation1984) of a landscape patch is influenced by both abiotic and biotic factors, which in turn constrain the potential vegetation types that can occupy the patch (Delcourt HR and Delcourt Citation1991). Grimm EC (Citation1984) proposes that the fire-probability pattern was substantially influenced by the occurrence of fires created by native Americans, the local distribution and occurrence of fire breaks, soil texture and moisture, the flammability of the existing vegetation type and its accumulation of fuel-load, and the likelihood of fire spread assessed by the areal extent and lateral continuity of the landscape patch. Along a transect from prairie to deciduous forest from south-western to central Minnesota, landscape patches with very different natural fire probabilities and wildfire regimes produce a landscape mosaic with a range of successional states following fire events. These states include fire-promoted prairie, fire-tolerant oak-scrub (Quercus macrocarpa) savannah and aspen (Populus tremuloides) stands, fire-infrequent mixed Quercus forest, and fire-intolerant forest of Acer, Tilia, and Ulmus (Bigwoods in ). Grimm EC (Citation1983, Citation1984) concludes that multiple stable states characterise the vegetation (at least the pre-settlement vegetation) of the prairie–forest border in the western Great Lakes region. The present-day composition of forest stands there cannot be reliably predicted based solely on the basis of abiotic factors of the environment today because historical legacies resulting from the long-term site history of disturbance and succession are a strong control on the landscape and its vegetation (; Berland et al. Citation2011).
Figure 20. (a) The ecological thresholds for the establishment of prairie, oak (Quercus) woodland, and mixed deciduous forest (Bigwoods) in central Minnesota plotted along a climatic gradient (e.g. precipitation) that influences fire frequency. Redrawn from Grimm EC (Citation1983). (b) Schematic representation of ecological thresholds for the establishment of prairie and woodland in central Minnesota. The horizontal axis is time; the vertical axis is a climatic gradient (e.g. mean annual precipitation). In the upper right quadrant (woodland only) above the woodland threshold for annual precipitation (Pw), only woodland will persist as a stable state once it is established. Either of two potentially stable states – woodland or prairie – can persist in areas with climate delimited by the thresholds for woodland (Pw) and prairie (Pp), while only prairie will persist as a stable state below the critical threshold for annual precipitation (Pp) and for increased fire recurrence (lower right quadrant: prairie only). Tw represents the minimum time required for woodland to invade prairie. The vegetation outcomes and pathways are summarised in the right-hand column. Redrawn from Grimm EC (Citation1983) and Delcourt HR and Delcourt (Citation1991).
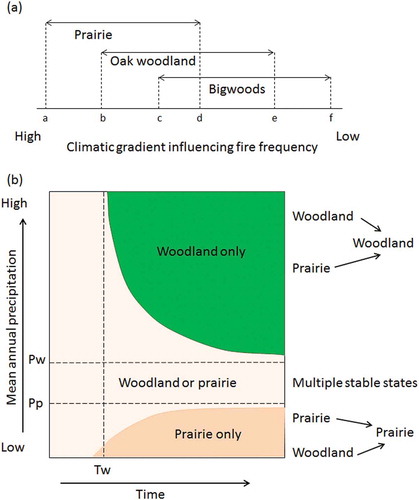
Fire palaeoecology is now a major area of research in Quaternary botany (e.g. Swetnam and Betancourt Citation1990; Bond and Keeley Citation2005; Marlon et al. Citation2012; Iglesias et al. Citation2015b). Techniques of charcoal analysis and characterisation are becoming more sophisticated (e.g. Jensen K et al. Citation2007Citation2007; Lynch et al. Citation2011) and many detailed long-term records of fire history are now available (e.g. Colombaroli et al. Citation2009; Tinner et al. Citation2009; Brown KJ and Giesecke Citation2014; Minckley and Long Citation2016; Fletcher et al. Citation2018; Moreno et al. Citation2018). As fire histories become more detailed (), interest is focusing on the interaction of fire, climate, vegetation, biomass and fuel-load, topography, land-use, and fauna (Tweiten et al. Citation2009, Citation2015; Gavin Citation2010; Mueller et al. Citation2014) and on rapid state shifts, alternative stable states, alternative transient states, abrupt changes (‘tipping points’; see Turney et al. Citation2016), and system dynamics.
Table 17. Selected examples of detailed fire histories reconstructed from charcoal in Quaternary botanical sequences with emphasis on interactions of fire with other variables, and rapid state shifts and system dynamics.
Theoretical ecologists, interested in ecosystem dynamics, and applied ecologists and managers have been exploring various aspects of catastrophic regime shifts in ecosystems such as tipping points and critical thresholds, and identifying early-warning signals for critical transitions (see above and ). Within Quaternary botany, pollen analysts have mainly focused on regime shifts and tipping points associated with interactions between climate and fire (see above). Palaeolimnologists are attempting to detect regime shifts or early-warning signals of a critical transition from aquatic biota and sediment geochemistry (). Beck et al. (Citation2018) study diatoms, pollen, geochemistry, and charcoal from the last 2400 years from a lake in Tasmania. Their data suggest that diatom composition changed at about 820 yr BP concomitant with increased fire disturbance in the lake’s catchment. Early-warning signals of increased compositional variance and rate-of-change occur in the diatom assemblages prior to this critical transition. Interestingly, the pollen records remain complacent to the fire disturbance until the shift in diatom composition. Disturbance taxa invade the vegetation and compositional variance and rate-of-change increase, suggesting the vegetation may be approaching a critical transition and the probable collapse of the local rainforest (Beck et al. Citation2018).
Table 18. Selected examples of recent theoretical and Quaternary studies on ecosystem dynamics, in particular regime shifts.
Palaeoecologists are also beginning to explore life-history and functional traits to aid in the interpretation of long-term records of, for example, pollen (Lacourse Citation2009; Brussel et al. Citation2018; van der Sande et al. Citation2019), plant macrofossils (Bhagwat and Willis Citation2008), and testate amoebae (e.g. Fournier et al. Citation2015; Marcisz et al. Citation2016; Connor et al. Citation2018a; Lamentowicz et al. Citation2019). Trait analysis of pollen data is greatly hampered by the fact that many pollen taxa can only be identified to genus (e.g. Pinus) or family level (e.g. Poaceae). The relevant numerical tools for robust and statistically significant trait analysis exist or are being developed but appropriate trait data are lacking for many of the fossil taxa. The concept of traits for a large family such as Poaceae is currently unresolved. Van der Sande et al. (Citation2019) use phylogenetic signals to derive genus-level and even family-level average trait values.
Disturbances play a key role in both short-term and long-term vegetational dynamics (e.g. White PS Citation1979; Delcourt HR et al. Citation1983; Pickett and White Citation1987; Delcourt HR and Delcourt Citation1991; Turner MG Citation2010; Newman Citation2019). Climate can influence the character, frequency, and magnitude of major perturbations that comprise the disturbance regime (White PS Citation1979). Herben et al. (Citation2016) derive from a large number of vegetation plots in central Europe, representing nearly 40 vegetation types, indicator values or indices for disturbance frequency and for disturbance severity for 1248 species. Disturbance frequency and intensity are both important in determining diversity-disturbance relationships (Miller AD et al. Citation2011). Such indices are being used in community and landscape ecology and in palaeoecology to estimate different aspects of the disturbance regime and their role in vegetation dynamics and to test the ‘intermediate-disturbance-diversity’ hypothesis (Kuneš et al. Citation2019).
The intermediate disturbance-diversity hypothesis of Connell (Citation1978) proposes a unimodal relationship between taxon richness and disturbance, with the peak of taxon richness at levels of intermediate disturbance. The robustness of this hypothesis has frequently been questioned (e.g. Millar and Woolfenden Citation1999; Roxburgh et al. Citation2004; Fox JW Citation2013) despite the many empirical demonstrations of a unimodal pattern (e.g. Mackey RL and Currie Citation2001; Sheil and Burslem Citation2013). Kuneš et al. (Citation2019) reconstruct disturbance and diversity dynamics in central Europe for 900-year time-windows over the last 12,000 years using pollen-stratigraphical data from over 90 sites, pollen richness estimated by rarefaction analysis, and the disturbance frequency indicator values of Herben et al. (Citation2016). Disturbance frequency is highest in the early and late Holocene and lowest in the mid Holocene. The disturbance–diversity relationship changes during the Holocene from a unimodal to a monotonic increasing pattern at about 4000 years ago. This change is interpreted as a result of land-use changes in the last 4000 years creating an increasingly patchy landscape that provided more habitats for disturbance-tolerant ‘ruderal’ taxa. Kuneš et al. (Citation2019) propose that the general hypothesis that taxon immigration, and hence the structure and composition of the regional taxon pool, determines biotic responses to external factors such as disturbance. This study illustrates the value of detailed palynological data covering broad temporal and spatial scales to test a critical ecological hypothesis and demonstrates that intermediate disturbance can result in unimodal or monotonic relationships with richness depending on factors such as the regional species pool.
The rigorous analysis of pollen-analytical and other palaeoecological stratigraphies in terms of abrupt extrinsic and intrinsic changes, critical thresholds, traits, disturbances, and change and stability demands fine-resolution data, excellent chronological control, and associated palaeoenvironmental reconstructions that are independent of the fossil biota being studied ecologically. Obtaining such data and reconstructions remains a major challenge (Thomas ZA Citation2016), as does the interpretation of such changes, critical thresholds, and stable states. The detailed studies by de Boer et al. (Citation2013a), Calder and Shuman (Citation2019), and Calder et al. (Citation2019) highlight this major interpretative challenge.
9.8. Antiquity of woodland types in Britain
How old are the woodland types that we see today? Jackson (Citation2006a) discusses the origin and termination of ecosystems in Late Quaternary time with particular reference to the western United States. He argues that new vegetation types originated when particular plant taxa became abundant or dominant in an area under particular environmental conditions (see also Jackson et al. Citation2009b). Such assemblages may terminate in the area gradually or abruptly when the dominant taxa are replaced by other taxa, usually in response to environmental change (including climate) or major disturbances (see subsection 11.3.8). Is such history the same in Britain?
The National Vegetation Classification (NVC) for Britain (Rodwell et al. Citation1991) describes and documents 18 woodland vegetation types today. Nine of these types produce characteristic pollen assemblages (Birks HJB Citation1973, Citation1982; Bradshaw RHW Citation1981), thereby allowing the recognition of these nine types in pollen-stratigraphical records (). In addition there are four common pollen assemblages dominated by tree pollen that do not have obvious widespread modern counterparts in British vegetation today although they occur very locally on steep slopes or in coppiced woodlands. Three of these assemblages involve abundant Corylus avellana pollen and the fourth is dominated by Tilia cordata pollen. Tilia cordata and some T. platyphyllos (Godwin Citation1975; Pigott Citation1981b; Waller MP and Early Citation2015) were probably major, if not dominant components of British woodland as far north as the central Lake District and north-east England (Birks HJB et al. Citation1975a). They never reached south-west England, Scotland, or Ireland (Birks HJB Citation1989). These 13 types () represent the major woodland assemblages present in Britain during the late Holocene prior to the widespread planting of exotic conifers in the British uplands in the middle and late twentieth century.
Table 19. Approximate age of modern British woodland assemblages inferred from pollen-analytical stratigraphies and macrofossil data. The assemblages are based on the dominant tree taxa as indicated from pollen and/or plant macrofossil taxa. To aid vegetation reconstruction, fossil tree-pollen values have been transformed by Andersen’s (Citation1970) general pollen representation factors as modified for British taxa (Edwards ME Citation1986). The antecedent pollen assemblage or vegetation type are based on available dated pollen data from Britain (Birks HJB Citation1989; Bennett and Birks Citation1990; Fyfe et al. Citation2010, Citation2013; Brewer et al. Citation2017). For details of the NVC types, see Rodwell et al. (Citation1991).
Several points emerge from examining the 13 woodland types. Virtually all arose at different times within Britain and many had antecedent assemblages that differed markedly in composition and often structure. Human disturbance has played a major part directly or indirectly in the origin of some of the woodland types in the last 3000–4000 years such as Fagus sylvatica (e.g. Godwin Citation1962; Thorley Citation1981; Waller MP and Hamilton Citation2000; Waller MP and Early Citation2015), Fraxinus excelsior (e.g. Pigott and Pigott Citation1969) woodlands, and Taxus baccata (e.g. Peglar Citation1993b). Fraxinus excelsior is interesting in that it occurred in damp Alnus woodland from about 6800 years ago but after about 3200 years ago it expanded into a new habitat to form the Fraxinus-dominated woodlands on the Carboniferous Limestone of the Pennines and Mendips. These woods are probably unique to Britain (and parts of Ireland). They almost certainly have an anthropogenic origin (e.g. Pigott Citation1969; Merton Citation1970) although ash was undoubtedly a native but local component in Alnus–Fraxinus stands within Quercus and Corylus–Quercus–Ulmus woodlands in the mid Holocene (Birks HJB Citation1989). Many of today’s woodland types that originated in the early or mid Holocene () occur in specialised habitats such as swamps and fen carrs (NVC W1–W3), shallow chalk soils (NVC W12–W15), dry sandy infertile soils (NVC W18), or shallow limestone soils often on steep slopes (NVC W8, W9). The woodland types that have no widespread modern counterpart probably occurred on damp to mesic soils with intermediate fertility, conditions that may have been strongly favoured by prehistoric people for clearance, settlement, and agriculture (Godwin Citation1975).
9.9. Extent of forest cover and landscape openness
9.9.1. Introduction
The extent of past forest cover and of landscape openness are currently topics of major concern in global ecology, Earth system science, and coupled climate–vegetation modelling because human activities have potentially influenced the climate system through deforestation and early agriculture long before humans started to emit CO2 from fossil fuel combustion (Ruddiman Citation2003, Citation2005, Citation2013a). Deforestation can affect climate at many scales from microclimate to global climate (Bala et al. Citation2007). The effects on global climate are influenced, in part, by the increased amounts of atmospheric CO2 from deforestation and by regional and local changes of land-surface properties (e.g. Strandberg et al. Citation2014). Such changes have a direct effect on the regional climate, including changes in albedo and energy fluxes between the land surface and the atmosphere (e.g. Pielke et al. Citation2011). Since forests generally have a lower reflectivity than unforested or deforested areas, the albedo effect from deforestation leads to lower regional temperatures. Reduced vegetation cover also results in reduced evapotranspiration that leads to higher air temperatures. However, the magnitude of the evapotranspiration changes depends on local factors such as soil-moisture availability (Strandberg et al. Citation2014). The impacts of changing forest cover and land-use over the last 8000 years on climate have been, and continue to be, extensively debated (e.g. Ruddiman Citation2003, Citation2005, Citation2013a; Oldfield Citation2005, Citation2008; Birks HJB Citation2008; Lenton Citation2016). Anthropogenic land-cover change is, after all, the most important transformation of the Earth system to occur in the pre-industrial Holocene, with potential impacts for carbon, water, and sediment cycles, biodiversity and the provision of ecosystem services, and regional and local climate (Klein Goldewijk et al. Citation2011; Kaplan et al. Citation2017). However, there are currently major differences between estimates of carbon budgets based on palaeoecological evidence and those based on models (e.g. Pedersen TF et al. Citation2003; Joos et al. Citation2004). These unresolved questions highlight the importance of considering climate–land-cover interactions and of comparing model-based simulations and estimates with empirical data for past land-cover change. Oldfield (Citation2005, Citation2008) emphasises that empirical approaches for estimating the extent of deforestation and carbon sequestration and release as a result of human activity need increased quantification and improved robustness (Birks HJB Citation2008). Similarly the possible effects of past land-use and land-cover changes on climate directly via changes in albedo, moisture retention, and dust fluxes on atmospheric gas concentrations and thus on climate are important topics within Earth system science where new, critical, and innovative approaches are being developed (e.g. Oldfield Citation2008; Jacobson MC et al. Citation2010; Lee et al. Citation2010; Schlesinger and Bernhardt Citation2013). Clearly the reconstructions of forest cover and of landscape openness using pollen-analytical data is an important contribution to these topics (Gaillard et al. Citation2008a, Citation2010).
9.9.2. Pollen-based reconstructions of forest-cover and landscape openness
Pollen analysts have been reconstructing forest composition from geographical arrays of pollen-stratigraphical data from many sites since the days of von Post (Citation1924, Citation1929, Citation1930) (e.g. Rudolph Citation1930; Firbas Citation1949; see Birks HJB and Berglund Citation2018 for a historical review). From the very beginnings of pollen analysis, palynologists have realised that there is no simple 1:1 relationship between pollen percentages and vegetation percentages (e.g. Hesselman Citation1916; von Post Citation1918; Fægri Citation1947; Fagerlind Citation1952; von Post Citation1967). Von Post in his 1916 lecture warned ‘as long as we have no indices to express the relative pollen productivity of the various trees, nor to express the different degrees to which their pollen is dispersed, we have no right to seek in the percentage figures an adequate expression of the composition of the forest communities’ (von Post Citation1967).
Iversen (Citation1947) proposed ‘correction factors’ or pollen-representation factors that could be applied to fossil pollen assemblages to derive transformed pollen values that might resemble the vegetation more closely than untransformed pollen values. This idea was formalised by Davis MB (Citation1963) in the concept of R-values and developed in detail by Andersen ST (Citation1970). Andersen distinguished between pollen-production factors (P-values) based on semi-absolute pollen and vegetation data and pollen-representation factors (R-values) based on relative pollen and vegetation data. He recognised, for the first time, the importance of pollen from outside the immediate pollen-source area, the background or PO component in estimating P-values. This study led directly to the so-called extended R-value models for relative percentage data (Parsons and Prentice Citation1981; Prentice and Parsons Citation1983; Prentice and Webb Citation1986; Prentice Citation1986a). These models incorporate the PO component from outside the sampled pollen-source area in modern pollen-percentage assemblages from small- to medium-sized lakes. These developments rapidly led to models of pollen deposition (Prentice Citation1985, Citation1988b; Sugita Citation1993, Citation1994) that attempt to quantify the pollen-source area and production and dispersal biases in pollen data and to predict the effect of basin size on the relative representation of different taxa. Simulations using these models (Sugita Citation1994, Citation1998) provide important insights into the spatial scale of pollen-based vegetation reconstructions. Sugita (Citation2007a, Citation2007b) presents a landscape-reconstruction algorithm (LRA) for the quantitative reconstruction of past vegetation composition and hence forest cover. The LRA consists of two parts: REVEALS and LOVE. REVEALS (Regional Estimates of VEgetation Abundance for Large Sites; Sugita Citation2007a) reconstructs vegetation composition in 104–105 km2 using pollen assemblages from large lakes (> 100 ha) that have small site-to-site variations even though the vegetation may be highly heterogeneous. LOVE (LOcal Vegetation Estimates; Sugita Citation2007b) incorporates the REVEALS estimates of regional vegetation composition into a quantitative model for reconstructing vegetation composition in small areas (1–103 km2) using pollen records from smaller sites. The LRA provides robust and repeatable quantitative estimates of the percentage cover of major trees and herbs at both the regional and local scales wherever pollen-production estimates – one of the most important parameters in the LRA models – have been estimated for the major taxa. The LRA is now being widely used to estimate past vegetation composition and forest cover at different spatial scales (e.g. Hellman et al. Citation2008; Gaillard et al. Citation2008b; Soepboer et al. Citation2010; Nielsen et al. Citation2012; Cui et al. Citation2013; Fyfe et al. Citation2013; Marquer et al. Citation2014, Citation2017; Åkesson et al. Citation2015; Hultberg et al. Citation2015; Abraham et al. Citation2016; Mehl and Hjelle Citation2016; Kaplan et al. Citation2017; Richer and Gearey Citation2017; Roberts N et al. Citation2018). An important development of particular value in incorporating pollen-based reconstructions of past land-cover into dynamic vegetation–climate models is the statistical creation of spatially continuous maps of past land cover from point estimates provided by REVEALS or LOVE (Pirzamanbein et al. Citation2014). Comparisons have recently been attempted between LRA-based reconstructions and two historical land-use scenarios (Kaplan et al. Citation2017). Neither land-use scenario matches the pollen-based reconstructions precisely, for a number of factors implicit in the land-use scenarios.
The LRA approach is stimulating methodological developments (e.g. Farrell et al. Citation2016), model improvements (e.g. Theuerkauf et al. Citation2012, Citation2016), critical testing of its assumptions (e.g. Conedera et al. Citation2006; Matthias et al. Citation2012; Matthias and Giesecke Citation2014; Theuerkauf et al. Citation2015; Baker AG et al. Citation2016; Mariani et al. Citation2016), and alternative approaches for the quantitative reconstruction of past vegetation composition and land-cover change (e.g. Bunting MJ and Middleton Citation2009; Paciorek and McLachlan Citation2009; Fyfe et al. Citation2010; Woodbridge et al. Citation2014; Dawson A et al. Citation2016; Goring et al. Citation2016; Matthes et al. Citation2016; Mrotzek et al. Citation2016; Paciorek et al. Citation2016; Binney et al. Citation2017; Bunting MJ et al. Citation2018; Theuerkauf and Couwenberg Citation2018). Approaches are also being developed to reconstruct quantitatively past fine-scale vegetation mosaic patterns through an extended down-scaling approach (Theuerkauf and Joosten Citation2009; Theuerkauf et al. Citation2014; Theuerkauf and Couwenberg Citation2017).
Several attempts have been made to reconstruct land-cover or woody plant-cover over large geographical areas using a combination of modern and fossil pollen data and modern satellite remote-sensing data from the Advanced Very High Resolution Radiometer (AVHRR; Williams and Jackson Citation2003). These AVHRR data are some of the longest continual series of images of the Earth’s surface and have been used to estimate land-cover and land-use change, net primary productivity, woody plant cover, leaf-area index, and other aspects of the Earth’s biosphere (Williams and Jackson Citation2003; Williams et al. Citation2011b). The approach involves (i) modern pollen data-sets calibrated against modern leaf-area index, woody plant cover, or other AVHRR products; and (ii) a network of fossil pollen sequences that are used in the reconstruction of temporal changes in leaf-area index (e.g. Gonzales et al. Citation2008; Williams et al. Citation2008; Herzschuh et al. Citation2010), total, conifer, and deciduous tree cover (e.g. Williams Citation2003; Tarasov et al. Citation2007; Williams et al. Citation2009, Citation2011b; Tian et al. Citation2016; Zanon et al. Citation2018), and normalised difference vegetation index (NDVI; e.g. D’Antoni and Schäbitz Citation1995). The same approach has also been used at the scale of a single site to reconstruct the cover of confiers, broadleaved trees, and open vegetation (Bezrukova et al. Citation2010).
The most detailed application of this approach to date is by Williams et al. (Citation2011b) in their study of variations in tree cover across the Northern Hemisphere forest–tundra ecotone. Their reconstructions show the expansion of forest following deglaciation and reveal significant hemispheric asymmetries in the Holocene position, steepness, and history of the ecotone. For example, in much of northern Asia, forests reached their maximum expansion during the early Holocene and then retreated, whereas in western Canada, forest expansion and infilling continued through the Holocene. The woody cover reconstructions are consistent with macrofossil-based reconstructions of northern tree-line dynamics but complement them by considering areas not only from the treeline limit (recorded by macrofossils) but also throughout the entire ecotone. Williams et al. (Citation2011b) combine their woody cover reconstruction with the Lund-Potsdam-Jena (LPJ) dynamic global vegetation model to reconstruct above-ground carbon sequestration. The model estimates that changes in northern forest density resulted in at least a 47.7 Gt C increase in above-ground carbon sequestration between 21,000 and 9000 yr BP, a 13.9 Gt C increase between 9000 and 6000 yr BP, and 3.5 Gt C loss of above-ground carbon after 6000 yr BP (Williams et al. Citation2011b). These estimates are consistent with atmospheric carbon-isotope measurements for the Holocene (Indermühle et al. Citation1999) which suggest carbon uptake by the terrestrial biosphere until about 6000 yr BP followed by small carbon releases from the terrestrial biosphere until 1000 yr BP (Williams et al. Citation2011b).
The drivers of changes in the Holocene carbon cycle remain unresolved (Foley et al. Citation1994; Bradshaw RHW and Sykes Citation2014), however, particularly the observed 20 ppmv increase in atmospheric CO2 over the last 8000 years (Indermühle et al. Citation1999; Williams et al. Citation2011b). Proposed mechanisms (Joos et al. Citation2004) include release from terrestrial vegetation, a delayed release of carbon from the oceans following an early Holocene pulse in terrestrial carbon uptake as vegetation cover increased, growth of coral reefs, other changes in the ocean carbon cycle, and early anthropogenic land-use (Foley et al. Citation1994; Ruddiman Citation2003, Citation2005, Citation2007, Citation2013b; Williams et al. Citation2011b).
The estimation of a 13.9 Gt C net sequestration by northern forests between 9000 and 6000 yr BP (Williams et al. Citation2011b) is equivalent to a 6.3 ppmv drawdown from the atmosphere, corresponding closely to the observed 8 ppmv decrease in atmospheric CO2 between 10,500 and 8200 yr BP (Indermühle et al. Citation1999) and supporting the hypothesis that this early-Holocene decrease in atmospheric CO2 was caused by the expansion and increasing growth of northern forests (Indermühle et al. Citation1999). Northern forests appear to have been a very minor contributor to the 20 ppmv rise in atmospheric CO2 between 8000 and 2000 yr BP, as they were taking carbon up until about 6000 yr BP. Thus the source of carbon for this 20 ppmv rise is more likely to be due to changes in the marine carbonate cycle (Joos et al. Citation2004; Elsig et al. Citation2009; Williams et al. Citation2011b). The early-Holocene anthropogenic hypothesis of Ruddiman (Citation2003, Citation2005, Citation2007) is not relevant here as northern forests experienced no substantial land clearance or supported any agriculture (Williams et al. Citation2011b).There are now many approaches at a range of spatial scales such as the LRA and the use of AVHRR data to reconstruct quantitatively vegetation composition, tree cover, and landscape openness. The reliability and robustness of these quantitative reconstructions have increased enormously since Iversen’s (Citation1947) and Davis’ (Citation1963) pioneering development of ‘correction factors’ and R-values, Palynologists now have a very powerful toolkit for reconstructing forest cover and for investigating forest dynamics, composition, and structure at a range of spatial scales including the continental and hemispherical scales.
9.10. Conclusions
Changes in pollen assemblages and, by inference, tree abundances and vegetation composition over thousands of years are the underlying ecological bases for pollen analysis as a tool in ecology. Palynology has contributed greatly to our knowledge of tree, forest, and landscape history and of broad-scale patterns of tree range changes and abundances. The processes behind these observed patterns are complex and remain poorly understood. They include environmental forcing, biotic interactions, neutral processes, and, in the Holocene, human impact and associated disturbances. Pollen analysis can provide abundant information at a range of spatial and temporal scales about vegetation composition, particularly forest composition but little about forest structure. Forest structure is a topic that is attracting much attention at present as a result of the currently controversial pasture-woodland hypothesis. There is, however, no palynological evidence to support the idea of any extensive early-Holocene pasture-woodland across lowland Europe, but there is a tendency among some advocates of the pasture-woodland idea not to question this idea but to reject the reliability of pollen analysis (King Citation2017; Farjon Citation2017a).
Pollen-analytical data are being used with varying degrees of success in a range of population, ecosystem, and biogeographical models to quantify rates of change and to test specific, ecological hypotheses about underlying processes. The limiting factor in many such modelling studies is the quality and quantity of the pollen-stratigraphical data and their associated chronological control. Our understanding of tree invasions, stand dynamics, and questions of stability, thresholds, and compositional change are also limited by available data and time controls. Major advances in reconstructing the extent of forest cover over broad geographical areas are providing important links between pollen analysis, global ecology, vegetation modelling, and Earth system science. Palynologists have ingeniously exploited the use of satellite remote-sensing data as a predictor of modern pollen assemblages (responses) to transform fossil pollen assemblages into estimates of past landscape variables such as net primary productivity, leaf-area index, or woody plant cover over broad areas.
Considerable advances have been made since the early days of pollen analysis as a tool to reconstruct forest history and to provide a fourth dimension to ecology. With the development of continental and global databases of pollen and other palaeoecological data and of appropriate numerical tools for data-mining of very large data-sets, considerable advances in understanding forest and tree dynamics can be expected in the coming decades.
This review now considers selected applications of Quaternary botany to topics of current interest in ecology and biogeography – human impact in tropical rainforests, conservation science, island biology, plant–animal interactions, and biodiversity changes.
10. Tropical palynology and human impact in tropical rainforests
10.1. Introduction
Quaternary pollen analysis began in Scandinavia in 1916 (von Post Citation1916) and had quickly spread through Europe and three other continents by the late 1930s (Birks HJB and Berglund Citation2018). The first palynological study of a tropical area was not until Selling’s (Citation1948, Citation1951) detailed reconstruction of the vegetational history of the Hawaiian Islands (Kaua’i, Moloka’i, West Mau’i). This major study was only possible through Selling’s (Citation1946, Citation1947) earlier very detailed pollen- and spore-morphological studies of the modern Hawaiian flora. In equatorial Africa early palynological studies centred on the East African Highlands (e.g. Hedberg Citation1954; Osmaston Citation1958; van Zinderen Bakker Citation1962, Citation1964; Coetzee Citation1967; Livingstone Citation1967, Citation1971) and later in the lowlands (Kendall Citation1969; see reviews by Livingstone Citation1975; Flenley Citation1979). Pioneering work in equatorial South America was primarily by van der Hammen and colleagues, as reviewed by van der Hammen (Citation1974), Flenley (Citation1979), and Hooghiemstra et al. (Citation2010), and by Colinvaux and colleagues as reviewed by Colinvaux (Citation2007). The pioneering work of Livingstone in Africa and Colinvaux in Amazonia is summarised by Bush and Gosling (Citation2018). The equatorial Indo-Malay region of south-east Asia was perhaps the last tropical forested area to be investigated palynologically with pioneering studies in New Guinea (Hope Citation1976; Walker and Flenley Citation1979), Sumatra (Morley Citation1976, Citation1982), and Australia (Kershaw Citation1970, Citation1971, Citation1975, Citation1976; Singh and Geissler Citation1985; Haberle Citation2005; see Flenley Citation1979, Citation1998 for reviews).
Although at the time that Flenley (Citation1979) wrote his masterly review of the history of Equatorial Rain Forest there were relatively few detailed pollen diagrams from equatorial areas, Flenley (Citation1979, p.126) concluded ‘that man has indeed been altering the vegetation of equatorial regions for a very long time and in diverse locations. His most striking effect on the vegetation appears to have been forest clearance, and his reason for this clearance has usually been in order to carry out some kind of agriculture. This has been going on since at least 3000 BP in Africa, 7000 BP1 in South and Central America, and possibly since 9000 BP or earlier in India and New Guinea. We have so far no real evidence as to whether these were independent developments of whether the idea of agriculture diffused from a single centre. In view of the different crops domesticated in each area, independent origin seems likely: on the other hand, the evidence of early agriculture in South-East Asia fits remarkably well with Sauer’s (Citation1952) diffusionist ideas’.
In the last 40 years, the number, scope, and detail of pollen-analytical investigations in equatorial areas have very greatly increased to include tropical islands such as Madagascar, Mauritius, and the Caribbean Islands (Birks HJB and Berglund Citation2018; see section 12). Bush et al. (Citation2011) present a thorough review of the (then) available data in their review of Tropical Rainforest Responses to Climatic Change. Although the emphasis of their review is tropical rainforest response to past and future climate change, Bush et al. (Citation2011) also consider the increasing evidence for human impact and agriculture on tropical systems in the Holocene, the main topic of this section.
In addition to studies on human impact in equatorial areas, major advances have been made thanks to detailed palynological studies in lowland Amazonia on the vegetational and ecological history of Amazonian forests over the last 170,000 years (e.g. Colinvaux et al. Citation1996; Mayle et al. Citation2000; Bush et al. Citation2004; D’Apolito et al. Citation2013), on the resilience of Amazonian forests over the last glacial-interglacial period (e.g. Bush Citation2017; Schiferl et al. Citation2017; Wang X et al. Citation2017), and on the testing (and refuting) the glacial refuge and aridity hypotheses for Amazonian speciation (e.g. Flenley Citation1993; Colinvaux et al. Citation2000, Citation2001; van der Hammen and Hooghiemstra Citation2000; Bush and de Oliveira Citation2006; Behling et al. Citation2010; Leite et al. Citation2016; Gomes da Rocha and Kaefer Citation2019). In addition there are now detailed pollen records covering the last glacial-interglacial cycle from, for example, Guatemala (Hodell et al. Citation2008; Correa-Metrio et al. Citation2012a, Citation2012b), Panama (Bush and Colinvaux Citation1990), the northern Andes (Hooghiemstra et al. Citation2006; Bogotá-Angel et al. Citation2011; Groot et al. Citation2013), Bolivia (Chepstow-Lusty et al. Citation2005), Bolivia/Peru (Gosling et al. Citation2008; Hanselman et al. Citation2011), and Brazil (Ledru et al. Citation2009). Urrego et al. (Citation2009, Citation2016) review recent palynological work in tropical South America and the tropical Andes and Flantua et al. (Citation2015, Citation2016a) summarise palynological data from Latin America, while Caballero-Rodríguez et al. (Citation2017) assess palynological data from Mexico.
10.2. How ‘virgin’ is virgin rainforest?
Willis et al. (Citation2004) address the question ‘how “virgin” is virgin rainforest?’ They show that palaeoecological data can provide insights not only into how extensive prehistoric human impacts in equatorial areas were but also the age and type of human activity being used. Some selected examples from three of the largest undisturbed rainforest blocks (Amazon basin, lowland Congo, Indo-Malay region) are summarised in .
Table 20. Selected examples of prehistoric human activities in the Amazon basin, lowland Congo basin, and Indo-Malaya region detected by palynological and associated palaeoecological studies with the approximate age of the activities and relevant references.
Early human impacts involving, inter alia, forest clearance, cultivation of Zea mays, and slash-and-burn activities have been widely documented from elsewhere in South America outside the vast Amazon basin. These areas include Panama (e.g. Bush et al. Citation1992; Bush and Colinvaux Citation1994), Costa Rica (e.g. Lane et al. Citation2009), Colombia (e.g. van der Hammen and Correal Urrego Citation1978; Berrío et al. Citation2002), Peru (e.g. Sublette Mosblech et al. Citation2012; Bush et al. Citation2015a; Matthews-Bird et al. Citation2017), Brazil (e.g. Barreto et al. Citation2017; Robinson M et al. Citation2018), Argentina (e.g. Castiñeira Latorre et al. Citation2017; Medina et al. Citation2017), and the Dominican Republic (Castilla-Beltrán et al. Citation2018; Hooghiemstra et al. Citation2018). Flantua et al. (Citation2016b) provide a comprehensive review of human land-use indicators in 68 pollen diagrams from South America for the last 2000 years. Their synthesis shows cultivation, mainly Zea mays, only occurred north of 30°S. Other valuable reviews on South American prehistory and human impacts include Denevan (Citation2003) Colinvaux (Citation2007), Dull et al. (Citation2010), and Balée (Citation2013) about Amazonia; Sarmiento (Citation2002) about Ecuador; Piperno (Citation2006) about Central America; and Beach et al. (Citation2015) and Battistel et al. (Citation2018) about the Maya lowlands of Mexico, Guatemala, Belize, and Honduras.
The examples in show that the earliest human activities detected using palaeoecological techniques are all in Indo-Malaya. Modern humans originated in Africa and had spread to south-east Asia by at least 75,000 years ago (Bae et al. Citation2017; Dennell Citation2017; Rutherford Citation2017; cf. McColl et al. Citation2018), whereas they did not reach South America until 10,000–20,000 years ago (Roosevelt et al. Citation1996; Corlett and Primack Citation2011; Rademaker et al. Citation2014; Dillehay et al. Citation2015). Although humans were in Indo-Malaya for over 75,000 years, possibly for 1.8 million years (Kershaw et al. Citation2011), and may have had the skill to manage vegetation using fire (e.g. Kusmartono et al. Citation2017), the ability to clear rainforest for agriculture appears to be a Holocene phenomenon (Kershaw et al. Citation2011; cf. Summerhayes et al. Citation2010). There is, however, evidence for the initiation of burning at about 45,000 years ago at Lynch’s Crater, north-east Queensland (Turney et al. Citation2001) with a sustained increase in Eucalyptus, a decline of Araucaria, and the disappearance of Dacrydium a few thousand years later. There is no evidence for sustained changes before this time even though the pollen record extends back to 230,000 years (Kershaw et al. Citation2007). Similar trends are detected in a marine core (ODP site 820) from the continental slope in the Coral Sea adjacent to the humid tropics of north-eastern Australia (Kershaw et al. Citation2011).
Rice-growing may have occurred as early as 16,000 years ago in the Yangtze Valley in China – an area Diamond (Citation1998, p.329) considers to be one of the ‘cradles of crop domestication’. Hilbert et al. (Citation2017) present evidence for the cultivation of rice at about 4000 BCE from the Monte Castelo shell-mound site in south-west Amazonia, along with maize (Zea mays) and squash (Cucurbita sp.) (see also Browne Ribeiro Citation2017). Several sites in upland Sumatra suggest that slash-and-burn (swiddening) occurred as early as 10,500 years ago and involved cultivation of dry (non-irrigated) rice or root-crops (Kershaw et al. Citation2011). New Guinea was a separate centre for early agriculture based on root-crops such as Colocasia (taro) beginning at least 9000 years ago (Haberle et al. Citation1991), although there is evidence for human occupation of New Guinea for at least 50,000 years (Summerhayes et al. Citation2010; Bergström et al. Citation2017).
In considering how ‘virgin’ virgin rainforest is, Willis et al. (Citation2004) identify three important implications for ecology and conservation biology from the palaeoecological records. First, they conclude that one can no longer regard land loss caused by previous human activities to be so small that it had no major ecological impact. Current estimates suggest that the major agent of deforestation (about 66% of the annual loss) today in tropical forests is still slash-and-burn farming (Myers Citation2002; Willis et al. Citation2004). Although the rate and extent of forest clearance are very much greater today than in the past, the ecological processes are, in many areas, comparable to prehistoric activities. Second, in many examples of prehistoric disturbance there was subsequent forest regeneration, suggesting that some tropical systems are not as fragile as is often thought and in fact may be surprisingly resilient (Bhagwat et al. Citation2012). Left for long enough, forests are likely to regenerate into secondary forest (Cole LES et al. Citation2014; Loughlin et al. Citation2018a). Third, palaeoecological data can provide estimates of the time required for such regeneration after abandonment (Cole LES et al. Citation2014: see below; Loughlin et al. Citation2018a). Such data can also provide information on forest composition before and after disturbance and what is missing from or has been added to the forest during regeneration (Willis et al. Citation2004; Nogué et al. Citation2018).
Based on 71 palynological studies in Central America, South America, Africa, and south-east Asia, Cole LES et al. (Citation2014) compiled data on 283 forest disturbance and recovery events such as disturbance source (4 types) and type of human activity (3 types) as well as a chronology. The pollen-assemblage recovery rates vary geographically, with median recovery rates of 141 years in Central America (35 disturbances), 162 years in Africa (29 disturbances), 325 years in South America (111 disturbances), and 415 years in south-east Asia (58 disturbances). Cole LES et al. (Citation2014) also suggest that an increasing frequency of disturbance events at a single site may increase pollen-assemblage recovery rates, suggesting some resilience in forests experiencing recurrent past disturbances. The reasons for the geographical variation in recovery rates are unclear but Cole LES et al. (Citation2014) suggest that the fastest rates of recovery in Central America and Africa may reflect the more extensive histories of disturbance in these areas. As shows, palynological evidence for human disturbances in Africa is often ambiguous (Brncic et al. Citation2007; Willis et al. Citation2013; Battistel et al. Citation2017; Ivory and Russell Citation2017). It is important to note that Cole LES et al. (Citation2014) consider many different disturbance types, not only human-induced (burning, clearance, agriculture) but also natural disturbances such as climate, precipitation, sea-level rise, and large infrequent disturbances such as hurricanes, fires, volcanic activity, and landslides.
In considering the possible impacts of prehistoric human activity on forests, it is important to try to estimate the spatial extent and intensity of human modification of the forests (Turner Citation1965; e.g. Bush et al. Citation2015b; Piperno et al. Citation2015). A potentially valuable approach is to apply pollen-representation models such as REVEALS (Sugita Citation2007a) in tropical situations to derive quantitative land-cover reconstructions in the way that Mariani et al. (Citation2017) have recently done for a cultural landscape in western Tasmania. There they show that untransformed pollen data greatly over-estimate the amount of rainforest cover and suggest that the landscape was largely treeless moorland dominated by Gymnoschoenus sphaerocephalus (button-grass) over the last 12,000 years, with a halving of rainforest cover about 4000 years ago due to anthropogenic burning in response to regional climate change. A recent Amazonian study (Whitney et al. Citation2019) illustrates the many problems of estimating forest cover from pollen assemblages, particularly at sites in mosaic and ecotonal landscapes, such as the forest–savanna transition in south-west Amazonia.
Besides studies on detecting and documenting prehistoric settlement areas (e.g. Fisher et al. Citation2017) and on the spatial scales and extent of prehistoric disturbances in tropical forests (Kelly et al. Citation2018), another important research priority is a closer integration of palaeoecology and archaeology (e.g. Heckmann et al. Citation2014; Mayle and Iriarte Citation2014; Barker et al. Citation2017; Roberts Citation2017; Xhauflair et al. Citation2017; de Souza et al. Citation2019) and more in situ palaeoecological studies (e.g. pollen, macrofossils, phytoliths) at habitation sites (e.g. Dickau et al. Citation2012; Hunt et al. Citation2012; Barker et al. Citation2017). Such studies in Bolivia (Dickau et al. Citation2012) reveal the widespread use of Zea mays along with Manihot esculenta (manioc, cassava), Dioscorea (yam), Arachis hypogaea (peanut), Arecaceae (palm fruits), and Gossypium (cotton), and, in Brazil (Hilbert et al. Citation2017), the cultivation of Oryza (rice). South-west Amazonia is postulated to have been a domestication centre for Capsicum baccatum (chilli) and Cucurbita maxima (squash) (Piperno Citation2011; Browne Ribeiro Citation2017).
Palynological studies provide means of testing hypotheses about aspects of tropical forest dynamics in relation to human impacts, such as why some trees form monodominant stands in highly diverse tropical forests. Bush and McMichael (Citation2016) use pollen data from 13 lakes in Amazonia to evaluate hypotheses about the hyperdominant Iriartea deltoidea, the commonest western Amazonian tree. They refute the hypothesis that its hyperdominance is a legacy of pre-Columbian human activity and that it gained its prominence due to major moisture changes at the Pleistocene–Holocene transition as its rise to prominence is time transgressive. They conclude that its rise to hyperdominance results primarily from increased moisture availability in the last 3000 years and that their findings are ‘consistent with the observation that communities in complex systems are ephemeral. The populations of even the most abundant species can change over a few tens of generations. The relative abundance of tree species, even in relatively stable ecosystems such as those of Amazonian floodplains, changes on ecological not evolutionary timescales’.
Tovar et al. (Citation2019) study a monodominant Gilbertiodendron dewevrei forest in the Republic of Congo. Using pollen and charcoal from a sediment sequence within the forest stand, they show that the stand has existed in situ for 2700 years in the absence of fire despite climatic fluctuations in the immediate region. Their results imply that this species may potentially be resilient to future climate changes.
10.3. Conclusions
As more and more basic palaeoecological data become available for tropical areas, it may be possible to extend the study of Levis et al. (Citation2017) on the legacy of pre-Columbian plant domestication on Amazonian forest composition. Levis et al. (Citation2017) overlaid known archaeological sites in Amazonia on the distributions and abundances of 85 woody species domesticated by pre-Columbian peoples. Domesticated species are five times more likely than non-domesticated species to be hyperdominant. Across Amazonia the relative abundance and richness of domesticated species increase in forests on and around archaeological sites. This analysis suggests that modern tree communities in Amazonia are structured to a large extent by a long history (‘legacy’) of plant domestication by pre-Columbian Amazonian people. Levis et al. (Citation2017) conclude that ‘[d]omestication shapes Amazonian forests’ (see also Levis et al. Citation2018). This view is challenged by McMichael et al. (Citation2017) who suggest that the data of Levis et al. (Citation2017) are spatially biased to sites in close proximity to areas of known post-Columbian human presence. There is continuing debate about the conclusions of Levis et al. (Citation2017) (e.g. Junqueira et al. Citation2017) and about the extent of pre-Columbian human influence in Amazonia (e.g. McMichael et al. Citation2017). These and other studies (e.g. Roberts et al. Citation2018; de Souza et al. Citation2019; McMichael and Bush Citation2019; Plumpton et al. Citation2019) and the ensuing debates show how important it is to unravel the human history of Amazonian forests, which is still commonly overlooked in ecological studies and strongly refutes the lingering but erroneous idea that Amazonian forests are untouched by humans. Ross and Rangel (Citation2011) reach similar conclusions in north-west Belize – ‘[t]he impacts of human land use, echoing across centuries of dispersal, colonization, disturbance, and biotic and abiotic interactions, can have important implications for understanding the current biodiversity patterns and processes’ (see also Ross Citation2011).
Palaeoecological studies in the last 30–40 years have greatly changed the views presented by Richards (Citation1952), who wrote in the first edition of his classic The Tropical Rain Forest that ‘[e]xtremely little is known of the past history of the rain-forest formations, but there is reason to believe that they have persisted, unchanged or changing exceedingly slowly, from a very remote period. … large areas of [rain forest] have been altogether uninhabited or inhabited only by food-gathering people with no more influence on the vegetation than any of the other animal inhabitants’ (Richards Citation1952, p.404). In the second edition of The Tropical Rain Forest, Richards (Citation1996) acknowledges that people have made clearings in tropical forests for various purposes from early prehistoric times, for example in Sumatra at least 4000 years ago, New Guinea 5000 years ago, and Ecuadorian Amazonia at least 6000 years ago. Corner (Citation1946) in his suggestions for botanical progress makes a characteristically provocative plea ‘I fear lest all the virgin lowland forest of the tropics may be destroyed before botany awakes’. Corner would have been shocked at the Willis et al. (Citation2004) note on ‘How “virgin” is virgin rainforest?’ and at how much has been found out in the last 70 years about human impact on tropical rainforests.
11. Conservation palaeoecology
11.1. Introduction
In the last 25 years, Quaternary palaeoecologists and pre-Quaternary ‘Deep-time’ palaeobiologists have increasingly urged conservation biologists to take account in their management plans of the long-term historical perspective on diversity changes, ecosystem dynamics, and biotic responses to environmental change that can be provided by palaeoecological studies. Relevant articles with this message can be found in . Many of the articles in and their calls for action on the part of conservationists have been published in literature primarily read by palaeoecologists or ecologists rather than by practising conservationists. There appear, not surprisingly, to be few indications that practising conservationists or managers are actually exploring or exploiting the long-term perspective provided by palaeoecology in their conservation or management strategies (Birks HJB Citation2012a; Saulnier-Talbot Citation2015; Kosnik Citation2018) – see also Driscoll and Lindenmayer (Citation2012), Anderson (Citation2014), Walsh et al. (Citation2015), and Bertuol‐Garcia et al. (Citation2018) for discussions on ‘The Great Divide’ between ecologists and conservation practitioners.
Table 21. Selected examples of publications on the potential contribution of Quaternary or ‘Deep-time’ palaeobiological studies to conservation by providing a historical perspective.
Here, I discuss selected palaeoecological studies involving pollen analysis and associated techniques such as charcoal analysis and palaeolimnology that directly relate to practical questions of conservation and management and outline future palaeoecological contributions to these critically important activities. The contributions are grouped into three broad types corresponding to the major shifts in conservation philosophy – (1) naturalness, protection, and management; (2) conservation in a changing world; and (3) ecosystem services and resources and natural capital – that have occurred in the last 40–50 years (Hunter et al. Citation2014; Mace Citation2014; Lawler et al. Citation2015; Colloff et al. Citation2017). Mace (Citation2014) terms these broad types ‘nature for itself’; ‘nature despite people’; and ‘nature for people’ and ‘people and nature’. For further palaeoecological examples that link directly to conservation, see .
Table 22. Further examples of Quaternary palaeobiological studies that link directly to conservation and management that are not discussed in the text.
This section draws extensively on Birks (Citation1996, Citation2012a); Willis and Birks (Citation2006); Jackson (Citation2006a, Citation2007, Citation2012a, Citation2013a); Froyd and Willis (Citation2008); Willis and Bhagwat (Citation2010); Willis et al. (Citation2010a, Citation2018); and Jeffers et al. (Citation2015).
11.2. Naturalness, protection, and management
These three aspects of nature conservation dominated conservation thinking and practice in the 1970s to early 1990s, at least in Britain. Their development and application are documented by Warren and Goldsmith (Citation1974, Citation1983) and Goldsmith and Warren (Citation1993).
11.2.1. Naturalness
Early applications of pollen analysis to conservation focussed on how ‘natural’ a particular site or vegetation type was (Oldfield Citation1969, Citation1970a; Birks Citation1996; Willis and Birks Citation2006). The concept of naturalness is one of the eleven criteria proposed by Ratcliffe (Citation1971, Citation1976, Citation1977, Citation1986) for evaluating the potential conservation value of a site within the approach of ‘nature for itself’ (Mace Citation2014). The term ‘natural’ strictly refers to areas that appear not to have been modified by human activities. It is questionable, in light of the wealth of pollen-analytical and other palaeoecological data now available world-wide (e.g. Birks HJB and Berglund Citation2018), if any such areas exist today. Naturalness is thus a relative term. In western Europe, for example, over 75% of the land has been totally transformed from its original state (Ratcliffe Citation1986). Nature conservation in western Europe is often concerned with the remaining 25% that supports semi-natural vegetation, defined as vegetation in which the dominant and constant taxa are undoubtedly native and the vegetation structure conforms to ideas (e.g. Jones Citation1945; Peterken Citation1996; Bradshaw et al. Citation2011; Peterken and Mountford Citation2017) about the structure of presumed natural vegetation (Ratcliffe Citation1977). Conservation value often increases with naturalness but as many habitats are partly anthropogenic, antiquity and lack of extensive disturbance can become key factors in evaluation (Ratcliffe Citation1986). The criterion of naturalness can thus be very contentious to apply (Anderson Citation1991, Citation1992; Götmark Citation1992) as it is usually difficult or impossible to assess reliably from present-day observations about the degree of modification, especially as the composition and structure of the truly natural vegetation is largely unknown (Ratcliffe Citation1977). A historical perspective from pollen-analytical studies can, in some instances, provide a direct means of assessing the extent of modification and some indications of the composition of the former natural vegetation (Birks Citation1996, Citation2012a).
The presumed naturalness of mires can be assessed by detailed peat- and pollen-stratigraphical studies (Birks Citation1996, Citation2012a). In perhaps the first application of palaeoecology to nature conservation, Oldfield (Citation1969, Citation1970a) investigated the recent history of Blelham Bog in the English Lake District. This was declared a National Nature Reserve because it represented ‘an almost unique example of Sphagnum bog developing from wet willow woodland’ (Pearsall in Oldfield Citation1970a). Detailed stratigraphical studies showed that the contemporary vegetation patterns, originally interpreted as reflecting the natural and progressive development of ombrogeneous conditions over telmatic peat still forming in the fen-carr woodland, are not the result of natural hydroseral succession but are the result of human interference (e.g. peat-cutting, artificial flooding, diversion of nearby streams, construction of a causeway, fencing) over the last two centuries (Oldfield Citation1970a). The presumed naturalness of this National Nature Reserve is not confirmed by these detailed palaeoecological studies.
In a contrasting study (Chambers et al. Citation1999) on upland moorland in Exmoor National Park, south-west England, palaeoecological investigations question the validity of proposed management plans based solely on contemporary observations. Species-poor Molinia caerulea vegetation was interpreted as a result of the recent invasion of heather moorland by Molinia. A management priority was thus to restore the presumed ‘natural’ Calluna vulgaris-dominated heathland. Pollen-analytical studies show, however, that the vegetation has alternated between Calluna and Molinia communities over the last 1000 years and appears to be part of the natural variability of the ecosystem rather than a recent human-induced degradation of the presumed ‘natural’ moorland (Chambers et al. Citation1999; Froyd and Willis Citation2008). Clearly a management plan based on present-day observations is inappropriate as it fails to consider the concept of a range of natural ecosystem variability (‘historical range of variability’ – Jackson Citation2012a). Palaeoecological studies on degraded upland blanket bogs in south Wales (e.g. Chambers et al. Citation2007a, Citation2007b, Citation2013; Chambers and Daniell Citation2011) and northern England (e.g. Chambers and Daniell Citation2011; McCarroll et al. Citation2016, Citation2017) have clearly shown how the long-term history of these mires can inform conservation practice and help to identify feasible targets for restoration.
Bryophyte-rich Quercus–Betula woods on acid soils on steep, rocky slopes in high rainfall areas of western Britain and Ireland (e.g. Kelly Citation1981; Edwards and Birks Citation1986) have long been assumed to be either ‘wildwood’ (sensu Rackham Citation2006) or closely related to the original ‘wildwood’ (Ratcliffe Citation1968). They support a remarkable luxuriance of bryophytes on trees, boulders, and the ground, including many southern Atlantic ferns and bryophytes (and epiphytic lichens) with Macaronesian–Tropical world distributions that reach their northernmost localities in these woodlands (Ratcliffe Citation1968; Preston Citation2015). These woods are thus of considerable international conservation importance. Local-scale pollen-analytical studies of small basins or humus profiles (‘stand-scale’ palynology sensu Bradshaw Citation2013) within stands of western oakwoods rich in Atlantic bryophytes in the English Lake District (Birks HJB Citation1993a; Bradshaw RHW et al. Citation2015), north Wales (Edwards ME Citation1986), south-west England (Bradshaw RHW et al. Citation2015), and south-west Ireland (Mitchell FJG Citation1988, Citation1990, Citation2013) all indicate that this very distinctive vegetation type has no long history and appears to have developed comparatively recently as a result of changes in land-use and woodland management. The history of their unique bryophyte and lichen flora is unknown. It is possible that these woods were never completely cleared because of the steep rocky terrain on which these woods persist. The bryophyte and lichen assemblages may therefore be, in part, relicts from pre-disturbance times (Edwards ME Citation1986; Birks HJB Citation1993a, Citation1996).
Management of these western oakwoods raises critical problems for conservationists (Palmer SCF et al. Citation2004). There is little or no tree regeneration today because of sheep and deer grazing. If areas are fenced, the field layer and tree seedlings grow up but the rare bryophytes in the ground-layer decline as a result of the field-layer expansion (Birks HJB Citation1996). Tree regeneration and a rich bryophyte assemblage appear incompatible. The palaeoecological perspective suggests that tree regeneration and the current woodland structure have largely resulted from management changes (Birks HJB Citation1996). Current management plans do not consider the role of land-use history or changes. Willis (Citation1993) discusses how palaeoecological studies highlight important implications for understanding the ecology of present-day woodlands in general and provides important insights relevant to management that cannot be obtained without recourse to palaeoecological record (cf. May 1994).
Stand-scale palynological studies of small hollows and humus profiles within boreal (Bradshaw RHW and Zackrisson Citation1990; Bradshaw RHW and Hannon Citation1992; Bradshaw RHW Citation1993a; Segerström et al. Citation1994; Josefsson et al. Citation2009), boreo-nemoral (Lindbladh et al. Citation2000; Bradshaw RHW and Hannon Citation2004, Citation2006; Molinari et al. Citation2005; Hannon et al. Citation2010, Citation2018; Bradshaw RHW et al. Citation2015), or nemoral forests (Lindbladh et al. Citation2003, Citation2008, Citation2013) in Sweden show the importance of land-use changes and natural disturbances (e.g. fire, wind-throw, insect attacks, floods, grazing) in influencing the long-term dynamics and present composition and structure of particular stands and for providing suggestions for future management (e.g. Björse and Bradshaw Citation1998). The structure and composition of Fiby Forest, a presumed ancient ‘old-growth’ forest in central Sweden are a result of changes in management and disturbance regimes a few centuries ago (Bradshaw RHW and Hannon Citation1992). As Bradshaw RHW and Hannon (Citation1992) conclude ‘many studies of forest dynamics and successional processes have been based in forest reserves, and it is therefore important to know the status of each forest in terms of the balance between human activity and natural processes, so that the nature of the dynamic processes occurring at the present time can be properly evaluated’.
The available, but admittedly limited, data from detailed local- or regional-scale palynological studies on the history of specific vegetation types or forest stands in many other parts of Europe () indicate that many types have originated in the late Holocene, usually closely associated with human activities and land-use changes. It appears that many modern vegetation types in Europe, central Asia, north-east America, Africa, and Australia may not have been in existence for more than 1000 – 2000 years at most (e.g. Janssen Citation1967, Citation1970; Birks HJB Citation1993a, Citation1996; Bond et al. Citation2008; Froyd and Willis Citation2008; Willis et al. Citation2008; Jackson Citation2013b; Connor et al. Citation2018b; see below and subsection 9.8). Many appear to have arisen through the direct or indirect influence of humans and their animals (Birks HJB Citation1996; Willis and Birks Citation2006; Pini et al. Citation2017). Dubois et al. (Citation2018) provide a comprehensive global synthesis of the first documented human impacts on aquatic systems. They demonstrate that the onset of human impacts on aquatic systems is highly variable in time and space and often appears several thousand years after the first human presence in an area.
Table 23. Selected examples of detailed local- or regional-scale palynological studies on the history of specific vegetation types in Europe, Asia, and United States.
Palynological studies have led to a re-assessment of the status of particular vegetation types in central Europe. For example, Picea abies-dominated forests at mid-elevations in the central European mountain ranges have long been thought to have originated from forestry activities. Szabó et al. (Citation2017) show that such forests have been an important component of the central European landscape for the last 9000 years. The unsuspected long-term continuity of various other vegetation types in central Europe such as wooded steppe on the Great Hungarian Plain (Magyari et al. Citation2010), grasslands and open woodlands in the Czech Republic (Kuneš et al. Citation2015), and species-rich semi-arid grasslands in Slovakia and the Czech Republic (Hájková et al. Citation2011) has also been established by detailed palynological and other palaeoecological studies. In the Sierra de Manatlan UNESCO Biosphere Reserve in western Mexico, Figueroa-Rangel et al. (Citation2008) demonstrate palynologically that the upland pine-dominated forests, long regarded as a product of secondary succession following human activities, are in fact of considerable antiquity (at least 4250 years) and have persisted as a result of interactions between aridity and natural fires. In the Eastern Arc Mountains of Tanzania, Finch and Marchant (Citation2011) show from pollen spectra that so-called ‘secondary montane grasslands’ have been present with unchanging composition for the past 13,000 years. Coupled with a lack of evidence for human activity, these grasslands may be a primary natural component of East African montane vegetation. Afromontane grasslands in the Drakensberg Escarpment (South Africa) have similarly been shown by pollen anlaysis to have been present for about 5000 years, suggesting long-term stability and a natural status despite a long history of human habitation in the area (Lodder et al. Citation2018). As McClenachan et al. (Citation2015) emphasise ‘surprising results revealed by historical sources are essential for ecology and conservation, providing new hypotheses that can be tested with additional data and new understandings of ecological dynamics that have immediate conservation implications’.
11.2.2. Fragility and other conservation-evaluation criteria
Pollen-analytical studies have also contributed to conservation-evaluation criteria beside naturalness (Birks HJB Citation1996). These include:
Assessing fragility (‘the degree of sensitivity of habitats, communities, and species to environmental change’) (Ratcliffe Citation1976, Citation1977). Fragility is an inherent property of a system and a system has a certain fragility irrespective of being exposed to extrinsic disturbances (Nilsson and Grelsson Citation1995). Inherently fragile systems include vegetation on oceanic islands prior to human colonisation (see subsection 12.2), grasslands and heathlands on nutrient-poor shallow soils, and valley and basin mires (Ratcliffe Citation1977)
Assessing the status, history, and ecology of particular taxa such as Alnus glutinosa in western Norway (Natlandsmyr and Hjelle Citation2016), Quercus and Tilia in southern Scandinavia (Lindbladh and Foster Citation2010; Hultberg et al. Citation2017), Tilia cordata in north-west England (Pigott and Huntley Citation1980), Pinus nigra in northern Spain (Morales-Molino et al. Citation2017), P. pinaster in Spain (Carrión et al. Citation2000), Abies pinsapo in Spain (Alba-Sánchez et al. Citation2019), A. alba in southern Italy (di Pasquale et al. Citation2014) Larix decidua in central Europe (Wagner S et al. Citation2015), A. alba in southern Europe (Tinner et al. Citation2013), P. palustris in the south-eastern US (Stambaugh et al. Citation2017), and Polylepis spp. in the tropical Andes (Valencia et al. Citation2018); of rare and/or endangered species such Picea omorika endemic to western Serbia and eastern Bosnia and Herzegovina (Finsinger et al. Citation2017a), the globally rare Sphagnum riparium (Gałka et al. Citation2018) and the rare S. austinii (= S. imbricatum) in Britain and Ireland (McClymont et al. Citation2008), the Neogene relict Prunus lusitanics in Iberia (Calleja et al. Citation2009), and Cedrus atlantica in the Rif Mountains of Morocco (Cheddadi et al. Citation2017; Abel Schaad et al. Citation2018); of disjunct populations such as Tsuga mertensiana in northern Idaho (Herring et al. Citation2018); or of ancient Gondwanan elements such as conifers in New Zealand (McGlone et al. Citation2017)
Establishing baseline or reference conditions at particular sites or vegetation types of interest () as a basis for management in the future, including restoration attempts
Developing management plans at the landscape scale in relation to natural disturbances such as wildfire (e.g. Wright Citation1974; Whitlock et al. Citation2003, Citation2010, Citation2018; Whitlock Citation2004; Conedera et al. Citation2009; Marlon et al. Citation2012; Higuera Citation2015; Colombaroli et al. Citation2017; Freeman et al. Citation2017; Kelly LT and Brotons Citation2017; Aakala et al. Citation2018; Hawthorne and Mitchell Citation2018; Carter VA et al. Citation2018b; Fyfe et al. Citation2018b) or human impact (e.g. Bradshaw RHW and Hannon Citation2006; Ekblom and Gillson Citation2017). Such plans require knowledge about the frequency of native fires and the intensity of such fires, and of the age, intensity, and extent of human impact. Palaeoecological studies can help provide such knowledge
Providing a historical perspective on socio-ecological systems and landscapes as a guide to evidence-based land-use and conservation management in the future (e.g. Davies Citation2007, Citation2009; Rhemtulla and Mladenoff Citation2007; Hanley et al. Citation2008, Citation2009b; Kirby Citation2012; Molinari and Montanari Citation2016; Catalan et al. Citation2017; Githumbi et al. Citation2018)
Deciphering the origin of landscape mosaics in relation to ecological factors including disturbances (e.g. Lynch Citation1998; Birks HJB Citation2012a; Hájková et al. Citation2018; Jamrichová et al. Citation2017)
Assessing the native and non-native status of species (e.g. Jackson Citation1997; Preston et al. Citation2004; Willis and Birks Citation2006; Crees and Turvey Citation2015; Stace and Crawley Citation2015; Downey and Richardson Citation2016; see below and subsection 12.4)
Providing a factual basis for ecological restoration, rewilding, wilding, translocation, and de-extinction attempts (see Box S3 for examples). Fisher M (Citation2013) and Pettorelli et al. (Citation2019) critically discuss what really is rewilding and the many ambiguities of the term along with terms such as ecological restoration, wilding, wilded, and natural processes.
Table 24. Selected examples of palaeoecological studies that contribute to establishing baseline or reference conditions at a particular site or vegetation type.
Although some suggested restoration, rewilding, wilding, and de-extinction ideas may currently seem rather fanciful and controversial, practical conservation today in much of Europe and parts of North America involves not only effective management but increasingly also ecosystem enhancement and restoration (Birks HJB Citation1996; Pereira and Navarro Citation2015; Suding et al. Citation2015; Thompson MSA et al. Citation2018). Ratcliffe (Citation1971, Citation1977, Citation1986) includes ‘potential value’ as a criterion in conservation evaluation and defines it as ‘in some instances, especially where good examples of certain types of ecosystem no longer exist, there are grounds for creating, by suitable management, a structure and composition regarded as desirable’ (Ratcliffe Citation1971). The question of ‘naturalistic grazing and re-wilding in Britain’ is explored in subsection 9.4.
11.2.3. Potential natural vegetation
Many vegetation scientists, particularly plant sociologists and vegetation cartographers, adhere to the concept of potential natural vegetation (PNV), for example in broad-scale vegetation maps (Küchler Citation1964; Bohn et al. Citation2000/2003; Somodi et al. Citation2017). The PNV concept replaced, to some degree, the climax concept popularised by Clements (Citation1936). Much controversy surrounded the climax concept (e.g. Selleck Citation1960; Kohler Citation2008) and in much vegetation science it was replaced by the PNV concept. PNV is defined by Tüxen (Citation1956) as ‘the hypothetical natural status of vegetation … that could be outlined for the present time or for a certain earlier period, by taking away human impact on vegetation … imaginable influences of climatic changes that could occur during long-term succession should be avoided’. Tüxen (Citation1956) recognised that the assumption that vegetation was in equilibrium with the current climate was necessary for the PNV to be operational (Chiarucci et al. Citation2010). Thus PNV must represent vegetation in the absence of human activity and recent climate change, and must represent mature vegetation (Küchler Citation1964, Citation1967; Härdtle Citation1995; Jackson Citation2013a).
The idea that the PNV concept is not supported by palynological data (e.g. Huntley and Birks Citation1983; Birks HJB Citation1986, Citation1993a; Jackson and Overpeck Citation2000; Shumilovskikh et al. Citation2017), by knowledge of how dynamic climate is over a wide range of temporal scales (e.g. Jackson et al. Citation2009b), and by the overriding impact of human activity on European vegetation has generated a lively discussion among vegetation scientists (e.g. Carrión and Fernández Citation2009; Carrión Citation2010; Chiarucci et al. Citation2010; Farris et al. Citation2010; Loidi et al. Citation2010; Somodi et al. Citation2012). The long-term role of human activity on European vegetation renders the PNV concept difficult, if not impossible to apply usefully or consistently (e.g. Chiarucci et al. Citation2010). Jackson (Citation2013a) explores with the eyes of both an ecologist and a palaeoecologist, the validity of the PNV concept in North America where there has been little or no human disturbance on vegetation in pre-Columbian times. Jackson (Citation2013a) concludes ‘[s]omething resembling PNV emerges at millennial temporal scales and at regional to subcontinental spatial scales. However, at finer spatial and temporal scales, actual vegetation often displays properties of inertia, contingency and hysteresis, most frequently because of climatic variability across multiple timescales and the episodic nature of disturbance and establishment. Thus, in the absence of human disturbance, the actual vegetation that develops at a site may not resemble a particular PNV ideal, but could instead represent one of any number of potential outcomes constrained by historically contingent processes. PNV may best be viewed as an artificial construct, with utility in some settings. Its utility may diminish and even be detrimental in a rapidly changing environment’ (see also Jackson (Citation2006a), Jackson et al. (Citation2009b), Jackson and Blois (Citation2015), and Rull (Citation2015) for elaborations of critical points in Jackson’s (Citation2013a) critique of the PNV concept). In light of all the currently available palaeoecological data not only from Europe and North America but also from the tropics (see section 10), oceanic islands (see section 12), and large continental areas, along with the unambiguous evidence for human impact on vegetation world-wide (Birks HJB and Berglund Citation2018), the PNV concept should be discontinued and replaced by what Ellis and Ramankutty (Citation2008) describe as ‘[p]utting people in the map: anthropogenic biomes of the world’.
Working on the Azores Islands, Rull et al. (Citation2017c) use a recently prepared map of the PNV (Elias RB et al. Citation2016) as a ‘null model’ to compare with past, present, or future vegetation cover. Predictions for past cover based on the map agree well, at least qualitatively, with available pollen data from just before human colonisation on Flores, Pica, and São Miguel (e.g. Connor et al. Citation2012, Citation2013). This agreement is gratifying as many defenders of the PNV concept dismiss pollen-analytical data as being unreliable, biased records of past vegetation patterns (e.g. Loidi et al. Citation2010), just as supporters of ‘naturalistic grazing and re-wilding in Britain’ and the Vera (Citation2000) pasture-woodland hypothesis dismiss pollen analysis (see subsection 9.4). As in many disputes, there is often much middle-ground between the PNV concept and the inherent variability of vegetation, environment, and historically contingent processes. Abraham et al. (Citation2016) compare quantitative vegetation reconstructions for the Czech Republic based on 87 pollen sequences with PNV estimates of vegetation composition based on Neuhäuslová et al. (Citation1998). They show that pollen-based reconstructions and PNV estimates are complementary and together they lead to a more integrated perspective on natural vegetation than PNV or pollen estimates alone.
All the palynological contributions discussed in this and the previous subsection (11.2.1) relate to the basic and important question in conservation, namely ‘what is natural?’ and the approach of ‘nature for itself’ (Mace Citation2014). A number of palaeoecological studies of a range of vegetation types and ecosystems has shown that answering the question what was or still is natural requires the long-term perspective provided by detailed and well-designed palaeoecological studies (e.g. Birks HJB Citation1996, Citation2012a; Willis and Birks Citation2006; Whitlock et al. Citation2018). However, conservation involves not only management for today but also planning for nature’s uncertain future in a rapidly changing world (Dearing et al. Citation2011a; van der Leeuw et al. Citation2011; Willis et al. Citation2012; Mace Citation2014).
11.3. Conservation palaeoecology in a changing world
11.3.1. Introduction
Contributions of palaeoecology to conservation biology broadened in the late 1990s and early 2000s following the publication of Conservation in a Changing World (Mace et al. Citation1998). This led to the increased recognition of the need for conservation to focus not only on protection and management of the status quo (‘nature for itself’) but also on the development and adoption of new conservation strategies in the face of climate change, increasing habitat-fragmentation and loss, impacts of invasive species, and landscape degradation (Corlett Citation2015; Ellis Citation2015) – ‘nature despite people’ (Mace Citation2014). The long-term perspective provided by Quaternary botany in response to this new paradigm in conservation and ecology (e.g. Mouquet et al. Citation2015) has focused on identifying (1) possible threats to biodiversity, baseline or reference conditions, and natural ecosystem variability; (2) ecological thresholds and resilience; (3) the role of historical legacies in ecosystem development; (4) biotic responses to future climate change; (5) impacts of invasive species; (6) novelty in past, present, and future systems; and (7) antiquity of vegetation types (e.g. Froyd and Willis Citation2008; Willis and Bhagwat Citation2010; Willis et al. Citation2010a; Vegas-Vilarrúbia et al. Citation2011; Birks HJB Citation2012a; Kidwell Citation2015; Barnosky et al. Citation2017).
11.3.2. Threats to biodiversity, baseline conditions, and natural ecosystem variability
The International Union for Conservation of Nature and Natural Resources (IUCN) has developed global Red Lists of threatened species, both plants and animals. These Lists show that human activities far outweigh natural threats to plant species and also to their habitats (Brummitt and Buchman Citation2010; Haddad et al. Citation2015; Isbell et al. Citation2017; Titeux et al. Citation2017). The ultimate impact of these threats is species extermination, first on a local and then a regional scale, and finally species extinction on a global – and thus total and irreversible – scale (Plotnick et al. Citation2016; Rivers Citation2017). Risks of loss due to human activities are currently estimated to be 1000 times higher than natural background rates of loss (De Vos et al. Citation2015). The current estimates of known plant extinctions is 571 species (Humphreys et al. Citation2019; Ledford Citation2019). These extinctions are concentrated on islands (e.g. Madagascar, Hawaiian Islands) and in species-rich areas such as South Africa, India, and Brazil. These estimates are at a rate 500 times higher than would be expected due to natural factors alone, but are less than the earlier estimates of De Vos et al. (Citation2015) by 50%. Any attempt to reduce the extinction risk and ultimately biodiversity loss requires a better understanding about what the threats are, where they are, what were the baseline conditions, and how to minimise the risks (Chape et al. Citation2005; Forest et al. Citation2007; Willis et al. Citation2007a; Joppa et al. Citation2016).
With international agreements such as the Convention of Biological Diversity on reducing the rate of biodiversity loss there is increasing emphasis on methods of biodiversity estimation and on detecting long-term trends in richness using palynological data (e.g. Willis et al. Citation2007b; Froyd and Willis Citation2008; Birks HJB et al. Citation2016a, Citation2016c; Felde et al. Citation2018; see section 14). Baseline or reference conditions against which current conditions can be assessed can be estimated from detailed palaeoecological studies (see above), where the baseline is often defined as being ‘the ecosystem present before human influence became pronounced on the landscape’ (Lindbladh et al. Citation2007). In practice, the reference-condition concept has been explored most fully by palaeolimnologists for lakes in response to the European Union Water Framework Directive which requires reference conditions (usually at 1850 CE) to be determined for all water bodies (e.g. Smol Citation1992, Citation2017; Battarbee Citation1999; Battarbee et al. Citation2005; Bennion and Simpson Citation2011; Weckström et al. Citation2015; Wiik et al. Citation2015; Wingard et al. Citation2017). The concept has been more rarely used in terrestrial systems (e.g. Davis MB Citation1989c; Swetnam et al. Citation1999; Litvaitis Citation2003; Bjorkman and Vellend Citation2010; Balaguer et al. Citation2014).
Ecological systems are not static entities, as is often implicitly assumed in baseline studies (Foster and Motzkin Citation1998; Jackson Citation2001, Citation2006a, Citation2013b; Jackson and Hobbs Citation2009; Jackson et al. Citation2009b) but can be highly dynamic systems responding to an ever-changing multivariate environment (Jackson and Overpeck Citation2000; Botkin et al. Citation2007; Allmon Citation2017). It is therefore important to define envelopes of ‘acceptable’ temporal variability (‘historical range of variability’ (Jackson Citation2012a) or ‘range of natural variability’ (Froyd and Willis Citation2008)) in terms of rates, magnitude, and frequency of system change (Millar and Woolfenden Citation1999; Seidl et al. Citation2016). Such envelopes of temporal variability can be constructed using, for example, fine-resolution pollen data (proxy for vegetation variability), charcoal data (proxy for fire variability) (Gavin et al. Citation2007; Cyr et al. Citation2009), and stable isotope and geochemical data (proxy for climate variability). Given such an envelope, it would be possible to identify what ecological state lies within an acceptable limit of variability. Such constructs are highly relevant in management and restoration studies (e.g. Landres et al. Citation1999; Froyd and Willis Citation2008; Jackson and Hobbs Citation2009; Jackson Citation2012a; Wiens JA et al. Citation2012; Wingard et al. Citation2017), as conservation managers increasingly recognise that establishing realistic management goals requires knowledge of the range of natural variability not only of the vegetation but also of climate and disturbance regimes (Froyd and Willis Citation2008). The concept of ‘range of natural variability’ can be directly linked to Quaternary ecological dynamics. The basic idea behind the concept is that ‘resilient ecosystems will be maintained if land management activities operate within the range of conditions that would be expected under the natural disturbance regime’ (Froyd and Willis Citation2008). This concept is implicit in many management plans for the coniferous forests of North America (e.g. Wright Citation1974; Whitlock et al. Citation2003; Gavin et al. Citation2007) based on detailed Holocene pollen and charcoal analyses of lake sediments. Long-term palaeoecological insights are essential to avoid erroneous management plans (Froyd and Willis Citation2008). For example, the view in Scandinavia that frequent low-intensity fires in Pinus sylvestris forests and less frequent but more intense fires in Picea abies stands (Fries C et al. Citation1997) reflect the natural fire-regime in Scandinavian boreal forests ignores the abundant palaeoecological evidence showing that many Picea-dominated forests have only developed recently and have remained largely fire-free since the diachronous invasions of Picea in Scandinavia (e.g. Bradshaw RHW and Hannon Citation1992; Ohlson and Tryterud Citation1999; Seppä et al. Citation2009b; Ohlson et al. Citation2011). This management plan is clearly inappropriate and may damage or even destroy the high biodiversity of lichens and invertebrates in many old-growth Picea forests. Available forest history over a range of time scales (e.g. Aakala et al. Citation2018) should be considered in management strategies (Froyd and Willis Citation2008; Cyr et al. Citation2009).
11.3.3. Ecological thresholds and resilience
This subsection on resilience and thresholds in conservation palaeoecology draws extensively on Froyd and Willis (Citation2008), Willis and Bhagwat (Citation2010), and Willis et al. (Citation2010a, Citation2018).
A topic of major importance within conservation in our changing world is ecological thresholds and resilience (Froyd and Willis Citation2008; Birks HJB Citation2012a; Gillson Citation2015; Reyer et al. Citation2015a, Citation2015b; Young AM et al. Citation2017; Willis et al. Citation2018). A threshold is ‘an abrupt change in an ecosystem and a switch from one stable state to another’ (Froyd and Willis Citation2008; see subsection 9.7). Thresholds define the limits to the amount of ‘acceptable’ change an ecosystem can withstand (Luck Citation2005). There can be problems in defining and using it in practical situations (Lindenmayer and Luck Citation2005). Closely related to the threshold concept is the concept of ecological resilience, namely ‘the ability of systems to absorb disturbance and still maintain the same relationships between populations (Holling Citation1973)’ (Gunderson Citation2000; Froyd and Willis Citation2008; Newsham and Bhagwat Citation2016; Darling and Côté Citation2018; see subsection 9.7). Resilience is thus ‘the magnitude of disturbance that can be tolerated before a system moves to another stable state (Gunderson and Holling Citation2001)’ (Froyd and Willis Citation2008; Newsham and Bhagwat Citation2016). An alternative view (Pimm Citation1991; Grimm V and Wissel Citation1997) is that resilience is the process of recovery following disturbance, not the ability to resist disturbance in the first place. Hodgson et al. (Citation2015) propose that as resilience is now so commonly used to refer to resistance or recovery, or both, it should assume the broadest definition. The concept of resilience therefore includes resistance, recovery, elasticity, return time, latitude, and precariousness (Hodgson et al. Citation2015) – see Standish et al. (Citation2014), Yeung and Richardson (Citation2016), Davies et al. (Citation2018), and Ingrisch and Bahn (Citation2018) for discussions on terminology and quantification of resilience and thresholds. Threshold and resilience concepts have, as with the baseline-condition concept, been mainly explored in palaeolimnology (e.g. Smol and Douglas Citation2007; Dearing Citation2008; Seddon et al. Citation2014b, Citation2017; Bennion et al. Citation2015, Citation2017; Smol Citation2017; Vermaire et al. Citation2017; Jeppesen et al. Citation2018). As discussed over 50 years ago by Smith AG (Citation1965) (see Birks HJB Citation1993b), these concepts are potentially relevant to the past dynamics of terrestrial systems (e.g. Grimm EC Citation1983; Cole KL Citation1985; Carrión et al. Citation2001a, Citation2001b; Carrión Citation2002; Morecroft et al. Citation2012; Aranbarri et al. Citation2014; Davies et al. Citation2017; Seddon et al. Citation2017).
Willis et al. (Citation2018) ask the key question – what makes a terrestrial ecosystem resilient? Understanding resilience requires comparative data-sets that span both space and time and address three major questions – where are the most resilient ecosystems; what attributes make them more resilient than others; and how close is an ecosystem to losing its resilience (Willis et al. Citation2018)? Finding suitable data-sets relevant to system resilience is difficult as many data do not cover the time required to record the response of a system to a perturbation, especially in forests with trees with long generation times. Here, following Willis et al. (Citation2018), I concentrate on tropical systems.
The most resilient tropical systems detected by Cole LES et al. (Citation2014) based on an analysis of 283 disturbances and recovery events recorded in 71 long-term pollen records are Central American tropical forests (see subsection 10.2). Working over a shorter time-scale of the past 20 years, Poorter et al. (Citation2016) show in their analysis of 1500 records from 45 Neotropical forests after clearance that seasonally dry forests appear to be less resilient than humid tropical lowland forests. Satellite imagery data (e.g. Moderate Resolution Imaging Spectroradiometer (MODIS)) can identify areas sensitive to climate fluctuations over the last 14 years. Resilient areas include the woody savannahs of the Brazilian Cerrado and the Sahel drylands, in contrast to vegetation in parts of West Africa and the Amazon basin (Huete Citation2016; Seddon et al. Citation2016) that appear to be highly sensitive to recent climatic perturbations.
Relatively little is known about what plant attributes provide resilience. RBG Kew (Citation2016) and Willis (Citation2017) present a global analysis of plant traits that may confer resilience to future perturbations. Climate appears to be important in influencing resilience (Côté and Darling Citation2010). In Neotropical dry forests, the regions with higher local rainfall and lower water deficit have the highest rates of above-ground recovery after clearance) (Poorter et al. Citation2016). Cole LES et al. (Citation2014) show that the more times a system had been disturbed, the faster it recovers, probably because the vegetation becomes dominated by forest species that can tolerate and respond quickly to disturbance. This finding based on long-term pollen data contrasts with the hypothesis that the number of times a system is disturbed, the less resilient it becomes as indicated by a reduction in recovery rate after each subsequent disturbance (Willis et al. Citation2018). The type of disturbance can also influence resilience and this varies between vegetation types. In West African tropical grasslands, fire buffers the ecosystem from forest encroachment and hence enhances grassland resilience (Laris et al. Citation2016). In contrast, frequent fires in savannah–tropical-forest ecotones can result in loss of resilience in the forests (Oliveras and Malhi Citation2016). Soil type and below-ground biota can also be important (e.g. Levine et al. Citation2016; Poorter et al. Citation2016; Allen K et al. Citation2017). The ‘insurance hypothesis’ (Hisano et al. Citation2018; Willis et al. Citation2018) proposes that more functionally diverse systems will be more resilient to environmental perturbations than functionally less diverse systems simply because the biodiverse systems have a large number of species to ‘replace’ functions previously performed by species being lost. Although this insurance hypothesis is supported at the community level, it may be refuted at the biogeographical scale of the African continent (Willis et al. Citation2018) where the highest tropical plant species richness (Linder Citation2014), and presumably highest functional diversity, occurs in areas most sensitive to climate perturbations (Seddon et al. Citation2016). Certain plant traits (e.g. woody density, rooting depth, leaf-area index) may make some systems more resilient (Willis Citation2017; Willis et al. Citation2018) than other systems (e.g. Greenwood S et al. Citation2017).
A critical question in conservation science is how close is a system to losing its resilience (Willis et al. Citation2018)? Several approaches have been developed to answer this question. For example, Hirota et al. (Citation2011) show that tropical systems in Africa, Australia, and South America may switch to a savannah type vegetation when forest cover is less than 60% (but see Ratajczak and Nippert Citation2012; van Nes et al. Citation2012). An alternative approach is to examine recovery rates after disturbances on the hypothesis that the closer a system is to a threshold, the slower the recovery rate will be (Holl et al. Citation2018; Willis et al. Citation2018). Verbesselt et al. (Citation2016) find that recovery rates slow down sharply once annual precipitation drops below 1500 mm in lowland evergreen tropical forests in South America, Africa, and south-east Asia, suggesting a tipping point or threshold is about to be crossed. In contrast, Levine et al. (Citation2016) working in Amazonian rainforests find evidence of gradual change to several transitional forest states in response to the lengthening of the dry season (Willis et al. Citation2018).
Resilience is a complex process, with many contributing and interacting factors such as soils, climate, below-ground processes, and history (Webb SL et al. Citation2000; Standish et al. Citation2014; Gillson Citation2015; Reyer et al. Citation2015a, Citation2015b; Timpane-Padgham et al. Citation2017; Willis et al. Citation2018). Cole LES et al. (Citation2014) show in their analysis of recovery rates after disturbance as recorded palynologically how complex the patterns are to interpret. Can pollen analysis contribute further to the detection and understanding of resilience in the context of conservation palaeoecology in our changing world?
A key question for conservation managers is if palaeoecological studies can identify critical thresholds and resilience in ecosystems (e.g. Côté and Darling Citation2010; Willis and Bhagwat Citation2010; Willis et al. Citation2010a; Birks HJB Citation2012a). Virah-Sawmy et al. (Citation2009a, Citation2010) explore long-term resilience and recovery of littoral forests on eastern Madagascar in relation to marine surges using a range of palaeoecological analyses (pollen, diatoms, geochemistry). They show that open upland Uapaca heath forest has low resilience to threshold events of marine storm surges and subsequent drought and that a switch from one stable state to another had occurred in open littoral forest (; Willis et al. Citation2010a). Closed littoral forest shows much greater resilience to repeated storm surges than open upland forest (). Identifying resilience in such systems (Willis et al. Citation2010a) is an important first step in developing management policies to try to combat future impacts associated with climate change and associated sea-level rise or changes in land-use practices (Gunderson Citation2000; Gunderson and Pritchard Citation2002; Dearing Citation2008).
Figure 21. Vegetation responses at two sites on eastern Madagascar (Fossa and Bassin) to an environmental perturbation (sea-level rise and subsequent drought) about 1000 years ago. Pollen assemblages at Fossa (left) recover towards the previous stable state (high ecological resilience) whereas at Bassin (right) the pollen assemblages continue to diversify (low ecological resilience). Each period shows a time-series of relative pollen proportions (vertical axis) (P continuous line) and a smoothed version (dashed line) based on a robust locally weighted polynomial model (span = 0.25). Heathland is Erica, Asteraceae, and Poaceae pollen, 'Forest' is the sum of pollen of littoral forest tree taxa at Fossa and the sum of pollen of open Uapaca forest tree taxa at Bassin. Recovery refers to a return to forest conditions (see Virah-Sawmy et al. Citation2009a, Citation2010 for details). The small inset panels in each large panel show a phase plot for the smoothed pollen data where the relative pollen abundance (P) is plotted against the local rate-of-change gradient (horizontal axis) (ΔP/Δt). The smoothed data were interpolated and resampled at uniform time intervals for the same number of points as in the original data-sets. The proximity of these points on the phase plots indicate the rate of change in system state, with arrows showing the direction of time from old to young. The dashed ovals enclose the stable forest state prior to the perturbation. The system appears to be moving towards full recovery at Fossa (high ecological resilience) whereas there are no signs of recovery at Bassin (low ecological resilience). Redrawn from Willis et al. (Citation2010a).
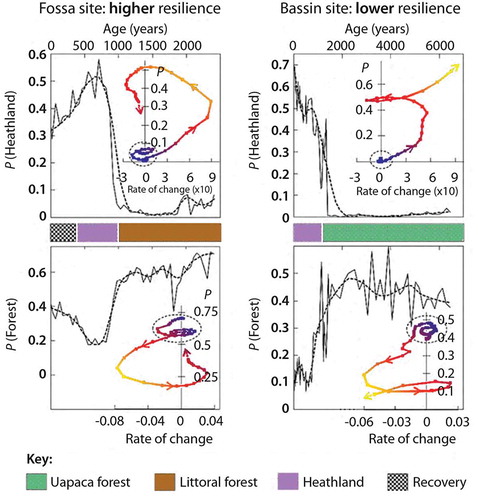
Virah-Sawmy et al. (Citation2009a, Citation2010) show that some littoral forest fragments provided more refugia for endemic taxa than others during environmental perturbations. One such taxon is the endemic evergreen forest tree Symphonia cf. verrucosa. The pollen records show that following climatic perturbations, Symphonia went extinct at one site but not at the other and that at both sites there was a close covariance between Symphonia pollen abundance and the presence of Erica and Myrica pollen (Virah-Sawmy et al. Citation2009b; Willis and Bhagwat Citation2010). To test alternative hypotheses for the drivers of Symphonia extinction (competition and/or facilitation) four population models were applied (Virah-Sawmy et al. Citation2009b). At the two sites, the best (in a statistical sense) model for the observed Symphonia pollen patterns was the competition model with Erica. At the nutrient-poor site, deficient in N and P (Mandena), Symphonia went extinct, whereas at the nutrient-rich site (St Luce) it survived. The model results, in conjunction with ecological observations, suggest that on nutrient-poor soils Erica gains a competitive advantage during climatic perturbations, reaches maximum carrying capacity, and out-competes Symphonia. In contrast, on nutrient-rich soils, Symphonia survives and exerts competitive effects on Erica even during a climatic perturbation (Virah-Sawmy et al. Citation2009b; Willis and Bhagwat Citation2010).
The message for conservation managers from these studies is to make conservation of littoral forest fragments on nutrient-rich sites a priority as well as the naturally occurring mosaic of heathlands around such fragments because they provide refugia for some species during intervals of storm surges and drought. Littoral forest fragments on nutrient-rich soils seem to be able to support the region’s important endemic taxa including Symphonia during extreme climatic events (Willis and Bhagwat Citation2010).
Pollen analysis has also been used to assess the long-term resilience of tropical systems in, for example, Indonesia (Biagioni et al. Citation2015; Hapsari et al. Citation2018) and India (Bhagwat et al. Citation2012; Nogué et al. Citation2018). There is a need to link detailed fine-resolution pollen-analytical data to long-term ecological data-sets (e.g. Morecroft et al. Citation2016; Müller et al. Citation2016) to further our understanding of resilience of vegetation and the factors that determine it.
A critical question for managers is to ascertain how close an ecological system of conservation concern is to a threshold – ‘thresholds of potential concern’ – and what makes a system resilient to a threshold event (Froyd and Willis Citation2008). Working in the savannah-dominated Kruger National Park of South Africa, Gillson and Duffin (Citation2007) use fine-scale pollen stratigraphies and modern pollen–vegetation relationships to address whether woody cover had decreased below 80% of its ‘highest ever value’ – a threshold of potential concern set by Park managers to define the upper and lower levels of acceptable variation in the ecosystem. Their results show that in the last 1400 years, estimated woody cover has remained at or above 20% of its highest ever value. Thus management intervention in this part of the Park is currently not needed. Interestingly, there are differences in the estimates of the highest ever value between sites, highlighting the importance of site-specific thresholds of potential concern.
11.3.4. Historical legacies
Ecologists are increasingly recognising the potential role in community assembly of neutral random processes such as dispersal, recruitment, and mortality (Hubbell Citation2001; Jackson and Blois Citation2015), in addition to extrinsic or exogenous environmental processes and intrinsic or endogenous interactions. Neutral communities have virtually unlimited membership, are non-equilibrium, and often bear historical imprints (Jackson and Blois Citation2015). Community composition may therefore be influenced by legacies of past demographic, dispersal, and disturbance events and community properties may drift as a result of singular events (Hubbell Citation2001; Essl et al. Citation2015; Jackson and Blois Citation2015). A question of direct relevance to conservation biologists and managers is over what time-scales is community composition contingent on historical events (‘legacies’ or ‘ecological memory’) (Ogle et al. Citation2015). Answers to this question require historical, including palaeoecological, data. There are many reports of land-use history, climate change, and disturbances such as fire in the last 100-200 years influencing present-day forest and grassland composition, biodiversity, and soil properties at a wide range of spatial scales (see Box S4 for examples). There is also evidence for the historical legacies of 500 years or more duration influencing tropical vegetation (e.g. Heckenberger et al. Citation2003, Citation2007; Willis et al. Citation2004; Glaser Citation2007; Junqueira et al. Citation2010; Levis et al. Citation2017; Whitney and Cárdenas Citation2017; see section 10).
Evidence for the importance of legacy effects on ecological systems over longer time-scales such as thousands of years is beginning to accumulate (see Box S4). Perhaps the most surprising is the demonstration at Lake El-Gygytgyn (Russian Far East) of legacies over glacial–interglacial time-scales (Herzschuh et al. Citation2016). Interglacial pollen assemblages and hence vegetation appear to be influenced by the vegetation as inferred from the pollen assemblages of the preceding glacial stage instead of the contemporary interglacial climate due to the combined effects of permafrost persistence, location of glacial-stage refugia, and fire (). By analogy with the past, today’s widespread Larix gmelinii and L. cajanderi forest on permafrost is not in equilibrium with today’s climate because the last glacial stage was severe with extensive permafrost and more thermophilous evergreen trees such as Picea obovata and Pinus sibirica were forced farther south than Larix spp. (). Historical legacies, ever-changing environments and disturbance regimes, disequilibrium between vegetation and climate, and novel or no-analogue ecosystems (see below) highlight the vital importance of a detailed historical perspective in designing conservation strategies in an ever-changing world.
Figure 22. A schematic representation of the glacial legacy or ‘ecological memory’ on interglacial vegetation models of Herzschuh et al. (Citation2016). During interglacials following mild glacial stages (left), NE Asia was colonised by evergreen trees (e.g. Picea, Pinus) from nearby glacial-stage refugia. In contrast, during interglacials following cold glacial stages (right) Larix spp. and deciduous shrubs were dominant in response to the combined effects of permafrost persistence and distant glacial-stage refugia of evergreen trees, and fire. This model implies that vegetation–climate disequilibrium can last for many millennia. Modified from Herzschuh et al. (Citation2016).
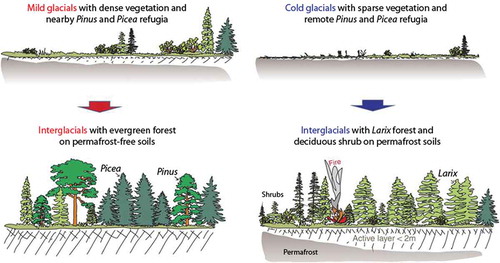
11.3.5. Impacts of future climate change
Considerable research effort is currently devoted to assessing the possible impacts of future climate change on biodiversity (see Box S5 for examples). Much of this involves bioclimate species-distribution modelling (e.g. Davis MB and Zabinski Citation1992; Franklin J Citation2010; Sinclair et al. Citation2010; Svenning et al. Citation2011; Warren DL Citation2012; Blois Citation2012a; Anderson RP Citation2013; Bahn and McGill Citation2013; Bradshaw RHW and Sykes Citation2014; Guisan et al. Citation2017; Morán-Ordóñez et al. Citation2017; Norberg A et al. Citation2019). This approach makes many assumptions (Warren DL et al. Citation2014; Suwal Citation2018) including that present-day distributions are in equilibrium with current climate (Pearson RG and Dawson Citation2003; Birks HJB et al. Citation2010; Araújo and Peterson Citation2012). Bradshaw RHW and Lindbladh (Citation2005) elegantly show that in southern Scandinavia, the southward spread of Picea abies over the last 4000 years was a complex response to regional climate change, whilst the northward spread and expansion of Fagus sylvatica appears to have been driven by human disturbance and fire and associated land-use changes. This example shows that distributional changes of two major forest trees were driven by different factors – regional climate (Picea) and human impact Fagus). Species-distribution modelling that assumes distributions are in equilibrium with climate could easily produce erroneous results in this case. Goring and Williams (Citation2017) show the compounding interactions of historical land-use and recent climate change on modern species–climate relationships that would underestimate species resilience to climate change (see also Gilbert B and O’Connor (Citation2013), HilleRisLambers et al. (Citation2013), Wisz et al. (Citation2013), and Epp et al. (Citation2018) for further considerations of the role of biotic interactions influencing climate change-induced range shifts).
The question of disequilibrium vegetation dynamics in response to climate change (past or future) was hotly debated in the 1980s and early 1990s (e.g. Birks HJB Citation1981b; Prentice Citation1983, Citation1986; Wright Citation1984; Ritchie Citation1986; Webb T Citation1986; Davis MB Citation1986a, Citation1989b). Webb T (Citation1986) proposed that vegetation has relatively short response times to climatic events of 500–1000 years duration and that there is a dynamic equilibrium with lags no greater than 1500 years during the Late Quaternary (Prentice et al. Citation1991). Such lags are large in the context of conservation planning in a rapidly changing world. Disequilibrium vegetation–climate relationships have recently emerged again as a major topic within biogeography and global-change biology (e.g. Corlett and Westcott Citation2013; Sax et al. Citation2013; Brown CD and Vellend Citation2014; Ash et al. Citation2017; Fordham et al. Citation2017; Blonder et al. Citation2017b, Citation2018; Huntley et al. Citation2018; Liang et al. Citation2018; Sandel Citation2019; Seeley et al. Citation2019). In an extensive review, Svenning and Sandel (Citation2013) note that ‘the general message from the paleobotanical literature is still one of multi-century migration lags in large-scale expansion following strong warming’. The role of ‘dispersal limitation’ over long time periods (thousands of years) and broad geographical areas (e.g. Europe) remains unclear (Normand et al. Citation2011). Svenning and Sandel (Citation2013) conclude that ‘there is emerging evidence that many plant species across a wide range of systems have not yet achieved equilibrium with climate following the onset of the current interglacial’ (see also Svenning and Skov Citation2004, Citation2007b; Birks HJB and Birks Citation2008; Svenning et al. Citation2008b, Citation2011; Normand et al. Citation2011; Ordonez Citation2013; Felde et al. Citation2018; Seeley et al. Citation2019). This conclusion contrasts with the implicit assumption of equilibrium conditions in species-distribution modelling (Pearson RG and Dawson Citation2003; Birks HJB et al. Citation2010; Franklin J Citation2010). At a finer temporal scale, Jackson and Sax (Citation2010) discuss immigration credit, extinction debt, and species turnover. They suggest that the time it takes for immigration (invasion) and extinction to be detectable following changes in climate could lead to short-term increases (invasion credit) or decreases (extinction debt) in local or regional diversity which might well change over longer time periods (see also Norberg J et al. Citation2012). Zhu et al. (Citation2012) show that in the eastern United States, there is no consistent evidence that tree population spread or range expansion are greatest in areas where climate has changed the most and suggest that there is no evidence for climate-mediated migration. This study highlights the problems of assessing the ecological risks posed by climate change.
A potentially valuable means of assessing biotic responses to climate change (past or future) is the climate-change velocity approach (Loarie et al. Citation2009; Ohlemüller Citation2011; Brito-Morales et al. Citation2018). This estimates the speed at which a given climate moves across a geographical area. Such climate velocities have been used to derive spatial trajectories for climate niches for the recent past (e.g. 1960–2009 CE) and the present and near future (e.g. 2006–2100 CE), to infer changes in species distributions, and to identify areas of potential change in biodiversity (e.g. Burrows et al. Citation2011, Citation2014). The approach has also been used over Late Quaternary time in North America (Ordonez and Williams Citation2013b) and South America (Correa-Metrio et al. Citation2013).
In the North American study, Ordonez and Williams (Citation2013b) estimate biotic velocities for 30 woody taxa from pollen data for the last 16,000 years and climate velocities from climate simulations for the same period. Biotic velocities are faster during periods of rapid temperature change (16,000–7000 yr BP) than during times of relative temperature stability (7000–1000 yr BP), with a consistent northward shift of both northern and southern range boundaries. Biotic velocities are faster for northern than for southern boundaries between 12,000 and 7000 yr BP, resulting in range expansion northwards. Interestingly, biotic velocities are as fast or faster than climate velocities for individual time periods. This study suggests that climate change paced the rate of range shifts of both the northern and southern boundaries but the northern boundaries were more sensitive. Similar sensitivities and pacings are expected in the near future (Ordonez and Williams Citation2013b).
Correa-Metrio et al. (Citation2013) use pollen data for the last 30,000 years from Mexico and 86,000 years from Guatemala to estimate temperature changes and their velocities. They show that climate velocities in Mesoamerica are at least four times slower than values reported for the last 50 years but twice as fast as those derived from recent climate models. These results suggest, given the high climate-change velocities, that taxon survival must have depended on microrefugial populations or long-term persistence of suppressed individuals. In contrast to the notion of stable climates being associated with high biodiversity, these results suggest that Mesoamerican tropical diversity was probably maintained by centennial-scale climate variability that limited competitive exclusion. Looking to the future, Correa-Metrio et al. (Citation2013) argue that as human activity has changed the landscape and removed many potential microrefugia and as climate change is occurring at a very high velocity, the risk of extirpation or extinction for tropical taxa is higher than at any time in the last 86,000 years.
Ecologists and biogeographers have imported the refugium concept (see subsection 2.2) into modern landscapes and global-change biology (Birks HJB Citation2015). The aim is to identify potential microrefugia against future climate change for particular species or vegetation types (e.g. Ashcroft et al. Citation2009; Ashcroft Citation2010; Keppel et al. Citation2012; Mackey B et al. Citation2012; Selwood et al. Citation2015; Lenoir et al. Citation2016; Morelli et al. Citation2016; Baumgartner et al. Citation2018; Gubler et al. Citation2018; Stralberg et al. Citation2018). Several approaches are used to predict future refugia but they nearly all involve studies of landscape-scale physiography, microclimatology of soil temperatures and moisture, species-distribution modelling, and palaeoecology (e.g. Saxon et al. Citation2005; Dobrowski et al. Citation2009; Ackerly et al. Citation2010; Graham CH et al. Citation2010; Scherrer and Körner Citation2010; Scherrer et al. Citation2011; Ashcroft and Gollan Citation2012, Citation2013; Ashcroft et al. Citation2012; Franklin J Citation2013; Potter et al. Citation2013; Keppel et al. Citation2015; Roberts DR and Hamann Citation2016; de Novaes Nascimento et al. Citation2019; Gubler et al. Citation2018). There are no means of evaluating how useful these predictions of potential refugia will be – only time will tell!
Other approaches to assessing the possible response of biota to future climate change have centred on discovering how biota responded to past climate changes reconstructed independently from the palaeoecological record (e.g. Davis MB Citation1989a, Citation1989b, Citation1989d, Citation2001a; Davis MB and Zabinski Citation1992; Bush Citation2002; Anderson NJ et al. Citation2006; MacDonald et al. Citation2008; Willis and Bhagwat Citation2009; Cole KL Citation2010; MacDonald Citation2010; Willis et al. Citation2010b, Citation2013; Willis and MacDonald Citation2011; Fang et al. Citation2013; Maiorano et al. Citation2013; Tinner et al. Citation2013; Iglesias et al. Citation2015a, Citation2018; Fordham et al. Citation2016). Despite the limitations and hidden assumptions of species-distribution modelling (Guisan and Thuiller Citation2005; Fitzpatrick and Hargrove Citation2009; Birks HJB et al. Citation2010; Hof et al. Citation2011; Araújo and Peterson Citation2012; Meier et al. Citation2012; Rapacciuolo et al. Citation2012; Suwal Citation2018), valuable contributions to conservation planning are emerging from a combination of modern biogeographical data, species-distribution modelling, and palaeoecological results (e.g. Willis and Birks Citation2006; Cole KL et al. Citation2011; Roberts DR and Hamann Citation2012; Henne et al. Citation2013; Macias Fauria and Willis Citation2013; Ruosch et al. Citation2016; Lima‐Ribeiro et al. Citation2017). The general approach proposed by Dawson TP et al. (Citation2011) in which palaeoecology, ecosystem (e.g. Shugart Citation1990) and species modelling, population analyses, field experiments and manipulations, etc. are considered together to predict biotic responses to future climate change seems, at present, to be the most useful and reliable approach (see also MacDonald Citation2010; Guisan and Rahbek Citation2011; Espíndola et al. Citation2012; Evans et al. Citation2016; Lovejoy and Hannah Citation2019).
11.3.6. Invasive species
Palaeoecology can provide insights into the key question faced by conservationists considering invasive species and the restoration of native biotic assemblages (Froyd and Willis Citation2008), namely is a species native? Palaeoecology provides a reliable approach to determine the native status of a species in a given area (Jackson Citation1997). For example, in their analysis of the British flora in relation to palaeobotanical data, Preston et al. (Citation2004) delimit 157 species as probable ‘archaeophytes’, i.e. species introduced to Britain by humans before 1500 CE. Many of these species (e.g. Capsella bursa-pastoris, Fumaria officinalis, Myosotis arvensis, Papaver rhoeas) were previously considered to be native or doubtfully native. The assignment of 157 species to archaeophyte status creates conservation problems because this ‘non-native’ label excludes them from the English Red Data Book (Stroh et al. Citation2014) of threatened or near-extinct species and automatically gives them lower conservation value than an equally rare or threatened native species (Willis and Birks Citation2006). Some archaeophytes are now very rare and have declined markedly in the last 50 years (e.g. Agrostemma githago (possibly extinct as an archaeophyte), Ranunculus arvensis).
It is unclear whether Pinus sylvestris, once an important native component of the Irish tree flora, has more than one remaining native occurrence in Ireland (McGeever and Mitchell Citation2016). It is, however, widely planted in semi-natural habitats in Ireland. Twenty Pinus sylvestris-dominated plots were surveyed by Roche et al. (Citation2009) and shown to contain 14.2% of the native Irish flora. Scots pine is well established and naturalised in a range of Irish semi-natural habitats. Some of its vegetation types are in habitats of international conservation importance, such as wooded raised bogs. Although considered a re-introduced species in Ireland and hence excluded from the Irish National Survey of Native Woodland and its associated community classification (Perrin et al. Citation2006) or afforded any special conservation in Ireland, Scots pine plays an important role in Irish biodiversity and vegetation. In some ways, its re-introduction falls within the concept of botanical ‘rewilding’ (see above) and has helped the restoration of some native vegetation types.
Determining native or non-native status is particularly critical on oceanic islands and palaeoecology has made important contributions to this topic (Willis and Birks Citation2006; Froyd and Willis Citation2008; see subsection 12.4). Assessing non-native status is becoming increasingly important in many aspects of conservation science (e.g. Webb SL et al. Citation2000; Ricciardi Citation2007; Ricciardi et al. Citation2017a, Citation2017b; Wagner V et al. Citation2017; Zenni et al. Citation2017) and global-change biology (e.g. Mack et al. Citation2000; Broennimann et al. Citation2007; Hulme et al. Citation2015; Thomas CD and Palmer Citation2015; Latombe et al. Citation2017; Nackley et al. Citation2017). Palaeoecology continues to provide key information about the status of species in particular areas.
11.3.7. No-analogue assemblages and future novel ecosystems
Vegetation re-sampling surveys (e.g. Hédl et al. Citation2017; Kapfer et al. Citation2017), although not palaeoecological studies, show that considerable changes in vegetation composition have occurred over the last 30–60 years in, for example, the Scottish Highlands (Britton et al. Citation2009, Citation2017a, Citation2017b; Ross LC et al. Citation2012; Flagmeier et al. Citation2014; Mitchell RJ et al. Citation2017). Overall there is a trend towards compositional taxonomic homogeneity, increased abundance of a few species (mostly graminoids), and a decline in the cover of other species, particularly herbs, lichens, and bryophytes. The causes for these changes are complex and are not at all well understood. Primary suspects are climate change, pollution deposition, land-use changes, and habitat loss, as well as interactions between these drivers. The overall effect has been a loss of various aspects of diversity at the local, community, and landscape scales, and the emergence of some vegetation types that were not present 50 or 60 years ago, so-called non-analogous or novel communities (sensu Keith et al. Citation2009a). Similar composition homogenisation has occurred widely during the last 50 – 100 years in, for example, woodlands in southern England (Keith et al. Citation2009b), Germany (Becker et al. Citation2017; Heinrichs and Schmidt Citation2017), Poland (Reczyńska and Świerkosz Citation2017), and the Czech Republic (Vild et al. Citation2017); mires in Sweden (Kapfer et al. Citation2011), Germany (Schweiger and Beierkuhnlein Citation2017), and the Czech Republic (Navrátilová et al. Citation2017); and grasslands in Germany (Koch M et al. Citation2017), Italy (Giarrizzo et al. Citation2017), and Oregon (Bernards and Morris Citation2017).
Keith et al. (Citation2009a) differentiate five types of non-analogous communities (Box S6). Palaeoecologists recognise no-analogue communities to 'consist of species that are extant today, but in combinations not found at present. “No-analog” is therefore shorthand for “no present analog” and can refer to both past and potential future communities' (Williams and Jackson Citation2007). As past communities cannot generally be seen directly but are inferred from fossil pollen and macrofossil assemblages, a palaeoecologist’s 'no-analogue' is strictly a pollen or macrofossil assemblage of taxa 'that are extant today, but in combinations not found at present' (see subsection 7.2).
Given the wide range of environmental (e.g. climate, soil, disturbance, land-use) and biotic (e.g. herbivory, invasive taxa, disease) that have occurred in the last 15,000 years and especially in the last 100 years, it is not surprising that no-analogue assemblages occurred in the past and are emerging today in response to the strong cocktail of climatic, environmental, and biotic changes (e.g. Urban MC et al. Citation2012; Mahony et al. Citation2017; Hobbs RJ et al. Citation2018). Westerling et al. (Citation2011) predict novel fire regimes and hence novel fire–climate–vegetation relationships in Yellowstone by the middle of this century.
The studies by Iversen (Citation1954, Citation1967, Citation1973) are perhaps the first to identify no-analogue fossil assemblages, in this case from the Danish late-glacial. With megaherbivores, open base-rich soils, and a climate with greater seasonality than today, the flora and vegetation consisted of taxa with very contrasting phytogeographical and ecological affinities (e.g. arctic, arctic-alpine, steppe, ruderal). No such assemblages occur widely today. No-analogue pollen assemblages are not uncommon in the late-glacial and early Holocene in many parts of the world (e.g. Cushing Citation1963; Birks HJB Citation1976, Citation1981a; Overpeck et al. Citation1992; Williams et al. Citation2001, Citation2004, 2013; Jackson and Williams Citation2004; Gill et al. Citation2009, Citation2012; Bridgewater et al. Citation2011; Correa-Metrio et al. Citation2012a; Gill Citation2014; Ordonez et al. Citation2016; Finsinger et al. Citation2017b; Birks HJB and Berglund Citation2018; see subsection 7.2).
Rapid and ongoing environmental and biotic changes are creating novelty in ecosystems almost everywhere, both when comparing contemporary systems to their historical baselines and when comparing predicted future systems to the present (e.g. Fitzpatrick and Hargrove Citation2009; Hobbs RJ et al. Citation2013, Citation2018; Reu et al. Citation2014; Radeloff et al. Citation2015; Alexander JM et al. Citation2016). Despite novel or no-analogue systems not being a feature confined to the recent past (Anthropocene) (‘Ecological novelty is not new’ – Jackson Citation2013b), the concept of ‘novel ecosystems’ and their implications for conservation and restoration (Jackson Citation2007; Hobbs RJ et al. Citation2009, Citation2018) have recently generated much debate amongst ecologists and conservationists (e.g. Thompson AR and Jackson Citation2013; Aronson et al. Citation2014; Hobbs et al. Citation2014a, Citation2014b; Corlett Citation2014; Murcia et al. Citation2014; Collier and Devitt Citation2016). Other than recognising non-analogue assemblages in the past or the future and attempting to disentangle what aspects of the past environment results in such novelties (Jackson Citation2013b; Urban MA et al. Citation2015; Ordonez et al. Citation2016), palaeoecology’s potential contribution to the problems of conserving nature in the face of ever-increasing novelty is to assess whether the development of novelty has been accelerating in the last 100–200 years, and to compare these rates with past rates of change (e.g. Finsinger et al. Citation2017b). As Jackson (Citation2013b) points out, a new kind of ecological novelty seems to be arising due to a massive acceleration in all the human activities that lead to novel ecosystems – introductions of exotic taxa, unprecedented rapid climate change and climate novelty (Fitzpatrick et al. Citation2018), ever-increasing human land-use and resource exploitation, alteration of global biogeochemical cycles, and all possible interactions (Vitousek et al. Citation1997; Bonan and Doney Citation2018). Novel biotic assemblages today are a result of some species moving (‘movers’ sensu Hobbs RJ et al. Citation2018) and other species staying put (‘stayers’ sensu Hobbs RJ et al. Citation2018) leading to an array of novel species interactions as species differ in their response that initiate movement or persistence (Hobbs RJ et al. Citation2018; see Graae et al. Citation2018 for an attempt to identify species responses). Jackson (Citation2013b) notes that ‘[e]cological novelty is widely perceived as a threat to conservation and restoration, and indeed it can be. However, it also comprises a reality and, more importantly, an opportunity. If we understand them better, we can leverage novel ecosystems and other aspects of ecological novelty to advantage in pursuing broad goals in conservation and climate-change adaptation’. Understanding past novel systems is thus an important part of our abilities to cope with ever-increasing ecological novelty now and in the future (Hobbs RJ et al. Citation2014b, Citation2018).
The increasing recognition of novelty in ecosystems is leading to changes in the use of historical baseline knowledge in restoration ecology (Higgs ES et al. Citation2014) including rewilding. Traditional restoration ecology has been driven by specific historically based targets, whereas a newer approach uses historical knowledge as a guide and not as a template, accepts multiple potential trajectories for ecosystems, and emphasises process over structure (Higgs ES et al. Citation2014). Restoration, including some forms of rewilding, in our rapidly changing world is becoming more and more important but also more and more challenging. Hobbs RJ et al. (Citation2018) emphasise that ‘[i]n a world of changing species distributions and assemblages, it will be increasingly important to understand how and why species move or stay and to deploy effective interventions that achieve desired conservation and restoration goals’.
11.3.8. Antiquity of vegetation types
Palaeoecology can provide unique insights into the antiquity of particular vegetation types on the basis of their dominant taxa (Jackson Citation2006a, Citation2013a; Liu et al. Citation2010). In his assessment of the antiquity of major western North American vegetation types Jackson (Citation2006a) discusses the range of environmental variation (‘historical range of variability’ – Jackson Citation2012a) a particular ecosystem may have experienced in the past and the environmental conditions under which the ecosystem developed. The range of environmental variation is scale-dependent, as a narrow time-span (e.g. 250–500 years) may underestimate the range of variation the ecosystem has sustained, whereas longer time-spans (1000–2000 years) naturally increase the range of variation experienced. Most vegetation types disappear when the time-span is increased to 15,000 years due to secular changes in Earth’s climate system (Jackson Citation2006a). Palaeoecological data can generally identify the time of origination and termination of particular vegetation types, at least at the broad landscape scale that regional-scale pollen-analytical data primarily sense. In general, different types originated at different times and almost all modern vegetation types are younger than 11,500 years, with many types developing in the last 3000–4000 years. There is considerable scope for extending this type of analysis to other geographical areas such as eastern North America, Europe, and the Mediterranean basin (e.g. Davis BAS et al. Citation2015; Fyfe et al. Citation2018a), and South America using the wealth of pollen data and associated chronological data stored in pollen databases such as Neotoma (Mitchell FJG Citation2011; Brewer et al. Citation2012; Grimm EC et al. Citation2013, Citation2018; Goring et al. Citation2015; Flantua et al. Citation2016b; Williams et al. Citation2018) and to look for spatial and temporal patterns in the origination and termination of vegetation types, in duration, and in historical ranges of variability (see subsection 9.8 for a preliminary analysis of the antiquity of British forest types). Such information is clearly of potential value for conservation planning and management.
11.4. Palaeoecology and ecosystem services
Following the publication of the Millennium Ecosystem Assessment (Citation2005) and the rapid adoption of the ecosystem-service concept, conservationists and managers are increasingly focussing on preserving and restoring ecosystems for their services (benefits that people receive from the natural functioning of healthy ecosystems: Mace Citation2014; Dee et al. Citation2017; Everard Citation2017). The shift in focus to ecosystem services has been very influential in conservation thinking at the ecosystem level in the last 5–10 years (Mace Citation2014). However, there has also been a recent change in emphasis from ‘managing nature to maximise the overall value of the human condition’ (‘nature for people’ framework – Mace Citation2014) to one that recognises the two-way dynamic relationships between people and nature (‘people and nature’ framework – Mace Citation2014). These ecosystem-based ideas (Colloff et al. Citation2017) require metrics to link nature to human well-being that explicitly identify and quantify benefits needed and received by people, so-called ‘natural capital’ (e.g. Costanza et al. Citation1997; Ekins et al. Citation2003; Mace Citation2014; Mace et al. Citation2015; Díaz et al. Citation2018; Haines-Young and Potschin Citation2018). Jackson (Citation2012b), Jeffers et al. (Citation2015), and Saulnier-Talbot (Citation2016) review the potential role of palaeoecological data in assessing the continuity of ecosystem services and goods over time. Behind ecosystem services (e.g. water supply, crops, climate regulation) and the resulting ecosystem goods (e.g. timber, soil-erosion protection) (Jeffers et al. Citation2015), there are basic underlying ecosystem processes that determine the quality and quantity of the final ecosystem services and goods (Montoya JM and Raffaelli Citation2010; Mace et al. Citation2012; Maron et al. Citation2017). Like all ecological processes these interact with biodiversity (Dee et al. Citation2017) and are vulnerable to climatic change over a range of temporal and spatial scales (e.g. Marland et al. Citation2003; Montoya JM and Raffaelli Citation2010; Jackson Citation2012b; Oliver et al. Citation2015; Isbell et al. Citation2017).
Several types of palaeoecological data can provide insights into the long-term variability of some of these ecosystem processes (e.g. nutrient cycling; soil formation (see section 3); biomass production) (Jeffers et al. Citation2015). Such data can assist in determining the resilience and persistence of ecosystem services over time and space to a range of environmental changes (cf. Jackson Citation2012b; Maron et al. Citation2017). These services include crops, water supply and quality, and climate regulation (Jeffers et al. Citation2015). There are also relevant palaeoecological data that can, with care, be interpreted as reflecting long-term changes in ecosystem goods such as timber, soil-erosion, and coastal protection (Jeffers et al. Citation2015). Examples of such studies involving pollen analysis include Paciorek and McLachlan (Citation2009 – biomass), Seppä et al. (Citation2009a – biomass), González et al. (Citation2010 – coastal protection), Higuera et al. (Citation2011 – timber), Prentice et al. (Citation2011 – climate regulation), Colombaroli and Tinner (Citation2013 – biodiversity provisions), Gosling and Williams (Citation2013 – water supply), and Macias Fauria and Willis (Citation2013 – crops). Multi-proxy studies, particularly palaeolimnology (Saulnier-Talbot Citation2016), extend the use of palaeoecological data in studying temporal patterns in ecosystem processes, services, and goods such as nutrient cycling (e.g. McLauchlan et al. Citation2013a, Citation2013b, Citation2019), soil erosion and catchment degradation (e.g. Dearing Citation2008; Dearing et al. Citation2008, Citation2012; Neff et al. Citation2008), and timber production and nutrient cycling in relation to disturbances (e.g. McLauchlan et al. Citation2014) including fire (e.g. Conedera et al. Citation2009; Higuera et al. Citation2011; Iglesias et al. Citation2015b; Morris et al. Citation2019) and infestations by defoliating insects (e.g. Morris et al. Citation2013, Citation2017; Lesk et al. Citation2017). Lake sediments and peat deposits provide multi-proxy records (‘archives’) of the development of the sites and their surroundings. Such histories are themselves ecosystem services (Millennium Ecosystem Assessment Citation2005; Geary and Fyfe Citation2016; Greiser and Joosten Citation2018; Haines-Young and Potschin Citation2018).
The application of a range of palaeoecological techniques to assess ecosystem services is a research area that will no doubt expand as techniques, project designs, and research questions improve and the links between palaeoecological data, ecosystem services, and cultural practices are more widely recognised and explored further (e.g. Marland et al. Citation2003; Dearing Citation2006; Costanza et al. Citation2007; Dearing et al. Citation2011b; Pearson S et al. Citation2015; Colombaroli et al. Citation2017; Law et al. Citation2017). However, this will perhaps not happen until the ecosystem-service concept has developed clearer definitions (e.g. Haines-Young and Potschin Citation2018) and the valuation of ecosystem services and natural capital today is approached in a more rigorous and transparent way than currently (e.g. Jax and Heink Citation2015; Costanza et al. Citation2017; Gunton et al. Citation2017; Keeler et al. Citation2019).
11.5. Conclusions
Effective evidence-based conservation in a rapidly changing world is a major challenge (Gillson et al. Citation2019) and must involve a huge range of disciplines, approaches, and techniques including palaeoecology and historical biogeography (Hamilton et al. Citation2011; Hobbs RJ et al. Citation2014b; Jackson Citation2016). Travis (Citation2003) warns that ‘[c]limate change and habitat destruction is a deadly anthropogenic cocktail’. Besides the enormous scientific, social, and economic challenges that conservation for the future is facing, there is the key question ‘[a]re conservation organizations configured for effective adaptation to global change?’ (Armsworth et al. Citation2015). The idea of translational ecology and hence also translational conservation (Schlesinger Citation2010; Chapin Citation2017; Enquist et al. Citation2017; Jackson et al. Citation2017) is very timely and relevant today. Translational ecology involves ecologists, stakeholders, and decision makers working together to develop research that addresses the ecological, sociological, and political contexts of a particular environmental problem or set of problems. As Enquist et al. (Citation2017) suggest ‘successful [translational ecology] increases the likelihood that ecological science will inform and improve decision making for environmental management and conservation’. Conservation palaeoecology presented here has a unique potential in providing a temporal perspective to environmental problems being addressed by translational ecologists and conservationists (Flessa Citation2017). Palaeoecology can contribute critical evidence for long-term sustainability through traditional practices of past ecosystem management as well as having the ability to test hindcast models against detailed long-term observational records. By adding the long-term perspective to conservation, palaeoecology – including Quaternary botany – can help develop flexible, adaptive, future strategies for conservation and management; shed light on potential thresholds; and provide vital information on the past responses of species to climate change (; Oldfield Citation2015).
12. Island palaeoecology
12.1. Introduction
Islands and their biota have long fascinated naturalists, ecologists, biogeographers, and evolutionary biologists since the times of Hooker (Citation1847a, Citation1847b, Citation1867), Darwin (Citation1845, Citation1859; Keynes Citation2003), Wallace (Citation1881 (reprinted 2017)), von Humboldt (Wulf Citation2015), and others (see Ziegler Citation2002; Whittaker RJ and Fernández-Palacios Citation2007; Bramwell and Caujapé-Castells Citation2011; Hanski Citation2016; Lasky et al. Citation2017). Islands have long been thought of as ‘laboratories for the experimental study of evolution’ (Deevey Citation1969) as elegantly exploited by, for example, Grant (Citation1997) and Grant and Grant (Citation2011, Citation2014) in their classic work on Darwin’s finches. The seductive equilibrium theory of island biogeography (MacArthur and Wilson Citation1967; Losos and Ricklefs Citation2009) has spawned a vast amount of observational and some experimental research on island biotas and their richness and diversity, on species-area relationships, and on testing the concept of equilibrium in which declining rates of species immigration balance the rising rates of species extinction (Gilbert FS Citation1980). Small ‘habitat-poor’ islands have lower species richness than large ‘habitat-rich’ islands, and near islands have more species than distant islands. Is there an equilibrium over time as proposed by MacArthur and Wilson (Citation1967)? Are the observed differences in richness between islands a result of a balanced equilibrium saturation, as the theory proposes? Alternatively, have the biota of distant islands not had sufficient time to reach equilibrium (Deevey Citation1969) or have some islands experienced greater extinctions than other due to factors unrelated to island area or distance from the mainland such as human exploitation (e.g. van der Geer et al. Citation2017)? As Deevey (Citation1969) comments ‘[t]he more one looks at this model … the more one wishes for some fossil evidence … One wishes for some kind of genuinely historical data that might constrain the bold assumptions about time-rates’. Fifty years since Deevey (Citation1969) made these comments, palaeoecologists have now studied many islands (see below). Island biologists have recently raised historically-based questions (e.g. How can palaeoecology contribute to the understanding of species arrival, establishment and spread on islands?) in the 50 fundamental questions after fifty years of The Theory of Island Biogeography (Patiño et al. Citation2017). This ‘roadmap for island biology’ notes that ‘palaeoecology is a field of emerging importance in island biogeography’ (Patiño et al. Citation2017), a view that reinforces Deevey’s (Citation1969) wish for historical data.
In a discussion of oceanic island conservation strategies, Cronk (1997) presents what he calls the 'diversity and stability “paradox” of island biotas'. Islands are often species-poor but show considerable biological interest and diversity in terms of morphologically unusual endemic genera and taxonomically isolated groups (e.g. Hooker Citation1867; Lems Citation1960, Citation1961; Lems and Holzapfel Citation1971; Bramwell and Bramwell Citation1974; Bramwell Citation1979; Carlquist Citation1992; Bramwell and Caujapé-Castells Citation2011). They appear compositionally stable (in some cases their biota may be millions of years old) and having an oceanic climate, they are largely buffered against extreme climatic events (e.g. Collins et al. Citation2013; Zhang et al. Citation2014). However, they are very susceptible to rapid change when subjected to exogenous disturbances, particularly human colonisation and exploitation (Cronk Citation1997) and invasive species (Simberloff Citation1986). The general patterns of 'proximate' and 'ultimate' diversity and stability on oceanic islands and on continental mainlands are summarised in .
Table 25. Comparison of ‘proximate’ and ‘ultimate’ diversity and stability on oceanic islands and continental mainlands (modified from Cronk Citation1997).
Island ecosystems worldwide have been greatly modified since their first colonisation by humans (e.g. Quammen Citation1996; Machado Citation2004). The small and isolated nature of populations of many island biota have rendered them particularly extinction-prone due to human activity and to competition from taxa introduced deliberately or accidentally by humans (e.g. Vitousek Citation1988; Cronk and Fuller Citation1995; Vitousek et al. Citation1995; Quammen Citation1996; Whittaker RJ et al. Citation2017). Even highly degraded islands may retain some of their biological interest by supporting small populations of endemic or relictual taxa (Cronk Citation1980, Citation1997). The very biological characteristics that make island biotas so unique () create major challenges in their conservation, management, or restoration (Cronk Citation1997; Nogué et al. Citation2017).
Since the pioneering palynological studies on the Hawaiian Islands of Kaua’i, Moloka’i, and West Maur’i by Selling (Citation1948, Citation1949, Citation1951; see also Fagerlind Citation1949; Fries M Citation1949), detailed stratigraphical records of pollen, macrofossils, and charcoal are now available from many islands. These records provide insights into vegetation stability prior to human colonisation; the timing of human colonisation, the extent of human activities, and plant extinctions; the distinction between native and non-native plant species on islands; the effects of extinction of vertebrate herbivores on island systems; long-term changes in fire and other disturbances; and baseline conditions for evidence-based restoration and management plants (e.g. Nogué et al. Citation2017). This review is divided into sections on stability prior to human colonisation; plant extinctions; identifying native and non-native species; faunal extinctions and their impacts and changes in disturbance regimes; and establishing baseline conditions. As habitat islands (e.g. ‘Sky islands’) are commonly included in island biogeography, I conclude by briefly reviewing what is known about the palaeoecology of the spectacular tepuis or ‘table mountains’ in Neotropical Venezuelan Guayana.
12.2. Stability prior to human colonisation
summarises palynological data from 37 representative oceanic islands or island groups in the different oceans around the world. All the pollen stratigraphies summarised in show a palynologically stable phase, generally of between 2000 and 8000 years, prior to the onset of human impact. This palynologically stable phase is interpreted to reflect relatively stable vegetation composition. As Colinvaux and Schofield (Citation1976) argue in connection with their long Galápagos pollen stratigraphies ‘the pollen diagrams suggest a stable vegetation in which species do not often become extinct. It is true that the [pollen] record does not include all species in the vegetation, but there is a record of a representative cross section. It cannot be argued that the record is exclusively of common species, which remain, while the rarities come and go in the manner of the MacArthur and Wilson paradigm, because the peculiar subset of species that leaves a pollen record in the lake includes both the common and the rare’.
Table 26. Selected examples of islands or island groups in different oceans where pollen-analytical studies show compositional stability prior to the onset of human impact and ecosystem modification. The approximate duration of the periods with stable pollen composition is given, along with the age for the onset of human activities and an indication of plant extinctions or exterminations of all types on each island. The duration of the stable phases are minimal as the pollen assemblages in some of the sequences may commence within the stable phase.
Tasmania () is an exception in having evidence for human impact (western Tasmania) dating back to the late-glacial where an ancient cultural landscape of moorland was created (Fletcher and Thomas Citation2010), whereas in the Cradle Mountain area of central Tasmania there is an 8000 year palynologically stable phase dominated by Nothofagus cunninghammii and Eucalyptus pollen (Stahle et al. Citation2016) and in the Inland Plains area of eastern Tasmania there is a 10,000-year palynologically stable phase of dominant Eucalyptus pollen (Jones PJ et al. Citation2017). It is not possible to detect palynologically human impact in the sub-Antarctic islands ().
There are some ecological surprises from the island palynological records within the stable phase. These include the former occurrence of Carpinus and Quercus (and subsequent regional extermination) on Tenerife (de Nascimento et al. Citation2009), the former abundance of Buxus balaerica and Corylus, and the occurrence of Abies, Betula, Quercus, Tilia, and Ulmus on Minorca and Mallorca (Yll et al. Citation1994, Citation1997; Pérez-Obiol et al. Citation1995; Welker et al. Citation2014), and the former occurrence and abundance of palms on several Pacific islands (Prebble and Dowe Citation2008), including the now totally extinct palm on Rapa Nui discussed below.
A striking feature of pollen diagrams from islands () is that virtually all the pollen taxa represented in the stratigraphic records are present from the very beginning of the sequence. This suggests that many, if not all, the associated plant taxa were present on many of the islands early in the Holocene. Given this observation and the fact that virtually no pollen taxa disappear from the records, all the available palynological data from islands support Colinvaux and Schofield’s (Citation1976) conclusion for the Galápagos pollen record that ‘[t]here was apparently no pattern of extinction and immigration as required by the MacArthur and Wilson paradigm’ and Cronk’s (Citation1997) () view that oceanic islands show stability over geological time, at least Quaternary time, in contrast to pollen records from continental mainlands.
These conclusions contrast markedly with the views of Kueffer et al. (Citation2014) who suggest that palaeoecological data show ‘how dynamic pre-human island ecosystems were. Vegetation on many islands fluctuated strongly in response to climate changes, contradicting the common opinion that islands experienced buffered climates with minimal fluctuations, providing a new perspective on the challenges for island species to persist in isolations’.
12.3. Plant extinctions and exterminations
Compared with the large number of known extinctions or exterminations of island vertebrates, especially birds, at or soon after human colonisation of oceanic islands, especially in the Pacific, Indian, and Southern Oceans (e.g. Burney and Flannery Citation2005; Lockwood JL et al. Citation2009; Tyrberg Citation2009; Turvey Citation2009a, Citation2009b; Hume Citation2017; Whittaker RJ et al. Citation2017), the number of known plant exterminations on oceanic islands is relatively small. Only eleven of the 37 islands summarised in witnessed some plant exterminations in the Holocene. Detecting plant extinction or extermination from palynological data is notoriously difficult (see section 5) due to the differential pollen representation of different plants (e.g. trees vs. low-growing herbs and climbers, anemophilous vs. zoophilous or entomophilous pollination syndromes) and the well-known problems of interpreting low scattered pollen occurrences as evidence of former presence (Birks HJB and Berglund Citation2018). Of the extinctions or exterminations indicated in , the vast majority occur at or soon after the onset of human impact. The exception is the extermination of Betula nana on the Færoe Islands in the early Holocene (Jóhansen Citation1968; Hannon et al. Citation2010), as a result, almost certainly, of climate change. Stumps of Betula pubescens dated to about 4500 BP occur at the Viking site of Argisbrekka on Eysturøy in the Færoe Islands (Jóhansen Citation1989; Hannon et al. Citation2001) but with very low pollen values. These wood remains are interpreted as records of a nearly sterile copse of trees surviving from a period of more favourable climate (Hannon et al. Citation2001) and which was exterminated by human activity and/or climatic deterioration (Jóhansen Citation1989; see also Lawson et al. Citation2005). Tree birch was also present on the Færoe Islands in the last interglacial (Bennike et al. Citation2018). Although the pollen record from the Kanaka Crater on Mauritius (de Boer et al. Citation2013a, Citation2013b) indicates the disappearance of pollen of Cycadaceae, Artemisia afra-type, Hydrocotyle-type, Alchornea-type, Laurembergia tetrandra, and several palm taxa during the Holocene, de Boer et al. (Citation2013a, Citation2013b) suggest that these taxa may have persisted elsewhere on the island and were not extirpated from Mauritius until human colonisation.
The vast majority of known regional or local Holocene exterminations on the Atlantic islands such as Shetland, Outer Hebrides, and Canary Islands () are of trees. Bennett (Citation1995) lists tree taxa that have been exterminated on 20 off-shore islands in the British Isles. The commonest exterminated taxon is Pinus sylvestris (11 out of 20 islands), followed by Alnus glutinosa (5 islands), and Quercus and Corylus avellana (4 islands each).
Little is known about pre-Holocene extinctions on islands due to the absence of Pleistocene deposits. The recent report of fossil seeds of Eurya stigmosa (Theaceae) from Early Pleistocene (1.3 million years ago) deposits on Madeira (Góis-Marques et al. Citation2019) documents the total extinction of this species, previously known only from continental Palaeogene and Neogene deposits.
Despite the large numbers of faunal extinctions or exterminations coinciding with human colonisation of Pacific Ocean Islands (e.g. Martin PS and Steadman Citation1999; Burney and Flannery Citation2005; Steadman Citation2006), relatively few plant extinctions or exterminations are recorded. At a low taxonomic level, faunal losses are concentrated in large-bodied representatives of certain genera or families (Lyons et al. Citation2004; Prebble and Dowe Citation2008). Plant losses show a similar trend with a large number in the Arecaceae (palms) across all five Arecaceae sub-families (Prebble and Dowe Citation2008). Pacific islands or island groups where there is evidence for regional palm extermination include the Hawaiian Islands, French Polynesia, Cook Islands, Norfolk Island (Australia), States of Micronesia, Rapa Nui, Fiji, and Poor Knight Islands (New Zealand) (Selling Citation1948; Prebble and Dowe Citation2008). Pritchardia is the genus that has been exterminated from most Pacific islands (Prebble and Dowe Citation2008). Exterminations are not, however, confined to palms. There is evidence for possible species extinctions (*) or regional or local exterminations of taxa in the Araliaceae*, Combretaceae, Cunoniaceae*, Elaeocarpaceae, Euphorbiaceae*, Fabaceae, Flacourtiaceae, Malvaceae*, Myoporaceae*, Myrsinaceae*, Myrtaceae, Pittosporaceae*, Santalaceae*, Sapotaceae, Rubiaceae, Rutaceae*, Ulmaceae, Urticaceae, and Xanthorrhoeaceae from French Polynesia, Cook Islands, Rapa Nui, Laysan (USA), and Juan Fernandez (Prebble and Dowe Citation2008; Cañellas-Boltà et al. Citation2014). The largest number of species extinctions or exterminations of non-palm families is on Rapa Nui (11 out of 19) followed by French Polynesia (5). In a recent analysis of the documented 571 plant extinctions globally (Humphreys et al. Citation2019; Ledford Citation2019), Hawaiian Islands have the largest number (75) of species extinctions.
Perhaps the best known recently extinct plant is the Rapa Nui palm that suffered total extinction at about 1200 CE. Pollen records from the various caldera lake sediments on Rapa Nui (e.g. Flenley et al. Citation1991; Azizi and Flenley Citation2008; Mann DG et al. Citation2008; Cañellas-Boltà et al. Citation2016) show that Arecaceae pollen was the dominant type for over 30,000 years until the time of human colonisation about 1200 CE. Macrofossils (endocarps), often gnawed by rats, found in a cave on the Poikes Peninsula suggest that the palm was a cocosoid and a member of the sub-tribe of Attaleinae within the Cocoseae tribe (Dransfield et al. Citation1984; Dransfield pers. comm. 2016). The endocarps are highly reminiscent of Jubaea chilensis but J. Dransfield (pers. comm. 2016) does ‘not believe them to be identical, but certainly very similar’. The fossil palm has been named Paschalococos disperta by Dransfield (Citation1991) but archaeologists, modellers, and non-palm experts continue to equate the extinct palm with the Chilean Jubaea chilensis that grows well in the Mediterranean climate of central Chilli today but is unlikely to thrive on Rapa Nui with its sub-tropical oceanic climate (Dransfield pers. comm. 2016). Delhon and Orliac (Citation2008) suggest on the basis of phytolith data that there may have been two species of palm growing on Rapa Nui. Clearly more work is needed and the matter of the identity of the Rapa Nui palm or palms remains partly unresolved.
There are, of course, many hypotheses to explain the extinction or extermination of plant species at or soon after the human colonisation of oceanic islands. These include deforestation, natural or deliberate fire, agriculture, habitat changes, over-grazing, soil degradation and erosion, regeneration failure, exploitation as a food source, disease, pests, competition from introduced plants, and damage by introduced animals. Most has been written about Rapa Nui (e.g. Dumont et al. Citation1998; Flenley and Bahn Citation2003; Cole A and Flenley Citation2008; Rull et al. Citation2010, Citation2018; Hunt TL and Lipo Citation2011; Rull Citation2016, Citation2019a, Citation2019b; West K et al. Citation2017) and clearly a combination of, for example, deforestation, using trunks for canoes, and the prevention of regeneration by herbivory may have played a part in the total extinction of its palm trees. There is good evidence that palm endocarps were gnawed by introduced rats (Dransfield et al. Citation1984; Diamond Citation2007; Hunt TL Citation2007) and it is possible that humans felled the palms to obtain the edible palm hearts as other food supplies ran out on the over-populated island (J. Dransfield pers. comm. 2016). Throughout the tropics, palms are felled for this purpose and in some areas (e.g. Madagascar) this represents one of the most severe threats for the survival of many palm species (Dransfield Citation1991).
12.4. Assessing native or non-native status
Oceanic islands have long been recognised as being highly vulnerable to invasions by non-native plants and animals (e.g. Vitousek Citation1988; Loope and Mueller-Dombois Citation1989; Lawesson Citation1990; Cronk and Fuller Citation1995; Williamson Citation1996). Isolated island groups such as the Galápagos Islands or the Hawaiian Islands have attracted much attention because of their unique flora and fauna containing many endemic taxa. Human colonisation initiated a cascade of ecological impacts on islands, introducing many non-native (alien) species. For example, the Galápagos Islands support about 500 native vascular-plant taxa and 700 presumed non-native taxa (Lawesson Citation1990), whereas on the Hawaiian Islands there are about 2690 taxa, of which 950 are thought to be non-native (Loope and Mueller-Dombois Citation1989; Bramwell and Caujapé-Castells Citation2011). The Juan Fernández (Robinson Crusoe) Islands vascular-plant flora consists of 441 taxa, of which 209 are native (125 are endemic) and the other 232 almost certainly non-native (Greimler et al. Citation2002). Despite much interest in island endemics, their ecology is not widely studied. Irl et al. (Citation2017) analyse the patterns of endemic rarity on La Palma in the Canary Islands and show that hotspots of endemic rarity are at high elevations and in unusual and rare climate combinations.
Species richness on oceanic islands has remained relatively unchanged for birds, with the large number of extinctions or exterminations (Hume Citation2017) being roughly equal to the number of introductions. For vascular plants, the number of species has increased dramatically, with the number of non-native taxa greatly exceeding the number of extinctions or exterminations of native taxa (Sax et al. Citation2002). Non-native plant additions to islands are highly non-random with an almost perfect 1:1 match between the number of established introduced aliens and native plant species on oceanic islands (Sax and Gaines Citation2008). Competition from introduced species has caused few native plant losses, in contrast to the extinction or extermination of many native animal species on islands by predation by introduced animals (Sax and Gaines Citation2008).
Cronk and Fuller (Citation1995) suggest six reasons why oceanic islands may be very vulnerable to invasions; (1) poverty of native species, (2) evolution in isolation, (3) early European colonisation, (4) small area and fine spatial scales, (5) cross-roads of international trade, and (6) ecological release (alien species may arrive without their natural pests).
Given the high proportion of presumed non-native aliens on oceanic islands, it is essential from a conservation management viewpoint to establish whether a particular taxon is native or not (Nogué et al. Citation2017). Establishing the native status of a taxon is often difficult (Willis and Birks Citation2006; see Webb DA Citation1985; Preston et al. Citation2004; Pearman Citation2007 for attempts with the well-studied British and Irish flora). In the absence of definitive documentary data, determining the status of a taxon on islands (and elsewhere) is often conjectural and derives from several factors – date and site of first recorded occurrence, particularly post-European colonisation; non-native designation in neighbouring areas; ability to modify habitats; the occurrence in a new area being a marked change in the current geographical range; and close association with humans (Nogué et al. Citation2017).
Pollen analysis and related palaeoecological techniques (plant macrofossils, phytoliths, ancient DNA) can help assess the provenance (native, historical or recent introduction) of a particular taxon on islands (e.g. Jackson Citation1997; Willis and Birks Citation2006; Nogué et al. Citation2017; Ficetola et al. Citation2018; see subsection 11.3.6). On the Galápagos Islands, palaeoecological studies show that nine species presumed to be either introduced after European discovery in 1535 CE and colonisation in 1597 CE or of doubtful native status (‘cryptogenic species’ Carlton Citation1996) are, in fact, native to the Islands (van Leeuwen et al. Citation2008; Coffey et al. Citation2011). One of the nine species, Hibiscus diversifolius, was considered to be a ‘habitat transformer’ and appeared to be expanding its range; it was thus a candidate for control or eradication (van Leeuwen et al. Citation2008; Nogué et al. Citation2017). Assessing its status by palaeobotanical studies changes its status and it is no longer a plant for control or eradication. Determining the non-native status of a taxon can also be established through palaeoecology. Wilmshurst et al. (Citation2015) show that the tree daisy Olearia lyallii, native and endemic to South Island, New Zealand, became established on the remote uninhabited sub-Antarctic Auckland Islands (e.g. Ewing Island) between ca. 1807 and 1810 CE, possibly due to habitat disturbance and fire associated with European sealers (). Olearia also occurs on the Snares Islands, coastal patches of Stewart Island, and the Titi and Solander Islands. When Hooker (Citation1844) first found it on Ewing Island off the main Auckland Islands in 1840 CE he presumed it was a remnant of a once more widely distributed forest or scrub. Pollen analysis () clearly shows Olearia became established on the island at about 1810 CE (Wilmshurst et al. Citation2015; Nogué et al. Citation2017).
Figure 23. Summary percentage pollen diagram from Ewing Island (sub-Antarctic Auckland Islands) with selected taxa plotted against depth with a calibrated age scale in years CE. Grey zone shows the time of earliest sealing activity in the region. Redrawn from Wilmshurst et al. (Citation2015).
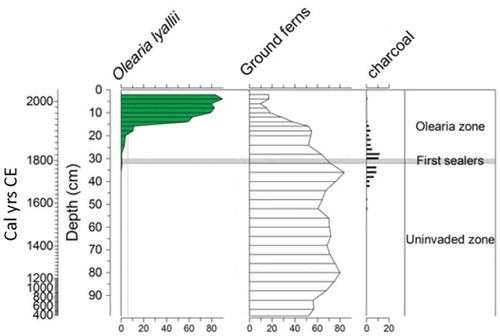
There are many species on islands that have uncertain ‘cryptic’ origins (‘cryptogenic species’ Carlton Citation1996). Pollen analysis can aid in determining the native or non-native status of such taxa, for example Selaginella kraussiania on the Azores whose distinctive spores occur in lake sediments on Flores Island for the last 3000 years, well before the Portuguese discovery and the Flemish settlement in the fifteenth century (van Leeuwen et al. Citation2005; Willis and Birks Citation2006).
Given the strong division of opinion about the harm that non-native plants can cause to native flora and vegetation (e.g. Liao et al. Citation2008; Simberloff Citation2014; Thomas CD and Palmer Citation2015; Hume Citation2017), a particularly interesting project on the recent impact of plants introduced to the Galápagos Islands would be an ecological resampling project in which floristic and vegetational data from different times are compared with the present vegetation. The Galápagos are perhaps unique in having floristic and vegetation descriptions at several times (e.g. Hooker Citation1847a, Citation1847b; Andersson NJ Citation1854, Citation1855; Robinson BL Citation1902; Stewart A Citation1911, Citation1915; Hamann Citation1981) dating from 1835 when Darwin visited the archipelago. Recent changes in vegetation over the last 180 years might be detected in such a resurvey. Rivas-Torres et al. (Citation2018) present a methodology to map native and invasive vegetation cover on the Galápagos using LandSat imagery, drones, dedicated satellites, and ground-truthing.
12.5. Establishing baseline conditions
As discussed in subsection 11.3.2, palaeoecological studies can provide unique information about baseline or reference conditions (e.g. Davis MB Citation1989c; Froyd and Willis Citation2008; Willis et al. Citation2010a; Gillson et al. Citation2011; Birks HJB Citation2012a). Similarly, palaeoecological studies on many oceanic islands can indicate baseline conditions prior to human colonisation and impact and baseline conditions prior to European colonisation and impact (e.g. Burney and Burney Citation1994; Prebble and Wilmshurst Citation2009; Horrocks et al. Citation2012, Citation2013, Citation2015; Wilmshurst et al. Citation2014, Citation2015; Nogué et al. Citation2017). Information from these different baselines can indicate the degree of change that has occurred, the level of intervention required to restore an ecosystem towards particular desired states (e.g. Burney Citation1997; Jackson and Hobbs Citation2009; Bush et al. Citation2014; Lyver et al. Citation2015; Nogué et al. Citation2017), and possible future trajectories of taxonomic similarity and biotic homogenisation (Rosenblad and Sax Citation2017). As human colonisation and impact on oceanic islands are relatively recent () compared to mainland areas, pre-human baselines can often be unambiguously identified and dated and provide data of direct relevance to conservation or restoration plans (Bush et al. Citation2014). The work by Burney and Burney (Citation2007) and Burney (Citation2010) illustrates the value of fossil pollen and other palaeoecological data from caves and wetlands on Kaua’i (Hawaiian Islands) (Burney et al. Citation2001; Burney and Kikuchi Citation2006) to infer the character and scale of anthropogenic ecological impacts and to derive a list of appropriate native plants for forest restoration attempts. Such data are also being used to develop management and interpretative programmes for centuries-old working cultural landscapes owned by the National Tropical Botanical Garden and by private individuals (Burney et al. Citation2002; Burney and Burney Citation2007; Nogué et al. Citation2017). Palaeoecological data from soils (e.g. δ13C, base saturation, resin-extractable P, remaining P) near the Kohala volcano on Hawai’i (Vitousek et al. Citation2004; Chadwick et al. Citation2007) show the contrasting agricultural and land-use activities and nutrient balances during the time of the Polynesian colonisers from about 800 CE until significant European contact in the late eigteenth century.
12.6. Vertebrate extinctions and introductions and their ecological impact
Of the 724 known vertebrate extinctions or exterminations in the last 500 years, over half are of island species (Turvey Citation2009b) and of the birds at least 90% are island dwellers (Tyrberg Citation2009). However, as Sax et al. (Citation2002) show, species number on an island has remained approximately constant since human colonisation with the number of losses being matched by the number of introductions.
Non-native herbivores (e.g. rabbits, feral goats) have greatly damaged the native flora and vegetation on many oceanic islands (e.g. Ficetola et al. Citation2018), whereas on other islands such herbivores may have enhanced biodiversity and/or cultural values (Nogué et al. Citation2017). Eradication or exclusion of introduced herbivores can have unexpected negative effects such as allowing an increase in rat populations and their impacts on nesting birds (Blackburn et al. Citation2004; Karels et al. Citation2008), seeds, and invertebrates or facilitating the spread and increase of invasive plants (Nogué et al. Citation2017).
Native herbivores also occur on oceanic islands and their decline, extermination, or extinction after human arrival has, on some islands, had strong and even drastic ecological impacts (Nogué et al. Citation2017). Palaeoecological techniques (pollen, Sporormiella dung-fungus spores, ancient DNA) can provide insights relevant to the management of native and non-native herbivores on islands by (1) determining whether large herbivores (e.g. tortoises, birds) have been eliminated in the past from some islands but introduced to others (Burney et al. Citation2003; Wood et al. Citation2011) and whether population changes have been natural or anthropogenically driven; (2) assessing the rate and patterns of herbivore introduction through time; and (3) establishing the long-term impact of changes in herbivore populations on island ecosystems (e.g. Wood et al. Citation2016; Nogué et al. Citation2017; Ficetola et al. Citation2018).
Sporormiella-type fungal spores (see subsection 13.2) have been invaluable in studying the decline of giant tortoise populations on the Galápagos Islands and revealing the previously unsuspected impact of this decline on wetland habitats (Froyd et al. Citation2014). Sphagnum bogs in the uplands of Santa Cruz have developed only over the last 500 years, and replaced small open-water areas. Sporormiella-type spores show that giant tortoises were once defaecating by these ponds and possibly helped maintain open-water conditions by wallowing. As the tortoise populations declined from 250,000 in the sixteenth century to 8000–14,000 in the 1970s (Froyd et al. Citation2014), a series of cascading ecological impacts occurred leading to the change from wetlands to Sphagnum bogs and the decline of several now rare or extinct species (e.g. Elatine sp., Utricularia foliosa, Ranunculus flagelliformis) (Coffey et al. Citation2012; Froyd et al. Citation2014). The key role that giant tortoises appear to have played as ‘ecological engineers’ has led to the management idea of using extant large tortoises in island ecosystems to replace extinct tortoises that may once have played such an engineering role (e.g. Hansen et al. Citation2010).
Following the mid-fifteenth century extinction of flightless moa birds in New Zealand, there was a 400 year period when large herbivores were absent. This period ended with the introduction of mammalian herbivores by European settlers in the ninteenth century. Wood and Wilmshurst (Citation2017) show, using pollen analysis, that ground-fern spores and Coprosma pollen increased during this 400-year ‘herbivore gap’. No other consistent pollen changes are detected. Wood and Wilmshurst (Citation2017) suggest that due to the relatively recent extinction of moas and the long life-span of many New Zealand trees, there may be an extinction debt and the long-term impacts of moa extinction may yet become apparent in the future. Extinction debt may also be important on other island systems, for example the Canary Islands (Otto et al. Citation2017).
The introduction of commensal mammals such as pigs, goats, sheep, cats, rats, and rabbits (the latter two possibly accidental introductions; e.g. Wilmshurst et al. Citation2008) to many islands often had drastic negative impacts on their flora and vegetation (e.g. Hunt TL Citation2007; Athens Citation2009; de Nascimento et al. Citation2009; Drake DR and Hunt Citation2009; Harris Citation2009; Nogué et al. Citation2017; Ficetola et al. Citation2018). These impacts include increased soil erosion, the extinction of some native fauna in Macaronesia (Illera et al. Citation2012), and the extinction of many small birds on Pacific Ocean islands (e.g. Taylor Citation1979; Steadman Citation2006; Hume Citation2017).
Mauritius is famous for its extinct flightless dodo (e.g. Cheke and Hume Citation2008). Detailed palaeoecological studies show that the dodo and two species of giant tortoise lived in dense populations in the coastal lowlands (Rijsdijk et al. Citation2015). A prolonged drought 4200 years ago caused a mass death in the Mare aux Songes, the major source of freshwater in the south-west of the island (Rijsdijk et al. Citation2009, Citation2011; de Boer et al. Citation2015). Over 100,000 giant tortoises and dodos died within 100 years when decreased precipitation resulted in algal blooms and increased salinity of the water. The reasons for the dodo’s final extinction between about 1662 and 1700 CE are probably human predation along with destruction of its forest habitat using fire (Gosling et al. Citation2017) and the introduction of pigs, macaques, and possibly rats predating nests (Cheke and Hume Citation2008).
The idea that plants with large-seeded fruits in the Americas and divaricating plants in New Zealand are anachronistic (sensu Barlow Citation2002; see subsection 13.3) due to the missing large vertebrates that had recently gone extinct (Martin PS Citation1969; Greenwood RM and Atkinson Citation1977; Janzen and Martin Citation1982; Janzen Citation1984; Pires et al. Citation2018) has been extended to the Mauritius endemic Tambalocoque or Dodo tree (Sideroxylon grandiflorum). This tree was thought to be dying out on Mauritius with only 13 individuals remaining in 1973, all estimated to be about 300 years old (Temple Citation1977). It was hypothesised that the tree depended on the dodo for its propagation and that its seeds would only germinate after passing through a dodo’s digestive tract (Temple Citation1977). With no dodos, the future of the tree was non-existent. This obligate-mutualism hypothesis is refuted by Witmer and Cheke (Citation1991) who show that the tree, although rare, has germinated since the demise of the dodo and that there are really several hundred extant individuals, not 13 as suggested by Temple (Citation1977).
It is not known if the reproduction of other plants on oceanic islands has been affected by the extinction of island vertebrates. The extinct Balearic mountain goat was the only large mammal on the Balearic Islands where it went extinct soon after human colonisation 4000–5000 years ago (Welker et al. Citation2014). Preserved coprolites of this goat contain abundant pollen and macrofossils of Buxus balearica. It is unclear if the demise of the goat was a result of the decline in B. balearica because of increasing aridity in the late Holocene or whether the goat so over-exploited B. balearica – a major component of its diet – that the goat could no longer survive.
The question of what caused the extinction of large vertebrates on large oceanic islands such as Madagascar is largely unresolved (e.g. Lyons et al. Citation2016a; Crowley et al. Citation2017; Kouvari and van der Geer Citation2018). Current hypotheses include over-hunting by humans; environmental change, particularly drought; fire; forest decline; competition with introduced animals; land-use changes; and interactions between these causes (Burney Citation1987a, Citation1987b, Citation1993, Citation1999; Crowley Citation2010; Virah-Sawmy et al. Citation2010; Wang L et al. Citation2019). One likely cause is pronounced climate desiccation between 1000 and 1200 CE, which may have triggered vegetation change and a decline in the megafauna. Hunting by humans, coping with a severe drought and competition with newly introduced cattle would have amplified the impacts on megafaunal populations leading to many extinctions in a relatively short period of time (Virah-Sawmy et al. Citation2010). Crowley et al. (Citation2017) and Hixon et al. (Citation2018), using δ15N isotopes from dated vertebrate bones, find no evidence for widespread aridification at this time. Crowley et al. (Citation2017) propose that a change in human land-use to a ‘more dedicated agro-pastoralist lifestyle’ at a time when Madagascar’s megafaunal populations were already in decline may have accelerated their inevitable loss.
Changes in other disturbance regimes, particularly fire, on islands have also been reconstructed from pollen-analytical and charcoal studies of lakes and mires (e.g. Higuera-Gundy et al. Citation1999; de Nascimento et al. Citation2009; McWethy D et al. Citation2010, Citation2014, Citation2017; Perry et al. Citation2012; Leys et al. Citation2014; see Nogué et al. Citation2017 for a detailed review). It is clear from these and other studies that fire regimes have changed through time, that both natural and human-induced fires have occurred, and that fire regimes have changed following human colonisation (e.g. McWethy DB et al. Citation2017).
12.7. Islands in the sky – tepui palaeoecology
Island biogeographical ideas have also been applied to a wide range of fragmented habitats (‘habitat islands’) on continents, such as forest patches, prairie remnants, lakes and ponds, caves, and mountain tops (‘islands in the sky’) The most spectacular fragmented habitat type is the high summit plateaux in Colombia, south-eastern Venezuela, Guyana, Suriname, French Guinea, and northernmost Brazil (tepuis) on top of vertical sandstone cliffs up to 1000 m high that separate the tepui summits from the surrounding lowlands (McPherson Citation2008; Huber et al. Citation2018). The elevation of the tepui summits range from 1500 m to 3014 m. Tepui summits above 1500 m form a distinctive but discontinuous biogeographical province called Pantepui (Huber Citation1995; McPherson Citation2008; Huber et al. Citation2018). It was originally thought to contain about 2000 species, of which 90–95% were endemic to the province (Maguire B Citation1970). The amount of apparent endemicity in any remote area often decreases as detailed botanical exploration increases. A later estimate for the Pantepui is 2322 species with 33% endemic taxa (Berry et al. Citation1995) but the latest estimate (Huber et al. Citation2018) is 2100 species with about 60% endemic taxa.
Tepuis () caught popular attention through William Hudson’s (Citation1904) Green Mansions and Arthur Conan Doyle’s (Citation1912) The Lost World books. The latter led to the ‘Lost World’ hypothesis which proposes that the Pantepui flora is relictual after millions of years of isolated evolution (Maguire B Citation1970; McPherson Citation2008). Can palynological studies help understand the origin of the endemic flora on the tepuis? Nogué et al. (Citation2009) present palynological results from a peat sequence at 2627 m elevation near the summit of the Eruoda tepui, north-east of the Chimautá massif (). The sequence covers the last 13,000 years. Like pollen records from oceanic islands prior to human colonisation, the Eruoda pollen sequence shows no palynological change with the dominant pollen taxon being Xyris, a genus that usually occurs on wet, often peaty soils (HJB Birks pers. obs.).
Figure 24. Mount Roraima, a spectacular tepui in Venezuela. Photograph MM from Switzerland (Wikimedia Commons).

Figure 25. Near the summit area of the Eruoda tepui, north-east of the Chimautá massif, Guyana Highlands where Nogué et al. (Citation2009) prepared a pollen sequence covering the last 13,000 years. Photo: Sandra Nogué-Bosch.

Using all the then available palaeoecological data from the tepuis, Rull (Citation2004) tests two hypotheses about the origin of the tepui flora – (1) the ‘Lost World’ hypothesis of a long history of evolution in isolation from the surrounding lowlands and (2) the vertical displacement hypothesis of repeated vertical movements during glacial-interglacial cycles, resulting in floristic mixing within the lowlands during glacial stages and genetic interchange between plateau summits (Steyermark and Dunsterville Citation1980; Huber Citation1988). Rull (Citation2004) concludes that there is support for both hypotheses with about half of the tepuis never being connected to the lowlands. In a more detailed biogeographical analysis involving GIS-based palaeotopographical reconstructions using a high-precision digital elevation model, Rull and Nogué (Citation2007) show that 70% of the flora with lower elevational levels today of ≤ 1500 m may have been able to move between tepuis and hence there could have been biotic interchange. Interestingly, the maximum numbers of single-tepui endemic taxa are confined to the very highest elevations where the possibility of biotic interchange via migration is minimal. Some endemics appear to have been able to migrate between tepuis, suggesting that topographical isolation alone is not enough to explain patterns of endemism. Factors such as tepui summit area and habitat heterogeneity or pre-Quaternary evolutionary patterns may also be important (Rull and Nogué Citation2007).
The results of these palaeoecological and biogeographical studies in the Guayanan Highlands raise important and challenging questions in conservation palaeoecology (Vegas-Vilarrúbia et al. Citation2011) and ecological dynamics (Rull et al. Citation2013) that warrant further detailed studies. It is striking that the available pollen records from tepuis all show little or no compositional change through the Holocene, as do oceanic islands prior to human colonisation and activities. Historically, oceanic islands and tepuis are similar except for the colonisation by humans on oceanic islands.
12.8. Conclusions
Palynological and other palaeoecological studies on islands provide no data to support the equilibrium theory of island biogeography over the time-scale of the Holocene. They show that pollen assemblages on oceanic islands and presumably the vegetation on these islands were stable in their composition and relative abundances over several thousand years prior to human colonisation when major ecological changes occurred. Palaeoecological evidence accords with Cronk’s (Citation1997) suggestion of high ultimate stability in geological time, particularly Quaternary time, and low proximate stability in ecological time (). Human activities following colonisation led, directly or indirectly, to several partial or regional extinctions. The introduction of non-native plants and animals often had deleterious impacts on the native island biota (high ultimate diversity and uniqueness changing to low proximate richness and uniqueness – Cronk Citation1997).
Kueffer et al. (Citation2014) in their ‘look towards the future’ in island biology conclude that 'palaeoecology is greatly expanding our understanding of island ecology’. Island palaeoecology has made considerable advances since the pioneering efforts of Selling (Citation1948, Citation1951) on the Hawaiian Islands and of Colinvaux and Schofield (Citation1976) on the Galápagos Islands. Much remains, however, to be discovered and understood about island biology in the past as a key to island biology in the future.
13. Non-pollen palynomorphs, Sporormiella-type spores, and megafaunal extinctions
13.1. Introduction
Recent advances in palynological analysis (e.g. van Geel Citation1986, Citation2001; van Geel and Aptroot Citation2006) have involved the careful documentation and, in some cases, taxonomic identification of so-called non-pollen palynomorphs (NPPs) that are commonly encountered during routine pollen analysis but which, until recently, have been largely ignored by palynologists (Birks HJB and Berglund Citation2018). If a fossil NPP can be identified and the ecology of the taxon is known, then that NPP may be a useful ecological indicator. An example is spores of the ascomycete Kretzschmaria deusta (formerly Ustulina deusta) that is parasitic on deciduous trees such as Fagus sylvatica (van Geel et al. Citation2013), Fraxinus excelsior (van Geel and Andersen Citation1988), and Tilia. Its fossil spore maxima have been interpreted as reflecting rainstorms and associated run-off (van Geel et al. Citation2013).
Non-pollen palynomorphs are being increasingly studied in different parts of the world (e.g. Cugny et al. Citation2010; Montoya E et al. Citation2010; Gelorini et al. Citation2011; van Geel et al. Citation2011; López-Vila et al. Citation2014; Loughlin et al. Citation2018b). Their interpretation often remains unclear because of a lack of ecological knowledge of the taxa concerned. One major exception is spores of dung fungi such as Sporormiella-type.
13.2. Sporormiella-type spores
Spores of dung fungi are currently attracting much attention as a means of inferring the past presence and abundance of large herbivores (megaherbivores) such as bison, mammoth (e.g. Davis OK Citation1987; Burney et al. Citation2003; Comandini and Rinaldi Citation2004; Robinson GS et al. Citation2005; Graham RW et al. Citation2016; Raczka et al. Citation2016), giant tortoise (see subsection 12.6), and other large (>45 kg) animals. Fossil spores of many coprophilous dung fungi have been identified (see Baker AG et al. Citation2013 for a compilation) but most studies concentrate on spores of Sporormiella-type, and, to a lesser extent, Sordaria-type and Podospora-type. It should be noted that Feranec et al. (Citation2011) warn that some critical taxonomic and ecological investigations are needed for the different Sporormiella-type spores. Fiedel (Citation2018) recently questions the robustness of dung fungal spores as an indicator of megafauna and Perrotti and van Asperen (Citation2019) provide a critical discussion about interpreting the presence and absence of such spores.
Since the identification of Sporormiella-type spores and the suggestion that the frequencies of such spores may, in part, reflect past megaherbivore abundances (Davis OK Citation1987), there have been several studies on the occurrence, abundance, and taphonomy of these spores in relation to faunal biomass today in an attempt to provide a factual basis for the interpretation of changing abundance of fossil spores and hence the reconstruction of past vegetation–faunal relationships and the assessment of the ecological impacts of Late Quaternary megafaunal (>100 kg; Gill Citation2014) extinctions (e.g. Davis OK and Shafer Citation2006; Cugny et al. Citation2010; Parker and Williams Citation2011; Gill et al. Citation2013; Baker AG et al. Citation2017). Such studies on the reliability of Sporormiella-type spores show that these spores have highest values today in areas with megaherbivores such as bison and that these spores are not dispersed far from their source. They thus appear to be reliable indicators of local megaherbivore presence (Raper and Bush Citation2009; Wood et al. Citation2011; Etienne et al. Citation2013; Raczka et al. Citation2016). Moreover, Baker AG et al. (Citation2017) (see also Wood and Wilmshurst Citation2013; Johnson CN et al. Citation2015) show that there is a significant monotonic relationship between spore accumulation rates (spores cm–2 yr–1) and local mammal biomass density (kg ha–1), indicating the potential of dung fungal spores in studying, for example, past plant–megaherbivore interactions (e.g. Gill et al. Citation2012; Bakker et al. Citation2016), herbivore extinction events (e.g. Rozas-Davila et al. Citation2016), and past pastoral activities (e.g. Schofield and Edwards Citation2011; Graham RW et al. Citation2016; Pini et al. Citation2017; Rey Citation2017; Rey et al. Citation2017).
A detailed study of late-glacial sediments at Appleman Lake, Indiana (Gill et al. Citation2009) involving pollen assemblages, charcoal, and Sporormiella-type spores () shows that megafaunal extinction (inferred from the decrease in Sporormiella-type spores) closely preceded an increased fire regime and the development of a pollen assemblage with abundant Fraxinus nigra-type, Ostrya/Carpinus, and Picea – an assemblage with no modern pollen analogue (see subsection 7.2). Gill et al. (Citation2009; see also Gill et al. Citation2012) propose that the loss of keystone megaherbivores may have altered ecosystem structure and function by the release of palatable hardwoods such as Fraxinus nigra-type and Ostrya/Carpinus from herbivore pressure and by increased fuel accumulation. Using the Sporormiella-type record as a proxy for megaherbivore populations, these faunal populations start to decline from 14,800 to 13,700 years ago (), well before their final extinctions at about 11,500 years ago. At about 11,900 years ago, Pinus and Quercus pollen values increase, reflecting the early-Holocene development of deciduous forests in northern Indiana. This study suggests that the loss of a broad guild of megaherbivore consumers led to a major restructuring of the vegetation and an enhanced fire regime (). Building on this study and more detailed work at Silver Lake, Ohio and Crystal Lake, Illinois, (Gill et al. Citation2012), Gill (Citation2014) develops a useful conceptual model for top-down controls on Late Quaternary vegetation dynamics when megaherbivores are present (). In this model, biotic interactions such as herbivory mediate climate-forced vegetation changes. She also provides a valuable and wide-ranging review of the ecological impacts of the Late Quaternary megaherbivore extinctions (Gill Citation2014; see also Malhi et al. Citation2016). These include changes in vegetation composition due to the release of plants from herbivory following the Late Quaternary extinctions (Gill et al. Citation2009, Citation2012); enhanced early-Holocene fire regimes relative to the Late Pleistocene due to build-up of landscape fuels (Gill et al. Citation2009); Pleistocene ecosystems may have been more open and/or patchy than present (Asner and Levick Citation2012; Bocherens Citation2018); the magnitude of megaherbivore impacts may have varied in relation to landscape and geographical position (lowlands, uplands) (Svenning Citation2002; Johnson CN et al. Citation2016); Late Quaternary extinctions may have affected the rate and spatial dynamics of biogeochemical cycling (Doughty et al. Citation2013; Bocherens Citation2018); many Holocene plants with large fleshy fruits may have become dispersal-limited relative to the Pleistocene due to the extinction of their natural seed dispersers, so-called ‘ecological anachronisms’ (Janzen and Martin Citation1982; Janzen Citation1984; Bocherens Citation2018; Galetti et al. Citation2018; Pires et al. Citation2018; see subsection 12.6); and Late Quaternary extinctions may have had cascading effects on small mammal assemblages (Blois et al. Citation2010). Other possible effects of the Late Quaternary extinctions include biome shifts in response to increased fire activity in Australia (Rule et al. Citation2012) and a possible abrupt decrease in atmospheric methane concentrations at the time of the extinction of the New World megafauna (Smith FA et al. Citation2010; cf. Brook and Severinghaus Citation2011).
Figure 26. Summary pollen and Sporormiella-type (Sporor.) spore stratigraphies (expressed as percentages of upland pollen sum) and charcoal concentrations at Appleman Lake, Indiana between 16,700 and 9000 years ago. The shaded part indicates when the pollen assemblage has no modern pollen analogue. The Sporormiella-type spore record is used as a proxy for megafaunal abundance. Modified from Gill et al. (Citation2009).
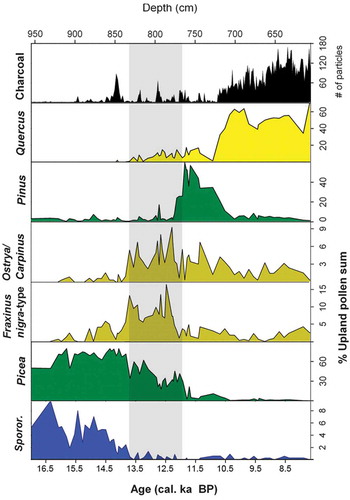
Figure 27. Conceptual model of top-down drivers of Late Quaternary vegetation dynamics mediated by biotic interaction. Arrow thickness reflects the hypothesised relative importance of each link. Dashed arrows represent poorly understood or studied relationships. Modified from Gill (Citation2014).
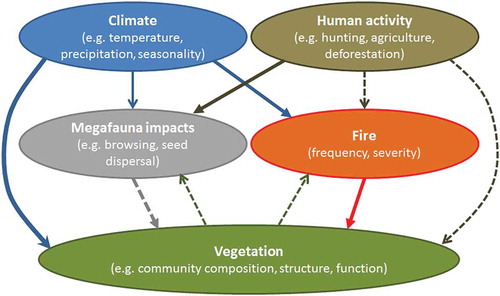
Interestingly, a recent detailed palynological study at Cupola Pond in the Ozark Mountains of Missouri (Jones RA et al. Citation2017) shows markedly no-analogue pollen assemblages from about 17,000 to 11,000 years ago with high values of Ambrosia-type, Carya, Fraxinus, Ostrya/Carpinus, Pinus, Picea, Quercus, and Ulmus pollen (see subsection 7.2). In contrast to the sites in Ohio and Illinois, Sporormiella-type spores are absent and charcoal remains are rare throughout, suggesting that megafauna and fire were not local drivers of disturbance, turnover, or no-analogue assemblages. The most likely explanation for such assemblages are no-analogue climatic conditions in terms of insolation values and continentality in the late-glacial and early Holocene (see Williams et al. Citation2001; Jackson and Williams Citation2004; Williams and Jackson Citation2007; Liu et al. Citation2013). No-analogue assemblages are discussed further in subsections 7.2 and 11.3.7.
Jeffers et al. (Citation2018) model drivers of ecosystem structure and function in the early Holocene and late-glacial at five sites in Britain and Ireland using pollen and dung fungal accumulation rates, sedimentary nitrogen (δ15N), and chironomid-based summer temperatures. In contrast to Gill’s (Citation2014) conceptual model () they find little evidence for megaherbivore control on vegetation composition and nitrogen availability.
13.3. Megafaunal extinctions
The causes of Late Pleistocene and early-Holocene (50,000/10,000 yrs BP) megafaunal (>45 kg) extinctions continue to attract considerable attention and discussion (). Clearly Quaternary botany by itself cannot resolve the cause(s) of these extinctions. What it can do is to describe if and when vegetation change occurred before, at, or after a megafaunal extinction event, as done by Gill et al. (Citation2009, Citation2012) in eastern North America, Wang Y et al. (Citation2017) on St Paul Island, Alaska, and Jeffers et al. (Citation2018) in Britain and Ireland. Three recent studies have attempted to disentangle the roles of humans, climate, vegetation change, and volcanic activity on megafaunal extinctions in Chile (Villavicencio et al. Citation2016), Peru (Rozas-Davila et al. Citation2016), and St Paul Island, Alaska (Graham RW et al. Citation2016). Results from the Chilean study in the Última Esperanza region of southern Patagonia suggest that a combination of human impact and climate-forced vegetation changes drove megafaunal extinctions in this area, with the balance of factors being taxon specific (Lorenzen et al. Citation2011). Competition between humans and large carnivores seems to be the most plausible hypothesis for the extinction of carnivores. In contrast, co-existence of humans with now-extinct horses, camels, and mylodonts for several thousand years conflicts with the scenario of a ‘blitzkreig’ overkill of the megafauna by humans. Vegetation change from cool grassland to Nothofagus forest inferred from pollen data corresponds with the disappearance of the American horse (Hippidion saldiasi) and the camel Lama cf. lama owenii. The later full development of Nothofagus forests and an increasing fire frequency coincide with the disappearance of the giant ground sloth and other mylodonts. The overall scenario is that climate-driven environmental change and reductions of open vegetation and herbivore populations increased their susceptibility to local extinction (Villavicencio et al. Citation2016; see also Raczka et al. Citation2017).
Table 27. Recent contributions to the debate about the causes of Late Pleistocene and early-Holocene megafaunal extinctions.
In contrast, the study from Lake Pachuca situated at an elevation of 3100 m in Peru (Rozas-Davila et al. Citation2016) is based on pollen, charcoal, diatoms, and Sporormiella-type spores and identifies a two-stage decline in Sporormiella-type (used as a megaherbivore proxy); the first at 21,000 years ago and the second between 16,800 and 15,000 years ago. There appears to have been a functional extinction about 15,800 yr BP, about 3000 years earlier than any known human occupation in the high Andes. Declining megaherbivore populations coincide with warm, wet intervals. This climatic instability and associated megafaunal population collapse initiates a rapid ecological cascade that results in novel no-analogue pollen assemblages with increases in woody taxa (e.g. Podocarpus, Acalypha, Polylepis), fire frequency, and taxa sensitive to trampling (e.g. Ericaceae, Melastomataceae). The overall emerging picture from this detailed study is that Andean megafanual populations collapsed due to positive feedbacks between habitat quality and type and climate change rather than due to human activity (Rozas-Davila et al. Citation2016).
St Paul Island (110 km2) lies 450 km from Alaska and the Aleutians. Woolly mammoth persisted on the island until about 5600 years ago as shown by sedimentary ancient DNA, radiocarbon dates on mammoth remains, and accumulation rates of Sporormiella-type, Sordaria-type, and Podospora-type fungal spores (Graham RW et al. Citation2016; Wang Y et al. Citation2017). Pollen assemblages suggest that the island vegetation was stable during the extinction window and there is no evidence of human presence on the island prior to the arrival of Russian whalers in 1787 CE. The mammoth extinction window coincides with declining freshwater resources and a drier climate between 7850 and 5600 years ago as inferred from sedimentary magnetic susceptibility, stable oxygen isotopes, and diatom and cladoceran assemblages in a sediment core from Lake Hill, a small freshwater lake near the centre of the island, and from stable nitrogen isotopes from mammoth remains (Graham RW et al. Citation2016; Wang Y et al. Citation2017; Wooller et al. Citation2018). All the evidence indicates that the mammoth population died out because of the synergistic effects of shrinking island area and freshwater scarcity caused by rising sea-levels and regional climate change (Graham RW et al. Citation2016). It is possible that the freshwater quality was also degraded by intense mammoth trampling and defaecation around the lake. The likely impacts of the megaherbivore extinction were reduced rates of lake-catchment erosion from the elimination of mammoth herds around the diminishing water-bodies and a shift to a more herbaceous vegetation. This study is not only one of the best-dated prehistoric faunal extinctions but it also highlights freshwater limitation as an overlooked driver of faunal extinction and the vulnerability of small island populations to subtle environmental change, even in the absence of human influence (Graham RW et al. Citation2016). Other aspects of the St Paul Island study are discussed in subsection 2.2 and the island biota, their history, and the island’s past environment are discussed in subsection 12.2 and in Wooller et al. (Citation2018)
13.4. Conclusions
Van Geel’s (Citation1986, Citation2001) pioneering and patient studies on non-pollen palynomorphs including Sporormiella-type spores are now playing a key role in deciphering vegetation–megaherbivore interactions in the Late Pleistocene. One hopes that as other NPPs are identified and their ecology is known, they will turn out to be such a valuable palaeoecological indicator as Sporormiella-type and other dung-fungus spores have been. A recent example of the rigorous quantitative analysis of NPPs in relation to pollen and macrofossils in the Danish Holocene as a guide to interpreting the NPP strategies is Enevold et al. (Citation2019).
14. Biodiversity trends within the Holocene
14.1. Introduction
The glacial–interglacial cycle (Iversen Citation1958; Andersen ST Citation1964, Citation1966) is a well-established metaphor for pollen-stratigraphical changes between a glacial stage and an interglacial stage and within an interglacial stage in Europe (see subsection 3.1; Birks HJB Citation1986; Birks HJB and Birks Citation2004; Birks HJB and Tinner Citation2016a, Citation2016b; Birks HJB and Berglund Citation2018). Given the current upsurge of interest amongst ecologists and biogeographers in biodiversity, in particular biodiversity change in the Anthropocene (ca. last 50–200 years) (e.g. Ellis et al. Citation2012; Schimel et al. Citation2013; Benito-Garzón et al. Citation2014; Wolkovitch et al. Citation2014; Young KR Citation2014; Corlett Citation2015; Dearing et al. Citation2015; Svenning et al. Citation2016a), can we estimate biodiversity trends in the current Holocene interglacial (ca. 11,700–200 years ago) using the conceptual framework used by McGill et al. (Citation2015) for the Anthropocene? Are the Anthropocene trends of McGill et al. (Citation2015) simply a continuation of trends in the Holocene (Birks HJB et al. Citation2016a)? The term biodiversity can mean many different aspects of biological organisation. Here, it refers to taxon richness and diversity.
McGill et al. (Citation2015) propose 15 measures of biodiversity trends within the Anthropocene despite the absence of any agreed definition of the onset of the Anthropocene. They consider biodiversity changes at four spatial scales (global, biogeographical, meta-community, local) and four measures of biodiversity trends – α-diversity (richness), spatial β-diversity (compositional or differentiation change in space), temporal β-diversity (compositional change in time), and biomass (a robust estimate of abundance that is often correlated with various ecosystem functions).
Pollen assemblages are the commonest and most abundant terrestrial fossil group in the Holocene and they mainly reflect broad-scale patterns of past flora and vegetation (Birks HJB and Gordon Citation1985; Felde et al. Citation2014a, Citation2014b) and, by inference, floristic and landscape richness and diversity at the meta-community or regional scale (Meltsov et al. Citation2013; Matthias et al. Citation2015; Birks HJB et al. Citation2016c; Felde et al. Citation2016a, Citation2018). Palynological richness (α-diversity) can be estimated by rarefaction analysis solved analytically (Birks HJB and Line Citation1992) or by repeated computer re-sampling without replacement to a constant base-sum (Felde et al. Citation2016a; Birks HJB et al. Citation2016c). The interpretation of such richness estimates has, until recently, been contentious (Odgaard Citation1999, Citation2001; Goring et al. Citation2013; Birks HJB et al. Citation2016c). Recent detailed studies show that modern palynological richness and diversity (see Birks HJB et al. Citation2016c for the distinction between richness and diversity in a palynological context) are, in part, a reflection of contemporary floristic α-richness and diversity (Felde et al. Citation2016a), and, in part, a reflection of landscape mosaic structure (spatial β-diversity) (Meltsov et al. Citation2013; Matthias et al. Citation2015). Spatial β-diversity of pollen assemblages can be estimated by diversity partitioning for a particular time (Blarquez et al. Citation2014; Felde et al. Citation2016a; Birks HJB et al. Citation2016c). Compositional turnover (temporal β-diversity) in fossil assemblages in commonly quantified as a rate-of-change (Birks HJB Citation2012b) or turnover (Birks HJB Citation2007) metric. Biomass changes can be roughly estimated from fossil pollen-accumulation rates calibrated to catchment biomass by modern accumulation rates (e.g. Seppä et al. Citation2009a, Citation2009b).
14.2. Holocene trends in northern and central Europe
Birks HJB et al. (Citation2016a) use Holocene pollen-stratigraphical data (0–11,500 yr BP) from over 50 sites in northern and central Europe (Birks HJB et al. Citation2016c) to estimate, for the meta-community scale (sensu McGill et al. Citation2015), temporal trends in the four types of biodiversity recognised by McGill et al. (Citation2015) (). Birks HJB et al. (Citation2016a) present general trends only for the four phases of the Holocene stage (protocratic, mesocratic, oligocratic, Homo sapiens; see subsection 3.1). These four phases do not have defined upper or lower boundaries (Birks HJB Citation1986) as they began and ended at different times at different sites depending on, for example, location, elevation, climate, bedrock geology, soil, and land-use history. They also present general trends for the last 200 years (Anthropocene), following Steffen et al.’s (Citation2007) concept of the ‘Great Acceleration’ beginning about 1800 CE.
Figure 28. Schematic summaries of the general major trends in the four types of diversity proposed by McGill et al. (Citation2015) at the meta-community scale of organisation (sensu McGill et al. Citation2015) within the protocratic, mesocratic, Homo sapiens, and oligocratic phases of the Holocene and the last 200 years ('Anthropocene' and 'Great acceleration') based on terrestrial palynological data from northern and central Europe. The vertical axis for all four diversity types runs from low (L) to high (H). The horizontal axis for the four phases and the last 200 years reflects time from oldest (left) to youngest (right). The trends are schematic trajectories of diversity change with the arrows representing the end-point in the different phases. Redrawn from Birks HJB et al. (Citation2016a).
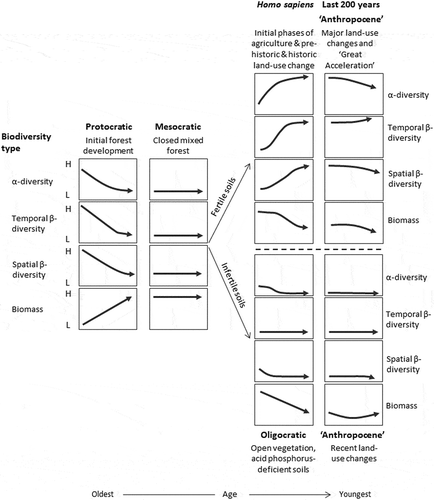
Richness (α-diversity) and β-diversity are initially high in the protocratic phase of the early Holocene () but they quickly decline as the diverse floras and complex fine-scale vegetational mosaic on recently deglaciated areas are replaced by a less fine-grained mosaic of grassland, heath, and open woodland (Birks HJB and Birks Citation2008). Biomass increases through this succession. During the forested mesocratic phase (), α- and β-diversity are relatively constant and low as the landscape mosaic was probably rather simple. Biomass is maximal. In northern and western oceanic areas with nutrient-poor soils (Birks HJB and Birks Citation2004), the oligocratic phase has low α- and β-diversity and decreasing biomass in a low-mosaic landscape of heath, bog, and open woodland (Birks HJB and Line Citation1992). In contrast, in the Homo sapiens phase () with human activity, forest clearance, and agriculture on fertile soils, α- and β-diversity increase and biomass decreases as complex mosaic landscapes develop (e.g. Peglar Citation1993b). In the very recent past (Anthropocene) there is a small decrease in α- and spatial β-diversity and biomass and a small increase in temporal β-diversity at sites on fertile soils. Sites on nutrient-poor soils show little change in α- or temporal β-diversity, and a very small decrease in spatial β-diversity and a recent increase in biomass, possibly in response to atmospheric nitrogen deposition (Birks HJB et al. Citation2016a).
Birks HJB et al. (Citation2016a) suggest that at sites on fertile soils, recent Anthropocene changes in biodiversity are not a simple continuation of trends initiated in the previous Homo sapiens phases (ca. last 200–5000 years; ). In contrast, Anthropocene trends in biodiversity at sites on infertile soils are primarily a continuation of trends that started in the oligocratic phase (ca. 200–4000 years ago), with the exception of a recent small decrease in β-diversity (biotic homogenisation) and a small increase in biomass. Some of these recent trends identified by Birks HJB et al. (Citation2016a) from terrestrial palynological data accord with the general trends suggested by McGill et al. (Citation2015) based on various types of organisms, inventories, surveys, except for trends in α-diversity with a decrease according to Birks HJB et al. (Citation2016a) and an increase hypothesised by McGill et al. (Citation2015). It is important to note that the analysis by Birks HJB et al. (Citation2016a) is based entirely on terrestrial regional-scale pollen-stratigraphical data from northern and central Europe whereas McGill et al. (Citation2015) present trends for all areas, habitats, and biota combined. The biodiversity patterns identified by Birks HJB et al. (Citation2016a) for northern and central Europe are likely to be different in different biogeographical regions such as the Alps, the Mediterranean basin, North America, and the tropics and in different ecological systems (e.g. aquatic, coastal). Interestingly the major changes in biodiversity trends based on regional terrestrial pollen assemblages in northern and central Europe are in the Homo sapiens or the oligocratic phases. In contrast, major biodiversity changes in aquatic systems in the same geographical areas are mainly in the last 200 years due to ‘acid-rain’ deposition, agricultural eutrophication, and other forms of pollution, although some biodiversity changes occur in lowland aquatic systems in the Homo sapiens phase (e.g. Peglar et al. Citation1989).
Felde et al. (Citation2018) investigate in detail pollen-biodiversity trends for the last 8000 years at 30 sites throughout Norway and two sites in Swedish Lapland. In contrast to all other areas in Europe (e.g. Birks HJB and Line Citation1992; Giesecke et al. Citation2012; Birks HJB et al. Citation2016a), diversity increases progressively through the Holocene, suggesting the continued addition of pollen taxa. Diversity changes at most sites in the same direction but at different rates and magnitudes. There are no clear spatial patterns (e.g. between northern and southern Norway) in these rates. The general patterns of increasing diversity over time are consistent with the hypothesis of Holocene dispersal limitation (Svenning and Skov Citation2007b; Willner et al. Citation2009). The rate of diversity-increase rises with the onset of the Homo sapiens phase in the last 2000–4000 years at 29 of the 32 sites (Felde et al. in preparation). It is not clear why the long-term diversity trends are different in Norway compared with elsewhere in Europe. A possible explanation is that as only the last 8000 years are considered by Felde et al. (Citation2018) to ensure comparability between all 32 sites, diversity changes in the early Holocene are missing.
The analyses by Birks HJB et al. (Citation2016a) and Felde et al. (Citation2018) illustrate the importance when analysing biodiversity trends in the recent past (Anthropocene) of considering such trends in the preceding Holocene, especially the last 4000–5000 years of the late Holocene. Detailed Late Quaternary studies can provide unique insights into biodiversity changes over a range of temporal and spatial scales and into shifting biodiversity patterns resulting from both environmental and anthropogenic drivers over the past 11,700 years (e.g. Hanley et al. Citation2008; Citation2009a; Connor et al. Citation2012; Giesecke et al. Citation2012, Citation2014b; Colombaroli et al. Citation2013; Ammann et al. Citation2013a; Reitalu et al. Citation2015; Hájek et al. Citation2016). Biodiversity change did not, as some modern ecologists and biogeographers seem to assume, begin in the Anthropocene, however it is defined (see, for example, Lewis and Maslin (Citation2015, Citation2018), Ruddiman (Citation2013b) and Ruddiman et al. (Citation2015) for contrasting views on defining the onset of the Anthropocene).
14.3. Conclusions
Although richness and diversity of pollen assemblages are imperfect and incomplete reflections of past vegetation or landscape richness and diversity, they document important biodiversity changes within the Holocene of northern and central Europe. There is clearly a need to explore Late Quaternary richness and diversity patterns in different biomes and geographical areas to assess pollen assemblage diversity changes more extensively through time.
15. Discussion and the future
Quaternary botany, mainly pollen analysis but also plant-macrofossil and charcoal analyses and palaeolimnology have made many important and unique contributions to ecology and biogeography. These include demonstrating the rapid dynamics of tree spreading and range expansion; the paucity of true extinctions compared to the large number of regional and local exterminations, the so-called ‘Quaternary conundrum’ (Botkin et al. Citation2007); the role of historical legacies in long-term ecological dynamics; the concepts of the potential niche and changing realised environmental space; the occurrence of novel assemblages in the past; human impacts on tropical systems; and answers to questions of direct relevance to conservation both on mainlands and islands.
In addition to the contributions of Quaternary botany to ecology and biogeography, it has provided many insights into floristic, vegetational, landscape, and environmental history. Pigott (Citation1984) comments that palynological investigations (in Britain) ‘have provided a detailed knowledge of the history of vegetation since the last glaciation and explanations of many present-day features of the distribution of plants, of vegetation and of soils. Such studies are an indispensable tool of the ecologist; from them we know, for example, which trees are truly native and we can reconstruct the composition of our native forests. In so doing we may appreciate that almost all our vegetation now is to a greater or lesser extent modified by human intervention.’
Over fifty years ago, the American ecologist and pollen analyst Paul Sears presented a very perspicuous view about the value of Quaternary palaeoecology: ‘Science can only help us if we use it to show how the present is a product of the past, and to remind us that we are now shaping our future. It is the privilege of the paleoecologist to reconstruct a [temporal ecological] continuum so convincing that the lesson will be heeded. … It may well be that [paleoecological] finding will, in the end, mean far more to the future of mankind than [we] now suspect’ (Sears Citation1964). The many contributions that Quaternary botany are making to conservation, global-change biology, limnology, biogeography, ecology, and Earth system science fully confirm Sear’s predictions of 1964 about the utility of palaeoecological research.
Birks HJB and Berglund (Citation2018) discuss the history and development of pollen analysis in the last 100 years in terms of a pioneering phase (1916–1950), a building phase (1951–1973), and a mature phase (1974–today). They ask ‘Where are we today?’ in response, in part, to Gill’s (Citation2013) provocative question ‘Is pollen analysis dead? Paleoecology in the era of Big Data’, or in other words has pollen analysis entered a degenerative phase? Birks HJB and Berglund (Citation2018) suggest that there are a very large and ever-increasing number of high-quality stratigraphical data-sets from many parts of the world; there are increasing numbers of syntheses and detailed narratives; but there are surprisingly few analytical studies that attempt to test specific hypotheses. Seddon et al. (Citation2014a) present 50 priority research questions in palaeoecology as a whole, many of which involve Quaternary botany, often in conjunction with other palaeoecological techniques. These questions fall into six themes: (1) human–environment interactions in the Anthropocene, (2) biodiversity, conservation, and novel ecosystems, (3) biodiversity over long time-scales, (4) ecosystem processes and biogeochemical cycling, (5) comparing, combining, and synthesising information from multiple records, and (6) new developments in palaeoecology. Many of these questions highlight the need for developing a common agenda for ecology and palaeoecology (Flessa and Jackson Citation2005a, Citation2005b). Attempting to answer the 50 questions of Seddon et al. (Citation2014a) and many other questions and developing a common agenda will challenge Quaternary botanists and palaeoecologists for years to come and will prevent pollen analysis and related techniques from entering a degenerative phase! Flenley’s (Citation2003) suggestions for new research directions in the twety-first century will similarly help to avoid any degenerative phase.
Following the example of Belovsky et al. (Citation2004) and its 10 suggestions to strengthen the science of ecology, I conclude with 10 suggestions that might help to increase the rigour of Quaternary botany and strengthen its links with ecology and biogeography. Some of these suggestions counteract some unfortunate recent trends in Quaternary botany (e.g. numbers 3–9), whereas others re-emphasise what has long been recognised as ‘best practice’ in the subject (e.g. numbers 1, 2, 10).
There is a great need for novel research questions, careful project design, and appropriate hypotheses to be tested (e.g. Edwards KJ Citation1983; Ritchie Citation1984, Citation1991, Citation1995; Walker Citation1990; Oldfield Citation1993; Birks HJB Citation1993a; MacDonald Citation1993b; Perry et al. Citation2016).
As pollen analysis and plant-macrofossil analysis are such time-consuming and labour-intensive activities, there is a strong need for careful site selection, pilot studies, and meticulous fieldwork and laboratory sampling (e.g. Birks HJB and Birks Citation1980; Berglund Citation1986; Last and Smol Citation2001).
There is a need for greater appreciation of past literature, particularly literature that is not in electronic form, for example many of the classic papers by Jessen, Rudolph, Firbas, Iversen, Fægri, Andersen, and others (see Birks HJB and Berglund Citation2018). Such an appreciation can help avoid some aspects of ‘ignorance creep’ (Blois Citation2012b; Jackson Citation2012c).
There is a need to integrate more fully empirical and theoretical aspects of pollen analysis, for example spatial and temporal scales (e.g. Oldfield Citation1970b; Bradshaw RHW Citation1994; Bennington et al. Citation2009), models of pollen representation (e.g. Jacobson GL and Bradshaw Citation1981; Jacobson GL Citation1988; Prentice Citation1988b; Sugita Citation1993, Citation1994, Citation2007a, Citation2007b; Jackson Citation1994; Jackson and Lyford Citation1999; Davis MB Citation2000; Edwards KJ et al. Citation2015), and ecological, biogeographical, and macroecological modelling (e.g. Bradshaw RHW and Sykes Citation2014; Rapacciuolo and Blois Citation2019).
There is a need for a closer integration of basic natural history, field botany, and ecology into Quaternary botany (Godwin Citation1961; Fægri Citation1966). Quaternary palaeoecology is part of ecology but carried out at spatial and temporal scales different from many ecological investigations (e.g. Walker Citation1982a; Bennett Citation1988b; Davis MB Citation1994; Rull Citation2009, Citation2014; Biondi Citation2014; Reitalu et al. Citation2014).
A recognition that palynological patterns may result not from a single cause but from multiple causes (Jackson and Blois Citation2015). The method of multiple working hypotheses (Chamberlin Citation1890) is as relevant today as it was over 100 years ago. Such patterns may reflect equilibrium conditions, non-equilibrium conditions, or historical legacies (Bradshaw RHW Citation1993b; Jackson and Blois Citation2015). Multi-proxy studies involving many proxies not only pollen and plant macrofossils but also proxies such as stable isotopes, chironomids, and sediment geochemistry (e.g. Flessa and Jackson Citation2005a) are essential to provide additional and independent lines of evidence in hypothesis testing and data interpretation (e.g. Birks HH and Birks Citation2006; Connor et al. Citation2018a).
There is a need for replication and greater consideration of the numerical properties of pollen-analytical and plant macrofsossil data (e.g. Fagerlind Citation1952; Birks HJB and Gordon Citation1985; Birks HJB Citation2014), such as their multivariate nature and stratigraphical ordering, and the inherent errors in estimating pollen percentages, concentrations or accumulation rates (Maher et al. Citation2012).
The data collected should be of the highest quality possible. Such quality depends on access to extensive and representative modern pollen, seed, and fruit reference collections (Seppä and Bennett Citation2003). Plant macrofossils should be used more often as they can greatly help refine identifications and can provide potential ecological information (e.g. Birks HH and Birks Citation2000; Birks HJB Citation2014).
Statistical or numerical methods should be regarded as tools to help answer palaeoecological questions. They are not an end in themselves, but a means to an end (Birks HJB et al. Citation2012). Stratigraphical palaeoecological data, most commonly expressed as percentages, require specific numerical methods that take account of the ‘closed’ nature of percentage data and the time ordering within and between stratigraphical sequences (Birks HJB Citation1998, Citation2012b).
Quaternary botany today is commonly part of multi-disciplinary studies (Bradshaw RHW and Sykes Citation2014). There is thus an ever-increasing need to follow and read a wide-ranging literature in, for example, archaeology, Quaternary geology, geochemistry, palaeoclimatology, palaeolimnology, ecology, biogeography, applied statistics, and ancient-DNA studies.
Quaternary botany and palaeoecology are rapidly developing fields with many new proxies and sources of biological or environmental information continuously being developed and improved (Flessa and Jackson Citation2005a; Smol Citation2008; Bradley Citation2015). Examples include molecular biomarkers (e.g. Guillemot et al. Citation2017), ancient DNA (e.g. Pedersen MW et al. Citation2016; Schmid et al. Citation2017; Lendvay et al. Citation2018), sediment geochemistry (e.g. Croudace and Rothwell Citation2015), stable-isotope analysis (e.g. Leng Citation2006), Posidonia oceanica mats as an underwater ‘peat’ record covering the last 5500 years (López‐Merino et al. Citation2017), high-elevation ice-caves in the central Pyrenees recording tree-line fluctuations through the Holocene (Leunda et al. Citation2019), and pollen preserved in laminated ice cores (Brugger et al. Citation2018, Citation2019). Further contributions to modern ecology from palaeoecology can thus be confidently expected in the coming decades. As Bennett (Citation1988b, p.718) suggests ‘[e]cologists need the Quaternary record to guide their perception of which aspects of plant ecology are relevant on which timescales. … palaeoecology need not be just a sink for information from descriptive ecology: it can contribute by adding to and vastly increasing the possibilities for ecological research. … The fossil record can describe what happened in the past, but experimental ecologists are needed to explainFootnote1 how it happened.’
Birks_GR_BoxesS1-S6.pdf
Download PDF (354.9 KB)Acknowledgements
I am greatly indebted to the following for many valuable, insightful, and challenging discussions about Quaternary botany and its relationships with ecology and biogeography over the last 50+ years: Brigitta Ammann, the late Svend Th Andersen, Keith Bennett, Shonil Bhagwat, Cajo ter Braak, Richard Bradshaw, Ed Cushing, John Dransfield, Mary Edwards,Vivian Felde, Suzette Flantua, the late John Flenley, the late Knut Fægri, Thomas Giesecke, Francis Gilbert, the late Harry Godwin, Allan Gordon, Eric Grimm, John-Arvid Grytnes, the late Johs Iversen, Ulrike Herzschuh, Henry Hooghiemstra, Brian Huntley, Steve Jackson, the late Roel Janssen, Lizzy Jeffers, Steve Juggins, Petr Kuneš, Henry Lamb, Andy Lotter, Sandra Nogué, Frank Oldfield, Donald Pigott, Colin Prentice, the late Michael Proctor, the late Oliver Rackham, the late Derek Ratcliffe, the late Jim Ritchie, the late Kamil Rybniček, Alistair Seddon, Heikki Seppä, Gavin Simpson, Alan Smith, Manuel Steinbauer, John Tallis, Richard Telford, Des Thompson, Willy Tinner, Chronis Tzedakis, the late Bill Watts, Tom Webb, Cathy Whitlock, Jack Williams, Will Williams, the late Herb Wright, and most of all, Hilary Birks and Kathy Willis. I received valuable comments on all or parts of the manuscript from Brigitta Ammann, Hilary Birks, Suzette Flantua, and Manuel Steinbauer and reviews and suggestions from Keith Bennett, Will Gosling, and Laszlo Nagy. I am extremely grateful to Cathy Jenks for her meticulous and skilful preparation of the manuscript. I appreciate the invitation from the Editorial Board to prepare this review and the patience and kindness of the editors Victor Resco de Dios and Laszlo Nagy in not imposing strict deadlines (or word limits!).
This manuscript was prepared with support from the Norwegian Research Council (project 249894/F20 – IGNEX) Interglacials and Glacials – Natural Experiments in Biodiversity Dynamics and from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (grant agreement No 741413 – HOPE) Humans on Planet Earth – Long-term impacts on biosphere dynamics. It is a contribution to the IGNEX and HOPE projects.
It is a personal honour to write this Grubb Review. I have known Peter Grubb for over 50 years. He taught me when I was an undergraduate 1963–66 and I learnt much from him about world botany and the many different approaches to ecology, and about always questioning ecological ‘truths’.
Disclosure statement
No potential conflicts of interest are known.
Supplemental material
Supplemental data for this article can be accessed here.
Additional information
Funding
Notes on contributors
H. John B. Birks
H. John B. Birks began pollen-analytical research in 1962. His Ph.D. (1966–69) combined modern ecology and pollen analysis in a palaeoecological study. After post-doctoral research in Minnesota in 1970, he started collaboration in 1971 with applied statisticians to develop techniques in quantitative palaeoecology. This collaboration continues to the present. In the late 1980s he extended his research into palaeolimnology and lake acidification. He has interests in all aspects of Quaternary palaeoecology. He also has a keen interest in alpine botany and he and his wife Hilary have explored mountains on all continents except Antarctica. To date, they have been on 94 such expeditions since 1965. He has published on palaeoecology, pollen analysis, vegetation history, palaeolimnology, applied statistics, quantitative palaeoecology, plant geography, community ecology, alpine botany, floristics, and bryology. He is Professor Emeritus at the University of Bergen and University College London and currently leads a European Research Council project on Humans on Planet Earth (HOPE) – Long-term impacts on biosphere dynamics (2018–2022).
Notes
1. Now estimated to be at least 12,000 BCE.
References
- Aakala T, Pasanen L, Helama S, Vakkari V, Drobyshev I, Seppä H, Kuuluvainen T, Stivrins N, Wallenius T, Vasander H et al. 2018. Multiscale variation in drought controlled historical forest fire activity in the boreal forests of eastern Fennoscandia. Ecol Monogr. 88(1):74–91.
- Aalto M, Eriksson B, Hirvas H. 1992. Naakenavaara interglacial - a till-covered peat deposit in western Finnish Lapland. Bull Geol Soc Finland. 64:169–181.
- Aario R. 1965. Die Fichtenverhäufigung in Lichte von C14-bestimmungen und die Altersverhaltnisse der Finnischen pollenzonen. C R Soc Géol Finlande. 37:215–231.
- Aartolahti T. 1966. Über die Einwanderlung und die Verhäufigung der Fichte in Finnland. Ann Bot Fennici. 3:368–379.
- Aartolahti T. 1967. Zur Rationellen Tilia-pollengrenze (To) in Finnland. Fennia. 97:1–30.
- Abel Schaad D, Iriarte E, López-Sáez JA, Pérez-Díaz S, Sabariego Ruiz S, Cheddadi R, Alba-Sánchez F. 2018. Are Cedrus atlantica forests in the Rif Mountains of Morocco heading towards local extinction? Holocene. 28:1023–1037. 0959683617752842.
- Abel Schaad D, Pulido F, López-Sáez JA, Alba Sánchez F, Nieto-Lugilde D, Franco Múgica F, Pérez Díaz S, Ruiz Zapata MB, Gil García MJ, Dorado Valiño M. 2014. Persistence of tree relicts in the Spanish Central System through the Holocene. LAZAROA. 35:107–131.
- Abraham V, Kuneš P, Petr L, Svitavská Svobodová H, Kozáková R, Jamrichová E, Švarcová MG, Pokorný P. 2016. A pollen-based quantitative reconstruction of the Holocene vegetation updates a perspective on the natural vegetation in the Czech Republic and Slovakia. Preslia. 88:409–434.
- Abrams PA. 2001. A world without competition. Nature. 412:858–859.
- Ackerly DD. 2003. Community assembly, niche conservatism, and adaptive evolution in changing environments. Int J Plant Sci. 164(3 Suppl.): S165–S184.
- Ackerly DD. 2015. The incomplete filling of the n-dimensional hypervolume. Bull Ecol Soc Am. 96(3):407–408.
- Ackerly DD, Loarie SR, Cornwell WK, Weiss SB, Hamilton H, Branciforte R, Kraft NJB. 2010. The geography of climate change: implications for conservation biogeography. Divers Distrib. 16(3):476–487.
- Adler PB, HilleRisLambers J, Levine JM. 2007. A niche for neutrality. Ecol Lett. 10(2):95–104.
- Ågren J, Schemske DW. 2012. Reciprocal transplants demonstrate strong adaptive differentiation of the model organism Arabidopsis thaliana in its native range. New Phytol. 194(4):1112–1122.
- Aitken SN, Yeaman S, Holliday JA, Wang T, Curtis-McLane S. 2008. Adaptation, migration or extirpation: climate change outcomes for tree populations. Evol Appl. 1(1):95–111.
- Åkesson C, Nielsen AB, Broström A, Persson T, Gaillard M-J, Berglund BE. 2015. From landscape description to quantification: a new generation of reconstructions provides new perspectives on Holocene regional landscapes of SE Sweden. Holocene. 25(1):178–193.
- Alba-Sánchez F, López-Sáez JA, Abel-Schaad D, Sabariego Ruiz S, Pérez-Díaz S, González-Hernández A, Linares JC. 2019. The impact of climate and land-use changes on the most southerly fir forests (Abies pinsapo) in Europe. Holocene. 29:1176–1188. 0959683619838043.
- Alba-Sánchez F, López-Sáez JA, Benito de Pando B, Linares JC, Nieto-Lugilde D, López-Merino L. 2010. Past and present potential distribution of the Iberian Abies species: a phytogeographic approach using fossil pollen data and species distribution models. Divers Distrib. 16(2):214–228.
- Alberto FJ, Aitken SN, Alía R, González-Martínez SC, Hänninen H, Kremer A, Lefèvre F, Lenormand T, Yeaman S, Whetten R et al. 2013. Potential for evolutionary responses to climate change – evidence from tree populations. Glob Chang Biol. 19(6):1645–1661.
- Alexander JM, Diez JM, Hart SP, Levine JM. 2016. When climate reshuffles competitors: a call for experimental macroecology. Trends Ecol Evol. 31(11):831–841.
- Alexander K, Allen MS, Butler J, Green E, Woods R. 2018. Britain’s natural landscapes - promoting improved understanding of the nature of the post-glacial vegeetation of lowland Britain. Br Wildl. 29:330–338.
- Alexander K, Butler J. 2018. The Knepp Vera conference. Br Wildl. 29:233–234.
- Allen K, Dupuy JM, Gei MG, Hulshof C, Medvigy D, Pizano C, Salgado-Negret B, Smith CM, Trierweiler A, van Bloem SJ et al. 2017. Will seasonally dry tropical forests be sensitive or resistant to future changes in rainfall regimes? Environ Res Lett. 12(2):023001.
- Allen MS, Butler K, Flenley J, Horrocks M. 2011. New pollen, sedimentary, and radiocarbon records from the Marquesas Islands, East Polynesia: implications for archaeological and palaeoclimate studies. Holocene. 21(3):473–484.
- Alley RB, Mayewski PA, Sowers T, Stuiver M, Taylor KC, Clark PU. 1997. Holocene climatic instability: a prominent, widespread event 8200 yr ago. Geology. 25(6):483–486.
- Allison TD, Moeller RE, Davis MB. 1986. Pollen in laminated sediments provides evidence for a mid-Holocene forest pathogen outbreak. Ecology. 67:1101–1105.
- Allmon WD. 2017. Speciation and shifting baselines: prospects for reciprocal illumination between evolutionary paleobiology and conservation biology. In: Dietl GP, Flessa KW, editors. Conservation paleobiology science and practice. Chicago: University of Chicago Press; p. 251–279.
- Almquist-Jacobson H. 1994. Interaction of the Holocene climate, water balance, vegetation, fire, and cultural land-use in Swedish Borderland [PhD Thesis]. Lund: University of Lund.
- Alonso D, Etienne RS, McKane AJ. 2006. The merits of neutral theory. Trends Ecol Evol. 21(8):451–457.
- Ammann B, Oldfield F, editors. 2000. Rapid warming project. Palaeogeogr Palaeoclimatol Palaeoecol. 159:191–366.
- Ammann B, van Leeuwen JFN, van der Knaap WO, Lischke H, Heiri O, Tinner W. 2013a. Vegetation responses to rapid warming and to minor climatic fluctuations during the Late-Glacial Interstadial (GI-1) at Gerzensee (Switzerland). Palaeogeogr Palaeoclimatol Palaeoecol. 391:40–59.
- Ammann B, van Raden UJ, Schwander J, Eicher U, Gilli A, Bernasconi SM, van Leeuwen JFN, Lischke H, Brooks SJ, Heiri O et al. 2013b. Responses to rapid warming at Termination 1a at Gerzensee (central Europe): primary succession, albedo, soils, lake development, and ecological interactions. Palaeogeogr Palaeoclimatol Palaeoecol. 391:111–131.
- Ammann B, von Grafenstein U, van Raden UJ. 2013c. Biotic responses to rapid warming about 14,685yr BP: introduction to a case study at Gerzensee (Switzerland). Palaeogeogr Palaeoclimatol Palaeoecol. 391:3–12.
- Andersen ST. 1961. Vegetation and its environment in Denmark in the Early Weichselian Glacial (Last Glacial). Danmarks Geol Unders II. 75:1–175.
- Andersen ST. 1964. Interglacial plant successions in the light of environmental changes. Report of the VIth International Congress on Quaternary, Warsaw 1961 Vol II: Palaeobotanical section. Łodź; p. 359–368.
- Andersen ST. 1966. Interglacial vegetation succession and lake development in Denmark. Palaeobotanist. 15:117–127.
- Andersen ST. 1970. The relative pollen productivity and pollen representation of north European trees, and correction factors for tree pollen spectra. Danmarks Geol Unders II. 96:1–99.
- Andersen ST. 1978. Local and regional vegetational development in eastern Denmark in the Holocene. Danmarks Geol Unders Årbog. 1976:5–27.
- Andersen ST. 1994. History of the terrestrial environment in the Quaternary of Denmark. Bull Geol Soc Den. 41:219–228.
- Andersen T, Carstensen J, Hernández-García E, Duarte CM. 2009. Ecological thresholds and regime shifts: approaches to identification. Trends Ecol Evol. 24(1):49–57.
- Anderson JE. 1991. A conceptual framework for evaluating and quantifying naturalness. Conserv Bio. 5:347–352.
- Anderson JE. 1992. Reply to Götmark. Conserv Bio. 6:459–460.
- Anderson NJ, Bugmann H, Dearing JA, Gaillard M-J. 2006. Linking palaeoenvironmental data and models to understand the past and to predict the future. Trends Ecol Evol. 21(12):696–704.
- Anderson P. 2014. Bridging the gap between applied ecological science and practical implementation in peatland restoration. J Appl Ecol. 51(5):1148–1152.
- Anderson RP. 2013. A framework for using niche models to estimate impacts of climate change on species distributions. Ann N Y Acad Sci. 1297(1):8–28.
- Anderson RS, Kaufman DS, Berg E, Schiff C, Daigle T. 2017. Holocene biogeography of Tsuga mertensiana and other conifers in the Kenai Mountains and Prince William Sound, south-central Alaska. Holocene. 27(4):485–495.
- Andersson G. 1902. Hasseln i Sverige fordom och nu. Sveriges Geol Undersökning Ser C. 3:1–168.
- Andersson G. 1909. The climate of Sweden in the Late-Quaternary period. Facts and theories. Sveriges Geol Undersökning Ser C Årbok. 3:1–88.
- Andersson NJ. 1854. Om Galápagos-öarnes vegetation. Stockholm.
- Andersson NJ. 1855. Enumeratio plantarum in Insulis Galapagensis hucusque observatorum. Kongliga Sven Vetenskaps Akademiens Handlingar. 1853:61–256.
- Angeler DG, Allen CR. 2016. Quantifying resilience. J Appl Ecol. 53(3):617–624.
- Aranbarri J, González-Sampériz P, Valero-Garcés B, Moreno A, Gil-Romera G, Sevilla-Callejo M, García-Prieto E, Di Rita F, Mata MP, Morellón M et al. 2014. Rapid climatic changes and resilient vegetation during the Lateglacial and Holocene in a continental region of south-western Europe. Glob Planet Change. 114:50–65.
- Araújo MB, Peterson AT. 2012. Uses and misuses of bioclimatic envelope modeling. Ecology. 93(7):1527–1539.
- Armsworth PR, Larson ER, Jackson ST, Sax DF, Simonin P, Blossey B, Green N, Klein ML, Lester L, Ricketts TH et al. 2015. Are conservation organizations configured for effective adaptation to global change? Front Ecol Environ. 13(3):163–169.
- Aronson J, Murcia C, Kattan GH, Moreno-Mateos D, Dixon K, Simberloff D. 2014. The road to confusion is paved with novel ecosystem labels: a reply to Hobbs et al. Trends Ecol Evol. 29(12):646–647.
- Ash JD, Givnish TJ, Waller DM. 2017. Tracking lags in historical plant species’ shifts in relation to regional climate change. Glob Chang Biol. 23(3):1305–1315.
- Ashcroft MB. 2010. Identifying refugia from climate change. J Biogeogr. 37:1407–1413.
- Ashcroft MB, Chisholm LA, French KO. 2009. Climate change at the landscape scale: predicting fine-grained spatial heterogeneity in warming and potential refugia for vegetation. Glob Chang Biol. 15(3):656–667.
- Ashcroft MB, Gollan JR. 2012. Fine-resolution (25 m) topoclimatic grids of near-surface (5 cm) extreme temperatures and humidities across various habitats in a large (200 × 300 km) and diverse region. Int J Climatol. 32(14):2134–2148.
- Ashcroft MB, Gollan JR. 2013. Moisture, thermal inertia, and the spatial distributions of near-surface soil and air temperatures: understanding factors that promote microrefugia. Agric Forest Meteorol. 176:77–89.
- Ashcroft MB, Gollan JR, Warton DI, Ramp D. 2012. A novel approach to quantify and locate potential microrefugia using topoclimate, climate stability, and isolation from the matrix. Glob Chang Biol. 18:1866–1879.
- Ashcroft MB, King DH, Ratymond B, Turnbull JD, Wasley J, Robinson SA. 2017. Moving beyond presence and absence when examining changes in species distributions. Glob Chang Biol. 23:2929–2940.
- Asner GP, Levick SR. 2012. Landscape effects of herbivores on treefall in African savannas. Ecol Lett. 15:1211–1217.
- Astudillo FJ. 2018. Environmental and historical archaeology of the Galápagos islands: archaeobotany of Hacienda El Progreso, 1870–1920. Veg Hist Archaeobot. doi:10.1007/s00334-00018-00668-00339.
- Athens JS. 2009. Rattus exulans and the catastrophic disappearance of Hawai’i’s native lowland forest. Biol Invasions. 11:1489–1501.
- Auer V. 1921. Zur Kenntris der Stratigrahie der mittelösterbotanischen Moore. Acta Forestalia Fennica. 18:1–40.
- Auer V. 1958. The Pleistocene of Fuego-Patagonia. Part II: the history of the flora and vegetation. Ann Acad Scientiarum Fenn Ser AIII. 50:1–239.
- Auer V. 1965. The Pleistocene of Fuego-Patagonia. Part IV: Bog profiles. Ann Acad Scientiarum Fenn Ser AIII. 80:1–160.
- Austin MP. 1990. Community theory and competition in vegetation. In: Grace JB, Tilman D, editors. Perspectives on plant competition. San Diego: Academic Press; p. 215–238.
- Austin MP. 1992. Modelling the environmental niche of plants: implications for plant community response to elevated CO2 levels. Aust J Bot. 40:615–630.
- Austin MP. 1999. A silent clash of paradigms: some inconsistencies in community ecology. Oikos. 86(1):170–178.
- Austin MP, Nicholls AO, Margules CR. 1990. Measurement of the realized quantitative niche: environmental niches of five Eucalyptus species. Ecol Monogr. 60:161–177.
- Austin MP, Pausas JG, Noble IR. 1997. Modelling environmental and temporal niches of eucalypts. In: Williams JE, Woinarski JCZ, editors. Eucalypt ecology: individuals to ecosystems. Cambridge: Cambridge University Press; p. 129–150.
- Ayres KR, Sayer CD, Skeate ER, Perrow MR. 2008. Palaeolimnology as a tool to inform shallow lake management: an example from Upton Great Broad, Norfolk, UK. Biodivers Conserv. 17(9):2153–2168.
- Azizi G, Flenley JR. 2008. The last glacial maximum climatic conditions on Easter Island. Quatern Int. 184:166–176.
- Backstrom AC, Garrard GE, Hobbs RJ, Bekessy SA. 2018. Grappling with the social dimensions of novel ecosystems. Front Ecol Environ. 16(2):109–117.
- Bae CJ, Douka K, Petraglia MD. 2017. On the origin of modern humans: Asian perspectives. Science. 358(6368):aai9067.
- Bagnoli F, Tsuda Y, Fineschi S, Bruschi P, Magri D, Zhelev P, Paule L, Simeone MC, González-Martínez SC, Vendramin GG. 2016. Combining molecular and fossil data to infer demographic history of Quercus cerris: insights on European eastern glacial refugia. J Biogeogr. 43(4):679–690.
- Bahn V, McGill BJ. 2013. Testing the predictive performance of distribution models. Oikos. 122(3):321–331.
- Baker AG, Bhagwat SA, Willis KJ. 2013. Do dung fungal spores make a good proxy for past distribution of large herbivores? Quat Sci Rev. 62:21–31.
- Baker AG, Perry C, Bhagwat SA, Vera FWM, Willis KJ. 2017. Quantification of population sizes of large herbivores and their long-term functional role in ecosystems using dung fungal spores. Methods Ecol Evol. 7(11):1273–1281.
- Baker AG, Zimny M, Keczynski A, Bhagwat SA, Willis KJ, Latałowa M. 2016. Pollen productivity estimates from old-growth forest strongly differ from those obtained in cultural landscapes: evidence from the Białowieża National Park, Poland. Holocene. 26(1):80–92.
- Baker RG. 1965. Late-glacial pollen and plant macrofossils from Spider Creek, southern St Louis county, Minnesota. Geol Soc Am Bull. 76:601–610.
- Bakker ES, Gill JL, Johnson CN, Vera FWM, Sandom CJ, Asner GP, Svenning J-C. 2016. Combining paleo-data and modern exclosure experiments to assess the impact of megafauna extinctions on woody vegetation. Proc Natl Acad Sci USA. 113(4):847–855.
- Bala G, Caldeira K, Wickett M. 2007. Combined climate and carbon-cycle effects of large-scale deforestation. Proc Natl Acad Sci USA. 104:6550–6555.
- Balaguer L, Escudero A, Martín-Duque JF, Mola I, Aronson J. 2014. The historical reference in restoration ecology: re-defining a cornerstone concept. Biol Conserv. 176:12–20.
- Balée W. 1988. Indigenous adaptation to Amazonian palm forests. Principes. 32:47–54.
- Balée W. 2013. Cultural forest of the Amazon - a historical ecology of people and their landscapes. Tuscaloosa: University of Alabama Press.
- Barker G, Hunt C, Barton H, Gosden C, Jones S, Lloyd-Smith L, Farr L, Nyirí B, O’Donnell S. 2017. The ‘cultured rainforests’ of Borneo. Quatern Int. 448:44–61.
- Barlow C. 2002. The ghosts of evolution: nonsensical fruit, missing partners, and other ecological anachronisms. New York: Basic Books.
- Barnard-Kubow KB, Debban CL, Galloway LF. 2015. Multiple glacial refugia lead to genetic structuring and the potential for reproductive isolation in a herbaceous plant. Am J Bot. 102(11):1842–1853.
- Barnosky AD, Bell CJ, Emslie SD, Goodwin HT, Mead JI, Repenning CA, Scott E, Shabel AB. 2004b. Exceptional record of mid-Pleistocene vertebrates helps differentiate climatic from anthropogenic ecosystem perturbations. Proc Natl Acad Sci USA. 101(25):9297–9302.
- Barnosky AD, Hadly EA, Gonzales P, Head J, Polly PD, Lawing AM, Eronen JT, Ackerly DD, Alex K, Biber E, et al. 2017. Merging paleobiology with conservation biology to guide the future of terrestrial ecosystems. Science. 355(6325):594.
- Barnosky AD, Koch PL, Feranec RS, Wing SL, Shabel AB. 2004a. Assessing the causes of late Pleistocene extinctions on the continents. Science. 306(5693):70–75.
- Barnosky AD, Lindsey EL, Villavicencio NA, Bostelmann E, Hadly EA, Wanket J, Marshall CR. 2015. Variable impact of late-Quaternary megafaunal extinction in causing ecological state shifts in North and South America. Proc Natl Acad Sci USA. 113(4):856–861.
- Barreto CF, de Freitas ADS, De Souza TCS, Vilela CG, Barth OM, Baptista Neto JA. 2017. A mid-Holocene vegetational and anthropogenic record in the Guanabara Bay region, Rio de Janeiro State, SE Brazil, assessed by palynological and charcoal data. Grana. 56(4):304–314.
- Barrow CJ. 1978. Postglacial pollen diagrams from South Georgia (sub Antarctic) and West Falkland Island (South Atlantic). J Biogeogr. 5:251–274.
- Barrow CJ. 1983a. Palynological studies in South Georgia. III. Three profiles from near King Edward Cove, Cumberland East Bay. Br Antarct Surv Bull. 58:43–60.
- Barrow CJ. 1983b. Palynological studies in South Georgia. IV. Three profiles from Barff Peninsula and Annenkov Island. Br Antarct Surv Bull. 58:61–70.
- Barrow CJ, Lewis RI. 1983. Palynological studies in South Georgia. II. Two profiles in Sphagnum Valley, Cumberland West Bay. Br Antarct Surv Bull. 58:15–42.
- Bartlein PJ, Anderson KH, Anderson PM, Edwards ME, Mock CJ, Thompson RS, Webb RS, Webb T, Whitlock C. 1998. Paleoclimatic simulations for North America over the past 21,000 years: features of the simulated climates and comparisons with paleoenvironmental data. Quat Sci Rev. 17:549–585.
- Bartlein PJ, Prentice IC. 1989. Orbital variations, climate and paleoecology. Trends Ecol Evol. 4(7):195–199.
- Battarbee RW. 1999. The importance of palaeolimnology to lake restoration. Hydrobiologia. 395/396:149–159.
- Battarbee RW. 2000. Palaeolimnological approaches to climate change, with special regard to the biological record. Quat Sci Rev. 19(1–5):107–124.
- Battarbee RW, Anderson NJ, Bennion H, Simpson GL. 2012. Combining limnological and palaeolimnological data to disentangle the effects of nutrient pollution and climate change on lake ecosystems: problems and potential. Freshw Biol. 57(10):2091–2106.
- Battarbee RW, Anderson NJ, Jeppesen E, Leavitt PR. 2005. Combining palaeolimnological and limnological approaches in assessing lake ecosystem response to nutrient reduction. Freshw Biol. 50:1772–1780.
- Battistel D, Argiriadis E, Kehrwald N, Spigariol M, Russell JM, Barbante C. 2017. Fire and human record at Lake Victoria, East Africa, during the Early Iron Age: did humans or climate cause massive ecosystem changes? Holocene. 27(7):997–1007.
- Battistel D, Roman M, Marchetti A, Kehrwald NM, Radaelli M, Balliana E, Toscano G, Barbante C. 2018. Anthropogenic impact in the Maya Lowlands of Petén, Guatemala, during the last 5500 years. J Quat Sci. 33(2):166–176.
- Baumgartner JB, Esperón-Rodríguez M, Beaumont LJ. 2018. Identifying in situ climate refugia for plant species. Ecography. doi:10.1111/ecog.03431.
- Bayliss-Smith T, Hviding E, Whitmore T. 2003. Rainforest composition and histories of human disturbance in Solomon Islands. Ambio. 32(5):346–352.
- Beach T, Luzzadder-Beach S, Cook D, Dunning N, Kennett DJ, Krause S, Terry R, Trein D, Valdez F. 2015. Ancient Maya impacts on the Earth’s surface: an early Anthropocene analog? Quat Sci Rev. 124:1–30.
- Beck KK, Fletcher MS, Gadd PS, Heijnis H, Saunders KM, Simpson GL, Zawadzki A. 2018. Variance and rate‐of‐change as early warning signals for a critical transition in an aquatic ecosystem state: a test case from Tasmania, Australia. J Geophys Res Biogeosci. 123(2):495–508.
- Beckage B, Gross LJ, Kauffman S. 2011. The limits to prediction in ecological systems. Ecosphere. 2 (11):art125.
- Becker T, Spanka J, Schröder L, Leuschner C. 2017. Forty years of vegetation change in former coppice-with-standards woodlands as a result of management change and N deposition. Appl Veg Sci. 20(2):304–313.
- Beer R, Kaiser F, Schmidt K, Ammann B, Carraro G, Grisa E, Tinner W. 2008. Vegetation history of the walnut forests in Kyrgyzstan (central Asia): natural or anthropogenic origin? Quat Sci Rev. 27(5–6):621–632.
- Behling H. 1996. First report on new evidence for the occurrence of Podocarpus and possible human presence at the mouth of the Amazon during the Late-glacial. Veg Hist Archaeobot. 5(3):241–246.
- Behling H, Berrío JC, Hooghiemstra H. 1999. Late Quaternary pollen records from the middle Caquetá river basin in central Colombian Amazon. Palaeogeogr Palaeoclimatol Palaeoecol. 145(1):193–213.
- Behling H, Bush MB, Hooghiemstra H. 2010. Biotic development of Quaternary Amazonia: a palynological perspective. In: Hoorn C, Wesselingh FP, editors. Amazonia: landscapes and species evolution. Chichester: Wiley-Blackwell; p. 335–345.
- Beier P, Hunter ML, Anderson M. 2015. Introduction (Conserving nature’s stage). Conserv Bio. 29(3):613–617.
- Belovsky GE, Botkin DB, Crowl TA, Cummins KW, Franklin JF, Hunter ML, Joern A, Lindenmayer DB, MacMahon JA, Margules CR et al. 2004. Ten suggestions to strengthen the science of ecology. BioScience. 54(4):345–351.
- Benito-Garzón M, Leadley PW, Fernández-Manjarrés JF. 2014. Assessing global biome exposure to climate change through the Holocene–Anthropocene transition. Global Ecol Biogeogr. 23(2):235–244.
- Bennett KD. 1983. Post-glacial population expansion of forest trees in Norfolk, UK. Nature. 303(5913):164–167.
- Bennett KD. 1984. The post-glacial history of Pinus sylvestris in the British Isles. Quat Sci Rev. 3:133–153.
- Bennett KD. 1985. The spread of Fagus grandifolia across Eastern North America during the last 18000 years. J Biogeogr. 12(2):147–164.
- Bennett KD. 1986. The rate of spread and population increase of forest trees during the Postglacial. Philos Trans R Soc B Bio Sci. 314(1167):523–531.
- Bennett KD. 1988a. Holocene geographic spread and population expansion of Fagus grandifolia in Ontario, Canada. J Ecol. 76:547–557.
- Bennett KD. 1988b. Post-glacial vegetation history: ecological considerations. In: Huntley B, Webb T, editors. Vegetation history. Dordrecht: Kluwer; p. 699–774.
- Bennett KD. 1988c. The power of movement in plants. Trends Ecol Evol. 13:339–340.
- Bennett KD. 1988d. Modelling Holocene changes in beech populations: a reply to Dexter et al. (1987). Rev Palaeobot Palynol. 56:361–364.
- Bennett KD. 1990. Models of plant population growth and analogies with reaction kinetics. Rev Palaeobot Palynol. 64:247–251.
- Bennett KD. 1993. Holocene forest dynamics with respect to southern Ontario. Rev Palaeobot Palynol. 79:69–81.
- Bennett KD. 1995. Insularity and the Quaternary tree and shrub flora of the British Isles. In: Preece RC, editor. Island Britain: a Quaternary perspective. Vol. 96. Cambridge: Geological Society Special Publication; p. 173–180.
- Bennett KD. 1997. Evolution and ecology: the pace of life. Cambridge: Cambridge University Press.
- Bennett KD. 2004. Continuing the debate on the role of Quaternary environmental change for macroevolution. Philos Trans R Soc B Bio Sci. 359(1442):295–303.
- Bennett KD, Birks HJB. 1990. Postglacial history of Alder (Alnus glutinosa (L) Gaertn) in the British Isles. J Quat Sci. 5(2):123–133.
- Bennett KD, Boreham S, Sharp MJ, Switsur VR. 1992. Holocene history of environment, vegetation and human settlement on Catta Ness, Lunnasting, Shetland. J Ecol. 80:241–273.
- Bennett KD, Fossitt JA, Sharp MJ, Switsur VR. 1990. Holocene vegetation and environmental history at Loch Lang, South Uist, Western Isles, Scotland. New Phytol. 114:281–298.
- Bennett KD, Lamb HF. 1988. Holocene pollen sequences as a record of competitive interactions among tree populations. Trends Ecol Evol. 3(6):141–144.
- Bennett KD, Tzedakis PC, Willis KJ. 1991. Quaternary refugia of north European trees. J Biogeogr. 18(1):103–115.
- Bennett KD, Willis KJ. 1995. The role of ecological factors in controlling vegetation dynamics on long temporal scales. Giornale Bot Ital. 129:243–254.
- Bennike O. 1990. The Kap København Formation: stratigraphy and palaeobotany of a Plio-Pleistocene sequence in Peary Land, North Greenland. Meddel Grønland. 23:1–85.
- Bennike O, Hedenäs L, Lemdahl G, Wiberg-Larsen P. 2018. A multiproxy macrofossil record of Eemian palaeoenvironments from Klaksvík, the Faroe Islands. Boreas. 47(1):106–113.
- Bennike O, Jensen JB, Lemke W. 2001. Late Quaternary records of Najas spp. (Najadaceae) from the southwestern Baltic region. Rev Palaeobot Palynol. 114(3):259–267.
- Bennington JB, DiMichele WA, Badgley C, Bambach RK, Barrett PM, Behrensmeyer AK, Bobe R, Burnham RJ, Daeschler EB, van Dam J et al. 2009. Critical issues of scale in paleoecology. Palaios. 24(1):1–4.
- Bennion H, Sayer CD, Clarke SJ, Davidson TA, Rose NL, Goldsmith B, Rawcliffe R, Burgess A, Clarke G, Turner SD et al. 2017. Sedimentary macrofossil records reveal ecological change in English lakes: implications for conservation. J Paleolimnol. doi:10.1007/s10933-10017-19941-10937.
- Bennion H, Simpson GL. 2011. The use of diatom records to establish reference conditions for UK lakes subject to eutrophication. J Paleolimnol. 45:469–488.
- Bennion H, Simpson GL, Goldsmith BJ. 2015. Assessing degradation and recovery pathways in lakes impacted by eutrophication using the sediment record. Front Ecol Evol. 3:94.
- Bergamin RS, Debastiani V, Joner DC, Lemes P, Guimarães T, Loyola RD, Müller SC. 2019. Loss of suitable climatic areas for Araucaria forests over time. Plant Ecol Divers. doi:10.1080/17550874.17552019.11618408.
- Berglund BE, editor 1986. Handbook of Holocene Palaeoecology and Palaeohydrology. Chichester (Reprinted 2003 by Blackburn Press, New Jersey): J Wiley & Sons.
- Berglund BE. 2003. Human impact and climate changes - synchronous events and a causal link? Quatern Int. 105:7–12.
- Berglund BE, Malmer N. 1971. Soil conditions and late-glacial stratigraphy. Geologiska Föreningens i Stockholm Förhandlingar. 93:11–45.
- Bergström A, Oppenheimer SJ, Mentzer AJ, Auckland K, Robson K, Attenborough R, Alpers MP, Koki G, Pomat W, Siba P et al. 2017. A Neolithic expansion, but strong genetic structure, in the independent history of New Guinea. Science. 357(6356):1160–1163.
- Berland A, Shuman B, Manson SM. 2011. Simulated importance of dispersal, disturbance, and landscape history in long-term ecosystem change in the Big Woods of Minnesota. Ecosystems. 14(3):398–414.
- Bernabo JC, Webb T. 1977. Changing patterns in the Holocene pollen record of northeastern North America: a mapped summary. Quat Res. 8:64–96.
- Bernards SJ, Morris LR. 2017. Influence of topography on long-term successional trajectories in canyon grasslands. Appl Veg Sci. 20(2):236–246.
- Berrío JC, Hooghiemstra H, Behling H, Botero P, van der Borg K. 2002. Late-Quaternary savanna history of the Colombian Llanos Orientales from Lagunas Chenevo and Mozambique: a transect synthesis. Holocene. 12(1):35–48.
- Berry PE, Holst BK, Yatskievych K, editors. 1995. Flora of the Venezuelan Guayana I. Introduction. St Louis: Missouri Botanical Garden Press.
- Bertelsmeier C, Ollier S. 2016. Questioning Holocene community shifts (Arising from Lyons et al. 2016). Nature. 537:E4.
- Bertrand R, Lenoir J, Piedallu C, Riofrío-Dillon G, de Ruffray P, Vidal C, Pierrat J-C, Gégout J-C. 2011. Changes in plant community composition lag behind climate warming in lowland forests. Nature. 479:517.
- Bertsch K. 1940. Geschichte des deutsches Waldes. Jena: Gustav Fischer.
- Bertsch K. 1953. Geschichte des deutsches Waldes. 3rd ed. Jena: Fischer.
- Bertuol‐Garcia D, Morsello C, El‐Hani CN, Pardini R. 2018. A conceptual framework for understanding the perspectives on the causes of the science–practice gap in ecology and conservation. Biol Rev. 93(2):1032–1055.
- Bezrukova EV, Tarasov PE, Solovieva N, Krivonogov SK, Riedel F. 2010. Last glacial–interglacial vegetation and environmental dynamics in southern Siberia: chronology, forcing and feedbacks. Palaeogeogr Palaeoclimatol Palaeoecol. 296(1):185–198.
- Bhagwat SA, Nogué S, Willis KJ. 2012. Resilience of an ancient tropical forest landscape to 7500 years of environmental change. Biol Conserv. 153:108–117.
- Bhagwat SA, Willis KJ. 2008. Species persistence in northerly glacial refugia of Europe: a matter of chance or biogeographical traits? J Biogeogr. 35:464–482.
- Bhaskar R, Dawson TE, Balvanera P. 2014. Community assembly and functional diversity along succession post-management. Funct Ecol. 28(5):1256–1265.
- Bhiry N, Filion L. 1996. Mid-Holocene hemlock decline in eastern North America linked with phytophagous insect activity. Quat Res. 45(3):312–320.
- Biagioni S, Wündsch M, Haberzettl T, Behling H. 2015. Assessing resilience/sensitivity of tropical mountain rainforests towards climate variability of the last 1500 years: the long-term perspective at Lake Kalimpaa (Sulawesi, Indonesia). Rev Palaeobot Palynol. 213:42–53.
- Binney HA, Edwards ME, Macias Fauria M, Lozhkin AV, Anderson P, Kaplan JO, Andreev AA, Bezrukova E, Blyakharchuk TA, Jankovska V et al. 2017. Vegetation of Eurasia from the last glacial maximum to present: key biogeographic patterns. Quat Sci Rev. 157:80–97.
- Biondi F. 2014. Paleoecology grand challenge. Front Ecol Evol. 2:50.
- Birks HH. 1984. Late-Quaternary pollen and plant macrofossil stratigraphy at Lochan an Druim, north-west Scotland. In: Haworth EY, Lund JWG, editors. Lake sediments and environmental history. Leicester: University of Leicester Press; p. 377–405.
- Birks HH. 2003. The importance of plant macrofossils in the reconstruction of Lateglacial vegetation and climate: examples from Scotland, western Norway, and Minnesota, USA. Quat Sci Rev. 22:453–473.
- Birks HH. 2008. The Late-Quaternary history of arctic and alpine plants. Plant Ecol Divers. 1:135–146.
- Birks HH, Ammann B. 2000. Two terrestrial records of rapid climate change during the glacial-Holocene transition (14,000-9,000 calendar years BP) from Europe. Proc Natl Acad Sci USA. 97:1390–1394.
- Birks HH, Battarbee RW, Birks HJB. 2000. The development of the aquatic ecosystem at Kråkenes Lake, western Norway, during the late glacial and early Holocene - a synthesis. J Paleolimnol. 23(1):91–114.
- Birks HH, Birks HJB. 2000. Future uses of pollen analysis must include plant macrofossils. J Biogeogr. 27:31–35.
- Birks HH, Birks HJB. 2006. Multi-proxy studies in palaeolimnology. Veg Hist Archaeobot. 15(4):235–251.
- Birks HH, Mathewes RW. 1978. Studies in the vegetational history of Scotland V. Late Devensian and early Flandrian pollen and macrofossil stratigraphy at Abernethy Forest, Inverness-shire. New Phytol. 80:455–484.
- Birks HJB. 1973. Past and Present Vegetation of the Isle of Skye - A Palaeoecological Study. Cambridge: Cambridge University Press.
- Birks HJB. 1976. Late-Wisconsinan vegetational history at Wolf Creek, central Minnesota. Ecol Monogr. 46:395–492.
- Birks HJB. 1981a. Late Wisconsin vegetational and climatic history at Kylen Lake, Northeastern Minnesota. Quat Res. 16:322–355.
- Birks HJB. 1981b. The use of pollen analysis in the reconstruction of past climates: a review. In: Wigley TML, Ingram MJ, Farmer G, editors. Climate and history. Cambridge: Cambridge University Press; p. 111–138.
- Birks HJB. 1982. Mid-Flandrian Forest history of Roudsea Wood National Nature Reserve, Cumbria. New Phytol. 90(2):339–354.
- Birks HJB. 1986. Late Quaternary biotic changes in terrestrial and limnic environments, with particular reference to north west Europe. In: Berglund BE, editor. Handbook of Holocene palaeoecology and palaeohydrology. Chichester: J Wiley & Sons; p. 3–65.
- Birks HJB. 1989. Holocene isochrone maps and patterns of tree-spreading in the British Isles. J Biogeogr. 16(6):503–540.
- Birks HJB. 1993a. Quaternary paleoecology and vegetation science - current contributions and possible future developments. Rev Palaeobot Palynol. 79(1–2):153–177.
- Birks HJB. 1993b. Precision, concepts, controversies: Alan Smith’s contributions to vegetational history and palaeoecology. In: Chambers FM, editor. Climate change and human impact on the landscape. London: Chapman & Hall; p. 5–11.
- Birks HJB. 1996. Contributions of Quaternary palaeoecology to nature conservation. J Veg Sci. 7(1):89–98.
- Birks HJB. 1998. Numerical tools in palaeolimnology - Progress, potentialities, and problems. J Paleolimnol. 20(4):307–332.
- Birks HJB. 2005. Mind the gap: how open were European primeval forests? Trends Ecol Evol. 20(4):154–156.
- Birks HJB. 2007. Estimating the amount of compositional change in late-Quaternary pollen-stratigraphical data. Veg Hist Archaeobot. 16:197–202.
- Birks HJB. 2008. Holocene climate research - progress, paradigms, and problems. In: Battarbee RW, Binney HA, editors. Natural climate variability and global warming: a Holocene perspective. Oxford: Wiley-Blackwell; p. 7–57.
- Birks HJB. 2012a. Ecological palaeoecology and conservation biology: controversies, challenges, and compromises. Int J Biodiver Sci Ecosyst Serv Manage. 8:292–304.
- Birks HJB. 2012b. Analysis of stratigraphical data. In: Birks HJB, Lotter AF, Juggins S et al., editors. Tracking environmental change using lake sediments volume 5: data handling and numerical techniques. Dordrecht: Springer; p. 355–378.
- Birks HJB. 2014. Challenges in the presentation and analysis of plant-macrofossil stratigraphical data. Veg Hist Archaeobot. 23(3):309–330.
- Birks HJB. 2015. Some reflections on the refugium concept and its terminology in historical biogeography, contemporary ecology and global-change biology. Biodiversity. 16(4):196–212.
- Birks HJB, Berglund BE. 2018. One hundred years of Quaternary pollen analysis 1916–2016. Veg Hist Archaeobot. 27(2):271–309.
- Birks HJB, Birks HH. 1980. Quaternary palaeoecology. London (Reprinted 2004 by Blackburn Press, New Jersey): Edward Arnold.
- Birks HJB, Birks HH. 2004. The rise and fall of forests. Science. 305(5683):484–485.
- Birks HJB, Birks HH. 2008. Biological responses to rapid climate changes at the Younger Dryas-Holocene transition at Kråkenes, western Norway. Holocene. 18:19–30.
- Birks HJB, Birks HH. 2016. How have studies of ancient DNA from sediments contributed to the reconstruction of Quaternary floras? New Phytol. 209:499–506.
- Birks HJB, Birks HH, Ammann B. 2016b. The fourth dimension of vegetation. Science. 354:412–413.
- Birks HJB, Deacon J, Peglar SM. 1975a. Pollen maps for the British Isles 5000 years ago. Proc R Soc B Bio Sci. 189(1094):87–105.
- Birks HJB, Felde VA, Bjune AE, Seppä H, Giesecke T. 2016c. Does pollen-assemblage richness reflect floristic richness? A review of recent developments and future challenges. Rev Palaeobot Palynol. 228:1–25.
- Birks HJB, Felde VA, Seddon AWR. 2016a. Biodiversity trends within the Holocene. Holocene. 26:994–1001.
- Birks HJB, Gordon AD. 1985. Numerical methods in Quaternary pollen analysis. London: Academic Press.
- Birks HJB, Heiri O, Seppä H, Bjune AE. 2010. Strengths and weaknesses of quantitative climate reconstructions based on Late-Quaternary biological proxies. Open Ecol J. 3:68–110.
- Birks HJB, Line JM. 1992. The use of rarefaction analysis for estimating palynological richness from Quaternary pollen-analytical data. Holocene. 2:1–10.
- Birks HJB, Lotter AF, Juggins S, Smol JP, editors. 2012. Tracking environmental change using lake sediments volume 5: data handling and numerical techniques. Dordrecht: Springer.
- Birks HJB, Peglar SM. 1979. Interglacial pollen spectra from Sel Ayre, Shetland. New Phytol. 83(2):559–575.
- Birks HJB, Saarnisto M. 1975. Isopollen maps and principal components analysis of Finnish pollen data for 4000, 6000, and 8000 years ago. Boreas. 4:77–96.
- Birks HJB, Tinner W. 2016a. European tree dynamics and invasions during the Quaternary. In: Krumm F, Quadt V, editors. Introduced tree species to European forests: challenges and opportunities. Luxembourg: Publication Office of the European Union; p. 21–43.
- Birks HJB, Tinner W. 2016b. Past forests of Europe. In: San Miguel Ayanz J, de Rigo D, Caudulio G et al., editors. European atlas of forest tree species. Luxembourg: Publication Office of the European Union; p. 36–39.
- Birks HJB, Webb T, Berti AA. 1975b. Numerical analysis of pollen samples from central Canada - Comparison of methods. Rev Palaeobot Palynol. 20(3):133–169.
- Birks HJB, Williams W. 1983. Late-Quaternary vegetational history of the Inner Hebrides. Proc R Soc Edinburgh B Biol Sci. 83:269–292.
- Birks HJB, Willis KJ. 2008. Alpines, trees, and refugia in Europe. Plant Ecol Divers. 1:147–160.
- Björck S. 1985. Deglaciation chronology and revegetation in northwestern Ontario. Can J Earth Sci. 22:850–871.
- Bjorkman AD, Vellend M. 2010. Defining historical baselines for conservation: Ecological changes since European settlement on Vancouver Island, Canada. Conserv Bio. 24(6):1559–1568.
- Björse G, Bradshaw RHW. 1998. 2000 years of forest dynamics in southern Sweden: suggestions for forest management. For Ecol Manage. 104(1–3):15–26.
- Bjune AE, Helvik I, Birks HJB. 2013. The Fagus sylvatica forests in the Larvik region, south-eastern Norway – their origin and history. Veg Hist Archaeobot. 22:215–229.
- Blaauw M, Bennett KD, Christen JA. 2010. Random walk simulations of fossil proxy data. Holocene. 20:645–649.
- Blackburn TM, Cassey P, Duncan RP, Evans KL, Gaston KJ. 2004. Avian extinction and mammalian introductions on oceanic islands. Science. 305(5692):1955–1958.
- Blarquez O, Carcaillet C, Frejaville T, Bergeron Y. 2014. Disentangling the trajectories of alpha, beta and gamma plant diversity of North American boreal ecoregions since 15,500 years. Front Ecol Evol. 2:6.
- Blois JL. 2012a. Recent advances using species distributional models to understand past distributions. Front Biogeogr. 3(4):123–124.
- Blois JL. 2012b. Stemming ‘ignorance creep’ in paleoecology and biogeography. Front Biogeogr. 4.3:93–94.
- Blois JL. 2013. Narrowing the estimates of species migration rates. Front Biogeogr. 5 (3):fb_19448.
- Blois JL, Gotelli NJ, Behrensmeyer AK, Faith JT, Lyons SK, Williams JW, Amatangelo KL, Bercovici A, Du A, Eronen JT et al. 2014. A framework for evaluating the influence of climate, dispersal limitation, and biotic interactions using fossil pollen associations across the late Quaternary. Ecography. 37(11):1095–1108.
- Blois JL, McGuire JL, Hadly EA. 2010. Small mammal diversity loss in response to late-Pleistocene climatic change. Nature. 465(7299):771–774.
- Blois JL, Williams JW, Fitzpatrick MC, Ferrier S, Veloz SD, He F, Liu ZY, Manion G, Otto-Bliesner B. 2013a. Modeling the climatic drivers of spatial patterns in vegetation composition since the Last Glacial Maximum. Ecography. 36(4):460–473.
- Blois JL, Williams JW, Fitzpatrick MC, Jackson ST, Ferrier S. 2013b. Space can substitute for time in predicting climate-change effects on biodiversity. Proc Natl Acad Sci USA. 110(23):9374–9379.
- Blois JL, Williams JW, Grimm EC, Jackson ST, Graham RW. 2011. A methodological framework for assessing and reducing temporal uncertainty in paleovegetation mapping from late-Quaternary pollen records. Quat Sci Rev. 30:1926–1939.
- Blonder B. 2016. Do hypervolumes have holes? Am Nat. 187(4):E93–E105.
- Blonder B. 2017. Hypervolume concepts in niche- and trait-based ecology. Ecography. 40:EV1–EV13.
- Blonder B, Babich Morrow C, Maitner B, Harris DJ, Lamanna C, Violle C, Enquist BJ, Kerkhoff AJ. 2017a. New approaches for delineating n-dimensional hypervolumes. Methods Ecol Evol. 9:305–319.
- Blonder B, Enquist BJ, Graae BJ, Kattge J, Maitner BS, Morueta-Holme N, Ordonez A, Šímová I, Singarayer J, Svenning J-C et al. 2018. Late Quaternary climate legacies in contemporary plant functional composition. Glob Chang Biol. 24(10):4827–4840.
- Blonder B, Lamanna C, Violle C, Enquist BJ. 2014. The n-dimensional hypervolume. Global Ecol Biogeogr. 23:595–605.
- Blonder B, Moulton DE, Blois JL, Enquist BJ, Graae BJ, Macias Fauria M, McGill B, Nogué S, Ordonez A, Sandel B et al. 2017b. Predictability in community dynamics. Ecol Lett. 20(3):293–306.
- Blytt A. 1881. Die Theorie der wechselnden kontinentalen und insularen Klimate. Englers Bot Jahrbüch 2:1–50.
- Bobek P, Svitavská Svobodová H, Werchan B, Švarcová MG, Kuneš P. 2018. Human-induced changes in fire regime and subsequent alteration of the sandstone landscape of Northern Bohemia (Czech Republic). Holocene. 28(3):427–443.
- Bocherens H. 2018. The rise of the Anthroposphere since 50,000 years: An ecological replacement of megaherbivores by humans in terrestrial ecosystems? Front Ecol Evol. 6:3.
- Bogotá-Angel RG, Groot MHM, Hooghiemstra H, Lourens L, van der Linden M, Berrío JC. 2011. Rapid climate change from north Andean Lake Fúquene pollen records driven by obliquity: implictions for a basin-wide biostratigraphic zonation for the last 284 ka. Quat Sci Rev. 30:3321–3337.
- Bohn U, Neuhäus R, Gollub G, Hettwer C, Neuhäuslová Z, Raus T, Schlütter H, Weber H, cartographers. 2000/2003. Karte der natürlichen Vegetation Europas/Map of the Natural Vegetation of Europe. Sclae 1:2,500,000. Münster: Bundesamt für Naturschutz.
- Bonan GB, Doney SC. 2018. Climate, ecosystems, and planetary futures: the challenge to predict life in Earth system models. Science. 359(533):e8328.
- Bond WJ, Keeley JE. 2005. Fire as a global ‘herbivore’: the ecology and evolution of flammable ecosystems. Trends Ecol Evol. 20(7):387–394.
- Bond WJ, Silander Jr JA, Ranaivonasy J, Ratsirarson J. 2008. The antiquity of Madagascar’s grasslands and the rise of C4 grassy biomes. J Biogeogr. 35(10):1743–1758.
- Bonuso N, Newton CR, Brower JC, Ivany LC. 2002. Statistical testing of community patterns: uppermost Hamilton Group, Middle Devonian (New York State, USA). Palaeogeogr Palaeoclimatol Palaeoecol. 185:1–24.
- Booth RK, Jackson ST, Gray CED. 2004. Paleoecology and high-resolution paleohydrology of a kettle peatland in upper Michigan. Quat Res. 61:1–13.
- Booth RK, Jackson ST, Sousa VA, Sullivan ME, Minckley TA, Clifford MJ. 2012. Multi-decadal drought and amplified moisture variability drove rapid forest community change in a humid region. Ecology. 93(2):219–226.
- Borregaard MK, Amorim IR, Borges PAV, Cabral JS, Fernández-Palacios JM, Field R, Heaney LR, Kreft H, Matthews TJ, Olesen JM et al. 2017. Oceanic island biogeography through the lens of the general dynamic model: assessment and prospect. Biol Rev. 92(2):830–853.
- Botkin DB, Saxe H, Araujo MB, Betts R, Bradshaw RHW, Cedhagen T, Chesson P, Dawson T, Etterson JR, Faith DP et al. 2007. Forecasting the effects of global warming on biodiversity. BioScience. 57:227–236.
- Bottema S. 1980. Palynological investigations on Crete. Rev Palaeobot Palynol. 31:193–217.
- Bottema S, Sarpaki A. 2003. Environmental change in Crete: a 9000-year record of Holocene vegetation history and the effect of the Santorini eruption. Holocene. 13(5):733–749.
- Boyd WE, Lentfer CJ, Parr J. 2005. Interactions between human activity, volcanic eruptions and vegetation during the Holocene at Garua and Numundo, West New Britain, PNG. Quat Res. 64(3):384–398.
- Boyer AG, Brenner M, Burney DA, Pandolfi JM, Savarese M, Dietl GP, Flessa KW. 2017. Chapter 15, Conservation paleobiology roundtable: from promise to application. In: Dietl GP, Flessa KW, editors. Conservation paleobiology science and practice. Chicago: University of Chicago Press; p. 291–302.
- Boyle JF. 2007. Loss of apatite caused irreversible early Holocene lake acidification. Holocene. 17:539–544.
- Brachi B, Villoutreix R, Faure N, Hautekèete N, Piquot Y, Pauwels M, Roby D, Cuguen J, Bergelson J, Roux F. 2013. Investigation of the geographical scale of adaptive phenological variation and its underlying genetics in Arabidopsis thaliana. Mol Ecol. 22(16):4222–4240.
- Bradley RS. 2015. Paleoclimatology. Reconstructing climates of the Quaternary. 3rd ed. Oxford: Academic Press.
- Bradshaw EG, Rasmussen P, Odgaard BV. 2005. Mid- to late-Holocene land-use change and lake development at Dallund Sø, Denmark: synthesis of multiproxy data, linking land and lake. Holocene. 15:1152–1162.
- Bradshaw RHW. 1981. Modern pollen-representation factors for woods in south-east England. J Ecol. 69(1):45–70.
- Bradshaw RHW. 1988. Spatially-precise studies of forest dynamics. In: Huntley B, Webb T, editors. Vegetation history. Dordrecht: Kluwer; p. 725–751.
- Bradshaw RHW. 1993a. Tree species dynamics and disturbance in 3 Swedish boreal forest stands during the last 2000 years. J Veg Sci. 4(6):759–764.
- Bradshaw RHW. 1993b. Forest response to Holocene climatic change: equilibrium or no-equilibrium? In: Chambers FM editor. Climate change and human impact on the landscape. London: Chapman & Hall; p. 57–65.
- Bradshaw RHW. 1994. Quaternary terrestrial sediments and spatial scale: the limits to interpretation. In: Traverse A, editor. Sedimentation of organic particles. Cambridge: Cambridge University Press; p. 239–252.
- Bradshaw RHW. 2013. Stand-scale palynology. In: Elias SA, Mock CJ, editors. Encyclopedia of Quaternary science. Amsterdam: Elsevier; p. 846–853.
- Bradshaw RHW, Hannon GE. 1992. Climatic change, human influence and disturbance regime in the control of vegetation dynamics within Fiby Forest, Sweden. J Ecol. 80(4):625–632.
- Bradshaw RHW, Hannon GE. 2004. The Holocene structure of north-west European temperate forest induced from palaeoecological data. In: Honnay O, Verheyen K, Bossuyt B et al., editors. Forest biodiversity: lessons from history for conservation. Wallingford: CABI; p. 11–25.
- Bradshaw RHW, Hannon GE. 2006. Long-term vegetation dynamics in southern Scandinavia and their use in managing landscapes for biodiversity. In: Agnoletti M, editor. The conservation of cultural landscapes. Wallingford: CABI; p. 94–107.
- Bradshaw RHW, Hannon GE, Lister AM. 2003. A long-term perspective on ungulate–vegetation interactions. For Ecol Manage. 181(1):267–280.
- Bradshaw RHW, Holmqvist BH, Cowling SA, Sykes MT. 2000. The effects of climate change on the distribution and management of Picea abies in southern Sweden. Can J Forest Res. 30:1993–1998.
- Bradshaw RHW, Jones CS, Edwards SJ, Hannon GE. 2015. Forest continuity and conservation value in western Europe. Holocene. 25(1):194–202.
- Bradshaw RHW, Josefsson T, Clear JL, Peterken GF. 2011. The structure and reproduction of the virgin forest: a review of Eustace Jones (1945). Scand J Forest Res. 26(S10):45–53.
- Bradshaw RHW, Kito N, Giesecke T. 2010. Factors influencing the Holocene history of Fagus. For Ecol Manage. 259(11):2204–2212.
- Bradshaw RHW, Lindbladh M. 2005. Regional spread and stand-scale establishment Fagus sylvatica and Picea abies in Scandinavia. Ecology. 86(7):1679–1686.
- Bradshaw RHW, Mitchell FJG. 1999. The palaeoecological approach to reconstructing former grazing-vegetation interactions. For Ecol Manage. 120(1–3):3–12.
- Bradshaw RHW, Sykes MT. 2014. Ecosystem dynamics. From the past to the future. Chichester: Wiley-Blackwell.
- Bradshaw RHW, Zackrisson O. 1990. A two thousand year history of a northern Swedish boreal forest stand. J Veg Sci. 1(4):519–528.
- Bradshaw WE, Holzapfel CM. 2006. Evolutionary response to rapid climate change. Science. 312:1477–1478.
- Bramwell D, editor. 1979. Plants and islands. London: Academic Press.
- Bramwell D, Bramwell Z. 1974. Wild flowers of the Canary Islands. Cheltenham: Stanley Thornes; p. 332.
- Bramwell D, Caujapé-Castells J. 2011. The biology of island floras. Cambridge: Cambridge University Press.
- Brede N, Sandrock C, Straile D, Spaak P, Jankowski T, Streit B, Schwenk K. 2009. The impact of human-made ecological changes on the genetic architecture of Daphnia species. Proc Natl Acad Sci USA. 106(12):4758–4763.
- Brewer S, Cheddadi R, de Beaulieu JL, Reille M, Data contributors. 2002. The spread of deciduous Quercus throughout Europe since the last glacial period. For Ecol Manage. 156:27–48.
- Brewer S, Giesecke T, Davis BAS, Finsinger W, Wolters S, Binney HA, de Beaulieu JL, Fyfe R, Gil-Romera G, Kühl N et al. 2017. Late-glacial and Holocene European pollen data. J Maps. 13(2):921–928.
- Brewer S, Hély-Alleaume C, Cheddadi R, de Beaulieu JL, Laurent J-M, le Cuziat J. 2006. Postglacial history of Atlantic oakwoods: context, dynamics and controlling factors. Bot J Scotl. 57:41–57.
- Brewer S, Jackson ST, Williams JW. 2012. Paleoecoinformatics: applying geohistorical data to ecological questions. Trends Ecol Evol. 27:104–112.
- Bridgewater P, Higgs ES, Hobbs RJ, Jackson ST. 2011. Engaging with novel ecosystems. Front Ecol Environ. 9:423.
- Brito-Morales I, García Molinos J, Schoeman DS, Burrows MT, Poloczanska ES, Brown CJ, Ferrier S, Harwood TD, Klein CJ, McDonald-Madden E et al. 2018. Climate velocity can inform conservation in a warming world. Trends Ecol Evol. 33(6):441–457.
- Britton AJ, Beale CM, Towers W, Hewison RJ. 2009. Biodiversity gains and losses: Evidence for homogenisation of Scottish alpine vegetation. Biol Conserv. 142:1728–1739.
- Britton AJ, Hester AJ, Hewison RL, Potts JM, Ross LC. 2017a. Climate, pollution and grazing drive long-term change in moorland habitats. Appl Veg Sci. 20(2):194–203.
- Britton AJ, Hewison RL, Mitchell RJ, Riach D. 2017b. Pollution and climate change drive long-term change in Scottish wetland vegetation composition. Biol Conserv. 210:72–79.
- Brncic TM, Willis KJ, Harris DJ, Washington R. 2007. Culture or climate? The relative influences of past processes on the composition of the lowland Congo rainforest. Philos Trans R Soc B Bio Sci. 362:229–242.
- Brock WA, Carpenter SR. 2010. Interacting regime shifts in ecosystems: implication for early warnings. Ecol Monogr. 80(3):353–367.
- Broennimann O, Treier UA, Müller-Schärer H, Thuiller W, Peterson AT, Guisan A. 2007. Evidence of climatic niche shift during biological invasion. Ecol Lett. 10(8):701–709.
- Brook EJ, Severinghaus JP. 2011. Methane and megafauna. Nat Geosci. 4:271–272.
- Brooker RW, Callaway RM, Cavieres LA, Kikvidze Z, Lortie CJ, Michalet R, Pugnaire FI, Valiente‐Banuet A, Whitham TG. 2009. Don’t diss integration: a comment on Ricklefs’s disintegrating communities. Am Nat. 174(6):919–927.
- Brown CD, Vellend M. 2014. Non-climatic constraints on upper elevational plant range expansion under climate change. Proc R Soc B Bio Sci. 281(1794):20141779.
- Brown KJ, Clark JS, Grimm EC, Donovan JJ, Mueller PG, Hansen BCS, Stefanova I. 2005. Fire cycles in North American interior grasslands and their relation to prairie drought. Proc Natl Acad Sci USA. 102(25):8865–8870.
- Brown KJ, Giesecke T. 2014. Holocene fire disturbance in the boreal forest of central Sweden. Boreas. 43(3):639–651.
- Brown SK, Blois JL. 2016. Ecological insights from ancient DNA. Encyclopedia of Life Sciences (eLS). Chichester: John Wiley & Sons.
- Browne Ribeiro AT. 2017. Rice cultivation in ancient Amazonia. Nat Ecol Evol. 1:1598–1599.
- Brugger SO, Gobet E, Blunier T, Morales-Molino C, Lotter AF, Fischer H, Schwikowski M, Tinner W. 2019. Palynological insights into global change impacts on Arctic vegetation, fire, and pollution recorded in Central Greenland ice. Holocene. doi:10.1177/0959683619838039.
- Brugger SO, Gobet E, Sigl M, Osmont D, Papina T, Rudaya N, Schwikowski M, Tinner W. 2018. Ice records provide new insights into climatic vulnerability of Central Asian forest and steppe communities. Glob Planet Change. 169:188–201.
- Brummitt NA, Buchman S, editors. 2010. Plants under pressure - a global assessment. The first report of the sampled red list index for plants. Kew: Royal Botanic Gardens.
- Brussel T, Minckley TA, Brewer SC, Long CJ. 2018. Community-level functional interactions with fire track long-term structural development and fire adaptation. J Veg Sci. 29(3):450–458.
- Buckley LB. 2013. Get real: putting models of climate change and species interactions in practice. Ann N Y Acad Sci. 1297(1):126–138.
- Bunting L, Leavitt PR, Simpson GL, Wissel B, Laird KR, Cumming BF, St. Amand A, Engstrom DR. 2016. Increased variability and sudden ecosystem state change in Lake Winnipeg, Canada, caused by 20th century agriculture. Limnol Oceanogr. 61(6):2090–2107.
- Bunting MJ, Farrell M, Bayliss A, Marshall P, Whittle A. 2018. Maps from mud—using the Multiple Scenario Approach to reconstruct land cover dynamics from pollen records: A case study of two Neolithic landscapes. Front Ecol Evol. 6:36.
- Bunting MJ, Gaillard M-J, Sugita S, Middleton R, Brostrom A. 2004. Vegetation structure and pollen source area. Holocene. 14:651–660.
- Bunting MJ, Middleton R. 2009. Equifinality and uncertainty in the interpretation of pollen data: the multiple scenario approach to reconstruction of past vegetation mosaics. Holocene. 19:799–803.
- Burke MJW, Grime JP. 1996. An experimental study of plant community invasibility. Ecology. 77(3):776–790.
- Burney DA. 1987a. Late Holocene vegetational change in central Madagascar. Quat Res. 28:130–143.
- Burney DA. 1987b. Late Quaternary stratigraphic charcoal records from Madagascar. Quat Res. 28:274–280.
- Burney DA. 1993. Late Holocene environmental change in arid southwestern Madagascar. Quat Res. 40:98–106.
- Burney DA. 1997. Tropical islands as paleoecological laboratories: gauging the consequences of human arrival. Hum Ecol. 25(3):437–457.
- Burney DA. 1999. Rates, patterns, and processes of landscape transformation and extinction in Madagascar. In: MacPhee RDE, editor. Extinctions in near time: causes, contexts, and consequences. New York: Kluwer/Plenum; p. 145–164.
- Burney DA. 2010. Back to the future in the Caves of Kaua’i: a scientist’s adventures in the dark. New Haven: Yale University Press.
- Burney DA, Burney LP. 1994. Holocene charcoal stratigraphy from Laguna Tortuguero, Puerto Rico, and the timing of human arrival on the island. J Archaeol Sci. 21:273–281.
- Burney DA, Burney LP. 2007. Paleoecology and “inter-situ” restoration on Kaua’i, Hawaii. Front Ecol Environ. 5(9):483–490.
- Burney DA, DeCandido RV, Burney LP, Kostel-Hughes FN, Stafford TW, James HF. 1995. A Holocene record of climate change, fire ecology and human activity from montaine Flat Top Bog, Maui. J Paleolimnol. 13:209–217.
- Burney DA, Flannery TF. 2005. Fifty millennia of catastrophic extinctions after human contact. Trends Ecol Evol. 20(7):395–401.
- Burney DA, James HF, Burney LP, Olson SL, Kikuchi WKP, Wagner WL, Burney M, McCloskey D, Kikuchi D, Grady FV et al. 2001. Fossil evidence for a diverse biota from Kaua’i and its transformation since human arrival. Ecol Monogr. 71(4):615–641.
- Burney DA, Kikuchi WKP. 2006. A millennium of human activity at Makauwahi Cave, Maha’ulepu, Kaua’i. Hum Ecol. 34(2):219–247.
- Burney DA, Robinson GS, Burney LP. 2003. Sporormiella and the late Holocene extinctions in Madagascar. Proc Natl Acad Sci USA. 100(19):10800–10805.
- Burney DA, Steadman DW, Martin PS. 2002. Evolution’s second chance. Wild Earth. Summer:12–15.
- Burrows MT, Schoeman DS, Buckley LB, Moore PD, Poloczanska ES, Brander KM, Brown C, Bruno JF, Duarte CM, Halpern BS et al. 2011. The pace of shifting climate in marine and terrestrial ecosystems. Science. 334(6056):652–655.
- Burrows MT, Schoeman DS, Richardson AJ, Molinos JG, Hoffmann A, Buckley LB, Moore PJ, Brown CJ, Bruno JF, Duarte CM et al. 2014. Geographical limits to species-range shifts are suggested by climate velocity. Nature. 507:492–495.
- Burthe SJ, Henrys PA, Mackay EB, Spears BM, Campbell R, Carvalho L, Dudley B, Gunn IDM, Johns DG, Maberly SC et al. 2016. Do early warning indicators consistently predict nonlinear change in long-term ecological data? J Appl Ecol. 53(3):666–676.
- Bush MB. 2002. Distributional change and conservation on the Andean flank: a palaeoecological perspective. Global Ecol Biogeogr. 11(6):463–473.
- Bush MB. 2017. News & Views: The resilience of Amazonian forests. Nature. 541:167–168.
- Bush MB, Colinvaux PA. 1988. A 7000-year pollen record from the Amazon lowland, Ecuador. Vegetatio. 76:141–154.
- Bush MB, Colinvaux PA. 1990. A pollen record of a complete glacial cycle from lowland Panama. J Veg Sci. 1:105–118.
- Bush MB, Colinvaux PA. 1994. Tropical forest disturbance: paleoecological records from Darien, Panama. Ecology 75(6):1761–1768.
- Bush MB, Correa-Metrio A, McMichael CH, Sully S, Shadik CR, Valencia BG, Guilderson T, Steinitz-Kannan M, Overpeck JT. 2016. A 6900-year history of landscape modification by humans in lowland Amazonia. Quat Sci Rev. 141:52–64.
- Bush MB, Correa-Metrio A, van Woesik R, Shadik CR, McMichael CNH. 2017. Human disturbance amplifies Amazonian El Niño–Southern Oscillation signal. Glob Chang Biol. 23(8):3181–3192.
- Bush MB, de Oliveira PE. 2006. The rise and fall of the Refugial Hypothesis of Amazonian Speciation: a paleoecological perspective. Biota Neotrop. 6(1):00106012006.
- Bush MB, de Oliveira PE, Colinvaux PA, Miller MC, Moreno JE. 2004. Amazonian paleoecological histories: one hill, three watersheds. Palaeogeogr Palaeoclimatol Palaeoecol. 214(4):359–393.
- Bush MB, Flenley JR, Gosling WD, editors. 2011. Tropical rainforest responses to climatic change. 2nd ed. Berlin: Springer.
- Bush MB, Gosling WD. 2018. In search of the ice age tropics, a tribute to Prof. Daniel Livingstone and Prof. Paul Colinvaux. Quat Res. 89(1):1–6.
- Bush MB, Hall AR. 1987. Flandrian Alnus: expansion or immigration? J Biogeogr. 14:479–481.
- Bush MB, McMichael CNH. 2016. Holocene variability of an Amazonian hyperdominant. J Ecol. 104(5):1370–1378.
- Bush MB, McMichael CNH, Piperno DR, Silman MR, Barlow J, Peres CA, Power M, Palace MW. 2015b. Anthropogenic influence on Amazonian forests in pre-history: An ecological perspective. J Biogeogr. 42:2277–2288.
- Bush MB, Piperno DR, Colinvaux PA. 1989. A 6000 year history of Amazonian maize cultivation. Nature. 340:303–305.
- Bush MB, Piperno DR, Colinvaux PA, de Oliveira PE, Krissek LA, Miller MC, Rowe WE. 1992. A 14300-yr paleoecological profile of a lowland tropical lake in Panama. Ecol Monogr. 62(2):251–275.
- Bush MB, Restrepo A, Collins AF. 2014. Galápagos history, restoration, and a shifted baseline. Restor Ecol. 22(3):296–298.
- Bush MB, Silman MR, de Toldeo MB, Listopad CMCS, Gosling WD, Williams CB, de Oliveira PE, Krisel C. 2007b. Holocene fire and occupation in Amazonia: records from two lake districts. Philos Trans R Soc B Bio Sci. 362:209–218.
- Bush MB, Silman MR, Listopad CMCS. 2007a. A regional study of Holocene climate change and human occupation in Peruvian Amazonia. J Biogeogr. 34:1342–1356.
- Bush MB, Sublette Mosblech NA, Church W. 2015a. Climate change and the agricultural history of a mid-elevation Andean montane forest. Holocene. 25(9):1522–1532.
- Caballero-Rodríguez D, Lozano-García S, Correa-Metrio A. 2017. Vegetation assemblages of central Mexico through the late Quaternary: modern analogs and compositional turnover. J Veg Sci. 28(3):504–514.
- Cain ML, Damman H, Muir A. 1998. Seed dispersal and the Holocene migration of woodland herbs. Ecol Monogr. 68(3):325–347.
- Cain ML, Milligan BG, Strand AE. 2000. Long-distance seed dispersal in plant populations. Am J Bot. 87(9):1217–1227.
- Calcote RR. 2003. Mid-Holocene climate and the hemlock decline: the range limit of Tsuga canadensis in the western Great Lakes region, USA. Holocene. 13:215–224.
- Calder WJ, Parker D, Stopka CJ, Jiménez-Moreno G, Shuman BN. 2015. Medieval warming initiated exceptionally large wildfire outbreaks in the Rocky Mountains. Proc Natl Acad Sci USA. 112(43):13261.
- Calder WJ, Shuman BN. 2017. Extensive wildfires, climate change, and an abrupt state change in subalpine ribbon forests, Colorado. Ecology 98(10):2585–2600.
- Calder WJ, Shuman BN. 2019. Detecting past changes in vegetation resilience in the context of a changing climate. Biol Lett. 15(3):20180768.
- Calder WJ, Stefanova I, Shuman B. 2019. Climate–fire–vegetation interactions and the rise of novel landscape patterns in subalpine ecosystems, Colorado. J Ecol.10.1111/1365-2745.13138.
- Calleja JA, Benito Garzón M, Sainz Ollero H. 2009. A Quaternary perspective on the conservation prospects of the Tertiary relict tree Prunus lusitanica L. J Biogeogr. 36(3):487–498.
- Callow P, editor 1998. The encyclopedia of ecology and environmental management. Oxford: Blackwell Science.
- Camill P, Umbanhowar CE, Teed R, Geiss CE, Aldinger J, Dvorak L, Kenning J, Limmer J, Walkup K. 2003. Late-glacial and Holocene climatic effects on fire and vegetation dynamics at the prarie–forest ecotone in south-central Minnesota. J Ecol. 91:822–836.
- Cañellas-Boltà N, Rull V, Sáez A, Margalef O, Pla-Rabes S, Valero-Garcés B, Giralt S. 2016. Vegetation dynamics at Raraku Lake catchment (Easter Island) during the past 34,000 years. Palaeogeogr Palaeoclimatol Palaeoecol. 446:55–69.
- Cañellas-Boltà N, Rull V, Sáez A, Prebble M, Margalef O. 2014. First records and potential palaeoecological significance of Dianella (Xanthorrhoeaceae), an extinct representative of the native flora of Rapa Nui (Easter Island). Veg Hist Archaeobot. 23(3):331–338.
- Carcaillet C, Blarquez O. 2017. Fire ecology of a tree glacial refugium on a nunatak with a view on Alpine glaciers. New Phytol. 216(4):1281–1290.
- Carcaillet C, Latil J-L, Abou S, Ali AA, Ghaleb B, Magnin F, Roiron P, Aubert S. 2018. Keep your feet warm? A cryptic refugium of trees linked to a geothermal spring in an ocean of glaciers. Glob Chang Biol. 24(6):2476–2487.
- Carey J. 2017. Putting fossils to work in hopes of restoration. Proc Natl Acad Sci USA. 114(33):8663–8666.
- Carlquist S. 1992. Hawaii. A natural history: geology, climate, native flora and fauna above the Shoreline. Lawai, Kauai: National Tropical Botanical Garden.
- Carlton JT. 1996. Biological invasions and cryptogenic species. Ecology. 77:1653–1655.
- Carrión JS. 2002. Patterns and processes of late Quaternary environmental change in a montane region of southwestern Europe. Quat Sci Rev. 21:2047–2066.
- Carrión JS. 2010. The concepts of potential natural vegetation (PNV) and other abstractions (trying to pick up fish with wet hands). J Biogeogr. 37(11):2213–2215.
- Carrión JS, Andrade A, Bennett KD, Navarro C, Mumuera M. 2001a. Crossing forest thresholds: inertia and collapse in a Holocene sequence from south-central Spain. Holocene. 11(6):635–653.
- Carrión JS, Fernández S. 2009. The survival of the ‘natural potential vegetation’ concept (or the power of tradition). J Biogeogr. 36(12):2202–2203.
- Carrión JS, Munuera M, Dupré M, Andrade A. 2001b. Abrupt vegetation changes in the Segura Mountains of southern Spain throughout the Holocene. J Ecol. 89:783–797.
- Carrión JS, Navarro C, Navarro J, Munuera M. 2000. The distribution of cluster pine (Pinus pinaster) in Spain as derived from palaeoecological data: relationships with phytosociological classification. Holocene. 10(2):243–252.
- Carroll FA, Hunt CO, Schembri PJ, Bonanno A. 2012. Holocene climate change, vegetation history and human impact in the Central Mediterranean: evidence from the Maltese Islands. Quat Sci Rev. 52:24–40.
- Carstensen J, Telford RJ, Birks HJB. 2013. Brief communications arising from ‘Diatom flickering prior to regime shift’. Nature. 498:E11.
- Carstensen J, Weydmann A. 2012. Tipping points in the arctic: eyeballing or statistical significance? Ambio. 41(1):34–43.
- Carter RN, Prince SD. 1981. Epidemic models used to explain biogeographical distribution limits. Nature. 293:644–645.
- Carter VA, Chiverrell RC, Clear JL, Kuosmanen N, Moravcová A, Svoboda M, Svobodová-Svitavská H, van Leeuwen JN, van der Knaap WO, Kuneš P. 2018b. Quantitative palynology informing conservation ecology in the Bohemian/Bavarian forests of central Europe. Front Plant Sci. 8(2268):a2268.
- Carter VA, Moravcová A, Chiverrell RC, Clear JL, Finsinger W, Dreslerová D, Halsall K, Kuneš P. 2018a. Holocene-scale fire dynamics of central European temperate spruce-beech forests. Quat Sci Rev. 191:15–30.
- Caseldine CJ, Geirsdóttir Á, Langdon PG. 2003. Efstadalsvatn - a multi-proxy study of a Holocene lacustrine sequence from NW Iceland. J Paleolimnol. 30:55–73.
- Castilla-Beltrán A, de Nascimento L, Fernández-Palacios JM, Fonville T, Whittaker RJ, Edwards ME, Nogué S. 2019. Late Holocene environmental change and the anthropization of the highlands of Santo Antão Island, Cabo Verde. Palaeogeogr Palaeoclimatol Palaeoecol. 524:101–117.
- Castilla-Beltrán A, Hooghiemstra H, Hoogland MLP, Pagán-Jiménez J, van Geel B, Field MH, Prins M, Donders T, Herrera Malatesta E, Ulloa Hung J et al. 2018. Columbus’ footprint in Hispaniola: A paleoenvironmental record of indigenous and colonial impacts on the landscape of the central Cibao Valley, northern Dominican Republic. Anthropocene. 22:66–80.
- Castiñeira Latorre C, Apolinaire E, Blasi AM, Bonomo M, Politis G, Bastourre L, Mari M. 2017. Pre-Hispanic settlements in hydrometeorologically susceptible areas during the late Holocene: The Upper Delta of the Paraná River Case. Holocene. 27(12):1801–1811.
- Catalan J, Ninot JM, Aniz MM, editors. 2017. High mountain conservation in a changing world. Cham: Springer Nature.
- Catullo RA, Ferrier S, Hoffmann AA. 2015. Extending spatial modelling of climate change responses beyond the realized niche: estimating, and accommodating, physiological limits and adaptive evolution. Global Ecol Biogeogr. 24:1192–1202.
- Cázares E, Trappe JM, Jumpponen A. 2005. Mycorrhiza-plant colonization patterns on a subalpine glacier foreland as a model system of primary succession. Mycorrhiza. 15:405–416.
- Chadwick OA, Kelly EF, Hotchkiss SC, Vitousek PM. 2007. Precontact vegetation and soil nutrient status in the shadow of Kohala Volcano, Hawaii. Geomorphology. 89:70–83.
- Chalk TB, Capron E, Drew M, Panagiotopoulos K. 2017. Interglacials of the 41 ka-world and the Mid-Pleistocene Transition. PAGES Mag. 25(3):155.
- Chamberlin TC. 1890. The method of multiple working hypotheses. Science. 15:92–96.
- Chambers FM, Cloutman EW, Daniell JRG, Mauquoy D, Jones PS. 2013. Long-term ecological study (palaeoecology) to chronicle habitat degradation and inform conservation ecology: an exemplar from the Brecon Beacons, South Wales. Biodivers Conserv. 22:719–736.
- Chambers FM, Daniell JRG. 2011. Conservation and habitat restoration of moorland and bog in the UK uplands: A regional, palaeoecological perspective. Pages News. 19(2):45–47.
- Chambers FM, Mauquoy D, Cloutman EW, Daniell JRG, Jones PS. 2007a. Recent vegetation history of Drygarn Fawr (Elenydd SSSI), Cambrian Mountains, Wales: implications for conservation management of degraded blanket mires. Biodivers Conserv. 16(10):2821–2846.
- Chambers FM, Mauquoy D, Gent A, Pearson F, Daniell JRG, Jones PS. 2007b. Palaeoecology of degraded blanket mire in South Wales: Data to inform conservation management. Biol Conserv. 137(2):197–209.
- Chambers FM, Mauquoy D, Todd PA. 1999. Recent rise to dominance of Molinia caerulea in environmentally sensitive areas: new perspectives from palaeoecological data. J Appl Ecol. 36:719–733.
- Chandler MEJ. 1923. The geological history of the genus Stratiotes: An account of the evolutionary changes which have occurred within the genus during Tertiary and Quaternary times. Q J Geol Soc London. 79:117–138.
- Chape S, Harrison J, Spalding M, Lysenko I. 2005. Measuring the extent and effectiveness of protected areas as an indicator for meeting global biodiversity targets. Philos Trans R Soc B Bio Sci. 360(1454):443–455.
- Chapin FS. 2017. Now is the time for translational ecology. Front Ecol Environ. 15:539.
- Chapin FS, Shaver GR. 1985. Individualistic growth response of tundra plant species to environmental manipulations in the field. Ecology 66(2):564–576.
- Chase JM. 2010. Stochastic community assembly causes higher biodiversity in more productive environments. Science. 328(5984):1388–1391.
- Chase JM, Myers JA. 2011. Disentangling the importance of ecological niches from stochastic processes across scales. Philos Trans R Soc B Bio Sci. 366(1576):2351–2363.
- Cheddadi R, Birks HJB, Tarroso P, Liepelt S, Gömöry D, Dullinger S, Meier ES, Hülber K, Maiorano L, Laborde H. 2014. Revisiting tree-migration rates: Abies alba (Mill.), a case study. Veg Hist Archaeobot. 23(2):113–122.
- Cheddadi R, de Beaulieu J-L, Jouzel J, Andrieu-Ponel V, Laurent J-M, Reille M, Raynaud D, Bar-Hen A. 2005. Similarity of vegetation dynamics during interglacial periods. Proc Natl Acad Sci USA. 102(39):13939–13943.
- Cheddadi R, Henrot A-J, François L, Boyer F, Bush MB, Carré M, Coissac E, de Oliveira PE, Ficetola F, Hambuckers A et al. 2017. Microrefugia, climate change, and conservation of Cedrus atlantica in the Rif Mountains, Morocco. Front Ecol Evol. 5:114.
- Cheddadi R, Vendramin GG, Litt T, Francois L, Kageyama M, Lorentz S, Laurent J-M, de Beaulieu JL, Sadori L, Jost A et al. 2006. Imprints of glacial refugia in the modern genetic diversity of Pinus sylvestris. Global Ecol Biogeogr. 15:271–282.
- Cheke AS, Hume JP. 2008. Lost land of the Dodo: The ecological history of Mauritius, Réunion, and Rodrigues. New Haven: Yale University Press.
- Chen Y. 1988. Early Holocene population expansion of some rainforest trees in the Lake Barrine Basin. Aust J Ecol. 13:225–233.
- Chepstow-Lusty A, Bush MB, Frogley MR, Baker PA, Fritz SC, Aronson J. 2005. Vegetation and climate change on the Bolivian Altiplano between 108,000 and 18,000 yr ago. Quat Res. 63(1):90–98.
- Chiarucci A, Araújo MB, Decocq G, Beierkuhnlein C, Fernández-Palacios JM. 2010. The concept of potential natural vegetation: an epitaph? J Veg Sci. 21(6):1172–1178.
- Chu C-J, Wang Y-S, Du G-Z, Maestre FT, Luo Y-J, Wang G. 2007. On the balance between niche and neutral processes as drivers of community structure along a successional gradient: Insights from alpine and sub-alpine meadow communities. Ann Bot. 100(4):807–812.
- Clapperton CM, Sugden DE, Birnie J, Wilson MJ. 1989. Late-glacial and Holocene glacier fluctuations and environmental change on South Georgia, Southern Ocean. Quat Res. 31:210–228.
- Clark JS. 1998. Why trees migrate so fast: confronting theory with dispersal biology and the paleorecord. Am Nat. 152:204–224.
- Clark JS. 2007. Models for ecological data - an introduction. Princeton: Princeton University Press.
- Clark JS, Fastie C, Hurtt G, Jackson ST, Johnson C, King GA, Lewis M, Lynch J, Pacala S, Prentice IC et al. 1998. Reid’s paradox of rapid plant migration. BioScience. 48:13–24.
- Clark JS, Grimm EC, Donovan JJ, Fritz SC, Engstrom DR, Almendinger JE. 2002. Drought cycles and landscape responses to past aridity on prairies of the northern Great Plains, USA. Ecology 83(3):595–601.
- Clark JS, Grimm EC, Lynch J, Mueller PG. 2001b. Effects of Holocene climate change on the C4 grassland/woodland boundary in the Northern Plains, USA. Ecology 82(3):620–636.
- Clark JS, Lewis M, Horvath L. 2001a. Dispersal and population spread. Am Nat. 157:537–554.
- Clark JS, Lewis M, McLachlan JS, Hille Ris Lambers J. 2003. Estimating population spread: what can we forecast and how well? Ecology 84(8):1979–1988.
- Clark JS, McLachlan JS. 2003. Stability of forest biodiversity. Nature. 423(6940):635–638.
- Clark JS, McLachlan JS. 2004. Neutral theory (communications arising): The stability of forest biodiversity. Nature. 427:696–697.
- Claussen M. 1998. On multiple solutions of the atmosphere–vegetation system in present‐day climate. Glob Chang Biol. 4(5):549–559.
- Clements FE. 1904. The development and structure of vegetation. Lincoln, Nebraska: The Botanical Survey of Nebraska.
- Clements FE. 1905. Research methods in ecology. Lincoln, Nebraska: University Publishing Co.
- Clements FE. 1916. Plant succession, an analysis of the development of vegetation. Washington: Carnegie Institution of Washington.
- Clements FE. 1936. Nature and structure of the climax. J Ecol. 24:252–284.
- Clifford MJ, Booth RK. 2015. Late-Holocene drought and fire drove a widespread change in forest community composition in eastern North America. Holocene. 25(7):1102–1110.
- Clobert J, Baguette M, Benton TG, Bullock JM, editors. 2012. Dispersal ecology and evolution. Oxford: Oxford University Press.
- Coetzee JA. 1967. Pollen analytical studies in east and southern Africa. In: van Zinderen Bakker EM, editor. Palaeoecology of Africa and of the surrounding islands & antarctic. Cape Town: AA Balkema; p. 1–146.
- Coffey EED, Froyd CA, Willis KJ. 2011. When is an invasive not an invasive? Macrofossil evidence of doubtful native plant species in the Galápagos Islands. Ecology 92(4):805–812.
- Coffey EED, Froyd CA, Willis KJ. 2012. Lake or bog? Reconstructing basline ecological conditions for the protected Galápagos Sphagnum peatbogs. Quat Sci Rev. 52:60–74.
- COHMAP Members. 1988. Climatic changes of the last 18,000 years: observations and model simulations. Science. 241:1043–1052.
- Cole A, Flenley J. 2008. Modelling human population change on Easter Island far-from-equilibrium. Quatern Int. 184:150–165.
- Cole KL. 1985. Past rates of change, species richness, and a model of vegetational inertia in the Grand Canyon, Arizona. Am Nat. 125(2):289–303.
- Cole KL. 1986. In defense of inertia. Am Nat. 127(5):727–728.
- Cole KL. 2010. Vegetation response to Early Holocene warming as an analog for current and future changes. Conserv Bio. 24(1):29–37.
- Cole KL, Ironside K, Eischeid J, Garfin G, Duffy PB, Toney C. 2011. Past and ongoing shifts in Joshua tree distribution support future modeled range contraction. Ecol App. 21(1):137–149.
- Cole LES, Bhagwat SA, Willis KJ. 2014. Recovery and resilience of tropical forests after disturbance. Nat Commun. 5:3906.
- Cole LES, Bhagwat SA, Willis KJ. 2015. Long-term disturbance dynamics and resilience of tropical peat swamp forests. J Ecol. 103:16–30.
- Coleman JS, McConnaughay KDM, Ackerly DD. 1994. Interpreting phenotypic variation in plants. Trends Ecol Evol. 9(5):187–191.
- Colinvaux PA. 2007. Amazon expeditions: My quest for the ice-age equator. New Haven: Yale University Press.
- Colinvaux PA, de Oliveira PE, Bush MB. 2000. Amazonian and neotropical plant communities on glacial time-scales: The failure of the aridity and refuge hypotheses. Quat Sci Rev. 19:141–169.
- Colinvaux PA, de Oliveira PE, Moreno JE, Miller MC, Bush MB. 1996. A long pollen record from lowland Amazonia: forest and cooling in glacial times. Science. 274:85–88.
- Colinvaux PA, Irion G, Räsänen ME, Bush MB, Nunes de Mello JAS. 2001. A paradigm to be discarded: geological and paleoecological data falsify the Haffer & Prance refuge hypothesis of Amazonian speciation. Amazoniana. 16(3/4):609–646.
- Colinvaux PA, Schofield EK. 1976. Historical ecology in the Galápagos Islands. I. A Holocene pollen record from El Junco Lake, Isla San Cristobel. J Ecol. 64:989–1012.
- Collier MJ, Devitt C. 2016. Novel ecosystems: challenges and opportunities for the Anthropocene. Anthropocene Rev. 3(3):231–424.
- Collignon AM, Favre JM. 2000. Contribution of the postglacial history at the western margin of Picea abies’ natural area using RAPD markers. Ann Bot. 85:713–722.
- Collins AF, Bush MB, Sachs JP. 2013. Microrefugia and species persistence in the Galápagos highlands: a 26,000-year paleoecological perspective. Front Genet. 4:269.
- Colloff MJ, Lavorel S, van Kerkhoff LE, Wyborn C, Fazey I, Gorddard R, Mace GM, Foden WB, Dunlop M, Prentice IC et al. 2017. Transforming conservation science and practice for a postnormal world. Conserv Bio. 31(5):1008–1017.
- Colombaroli D, Beckmann M, van der Knaap WO, Curdy P, Tinner W. 2013. Changes in biodiversity and vegetation composition in the central Swiss Alps during the transition from pristine forest to first farming. Divers Distrib 19:157–170.
- Colombaroli D, Marchetto A, Tinner W. 2007. Long-term interactions between climate, Mediterranean vegetation and the fire regime at Lago di Massaciuccoli (Tuscany, Italy). J Ecol. 95:755–770.
- Colombaroli D, Tinner W. 2013. Determining the long-term changes in biodiversity and provisioning services along a transect from central Europe to the Mediterranean. Holocene. 23:1625–1634.
- Colombaroli D, Tinner W, van Leeuwen JFN, Noti R, Vescovi E, Vannière B, Magny M, Schmidt R, Bugmann H. 2009. Response of broadleaved evergreen Mediterranean forest vegetation to fire disturbance during the Holocene: insights from the peri‐Adriatic region. J Biogeogr. 36(2):314–326.
- Colombaroli D, Whitlock C, Tinner W, Conedera M. 2017. Paleo records as a guide for ecosystem management and biodiversity conservation. PAGES Mag. 25(2):77–79.
- Colwell RK, Rangel TF. 2009. Hutchinson’s duality: the once and future niche. Proc Natl Acad Sci USA. 106:19651–19658.
- Comandini O, Rinaldi AC. 2004. Tracing megafaunal extinctins with dung fungal spores. Mycologist. 18(4):140–142.
- Combourieu-Nebout N, Bertini A, Russo-Ermolli E, Peyron O, Klotz S, Montade V, Fauquette S, Allen J, Fusco F, Goring SJ et al. 2015. Climate changes in the central Mediterranean and Italian vegetation dynamics since the Pliocene. Rev Palaeobot Palynol. 218:127–147.
- Conedera M, Tinner W, Crameri S, Torriami D, Herold A. 2006. Taxon-related pollen source areas for lake basins in the southern Alps: an empirical approach. Veg Hist Archaeobot. 15(4):263–272.
- Conedera M, Tinner W, Neff C, Meurer M, Dickens AF, Krebs P. 2009. Reconstructing past fire regimes: methods, applications, and relevance to fire management and conservation. Quat Sci Rev. 28:555–576.
- Connell JH. 1978. Diversity in tropical rain forests and coral reefs. Science. 199:1301–1310.
- Connell JH, Tracey JG, Webb LJ. 1984. Compensatory recruitment, growth, and mortality as factors maintaining rain forest tree diversity. Ecol Monogr. 54(2):141–164.
- Connor SE, Colombaroli D, Confortini F, Gobet E, Ilyashuk BP, Ilyashuk EA, van Leeuwen JFN, Lamentowicz M, van der Knaap WO, Malysheva E et al. 2018a. Long‐term population dynamics: theory and reality in a peatland ecosystem. J Ecol. 106(1):333–346.
- Connor SE, Schneider L, Trezise J, Rule S, Barrett RL, Zawadzki A, Haberle SG. 2018b. Forgotten impacts of European land-use on riparian and savanna vegetation in northwest Australia. J Veg Sci. 29(3):427–437.
- Connor SE, van der Knaap WO, van Leeuwen JFN, Kuneš P. 2013. Holocene palaeoclimate and palaeovegetation of the islands of Flores and Pico. In: Fernández Palacios JM, de Nascimento L, Hernández JC et al., editors, pp. 149–162. Climate change perspectives from the Atlantic: Past, present, and future. San Cristóbal de La Laguna: Servicio de Publicaciones de la Universidad de La Laguna.
- Connor SE, van Leeuwen JFN, Rittenour TM, van der Knaap WO, Ammann B, Björck S. 2012. The ecological impact of oceanic island colonization - a palaeoecological perspective from the Azores. J Biogeogr. 39:1007–1023.
- Contamin R, Ellison AM. 2009. Indicators of regime shifts in ecological systems: what do we need to know and when do we need to know it? Ecol App. 19(3):799–816.
- Cook ER, Anchukaitis KJ, Buckley BM, D’Arrigo RD, Jacoby GC, Wright WE. 2010a. Asian monsoon failure and megadrought during the last millennium. Science. 328(5977):486–489.
- Cook ER, Seager R, Heim RR, Vose RS, Herweijer C, Woodhouse C. 2010b. Megadroughts in North America: placing IPCC projections of hydroclimatic change in a long‐term palaeoclimate context. J Quat Sci. 25(1):48–61.
- Cooper A, Turney CSM, Hughen KA, Brook BW, McDonald HG, Bradshaw CJA. 2015. Abrupt warming events drove Late Pleistocene Holarctic megafaunal turnover. Science. 349(6248):602–606.
- Corlett RT. 2014. New approaches to novel ecosystems. Trends Ecol Evol. 29(3):137–138.
- Corlett RT. 2015. The Anthropocene concept in ecology and conservation. Trends Ecol Evol. 30(1):36–41.
- Corlett RT, Primack RB. 2011. Tropical rain forests - an ecological and biogeographical comparison. Chichester: Wiley-Blackwell.
- Corlett RT, Westcott DA. 2013. Will plant movements keep up with climate change? Trends Ecol Evol. 28(8):482–488.
- Corner EJH. 1946. Suggestions for botanical progress. New Phytol. 45:185–192.
- Correa-Metrio A, Bush MB, Cabrera KR, Sully S, Brenner M, Hodell DA, Escobar J, Guilderson TP. 2012a. Rapid climate change and no-analog vegetation in lowland Central America during the last 86,000 years. Quat Sci Rev. 38:63–75.
- Correa-Metrio A, Bush MB, Hodell DA, Brenner M, Escobar J, Guilderson TJ. 2012b. The influence of abrupt climate change on the ice-age vegetation of the Central American lowlands. J Biogeogr. 39(3):497–509.
- Correa-Metrio A, Bush MB, Lozano-García S, Sosa-Nájera S. 2013. Millennial-scale temperature change velocity in the continental northern neotropics. PLoS One. 8(12):e81958.
- Correa-Metrio A, Meave JA, Lozano-García S, Bush MB. 2014. Environmental determinism and neutrality in vegetation at millennial time scales. J Veg Sci. 25(3):627–635.
- Cossart E, Fort M, Bourlès D, Braucher R, Perrier R, Siame L. 2012. Deglaciation pattern during the Lateglacial/Holocene transition in the southern French Alps. Chronological data and geographical reconstruction from the Clarée Valley (upper Durance catchment, southeastern France). Palaeogeogr Palaeoclimatol Palaeoecol. 315 (SupplementC):109–123.
- Costanza R, d’Arge R, de Groot R, Farber S, Grasso M, Hannon B, Limburg K, Naeem S, O’Neill RV, Paruelo J et al. 1997. The value of the world’s ecosystem services and natural capital. Nature. 387(6630):253–260.
- Costanza R, de Groot R, Braat L, Kubiszewski I, Fioramonti L, Sutton P, Farber S, Grasso M. 2017. Twenty years of ecosystem services: How far have we come and how far do we still need to go? Ecosyst Serv. 28(Part A):1–16.
- Costanza R, Graumlich LJ, Steffen W, editors. 2007. Sustainability or collapse? An integrated history and future of people on earth. Cambridge, Massachusetts: MIT Press.
- Côté IM, Darling ES. 2010. Rethinking ecosystem resilience in the face of climate change. PLoS Biol. 8(7):e1000438.
- Coutts SR, Helmstedt KJ, Bennett JR. 2018. Invasion lags: The stories we tell ourselves and our inability to infer process from pattern. Divers Distrib. 24(2):244–251.
- Cowles HC. 1911. The causes of vegetative cycles. Bot Gaz. 51(3):161–183.
- Cowling SA. 1999. Simulated effects of low atmospheric CO2 on structure and composition of North American vegetation at the Last Glacial Maximum. Global Ecol Biogeogr. 8:81–93.
- Cowling SA, Sykes MT. 1999. Physiological significance of low atmospheric CO2 for plant-climate interactions. Quat Res. 52:237–242.
- Cowling SA, Sykes MT, Bradshaw RHW. 2001. Palaeovegetation-model comparisons, climate change and tree succession in Scandinavia over the past 1500 years. J Ecol. 89(2):227–236.
- Craine JM, McLauchlan KK. 2004. The influence of biotic drivers on North American palaeorecords: alternatives to climate. Holocene. 14(5):787–791.
- Crausbay SD, Higuera PE, Sprugel DG, Brubaker LB. 2017. Fire catalyzed rapid ecological change in lowland coniferous forests of the Pacific Northwest over the past 14,000 years. Ecology. 98(9):2356–2369.
- Crawley MJ. 1987. What makes a community invisible. In: Gray AJ, Crawley MJ, Edwards PJ, editors. Colonization, succession and stability. Oxford: Blackwell; p. 429–453.
- Crawley MJ. 1989. Chance and timing in biological invasions. In: Drake JA, Mooney HA, di Castri F et al., editors. Biological invasions: a global perspective. Chichester: Wiley; p. 407–424.
- Crees JJ, Turvey ST. 2015. What constitutes a ‘native’ species? Insights from the Quaternary faunal record. Biol Conserv. 186:143–148.
- Cronk QCB. 1980. Extinction and survival in the endemic vascular flora of Ascension Island. Biol Conserv. 17:207–219.
- Cronk QCB. 1997. Islands: stability, diversity, conservation. Biodivers Conserv. 6:477–493.
- Cronk QCB, Fuller JL. 1995. Plant invaders. London: Chapman and Hall.
- Croudace IW, Rothwell RG, editors. 2015. Micro-XRF studies of sediment cores. Dordrecht: Springer.
- Crowley BE. 2010. A refined chronology of prehistoric Madagascar and the demise of the megafauna. Quat Sci Rev. 29(19):2591–2603.
- Crowley BE, Godfrey LR, Bankoff RJ, Perry GH, Culleton BJ, Kennett DJ, Sutherland MR, Samonds KE, Burney DA. 2017. Island-wide aridity did not trigger recent megafaunal extinctions in Madagascar. Ecography. 40(8):901–912.
- Cugny C, Mazier F, Galop D. 2010. Modern and fossil non-pollen palynomorphs from the Basque mountains (western Pyrenees, France): the use of coprophilous fungi to reconstruct pastoral activity. Veg Hist Archaeobot. 19:391–408.
- Cui Q-Y, Gaillard M-J, Lemdahl G, Sugita S, Greisman A, Jacobson GL, Olsson F. 2013. The role of tree composition in Holocene fire history of the hemiboreal and southern boreal zones of southern Sweden, as revealed by the application of the Landscape Reconstruction Algorithm: Implications for biodiversity and climate-change issues. Holocene. 23(12):1747–1763.
- Curtis JT. 1959. The vegetation of Wisconsin. Madison: University of Wisconsin Press.
- Cushing EJ. 1963. Late-Wisconsin pollen stratigraphy in east-central Minnesota [PhD thesis]. Minnesota: University of Minnesota.
- Cushing EJ. 1965. Problems in the Quaternary phytogeography of the Great Lakes region. In: Wright HE, Frey DG, editors. The Quaternary of the United States. Princeton, (NJ): Princeton University Press; p. 403–416.
- Cyr D, Gauthier S, Bergeron Y, Carcaillet C. 2009. Forest management is driving the eastern North American boreal forest outside its natural range of varaibility. Front Ecol Environ. 7(10):519–524.
- D’Amen M, Rahbek C, Zimmermann NE, Guisan A. 2017. Spatial predictions at the community level: from current approaches to future frameworks. Biol Rev. 92(1):169–187.
- D’Antoni HL, Schäbitz F. 1995. Remote sensing and Holocene vegetation: history of global change. World Resour Rev. 7(2):282–288.
- D’Apolito C, Absy ML, Latrubesse EM. 2013. The Hill of Six Lakes revisited: new data and re-evaluation of a key Pleistocene Amazon site. Quat Sci Rev. 76:140–155.
- Dahl E. 1998. The phytogeography of Northern Europe (British Isles, Fennoscandia and adjacent areas). Cambridge: Cambridge University Press.
- Dakos V, Carpenter SR, van Nes EH, Scheffer M. 2015. Resilience indicators: prospects and limitations for early warnings of regime shifts. Philos Trans R Soc B Bio Sci. 370(1659):20130263.
- Dale VH, Joyce LA, McNulty S, Neilson RP, Ayres MP, Flannigan MD, Hanson PJ, Irland LC, Lugo AE, Petereson CJ et al. 2001. Climate change and forest disturbances. BioScience. 51(9):723–734.
- Daniau A-L, Desprat S, Aleman JC, Bremond L, Davis B, Fletcher W, Marlon JR, Marquer L, Montade V, Morales-Molino C et al. 2019. Terrestrial plant microfossils in palaeoenvironmental studies, pollen, microcharcoal and phytolith. Towards a comprehensive understanding of vegetation, fire and climate changes over the past one million years. Rev Micropaléontol.10.1016/j.revmic.2019.1002.1001.
- Dansereau P, Cantlon JE, Egler FE, Gimingham CH, Guinochet M, Leith H, Monk CD, Robbins RG, Vasilevich VI, McIntosh RP. 1968. The continuum concept of vegetation: responses. Bot Rev. 34(3):253–332.
- Darling ES, Côté IM. 2018. Seeking resilience in marine ecosystems. Science. 359(6379):986–987.
- Darwin C. 1845. A naturalist’s voyage round the world. London: John Murray.
- Darwin C. 1859. On the origin of species by means of natural selection, or the preservation of favoured races in the struggle for life. London: John Murray.
- Dau JHC. 1829. Allerunterhänigster Bericht an die Königliche Dänische Rentekammer über die Torfmoore Seelands. Copenhagen: Gyldendahl.
- Davies AL. 2007. Upland agriculture and environmental risk: a new model of upland land-use based on high spatial-resolution palynological data from West Affric, NW Scotland. J Archaeol Sci. 34:2053–5063.
- Davies AL. 2009. Review of the historical environmental changes in the UK uplands relevant to management and policy. Stirling: University of Stirling.
- Davies AL. 2016a. Book review: Looking back to look forward. Trends Ecol Evol. 31(1):11–12.
- Davies AL. 2016b. Late Holocene regime shifts in moorland ecosystems: high resolution data from the Pennines, UK. Veg Hist Archaeobot. 25(3):207–219.
- Davies AL, Bunting MJ. 2010. Applications of palaeoecology in conservation. Open Ecol J. 3:54–67.
- Davies AL, Colombo S, Hanley N. 2014. Improving the application of long-term ecology in conservation and land management. J Appl Ecol. 51(1):63–70.
- Davies AL, Smith MA, Froyd CA, McCulloch RD. 2017. Microclimate variability and long-term persistence of fragmented woodland. Biol Conserv. 213:95–105.
- Davies AL, Streeter R, Lawson IT, Roucoux KH, Hiles W. 2018. The application of resilience concepts in palaeoecology. Holocene. 28(9):1523–1534.
- Davis BAS, Collins PM, Kaplan JO. 2015. The age and post-glacial development of the modern European vegetation: a plant functional approach based on pollen data. Veg Hist Archaeobot. 24(2):303–317.
- Davis BAS, Zanon M, EMPD project members. 2013. The European Modern Pollen Database (EMPD) project. Veg Hist Archaeobot. 22:521–530.
- Davis MA. 2009. Invasion biology. Oxford: Oxford University Press.
- Davis MB. 1963. On the theory of pollen analysis. Am J Sci. 261:897–912.
- Davis MB. 1976. Pleistocene biogeography of temperate deciduous forests. Geosci Man. 13:13–26.
- Davis MB. 1978. Climatic interpretation in Quaternary sediments. In: Walker D, Guppy JC, editors. Biology and Quaternary environments. Canberra: Australian Academy of Science; p. 35–51.
- Davis MB. 1981a. Quaternary history and the stability of forest communities. In: Shugart HH, West DC, Botkin DB, editors. Forest succession. New York: Springer; p. 132–177.
- Davis MB. 1981b. Outbreaks of forest pathogens in Quaternary history. Proceedings of the Fourth International Palynological Conference. Lucknow; p. 216–228.
- Davis MB. 1983a. Quaternary history of deciduous forests of eastern North America and Europe. Ann Missouri Bot Garden. 70:550–563.
- Davis MB. 1983b. Holocene vegetational history of the eastern United States. In: Wright HE, editor. Late-Quaternary environments of the United States, volume 2: the Holocene. Minneapolis: University of Minnesota Press; p. 166–181.
- Davis MB. 1986a. Climatic instability, time lags, and community disequilibrium. In: Diamond J, Case TJ, editors. Community Ecology. New York: Harper & Row; p. 269–284.
- Davis MB, editor. 1986b. Vegetation-climate equilibrium. Vegetatio. Vol. 67:65–141.
- Davis MB. 1987. Invasions of forest communities during the Holocene: beech and hemlock in the Great Lakes region. In: Gray AJ, Crawley MJ, Edwards PJ, editors. Colonization, Succession and Stability. Oxford: Blackwell Scientific Publishing; p. 373–393.
- Davis MB. 1989a. Lags in vegetation response to greenhouse warming. Clim Change. 15:75–82.
- Davis MB. 1989b. Research questions posed by the paleoecological record of global change. In: Bradley RS, editor. Global changes of the past. Boulder (CO): UCAR/Office for Interdisciplinary Earth Studies; p. 385–398.
- Davis MB. 1989c. Retrospective studies. In: Likens GE, editor. Long-term studies in ecology: approaches and alternatives. New York: Springer; p. 71–89.
- Davis MB. 1989d. Insights from paleoecology on global change. Ecol Soc Am Bull. 70(7):222–228.
- Davis MB. 1994. Ecology and paleoecology begin to merge. Trends Ecol Evol. 9(10):357–358.
- Davis MB. 2000. Palynology after Y2K - understanding the source area of pollen in sediments. Annu Rev Earth Planet Sci. 28:1–18.
- Davis MB. 2001a. Chapter 1.12, Past and future forest response to rapid climate change. In: Schulze E-D, Heimann M, Harrison S et al., editors. Global biogeochemical cycles in the climate system. London: Academic Press; p. 167–175.
- Davis MB. 2001b. Climate change and steady state in temperate hardwood forests. In: Press MC, Huntly NJ, Levin S, editors. Ecology: achievement and challenge. Oxford: Blackwell Science; p. 211–225.
- Davis MB, Botkin DB. 1985. Sensitivity of cool-temperate forests and their fossil pollen record to rapid temperature change. Quat Res. 23(3):327–340.
- Davis MB, Calcote RR, Sugita S, Takahara H. 1998. Patchy invasion and the origin of a hemlock-hardwoods forest mosaic. Ecology. 79(8):2641–2659.
- Davis MB, Schwartz MW, Woods K. 1991. Detecting a species limit from pollen in sediments. J Biogeogr. 18(6):653–668.
- Davis MB, Shaw RG. 2001. Range shifts and adaptive responses to Quaternary climate change. Science. 292(5517):673–679.
- Davis MB, Shaw RG, Etterson JR. 2005. Evolutionary responses to changing climate. Ecology 86(7):1704–1714.
- Davis MB, Sugita S. 1997. Reinterpreting the fossil pollen record of Holocene tree migration. In: Huntley B, Cramer W, Morgan AV et al., editors. Past and future rapid environmental changes the spatial and evolutionary responses of terrestrial biota. Dordrecht: Springer; p. 181–193.
- Davis MB, Sugita S, Calcote RR, Frelich LE. 1992. Effects of invasion by Tsuga canadensis on a North American forest ecosystem. In: Teller A, Mathy P, Jeffers JNR, editors. Responses of forest ecosystems to environmental changes. London: Elsevier Applied Science; p. 34–44.
- Davis MB, Woods KD, Webb SL, Futyma RP. 1986. Dispersal versus climate: expansion of Fagus and Tsuga into the Upper Great Lakes region. Vegetatio. 67:93–103.
- Davis MB, Zabinski C. 1992. Changes in geographical range resulting from greenhouse warming: effects on biodiversity in forests. In: Peters RL, Lovejoy TE, editors. Global warming and biological diversity. New Haven: Yale University Press; p. 297–308.
- Davis OK. 1987. Spores of the dung fungus Sporormiella: increased abundance in historic sediments and before Pleistocene megafaunal extinction. Quat Res. 28:290–294.
- Davis OK, Shafer DS. 2006. Sporormiella fungal spores, a palynological means of detecting herbivore density. Palaeogeogr Palaeoclimatol Palaeoecol. 237:40–50.
- Davis RB, Jacobson GL. 1985. Late glacial and early Holocene landscapes in northern New England and adjacent areas of Canada. Quat Res. 23:341–368.
- Dawson A, Paciorek CJ, McLachlan JS, Goring SJ, Williams JW, Jackson ST. 2016. Quantifying pollen-vegetation relationships to reconstruct ancient forests using 19th-century forest composition and pollen data. Quat Sci Rev. 137:156–175.
- Dawson TP, Jackson ST, House JI, Prentice IC, Mace GM. 2011. Beyond predictions: biodiversity conservation in a changing climate. Science. 332:53–58.
- de Beaulieu J-L, Andrieu-Ponel V, Cheddadi R, Guiter F, Ravazzi C, Reille M, Rossi S. 2006. Apport des longues séquences lacustres à la connaissance des variations des climats et des paysages pléistocènes. Comptes Rendus Palevol. 5(1):65–72.
- de Beaulieu J-L, Andrieu-Ponel V, Reille M, Grüger E, Tzedakis C, Svitavská Svobodova H. 2001. An attempt at correlation between the Velay pollen sequence and the Middle Pleistocene stratigraphy from central Europe. Quat Sci Rev. 20(16):1593–1602.
- de Beaulieu J-L, Brugiapaglia E, Joannin S, Guiter F, Zanchetta G, Wulf S, Peyron O, Bernardo L, Didier J, Stock A et al. 2017. Lateglacial-Holocene abrupt vegetation changes at Lago Trifoglietti in Calabria, Southern Italy: The setting of ecosystems in a refugial zone. Quat Sci Rev. 158 (SupplementC):44–57.
- de Boer EJ, Hooghiemstra H, Florens FBV, Baider C, Engels S, Dakos V, Blaauw M, Bennett KD. 2013a. Rapid succession of plant associations on the small ocean island of Mauritius at the onset of the Holocene. Quat Sci Rev. 68:114–125.
- de Boer EJ, Slaikovska M, Hooghiemstra H, Rijsdijk KF, Vélez MI, Prins M, Baider C, Florens FBV. 2013b. Multi-proxy reconstruction of environmental dynamics and colonization impacts in the Mauritian uplands. Palaeogeogr Palaeoclimatol Palaeoecol. 383:42–51.
- de Boer EJ, Vélez MI, Rijsdijk KF, de Louw PGB, Vernimmen TJJ, Visser PM, Tjallingii R, Hooghiemstra H. 2015. A deadly cocktail: how a drought around 4200 cal. yr BP caused mass mortality events at the famous ‘dodo swamp’ in Mauritius. Holocene. 25(5):758–771.
- de Lafontaine G, Amasifuen Guerra CA, Ducousso A, Petit RJ. 2014a. Cryptic no more: soil macrofossils uncover Pleistocene forest microrefugia within a periclacial desert. New Phytol. 204:715–729.
- de Lafontaine G, Amasifuen Guerra CA, Ducousso A, Sánchez-Goñi M-F, Petit RJ. 2014b. Beyond skepticism: uncovering cryptic refugia using multiple lines of evidence. New Phytol. 204:450–454.
- de Nascimento L, Nogué S, Criado C, Ravazzi C, Whittaker RJ, Willis KJ, Fernández-Palacios JM. 2016. Reconstructing Holocene vegetation on the island of Gran Canaria before and after human colonization. Holocene. 26(1):113–125.
- de Nascimento L, Willis KJ, Fernández-Palacios JM, Criado C, Whittaker RJ. 2009. The long-term ecology of the lost forests of La Laguna, Tenerife (Canary Islands). J Biogeogr. 36:499–514.
- de Novaes Nascimento M, Laurenzi AG, Valencia BG, Van R, Bush MB. 2019. A 12,700-year history of paleolimnological change from an Andean microrefugium. Holocene. 29:231–243.
- de Souza JG, Robinson M, Maezumi SY, Capriles J, Hoggarth JA, Lombardo U, Novello VF, Apaéstegui J, Whitney B, Urrego D et al. 2019. Climate change and cultural resilience in late pre-Columbian Amazonia. Nat Ecol Evol. doi:10.1038/s41559-41019-40924-41550.
- De Vos JM, Joppa LN, Gittleman JL, Stephens PR, Pimm SL. 2015. Estimating the normal background rate of species extinction. Conserv Bio. 29(2):452–462.
- de Witte LC, Stöcklin J. 2010. Longevity of clonal plants: why it matters and how to measure it. Ann Bot. 106(6):859–870.
- Dearing JA. 2006. Climate-human-environment interactions: resolving our past. Clim Past. 2:187–203.
- Dearing JA. 2008. Landscape change and resilience theory: a palaeoenvironmental assessment from Yunnan, SW China. Holocene. 18:117–127.
- Dearing JA, Acma B, Bub S, Chambers FM, Chen X, Cooper J, Crook D, Dong XH, Dotterweich M, Edwards ME et al. 2015. Social-ecological systems in the Anthropocene: The need for integrating social and biophysical records at regional scales. Anthropocene Rev. 2(3):220–246.
- Dearing JA, Dotterweich M, Foster T. 2011b. Editorial: Integrative palaeoscience for sustainable management. Pages News. 19(2):43.
- Dearing JA, Dotterweich M, Foster T, Newman L, von Gunten L, editors. 2011a. Integrative Paleoscience for Sustainable Management. Pages News. 19(2):41–92.
- Dearing JA, Jones RT, Shen J, Yang X, Boyle JF. 2008. Using multiple archives to understand past and present climate-human-environment interactions: the Lake Erhai catchment, Yunnan Province, China. J Paleolimnol. 40:3–31.
- Dearing JA, Yang X, Dong X, Zhang E, Chen X, Langdon PG, Zhang K, Zhang W, Dawson TP. 2012. Extending the timescale and range of ecosystem services through paleoenvironmental analyses, exemplified in the lower Yangtze basin. Proc Natl Acad Sci USA. 109(18):E1111–E1120.
- Dee LE, de Lara M, Costello C, Gaines SD. 2017. To what extent can ecosystem services motivate protecting biodiversity? Ecol Lett. 20:935–946.
- Deevey ES. 1969. Coaxing history to conduct experiments. BioScience. 19:40–43.
- Deevey ES. 1984. Stress, strain and stability of lacustrine ecosystems. In: Haworth EY, Lund JWG, editors. Lake sediments and environmental history. Leicester: Leicester University Press; p. 203–229.
- Delcourt HR. 2002. Forests in Peril. Tracking deciduous trees from ice-age refuges into the greenhouse world. Blacksburg, Virginia: McDonald & Woodward.
- Delcourt HR, Delcourt PA. 1988. Quaternary landscape ecology: relevant scales in space and time. Landsc Ecol. 2(1):23–44.
- Delcourt HR, Delcourt PA. 1991. Quaternary ecology. A paleoecological perspective. London: Chapman & Hall.
- Delcourt HR, Delcourt PA, Webb T. 1983. Dynamic plant ecology: the spectrum of vegetational change in space and time. Quat Sci Rev. 1(3):153–175.
- Delcourt PA, Delcourt HR. 1987. Long-term Forest dynamics in the temperate zone. New York: Springer.
- Delcourt PA, Delcourt HR. 1998. Paleoecological insights on conservation of biodiverstiy: a focus on species, ecosystems, and landscapes. Ecol App. 8(4):921–934.
- Delhon C, Orliac C. 2008. The vanished palm trees of Easter Island: new radiocarbon and phytolith data. VII International Conference on Easter Island and the Pacific; Visby, Sweden, 20–25 August 2007.
- deMenocal P, Ortiz J, Guilderson TJ, Adkins J, Sarnthein M, Baker L, Yarusinsky M. 2000. Abrupt onset and termination of the African Humid Period:: rapid climate responses to gradual insolation forcing. Quat Sci Rev. 19(1):347–361.
- Denevan WM. 2003. Cultivatied landscapes of native Amazonia and the Andes. Oxford: Oxford University Press.
- Denham TP, Haberle SG, Lentfer C, Fullagar R, Field J, Therin M, Porch N, Winsborough B. 2003. Origins of agriculture at Kuk swamp in the Highlands of New Guinea. Science. 301(5630):189–193.
- Dennell R. 2017. Human colonization of Asia in the Late Pleistocene: The history of an invasive species. Curr Anthropol. 58(Suppl 17):S383–S396.
- Dering M, Kosiński P, Wyka TP, Pers–Kamczyc E, Boratyński A, Boratyńska K, Reich PB, Romo A, Zadworny M, Żytkowiak R et al. 2017. Tertiary remnants and Holocene colonizers: Genetic structure and phylogeography of Scots pine reveal higher genetic diversity in young boreal than in relict Mediterranean populations and a dual colonization of Fennoscandia. Divers Distrib. 23(5):540–555.
- Dexter F, Banks HT, Webb T. 1987. Modeling Holocene changes in the location and abundance of beech populations in eastern North America. Rev Palaeobot Palynol. 50:273–292.
- Di Febbraro M, Carotenuto F, Castiglione S, Russo D, Loy A, Maiorano L, Raia P. 2017. Does the jack of all trades fare best? Survival and niche width in Late Pleistocene megafauna. J Biogeogr. 44(12):2828–2838.
- di Pasquale G, Allevato E, Cocchiararo A, Moser D, Pacciarelli M, Saracino A. 2014. Late Holocene persistence of Abies alba in low-mid altitude deciduous forests of central and southern Italy: new perspectives from charcoal data. J Veg Sci. 25:1299–1310.
- Diamond JM. 1975. Assembly of species communities. In: Cody ML, Diamond JM, editors. Ecology and evolution of communities. Cambridge: Harvard University Press; p. 342–444.
- Diamond JM. 1998. Guns, germs, and steel. London: Vintage.
- Diamond JM. 2007. Easter Island revisited. Science. 317:1692–1694.
- Díaz S, Pascual U, Stenseke M, Martín-López B, Watson RT, Molnár Z, Hill R, Chan KMA, Baste IA, Brauman KA et al. 2018. Assessing nature’s contributions to people. Science. 359(6373):270.
- Dickau R, Bruno MC, Iriarte J, Prümers H, Jaimes Betancourt C, Holst I, Mayle FE. 2012. Diversity of cultivars and other plant resources used at habitation sites in the Llanos de Mojos, Beni, Bolivia: evidence from macrobotanical remains, starch grains, and phytoliths. J Archaeol Sci. 39(2):357–370.
- Dickie IA, Martinez-Garciá LB, Koele N, Grelet G-A, Tylianakis JM, Peltzer DA, Richardson SJ. 2013. Mycorrhizas and mycorrhizal fungal communities throughout ecosystem development. Plant Soil. 367:11–39.
- Dietl GP. 2016a. Different worlds. Nature. 529:29–30.
- Dietl GP. 2016b. Brave New World of conservation paleobiology. Front Ecol Evol. 4:21.
- Dietl GP, Flessa KW, editors. 2009. Conservation Paleobiology. Using the Past to Manage for the Future. Vol. 15. New York: The Paleontological Society Papers.
- Dietl GP, Flessa KW. 2011. Conservation paleobiology: putting the dead to work. Trends Ecol Evol. 26(1):30–37.
- Dietl GP, Flessa KW, editors. 2017. Conservation paleobiology: science and practice. Chicago: University of Chicago Press.
- Dietl GP, Kidwell SM, Brenner M, Burney DA, Flessa KW, Jackson ST, Koch PL. 2015. Conserving paleobiology: leveraging knowledge of the past to inform conservation and restoration. Annu Rev Earth Planet Sci. 43:79–103.
- Dillehay TD, Ocampo C, Saavedra J, Sawakuchi AO, Vega RM, Pino M, Collins MB, Scott Cummings L, Arregui I, Villagran XS et al. 2015. New archaeological evidence for an early human presence at Monte Verde, Chile. PLoS One. 10(11):e0141923.
- DiMichele WA. 1994. Ecological patterns in time and space. Paleobiology. 20(2):89–92.
- DiMichele WA, Phillips TL. 1996. Climate change, plant extinctions and vegetational recovery during the Middle–Late Pennsylvanian transition: the case of tropical peat-forming envionments in North America. In: Hart MB, editor. Biotic recovery from mass extinction events. London: Geological Society Special Publication No. 102; p. 201–221.
- Dippery JK, Tissue DT, Thomas RB, Strain BR. 1995. Effects of low and elevated CO2 on C3 and C4 annuals. Oecologia. 101(1):13–20.
- Dobrowski SZ. 2011. A climatic basis for microrefugia: the influence of terrain on climate. Glob Chang Biol. 17:1022–1035.
- Dobrowski SZ, Abatzoglou JT, Greenberg JA, Schladow SG. 2009. How much influence does landscape-scale physiography have on air temperature in a mountain environment? Agric Forest Meteorol. 149(10):1751–1758.
- Donner JJ. 1963. The zoning of the post-glaial pollen diagrams in Finland and the main changes in the forest composition. Acta Botan Fenni. 65:2–40.
- Donner JJ. 1995. The Quaternary history of scandinavia. Cambridge: Cambridge University Press.
- Douda J, Doudová J, Drasnarová A, Kuneš P, Hadincová V, Krak K, Zákravský P, Mandák B. 2014. Migration patterns of subgenus Alnus in Europe since the Last Glacial Maximum: a systematic review. PLoS One. 9 (2):e88709.
- Doughty CE, Wolf A, Malhi Y. 2013. The legacy of the Pleistocene megafauna extinctions on nutrient availability in Amazonia. Nat Geosci. 6:761–764.
- Doweld AB. 2016. New names of fossil Hemitrapa and Trapa (Lythraceae). Phytotaxa. 252(3):228–230.
- Downey PO, Richardson DM. 2016. Alien plant invasions and native plant extinctions: a six-threshold framework. AoB Plants. 8:plw047.
- Doyle AC. 1912. The lost world. London: Hodder and Stoughton.
- Drake DR, Hunt TL. 2009. Invasive rodents on islands: integrating historical and contemporary ecology. Biol Invasions. 11:1483–1487.
- Drake JM, Griffen BD. 2010. Early warning signals of extinction in deteriorating environments. Nature. 467:456–459.
- Dransfield J. 1991. Arecaceae Paschalococos disperta J. Dranf. Palmarum Hortus Francofurtensis. 3:64.
- Dransfield J, Flenley JR, King SM, Harkness DD, Rapu S. 1984. A recently extinct palm from Easter Island. Nature. 312(5996):750–752.
- Driscoll DA, Lindenmayer DB. 2012. Framework to improve the application of theory in ecology and conservation. Ecol Monogr. 82(1):129–147.
- Drzymulska D. 2018. On the history of Brasenia Schreb. in the European Pleistocene. Veg Hist Archaeobot. 27(3):527–534.
- Dubois N, Saulnier-Talbot É, Mills K, Gell P, Battarbee RW, Bennion H, Chawchai S, Dong X, Francus P, Flower R et al. 2018. First human impacts and responses of aquatic systems: A review of palaeolimnological records from around the world. Anthropocene Rev. 5(1):38–68.
- Dull RA, Nevle RJ, Woods WI, Bird DK, Avnery S, Denevan WM. 2010. The Columbian encounter and the little ice age: abrupt land use change, fire, and greenhouse forcing. Ann Assoc Am Geogr. 100(4):755–771.
- Dumont HJ, Cocquyt C, Fontugne M, Arnold M, Reyss J-L, Bloemndal J, Oldfield F, Steenbergen CLM, Korthals HJ, Zeeb BA. 1998. The end of moai quarrying and its effect on Lake Rano Raraku, Easter Island. J Paleolimnol. 20:409–422.
- Duval M, Arnold LJ, Parés JM, Hoffmann DL. 2015. The Jaramillo Subchron and the Early-Middle Pleistocene transition in continental records from a multidisciplinary perspective. Quatern Int. 389:1–6.
- Dyke AS. 2005. Late Quaternary vegetation history of northern North America based on pollen, macrofossil, and faunal remains. Géogr Phys Quat. 59(2–3):211–262.
- Edwards KJ. 1983. Quaternary palynology: consideration of a discipline. Prog Phys Geog. 7(1):113–125.
- Edwards KJ, Bennett KD, Davies AL. 2018. Palaeoecological perspectives on Holocene environmental change in Scotland. Earth Environ Sci Trans R Soc Edinburgh. 10.1017/S1755691018000208.
- Edwards KJ, Fyfe RM, Hunt CO, Schofield JE. 2015. Moving forwards? Palynology and the human dimension. J Archaeol Sci. 56:117–132.
- Edwards KJ, Fyfe RM, Jackson ST. 2017. The first 100 years of pollen analysis. Nat Plants. 3:17001.
- Edwards ME. 1986. Disturbance histories of four Snowdonian woodlands and their relation to Atlantic bryophyte distributions. Biol Conserv. 37:301–320.
- Edwards ME, Birks HJB. 1986. Vegetation and ecology of four western oakwoods (Blechno Quercetum petraea Br. Bl. et Tx. 1952) in North Wales. Phytocoenologia. 14:237–262.
- Edwards ME, Franklin-Smith L, Clarke C, Baker J, Hill S, Gallagher K. 2015. The role of fire in the mid-Holocene arrival and expansion of lodgepole pine (Pinus contorta var. latifolia Engelm. ex S. Watson) in Yukon Holocene. 25(1):64–78.
- Ehleringer JR, Cerling TE, Helliker BR. 1997. C4 photosynthesis, atmospheric CO2, and climate. Oecologia. 112(3):285–299.
- Eiserhardt WL, Borchsenius F, Plum CM, Ordonez A, Svenning J-C. 2015. Climate-driven extinctions shape the phylogenetic structure of temperate tree floras. Ecol Lett. 18(3):263–272.
- Ekblom A, Gillson L. 2017. The importance of paleoecology in the conservation and restoration of cultural landscapes. PAGES Mag. 25(2):88–89.
- Ekins P, Simon S, Deutsch L, Folke C, de Groot R. 2003. A framework for the practical application of the concepts of critical natural capital and strong sustainability. Ecol Econ. 44:165–185.
- Elias MK. 1935. Tertiary grasses and other prairie vegetation from the High Plains of North America. Am J Sci. 29:24–33.
- Elias RB, Gil A, Silva L, Fernández-Palacios JM, Azevedo EB, Reis F. 2016. Natural zonal vegetation of the Azores Islands: characterization and potential distribution. Phytocoenologia. 46:107–123.
- Eliot C. 2007. Method and metaphysics in Clements’s and Gleason’s ecological explanations. Stud Hist Philos Sci C. 38(1):85–109.
- Elith J, Leathwick JR. 2009. Species distribution models: ecological explanation and prediction across space and time. Annu Rev Ecol Evol Syst. 40:677–697.
- Ellenberg H. 1988. Vegetation ecology of central Europe. Cambridge: Cambridge University Press.
- Ellenberg H, Weber HE, Düll R, Wirth V, Werner W, Paulissen D. 1991. Zeigerwerte von Pflanzen in Mitteleuropa. Scr Geobotanica. 18:1–248.
- Ellis EC. 2015. Ecology in an anthropogenic biosphere. Ecol Monogr 85(3):287–331.
- Ellis EC, Antill EC, Kreft H. 2012. All is not loss: plant biodiversity in the Anthropocene. PLoS One. 7(1):e30535.
- Ellis EC, Ramankutty N. 2008. Putting people in the map: anthropogenic biomes of the world. Front Ecol Environ. 6(8):439–447.
- Elmendorf SC, Henry GHR, Hollister RD, Björk RG, Bjorkman AD, Callaghan TV, Collier LS, Cooper EJ, Cornelissen JHC, Day TA et al. 2012. Global assessment of experimental climate warming on tundra vegetation: heterogeneity over space and time. Ecol Lett. 15(2):164–175.
- Elsig J, Schmitt J, Leuenberger D, Schneider R, Eyer M, Leuenberger M, Joos F, Fischer H, Stocker TF. 2009. Stable isotope constraints on Holocene carbon cycle changes from an Antarctic ice core. Nature. 461:507.
- Elton CS. 1958. The ecology of invasions by plants and animals. London: Methuen.
- Enevold R, Rasmussen P, Løvschal M, Olsen J, Odgaard BV. 2019. Circumstantial evidence of non-pollen palynomorph palaeoecology: a 5,500 year NPP record from forest hollow sediments compared to pollen and macrofossil inferred palaeoenvironments. Vegetation history and archaeobotany. 28:105–121.
- Enquist CAF, Jackson ST, Garfin GM, Davis FW, Gerber LR, Littell JA, Tank JL, Terando AJ, Wall TU, Halpern BS et al. 2017. Foundations of translational ecology. Front Ecol Environ. 15(10):541–550.
- Epp LS, Kruse S, Kath NJ, Stoof-Leichsenring KR, Tiedemann R, Pestryakova LA, Herzschuh U. 2018. Temporal and spatial patterns of mitochondrial haplotype and species distributions in Siberian larches inferred from ancient environmental DNA and modeling. Sci Rep. 8(1):17436.
- Erdman C, Emerson JW. 2007. bcp: An R package for performing a Bayesian analysis of change point problems. J Stat Softw. 23(3). 10.18637/jss.v023.i03
- Erdtman G. 1943. An introduction to pollen analysis. Waltham, MA: Chronica Botanica Company.
- Erdtman G, Berglund BE, Praglowski JR. 1961. An introduction to a scandinavian pollen flora. Stockholm: Almqvist & Wiksell.
- Erichsen EO, Budde KB, Sagheb-Talebi K, Bagnoli F, Vendramin GG, Hansen OK. 2018. Hyrcanian forests—Stable rear-edge populations harbouring high genetic diversity of Fraxinus excelsior, a common European tree species. Divers Distrib. 24(11):1521–1533.
- Eroglu D, Marwan N, Stebich M, Kurths J. 2018. Multiplex recurrence networks. Phy Rev. E97:012312.
- Espíndola A, Pellissier L, Maiorano L, Hordijk W, Guisan A, Alvarez N. 2012. Predicting present and future intra-specific genetic structure through niche hindcasting across 24 millennia. Ecol Lett. 15(7):649–657.
- Essl F, Dullinger S, Rabitsch W, Hulme PE, Pyšek P, Wilson JRU, Richardson DM. 2015. Historical legacies accumulate to shape future biodiversity in an era of rapid global change. Divers Distrib. 21(5):534–547.
- Etienne D, Wilhelm B, Sabatier P, Reyss J-L, Arnaud F. 2013. Influence of sample location and livestock numbers of Sporormiella concentrations and accumulation rates in surface sediments of Lake Allos, French Alps. J Paleolimnol. 49:117–127.
- Etterson JR, Franks SJ, Mazer SJ, Shaw RG, Gorden NLS, Schneider HE, Weber JJ, Winkler KJ, Weis AE. 2016. Project baseline: An unprecedented resource to study plant evolution across space and time. Am J Bot. 103(1):164–173.
- Etterson JR, Shaw RG. 2001. Constraint to adaptive evolution in response to global warming. Science. 294(5540):151–154.
- Evans MEK, Merow C, Record S, McMahon SM, Enquist BJ. 2016. Towards process-based range modeling of many species. Trends Ecol Evol. 31(11):860–871.
- Everard M. 2017. Ecosystem services. London: Routledge.
- Ewers RM, Didham RK, Pearse WD, Lefebvre V, Rosa IMD, Carreiras JMB, Lucas RM, Reuman DC. 2013. Using landscape history to predict biodiversity patterns in fragmented landscapes. Ecol Lett. 16:1221–1233.
- Ezquerra FJ, Cañas I, Oria-de-Rueda JA, Rubiales JM. 2019. Late-Holocene fossil evidence and the interpretation of the vegetation in NW Iberia: management issues in the light of palaeoecological findings. Veg Hist Archaeobot. 28(1):35–50.
- Fægri K. 1940. Quartärgeologicshe Untersuchungen im westlichen Norwegen. II. Zur Spätquartären Geschichte Jærens. Bergens Museums Årbok 1939–40. p. 1–120.
- Fægri K. 1947. Heterodokse tanker om pollenanalysen. Geologiska Föreningens i Stockholm Förhandlingar. 69:55–66.
- Fægri K. 1949. Studies on the Pleistocene of western Norway, IV. On the immigration of Picea abies (L.) Karst. Bergens Universitetet Årbok. Bergen: University of Bergen; p. 1–52.
- Fægri K. 1963. Problems of immigration and dispersal of the Scandinavian Flora. In: Löve A, Löve D, editors. North atlantic biota and their history. New York: Macmillan; p. 221–237.
- Fægri K. 1966. Some problems of representivity in pollen analysis. Palaeobotanist. 15:135–140.
- Fægri K. 1981. Some Pages of the history of pollen analysis. Striae. 14:42–47.
- Fægri K, Iversen J. 1950. Text-book of modern pollen analysis. Copenhagen: Munksgaard.
- Fagerlind F. 1949. Some reflections on the history of the climate and vegetation of the Hawaiian Islands. Sven Bot Tidskr. 43(1):73–81.
- Fagerlind F. 1952. The real signification of pollen diagrams. Bot Notiser. 105:185–224.
- Fang K, Morris JL, Salonen JS, Miller PA, Renssen H, Sykes MT, Seppä H. 2013. How robust are Holocene treeline simulations? A model–data comparison in the European Arctic treeline region. J Quat Sci. 28(6):595–604.
- Farjon A. 2017a. The vera conference. Br Wildl. 29:155–156.
- Farjon A. 2017b. Ancient oaks in the english landscape. Kew: Royal Botanic Gardens.
- Farrell M, Bunting MJ, Middleton R. 2016. Replicability of data collected for empirical estimation of relative pollen productivity. Rev Palaeobot Palynol. 232:1–13.
- Farris E, Filibeck G, Marignani M, Rosati L. 2010. The power of potential natural vegetation (and of spatial-temporal scale): a response to Carrión & Fernández (2009). J Biogeogr. 37(11):2211–2213.
- Fauth JE, Bernardo J, Camara M, Resetarits WJ, van Buskirk J, McCollum SA. 1996. Simplifying the jargon of community ecology: a conceptual approach. Am Nat. 147(2):282–286.
- Feeley KJ, Silman MR. 2010. Biotic attrition from tropical forests correcting for truncated temperature niches. Glob Chang Biol. 16(6):1830–1836.
- Felde VA, Bjune AE, Grytnes J-A, Birks HJB. 2014a. A comparison of novel and traditional numerical methods for the analysis of modern pollen assemblages from major vegetation-landform types. Rev Palaeobot Palynol. 210:22–36.
- Felde VA, Grytnes J-A, Bjune AE, Peglar SM, Birks HJB. 2018. Are diversity trends in western Scandinavia influenced by post-glacial dispersal limitation? J Veg Sci. 29(3):360–370.
- Felde VA, Peglar SM, Bjune AE, Grytnes J-A, Birks HJB. 2014b. The relationship between vegetation composition, vegetation zones and modern pollen assemblages in Setesdal, southern Norway. Holocene. 24:985–1001.
- Felde VA, Peglar SM, Bjune AE, Grytnes J-A, Birks HJB. 2016a. Modern pollen–plant richness and diversity relationships exist along a vegetational gradient in southern Norway. Holocene. 26(2):163–175.
- Feranec RS, Miller NG, Lothrop JC, Graham RW. 2011. The Sporormiella proxy and end-Pleistocene megafaunal extinction: a perspective. Quatern Int. 245:333–338.
- Fernández Palacios JM. 2016. Shaped by sea-level shifts. Nature. 532:42–43.
- Ferrier S, Drielsma M, Manion G, Watson G. 2002. Extended statistical approaches to modelling spatial pattern in biodiversity in northeast New South Wales. II. Community-level modelling. Biodivers Conserv. 11(12):2309–2338.
- Ferrier S, Guisan A. 2006. Spatial modelling of biodiversity at the community level. J Appl Ecol. 43(3):393–404.
- Ferrier S, Manion G, Elith J, Richardson K. 2007. Using generalized dissimilarity modelling to analyse and predict patterns of beta diversity in regional biodiversity assessment. Divers Distrib. 13(3):252–264.
- Feurdean A, Bhagwat SA, Willis KJ, Birks HJB, Lischke H, Hickler T. 2013. Tree migration-rates: narrowing the gap between inferred post-glacial rates and projected rates. PLoS One. 8(8):e71797.
- Feurdean A, Willis KJ. 2008. Long-term variability of Abies alba in NW Romania: implications for its conservation management. Divers Distrib. 14(6):1004–1017.
- Ficetola GF, Poulenard J, Sabatier P, Messager E, Gielly L, Leloup A, Etienne D, Bakke J, Malet E, Fanget B et al. 2018. DNA from lake sediments reveals long-term ecosystem changes after a biological invasion. Sci Adv 4 (5):eaar4292.
- Fiedel SJ. 2018. The spore conundrum: Does a dung fungus decline signal humans’ arrival in the Eastern United States? Quatern Int. 466:247–255.
- Field MH, Gibson SM, Gibbard PL. 2017. East–West European Middle Pleistocene correlation – the contribution of the first British record of Aracites interglacialis Wieliczk. Acta Palaeobot. 57(1):101–108.
- Field MH, Lewis SG. 2019. The first Pleistocene fossil records of Urtica kioviensis Rogow. (Urticaceae) and Potamogeton sukaczevii Wieliczk. (Potamogetonaceae) in the British Isles. Veg Hist Archaeobot. 28:1–8.
- Field MH, Velichkevich FY, Andrieu-Ponel V, Woltz P. 2000. Significance of two new Pleistocene plant records from western Europe. Quat Res. 54(2):253–263.
- Figueroa-Rangel BL, Willis KJ, Olvera-Vargas M. 2008. 4200 years of pine-dominated upland forest dynamics in west-central Mexico: human or natural legacy? Ecology. 89:1893–1907.
- Finch J, Marchant R. 2011. A palaeoecological investigation into the role of fire and human activity in the development of montane grasslands in East Africa. Veg Hist Archaeobot. 20:109–124.
- Fineschi S, Salvini D, Taurchini D, Carnevale S, Vendramin GG. 2003. Chloroplast DNA variation of Tilia cordata (Tiliaceae). Can J Forest Res. 33:2503–2508.
- Finsinger W, Colombaroli D, De Beaulieu J-L, Valsecchi V, Vannière B, Vescovi E, Chapron E, Lotter AF, Magny M, Tinner W. 2010. Early to mid-Holocene climate change at Lago dell’Accesa (central Italy): climate signal or anthropogenic bias? J Quat Sci. 25(8):1239–1247.
- Finsinger W, Fevre J, Orbán I, Pál I, Vincze I, Hubay K, Birks HH, Braun M, Tóth M, Magyari EK. 2018. Holocene fire-regime changes near the treeline in the Retezat Mts. (Southern Carpathians, Romania). Quatern Int. 477:94–105.
- Finsinger W, Giesecke T, Brewer S, Leydet M. 2017b. Emergence patterns of novelty in European vegetation assemblages over the past 15 000 years. Ecol Lett. 20(3):336–346.
- Finsinger W, Morales-Molino C, Gałka M, Valsecchi V, Bojovic S, Tinner W. 2017a. Holocene vegetation and fire dynamics at Crveni Potok, a small mire in the Dinaric Alps (Tara National Park, Serbia). Quat Sci Rev. 167:63–77.
- Finsinger W, Schwörer C, Heiri O, Morales-Molino C, Ribolini A, Giesecke T, Haas JN, Kaltenrieder P, Magyari EK, Ravazzi C et al. 2019. Fire on ice and frozen trees? Inappropriate radiocarbon dating leads to unrealistic reconstructions. New Phytol. 222(2):657–662.
- Firbas F. 1934. Über die Bestimmung der Walddichte und der Vegetation waldloser Gebeite mit Hilfe der Pollenanalyse. Planta. 22(1):109–145.
- Firbas F. 1949. Spätz und Nacheiszeitiliche Waldgeschichte Mitteleuropas Nördlich der Alpen. I. Jena: Gustav Fischer.
- Fischer JM, Frost TM, Ives AR. 2001. Compensatory dynamics in zooplankton community responses to acidification: measurement and mechanisms. Ecol App. 11(4):1060–1072.
- Fisher CT, Cohen AS, Fernández-Diaz JC, Leisz SJ. 2017. The application of airborne mapping LiDAR for the documentation of ancient cities and regions in tropical regions. Quatern Int. 448:129–138.
- Fisher M. 2012. A review of naturalistic grazing versus natural processes. Self-willed land. [accessed 2018 May 3]. http://www.self-willed-land.org.uk/rep_res/EWD11_MARK_FISHERv3.pdf.
- Fisher M. 2013. What is rewilding? Self-willed land. [accessed 2018 March 9]. www.self-willed-land.org.uk/articles/what_rewilding.htm.
- Fitzpatrick MC, Blois JL, Williams JW, Nieto‐Lugilde D, Maguire KC, Lorenz DJ. 2018. How will climate novelty influence ecological forecasts? Using the Quaternary to assess future reliability. Glob Chang Biol.10.1111/gcb.14138.
- Fitzpatrick MC, Hargrove WW. 2009. The projection of species distribution models and the problem of non-analog climate. Biodivers Conserv. 18(8):2255.
- Fitzpatrick MC, Sanders NJ, Normand S, Svenning J-C, Ferrier S, Gove AD, Dunn RR. 2013. Environmental and historical imprints on beta diversity: insights from variation in rates of species turnover along gradients. Proc R Soc B Bio Sci. 280(1768). 10.1098/rspb.2013.1201
- Flagmeier M, Long DG, Genney DR, Hollingsworth PM, Ross LC, Woodin SJ. 2014. Fifty years of vegetation change in oceanic-montane liverwort-rich heath in Scotland. Plant Ecol Divers. 7(3):457–470.
- Flantua SGA, Blaauw M, Hooghiemstra H. 2016a. Geochronological database and classification system for age uncertainties in Neotropical pollen records. Clim Past. 12(2):387–414.
- Flantua SGA, Hooghiemstra H, Grimm EC, Behling H, Bush MB, González-Arango C, Gosling WD, Ledru M-P, Lozano-García S, Moldonado A et al. 2015. Updated site compilation of the Latin American Pollen Database. Rev Palaeobot Palynol. 223:104–115.
- Flantua SGA, Hooghiemstra H, Vuille M, Behling H, Carson JF, Gosling WD, Hoyos I, Ledru MP, Montoya E, Mayle F et al. 2016b. Climate variability and human impact in South America during the last 2000 years: synthesis and perspectives from pollen records. Clim Past. 12(2):483–523.
- Flenley JR. 1979. The Equatorial Rain Forest: a geological history. London: Butterworth.
- Flenley JR. 1988. Palynological evidence for land-use changes in south-east Asia. J Biogeogr. 15:185–197.
- Flenley JR. 1993. The origins of diversity in tropical rain forests. Trends Ecol Evol. 8(4):119–120.
- Flenley JR. 1998. Tropical forests under the climates of the last 30,000 years. Clim Change. 39(2–3):177–197.
- Flenley JR. 2003. Some prospects for lake sediment analysis in the 21st century. Quatern Int. 105:77–80.
- Flenley JR, Bahn PG. 2003. The Enigmas of Easter Island. Oxford: Oxford University Press.
- Flenley JR, Butler K. 2001. Evidence for continued disturbance of upland rainforest in Sumatra for the last 7000 years of an 11000 year record. Palaeogeogr Palaeoclimatol Palaeoecol. 171:289–305.
- Flenley JR, King ASM, Teller JY, Prentice ME, Jackson J, Chew C. 1991. The Late Quaternary vegetational and climatic history of Easter Island. J Quat Sci. 6(2):85–115.
- Flenley JR, Maloney BK, Ford D, Hallam G. 1975. Trapa natans in the British Flandrian. Nature. 257:39–41.
- Flessa KW. 2017. Putting the dead to work: translational paleoecology. In: Dietl GP, Flessa KW, editors. Conservation paleobiology science and practice. Chicago: University of Chicago Press; p. 283–289.
- Flessa KW, Jackson ST. 2005a. The geological record of ecological dynamics. Understanding the biotic effects of future environmental change. Washington, D.C.: National Research Council of the National Academies.
- Flessa KW, Jackson ST. 2005b. Forging a common agenda for ecology and paleoecology. BioScience. 55:1030–1031.
- Fletcher M-S, Bowman DMJS, Whitlock C, Mariani M, Stahle L. 2018. The changing role of fire in conifer-dominated temperate rainforest through the last 14,000 years. Quat Sci Rev. 182:37–47.
- Fletcher M-S, Thomas I. 2010. The origin and temporal development of an ancient cultural landsacpe. J Biogeogr. 37:2183–2196.
- Fletcher M-S, Wolfe BB, Whitlock C, Pompeani DP, Heijnis H, Haberle SG, Gadd PS, Bowman DMJS. 2014a. The legacy of mid-Holocene fire on a Tasmanian montane landscape. J Biogeogr. 41:476–488.
- Fletcher M-S, Wood SW, Haberle SG. 2014b. A fire-driven shift from forest to non-forest: evidence for alternative stable states? Ecology. 95(9):2504–2513.
- Foley JA, Kutzbach JE, Coe MT, Levis S. 1994. Feedbacks between climate and boreal forest during the Holocene epoch. Nature. 371:52–54.
- Folke C, Carpenter SR, Walker B, Scheffer M, Elmqvist T, Gunderson L, Holling CS. 2004. Regime shifts, resilience, and biodiversity in ecosystem management. Annu Rev Ecol Evol Syst. 35(1):557–581.
- Forbes CJ, Gillson L, Hoffman MT. 2018. Shifting baselines in a changing world: Identifying management targets in endangered heathlands of the Cape Floristic Region, South Africa. Anthropocene. 22:81–93.
- Forcier LK. 1975. Reproductive strategies and the co-occurrence of climax tree species. Science. 189:808–810.
- Fordham DA, Akçakaya HR, Alroy J, Saltré F, Wigley TML, Brook BW. 2016. Predicting and mitigating future biodiversity loss using long-term ecological proxies. Nat Clim Chang. 6:909.
- Fordham DA, Saltré F, Haythorne S, Wigley TML, Otto-Bliesner BL, Chan KC, Brook BW. 2017. PaleoView: a tool for generating continuous climate projections spanning the last 21 000 years at regional and global scales. Ecography. 40(11):1348–1358.
- Forest F, Grenyer R, Rouget M, Davies TJ, Cowling RM, Faith DP, Balmford A, Manning JC, Procheş Ş, van der Bank M et al. 2007. Preserving the evolutionary potential of floras in biodiversity hotspots. Nature. 445:757.
- Forman RTT, Godron M. 1986. Landscape ecology. New York: Wiley.
- Fortes GG, Speller CF, Hofreiter M, King TE. 2013. Phenotypes from ancient DNA: Approaches, insights and prospects. Bioessays. 35(8):690–695.
- Foster DR, Knight DH, Franklin JF. 1998. Landscape patterns and legacies resulting from large, infrequent forest disturbances. Ecosystems. 1:497–510.
- Foster DR, Motzkin G. 1998. Ecology and conservation in the cultural landscape of New England: lessons from nature’s history. Northeast Nat. 5(2):111–126.
- Foster DR, Motzkin G. 2003. Interpeting and conserving the openland habitats of coastal New England: insights from landscape history. For Ecol Manage. 185:127–150.
- Foster DR, Orwig DA, McLachlan JS. 1996. Ecological and conservation insights from reconstructive studies of temperate old-growth forests. Trends Ecol Evol. 11:419–424.
- Foster DR, Oswald WW, Kaison EK, Doughty ED, Hansen BCS. 2006. A climatic driver for abrupt mid-Holocene vegetation dynamics and the hemlock decline in New England. Ecology. 87:2959–2966.
- Foster DR, Schoonmaker PK, Pickett STA. 1990. Insights from paleoecology to community ecology. Trends Ecol Evol. 5(4):119–122.
- Fournier B, Lara E, Jassey VEJ, Mitchell EAD. 2015. Functional traits as a new approach for interpreting testate amoeba palaeo-records in peatlands and assessing the causes and consequences of past changes in species composition. Holocene. 25(9):1375–1383.
- Fox JF. 1977. Alternation and co-existence of tree species. Am Nat. 111:69–89.
- Fox JW. 2013. The intermediate disturbance hypothesis should be abandoned. Trends Ecol Evol. 28(2):86–92.
- Franklin J. 2010. Mapping species distributions - spatial inference and prediction. Cambridge: Cambridge University Press.
- Franklin J. 2013. Species distribution models in conservation biogeography: developments and challenges. Divers Distrib. 19:1217–1223.
- Franklin SB, Tolonen M. 2000. Temporally-explicit models of fire and forest. Plant Ecol. 146:145–168.
- Franks SJ, Sim S, Weis AE. 2007. Rapid evolution of flowering time by an annual plant in response to a climate fluctuation. Proc Natl Acad Sci USA. 104(4):1278–1282.
- Franks SJ, Weber JJ, Aitken SN. 2014. Evolutionary and plastic responses to climate change in terrestrial plant populations. Evol Appl. 7(1):123–139.
- Freeman J, Kobziar L, White Rose E, Cropper W. 2017. A critique of the historical-fire-regime concept in conservation. Conserv Bio. 31(5):976–985.
- Frégeau M, Payette S, Grondin P. 2015. Fire history of the central boreal forest in eastern North America reveals stability since the mid-Holocene. Holocene. 25(12):1912–1922.
- Fries C, Johansson O, Pettersson B, Simonson P. 1997. Silvicultural models to maintain and restore natural stand structures in Swedish boreal forests. For Ecol Manage. 94(1–3):79–103.
- Fries M. 1949. Anmälanden och kritiker. Olof H Selling: On the Late Quaternary history of the Hawaiian vegetation. Studies in Hawaiian pollen statistics, part III. 1948. Geologiska Föreningens i Stockholm Förhandlingar. 71(2):347–355.
- Fries M. 1967. Lennart von Post’s pollen diagram series of 1916. Rev Palaeobot Palynol. 4:9–13.
- Fröhlich J. 1930. Der südosteuropäische Urwald und seine Überführung in Wirtschaftswald. Centralblatt für das Gesamte Forstwesen. 56:49–68.
- Froyd CA. 2005. Fossil stomata reveal early pine presence in Scotland: implications for postglacial colonisation analyses. Ecology. 86:579–586.
- Froyd CA, Coffey EED, van der Knaap WO, van Leeuwen JFN, Tye A, Willis KJ. 2014. The ecological consequences of megafaunal loss: giant tortoises and wetland biodiversity. Ecol Lett. 17:144–154.
- Froyd CA, Willis KJ. 2008. Emerging issues in biodiversity and conservation management: the need for a palaeoecological perspective. Quat Sci Rev. 27:1723–1732.
- Fukami T, Nakajima M. 2011. Community assembly: alternative stable states or alternative transient states? Ecol Lett. 14(10):973–984.
- Fuller JL. 1997. Holocene forest dynamics in southern Ontario, Canada: fine-resolution pollen data. Can J Bot. 75:1714–1727.
- Fuller JL. 1998. Ecological impact of the mid-Holocene hemlock decline in southern Ontario, Canada. Ecology. 79(7):2337–2351.
- Fyfe RM. 2007. The importance of local-scale openness within regions dominated by closed woodland. J Quat Sci. 22(6):571–578.
- Fyfe RM. 2018. ‘Natural’ vegetation in Britain: the pollen-eye view. Br Wildl. 29:339–349.
- Fyfe RM, de Beaulieu JL, Binney HA, Bradshaw RHW, Brewer S, Le Flao A, Finsinger W, Gaillard MJ, Giesecke T, Gil-Romera G et al. 2009. The European Pollen Database: past efforts and current activities. Veg Hist Archaeobot. 18:417–424.
- Fyfe RM, Ombashi H, Davies HJ, Head K. 2018b. Quantified moorland vegetation and assessment of the role of burning over the past five millennia. J Veg Sci. 29(3):393–403.
- Fyfe RM, Roberts N, Woodbridge J. 2010. A pollen-based pseudobiomisation approach to anthropogenic land-cover change. Holocene. 20(7):1165–1171.
- Fyfe RM, Twiddle CL, Sugita S, Gaillard M-J, Barratt P, Caseldine CJ, Dodson J, Edwards KJ, Farrell M, Froyd CA et al. 2013. The Holocene vegetation cover of Britain and Ireland: overcoming problems of scale and discerning patterns of openness. Quat Sci Rev. 73:132–148.
- Fyfe RM, Woodbridge J, Roberts CN. 2018a. Trajectories of change in Mediterranean Holocene vegetation through classification of pollen data. Veg Hist Archaeobot. 27(2):351–364.
- Fyfe RM, Woodbridge J, Roberts N. 2015. From forest to farmland: pollen-inferred land cover change across Europe using the pseudobiomization approach. Glob Chang Biol. 21:1197–1212.
- Gaillard M-J, Berglund BE, Birks HJB, Edwards KJ, Bittmann F. 2018. “Think horizontally, act vertically”: the centenary (1916–2016) of pollen analysis and the legacy of Lennart von Post. Veg Hist Archaeobot. 27(2):267–269.
- Gaillard M-J, Sugita S, Bunting J, Dearing J, Bittmann F. 2008a. Human impact on terrestrial ecosystems, pollen calibration and quantitative reconstruction of past land-cover. Veg Hist Archaeobot. 17(5):415–418.
- Gaillard M-J, Sugita S, Bunting MJ, Middleton R, Broström A, Caseldine C, Giesecke T, Hellman SEV, Hicks S, Hjelle KL et al. 2008b. The use of modelling and simulation approach in reconstructing past landscapes from fossil pollen data: a review and results from the POLLANDCAL network. Veg Hist Archaeobot. 17:419–443.
- Gaillard M-J, Sugita S, Mazier F, Kaplan JO, Trondman A-K, Broström A, Hickler T, Kjellström E, Kunes F, Lemmen C et al. 2010. Holocene land-cover reconstructions for studies on land cover-climate feedbacks. Clim Past. 6:307–346.
- Gajewski K. 1987. Climatic impacts on the vegetation of eastern North America during the past 2000 years. Vegetatio. 68:179–190.
- Galetti M, Moleón M, Jordano P, Pires MM, Guimarães PR, Pape T, Nichols E, Hansen D, Olesen JM, Munk M et al. 2018. Ecological and evolutionary legacy of megafauna extinctions. Biol Rev. 93(2):845–862.
- Gałka M, Galloway JM, Lemonis N, Mazei YA, Mitchell EAD, Morse PD, Patterson RT, Tsyganov AN, Wolfe SA, Swindles GT. 2018. Palaeoecology of Sphagnum riparium (Ångström) in Northern Hemisphere peatlands: Implications for peatland conservation and palaeoecological research. Rev Palaeobot Palynol. 254:1–7.
- Garcia C, Klein EK, Jordano P. 2017. Dispersal processes driving plant movement: challenges for understanding and predicting range shifts in a changing world. J Ecol. 105:1–5.
- Garzón MB, Sanchez de Dios R, Ollero HS. 2007. Predictive modelling of tree species distribution on the Iberian Peninsula during the alst glacial maximum and mid-Holocene. Ecography. 30:120–134.
- Gaudreau DC, Webb T. 1985. Late-Quaternary pollen stratigraphy and isochrone maps for the northeastern United States. In: Bryant VM, Holloway RG, editors. Pollen records of Late-Quaternary sediments. Dallas: American Association of Stratigraphic Palynologists Foundation; p.245–280.
- Gaüzère P, Iversen LL, Barnagaud J-Y, Svenning J-C, Blonder B. 2018. Empirical predictability of community responses to climate change. Front Ecol Evol. 6:186.
- Gavin DG. 2010. Ecological complexity at the forest–grassland transition revealed by lake sediment records. Front Biogeogr. 2(1):2–3.
- Gavin DG, Fitzpatrick MC, Gugger PF, Heath KD, Rodriguez-Sanchez F, Dobrowski SZ, Hampe A, Hu FS, Ashcroft MB, Bartlein PJ et al. 2014. Climate refugia: joint inference from fossil records, species distribution models and phylogeography. New Phytol. 204(1):37–54.
- Gavin DG, Hallett DJ, Hu FS, Lertzman KP, Pritchard SJ, Brown KJ, Lynch JA, Bartlein PJ, Peterson DL. 2007. Forest fire and climate change in western North America: insights from sediment charcoal records. Front Ecol Environ. 5:499–506.
- Gavin DG, Hu FS, Walker IR, Westover KS. 2009. The northern inland temperate rainforest of Biritsh Columbia: old forests with a young history? Northwest Sci. 83(1):70–78.
- Gavin DG, Oswald WW, Wahl ER, Williams JW. 2003. A statistical approach to evaluating distance metrics and analog assignments for pollen records. Quat Res. 60(3):356–367.
- Geary B, Fyfe R. 2016. Peatlands as knowledge archives. In: Bonn A, Allott T, Evans M et al., editors. Peatland restoration and ecosystem services: science, policy, and practice. Cambridge: Cambridge University Press; p. 95–113.
- Gell P. 2010. With the benefit of hindsight: the utility of palaeoecology in wetland condition assessment and identification of restoration targets. In: Batty LC, Hallberg KB, editors. Ecology of industrial pollution. Cambridge: Cambridge University Presss; p. 162–188.
- Gell P. 2017. Using paleoecology to understand natural ecological character in Ramsar wetlands. PAGES Mag. 25(2):86–87.
- Gelorini V, Verbeken A, van Geel B, Cocquyt C, Verschuren D. 2011. Modern non-pollen palynomorphs from East African lake sediments. Rev Palaeobot Palynol. 164(3):143–173.
- Giarrizzo E, Burrascano S, Chiti T, de Bello F, Lepš J, Zavattero L, Blasi C. 2017. Re-visiting historical semi-natural grasslands in the Apennines to assess patterns of changes in species composition and functional traits. Appl Veg Sci. 20(2):247–258.
- Gienapp P, Teplitsky C, Alho JS, Mills JA, Merilä J. 2008. Climate change and evolution: disentangling environmental and genetic responses. Mol Ecol. 17(1):167–178.
- Giesecke T. 2004. The Holocene spread of spruce in Scandinavia [PhD thesis]. Uppsala: University of Uppsala.
- Giesecke T. 2005a. Moving front or population expansion: how did Picea abies (L) Karst. become frequent in central Sweden? Quat Sci Rev. 4:2495–2509.
- Giesecke T. 2005b. Holocene dynamics of the southern boreal forest in Sweden. Holocene. 15:858–872.
- Giesecke T. 2013. Changing plant distributions and abundances. In: Elias SA, editor. Encyclopedia of Quaternary science. Amsterdam: Elsevier; p. 854–860.
- Giesecke T. 2016. Did thermophilous trees spread into central Europe during the Late Glacial? New Phytol. 212:15–18.
- Giesecke T, Ammann B, Brande A. 2014b. Palynological richness and evenness: insights from the taxa accumulation curve. Veg Hist Archaeobot. 23:217–228.
- Giesecke T, Bennett KD. 2004. The Holocene spread of Picea abies (L.) Karst. in Fennoscandia and adjacent areas. J Biogeogr. 31(9):1523–1548.
- Giesecke T, Bennett KD, Birks HJB, Bjune AE, Bozilova E, Feurdean A, Finsinger W, Froyd CA, Pokorný P, Rösch M et al. 2011. The pace of Holocene vegetation change – testing for synchronous developments. Quat Sci Rev. 30(19–20):2805–2814.
- Giesecke T, Bjune AE, Chiverrell RC, Seppä H, Ojala AEK, Birks HJB. 2008. Exploring Holocene continentality changes in Fennoscandia using present and past tree distributions. Quat Sci Rev. 27(13):1296–1308.
- Giesecke T, Brewer S. 2018. Notes on the post-glacial spread of abundant European tree taxa. Veg Hist Archaeobot. 27(2):337–349.
- Giesecke T, Brewer S, Finsinger W, Leydet M, Bradshaw RHW. 2017. Patterns and dynamics of European vegetation change over the last 15000 years. J Biogeogr. 44:1441–1456.
- Giesecke T, Davis BAS, Brewer S, Finsinger W, Wolters S, Blaauw M, de Beaulieu JL, Binney HA, Fyfe RM, Gaillard MJ et al. 2014a. Towards mapping the late Quaternary vegetation change of Europe. Veg Hist Archaeobot. 23(1):75–86.
- Giesecke T, de Beaulieu J-L, Leydet-Barbier M. 2016. The European Pollen Database: Research tool and community. Pages News. 24(1):48.
- Giesecke T, Hickler T, Kunkel T, Sykes MT, Bradshaw RHW. 2007. Towards an understanding of the Holocene distribution of Fagus sylvatica L. J Biogeogr. 34:118–131.
- Giesecke T, Miller PA, Sykes MT, Ojala AEK, Seppä H, Bradshaw RHW. 2010. The effect of past changes in inter-annual temperature variability on tree distribution limits. J Biogeogr. 37(7):1394–1405.
- Giesecke T, Wolters S, Jahns S, Brande A. 2012. Exploring Holocene changes in palynological richness in northern Europe - Did postglacial immigration matter? PLoS One. 7:e51624.
- Gilbert B, O’Connor MI. 2013. Climate change and species interactions: beyond local communities. Ann N Y Acad Sci. 1297(1):98–111.
- Gilbert FS. 1980. The equilibrium theory of island biogeography: fact or fiction? J Biogeogr. 7:209–235.
- Gill JL. 2013. Is pollen analysis dead? Paleoecology in the era of Big Data. The Contemplative Mammoth. [accessed 2013 July 10].
- Gill JL. 2014. Ecological impacts of the late Quaternary megaherbivore extinctions. New Phytol. 201:1163–1169.
- Gill JL, Blois JL, Benito B, Dobrowski S, Hunter ML, McGuire JL. 2015. A 2.5-million-year perspective on coarse-filter strategies for conserving nature’s stage. Conserv Bio. 29(3):640–648.
- Gill JL, McLauchlan KK, Skibbe AM, Goring SJ, Zirbel CR, Williams JW. 2013. Linking abundances of the dung fungus Sporormiella to the density of bison: implications for assessing grazing by megaherbivores in palaeorecords. J Ecol. 101(5):1125–1136.
- Gill JL, Williams JW, Jackson ST, Donnelly JP, Schellinger GC. 2012. Climatic and megaherbiovry controls on late-glacial vegetation dynamics: a new, high-resolution, multi-proxy record from Silver Lake, Ohio. Quat Sci Rev. 34:66–80.
- Gill JL, Williams JW, Jackson ST, Lininger KB, Robinson GS. 2009. Pleistocene megafaunal collapse, novel plant communities, and enhanced fire regimes in North America. Science. 326:1100–1103.
- Giller PS, Gee JHR. 1987. The analysis of community organization: the influence of equilibrium, scale and terminology. In: Gee JHR, Giller PS, editors. Organization of communities past and present. Oxford: Blackwell Scientific Publications; p. 519–542.
- Gillingham PK, Huntley B, Kunin WE, Thomas CD. 2012. The effect of spatial resolution on projected responses to climate warming. Divers Distrib. 18(10):990–1000.
- Gillson L. 2009. Landscapes in time and space. Landsc Ecol. 24:149–155.
- Gillson L. 2015. Biodiversity conservation and environmental change. Using palaeoecology to manage dynamic landscapes in the Anthropocene. Oxford: Oxford University Press.
- Gillson L, Biggs H, Smit IPJ, Virah-Sawmy M, Rogers K. 2019. Finding common ground between adaptive management and evidence-based approaches to biodiversity conservation. Trends Ecol Evol. 34:31–44.
- Gillson L, Duffin KI. 2007. Thresholds of potential concern as benchmarks in the management of African savannahs. Philos Trans R Soc B Bio Sci. 362:309–319.
- Gillson L, Ekblom A, Willis KJ, Froyd CA. 2008. Holocene palaeo-invasions: the link between pattern, process and scale in invasion ecology. Landsc Ecol. 23:757–769.
- Gillson L, Gell P, von Gunten L, editors. 2017. Sustaining earth’s biodiversity. PAGES Mag. 25(2): 76–130.
- Gillson L, Ladle RJ, Araújo MB. 2011. Baselines, patterns and process. In: Ladle RJ, Whittaker RJ, editors. Conservation biology. Chichester: Wiley-Blackwell; p. 31–44.
- Gillson L, Marchant R. 2014. From myopia to clarity: sharpening the focus of ecosystem management through the lens of palaeoecology. Trends Ecol Evol. 29(6):317–325.
- Githumbi EN, Kariuki R, Shoemaker A, Courtney-Mustaphi CJ, Chuhilla M, Richer S, Lane P, Marchant R. 2018. Pollen, people and place: multidisciplinary perspectives on ecosystem change at Amboseli, Kenya. Front Earth Sci. 5:a113.
- Glaser B. 2007. Prehistorically modified soils of central Amazonia: a model for sustainable agriculture in the twenty-first century. Philos Trans R Soc B Bio Sci. 362(1478):187.
- Gleason HA. 1917. The structure and development of the plant association. Bull Torrey Bot Club. 43:463–481.
- Gleason HA. 1923. The vegetational history of the Middle West. Ann Assoc Am Geogr. 12:39–85.
- Gleason HA. 1926. The individualistic concept of the plant association. Bull Torrey Bot Club. 53(1):7–26.
- Gleason HA. 1927. Further views on the succession-concept. Ecology. 8(3):299–326.
- Gleason HA. 1939. The individualistic concept of the plant association. Am Midl Nat. 21(1):92–110.
- Godwin H. 1923. Dispersal of pond floras. J Ecol. 11(2):160–164.
- Godwin H. 1934a. Pollen analysis: an outline of the problems and potentialities of the method. Part I: Technique and interpretation. New Phytol. 33(4):278–305.
- Godwin H. 1934b. Pollen analysis: an outline of the problems and potentialities of the method. Part II: General applications of pollen analysis. New Phytol. 33(5):325–358.
- Godwin H. 1940. Pollen analysis and forest history of England and Wales. New Phytol. 39(4):370–400.
- Godwin H. 1961. Introduction to symposium on Quaternary ecology 1959. Proc Linn Soc London. 172:25–29.
- Godwin H. 1962. Vegetational history of the Kentish Chalk Downs as seen at Wingham and Frogholt. Veröffentlichungen des Geobotanischen Institutes der Eidgenossische Technische Hochschule, Stiftung Rübel in Zurich. 37:83–99.
- Godwin H. 1966. Introductory address. In: Sawyer JS, editor. World climate from 8000 to 0 BC. London: Royal Meteorological Society; p. 3–14.
- Godwin H. 1975. The history of the British Flora. A factual basis for phytogeography. 2nd ed. Cambridge: Cambridge University Press.
- Góis-Marques CA, Mitchell RL, de Nascimento L, Fernández-Palacios JM, Madeira J, Menezes de Sequeira M. 2019. Eurya stigmosa (Theaceae), a new and extinct record for the Calabrian stage of Madeira Island (Portugal): 40Ar/39Ar dating, palaeoecological and oceanic island palaeobiogeographical implications. Quat Sci Rev. 206:129–140.
- Goldsmith FB, Warren A, editors. 1993. Conservation in progress. Chichester: J Wiley.
- Gomes da Rocha D, Kaefer IL. 2019. What has become of the refugia hypothesis to explain biological diversity in Amazonia? Ecol Evol. 9(7):4302–4309.
- Gonzales LM, Grimm EC, Williams JW, Nordheim EV. 2009a. A modern plant-climate research dataset for modelling eastern North American plant taxa. Grana. 48:1–18.
- Gonzales LM, Williams JW, Grimm EC. 2009b. Expanded response-surfaces: a new method to reconstruct paleoclimates from fossil pollen assemblages that lack modern analogues. Quat Sci Rev. 28:3315–3332.
- Gonzales LM, Williams JW, Kaplan JO. 2008. Variations in leaf area index in northern and eastern North America over the past 21,000 years: a data-model comparison. Quat Sci Rev. 27:1453–1466.
- González C, Urrego LE, Martínez JI, Polanía J, Yokoyama Y. 2010. Mangrove dynamics in the southwestern Caribbean since the ‘Little Ice Age’: A history of human and natural disturbances. Holocene. 20(6):849–861.
- Gorham E. 1957. The development of peatlands. Q Rev Biol. 32:145–166.
- Goring SJ, Dawson A, Simpson GL, Ram K, Graham RW, Grimm EC, Williams JW. 2015. Neotoma: A programmatic interface to the neotoma paleoecological database. Open Quat. 1(2):1–17.
- Goring SJ, Lacourse T, Pellatt MG, Mathewes RW. 2013. Pollen assemblage richness does not reflect regional plant species richness: a cautionary tale. J Ecol. 101:1137–1145.
- Goring SJ, Mladenoff DJ, Cogbill CV, Record S, Paciorek CJ, Jackson ST, Dietze MC, Dawson A, Matthes JH, McLachlan JS et al. 2016. Novel and lost forests in the upper midwestern United States, from new estimates of settlement-era composition, stem density, and biomass. PLoS One. 11(12):e0151935.
- Goring SJ, Williams JW. 2017. Effect of historical land-use and climate change on tree-climate relationships in the upper Midwestern United States. Ecol Lett. 20(4):461–470.
- Gosling WD, Bush MB, Hanselman JA, Chepstow-Lusty A. 2008. Glacial-interglacial changes in moisture balance and the impact on vegetation in the southern hemisphere tropical Andes (Bolivia/Peru). Palaeogeogr Palaeoclimatol Palaeoecol. 259(1):35–50.
- Gosling WD, de Kruif J, Norder SJ, de Boer EJ, Hooghiemstra H, Rijsdijk KF, McMichael CNH. 2017. Mauritius on fire: Tracking historical human impacts on biodiversity loss. Biotropica. 49(6):778–783.
- Gosling WD, Williams JJ. 2013. Ecosystem service provision sets the pace for pre-Hispanic societal development in the central Andes. Holocene. 23(11):1619–1624.
- Gotelli NJ. 2004. A taxonomic wish-list for community ecology. Philos Trans R Soc B Bio Sci. 359:585–597.
- Gotelli NJ, Graves GR. 1996. Null Models in Ecology. Washington: Smithsonian Institute.
- Götmark F. 1992. Naturalness as an evaluation criterion in nature conservation: a response to Anderson. Conserv Bio. 6:455–458.
- Gould J. 2018. Historic ecosystem change. Br Wildl. 29:312.
- Graae BJ, Vandvik V, Armbruster WS, Eiserhardt WL, Svenning J-C, Hylander K, Ehrlén J, Speed JDM, Klanderud K, Bråthen KA et al. 2018. Stay or go – how topographic complexity influences alpine plant population and community responses to climate change. Perspect Plant Ecol Evol Syst. 30:41–50.
- Graham CH, VanDerWal J, Phillips SJ, Moritz C, Williams SE. 2010. Dynamic refugia and species persistence: tracking spatial shifts in habitat through time. Ecography. 33:1062–1069.
- Graham RW. 1988. The role of climatic change in the design of biological reserves: the palaeoecological perspective for conservation biology. Conserv Bio. 2:391–394.
- Graham RW, Belmecheri S, Choy K, Culleton BJ, Davies LJ, Froese D, Heintzmann PD, Hritz C, Kapp JD, Newsom LA et al. 2016. Timing and causes of mid-Holocene mammoth extinction on St. Paul Island, Alaska. Proc Natl Acad Sci USA. 113(33):9310–9314.
- Graham RW, Grimm EC. 1990. Effects of global climate change on the patterns of terrestrial biological communities. Trends Ecol Evol. 5(9):289–292.
- Granoszewski W. 2003. Late Pleistocene vegetation history and climatic changes at Horoszki Duże, eastern Poland: a palaeobotanical study. Acta Palaeobot Suppl 4:3–95.
- Grant PR, editor 1997. Evolution on islands. Oxford: Oxford University Press.
- Grant PR, Grant BR. 2011. How and why species multiply: The radiation of Darwin’s Finches. Princeton: Princeton University Press.
- Grant PR, Grant BR. 2014. 40 years of evolution: Darwin’s Finches on Daphne major. Princeton: Princeton University Press.
- Gravel D, Canham CD, Beaudet M, Messier C. 2006. Reconciling niche and neutrality: the continuum hypothesis. Ecol Lett. 9(4):399–409.
- Gray ST, Betancourt JL, Jackson ST, Eddy RG. 2006. Role of multidecadal climatic variability in a range extension of pinyon pine. Ecology. 87(5):1124–1130.
- Grayson DK, Meltzer DJ. 2015. Revisiting Paleoindian exploitation of extinct North American mammals. J Archaeol Sci. 56:177–193.
- Green DG. 1982. Fire and stability in the postglacial forests of southwest Nova Scotia. J Biogeogr. 9:29–40.
- Green DG. 1983. The ecological interpretation of fine resolution pollen records. New Phytol. 94:459–477.
- Greenwood RM, Atkinson IAE. 1977. Evolution of divaricating plants in New Zealand in relation to moa browsing. Proc N Z Ecol Soc. 24:21–33.
- Greenwood S, Ruiz‐Benito P, Martínez‐Vilalta J, Lloret F, Kitzberger T, Allen CD, Fensham R, Laughlin DC, Kattge J, Bönisch G et al. 2017. Tree mortality across biomes is promoted by drought intensity, lower wood density and higher specific leaf area. Ecol Lett. 20(4):539–553.
- Greimler J, Stuessy TF, Swenson U, Baeza CM, Matthei O. 2002. Plant invasions on an oceanic archipelago. Biol Invasions. 4:73–85.
- Greiser C, Joosten H. 2018. Archive value: measuring the palaeo-information content of peatlands in a conservation and compensation perspective. Int J Biodiver Sci Ecosyst Serv Manage. 14(1):209–220.
- Griesemer JR. 1992. Niche: historical perspectives. In: Fox-Keller E, Lloyd EA, editors. Key words in evolutionary biology. Cambridge: Harvard University Press; p. 231–240.
- Grimm EC. 1983. Chronology and dynamics of vegetation change in the prairie‐woodland region of southern Minnesota, USA New Phytol 93(2):311–350.
- Grimm EC. 1984. Fire and other factors controlling the Big Woods vegetation of Minnesota in the mid-19th century. Ecol Monogr. 54(3):291–311.
- Grimm EC. 2001. Trends and palaeoecological problems in the vegetation and climate history of the northern Great Plains, USA. Bio Environ Proc R Irish Acad. 101B(1–2):47–64.
- Grimm EC. 2008. Neotoma: an ecosystem database for the Pliocene, Pleistocene, and Holocene. Vol. E Series 1. Springfield, Illinois: Illinois State Museum Scientific Papers.
- Grimm EC, Blois JL, Giesecke T, Graham RW, Smith AJ, Williams JW. 2018. Constituent databases and data stewards in the neotoma paleoecology database: history, growth, and new directions. PAGES Mag. 26(2):64–65.
- Grimm EC, Bradshaw RHW, Brewer S, Flantua S, Giesecke T, Lézine A-M, Takahara H, Williams JW. 2013. Databases and their application. In: Elias SA, Mock CJ, editors. Encyclopedia of Quaternary science. 2nd ed. Amsterdam: Elsevier; p. 831–838.
- Grimm EC, Donovan JJ, Brown KJ. 2011. A high-resolution record of climate variability and landscape response from Kettle Lake, northern Great Plains, North America. Quat Sci Rev. 30:2626–2650.
- Grimm EC, Jacobson GL. 2004. Late-Quaternary vegetation history of the eastern United States. In: Gillispie AL, Porter SC, Atwater BF, editors. The Quaternary period in the United States. Amsterdam: Elsevier; p. 381–402.
- Grimm V, Wissel C. 1997. Babel, or the ecological stability discussions: an inventory and analysis of terminology and a guide for avoiding confusion. Oecologia. 109(3):323–334.
- Grinnell J. 1917. The niche-relationships of the Californian Thrasher. Auk. 34:427–433.
- Grinnell J. 1924. Geography and evolution. Ecology 5:225–229.
- Grivet D, Petit RJ. 2003. Chloroplast DNA phylogeography of the hornbeam in Europe: evidence for a bottleneck at the outset of postglacial colonization. Conserv Genet. 4(1):47–56.
- Groot MHM, Hooghiemstra H, Berrío JC, Giraldo C. 2013. North Andean environmental and climatic change at orbital to submillennial time-scales: Vegetation, water levels and sedimentary regimes from Lake Fúquene 130-27 ka. Rev Palaeobot Palynol. 197:186–204.
- Grubb PJ. 1977. The maintenance of species-richness in plant communities: The importance of the regeneration niche. Biol Rev. 52:107–145.
- Gubler M, Henne PD, Schworer C, Lotter AF, Tinner W. 2018. Microclimate gradients provide evidence for a glacial refugium for temperate trees in a sheltered hilly landscape of northern Italy. J Biogeogr. 45(11):2564–2575.
- Guilday JE. 1967. Differential extinction during Late-Pleistocene and recent times. In: Martin PS, Wright HE, editors. Pleistocene extinctions. New Haven: Yale University Press; p. 121–140.
- Guillemot T, Bichet V, Gauthier E, Zocatelli R, Massa C, Richard H. 2017. Environmental responses of past and recent agropastoral activities on south Greenlandic ecosystems through molecular biomarkers. Holocene. 27(6):783–795.
- Guisan A, Rahbek C. 2011. SESAM – a new framework integrating macroecological and species distribution models for predicting spatio‐temporal patterns of species assemblages. J Biogeogr. 38(8):1433–1444.
- Guisan A, Thuiller W. 2005. Predicting species distribution: offering more than simple habitat models. Ecol Lett. 8:993–1009.
- Guisan A, Thuiller W, Zimmermann NE. 2017. Habitat suitability and distribution models. Cambridge: Cambridge University Press.
- Gunderson LH. 2000. Ecological resilience - in theory and application. Annu Rev Ecol Syst. 31:425–439.
- Gunderson LH, Holling CS, editors. 2001. Panarchy. Understanding transformation in human and natural systems. Washington DC: Island Press.
- Gunderson LH, Pritchard L. 2002. Resilience and the behaviour of large-scale systems. Washington DC: Island Press.
- Gunton RM, van Asperen EN, Basden A, Bookless D, Araya Y, Hanson DR, Goddard MA, Otieno G, Jones GO. 2017. Beyond ecosystem services: valuing the invaluable. Trends Ecol Evol. 32(4):249–257.
- Gustavsson G, Lemdahl G, Gaillard M-J. 2009. Abrupt forest ecosystem change in SW Sweden during the late Holocene. Holocene. 19(5):691–702.
- Haberle SG. 2003. Late Quaternary vegetation dynamics and human impact on Alexander Selkirk Island, Chile. J Biogeogr. 30:239–255.
- Haberle SG. 2005. A 23,000-yr pollen record from Lake Euramoo, wet tropics of NE Queensland, Australia. Quat Res. 64(3):343–356.
- Haberle SG, Hope GS, Fretes Y. 1991. Environmental changes in the Baliem Valley, montane Irian Java, Republic of Indonesia. J Biogeogr. 18:25–40.
- Haddad NM, Brudvig LA, Clobert J, Davies KF, Gonzalez A, Holt RD, Lovejoy TE, Sexton JO, Austin MP, Collins CD et al. 2015. Habitat fragmentation and its lasting impact on Earth’s ecosystems. Sci Adv. 1(2):e1500052.
- Hafsten U. 1951. A pollen analytical investigation of two peat deposits from Tristan da Cunha. Research Report of Norwegian Scientific Expedition to Tristan da Cunha 1937–1938.
- Hafsten U. 1960. Pleistocene development of vegetation and climate in Tristan da Cunha and Gough Island. Årbok Univ Bergen Mat Nat Ser. 1960(20):1–48.
- Haines-Young R, Potschin M. 2018. Common international classification of ecosystem servies (CICES) V5.1. Guidance on the application of the revised structure. Nottingham: Fabis Consulting Ltd.
- Hairston NG, Kearns CM, Perry DL, Effler SW. 2005. Species‐specific Daphnia phenotypes: A history of industrial pollution and pelagic ecosystem response. Ecology. 86(7):1669–1678.
- Hairston NG, Lampert W, Cáceres CE, Holtmeier CL, Weider LJ, Gaedke U, Fischer JM, Fox JA, Post DM. 1999. Rapid evolution revealed by dormant eggs. Nature. 401:446.
- Hais M, Komprdová K, Ermakov N, Chytrý M. 2015. Modelling the Last Glacial Maximum environments for a refugium of Pleistocene biota in the Russian Altai Mountains, Siberia. Palaeogeogr Palaeoclimatol Palaeoecol. 438:135–145.
- Hájek M, Dudová L, Hájková P, Rolecek J, Moutelíková J, Jamrichová E, Horsák M. 2016. Contrasting Holocene environmental histories may explain patterns of species richness and rarity in a central European landscape. Quat Sci Rev. 133:48–61.
- Hájková P, Jamrichová E, Petr L, Dudová L, Roleček J, Gálová A, Dresler P, Novák J, Hájek M. 2018. Persistence of a vegetation mosaic in a peripheral region: could turbulent medieval history disrupt Holocene continuity of extremely species-rich grasslands? Veg Hist Archaeobot. 27:591–910.
- Hájková P, Rolecek J, Hájek M, Horsák M, Fajmon K, Polák M, Jamrichová E. 2011. Prehistoric origin of the extremely species-rich semi-dry grasslands in the Bílé Karpaty Mts (Czech Republic and Slovakia). Preslia. 83:185–204.
- Hällfors MH, Aikio S, Fronzek S, Hellmann JJ, Ryttäri T, Heikkinen RK. 2016. Assessing the need and potential of assisted migration using species distribution models. Biol Conserv. 196:60–68.
- Hällfors MH, Aikio S, Schulman LE. 2017. Quantifying the need and potential of assisted migration. Biol Conserv. 205 (SupplementC):34–41.
- Hamann O. 1981. Plant communities of the Galápagos Islands. Dansk Bot Ark. 34(2):1–163.
- Hamilton A, Watson F, Davies AL, Hanley N. 2011. Interdisciplinary conversations: the collective model. In: Sörlin S, Warde P, editors. Nature’s end history and the environment. Palgrave: Macmillan; p. 162–187.
- Hampe A, Arroya J. 2002. Recruitment and regeneration in populations of an endangered South Iberian Tertiary relict tree. Biol Conserv. 107:263–271.
- Hampe A, Jump AS. 2011. Climate relicts: past, present, future. Annu Rev Ecol Evol Syst. 42(1):313–333.
- Hampe A, Petit RJ. 2005. Conserving biodiversity under climate change: the rear edge matters. Ecol Lett. 8:461–467.
- Hanley N, Davies A, Angelopoulos K, Hamilton A, Ross A, Tinch D, Watson F. 2008. Economic determinants of biodiversity change over a 400-year period in the Scottish uplands. J Appl Ecol. 45:1557–1565.
- Hanley N, Ready R, Colombo S, Watson F, Stewart M, Bergmann EA. 2009b. The impacts of knowledge of the past on preferences for future landscape change. J Environ Manage. 90:1404–1412.
- Hanley N, Tinch D, Angelopoulos K, Davies A, Barbier EB, Watson F. 2009a. What drives long-run biodiversity change? New insights from combining economics, palaeoecology and environmental history. J Environ Econ Manage. 57:5–20.
- Hannon GE, Bradshaw RHW. 2000. Impacts and timing of the first human settlement on vegetation of the Færoe Islands. Quat Res. 54:404–413.
- Hannon GE, Bradshaw RHW, Bradshaw EG, Snowball I, Wastegård S. 2005. Climate change and human settlement as drivers of Late Holocene vegetational change in the Færoe Islands. Holocene. 15:639–647.
- Hannon GE, Halsall K, Molinari C, Boyle JF, Bradshaw RHW. 2018. The reconstruction of past forest dynamics over the last 13,500 years in SW Sweden. Holocene. 28(11):1791–1800.
- Hannon GE, Niklasson M, Brunet J, Eliasson P, Lindbladh M. 2010. How long has the ‘hotspot’ been ‘hot’? Past stand-scale structures at Siggaboda nature reserve in southern Sweden. Biodivers Conserv. 19(8):2167–2187.
- Hannon GE, Wastegård S, Bradshaw EG, Bradshaw RHW. 2001. Human impact and landscape degradation on the Faroe Islands. Bio Environ Proc R Irish Acad 101B(1–2):129–139.
- Hanselman JA, Bush MB, Gosling WD, Collins A, Knox C, Baker PA, Fritz SC. 2011. A 370,000-year record of vegetation and fire history around Lake Titicaca (Bolivia/Peru). Palaeogeogr Palaeoclimatol Palaeoecol. 305(1):201–214.
- Hansen DM, Donlan CJ, Giriffiths CJ, Campbell KJ. 2010. Ecological history and latent conservation potential: large and giant tortoises as a model for taxon substitutions. Ecography. 33:272–284.
- Hansen DM, Galetti M. 2009. The forgotten megafauna. Science. 324:42–43.
- Hanski I. 2016. Messages from Islands. A global biodiversity tour. Chicago: University of Chicago Press.
- Hansson SO, Helgesson G. 2003. What is stability? Synthese. 136:219–235.
- Hao Q, de Lafontaine G, Guo D, Gu H, Hu FS, Han Y, Song Z, Liu H. 2018. The critical role of local refugia in postglacial colonization of Chinese pine: joint inferences from DNA analyses, pollen records, and species distribution modeling. Ecography. 41(4):592–606.
- Hapsari KA, Biagioni S, Jennerjahn TC, Reimer P, Saad A, Sabiham S, Behling H. 2018. Resilience of a peatland in Central Sumatra, Indonesia to past anthropogenic disturbance: Improving conservation and restoration designs using palaeoecology. J Ecol. 106(6):2473–2490.
- Härdtle W. 1995. On the theoretical concept of the potential natural vegetation and proposals for an up-to-date modification. Folia Geobot Phytotaxonomica. 30:263–276.
- Harley JL, Harley EL. 1987. A check-list of mycorrhiza in the British flora. New Phytol. 105 (suppl.):1–102.
- Harper JL. 1967. A Darwinian approach to plant ecology. J Ecol. 55:247–270.
- Harris DB. 2009. Review of negative effects of introduced rodents on small mammals on islands. Biol Invasions. 11(7):1611–1630.
- Harrison SP, Prentice IC. 2003. Climate and CO2 controls on global vegetation distribution at the last glacial maximum: analysis based on palaeovegetation data, biome modelling and palaeoclimate simulations. Glob Chang Biol. 9:983–1004.
- Haskell J. 2001. The latitudinal gradient of diversity through the Holocene as recorded by fossil pollen in Europe. Evol Ecol Res. 3:345–360.
- Havrdová A, Douda J, Krak K, Vít P, Hadincová V, Zákravský P, Mandák B. 2015. Higher genetic diversity in recolonized areas than in refugia of Alnus glutinosa triggered by continent-wide lineage admixture. Mol Ecol. 24(18):4759–4777.
- Hawthorne D, Mitchell FJG. 2018. Investigating patterns of wildfire in Ireland and their correlation with regional and global trends in fire history. Quatern Int. 488:58–66.
- Hayashida FM. 2005. Archaeology, ecological history, and conservation. Annu Rev Anthropol. 34:43–65.
- Haynes CV, Eyles CH, Pavlish LA, Ritchie JC, Rybak M. 1989. Holocene palaeoecology of the eastern Sahara; Selima Oasis. Quat Sci Rev. 8(2):109–136.
- He X, Orlóci L. 1998. Anderson Pond revisited: the late-Quaternary vegetation process. Abstr Bot. 22:81–93.
- Head MJ, Gibbard PL. 2015. Early–Middle Pleistocene transitions: Linking terrestrial and marine realms. Quatern Int. 389:7–46.
- Heckenberger MJ, Kuikuro A, Kuikuro UT, Russell JC, Schmidt M, Fausto C, Franchetto B. 2003. Amazonia 1492: Pristine forest or cultural parkland? Science. 301(5640):1710.
- Heckenberger MJ, Russell JC, Toney JR, Schmidt MJ. 2007. The legacy of cultural landscapes in the Brazilian Amazon: implications for biodiversity. Philos Trans R Soc B Bio Sci. 362(1478):197–208.
- Heckmann M, Muiruri V, Marchant R. 2014. Human–environment interactions in an agricultural landscape: a 1400-yr sediment and pollen record from North Pare, NE Tanzania. Palaeogeogr Palaeoclimatol Palaeoecol. 406:49–61.
- Hedberg O. 1954. A pollen-analytical reconnaissance in tropical East Africa. Oikos. 5(2):137–166.
- Hédl R, Bernhardt-Römermann M, Grytnes J-A, Jurasinski G, Ewald J. 2017. Resurvey of historical vegetation plots: a tool for understanding long-term dynamics of plant communities. Appl Veg Sci. 20:161–163.
- Heinrichs S, Schmidt W. 2017. Biotic homogenization of herb layer composition between two contrasting beech forest communities on limestone over 50 years. Appl Veg Sci. 20(2):271–281.
- Heiri C, Bugmann H, Tinner W, Heiri O, Lischke H. 2006. A model-based reconstruction of Holocene treeline dynamics in the Central Swiss Alps. J Ecol. 94(1):206–216.
- Hellman SEV, Gaillard M-J, Broström A, Sugita S. 2008. The REVEALS model, a new tool to estimate past regional plant abundance from pollen data in large lakes: validation in southern Sweden. J Quat Sci. 23(1):21–42.
- Hempel S, Götzenberger L, Kühn I, Michalski SG, Rillig MC, Zobel M, Moora M. 2013. Mycorrhizas in the central European flora: relationships with plant life history traits and ecology. Ecology. 94(6):1389–1399.
- Hengeveld R. 1989. Dynamics of Biological Invasions. London: Chapman and Hall.
- Henn JJ, Buzzard V, Enquist BJ, Halbritter AH, Klanderud K, Maitner BS, Michaletz ST, Pötsch C, Seltzer L, Telford RJ et al. 2018. Intraspecific trait variation and phenotypic plasticity mediate alpine plant species response to climate change. Front Plant Sci. 9(1548).
- Henne PD, Elkin C, Colombaroli D, Samartin S, Bugmann H, Heiri O, Tinner W. 2013. Impacts of changing climate and land use on vegetation dynamics in a Mediterranean ecosystem: insights from paleoecology and dynamic modeling. Landsc Ecol. 28(5):819–833.
- Henne PD, Elkin C, Franke J, Colombaroli D, Calò C, La Mantia T, Pasta S, Conedera M, Dermody O, Tinner W. 2015. Reviving extinct Mediterranean forest communities may improve ecosystem potential in a warmer future. Front Ecol Environ. 13(7):356–362.
- Henne PD, Elkin CM, Bugmann HKM, Tinner W. 2011. Did soil development limit spruce (Picea abies) expansion in the Central Alps during the Holocene? Testing a palaeobotanical hypothesis using a dynamic landscape model. J Biogeogr. 38:933–949.
- Herben T, Chytrý M, Klimešová J. 2016. A quest for species‐level indicator values for disturbance. J Veg Sci. 27(3):628–636.
- Herring EM, Gavin DG, Dobrowski SZ, Fernandez M, Hu FS. 2018. Ecological history of a long-lived conifer in a disjunct population. J Ecol. 106(1):319–332.
- Herzschuh U, Birks HJB, Laepple T, Andreev AA, Melles M, Brigham-Grette J. 2016. Glacial legacies on interglacial vegetation at the Pliocene-Pleistocene transition in Asia. Nat Commun. 7:11967.
- Herzschuh U, Birks HJB, Ni J, Zhao Y, Liu H, Liu X, Grosse G. 2010. Holocene land-cover changes on the Tibetan Plateau. Holocene. 20:91–104.
- Hesselman H. 1916. Yttrande med anledning av L. von Post’s föredrag om skogsträdpollen i sydsvenska torfmosselagerfölder. Geologiska Föreningens i Stockholm Förhandlingar. 38:390–392.
- Hewitt GM. 1993. Chapter 7, Postglacial distribution and species substructure: lessons from pollen, insects and hybrid zones. In: Lees DR, Edwards D, editors. Evolutionary patterns and processes. London: Linnean Society of London/Academic Press; p. 97–123.
- Hewitt GM. 1996. Some genetic consequences of ice ages, and their role in divergence and speciation. Biol J Linn Soc. 58:247–276.
- Hewitt GM. 1999. Post-glacial re-colonization of European biota. Biol J Linn Soc. 68:87–112.
- Hewitt GM. 2000. The genetic legacy of the Quaternary ice ages. Nature. 405:907–913.
- Hewitt GM. 2001. Speciation, hybrid zones and phylogeography — or seeing genes in space and time. Mol Ecol. 10(3):537–549.
- Hewitt N, Klenk N, Smith AL, Bazely DR, Yan N, Wood S, MacLellan JI, Lipsig-Mumme C, Henriques I. 2011. Taking stock of the assisted migration debate. Biol Conserv. 144(11):2560–2572.
- Higgins SI, Nathan R, Cain ML. 2003. Are long-distance dispersal events in plants usually caused by nonstandard means of dispersal? Ecology. 84(8):1945–1956.
- Higgins SI, Richardson DM. 1999. Predicting plant migration rates in a changing world: The role of long-distance dispersal. Am Nat. 153(5):464–475.
- Higgs EC, Jackson ST. 2017. The role of history in ecological restoration. In: Allison S, Murphy SD, editors. Routledge handbook of ecological and environmental restoration. London: Routledge; p. 66–75.
- Higgs ES, Falk DA, Guerrini A, Hall M, Harris J, Hobbs RJ, Jackson ST, Rhemtulla JM, Throop W. 2014. The changing role of history in restoration ecology. Front Ecol Environ. 12(9):499–506.
- Higuera-Gundy A, Brenner M, Hodell DA, Curtis JH, Leyden BW, Binford MW. 1999. A 10,300 14C yr record of climate and vegetation change from Haiti. Quat Res. 52:159–170.
- Higuera PE. 2015. Taking time to consider the causes and consequences of large wildfires. Proc Natl Acad Sci USA. 112(43):13137–13138.
- Higuera PE, Whitlock C, Gage JA. 2011. Linking tree-ring and sediment-charcoal records to reconstruct fire occurrence and area burned in subalpine forests of Yellowstone National Park, USA. Holocene. 21(2):327–341.
- Hilbert L, Neves EG, Pugliese F, Whitney BS, Shock M, Veasey E, Zimpel CA, Iriarte J. 2017. Evidence for mid-Holocene rice domestication in the Americas. Nat Ecol Evol. 1(11):1693–1698.
- Hilborn R, Mangel M. 1997. The ecological detective - confronting models with data. New Jersey: Princeton University Press.
- Hill JK, Griffiths HM, Thomas CD. 2011. Climate change and evolutionary adaptations at species’ range margins. Annu Rev Entomol. 56(1):143–159.
- Hill MO, Carey PD. 1997. Prediction of yield in the Rothamsted Park Grass Experiment by Ellenberg indicator values. J Veg Sci. 8:579–586.
- Hill MO, Preston CD, Roy DB. 2004. PLANTATT - Attributes of British and Irish Plants: status, size, life history, geography and habitats. Huntingdon: Centre for Ecology & Hydrology.
- HilleRisLambers J, Harsch MA, Ettinger AK, Ford KR, Theobald EJ. 2013. How will biotic interactions influence climate change–induced range shifts? Ann N Y Acad Sci. 1297(1):112–125.
- Hintikka V. 1963. Über das Grossklima einiger Pflanzenareale in zwei Klimakoordinatensystemen dargestellt. Ann Bot Soc Zool Bot Fenn Vanamo. 34(5):1–64.
- Hirota M, Holmgren M, Van Nes EH, Scheffer M. 2011. Global resilience of tropical forest and savanna to critical transitions. Science. 334(6053):232–235.
- Hisano M, Searle EB, Chen HYH. 2018. Biodiversity as a solution to mitigate climate change impacts on the functioning of forest ecosystems. Biol Rev. 93(1):439–456.
- Hixon SW, Elliott Smith EA, Crowley BE, Perry GH, Randrianasy J, Ranaivoarisoa JF, Kennett DJ, Newsome SD. 2018. Nitrogen isotope (δ15N) patterns for amino acids in lemur bones are inconsistent with aridity driving megafaunal extinction in south-western Madagascar. J Quat Sci. 33(8):958–968.
- Hobbs RJ, Higgs ES, Hall CM, editors. 2013. Novel ecosystems: intervening in the new ecological world order. Chichester: Wiley-Blackwell.
- Hobbs RJ, Higgs ES, Hall CM, Bridgewater P, Chapin FS, Ellis EC, Ewel JJ, Hallett LM, Harris J, Hulvey KB et al. 2014b. Managing the whole landscape: historical, hybrid, and novel ecosystems. Front Ecol Environ. 12(10):557–564.
- Hobbs RJ, Higgs ES, Harris JA. 2009. Novel ecosystems: implications for conservation and restoration. Trends Ecol Evol. 24(11):599–605.
- Hobbs RJ, Higgs ES, Harris JA. 2014a. Novel ecosystems: concept or inconvenient reality? A response to Murcia et al. Trends Ecol Evol. 29(12):645–646.
- Hobbs RJ, Valentine LE, Standish RJ, Jackson ST. 2018. Movers and stayers: novel assemblages in changing environments. Trends Ecol Evol. 33(2):116–128.
- Hobbs WO, Ramstack Hobbs JM, LaFrançois T, Zimmer KD, Theissen KM, Edlund MB, Michelutti N, Butler MG, Hanson MA, Carlson TJ. 2012. A 200‐year perspective on alternative stable state theory and lake management from a biomanipulated shallow lake. Ecol App. 22(5):1483–1496.
- Hodder KH, Buckland PC, Kirby KJ, Bullock JM. 2009. Can the mid-Holocene provide records for rewilding the landscape in Britain. Br Wildl. 20(5 Special Supplement):4–15.
- Hodell DA, Anselmetti FS, Ariztegui D, Brenner M, Curtis JH, Gilli A, Grzesik DA, Guilderson TJ, Müller AD, Bush MB et al. 2008. An 85-ka record of climate change in lowland Central America. Quat Sci Rev. 27(11):1152–1165.
- Hodgson D, McDonald JL, Hosken DJ. 2015. What do you mean, ‘resilient’? Trends Ecol Evol. 30(9):503–506.
- Høeg HI. 1978. The immigration of Picea abies to southeasten Norway with special regard to Telemark (a preliminary report). Norw J Bot. 25:19–21.
- Hoegh-Goldberg O, Hughes L, McIntyre S, Lindenmayer DB, Parmesan C, Possingham HP, Thomas CD. 2008. Assisted colonization and rapid climate change. Science. 321:345–346.
- Hof C, Levinsky I, Araújo MB, Rahbek C. 2011. Rethinking species’ ability to cope with rapid climate change. Glob Chang Biol. 17:2987–2990.
- Hoffmann AA, Sgrò CM. 2011. Climate change and evolutionary adaptation. Nature. 470:479–485.
- Hofreiter M, Paijmans JLA, Goodchild H, Speller, C.F., Barlow A, Fortes GG, Thomas JA, Ludwig A, Collins MJ. 2014. The future of ancient DNA: Technical advances and conceptual shifts. Bioessays. 37(3):284–293.
- Hofreiter M, Stewart JR. 2009. Ecological change, range fluctuations and population dynamics during the Pleistocene. Curr Biol. 19(14):R584–R594.
- Hofstetter S, Tinner W, Valsecchi V, Carraro G, Conedera M. 2006. Lateglacial and Holocene vegetation history in the Insubrian Southern Alps—New indications from a small-scale site. Veg Hist Archaeobot. 15(2):87–98.
- Holl KD, Reid JL, Oviedo-Brenes F, Kulikowski A, Zahawi RA. 2018. Rules of thumb for predicting tropical forest recovery. Appl Veg Sci. 21(4):669–677.
- Holling CS. 1973. Resilience and stability of ecological systems. Annu Rev Ecol Syst. 4:1–23.
- Holm SR, Svenning J-C. 2014. 180,000 years of climate change in Europe: avifaunal responses and vegetation implications. PLoS One. 9(4):e94021.
- Holmes JA. 2008. How the Sahara became dry. Science. 320(5877):752.
- Holt RD. 1990. The microevolutionary consequences of climate change. Trends Ecol Evol. 5(9):311–315.
- Holt RD. 2009. Bringing the Hutchinsonian niche into the 21st century: Ecological and evolutionary perspectives. Proc Natl Acad Sci USA. 106(Supplement 2):19659.
- Honnay O, Verheyen K, Butaye J, Jacquemyn H, Bossuyt B, Hermy M. 2002. Possible effects of habitat fragmentation and climate change on the range of forest plant species. Ecol Lett. 5(4):525–530.
- Hooghiemstra H, Olijhoek T, Hoogland M, Prins M, van Geel B, Donders T, Gosling WD, Hofman CA. 2018. Columbus’ environmental impact in the new world: land use change in the Yaque River valley, Dominican Republic. Holocene. 28(11):1818–1835.
- Hooghiemstra H, van Geel B, Cleef AM. 2010. Prof. Dr. Thomas van der Hammen (1924–2010). Rev Palaeobot Palynol. 162:116–118.
- Hooghiemstra H, Wijninga VM, Cleef AM. 2006. The paleobotanical record of Colombia: implications for biogeography and biodiversity. Ann Missouri Bot Garden. 93:297–324.
- Hooker JD. 1844. The botany of the antarctic voyage of HMS discovery ships ‘Erebus’ and ‘Terror’ in the year 1839–1843, under the command of captain Sir James Cook. Vol. 1. Flora Antarctica. Part 1 Botany of Lord Auckland’s Group and Campbell’s Island. London: Reeve.
- Hooker JD. 1847a. An enumeration of the plants of the Galápagos Archipelago; with descriptions of those which are new. Trans Linn Soc Lond. 20:163–233.
- Hooker JD. 1847b. On the vegetation of the Galapagos Archpelago as compared with that of some other tropical islands and the continent of America. Trans Linn Soc Lond. 20:235–262.
- Hooker JD. 1867. Insular floras. Gard Chron Agric Gaz. 1867:6–7, 27, 50–51, 75–76.
- Hope GS. 1976. The vegetational history of Mt Wilhelm, Papua New Guinea. J Ecol. 64:627–663.
- Horrocks M, Baisden WT, Harper MA, Marra M, Flenley J, Feek D, Haoa-Cardinali S, Keller ED, Nualart LG, Gorman TE. 2015. A plant microfossil record of Late Quaternary environments and human activity from Rano Aroi and surroundings, Easter Island. J Paleolimnol. 54(4):279–303.
- Horrocks M, Baisden WT, Nieuwoudt MK, Flenley J, Feek D, González Nualart L, Haoa-Cardinali S, Edmunds Gorman T. 2012. Microfossils of polynesian cultigens in lake sediment cores from Rano Kau, Easter Island. J Paleolimnol. 47(2):185–204.
- Horrocks M, Marra M, Baisden WT, Flenley J, Feek D, González Nualart L, Haoa-Cardinali S, Edmunds Gorman T. 2013. Pollen, phytoliths, arthropods and high-resolution 14C sampling from Rano Kau, Easter Island: evidence for late Quaternary environments, ant (Formicidae) distributions and human activity. J Paleolimnol. 50(4):417–432.
- Hotchkiss S. 2003. Quaternary history from the U.S. tropics. In: Gillespie AR, Porter SC, Atwater BF, editors. The Quaternary period in the United States. Amsterdam: Elsevier; p. 441–457.
- Hotchkiss S, Juvik JO. 1999. A late-Quaternary pollen record from Ka’au Crater, O’ahu, Hawai’i. Quat Res. 52:115–128.
- Hotchkiss SC, Calcote R, Lynch EA. 2007. Response of vegetation and fire to Little Ice Age climate change: regional continuity and landscape heterogeneity. Landsc Ecol. 22:25–41.
- Hothorn T, Hornik K, Zeileis A. 2006. Unbiased recursive partitioning: a conditional inference framework. J Comput Graphical Stat. 15(3):651–674.
- Howard C, Stephens PA, Pearce-Higgins JW, Gregory RD, Willis SG. 2014. Improving species distribution models: the value of data on abundance. Methods Ecol Evol. 5:506–513.
- Howe S, Webb T. 1983. Calibrating pollen data in climatic terms: Improving the methods. Quat Sci Rev. 2(1):17–51.
- Hu FS, Hampe A, Petit RJ. 2009. Paleoecology meets genetics: deciphering past vegetational dynamics. Front Ecol Environ. 7:371–379.
- Huang YJ, Jacques FMB, Su T, Ferguson DK, Tang H, Chen W, Zhou Z. 2015. Distribution of Cenozoic plant relicts in China explained by drought in dry season. Sci Rep. 5:14212.
- Hubbell SP. 2001. The unified neutral theory of biodiversity and biogeography. Princeton: Princeton University Press.
- Huber O. 1988. Guayana highlands versus Guayana lowlands, a reappraisal. Taxon. 37:595–614.
- Huber O. 1995. Geographical and physical features. In: Berry PE, Holst BK, Yatskievych K, editors. Flora of the Venezuelan Guayana I Introduction. St Louis: Missouri Botanical Garden Press; p. 1–61.
- Huber O, Prance GT, Kroonenberg B, Antonelli A. 2018. The tepuis of the guinea highlands. In: Hoorn C, Perrigo A, Antonelli A, editors. Mountains, climate and biodiversity. Oxford: Wiley Blackwell; p. 339–353.
- Huckerby E, Oldfield F. 1976. The Quaternary vegetational history of the French Pays Basque, II: Plant macrofossils and additional pollen-analytical data. New Phytol. 77:499–526.
- Hudson WH. 1904. Green mansions. London: Duckworth.
- Huete A. 2016. Vegetation’s responses to climate variability. Nature. 531:181.
- Hulme PE, Pauchard A, Pyšek P, Vilà M, Alba C, Blackburn TM, Bullock JM, Chytrý M, Dawson W, Dunn AM et al. 2015. Challenging the view that invasive non-native plants are not a significant threat to the floristic diversity of Great Britain. Proc Natl Acad Sci USA. 112(23):E2988–E2989.
- Hultberg T, Gaillard M-J, Grundmann B, Lindbladh M. 2015. Reconstruction of past landscape openness using the Landscape Reconstruction Algorithm (LRA) applied on three local pollen sites in a southern Swedish biodiversity hotspot. Veg Hist Archaeobot. 24(2):253–266.
- Hultberg T, Lagerås P, Björkman L, Sköld E, Jacobson GL, Hedwall P-O, Lindbladh M. 2017. The late-Holocene decline of Tilia in relation to climate and human activities – pollen evidence from 42 sites in southern Sweden. J Biogeogr. 44(10):2398–2409.
- Hume JP. 2017. Extinct birds. 2nd ed. London: Bloomsbury Natural History.
- Humphreys AM, Govaerts R, Ficinski SZ, Lughadha EN, Vorontsova MS. 2019. Global dataset shows geography and life form predict modern plant extinction and rediscovery. Nat Ecol Evol. doi:10.1038/s41559-41019-40906-41552.
- Hunt CO, Gilbertson DD, Rushworth G. 2012. A 50,000-year record of late Pleistocene tropical vegetation and human impact in lowland Borneo. Quat Sci Rev. 37:61–80.
- Hunt TL. 2007. Rethinking Easter Island’s ecological catastrophe. J Archaeol Sci. 34:485–502.
- Hunt TL, Lipo CP. 2006. Late colonization of Easter Island. Science. 311:1603–1606.
- Hunt TL, Lipo CP. 2011. The statues that walked. New York: Simon and Schuster.
- Hunter ML. 2007. Climate change and moving species: furthering the debate on assisted colonization. Conserv Bio. 21(5):1356–1358.
- Hunter ML, Jacobson GL, Webb T. 1988. Paleoecology and the coarse-filter approach to maintaining biological diversity. Conserv Bio. 2:375–385.
- Hunter ML, Redford KH, Lindenmayer DB. 2014. The complementary niches of anthropocentric and biocentric conservationists. Conserv Bio. 28(3):641–645.
- Huntley B. 1988. Europe. In: Huntley B, Webb T, editors. Vegetation history. Dordrecht: Kluwer; p. 341–383.
- Huntley B. 1990a. Dissimilarity mapping between fossil and contemporary pollen spectra in Europe for the past 13,000 years. Quat Res. 33(3):360–376.
- Huntley B. 1990b. European post-glacial forests: compositional changes in response to climatic change. J Veg Sci. 1(4):507–518.
- Huntley B. 1991. How plants respond to climate change: migration rates, indivdualism and the consequences for plant communities. Ann Bot. 67(Suppl.1):15–22.
- Huntley B. 1996. Quaternary palaeoecology and ecology. Quat Sci Rev. 15:591–606.
- Huntley B. 2014. Extreme temporal interpolation of sparse data is not a sufficient basis to substantiate a claim to have uncovered Pleistocene forest microrefugia. New Phytol. 204:447–449.
- Huntley B, Allen JRM, Bennie JJ, Collingham YC, Miller PA, Suggitt AJ. 2018. Climatic disequilibrium threatens conservation priority forests. Conserv Lett. 11(1):e12349.
- Huntley B, Allen JRM, Collingham YC, Hickler T, Lister AM, Singarayer J, Stuart AJ, Sykes MT, Valdes PJ. 2013. Millennial climatic fluctuations are key to the structure of last glacial ecosystems. PLoS One. 8(4):e61963.
- Huntley B, Barnard P, Altwegg R, Chambers L, Coetzee BWT, Gibson L, Hockey PAR, Hole DG, Midgley GF, Underhill LG et al. 2010. Beyond bioclimatic envelopes: dynamic species’ range and abundance modelling in the context of climatic change. Ecography. 33(3):621–626.
- Huntley B, Birks HJB. 1983. An atlas of past and present pollen maps for europe: 0-13000 years ago. Cambridge: Cambridge University Press.
- Huntley B, Webb T, editors. 1988. Vegetation history. Dordrecht: Kluwer Academic Publishers.
- Huntley B, Webb T. 1989. Migration: species’ response to climatic variations caused by changes in the Earth’s orbit. J Biogeogr. 16:5–19.
- Hutchinson GE. 1957. Concluding remarks. Cold Spring Harb Symp Quant Biol. 22:415–427.
- Hutchinson GE. 1978. An Introduction to Population Ecology. New Haven: Yale University Press.
- Huttunen P, Tolonen M. 1972. Pollen-analytical studies of prehistoric agriculture in Northern Ångermanland. Early Norrland. 1:9–34.
- Iglesias V, Krause TR, Whitlock C. 2015a. Complex response of white pines to past environmental variability increases understanding of future vulnerability. PLoS One. 10(4):e0124439.
- Iglesias V, Whitlock C, Krause TR, Baker RG. 2018. Past vegetation dynamics in the Yellowstone region highlight the vulnerability of mountain systems to climate change. J Biogeogr. 45(8):1768–1780.
- Iglesias V, Yospin GI, Whitlock C. 2015b. Reconstruction of fire regimes through integrated palaeoecological proxy data and ecological modeling. Front Plant Sci. 5:art785.
- Illera JC, Rando JC, Richardson DS, Emerson BC. 2012. Age, origins and extinctions of the avifauna of Macaronesia: a synthesis of phylogenetic and fossil information. Quat Sci Rev. 50:14–22.
- Imbrie J, Imbrie KP. 1979. Ice Ages: Solving the Mystery. Short Hills, New Jersey: Enslow Publishers.
- Indermühle A, Stocker TF, Joos F, Fischer H, Smith HJ, Wahlen M, Deck B, Mastroianni D, Tschumi J, Blunier T et al. 1999. Holocene carbon-cycle dynamics based on CO2 trapped in ice at Taylor Dome, Antarctica. Nature. 398:121.
- Ingrisch J, Bahn M. 2018. Towards a comparable quantification of resilience. Trends Ecol Evol. 33(4):251–259.
- Irl SDH, Schweiger AH, Medina FM, Fernández-Palacios JM, Harter DEV, Jentsch A, Provenzale A, Steinbauer MJ, Beierkuhnlein C. 2017. An island view of endemic rarity—Environmental drivers and consequences for nature conservation. Divers Distrib. 23(10):1132–1142.
- Isbell F, Gonzalez A, Loreau M, Cowles J, Diaz S, Hector A, Mace GM, Wardle DA, O’Connor MI, Duffy JE et al. 2017. Linking the influence and dependence of people on biodiversity across scales. Nature. 546(7656):65–72.
- Iversen J. 1944. Viscum, Hedera and Ilex as climate indicators. A contribution to the study of the post-glacial temperature climate. Geologiska Föreningens i Stockholm Förhandlingar. 66(3):463–483.
- Iversen J. 1947. Geologklubben vid Stockholms Högskola. Nordiskt kvartärgeologiskt möte den 5–9 november 1945. Geologiska Föreningens i Stockholm Förhandlingar. 69(2):205–252.
- Iversen J. 1950. Book review: Late and post-glacial forest history in central Europe. Oikos. 2:315–318.
- Iversen J. 1954. The Late-glacial flora of Denmark and its relation to climate and soil. Danmarks Geol Unders II. 80:87–119.
- Iversen J. 1958. The bearing of glacial and interglacial epochs on the formation and extinction of plant taxa. Syst Today Uppsala Univ Årsskr 6:210–215.
- Iversen J. 1960. Problems of the early post-glacial forest development in Denmark. Danmarks Geol Unders IV. 4(3):1–32.
- Iversen J. 1967. Naturens Udvikling siden sidste Istid. Danmarks Natur. 1:345–445.
- Iversen J. 1973. The development of Denmark’s nature since the last glacial. Danmarks Geol Unders V. 7-C:1–126 (English translation of Iversen 1967).
- Ivory SJ, Russell J. 2017. Lowland forest collapse and early human impacts at the end of the African Humid Period at Lake Edward, equatorial East Africa. Quat Res. 89(1):7–20.
- Iwaschkewitsch BA. 1929. Die wichstigen eigenarten der struktur und der entwicklung der urwaldsbestände. Proceedings of the 7th International Congress of Forestry Experimental Stations, Stockholm; p. 129–147.
- Jackson ST. 1994. Pollen and spores in Quaternary lake sediments as sensors of vegetation composition: theoretical models and empirical evidence. In: Traverse A, editor. Sedimentation of organic particles. Cambridge: Cambridge University Press; p. 253–286.
- Jackson ST. 1997. Documenting natural and human-caused plant invasions using paleoecological methods. In: Luken JO, Thieret JW, editors. Assessment and management of plant invasions. New York: Springer-Verlag; p. 37–55.
- Jackson ST. 2000. Out of the garden and into the cooler? A Quaternary perspective on deep-time paleoecology. Paleontol Soc Pap. 6:287–308.
- Jackson ST. 2001. Finding your way back home, or at least learning how it looked. Divers Distrib. 7:301–305.
- Jackson ST. 2006a. Vegetation, environment, and time: the origination and termination of ecosystems. J Veg Sci. 17:549–557.
- Jackson ST. 2006b. Forest genetics in space and time. New Phytol. 171:1–3.
- Jackson ST. 2007. Looking forward from the past: history, ecology, and conservation. Front Ecol Environ. 5(9):455.
- Jackson ST. 2012a. Conservation and resource management in a changing world: extending the historical range of variation beyond the baseline. In: Wiens JA, Hayward GD, Safford HD et al., editors. Historical environmental variation in conservation and natural resource management. Chichester: Wiley-Blackwell; p. 92–109.
- Jackson ST. 2012b. Ecosystems - How do ecosystems and associated services respond to climatic change? The past. PAGES News. 20:41.
- Jackson ST. 2012c. Representation of flora and vegetation in Quaternary fossil assemblages: known and unknown knowns and unknowns. Quat Sci Rev. 49:1–15.
- Jackson ST. 2013a. Natural, potential and actual vegetation in North America. J Veg Sci. 24:772–776.
- Jackson ST. 2013b. Perspective: ecological novelty is not new. In: Hobbs RJ, Higgs ES, Hall CM, editors. Novel ecosystems: intervening in the new ecological world order. Chichester: Wiley-Blackwell; p. 63–65.
- Jackson ST. 2016. Reinventing conservation – again. Front Ecol Environ. 14(10):519–519.
- Jackson ST, Betancourt JL, Booth RK, Gray ST. 2009b. Ecology and the ratchet of events: Climate variability, niche dimensions, and species distributions. Proc Natl Acad Sci USA. 106:19685–19692.
- Jackson ST, Betancourt JL, Lyford ME, Gray ST, Rylander KA. 2005. A 40,000‐year woodrat‐midden record of vegetational and biogeographical dynamics in north‐eastern Utah, USA. J Biogeogr. 32(6):1085–1106.
- Jackson ST, Blois JL. 2015. Community ecology in a changing environment: Perspectives from the Quaternary. Proc Natl Acad Sci USA. 112(16):4915–1921.
- Jackson ST, Booth RK. 2002. The role of late Holocene climate variability in the expansion of yellow birch in the western Great Lakes region. Divers Distrib. 8:275–284.
- Jackson ST, Booth RK. 2013. Validation of pollen studies. In: Elias SA, Mock CJ, editors. Encyclopedia of Quaternary science. 2nd ed. Amsterdam: Elsevier; p. 725–732.
- Jackson ST, Booth RK, Reeves K, Andersen JJ, Minckley TA, Jones RA. 2014. Inferring local to regional changes in forest composition from Holocene macrofossils and pollen of a small lake in central Upper Michigan. Quat Sci Rev. 98:60–73.
- Jackson ST, Garfin GM, Enquist CAF. 2017. Toward an effective practice of translational ecology. Front Ecol Environ. 15:540.
- Jackson ST, Givens CR. 1994. Late Wisconsinan vegetation and environment of the Tunica Hills Region, Louisiana/Mississippi. Quat Res. 41:316–325.
- Jackson ST, Gray ST, Shuman B. 2009a. Paleoecology and resource management in a dynamic landscape: case studies from the Rocky Mountain headwaters. In: Dietl GP, Flessa KW, editors. Conservation paleobiology: using the past to manage for the future. New York: The Paleontological Society Papers; p. 61–80.
- Jackson ST, Grimm EC, Thompson RS. 2000a. Database resources in Quaternary paleobotany. SIDA Bot Miscellany. 18:113–120.
- Jackson ST, Hobbs RJ. 2009. Ecological restoration in the light of ecological history. Science. 325:567–569.
- Jackson ST, Lyford ME. 1999. Pollen dispersal models in Quaternary plant ecology: Assumptions, parameters, and prescriptions. Bot Rev. 65(1):39–75.
- Jackson ST, Overpeck JT. 2000. Responses of plant populations and communities to environmental changes of the late Quaternary. Paleobiology. 26 (Supplement 4): 194–220.
- Jackson ST, Sax DF. 2010. Balancing biodiversity in a changing environment: extinction debt, immigration credit and species turnover. Trends Ecol Evol. 25(3):153–160.
- Jackson ST, Webb RS, Anderson KH, Overpeck JT, Webb T, Williams JW, Hansen BCS. 2000b. Vegetation and environment in eastern North America during the last glacial maximum. Quat Sci Rev. 19:489–508.
- Jackson ST, Weng CY. 1999. Late Quaternary extinction of a tree species in eastern North America. Proc Natl Acad Sci USA. 96(24):13847–13852.
- Jackson ST, Whitehead DR. 1991. Holocene vegetation patterns in the Adirondack Mountains. Ecology. 72:641–653.
- Jackson ST, Williams JW. 2004. Modern analogs in Quaternary paleoecology: Here today, gone yesterday, gone tomorrow? Annu Rev Earth Planet Sci. 32:495–537.
- Jacobson GL. 1979. The palaeoecology of white pine (Pinus strobus) in Minnesota. J Ecol. 67(2):697–726.
- Jacobson GL. 1988. Ancient permanent plots: sampling in paleovegetational studies. In: Huntley B, Webb T, editors. Vegetation history. Dordrecht: Kluwer Academic Publications; p. 3–16.
- Jacobson GL, Bradshaw RHW. 1981. The selection of sites for paleovegetational studies. Quat Res. 16(1):80–96.
- Jacobson GL, Webb T, Grimm EC. 1987. Patterns and rates of vegetation change during the deglaciation of eastern North America. In: Ruddiman WF, Wright HE, editors. North America and adjacent oceans during the last deglaciation. Boulder (CO): Geological Society of America; p. 277–288.
- Jacobson MC, Charlson RJ, Rodhe H, Orians GH, editors. 2010. Earth system science: from biogeochemical cycles to global changes. Cambridge, Massachusetts: Academic Press.
- James FC, Johnston RF, Wamer NO, Niemi GJ, Boecklen WJ. 1984. The Grinnellian niche of the wood thrush. Am Nat. 124:17–30.
- Jamrichová E, Petr L, Jiménez-Alfaro B, Jankovská V, Dudová L, Pokorný P, Kołaczek P, Zernitskaya V, Čierniková M, Břízová E et al. 2017. Pollen-inferred millennial changes in landscape patterns at a major biogeographical interface within Europe. J Biogeogr. 44(10):2386–2397.
- Jamrichová E, Szabó P, Hédl R, Kuneš P, Bobek P, Pelánková B. 2012. Continuity and change in the vegetation of a central European oakwood. Holocene. 23(1):46–56.
- Janská V, Jiménez-Alfaro B, Chytrý M, Divíšek J, Anenkhonov O, Korolyuk A, Lashchinskyi N, Culek M. 2017. Palaeodistribution modelling of European vegetation types at the Last Glacial Maximum using modern analogues from Siberia: Prospects and limitations. Quat Sci Rev. 159:103–115.
- Janssen CR. 1967. A post‐glacial pollen diagram from a small Typha swamp in northwestern Minnesota, interpreted from pollen indicators and surface samples. Ecol Monogr. 37:145–172.
- Janssen CR. 1970. Problems in the recognition of plant communities in pollen diagrams. Vegetatio. 20:187–198.
- Janssen CR. 1981. On the reconstruction of past vegetation by pollen analysis: a review. Proc IVth Int Palynological Conf Lucknow (1976–1977). 3:163–172.
- Janssen CR, Törnqvist TE. 1991. The role of scale in the biostratigraphy and chronostratigraphy of the Holocene Series in The Netherlands. Holocene. 1(2):112–120.
- Jansson R, Dynesius M. 2002. The fate of clades in a world of recurrent climatic change: Milankovitch oscillations and evolution. Annu Rev Ecol Syst. 33:741–777.
- Janzen DH. 1984. Dispersal of small seeds by big herbivores: foliage is the fruit. Am Nat. 123:338–350.
- Janzen DH, Martin PS. 1982. Neotropical anachronisms: the fruit the Gomphotheres ate. Science. 215:19–27.
- Jax K, Heink U. 2015. Searching for the place of biodiversity in the ecosystem services discourse. Biol Conserv. 191:198–205.
- Jeffers ES, Bonsall MB, Brooks SJ, Willis KJ. 2011b. Abrupt environmental changes drive shifts in tree–grass interaction outcomes. J Ecol. 99:1063–1070.
- Jeffers ES, Bonsall MB, Watson JE, Willis KJ. 2012. Climate change impacts on ecosystem functioning: evidence from an Empetrum heathland. New Phytol. 193:150–164.
- Jeffers ES, Bonsall MB, Willis KJ. 2011a. Stability in ecosystem functioning across a climatic threshold and contrasting forest regimes. PLoS One. 6:e16134.
- Jeffers ES, Nogué S, Willis KJ. 2015. The role of palaeoecological records in assessing ecosystem services. Quat Sci Rev. 112:17–32.
- Jeffers ES, Whitehouse NJ, Lister A, Plunkett G, Barratt P, Smyth E, Lamb P, Dee MW, Brooks SJ, Willis KJ et al. 2018. Plant controls on Late Quaternary whole ecosystem structure and function. Ecol Lett. 21(6):814–825.
- Jeffries M. 1989. Measuring Talling’s ‘element of chance in pond populations’. Freshw Biol. 21:383–393.
- Jensen K, Lynch EA, Calcote R, Hotchkiss SC. 2007. Interpretation of charcoal morphotypes in sediments from Ferry Lake, Wisconsin, USA: do different plant fuel sources produce distinctive charcoal morphotypes? Holocene. 17(7):907–915.
- Jensen PB. 1910. Studier over Skovtrærnes Forhold til Lyset. Tidsskr Skovvæsen. 22 B:1–116.
- Jensen PB. 1949. Causal plant-geography. Kongelige Danske Videnskabernes Selskab Biologiske Medd. 21(3):1–19.
- Jeppesen E, Appelt M, Hastrup K, Grønnow B, Mosbech A, Smol JP, Davidson TA. 2018. Living in an oasis: rapid transformations, resilience, and resistance in the North Water Area societies and ecosystems. Ambio. 47(2):296–309.
- Jessen K. 1920. Moseundersøgelser i det nordøstlige Sjælland. Danmarks Geol Unders II. 34:1–268.
- Jessen K. 1949. Studies in late Quaternary deposits and flora-history of Ireland. Proc R Irish Acad B. 52:85–290.
- Jessen K, Andersen ST, Farrington A. 1959. The interglacial deposit near Gort, Co. Galway, Ireland. Proc R Irish Acad B. 60:3–77.
- Jóhansen J. 1968. A pollen diagram from the Færoe Islands showing the former presence of Betula nana. Ann Soc Scientiarum Færoensis. 16:119–128.
- Jóhansen J. 1975. Pollen diagrams from the Shetland and Færoe Islands. New Phytol. 75:369–387.
- Jóhansen J. 1985. Studies in the vegetational history of the Faroe and Shetland Islands. Ann Soc Scientiarum Færoensis. S11:1–117.
- Jóhansen J. 1989. Survey of geology, climate and vegetational history. In: Højgaard A, Jóhansen J, Odum S, editors. A century of tree-planting in the Faroe Islands. Føroya: Fróoskarparfelg; p. 11–15.
- Johnson CN, Rule S, Haberle SG, Kershaw AP, McKenzie GM, Brook BW. 2016. Geographic variation in the ecological effects of extinction of Australia’s Pleistocene megafauna. Ecography. 39:109–116.
- Johnson CN, Rule S, Haberle SG, Turney CSM, Kershaw AP, Brook BW. 2015. Using dung fungi to interpret decline and extinction of megaherbivores: problems and solutions. Quat Sci Rev. 110:107–113.
- Johnson WC, Webb T. 1989. The role of blue jays (Cyanocitta cristata L.) in the postglacial dispersal of fagaceous trees in eastern North America. J Biogeogr. 16:561–571.
- Johnstone JF, McIntire EJB, Pedersen EJ, King G, Pisaric MJF. 2010. A sensitive slope: estimating landscape patterns of forest resilience in a changing climate. Ecosphere. 1 (6):art14.
- Jones EW. 1945. The structure and reproduction of the virgin forest of the north temperate zone. New Phytol. 44(2):130–148.
- Jones HG, Vaughan RA. 2010. Remote sensing of vegetation. Oxford: Oxford University Press.
- Jones PJ, Thomas I, Fletcher M-S. 2017. Long-term environmental change in eastern Tasmania: Vegetation, climate and fire at Stoney Lagoon. Holocene. 27(9):1340–1349.
- Jones RA, Williams JW, Jackson ST. 2017. Vegetation history since the last glacial maximum in the Ozark highlands (USA): A new record from Cupola Pond, Missouri. Quat Sci Rev. 170 (SupplementC):174–187.
- Joos F, Gerber S, Prentice IC, Otto-Bliesner BL, Valdes PJ. 2004. Transient simulations of Holocene atmospheric carbon dioxide and terrestrial carbon since the Last Glacial Maximum. Global Biogeochem Cycles. 18:GB2002.
- Joppa LN, O’Connor B, Visconti P, Smith C, Geldmann J, Hoffmann M, Watson JEM, Butchart SHM, Virah-Sawmy M, Halpern BS et al. 2016. Filling in biodiversity threat gaps. Science. 352(6284):416–418.
- Jordano P. 2017. What is long-distance dispersal? And a taxonomy of dispersal events. J Ecol. 105:75–84.
- Josefsson T, Hörnberg G, Östlund L. 2009. Long-term human impact and vegetation changes in a boreal forest reserve: Implications for the use of protected areas as ecological references. Ecosystems. 12(6):1017–1036.
- Jump AS, Peñuelas J. 2005. Running to stand still: adaptation and the response of plants to rapid climate change. Ecol Lett. 8(9):1010–1020.
- Junqueira AB, Levis C, Bongers F, Peña-Claros M, Clement CR, Costa F, ter Steege H. 2017. Response to comment on “Persistent effects of pre-Columbian plant domestication on Amazonian forest composition”. Science. 358 (6361):eaan8837.
- Junqueira AB, Shepard GH, Clement CR. 2010. Secondary forests on anthropogenic soils in Brazilian Amazonia conserve agrobiodiversity. Biodivers Conserv. 19(7):1933–1961.
- Juřičková L, Horáčková J, Lozek V. 2014. Direct evidence of central European forest refugia during the last glacial period based on mollusc fossils. Quat Res. 82:222–228.
- Juřičková L, Pokorný P, Hošek J, Horáčková J, Květoň J, Zahajská P, Jansová A, Ložek V. 2018. Early postglacial recolonisation, refugial dynamics and the origin of a major biodiversity hotspot. A case study from the Malá Fatra mountains, Western Carpathians, Slovakia. Holocene. 28(4):583–594.
- Kaltenrieder P, Belis CA, Hofstetter S, Ammann B, Ravazzi C, Tinner W. 2009. Environmental and climatic conditions at a potential Glacial refugial site of tree species near the Southern Alpine glaciers. New insights from multiproxy sedimentary studies at Lago della Costa (Euganean Hills, Northeastern Italy). Quat Sci Rev. 28(25):2647–2662.
- Kaltenrieder P, Procacci G, Vannière B, Tinner W. 2010. Vegetation and fire history of the Euganean Hills (Colli Euganei) as recorded by Lateglacial and Holocene sedimentary series from Lago della Costa (northeastern Italy). Holocene. 20(5):679–695.
- Kapfer J, Gunnarsson U, Grytnes J-A, Birks HJB. 2011. Fine-scale changes in vegetation composition in a boreal mire over 50 years. J Ecol. 99:1179–1189.
- Kapfer J, Hédl R, Jurasinski G, Kopecký M, Schei FH, Grytnes J-A. 2017. Resurveying historical vegetation data – opportunities and challenges. Appl Veg Sci. 20(2):164–171.
- Kaplan JO, Krumhardt KM, Gaillard M-J, Sugita S, Trondman A-K, Fyfe R, Marquer L, Mazier F, Nielsen AB. 2017. Constraining the deforestation history of Europe: evaluation of historical land use scenarios with pollen-based land cover reconstructions. Land. 6(4):91.
- Karels TJ, Dobson FS, Trevino HS, Skibiel AL. 2008. The biogeography of avian extinctions on oceanic islands. J Biogeogr. 35(6):1106–1111.
- Katz NJ, Katz SV, Kipiani MG. 1965. Atlas and Keys of fruits and seeds occurring in the quaternary deposits of the USSR. Moscow: Commission for Investigations of the Quaternary Period, Academy of Sciences of the USSR.
- Kealhofer L. 2003. Looking into the gap: land use and the tropical forests of southern Thailand. Asian Perspect. 42:72–95.
- Kealhofer L, Penny D. 1998. A combined pollen and phytolith record for fourteen thousand years of vegetation change in northeastern Thailand. Rev Palaeobot Palynol. 103:83–93.
- Keeler BL, Dalzell BJ, Gourevitch JD, Hawthorne PL, Johnson KA, Noe RR. 2019. Putting people on the map improves the prioritization of ecosystem services. Front Ecol Environ. 17(3):151–156.
- Keith SA, Newton AC, Herbert RJH, Morecroft MD, Bealey CE. 2009a. Non-analogous community formation in response to climate change. J Nat Conserv. 17:228–235.
- Keith SA, Newton AC, Morecroft MD, Bealey CE, Bullock JM. 2009b. Taxonomic homogenization of woodland plant communities over 70 years. Proc R Soc B Bio Sci. 276(1672):3539–3544.
- Kelly CK, Bowler MG, Pybus O, Harvey PH. 2008. Phylogeny, niches, and relative abundance in natural communities. Ecology. 89(4):962–970.
- Kelly D. 1981. The native forest vegetation of Killarney, southwest Ireland: an ecological account. J Ecol. 69:437–472.
- Kelly LT, Brotons L. 2017. Using fire to promote biodiversity. Science. 355(6331):1264–1265.
- Kelly TJ, Lawson IT, Roucoux KH, Baker TR, Honorio‐Coronado EN, Jones TD, Rivas Panduro S. 2018. Continuous human presence without extensive reductions in forest cover over the past 2500 years in an aseasonal Amazonian rainforest. J Quat Sci. 33(4):369–379.
- Kendall RL. 1969. An ecological history of the Lake Victoria Basin. Ecol Monogr. 39(2):121–176.
- Keppel G, Mokany K, Wardell-Johnson GW, Phillips BL, Welbergen JA, Reside AE. 2015. The capacity of refugia for conservation planning under climate change. Front Ecol Environ. 13(2):106–112.
- Keppel G, Van Niel KP, Wardell-Johnson GW, Yates CJ, Byrne M, Mucina L, Schut AGT, Hopper SD, Franklin SE. 2012. Refugia: identifying and understanding safe havens for biodiversity under climate change. Global Ecol Biogeogr. 21:393–404.
- Kerfoot WC, Robbins JA, Weider LJ. 1999. A new approach to historical reconstruction: Combining descriptive and experimental paleolimnology. Limnol Oceanogr. 44(5):1232–1247.
- Kerfoot WC, Weider LJ. 2004. Experimental paleoecology (resurrection ecology): Chasing Van Valen’s Red Queen hypothesis. Limnol Oceanogr. 49(4 part 2): 1300–1316.
- Kershaw AP. 1970. A pollen diagram from Lake Euramoo, north-east Queensland, Australia. New Phytol. 69(3):785–805.
- Kershaw AP. 1971. A pollen diagram from Quincan Crater, north-east Queensland, Australia. New Phytol. 70(4):669–681.
- Kershaw AP. 1975. Stratigraphy and pollen analysis of Bromfield Swamp, north eastern Queensland, Australia. New Phytol. 75(1):173–191.
- Kershaw AP. 1976. A late Pleistocene and Holocene pollen diagram from Lynch’s Crater, northeastern Queensland, Australia. New Phytol. 77(2):469–498.
- Kershaw AP. 1984. Late Cenozoic plant extinctions in Australia. In: Martin PS, Klein RG, editors. Quaternary extinctions: a prehistoric revolution. Arizona: University of Arizona Press; p. 691–707.
- Kershaw AP, Bretherton SC, van der Kaars S. 2007. A complete pollen record of the last 230 ka from Lynch’s Crater, north-eastern Australia. Palaeogeogr Palaeoclimatol Palaeoecol. 251:23–45.
- Kershaw AP, McGlone MS. 1996. The quaternary history of southern conifers. In: Enright NJ, Hill RS, editors. Ecology of the southern conifers. Washington: Smithsonian Institute Press; p. 30–63.
- Kershaw AP, van der Kaars S, Flenley JR. 2011. The Quaternary history of Far Eastern rainforests. In: Bush MB, Flenley JR, Gosling WD, editors. Tropical rainforest responses to climate change. 2nd ed. Berlin: Springer; p. 85–123.
- Keynes R. 2003. Fossils, finches, fuegians: Charles Darwin’s adventures and discoveries on the beagle. London: HarperCollins.
- Kidwell SM. 2015. Biology in the Anthropocene: Challenges and insights from young fossil records. Proc Natl Acad Sci USA. 112(16):4922.
- Kienast F, Ashastina K, Troeva E. 2018. Phylogeography of a west-Beringian endemic plant: An ancient seed of Stellaria jacutica Schischk detected in permafrost deposits of the last interglacial. Rev Palaeobot Palynol. 259:48–54.
- King M. 2017. The Knepp Vera conference: the case for creating new wood pastures. Br Wildl. 29:27–33.
- Kirby KJ. 2003. What might British forest-landscapes driven by large herbivores look like? Peterborough: English Nature.
- Kirby KJ. 2004a. A model of a British forest-landscape driven by large herbivore activity. Forestry. 77:406–420.
- Kirby KJ. 2012. A view from the past to the future. In: Wiens JA, Hayward GD, Safford HD et al., editors. Historical environmental variations in conservation and natural resource management. Chichester: John Wiley & Sons; p. 281–288.
- Kirby KJ, Baker A. 2013. The dynamics of pre-Neolithic European landscapes and their relevance to modern conservation. In: Rotherham ID, editor. Trees, forest landscape and grazing animals. London: Routledge; p. 87–98.
- Kirby KJ, Branson A, editors. 2009. Naturalistic grazing and re-wilding in Britain. Perspectives from the past and future directions. Br Wildl 20(5);1–63. Special Supplement.
- Kito N, Takimoto F. 1999. Population growth and migration rate of Fagus crenata during the Holocene in southwestern Hokkaido, Japan. Quat Res. 38:297–311.
- Klein Goldewijk K, Beusen A, van Drecht G, de Vos M. 2011. The HYDE 3.1 spatially explicit database of human-induced global land-use change over the past 12,000 years. Global Ecol Biogeogr. 20(1):73–86.
- Kleinbauer I, Dullinger S, Peterseil J, Essl F. 2010. Climate change might drive the invasive tree Robinia pseudacacia into nature reserves and endangered habitats. Biol Conserv. 143(2):382–390.
- Koch M, Schröder B, Günther A, Albrecht K, Pivarci R, Jurasinski G. 2017. Taxonomic and functional vegetation changes after shifting management from traditional herding to fenced grazing in temperate grassland communities. Appl Veg Sci. 20(2):259–270.
- Koch PL, Barnosky AD. 2006. Late Quaternary extinctions: State of the debate. Annu Rev Ecol Evol Syst. 37:215–250.
- Kohler RE. 2008. Plants and pigeonholes: classification as a practice in American ecology. Hist Stud Nat Sci. 38(1):77–108.
- Kolstrup E. 2007. Water-borne macroscopic plant particle transport through central and northern Europe during warming phases: a hypothetical spreading mechanism for climatic pioneers. Geologiska Föreningens i Stockholm Förhandlingar. 129:307–313.
- Kondratiene O, Šeiriene V. 2003. Vegetation and climate of the Butënai Interglacial (Holsteinian) in Lithuania. Geol Q. 47(2):139–148.
- Körner C. 2003. Alpine plant life. Berlin: Springer.
- Körner C. 2005. An introduction to the functional diversity of temperate forest trees. In: Scherer-Lorenzen M, Körner C, Schulze E-D, editors. Forest diversity and function: temperate and boreal systems. Berlin: Springer; p. 13–37.
- Körner C. 2012. Alpine Treelines. Basel: Springer.
- Körner C, Paulsen J. 2004. A world-wide study of high altitude treeline temperatures. J Biogeogr. 31(5):713–732.
- Kosnik MA. 2018. Holocene book review: Dietl & Flessa (eds), Conservation Paleobiology. Holocene. doi:10.1177/0959683618770752.
- Kouvari M, van der Geer AAE. 2018. Biogeography of extinction: The demise of insular mammals from the Late Pleistocene till today. Palaeogeogr Palaeoclimatol Palaeoecol. doi:10.1016/j.palaeo.2018.1006.1008.
- Kratz TK, Benson BJ, Blood ER, Cunningham GL, Dahlgren RA. 1991. The influence of landscape position on temporal variability in four North American ecosystems. Am Nat. 138(2):355–378.
- Krause TR, Whitlock C. 2017. Climatic and non-climatic controls shaping early postglacial conifer history in the northern Greater Yellowstone Ecosystem, USA. J Quat Sci. 32(7):1022–1036.
- Krebs P, Conedera M, Pradella M, Torriani D, Felber M, Tinner W. 2004. Quaternary refugia of the sweet chestnut (Castanea sativa Mill.): an extended palynological approach. Veg Hist Archaeobot. 13(3):145–160.
- Krebs P, Pezzatti GB, Beffa G, Tinner W, Conedera M. 2019. Revising the sweet chestnut (Castanea sativa Mill.) refugia history of the last glacial period with extended pollen and macrofossil evidence. Quat Sci Rev. 206:111–128.
- Kreyling J, Jentsch A, Beierkuhnlein C. 2011. Stochastic trajectories of succession initiated by extreme climatic events. Ecol Lett. 14(8):758–764.
- Kröpelin S, Verschuren D, Lézine A-M, Eggermont H, Cocquyt C, Francus P, Cazet JP, Fagot M, Rumes B, Russell JM et al. 2008. Climate-driven ecosystem succession in the Sahara: the past 6000 years. Science. 320(5877):765.
- Küchler AW. 1964. Potential natural vegetation of the conterminous United States. New York: American Geographical Society Special Publication 26.
- Küchler AW. 1967. Vegetation mapping. New York: Ronald Press.
- Kueffer C, Drake DR, Fernández-Palacios JM. 2014. Island biology: looking towards the future. Biol Lett. 10(10):20140719.
- Kullman L. 1998. Palaeoecological, biogeographical and palaeoclimatological implications of early Holocene immigration of Larix sibirica Ledeb. into the Scandes mountains, Sweden. Global Ecol Biogeogr. 7(3):181–188.
- Kullman L. 2017. Melting glaciers in the Swedish Scandes provide new insights into palaeotreeline performance. Int J Curr Multidiscip Stud. 3(3):607–618.
- Kuneš P, Abraham V. 2017. History of Czech vegetation since the Late Pleistocene. In: Chytrý M, Danihelka J, Kaplan Z et al., editors, pp. 193-227. Flora and vegetation of the Czech Republic. Cham: Springer.
- Kuneš P, Abraham V, Herben T. 2019. Changing disturbance-diversity relationships in temperate ecosystems over the past 12000 years. J Ecol. doi:10.1111/1365-2745.13136.
- Kuneš P, Odgaard BV, Gaillard MJ. 2011. Soil phosphorus as a control of productivity and openness in temperate interglacial forest ecosystems. J Biogeogr. 38:2150–2164.
- Kuneš P, Pelánková B, Chytrý M, Jankovská V, Pokorný P, Libor P. 2008. Interpretation of the last-glacial vegetation of eastern-central Europe using modern analogues from southern Siberia. J Biogeogr. 35:2223–2236.
- Kuneš P, Svitavská Svobodová H, Kolář J, Hajnalová M, Abraham V, Macek M, Tkáč P, Szabó P. 2015. The origin of grasslands in the temperate forest zone of east-central Europe: long-term legacy of climate and human impact. Quat Sci Rev. 116:15–27.
- Kuper R, Kröpelin S. 2006. Climate-controlled Holocene occupation in the Sahara: motor of Africa’s evolution. Science. 313(5788):803–807.
- Kupryjanowicz M. 2008. Vegetation and climate of the Eemian and early Vistulian Lakeland in northern Podlasie. Acta Palaeobot. 48(1):3–130.
- Kupryjanowicz M, Fiłoc M, Czerniawska D. 2018c. Occurrence of slender naiad (Najas flexilis (Willd.) Rostk. & W. L. E. Schmidt) during the Eemian Interglacial – An example of a palaeolake from the Hieronimowo site, NE Poland. Quatern Int. 467:117–130.
- Kupryjanowicz M, Granoszewski W, Fiłoc M. 2018b. New finds of Eemian Tilia tomentosa Moench macroremais in NE Poland, and the reconstructed European range of this species during the last interglacial. Quatern Int. 467:107–116.
- Kupryjanowicz M, Nalepka D, Pidek IA, Walanus A, Balwierz Z, Bińka K, Fiłoc M, Granoszewski W, Kołaczek P, Majecka A et al. 2018a. The east-west migration of trees during the Eemian Interglacial registered on isopollen maps of Poland. Quatern Int. 467:178–191.
- Kusmartono VPR, Hindarto I, Herwanto E. 2017. Late Pleistocene to recent: Human activities in the deep interior equatorial rainforest of Kalimantan, Indonesian Borneo. Quatern Int. 448:82–94.
- Kutzbach JE, Guetter PJ. 1986. The influence of changing orbital parameters and surface boundary conditions on climate simulations for the last 16,000 years. J Atmos Sci. 41:1–34.
- Kuzmicheva EA, Smyshlyaeva OI, Vasyukov DD, Khasanov BF, Krylovich OA, Okuno M, West DL, Hatfield VL, Savinetsky AB. 2019. A 7300-yr-old environmental history of seabird, human, and volcano impacts on Carlisle Island (the Islands of Four Mountains, eastern Aleutians, Alaska). Quat Res. 91(3):934–952.
- Lacourse T. 2009. Environmental change controls postglacial forest dynamics through interspecific differences in life-history traits. Ecology. 90:2149–2160.
- LaDeau S. 2010. Advances in modeling highlight a tension between analytical accuracy and accessibility. Ecology. 91(12):3488–3492.
- Laliberté E, Grace JB, Huston MA, Lambers H, Teste FP, Turner BL, Wardle DA. 2013. How does pedogenesis drive plant diversity? Trends Ecol Evol. 28(6):331–340.
- Lallensack R. 2018. Evology. Nature. 554:19–21.
- Lambers H, Raven JA, Shaver GR, Smith SE. 2007. Plant nutrient-acquisition strategies change with soil age. Trends Ecol Evol. 23(2):95–103.
- Lamentowicz M, Gałka M, Marcisz K, Słowiński M, Kajukało-Drygalska K, Dayras MD, Jassey VEJ. 2019. Unveiling tipping points in long-term ecological records from Sphagnum-dominated peatlands. Biol Lett. 15(4):20190043.
- Landres PB, Morgan P, Swanson SJ. 1999. Overview of the use of natural variability concepts in managing ecological systems. Ecol App. 9:1179–1188.
- Lane CS, Horn SP, Taylor ZP, Mora CI. 2009. Assessing the scale of prehistoric human impact in the neotropics using stable carbon isotope analyses of lake sediments: a test case from Costa Rica. Lat Am Antiq. 20(1):120–133.
- Lang G. 1992. Some aspects of European late- and post-glacial flora history. Acta Botan Fenni. 144:1–17.
- Lang G. 1994. Quartäre Vegetationsgeschichte Europas. Jena: Gustav Fischer Verlag.
- Lanner RM. 2007. The Bristlecone pine: a natural history of the world’s oldest trees. Missoula, Montana: Mountain Press.
- Laris P, Dadashi S, Jo A, Wechsler S. 2016. Buffering the savanna: fire regimes and disequilibrium ecology in West Africa. Plant Ecol. 217(5):583–596.
- Lasky JR, Des Marais DL, McKay JK, Richards JH, Juenger TE, Keitt TH. 2012. Characterizing genomic variation of Arabidopsis thaliana: the roles of geography and climate. Mol Ecol. 21(22):5512–5529.
- Lasky JR, Keitt TH, Weeks BC, Economo EP. 2017. A hierarchical model of whole assemblage island biogeography. Ecography. 40(8):982–990.
- Last WM, Smol JP, editors. 2001. Tracking Environmental Change Using Lake Sediments. Volume 1: Basin Analysis, Coring, and Chronological Techniques. Dordrecht: Kluwer Academic Publishers.
- Latałowa M, Święta-Musznicka J, Słowiński M, Pędziszewska A, Noryśkiewicz AM, Zimny M, Obremska M, Ott F, Stivrins N, Pasanen L et al. 2019. Abrupt Alnus population decline at the end of the first millennium CE in Europe – The event ecology, possible causes and implications. Holocene. 29:1335–1349. 0959683619846978.
- Latham RE, Ricklefs RE. 1993. Global patterns of tree species richness in moist forests: energy-diversity theory does not account for variation in species richness. Oikos. 67:325–333.
- Latombe G, Pyšek P, Jeschke JM, Blackburn TM, Bacher S, Capinha C, Costello MJ, Fernández M, Gregory RD, Hobern D et al. 2017. A vision for global monitoring of biological invasions. Biol Conserv. 213(Part B):295–308.
- Lau JA, Shaw RG, Reich PB, Tiffin P. 2014. Indirect effects drive evolutionary responses to global change. New Phytol. 201(1):335–343.
- Lavine M. 2010. Living dangerously with big fancy models. Ecology. 91:3487.
- Law A, Gaywood MJ, Jones KC, Ramsay P, Willby NJ. 2017. Using ecosystem engineers as tools in habitat restoration and rewilding: beaver and wetlands. Sci Total Environ. 605–606:1021–1030.
- Lawesson JE. 1990. Alien plants in the Galapagos Islands, a summary. In: Lawesson JE, Hammann O, Reck G et al., editors. Botanical research and management in galapagos. St Louis: Monographs in Systematic Botany from the Missouri Botanical Garden; p. 15–20.
- Lawler JJ, Ackerly DD, Albano CM, Anderson MG, Dobrowski SZ, Gill JL, Heller NE, Pressey RL, Sanderson EW, Weiss SB. 2015. The theory behind, and the challenges of, conserving nature’s stage in a time of rapid change. Conserv Bio. 29(3):618–629.
- Lawson IT, Church MJ, McGovern TH, Arge SV, Woollet J, Edwards KJ, Gathorne-Hardy FJ, Dugmore AJ, Cook GT, Mairs K-A et al. 2005. Historical ecology on Sandøy, Færoe Islands: Palaeoenvironmental and archaeological perspectives. Hum Ecol. 33(5):651–684.
- Lawson IT, Edwards KJ, Church MJ, Newton AJ, Cook GT, Gathorne-Hardy FJ, Dugmore AJ. 2008. Human impact on an island ecosystem: pollen data from Sandøy, Færoe Islands. J Biogeogr. 35:1130–1152.
- Lawson IT, Gathorne-Hardy FJ, Church MJ, Newton AJ, Edwards KJ, Dugmore AJ, Einarsson Á. 2007. Environmental impacts of the Norse settlement: palaeoenvironmental data from Mývatnssveit, northern Iceland. Boreas. 36:1–19.
- Lawton JH. 1999. Are there general laws in ecology? Oikos. 84(2):177–192.
- Ledford H. 2019. World’s largest plant survey reveals alarming extinction rate. Nature. 570(7760):148–149.
- Ledru M-P, Mourguiart P, Riccomini C. 2009. Related changes in biodiversity, insolation and climate in the Atlantic rainforest since the last interglacial. Palaeogeogr Palaeoclimatol Palaeoecol. 271(1):140–152.
- Lee RK, Kasting JF, Crane RG. 2010. The Earth System. 3rd ed. New York: Prentice Hall.
- Legendre P, Birks HJB. 2012. Chapter 8, From classical to canonical ordination. In: Birks HJB, Lotter AF, Juggins S et al., editors. Tracking environmental change using lake sediments volume 5: data handling and numerical techniques. Dordrecht: Springer; p. 201–248.
- Leibold MA. 1996. The niche concept revisited: mechanistic models and community context. Ecology. 76:1371–1382.
- Leimu R, Fischer M. 2008. A meta-analysis of local adaptation in plants. PLoS One. 3(12):e4010.
- Leite YLR, Costa LP, Loss AC, Rocha RG, Batalha-Filho H, Bastos AC, Quaresma VS, Fagundes V, Paresque R, Passamani M et al. 2016. Neotropical forest expansion during the last glacial period challenges refuge hypothesis. Proc Natl Acad Sci USA. 113(4):1008–1013.
- Lele SR. 2010. Model complexity and information in the data: could it be a house built on sand? Ecology. 91(12):3493–3496.
- Lems K. 1960. Botanical notes on the Canary Islands II. The evolution of plant forms in the Islands: Aeonium. Ecology. 41(1):1–17.
- Lems K. 1961. Botanical notes on the Canary Islands III. The life form spectrum and its interpretation. Ecology. 42(3):569–572.
- Lems K, Holzapfel CM. 1971. Adaptation of growth form in Echium leucophaeum (Boraginaceae). Ecology. 52(3):499–506.
- Lendvay B, Bálint M, Pál I, Vincze I, Orbán I, Magyari EK. 2018. Plant macrofossils from lake sediment as the material to assess ancient genetic diversity: Did deforestation influence Norway spruce (Picea abies) in the South Carpathians? Quatern Int. 477:106–116.
- Leng MJ, editor 2006. Isotopes in Palaeoenvironmental Research. Dordrecht: Springer.
- Lenoir J, Gégout JC, Guisan A, Vittoz P, Wohlgemuth T, Zimmermann NE, Dullinger S, Pauli H, Willner W, Svenning JC. 2010. Going against the flow: potential mechanisms for unexpected downslope range shifts in a warming climate. Ecography. 33(2):295–303.
- Lenoir J, Gégout JC, Marquet PA, de Ruffray P, Brisse H. 2008. A significant upward shift in plant species optimum elevation during the 20th century. Science. 320(5884):1768–1771.
- Lenoir J, Hattab T, Pierre G. 2016. Climatic microrefugia under anthropogenic climate change: implications for species redistribution. Ecography. 40(2):253–266.
- Lenoir J, Svenning J-C. 2013. Latitudinal and elevational range shifts under contemporary climate change. In: Levin SA, editor. Amsterdam: Elsevier: Encyclopedia of biodiversity. 2nd ed. p. 599–611.
- Lenoir J, Svenning J-C. 2015. Climate-related range shifts – a global multidimensional synthesis and new research directions. Ecography. 38(1):15–28.
- Lenton TM. 2016. Earth System Science. Oxford: Oxford University Press.
- Leopold EB. 1967. Late-Cenozoic patterns of plant extinction. In: Martin PS, Wright HE, editors. Pleistocene Extinctions. New Haven: Yale University Press; p. 203–246.
- Lepais O, Muller SD, Ben Saad-Liman S, Benslama M, Rhazi L, Belouahem-Abed D, Daoud-Bouattour A, Gammar AM, Ghrabi-Gammar Z, Bacles CFE. 2013. High genetic diversity and distinctiveness of rear-edge climate relicts maintained by ancient tetraploidisation for Alnus glutinosa. PLoS One. 8 (9):e75029.
- Leroy SAG, Arpe K. 2007. Glacial refugia for summer-green trees in Europe and south-west Asia as proposed by ECHAM3 time-slice atmospheric model simulations. J Biogeogr. 34:2115–2128.
- Lesk C, Coffel E, D’Amato AW, Dodds K, Horton R. 2017. Threats to North American forests from southern pine beetle with warming winters. Nat Clim Chang. 7:713–718.
- Lesser MR, Jackson ST. 2011. Reliability of macrofossils in woodrat (Neotoma) middens for detecting low-density tree populations. Paleobiology. 37:603–615.
- Lesser MR, Jackson ST. 2012. Making a stand: five centuries of population growth in colonizing populations of Pinus ponderosa. Ecology. 93(5):1071–1081.
- Lesser MR, Jackson ST. 2013. Contributions of long-distance dispersal to population growth in colonising Pinus ponderosa populations. Ecol Lett. 16:380–389.
- Leunda M, González-Sampériz P, Gil-Romera G, Bartolomé M, Belmonte-Ribas Á, Gómez-García D, Kaltenrieder P, Rubiales JM, Schwörer C, Tinner W et al. 2019. Ice cave reveals environmental forcing of long-term Pyrenean tree line dynamics. J Ecol. 107(2):814–828.
- Levine NM, Zhang K, Longo M, Baccini A, Phillips OL, Lewis SL, Alvarez-Dávila E, Segalin de Andrade AC, Brienen RJW, Erwin TL et al. 2016. Ecosystem heterogeneity determines the ecological resilience of the Amazon to climate change. Proc Natl Acad Sci USA. 113(3):793–797.
- Levins R, Lewontin R. 1980. Dialectics and reductionsim in ecology. Synthese. 43:47–78.
- Levis C, Costa FRC, Bongers F, Peña-Claros M, Clement CR, Junqueira AB, Neves EG, Tamanaha EK, Figueiredo FOG, Salomão RP et al. 2017. Persistent effects of pre-Columbian plant domestication on Amazonian forest composition. Science. 355(6324):925–931.
- Levis C, Flores BM, Moreira PA, Luize BG, Alves RP, Franco-Moraes J, Lins J, Konings E, Peña-Claros M, Bongers F et al. 2018. How people domesticated Amazonian forests. Front Ecol Evol. 5: art171.
- Lewis SL, Maslin MA. 2015. Defining the anthropocene. Nature. 519:171–180.
- Lewis SL, Maslin MA. 2018. The human planet - how we created the anthropocene. UK: Pelican.
- Leys B, Finsinger W, Carcaillet C. 2014. Historical range of fire frequency is not the Achilles’ heel of the Corsican black pine ecosystem. J Ecol. 102(2):381–395.
- Liang Y, Duveneck MJ, Gustafson EJ, Serra-Diaz JM, Thompson JR. 2018. How disturbance, competition, and dispersal interact to prevent tree range boundaries from keeping pace with climate change. Glob Chang Biol. 24(1):e335–e351.
- Liao C, Peng R, Luo Y, Zhou X, Wu X, Fang C, Chen J, Li B. 2008. Altered ecosystem carbon and nitrogen cycles by plant invasion: a meta-analysis. New Phytol. 177(3):706–714.
- Libby WF. 1965. Radiocarbon dating. Chicago: University of Chicago Press.
- Libby WF, Arnold JR. 1949. Age determinations by radiocarbon content: Checks with samples of known age. Science. 11:678–680.
- Lichti-Federovich S, Ritchie JC. 1968. Recent pollen assemblages from the Western Interior of Canada. Rev Palaeobot Palynol. 7(4):297–344.
- Liepelt S, Cheddadi R, De Beaulieu JL, Fady B, Gömöry D, Hussendörfer E, Konnert M, Litt T, Longauer R, Terhürne-Berson R et al. 2009. Postglacial range expansion and its genetic imprints in Abies alba (Mill.) - a synthesis from palaeobotanic and genetic data. Rev Palaeobot Palynol. 153:139–149.
- Lima‐Ribeiro MS, Moreno AKM, Terribile LC, Caten CT, Loyola R, Rangel TF, Diniz‐Filho JAF. 2017. Fossil record improves biodiversity risk assessment under future climate change scenarios. Divers Distrib. 23(8):922–933.
- Limburg PA, Weider LJ. 2002. ‘Ancient’ DNA in the resting egg bank of a microcrustacean can serve as a palaeolimnological database. Proc R Soc B Bio Sci. 269(1488):281–287.
- Lindbladh M. 1999. The influence of former land-use on vegetation and biodiversity in the boreo-nemoral zone of Sweden. Ecography. 22:485–498.
- Lindbladh M, Bradshaw RHW, Holmqvist BH. 2000. Pattern and process in south Swedish forests during the last 3000 years, sensed at stand and regional scales. J Ecol. 88(1):113–128.
- Lindbladh M, Brunet J, Hannon G, Niklasson M, Eliasson P, Eriksson GR, Ekstrand A. 2007. Forest history as a basis for ecosystem restoration - a multidisciplinary case study in a south Swedish temperate landsape. Restor Ecol. 15:284–295.
- Lindbladh M, Foster DR. 2010. Dynamics of long-lived foundation species: the history of Quercus in southern Scandinavia. J Ecol. 98(6):1330–1345.
- Lindbladh M, Fraver S, Edvardsson J, Felton A. 2013. Past forest composition, structures and processes - How paleoecology can contribute to forest conservation. Biol Conserv. 168:116–127.
- Lindbladh M, Niklasson M, Karlsson M, Björkman L, Churski M. 2008. Close anthropogenic control of Fagus sylvatica establishment and expansion in a Swedish protected landscape – implications for forest history and conservation. J Biogeogr. 35(4):682–697.
- Lindbladh M, Niklasson M, Nilsson SG. 2003. Long-time record of fire and open canopy in a high biodiversity forest in southeast Sweden. Biol Conserv. 114(2):231–243.
- Lindenmayer DB, Luck GW. 2005. Synthesis: Thresholds in conservation and management. Biol Conserv. 124:351–354.
- Linder HP. 2014. The evolution of African plant diversity. Front Ecol Evol. 2:38.
- Lindner L, Morks S, Nita M. 2013. Climatostratigraphy of interglacials in Poland: Middle and Upper Pleistocene lower boundaries from a Polish perspective. Quatern Int. 292:113–123.
- Lisitsyna OV, Giesecke T, Hicks S. 2011. Exploring pollen percentage threshold values as an indication for the regional presence of major European trees. Rev Palaeobot Palynol. 166(3–4):311–324.
- Litvaitis JA. 2003. Are pre-Columbian conditions relevant baselines for managed forests in the northeastern United States? For Ecol Manage. 185:113–126.
- Liu Y, Andersen JJ, Williams JW, Jackson ST. 2013. Vegetation history in central Kentucky and Tennessee (USA) during the last glacial and deglacial periods. Quat Res. 79(2):189–198.
- Liu Y, Jackson ST, Brewer S, Williams JW. 2010. Assessing antiquity and turnover of terrestrial ecosystems in eastern North America using fossil pollen data: a preliminary study. IOP Conf Ser Earth Environ Sci. 9:012005.
- Livingstone DA. 1967. Postglacial vegetation of the Ruwenzori Mountains in equatorial Africa. Ecol Monogr. 37(1):25–52.
- Livingstone DA. 1971. A 22,000-year pollen record from the plateau of Zambia. Limnol Oceanogr. 16(2):349–356.
- Livingstone DA. 1975. Late Quaternary climatic change in Africa. Annu Rev Ecol Syst. 6(1):249–280.
- Ljung K. 2007. Holocene climate and environmental dynamics on the Tristan da Cunha island group, South Atlantic [PhD thesis]. Lund: Lund University.
- Ljung K, Björck S. 2011. A pollen record of the last 450 years from a lowland peat bog on Tristan da Cunha, South Atlantic, implying early anthropogenic influence. J Quat Sci. 26(7):688–693.
- Loarie SR, Duffy PB, Hamilton H, Asner GP, Field CB, Ackerly DD. 2009. The velocity of climate change. Nature. 462:1052.
- Lockwood JG. 2001. Abrupt and sudden climatic transitions and fluctuations: a review. Int J Climatol. 21(9):1153–1179.
- Lockwood JL, Blackburn TM, Cassey P, Olden JD. 2009. The shape of things to come: non-native mammalian predators and the fate of island biodiversity. In: Turvey ST, editor. Holocene extinctions. Oxford: Oxford University Press; p. 235–247.
- Lodder J, Hill TR, Finch JM. 2018. A 5000-yr record of Afromontane vegetation dynamics from the Drakensberg Escarpment, South Africa. Quatern Int. 470:119–129.
- Loidi J, del Arco M, Pérez de Paz PL, Asensi A, Díez Garretas B, Costa M, Díaz González T, Fernández-González F, Izco J, Penas Á et al. 2010. Understanding properly the `potential natural vegetation’ concept. J Biogeogr. 37(11):2209–2211.
- Loope LL, Mueller-Dombois D. 1989. Characteristics of invaded islands, with special reference to Hawaii. In: Drake JA, Mooney HA, di Castri F et al., editors. Biological invasions - a global perspective. Chichester: Wiley; p. 257–280.
- López-Vila J, Montoya E, Cañellas-Boltà N, Rull V. 2014. Modern non-pollen palynomorphs sedimentation along an elevational gradient in the south-central Pyrenees (southwestern Europe) as a tool for Holocene paleoecological reconstruction. Holocene. 24(3):327–345.
- López‐Merino L, Colás‐Ruiz NR, Adame MF, Serrano O, Martínez Cortizas A, Mateo MA. 2017. A six thousand‐year record of climate and land‐use change from Mediterranean seagrass mats. J Ecol. 105(5):1267–1278.
- Lorenzen ED, Nogués-Bravo D, Orlando L, Weinstock J, Binladen J, Marske KA, Ugan A, Borregaard MK, Gilbert MTP, Nielsen R, Ho SYW, et al. 2011. Species-specific responses of Late Quaternary megafauna to climate and humans. Nature. 479:359–364.
- Lortie CJ, Brooker RW, Choler P, Kikvidze Z, Michalet R, Pugnaire FI, Callaway RM. 2004. Rethinking plant community theory. Oikos. 107(2):433–438.
- Losos JB, Ricklefs RE, editors. 2009. The theory of island biogeography revisited. Princeton: Princeton University Press.
- Lotka AJ. 1907. Studies on the mode of material aggregates. Am J Sci. 174:199–216.
- Lotter AF, Birks HJB. 2003b. The Holocene palaeolimnology of Sägistalsee and its environmental history - a synthesis. J Paleolimnol. 30:333–342.
- Loughlin NJD, Gosling WD, Montoya E. 2018b. Identifying environmental drivers of fungal non-pollen palynomorphs in the montane forest of the eastern Andean flank, Ecuador. Quat Res. 89(1):119–133.
- Loughlin NJD, Gosling WD, Mothes P, Montoya E. 2018a. Ecological consequences of post-Columbian indigenous depopulation in the Andean–Amazonian corridor. Nat Ecol Evol. 2(8):1233–1236.
- Lovejoy TE, Hannah L, editors. 2019. Biodiversity and climate change: transforming the biosphere. New Haven: Yale University Press.
- Lowe A, Unsworth C, Gerber S, Davies S, Munro RC, Kelleher C, King A, Brewer S, White A, Cottrell J. 2006. Route, speed and mode of oak postglacial colonisation across the British Isles: integrating molecular ecology, palaeoecology and modelling approaches. Bot J Scotl. 57:59–81.
- Luck GW. 2005. An introduction to ecological thresholds. Biol Conserv. 124:299–300.
- Lumibao CY, Hoban SM, McLachlan J. 2017. Ice ages leave genetic diversity ‘hotspots’ in Europe but not in Eastern North America. Ecol Lett. 20(11):1459–1468.
- Luouys J, Wilkinson DM, Bishop LC. 2012. Ecology needs a paleontological perspective. In: Louys J, editor. Paleontology in ecology and conservation. Berlin: Springer-Verlag; p. 23–38.
- Lyford ME, Jackson ST, Betancourt JL, Gray ST. 2003. Influence of landscape structure and climate variability on a late Holocene plant migration. Ecol Monogr 73:567–583.
- Lynch EA. 1998. Origin of a park-forest vegetation mosaic in the Wind River Range, Wyoming. Ecology. 79(4):1320–1338.
- Lynch EA, Calcote R, Hotchkiss SC. 2006. Late-Holocene vegetation and fire history from Ferry Lake, northern Wisconsin, USA. Holocene. 16(4):495–504.
- Lynch EA, Calcote R, Hotchkiss SC, Tweiten M. 2014. Presence of lakes and wetlands decreases resilience of jack pine ecosystems to late-Holocene climatic changes. Can J Forest Res. 44(11):1331–1343.
- Lynch EA, Hotchkiss SC, Calcote R. 2011. Charcoal signatures defined by multivariate analysis of charcoal records from 10 lakes in northwest Wisconsin (USA). Quat Res. 75(1):125–137.
- Lyons SK, Amatangelo KL, Behrensmeyer AK, Bercovici A, Blois JL, Davis M, DiMichele WA, Du A, Eronen JT, Faith JT et al. 2016b. Holocene shifts in the assembly of plant and animal communities implicate human impacts. Nature. 529:80–83.
- Lyons SK, Miller JH, Fraser D, Smith FA, Boyer A, Lindsey E, Mychajliw AM. 2016a. The changing role of mammal life histories in Late Quaternary extinction vulnerability on continents and islands. Biol Lett. 12(6):20160342.
- Lyons SK, Smith FA, Brown JH. 2004. Of mice, mastodons and men: human-mediated extinctions on four continents. Evol Ecol Res. 6:339–358.
- Lytle DE. 2005. Palaeoecological evidence of state shifts between forest and barrens on a Michigan sand plain, USA. Holocene. 15:821–836.
- Lytle DE, Wahl ER. 2005. Palaeoenvironmental reconstructions using the modern analogue technique: the effects of sample size and decision rules. Holocene. 15(4):554–566.
- Lyver POB, Wilmshurst JM, Wood JR, Jones CJ, Fromont M, Bellingham PJ, Stone C, Sheehan M, Moller H. 2015. Looking back for the future: Local knowledge and palaeoecology inform biocultural restoration of coastal ecosystems in New Zealand. Hum Ecol. 43(5):681–695.
- MacArthur RH, Wilson EO. 1967. The theory of island biogeography. Princeton: Princeton University Press.
- MacDonald GM. 1993a. Fossil pollen analysis and the reconstruction of plant invasions. Adv Ecol Res. 24:67–110.
- MacDonald GM. 1993b. Methodological falsification and the interpretation of paleoecological records - the cause of the early Holocene birch decline in western Canada. Rev Palaeobot Palynol. 79(1–2):83–97.
- MacDonald GM. 2010. Global warming and the Arctic: a new world beyond the reach of the Grinnellian niche? J Exp Biol. 213(6):855–861.
- MacDonald GM, Beilman DW, Kuzmin YV, Orlova LA, Kremenetski KV, Shapiro B, Wayne RK, van Valkenburgh B. 2012. Pattern of extinction of the woolly mammoth in Beringia. Nat Commun. 3:893.
- MacDonald GM, Bennett KD, Jackson ST, Parducci L, Smith FA, Smol JP, Willis KJ. 2008. Impacts of climate change on species, populations and communities: palaeobiogeographical insights and frontiers. Prog Phys Geog. 32(2):139–172.
- MacDonald GM, Cwynar LC. 1991. Post-glacial population growth rates of Pinus contorta ssp. latifolia in western Canada. J Ecol. 79:417–429.
- Mace GM. 2014. Whose conservation? Science. 345(6204):1558–1560.
- Mace GM, Balmford A, Ginsberg JR, editors. 1998. Conservation in a changing world. Cambridge: Cambridge University Press.
- Mace GM, Hails RS, Cryle P, Harlow J, Clarke SJ. 2015. Towards a risk register for natural capital. J Appl Ecol. 52(3):641–653.
- Mace GM, Norris G, Fitter AH. 2012. Biodiversity and ecosystem services: a multi-layered relationship. Trends Ecol Evol. 27:19–26.
- MacGintie HD. 1953. Fossil beds of the Florissant Beds, Colorado. Washington DC: Carnegie Institution Publication, 599.
- Machado A. 2004. An index of naturalness. J Nat Conserv. 12(2):95–110.
- Macias Fauria M, Willis KJ. 2013. Landscape planning for the future: using fossil records to independently validate bioclimatic envelope models for economically valuable tree species in Europe. Global Ecol Biogeogr. 22(3):318–333.
- Mack RN, Simberloff D, Lonsdale WM, Evans H, Clout M, Bazzaz FA. 2000. Biotic invasions: causes, epidemiology, global consequences, and control. Ecol App. 10(3):689–710.
- Mackey B, Berry S, Hugh S, Ferrier S, Harwood TD, Williams KJ. 2012. Ecosystem greenspots: identifying potential drought, fire, and climate-change micro-refuges. Ecol App. 22(6):1852–1864.
- Mackey RL, Currie DJ. 2001. The diversity–disturbance relationship: is it generally strong and peaked? Ecology. 82(12):3479–3492.
- Maezumi SY, Robinson M, de Souza JG, Urrego DH, Schaan D, Alves D, Iriarte J. 2018b. New insights from pre-Columbian land use and fire management in Amazonian dark earth forests. Front Ecol Evol. 6:111.
- Maezumi SY, Whitney BS, Mayle FE, de Souza JG, Iriarte J. 2018a. Reassessing climate and pre-Columbian drivers of paleofire activity in the Bolivian Amazon. Quatern Int. 488:81–94.
- Magri D. 1989. Interpreting long-term exponential growth of plant populations in a 250,000-year pollen record from Valle di Castiglione (Roma). New Phytol. 112:123–128.
- Magri D. 2008. Patterns of post-glacial spread and the extent of glacial refugia of European beech (Fagus sylvatica). J Biogeogr. 35:450–463.
- Magri D. 2010. Persistence of tree taxa in Europe and Quaternary climate changes. Quatern Int. 219:145–151.
- Magri D, Agrillo E, Di Rita F, Furlanetto G, Pini R, Ravazzi C, Spada F. 2015. Holocene dynamics of tree taxa populations in Italy. Rev Palaeobot Palynol. 218:267–284.
- Magri D, Di Rita F, Aranbarri J, Fletcher W, González-Sampériz P. 2017. Quaternary disappearance of tree taxa from Southern Europe: Timing and trends. Quat Sci Rev. 163:23–55.
- Magri D, Palombo MR. 2013. Early to Middle Pleistocene dynamics of plant and mammal communities in south-west Europe. Quatern Int. 288:63–72.
- Magri D, Vendramin GG, Comps B, Dupanloup I, Geburek T, Gömöry D, Latałowa M, Litt T, Paule L, Roure JM et al. 2006. A new scenario for the Quaternary history of European beech populations: palaeobotanical evidence and genetic consequences. New Phytol. 171:199–221.
- Maguire B. 1970. On the flora of the Guayana Highland. Biotropica. 2:85–100.
- Maguire KC, Nieto-Lugilde D, Blois JL, Fitzpatrick MC, Williams JW, Ferrier S, Lorenz DJ. 2016. Controlled comparison of species- and community-level models across novel climates and communities. Proc R Soc B Bio Sci. 283:20152817.
- Maguire KC, Nieto-Lugilde D, Fitzpatrick MC, Williams JW, Blois JL. 2015. Modeling species and community responses to past, present, and future episodes of climatic and ecological change. Annu Rev Ecol Evol Syst. 46:343–368.
- Magyari EK, Chapman JC, Passmore DG, Allen JRM, Huntley JP, Huntley B. 2010. Holocene persistence of wooded steppe in the Great Hungarian Plain. J Biogeogr. 37(5):915–935.
- Magyari EK, Vincze I, Orbán I, Bíró T, Pál I. 2018. Timing of major forest compositional changes and tree expansions in the Retezat Mts during the last 16,000 years. Quatern Int. 477:40–58.
- Maher LJ, Heiri O, Lotter AF. 2012. Assessment of uncertainties associated with palaeolimnological laboratory methods and microfossil analysis. In: Birks HJB, Lotter AF, Juggins S et al., editors. Tracking environmental change using lake sediments volume 5: data handling and numerical techniques. Dordrecht: Springer; p. 143–166.
- Mahony CR, Cannon AJ, Wang T, Aitken SN. 2017. A closer look at novel climates: new methods and insights at continental to landscape scales. Glob Chang Biol. 23(9):3934–3955.
- Mai DH. 1995. Tertiäre Vegetationsgeschichte Europas. Jena: Gustav Fischer Verlag.
- Maiorano L, Cheddadi R, Zimmermann NE, Pellissier L, Petitpierre B, Pottier J, Laborde H, Hurdu BI, Pearman PB, Psomas A et al. 2013. Building the niche through time: using 13,000 years of data to predict the effects of climate change on three tree species in Europe. Global Ecol Biogeogr. 22(3):302–317.
- Malanson GP, Cairns DM. 1997. Effects of dispersal, population delays, and forest fragmentation on tree migration rates. Plant Ecol. 131:67–79.
- Malhi Y, Doughty CE, Galetti M, Smith FA, Svenning J-C, Terborgh JW. 2016. Megafauna and ecosystem function from the pleistocene to the Anthropocene. Proc Natl Acad Sci USA. 113(4):838–846.
- Maloney BK. 1980. Pollen analytical evidence for early forest clearance in North Sumatra. Nature. 287:324–326.
- Maloney BK. 1985. Man’s impact on the rainforests of West Malesia: the palynological record. J Biogeogr. 12(6):537–558.
- Mamakowa K. 1989. Late Middle Polish Glaciation, Eemian, and Early Vistulian vegetation at Imbramowice near Wroclaw and the pollen stratigraphy of this part of the Pleistocene in Poland. Acta Palaeobot. 29:11–176.
- Mamakowa K, Velichkevich FY. 1993a. Aracites interglacialis Wieliczk. - extinct plant found in the floras of the Mazovian (Alexandrian, Likhvinian) interglacial in Poland, Belarus, Russia, and the Ukraine. Acta Palaeobot. 33(2):321–341.
- Mamakowa K, Velichkevich FY. 1993b. Exotic plants in the floras of the Mazovian (Alexandrian) interglacial of Poland and Belarus. Acta Palaeobot. 33(2):305–319.
- Mandák B, Havrdová A, Krak K, Hadincová V, Vít P, Zákravský P, Douda J. 2016. Recent similarity in distribution ranges does not mean a similar postglacial history: a phylogeographical study of the boreal tree species Alnus incana based on microsatellite and chloroplast DNA variation. New Phytol. 210(4):1395–1407.
- Mander L, Rodrigues JM, Mueller PG, Jackson ST, Punyasena SW. 2014. Identifying the pollen of an extinct spruce species in the Late Quaternary sediments of the Tunica Hills regions, south-eastern United States. J Quat Sci. 29(7):711–721.
- Mangel M. 2006. The theoretical biologist’s Toolbox. Cambridge: Cambridge University Press.
- Mann DG, Edwards J, Chase JM, Beck W, Reanier R, Mass M, Finney B, Loret J. 2008. Drought, vegetation change, and human history on Rapa Nui (Isla de Pascua, Easter Island). Quat Res. 69:16–28.
- Mann DH, Groves P, Gaglioti BV, Shapiro BA. 2019. Climate-driven ecological stability as a globally shared cause of late quaternary megafaunal extinctions: the Plaids and Stripes Hypothesis. Biol Rev. 94(1):328–352.
- Marchant R, Brewer S, Webb T, Turvey ST. 2009. Holocene deforestation: a history of human-environmental interactions, climate change, and extinction. In: Turvey ST, editor. Holocene extinctions. Oxford: Oxford University Press; p. 213–233.
- Marchant R, Courtney-Mustaphi C, Githumbi E. 2017. Entangled ecosystem-people-animal interactions: perspectives from the East African savannas. PAGES Mag. 25(2):80–81.
- Marcisz K, Colombaroli D, Jassey VEJ, Tinner W, Kołaczek P, Gałka M, Karpińska-Kołaczek M, Słowiński M, Lamentowicz M. 2016. A novel testate amoebae trait-based approach to infer environmental disturbance in Sphagnum peatlands. Sci Rep. 6:33907.
- Måren IE, Kapfer J, Aarrestad PA, Grytnes J-A, Vandvik V. 2018. Changing contributions of stochastic and deterministic porcesses in community assembly over a successional gradient. Ecology. 99(1):148–157.
- Mariani M, Connor SE, Fletcher M-S, Theuerkauf M, Kuneš P, Jacobsen G, Saunders KM, Zawadzki A. 2017. How old is the Tasmanian cultural landscape? A test of landscape openness using quantitative land-cover reconstructions. J Biogeogr. 44(10):2410–2420.
- Mariani M, Connor SE, Theuerkauf M, Kunes P, Fletcher M-S. 2016. Testing quantitative pollen dispersal models in animal-pollinated vegetation mosaics: An example from temperate Tasmania, Australia Quat Sci Rev. 154:214–225.
- Mariani M, Fletcher M-S, Haberle S, Chin H, Zawadzki A, Jacobsen G. 2019. Climate change reduces resilience to fire in subalpine rainforests. Glob Chang Biol. 25(6):2030–2042.
- Markgraf F, Dengler A. 1931. Aus den Südosteuropäischen Urwälden. I. Die Wälder Albaniens. Z Forst Jagdwesen. 63:1–31.
- Markgraf V. 1986. Plant inertia reassessed. Am Nat. 127(5):725–726.
- Marland G, Pielke RA, Apps M, Avissar R, Betts RA, Davis KJ, Frumhoff PC, Jackson ST, Joyce LA, Kauppi P et al. 2003. The climatic impacts of land surface change and carbon management, and the implications for climate-change mitigation policy. Clim Policy. 3(2):149–157.
- Marlon JR, Bartlein PJ, Gavin DG, Long CJ, Anderson RS, Briles CE, Brown KJ, Colombaroli D, Hallett DJ, Power MJ et al. 2012. Long-term perspective on wildfires in the western USA. Proc Natl Acad Sci USA. 109(9):E535–E543.
- Maron M, Mitchell MGE, Runting RK, Rhodes JR, Mace GM, Keith DA, Watson JEM. 2017. Towards a threat assessment framework for ecosystem services. Trends Ecol Evol. 32(4):240–248.
- Marquer L, Gaillard M-J, Sugita S, Poska A, Trondman A-K, Mazier F, Nielsen AB, Fyfe RM, Jönsson AM, Smith B et al. 2017. Quantifying the effects of land use and climate on Holocene vegetation in Europe. Quat Sci Rev. 171 (SupplementC):20–37.
- Marquer L, Gaillard M-J, Sugita S, Trondman A-K, Mazier F, Nielsen AB, Fyfe RM, Odgaard BV, Alenius T, Birks HJB et al. 2014. Holocene changes in vegetation composition in northern Europe: why quantitative pollen-based vegetation reconstructions matter. Quat Sci Rev. 90:199–216.
- Marsicek JP, Shuman B, Brewer S, Foster DR, Oswald WW. 2013. Moisture and temperature changes associated with the mid-Holocene Tsuga decline in the northeastern United States. Quat Sci Rev. 80 (SupplementC):129–142.
- Marta S, Mattoccia M, Sbordoni V. 2013. Modelling landscape dynamics in a glacial refugium – or the spatial and temporal fluctuations of tree line altitudes. J Biogeogr. 40(9):1767–1779.
- Martín C, Para T, Clemente-Muñoz M, Hernandez-Bermejo E. 2008. Genetic diversity and structure of the endangered Betula pendula subsp. fontqueri populations in the south of Spain. Silva Fennica. 42:487–498.
- Martin PH, Canham CD, Marks PL. 2009. Why forests appear resistant to exotic plant invasions: intentional introductions, stand dynamics, and the role of shade tolerance. Front Ecol Environ. 7(3):142–149.
- Martin PS. 1958. Pleistocene ecology and biogeography of North America. In: Hubbs CL, editor. Zoogeography. Washington DC: American Association for the Advancement of Science; p. 375–420.
- Martin PS. 1969. Wanted: a suitable herbivore. Nat Hist. 78:35–39.
- Martin PS, Steadman DW. 1999. Prehistoric extinctions on islands and continents. In: MacPhee RDE, editor. Extinctions in near time. New York: Kluwer; p. 17–55.
- Martinetto E. 1999. Chronological framing of pliocene to early pleistocene plant macrofossil assemblages from northern Italy. Acta Palaeobot Suppl 2:503–511.
- Martinetto E, Momohara A, Bizzarri R, Baldanza A, Delfino M, Esu D, Sardella R. 2017. Late persistence and deterministic extinction of “humid thermophilous plant taxa of East Asian affinity” (HUTEA) in southern Europe. Palaeogeogr Palaeoclimatol Palaeoecol. 467:211–231.
- Martinetto E, Tema E, Irace A, Violanti D, Ciuto M, Zanella E. 2018. High-diversity European palaeoflora favoured by early Pliocene warmth: New chronological constraints from the Ca′ Viettone section, NW Italy. Palaeogeogr Palaeoclimatol Palaeoecol. 496:248–267.
- Maslin MA, Brierley CM. 2015. The role of orbital forcing in the Early Middle Pleistocene Transition. Quatern Int. 389:47–55.
- Matesanz S, Gianoli E, Valladares F. 2010. Global change and the evolution of phenotypic plasticity in plants. Ann N Y Acad Sci. 1206(1):35–55.
- Matlack GR. 1994. Plant species migration in a mixed-history forest landscape in eastern North America. Ecology. 75(5):1491–1502.
- Matthes JH, Goring SJ, Williams JW, Dietze MC. 2016. Benchmarking historical CMIP5 plant functional types across the upper midwest and northeastern United States. J Geophys Res Biogeosci. 121(2):523–535.
- Matthews-Bird F, Valencia BG, Church W, Peterson LC, Bush MB. 2017. A 2000-year history of disturbance and recovery at a sacred site in Peru’s northeastern cloud forest. Holocene. 27(11):1707–1719.
- Matthews JA. 1994. The ecology of recently-deglaciated terrain. Cambridge: Cambridge University Press.
- Matthews JA. 1996. Classics in physical geography revisited. Prog Phys Geog. 20(2):193–203.
- Matthias I, Giesecke T. 2014. Insights into pollen source area, transport and deposition from modern pollen accumulation rates in lake sediments. Quat Sci Rev. 87:12–23.
- Matthias I, Nielsen AB, Giesecke T. 2012. Evaluating the effect of flowering age and forest structure on pollen productivity estimates. Veg Hist Archaeobot. 12:471–484.
- Matthias I, Semmler MSS, Giesecke T. 2015. Pollen diversity captures landscape structure and diversity. J Ecol. 103:880–890.
- Mauve K. 1931. Über Bestandesaufblau, Zuwachsverhaltnisse und Verjüngung im galizischen Karpaten-Urwald. Mitt aus Forstwirtsch Forstwissenschaft. 2:257–311.
- Maxwell H. 1915. Trees: a woodland notebook. Glasgow: Maclehose.
- May RM. 1994. Paleoecology and ecology. Trends Ecol Evol. 9(9):345–345.
- Mayle FE, Burbridge R, Killeen TJ. 2000. Millennial-scale dynamics of southern Amazonian rain forests. Science. 290(5500):2291–2294.
- Mayle FE, Iriarte J. 2014. Integrated palaeoecology and archaeology - a powerful approach for understanding pre-Columbian Amazonia. J Archaeol Sci. 51:54–64.
- Mbida CM, van Neer W, Doutrelepont H, Vrydaghs L. 2000. Evidence for banana cultivation and animal husbandry during the first millennium BC in the forest of southern Cameroon. J Archaeol Sci. 27(2):151–162.
- McCarroll J, Chambers FM, Webb JC, Thom T. 2016. Using palaeoecology to advise peatland conservation: An example from West Arkengarthdale, Yorkshire, UK. J Nat Conserv. 30:90–102.
- McCarroll J, Chambers FM, Webb JC, Thom T. 2017. Application of palaeoecology for peatland conservation at Mossdale Moor, UK. Quatern Int. 432:39–47.
- McClenachan L, Cooper AB, McKenzie MG, Drew JA. 2015. The importance of surprising results and best practices in historical ecology. BioScience. 65(9):932–939.
- McClymont EL, Mauquoy D, Yeloff D, Broekens P, van Geel B, Charman DJ, Pancost RD, Chambers FM, Evershed RP. 2008. The disappearance of Sphagnum imbricatum from Butterburn Flow, UK. Holocene. 18(6):991–1002.
- McColl H, Racimo F, Vinner L, Demeter F, Gakuhari T, Moreno-Mayar JV, van Driem G, Gram Wilken U, Seguin-Orlando A, de la Fuente Castro C et al. 2018. The prehistoric peopling of Southeast Asia. Science. 361(6397):88–92.
- McElwain JC. 2018. Paleobotany and global change: Important lessons for species to biomes from vegetation responses to past global change. Annu Rev Plant Biol. 69(1):761–787.
- McElwain JC, Punyasena SW. 2007. Mass extinction events and the plant fossil record. Trends Ecol Evol. 22(10):548–557.
- McGeever AH, Mitchell FJG. 2016. Re-defining the natural range of Scots Pine (Pinus sylvestris L.): a newly discovered microrefugia in western Ireland. J Biogeogr. 43:2199–2208.
- McGill BJ. 2003. A test of the unified neutral theory of biodiversity. Nature. 422:881–885.
- McGill BJ, Dornelas M, Gotelli NJ, Magurran AE. 2015. Fifteen forms of biodiversity trend in the anthropocene. Trends Ecol Evol. 30(2):104–113.
- McGlone MS, Richardson SJ, Burge OR, Perry GLW, Wilmshurst JM. 2017. Palynology and the ecology of the New Zealand conifers. Front Earth Sci. 5:a94.
- McIntosh RP. 1967. The continuum concept of vegetation. Bot Rev. 33:99–187.
- McIntosh RP. 1975. HA Gleason - ‘individualistic ecologist’ 1882–1975. His contributions to ecological theory. Bull Torrey Bot Club. 102:253–273.
- McIntosh RP. 1980. The background and some current problems of theoretical ecology. Synthese. 43:195–255.
- McIntosh RP. 1987. Pluralism in ecology. Annu Rev Ecol Syst. 18:321–341.
- McIntosh RP. 1993. The continuum continued: John T Curtis’ influence in ecology. In: Fralish JS, McIntosh RP, Loucks OL, editors. John T Curtis: fifty years of wisconsin plant ecology. Madison: Wisconsin Academy of Science, Arts and Letters; p. 95–122.
- McIntosh RP. 1998. The myth of community as organism. Perspect Biol Med. 41(3):426–438.
- McLachlan JS, Clark JS. 2004. Reconstructing historical ranges with fossil data at continental scales. For Ecol Manage. 197:139–147.
- McLachlan JS, Clark JS, Manos PS. 2005. Molecular indicators of tree migration capacity under rapid climate change. Ecology. 86:2088–2098.
- McLachlan JS, Hellmann JJ, Schwartz MW. 2007. A framework for debate of assisted migration in an era of climate change. Conserv Bio. 21(2):297–302.
- McLauchlan KK, Higuera PE, Gavin DG, Perakis SS, Mack MC, Alexander H, Battles J, Biondi F, Buma B, Colombaroli D et al. 2014. Reconstructing disturbances and their biogeographical consequences over multiple timescales. BioScience. 64(2):105–116.
- McLauchlan KK, Lascu I, Mellicant E, Scharping RJ, Williams JJ. 2019. Influences of forested and grassland vegetation on late Quaternary ecosystem development as recorded in lacustrine sediments. Quat Res. 92(1):201–215.
- McLauchlan KK, Williams JJ, Craine JM, Jeffers ES. 2013a. Changes in global nitrogen cycling during the Holocene epoch. Nature. 495:352–355.
- McLauchlan KK, Williams JJ, Engstrom DR. 2013b. Nutrient cycling in the palaeorecord: Fluxes from terrestrial to aquatic ecosystems. Holocene. 23(11):1635–1643.
- McLaughlin BC, Ackerly DD, Klos PZ, Natali J, Dawson TE, Thompson SE. 2017. Hydrologic refugia, plants, and climate change. Glob Chang Biol. 23(8):2941–2961.
- McMichael CNH, Bush MB. 2019. Spatiotemporal patterns of pre-Columbian people in Amazonia. Quat Res. 92(1):53–69.
- McMichael CNH, Feeley KJ, Dick CW, Piperno DR, Bush MB. 2017. Comment on “Persistent effects of pre-Columbian plant domestication on Amazonian forest composition”. Science. 358 (6361):eaan8347.
- McMichael CNH, Piperno DR, Neves EG, Bush MB, Almeida FO, Mongeló G, Eyjolfsdottir MB. 2015. Phytolith assemblages along a gradient of ancient human disturbance in western Amazonia. Front Ecol Evol. 3:141.
- McPherson S. 2008. Lost worlds of the guiana highlands. Poole: Redfern Natural History.
- McVean DN. 1955. Ecology of Alnus glutinosa (L.) Gaertn. I. Fruit formation. J Ecol. 43(1):46–60.
- McWethy D, Whitlock C, Wilmshurst JM, McGlone MS, Fromont M, Li X, Dieffenbacher-Krall AC, W.O. Hobbs, Fritz SC, Cook ER. 2010. Rapid landscape transformation in South Island, New Zealand, following initial Polynesian settlement. Proc Natl Acad Sci USA. 107(50):21343–21348.
- McWethy DB, Haberle SG, Hopf F, Bowman DMJS. 2017. Aboriginal impacts on fire and vegetation on a Tasmanian island. J Biogeogr. 44(6):1319–1330.
- McWethy DB, Wilmshurst JM, Whitlock C, Wood JR, McGlone MS. 2014. A high-resolution chronology of rapid forest transitions following Polynesian arrival in New Zealand. PLoS One. 9:e111328.
- Médail F, Diadema K. 2009. Glacial refugia influence plant diversity patterns in the Mediterranean Basin. J Biogeogr. 36:1333–1345.
- Medina ME, Grill S, Fernandez AL, López ML. 2017. Anthropogenic pollen, foraging, and crops during Sierras of Córdoba Late Prehispanic Period (Argentina). Holocene. 27(11):1769–1780.
- Mehl IK, Hjelle KL. 2016. From deciduous forest to open landscape: application of new approaches to help understand cultural landscape development in western Norway. Veg Hist Archaeobot. 25:153–176.
- Meier ES, Lischke H, Schmatz DR, Zimmermann NE. 2012. Climate, competition and connectivity affect future migration and ranges of European trees. Global Ecol Biogeogr. 21(2):164–178.
- Meltsov V, Poska A, Reitalu T, Sammul M, Kull T. 2013. The role of landscape structure in determining palynological and floristic richness. Veg Hist Archaeobot. 22:39–49.
- Menke B. 1970. Ergebnisse der Pollenanalyse zur Pleistozãn-Stratigraphie und zur Pliozãn-Grenze in Schlewig-Holstein. Eiszeitalter Ggw. 21:5–21.
- Merilä J, Hendry AP. 2014. Climate change, adaptation, and phenotypic plasticity: the problem and the evidence. Evol Appl. 7(1):1–14.
- Merton LFH. 1970. The history and status of the woodlands of the Derbyshire limestone. J Ecol. 58:723–744.
- Michalak JL, Lawler JJ, Roberts DR, Carroll C. 2018. Distribution and protection of climatic refugia in North America. Conserv Bio. 32(6):1414–1425.
- Miki S. 1959. Evolution of Trapa from ancestral Lythrum through Hemitrapa. Proc Jpn Acad. 35(6):289–294.
- Mildenhall DC. 1980. New Zealand late Cretaceous and Cenozoic plant biogeography: a contribution. Palaeogeogr Palaeoclimatol Palaeoecol. 31:197–233.
- Millar CI, Woolfenden W. 1999. The role of climate change in interpreting historic variability. Ecol App. 9:1207–1216.
- Millennium Ecosystem Assessment. 2005. Ecosystems and human well-being: current state and trends. Washington, DC: Island Press.
- Miller AD, Roxburgh SH, Shea K. 2011. How frequency and intensity shape diversity-disturbance relationships. Proc Natl Acad Sci USA. 108:5643–5648.
- Miller DM, Miller IM, Jackson ST. 2014. Biogeography of Pleistocene conifer species from the Ziegler Reservoir fossil site, Snowmass Village, Colorado. Quat Res. 82:567–574.
- Miller KM, McGill BJ. 2018. Land use and life history limit migration capacity of eastern tree species. Global Ecol Biogeogr. 27(1):57–67.
- Miller PA, Giesecke T, Hickler T, Bradshaw RHW, Smith B, Seppä H, Valdes PJ, Sykes MT. 2008. Exploring climatic and biotic controls on Holocene vegetation change in Fennoscandia. J Ecol. 96:247–259.
- Miller U, Robertson A-M. 1979. Biostratigraphical investigations in the Anundsjö region, Ångermanland, northern Sweden. Early Norrland. 12:1–76.
- Miller W. 1993. Models of recurrent fossil assemblages. Lethaia. 26:182–183.
- Minckley TA, Booth RK, Jackson ST. 2011. Response of arboreal pollen abundance to late-Holocene drought events in the Upper Midwest, USA. Holocene. 22(5):531–539.
- Minckley TA, Felstead NJ, Gonzalez S. 2019. Novel vegetation and establishment of Chihuahuan Desert communities in response to late Pleistocene moisture availability in the Cuatrociénegas Basin, NE Mexico. Holocene. 29(3):457–466.
- Minckley TA, Long CJ. 2016. Paleofire severity and vegetation change in the Cascade Range, Oregon, USA. Quat Res. 85(2):211–217.
- Minckley TA, Shriver RK, Shuman B. 2012. Resilience and regime change in a southern Rocky Mountain ecosystem during the past 17000 years. Ecol Monogr. 82(1):49–68.
- Missa O. 2005. The Unified Neutral Theory of biodiversity and biogeography: alive and kicking. Bull Br Ecol Soc. 36(2):12–17.
- Mitchell FJG. 1988. The vegetational history of the Killarney Oakwoods, SW Ireland: evidence from fine spatial resolution pollen analysis. J Ecol. 76:415–436.
- Mitchell FJG. 1990. The impact of grazing and human disturbance on the dynamics of woodland in SW Ireland. J Veg Sci. 1:245–254.
- Mitchell FJG. 2005. How open were European primeval forests? Hypothesis testing using palaeoecological data. J Ecol. 93(1):168–177.
- Mitchell FJG. 2011. Exploring vegetation in the fourth dimension. Trends Ecol Evol. 26(1):45–52.
- Mitchell FJG. 2013. Long-term changes and drivers of biodiversity in Atlantic oakwoods. For Ecol Manage. 307:1–6.
- Mitchell GF. 1954. The late-glacial flora of Ireland. Danmarks Geol Unders II. 80:73–86.
- Mitchell RJ, Hewison RL, Britton AJ, Brooker RW, Cummins RP, Fielding DA, Fisher JM, Gilbert DJ, Hester AJ, Hurskainen S et al. 2017. Forty years of change in Scottish grassland vegetation: Increased richness, decreased diversity and increased dominance. Biol Conserv. 212:327–336.
- Moe D. 1970. Post-glacial immigration of Picea abies into Fennoscandia. Bot Notiser. 123 (1):61–66.
- Molinari C, Bradshaw RHW, Risbøl O, Lie M, Ohlson M. 2005. Long-term vegetational history of a Picea abies stand in south-eastern Norway: Implications for the conservation of biological values. Biol Conserv. 126(2):155–165.
- Molinari C, Montanari C. 2016. Interdisciplinary approach for reconstructing an alder-based historical agricultural practice of the Eastern Ligurian Apennines (NW Italy). Environ Archaeol. 21(1):31–44.
- Molisch H. 1938. The Longevity of plants (translated by E.H. Fulling). Lancaster, Pennsylvania: Science Press.
- Monegato G, Ravazzi C, Culiberg M, Pini R, Bavec M, Calderoni G, Jez J, Perego R. 2015. Sedimentary evolution and persistence of open forests between the south-eastern Alpine fringe and the Northern Dinarides during the Last Glacial Maximum. Palaeogeogr Palaeoclimatol Palaeoecol. 436:23–40.
- Montoya E, Rull V, van Geel B. 2010. Non-pollen palynomorphs from surface sediments along an altitudinal transect of the Venezuelan Andes. Palaeogeogr Palaeoclimatol Palaeoecol. 297(1):169–183.
- Montoya JM, Raffaelli D. 2010. Climate change, biotic interactions and ecosystem services. Philos Trans R Soc B: Bio Sci. 365(1549):2013–2018.
- Morales-Molino C, Tinner W, García-Antón M, Colombaroli D. 2017. The historical demise of Pinus nigra forests in the Northern Iberian Plateau (south-western Europe). J Ecol. 105(3):634–646.
- Morán-Ordóñez A, Lahoz-Monfort JJ, Elith J, Wintle BA. 2017. Evaluating 318 continental-scale species distribution models over a 60-year prediction horizon: what factors influence the reliability of predictions? Global Ecol Biogeogr. 26(3):371–384.
- Moran JM. 1973. The late-glacial retreat of ‘Arctic’ air as suggested by the onset of Picea decline. Prof Geogr. 25:373–376.
- Morecroft MD, Bealey CE, Scott WA, Taylor ME. 2016. Interannual variability, stability and resilience in UK plant communities. Ecol Indic. 68:63–72.
- Morecroft MD, Crick HQP, Duffield SJ, Macgregor NA. 2012. Resilience to climate change: translating principles into practice. J Appl Ecol. 49(3):547–551.
- Morelli TL, Daly C, Dobrowski SZ, Dulen DM, Ebersole JL, Jackson ST, Lundquist JD, Millar CI, Maher SP, Monahan WB et al. 2016. Managing climate change refugia for climate adaptation. PLoS One. 11(8):e0159909.
- Moreno PI, Vilanova I, Villa-Martínez RP, Francois JP. 2018. Modulation of fire regimes by vegetation and site type in southwestern Patagonia since 13 ka. Front Ecol Evol. 6:34.
- Morley RJ. 1976. Vegetation change in West Malesia during the Late Quaternary period [PhD thesis]. Kingston-upon-Hull: University of Hull.
- Morley RJ. 1982. A palaeoecological interpretation of a 10,000 year pollen record from Danau Padang, central Sumatra, Indonesia. J Biogeogr. 9(2):151–190.
- Morris JL, Cottrell S, Fettig CJ, DeRose RJ, Mattor KM, Carter VA, Clear JL, Clement J, Hansen WD, Hicke JA et al. 2018. Bark beetles as agents of change in social–ecological systems. Front Ecol Environ. 16(S1):S34–S43.
- Morris JL, Cottrell S, Fettig CJ, Hansen WD, Sherriff RL, Carter VA, Clear JL, Clement J, DeRose RJ, Hicke JA et al. 2017. Managing bark beetle impacts on ecosystems and society: priority questions to motivate future research. J Appl Ecol. 54(3):750–760.
- Morris JL, DeRose RJ, Brussel T, Brewer S, Brunelle A, Long JN. 2019. Stable or seral? Fire-driven alternative states in aspen forests of western North America. Biol Lett. 15(6):20190011.
- Morris JL, le Roux PC, Macharia AN, Brunelle A, Hebertson EG, Lundeen ZJ. 2013. Organic, elemental, and geochemical contributions to lake sediment deposits during severe spruce beetle (Dendroctonus rufipennis) disturbances. For Ecol Manage. 289:78–89.
- Mortensen MF, Birks HH, Christensen C, Holm J, Noe-Nygaard N, Odgaard BV, Olsen J, Rasmussen KL. 2011. Lateglacial vegetation development in Denmark - new evidence based on macrofossils and pollen from Slotseng, a small-scale site in southern Jutland. Quat Sci Rev. 30:2534–2550.
- Mortensen MF, Henriksen PS, Christensen C, Petersen PV, Olsen J. 2014. Vegetation development in south-east Denmark during the Weichselian Late Glacial: palaeoenvironmental studies close to the Palaeolithic site of Hasselø. Danish J Archaeol. 3(1):33–51.
- Mosbrugger V, Utescher T, Dilcher DL. 2005. Cenozoic continental climatic evolution of Central Europe. Proc Natl Acad Sci USA. 102(42):14964–14969.
- Motzkin G, Foster DR. 2002. Grasslands, heathlands, and shrublands in coastal New England: historical interpretations and approaches to conservation. J Biogeogr. 29:1569–1590.
- Mouquet N, Lagadeuc Y, Devictor V, Doyen L, Duputié A, Eveillard D, Faure D, Garnier E, Gimenez O, Huneman P et al. 2015. Predictive ecology in a changing world. J Appl Ecol. 52(5):1293–1310.
- Mrotzek A, Couwenberg J, Theuerkauf M, Joosten H. 2016. MARCO POLO - A new and simple tool for pollen-based stand-scale vegetation reconstruction. Holocene. 27(3):321–330.
- Mueller JR, Long CJ, Williams JJ, Nurse A, McLauchlan KK. 2014. The relative controls on forest fires and fuel source fluctuations in the Holocene deciduous forests of southern Wisconsin, USA. J Quat Sci. 29(6):561–569.
- Muggeo VMR. 2003. Estimating regression models with unknown break-points. Stat Med. 22:3055–3071.
- Müller F, Bergmann M, Dannowski R, Dippner JW, Gnauck A, Haase P, Jochimsen MC, Kasprzak P, Kröncke I, Kümmerlin R et al. 2016. Assessing resilience in long-term ecological data sets. Ecol Indic. 65:10–43.
- Muñoz Sobrino C, García-Moreiras I, Gómez-Orellana L, Iriarte-Chiapusso MJ, Heiri O, Lotter AF, Ramil-Rego P. 2018. The last hornbeam forests in SW Europe: new evidence on the demise of Carpinus betulus in NW Iberia. Veg Hist Archaeobot. 27(4):551–576.
- Murcia C, Aronson J, Kattan GH, Moreno-Mateos D, Dixon K, Simberloff D. 2014. A critique of the ‘novel ecosystem’ concept. Trends Ecol Evol. 29(10):548–553.
- Myers N. 2002. Biodiversity and biodepletion: the need for a paradigm shift. In: O’Riordan T, S. Stoll-Kleemann, editors. Protecting the protected: managing biodiversity for sustainability. Cambridge: Cambridge University Press; p. 46–60.
- Nackley LL, West AG, Skowno AL, Bond WJ. 2017. The nebulous ecology of native invasions. Trends Ecol Evol. 32(11):814–824.
- Nagy L, Grabherr G. 2009. The biology of alpine habitats. Oxford: Oxford University Press.
- Nathan R. 2006. Long-distance dispersal of plants. Science. 313(5788):786.
- Nathan R, Katul GG, Horn HS, Thomas SM, Oren R, Avissar R, Pacala SW, Levin SA. 2002. Mechanisms of long-distance dispersal of seeds by wind. Nature. 418(6896):409–413.
- Nathan R, Safriel UN, Noy-Meir I. 2001. Field validation and sensitivity analysis of a mechanistic model for tree seed dispersal by wind. Ecology. 82(2):374–388.
- Nathan R, Schurr FM, Spiegel O, Steinitz O, Trakhtenbrot A, Tsoar A. 2008. Mechanisms of long-distance seed dispersal. Trends Ecol Evol. 23(11):638–647.
- Nathorst AG. 1870. Om några arktiska växtlämningar i en sötvattenslera vid Alnarp i Skåne. Lunds Univ Årsskr. 7:17.
- Nathorst AG. 1892. Über den gegenwärtigen Standpunkt unserer Kenntnis von dem Vorkommen fossiler Glazialpflanzen. Sven Vetenskaps Akad Handlingar 17 Ser III. 5:32.
- Natlandsmyr B, Hjelle KL. 2016. Long-term vegetation dynamics and land-use history: Providing a baseline for conservation strategies in protected Alnus glutinosa swamp woodlands. For Ecol Manage. 372:78–92.
- Navrátilová J, Hájek M, Navrátil J, Hájková P, Frazier RJ. 2017. Convergence and impoverishment of fen communities in a eutrophicated agricultural landscape of the Czech Republic. Appl Veg Sci. 20(2):225–235.
- Neff JC, Ballantyne AP, Farmer GL, Mahowald NM, Conroy JL, Landry CC, Overpeck JT, Painter TH, Lawrence CR, Reynolds RL. 2008. Increasing eolian dust deposition in the western United States linked to human activity. Nat Geosci. 1(3):189–195.
- Neilson RP, Pitelka LF, Solomon AM, Nathan R, Midgley GF, Fragoso JM, Lischke H, Thompson K. 2005. Forecasting regional to global plant migration in response to climate change. BioScience. 55(9):749–759.
- Neilson RP, Wullstein LH. 1983. Biogeography of two southwest American oaks in relation to atmospheric dynamics. J Biogeogr. 10(4):275–297.
- Nelson DM, Hu FS. 2008. Patterns and drivers of Holocene vegetational change near the prairie–forest ecotone in Minnesota: revisiting McAndrews’ transect. New Phytol. 179(2):449–459.
- Nelson EC. 2009. Erica scoparia and Erica spiculifolia (formerly Bruckenthalia spicifolia) in interglacial floras in Ireland and Britain: confused nomenclature leading to misidentification of fossilized reeds. Quat Sci Rev. 28:381–383.
- Neuhäuslová Z, Blažková D, Grulich V, Husová M, Chytrý M, Jeník J, Jirásek J, Kolbek J, Kropáč Z, Ložek V et al. 1998. Map of Potential Natural Vegetation of the Czech Republic. Explanatory Text. Prgaue: Academia.
- Neustadt MI. 1959. Geschichte der vegetation der USSR in Holozän. Grana Palynol. 2:67–76.
- Newman EA. 2019. Disturbance ecology in the Anthropocene. Front Ecol Evol. 7: art147.
- Newsham A, Bhagwat SA. 2016. Conservation and development. London: Routledge.
- Nicolson M. 1990. Henry Allan Gleason and the individualistic hypothesis: the structure of a botanist’s career. Bot Rev. 56(2):91–161.
- Nicolson M. 2001. “Towards establishing ecology as a science instead of an art”: the work of John T. Curtis on the plant community continuum. Web Ecol. 2:1–6.
- Nicolson M, McIntosh RP. 2002. H.A. Gleason and the individualistic hypothesis revisited. Bull Ecol Soc Am. 83:133–142.
- Nicotra AB, Atkin OK, Bonser SP, Davidson AM, Finnegan EJ, Mathesius U, Poot P, Purugganan MD, Richards CL, Valladares F et al. 2010. Plant phenotypic plasticity in a changing climate. Trends Plant Sci. 15(12):684–692.
- Nielsen AB, Giesecke T, Theuerkauf M, Feeser I, Behre K-E, Beug H-J, Chen S-H, Christiansen J, Dörfler W, Endtmann E et al. 2012. Quantitative reconstructions of changes in regional openness in north-central Europe reveal new insights into old questions. Quat Sci Rev. 47:131–149.
- Nieto-Lugilde D, Maguire KC, Blois JL, Williams JW, Fitzpatrick MC. 2018. Multiresponse algorithms for community-level modeling: review of theory, applications, and comparison to species distribution models. Methods Ecol Evol. 9(4):834–848.
- Nikitin VP. 1957. Pliocenovye i Cetverticnye Flory Voronezskoj Oblasti. Moscow: Academy of Sciences of the USSR.
- Nikitin VP. 1979. Nekotorye novye vidy rasteniy v Neogenovych florach Severo-Vostoka SSSR. In: Shilo ON, Baranova YP, editors. Kontinental’nye Tretichnya Tolschi Severo-Vostoka Azii (Stratigrafiya, Korrelyatsiya, Paleoklimaty) (Continental Tertiary deposits of North-East Asia: Stratigraphy, correlation, paleoclimate). Moscow: Academy of Sciences of the USSR; p. 125–130.
- Nilsson C, Grelsson G. 1995. The fragility of ecosystems: a review. J Appl Ecol. 32:677–692.
- Nogué S, de Nascimento L, Fernández-Palacios JM, Whittaker RJ, Willis KJ. 2013. The ancient forests of La Gomera, Canary Islands, and their sensitivity to environmental change. J Ecol. 101:368–377.
- Nogué S, de Nascimento L, Froyd CA, Wilmshurst JM, de Boer EJ, Coffey EED, Whittaker RJ, Fernández-Palacios JM, Willis KJ. 2017. Island biodiversity conservation needs palaeoecology. Nat Ecol Evol. 1:0181.
- Nogué S, Rull V, Montoya E, Huber O, Vegas-Vilarrúbia T. 2009. Paleoecology of the Guayana Highlands (northern South America): Holocene pollen record from the Eruoda-tepui, in the Chimantá massif. Palaeogeogr Palaeoclimatol Palaeoecol. 281:165–173.
- Nogué S, Tovar C, Bhagwat SA, Finsinger W, Willis KJ. 2018. Exploring the ecological history of a tropical agroforestry landscape using fossil pollen and charcoal analysis from four sites in Western Ghats, India. Ecosystems. 21(1):45–55.
- Nogués-Bravo D, Rodríguez-Sánchez F, Orsini L, de Boer E, Jansson R, Morlon H, Fordham DA, Jackson ST. 2018. Cracking the code of biodiversity responses to past climate change. Trends Ecol Evol. 33(10):765–776.
- Nogués-Bravo D, Veloz S, Holt BG, Singarayer J, Valdes P, Davis B, Brewer SC, Williams JW, Rahbek C. 2016. Amplified plant turnover in response to climate change forecast by late quaternary records. Nat Clim Chang. 6:1115.
- Norberg A, Abrego N, Blanchet FG, Adler FR, Anderson BJ, Anttila J, Araújo MB, Dallas T, Dunson D, Elith J et al. 2019. A comprehensive evaluation of predictive performance of 33 species distribution models at species and community levels. Ecol Monogr. 89. p. e01370.
- Norberg J, Urban MC, Vellend M, Klausmeier CA, Loeuille N. 2012. Eco-evolutionary responses of biodiversity to climate change. Nat Clim Chang. 2:747–751.
- Nordlund C. 2014. Peat bogs as geological archives: Lennart von Post et al. and the development of quantitative pollen analysis during World War I. Earth Sci Rev. 33(2):187–200.
- Normand S, Ricklefs RE, Skov J, Bladt O, Tackenberg O, Svenning J-C. 2011. Postglacial migration supplements climate in determining plant species ranges in Europe. Proc R Soc B Bio Sci. 278:3644–3653.
- Norris JR, Betancourt JL, Jackson ST. 2016. Late Holocene expansion of ponderosa pine (Pinus ponderosa) in the central Rocky Mountains. J Biogeogr. 43:778–790.
- Noti R, van Leeuwen JFN, Colombaroli D, Vescovi E, Pasta S, La Mantia T, Tinner W. 2009. Mid- and late-Holocene vegetation and fire history at Biviere di Gela, a coastal lake in southern Sicily, Italy. Veg Hist Archaeobot. 18:371–387.
- Novenko EY, Tsyganov AN, Rudenko OV, Volkova EV, Zuyganova IS, Babeshko KV, Olchev AV, Losbenev NI, Payne RJ, Mazei YA. 2016. Mid- and late-Holocene vegetation history, climate and human impact in the forest-steppe ecotone of European Russia: new data and a regional synthesis. Biodivers Conserv. 25(12):2453–2472.
- Nowak CL, Nowak RS, Tausch RJ, Wigand PE. 1994. A 30 000 year record of vegetation dynamics at a semi-arid locale in the Great Basin. J Veg Sci. 5:579–590.
- O’Dea A, Dillon EM, Altieri AH, Lepore ML. 2017. Look to the past for an optimistic future. Conserv Bio. 31(6):1221–1222.
- Odgaard BV. 1994. The Holocene vegetation history of northern West Jutland, Denmark. Opera Bot. 123:1–171.
- Odgaard BV. 1999. Fossil pollen as a record of past biodiversity. J Biogeogr. 26:7–17.
- Odgaard BV. 2001. Palaeoecological perspectives on pattern and process in plant diversity and distribution adjustments: a comment on recent developments. Divers Distrib. 7:197–201.
- Odion DC, Moritz MA, DellaSala DA. 2010. Alternative community states maintained by fire in the Klamath Mountains, USA. J Ecol. 98(1):96–105.
- Ogle K, Barber JJ, Barron-Gafford GA, Patrick Bentley L, Young JM, Huxman TE, Loik ME, Tissue DT. 2015. Quantifying ecological memory in plant and ecosystem processes. Ecol Lett. 18(3):221–235.
- Ohlemüller R. 2011. Running out of climate space. Science. 334:613–614.
- Ohlemüller R, Gritti ES, Sykes MT, Thomas CD. 2006. Towards European climate risk surfaces: the extent and distribution of analogous and non-analogous cliamtes 1931–2100. Global Ecol Biogeogr. 15:395–405.
- Ohlemüller R, Huntley B, Normand S, Svenning JC. 2011. Potential source and sink locations for climate‐driven species range shifts in Europe since the Last Glacial Maximum. Global Ecol Biogeogr. 21(2):152–163.
- Ohlson M, Brown KJ, Birks HJB, Grytnes J-A, Hörnberg G, Niklasson M, Seppä H, Bradshaw RHW. 2011. Invasion of Norway spruce diversifies the fire regime in boreal European forests. J Ecol. 99(2):395–403.
- Ohlson M, Tryterud E. 1999. Long-term spruce forest continuity - a challenge for a sustainable Scandinavian forestry. For Ecol Manage. 124:27–34.
- Olajos F, Bokma F, Bartels P, Myrstener E, Rydberg J, Öhlund G, Bindler R, Wang XR, Zale R, Englund G. 2018. Estimating species colonization dates using DNA in lake sediment. Methods Ecol Evol. 9(3):535–543.
- Oldfield F. 1969. Pollen analysis and history of land use. Adv Sci. 25:298–311.
- Oldfield F. 1970a. The ecological history of Blelham bog national nature reserve. In: Walker D, West RG, editors. Studies in the vegetational history of the British Isles. Cambridge: Cambridge University Press; p. 141–157.
- Oldfield F. 1970b. Some aspects of scale and complexity in pollen-analytically based palaeoecology. Pollen Spores. 12(2):163–171.
- Oldfield F. 1993. Forward to the past: changing approaches to Quaternary palaeoecology. In: Chambers FM, editor. Climate change and human impact on the landscape. London: Chapman & Hall; p. 13–21.
- Oldfield F. 2005. Environmental change: key issues and alternative approaches. Cambridge: Cambridge University Press.
- Oldfield F. 2008. The role of people in the Holocene. In: Battarbee RW, Binney HA, editors. Natural climate variability and global warming: a holocene perspective. Oxford: Wiley-Blackwell; p. 58–97.
- Oldfield F. 2015. Book review 9 - biodiversity conservation and environmental change. [accessed 2018 March 9]. http://www.theanthropocenereview.com/2015/09/.
- Oldfield F, Appleby PG, Thompson R. 1980. Palaeoecological studies of lakes in the highlands of Papua New Guinea. I. The chronology of sedimentation. J Ecol. 68:457–477.
- Oldfield F, Huckerby E. 1979. The Quaternary palaeobotany of the French pays basque: a summary. Pollen Spores. 21:337–360.
- Oliver TH, Heard MS, Isaac NJB, Roy DB, Procter D, Eigenbrod F, Freckleton RP, Hector A, Orme CDL, Petchey OL et al. 2015. Biodiversity and resilience of ecosystem functions. Trends Ecol Evol. 30(11):673–684.
- Oliveras I, Malhi Y. 2016. Many shades of green: the dynamic tropical forest–savannah transition zones. Philos Trans R Soc B Bio Sci. 371(1703):20150308.
- Orbán I, Birks HH, Vincze I, Finsinger W, Pál I, Marinova E, Jakab G, Braun M, Hubay K, Bíró T et al. 2018. Treeline and timberline dynamics on the northern and southern slopes of the Retezat Mountains (Romania) during the late glacial and the Holocene. Quatern Int. 477:59–78.
- Ordonez A. 2013. Realized climatic niche of North American plant taxa lagged behind climate during the end of the Pleistocene. Am J Bot. 100(7):1255–1265.
- Ordonez A, Martinuzzi S, Radeloff VC, Williams JW. 2014. Combined speeds of climate and land-use change of the conterminous US until 2050. Nat Clim Chang. 4:811–816.
- Ordonez A, Williams JW. 2013a. Projected climate reshuffling based on multivariate climate-availability, climate-analog, and climate-velocity analyses: implications for community disaggregation. Clim Change. 119(3):659–675.
- Ordonez A, Williams JW. 2013b. Climatic and biotic velocities for woody taxa distributions over the last 16000 years in eastern North America. Ecol Lett. 16:773–781.
- Ordonez A, Williams JW, Svenning J-C. 2016. Mapping climatic mechanisms likely to favour the emergence of novel communities. Nat Clim Chang. 6(12):1104–1109.
- Orlando L, Cooper A. 2014. Using ancient DNA to understand evolutionary and ecological processes. Annu Rev Ecol Evol Syst. 45:573–598.
- Orr HA. 2005. The genetic theory of adaptation: a brief history. Nat Rev Genet. 6:119–127.
- Orsini L, Marshall H, Cuenca Cambronero M, Chaturvedi A, Thomas KW, Pfrender ME, Spanier KI, De Meester L. 2016. Temporal genetic stability in natural populations of the waterflea Daphnia magna in response to strong selection pressure. Mol Ecol. 25(24):6024–6038.
- Orsini L, Mergeay J, Vanoverbeke J, de Meester L. 2013a. The role of selection in driving landscape genomic structure of the waterflea Daphnia magna. Mol Ecol. 22(3):583–601.
- Orsini L, Schwenk K, de Meester L, Colbourne JK, Pfrender ME, Weider LJ. 2013b. The evolutionary time machine: using dormant propagules to forecast how populations can adapt to changing environments. Trends Ecol Evol. 28(5):274–282.
- Orsini L, Spanier KI, de Meester LUC. 2012. Genomic signature of natural and anthropogenic stress in wild populations of the waterflea Daphnia magna: validation in space, time and experimental evolution. Mol Ecol. 21(9):2160–2175.
- Oslisly R. 2001. The history of human settlement in the middle Ogooué valley (Gabon): implications for the environment. In: Weber B, White LJT, Vedder A et al., editors. African Rain Forest ecology and conservation. New Haven: Yale University Press; p. 101–118.
- Oslisly R, White L, Bentaleb I, Favier C, Fontugne M, Gillet J-F, Sebag D. 2013. Climatic and cultural changes in the west Congo Basin forests over the past 5000 years. Philos Trans R Soc B Bio Sci. 368(1625):20120304.
- Osmaston HA. 1958. Pollen analysis in the study of the past vegetatio and climate of Rwenzori and its neighbourhood [BSc thesis]. Oxford: University of Oxford.
- Oswald WW, Brubaker LB, Hu FS, Gavin DG. 2003. Pollen-vegetation calibration for tundra communities in the Arctic Foothills, northern Alaska. J Ecol. 91(6):1022–1033.
- Otto R, Garzón-Machado V, del Arco M, Fernández-Lugo S, de Nascimento L, Oromí P, Báez M, Ibáñez M, Alonso MR, Fernández-Palacios JM. 2017. Unpaid extinction debts for endemic plants and invertebrates as a legacy of habitat loss on oceanic islands. Divers Distrib. 23(9):1031–1041.
- Overpeck JT, Webb RS, Webb T. 1992. Mapping eastern North American vegetation change of the past 18 ka: no-analogs and the future. Geology. 20:1071–1074.
- Overpeck JT, Webb T, Prentice IC. 1985. Quantitative interpretation of fossil pollen spectra - dissimilarity coefficients and the method of modern analogs. Quat Res. 23:87–108.
- Paciorek CJ, Goring SJ, Thurman AL, Cogbill CV, Williams JW, Mladenoff DJ, Peters JA, Zhu J, McLachlan JS. 2016. Statistically-estimated tree composition for the northeastern United States at Euro-American settlement. PLoS One. 11(2):e0150087.
- Paciorek CJ, McLachlan JS. 2009. Mapping ancient forests: Bayesian inference for spatio-temporal trends in forest composition using the fossil pollen proxy record. J Am Stat Assoc. 104(486):608–622.
- Pakeman RJ. 2001. Plant migration rates and seed dispersal mechanisms. J Biogeogr. 28(6):795–800.
- Palmer MW, White PS. 1994. On the existence of ecological communities. J Veg Sci. 5:279–282.
- Palmer SCF, Mitchell RJ, Truscott AM, Welch D. 2004. Regeneration failure in Atlantic oakwoods: the roles of ungulate grazing and invertebrates. For Ecol Manage. 192(2):251–265.
- Palmgren A. 1929. Chance as an element in plant geography. Proc Int Congr Plant Sci. 1:591–602.
- Parducci L, Bennett KD. 2017. The real significance of ancient DNA. Am J Bot. 104(6):800–802.
- Parducci L, Bennett KD, Ficetola GF, Alsos IG, Suyama Y, Wood JR, Pedersen MW. 2017. Ancient plant DNA in lake sediments. New Phytol. 214(3):924–942.
- Parker NE, Williams JW. 2011. Influences of climate, cattle density, and lake morphology on Sporormiella abundances in modern lake sediments in the US Great Plains. Holocene. 22(4):475–483.
- Parmesan C. 2006. Ecological and evolutionary responses to recent climate change. Annu Rev Ecol Evol Syst. 37(1):637–669.
- Parshall T. 2002. Late Holocene stand-scale invasion by hemlock (Tsuga canadensis) at its western range limit. Ecology. 83(5):1386–1398.
- Parsons RW, Prentice IC. 1981. Statistical approaches to R-values and the pollen-vegetation relationship. Rev Palaeobot Palynol. 32(2–3):127–152.
- Past Interglacials Working Group of PAGES. 2016. Interglacials of the last 800,000 years. Rev Geophys. 54:162–219.
- Patiño J, Whittaker RJ, Borges PAV, Fernández-Palacios JM, Ah-Peng C, Araújo MB, Ávila SP, Cardoso P, Cornuault J, de Boer EJ et al. 2017. A roadmap for island biology: 50 fundamental questions after 50 years of The Theory of Island Biogeography. J Biogeogr. 44(5):963–983.
- Pearman DA. 2007. ‘Far from any house’ - assessing the status of doubtfully native species in the flora of the British Isles. Watsonia. 26:271–290.
- Pearsall WH. 1959. The ecology of invasion: ecological stability and instability. New Biol. 29:95–101.
- Pearson RG. 2006. Climate change and the migration capacity of species. Trends Ecol Evol. 21(3):111–113.
- Pearson RG, Dawson TP. 2003. Predicting the impacts of climate change on the distribution of species: are bioclimatic envelope models useful? Global Ecol Biogeogr. 12:361–371.
- Pearson S, Lynch AJJ, Plant R, Cork S, Taffs K, Dodson J, Maynard S, Gergis J, Gell P, Thackway R et al. 2015. Increasing the understanding and use of natural archives of ecosystem services, resilience and thresholds to improve policy, science and practice. Holocene. 25(2):366–378.
- Peat HJ, Fitter AH. 1993. The distribution of arbuscular mycorrhizas in the British flora. New Phytol. 125:845–854.
- Pedersen MW, Ruter A, Schweger C, Friebe H, Staff RA, Kjeldsen KK, Mendoza MLZ, Beaudoin AB, Zutter C, Larsen NK et al. 2016. Postglacial viability and colonization in North America’s ice-free corridor. Nature. 537:45–49.
- Pedersen TF, Francois R, Francois L, Alverson KD, McManus J. 2003. The late Quaternary history of biogeochemical cycling of carbon. In: Alverson KD, Bradley RS, Pedersen TF, editors. Paleoclimate, global change and the future. Berlin: Springer; p. 63–79.
- Pederson N, Dyer JM, McEwan RW, Hessl AE, Mock CJ, Orwig DA, Rieder HE, Cook BI. 2014. The legacy of episodic climatic events in shaping temperate, broadleaf forests. Ecol Monogr. 84(4):599–620.
- Peglar SM. 1993a. The mid-Holocene Ulmus decline at Diss Mere, Norfolk, UK: a year-by-year pollen stratigraphy from annual laminations. Holocene. 3 (1):1–13.
- Peglar SM. 1993b. The development of the cultural landscape around Diss Mere, Norfolk, UK, during the past 7000 years. Rev Palaeobot Palynol. 76:1–47.
- Peglar SM, Birks HJB. 1993. The mid-Holocene Ulmus fall at Diss Mere, south-east England - disease and human impact? Veg Hist Archaeobot. 2:61–68.
- Peglar SM, Fritz SC, Birks HJB. 1989. Vegetation and land-use history at Diss, Norfolk, UK. J Ecol. 77(1):203–222.
- Peltzer DA, Wardle DA, Allison VJ, Baisden WT, Bardgett RD, Chadwick OA, Condron LM, Parfitt RL, Porder S, Richardson SJ et al. 2010. Understanding ecosystem retrogression. Ecol Monogr. 80(4):509–529.
- Peñalba MC, Payette S. 1997. Late-Holocene expansion of eastern larch (Larix laricina [Du Roi] K. Koch) in northwestern Québec. Quat Res. 48(1):114–121.
- Pereira HM, Navarro L, editors. 2015. Rewilding European Landscapes. Berlin: Springer.
- Pérez-Obiol R, Sadori L. 2007. Similarities and dissimilarities, synchronisms and diachronisms in the Holocene vegetation history of the Balearic Islands and Sicily. Veg Hist Archaeobot. 16(4):259–265.
- Pérez-Obiol R, Yll EI, Pantaleon-Cano J, Roure JM. 1995. Historia de Buxus y Corylus en las Islas Baleares durante el Holoceno. In: Ramil-Rego P, Fernández Rodríguez C, Rodríguez Guitían M, editors. Biogeografia Pleistocena - Holocena de la Peninsula Ibérica. Santiago: Xunta de Galicia; p. 87–97.
- Perrin PM, Martin JR, Barron SJ, Roche JR. 2006. A cluster analysis approach to classifying Irish native woodlands. Bio Environ Proc R Irish Acad. 106B:261–279.
- Perrotti AG, van Asperen EN. 2019. Dung fungi as a proxy for megaherbivores: opportunities and limitations for archaeological applications. Veg Hist Archaeobot. 28(1):93–104.
- Perry GLW, Wainwright J, Etherington TR, Wilmshurst JM. 2016. Experimental simulation: using generative modeling and palaeoecological data to understand human-environment interactions. Front Ecol Evol. 4:109.
- Perry GLW, Wilmshurst JM, McGlone MS, Napier A. 2012. Reconstructing spatial vulnerability to forest loss by fire in pre-historic New Zealand. Global Ecol Biogeogr. 21(10):1029–1041.
- Persson C. 1975. Speculations on the immigration of spruce in Sweden. Geologiska Föreningens i Stockholm Förhandlingar. 97:292–294.
- Peterken GF. 1996. Natural Woodland. Ecology and conservation in northern temperate regions. Cambridge: Cambridge University Press.
- Peterken GF, Mountford E. 2017. Woodland development: a long-term study of Lady Park Wood. Wallingford: CABI.
- Peterson BJ, Graves WR. 2016. Chloroplast phylogeography of Dirca palustris L. indicates populations near the glacial boundary at the Last Glacial Maximum in eastern North America. J Biogeogr. 43:314–327.
- Petit RJ, Bialozyt R, Brewer S, Cheddadi R, Comps B. 2001. From spatial patterns of genetic diversity to postglacial migration processes in forest trees. In: Silvertown J, Antonovics J, editors. Integrating ecology and evolution in a spatial context. Oxford: Blackwell Science; p. 295–318.
- Petit RJ, Bialozyt R, Garnier-Géré P, Hampe A. 2004. Ecology and genetics of tree invasions: from recent introductions to Quaternary migrations. For Ecol Manage. 197:117–137.
- Petit RJ, Brewer S, Bordács S, Burg K, Cheddadi R, Coart E, Cottrell J, Csaikl UM, van Dam B, Deans JD et al. 2002b. Identification of refugia and post-glacial colonisation routes of European white oaks based on chloroplast DNA and fossil pollen evidence. For Ecol Manage. 156:49–74.
- Petit RJ, Csaikl UM, Bordács S, Burg K, Coart E, Cottrell J, van Dam B, Deans JD, Dumolin-Lapègue S, Fineschi S et al. 2002a. Chloroplast DNA variation in European white oaks. Phylogeography and patterns of diversity based on data from over 2600 populations. For Ecol Manage. 156:5–26.
- Petit RJ, Hampe A, Cheddadi R. 2005. Climate changes and tree phylogeography in the Mediterranean. Taxon. 54(4):877–885.
- Petit RJ, Hu FS, Dick CW. 2008. Forests of the past: a window to future changes. Science. 320:1450–1452.
- Petit RJ, Pineau E, Demesure B, Bailieri R, Ducousso A, Kramer A. 1997. Chloroplast DNA footprints of postglacial recolonization by oaks. Proc Natl Acad Sci USA. 94:9996–10001.
- Pettorelli N, Durant SM, du Toit JT, editors. 2019. Rewilding. Cambridge: Cambridge University Press.
- Pfeiffer M, van Leeuwen JFN, van der Knaap WO, Kaplan JO. 2013. The effect of abrupt climatic warming on biogeochemical cycling and N2O emisions in a terrestrial ecosystem. Palaeogeogr Palaeoclimatol Palaeoecol. 391:74–83.
- Phillips BL, Brown GP, Travis JMJ, Shine R. 2008. Reid’s paradox revisited: the evolution of dispersal kernels during range expansion. Am Nat. 172(S1):S34–S48.
- Pickett STA, White PS, editors. 1987. The ecology of natural disturbance and patch dynamics. Orlando: Academic Press.
- Pielke RA, Pitman A, Niyogi D, Mahmood R, McAlpine C, Hossain F, Goldewijk KK, Nair U, Betts R, Fall S et al. 2011. Land use/land cover changes and climate: modeling analysis and observational evidence. Clim Change. 2(6):828–850.
- Pigott CD. 1969. The status of Tilia cordata and T. platyphyllos on Derbyshire limestone. J Ecol. 57:491–504.
- Pigott CD. 1975. Natural regeneration of Tilia cordata in relation to forest structure in the forest of Białowieża, Poland. Philos Trans R Soc B Bio Sci. 270:151–179.
- Pigott CD. 1981a. Nature of seed sterility and natural regeneration of Tilia cordata near its northern limit in Finland. Ann Bot Fennici. 18:255–263.
- Pigott CD. 1981b. The status, ecology and conservation of Tilia platyphyllos in Britain. In: Synge H, editor. The biological aspects of rare plant conservation. Chichester: J. Wiley; p. 305–317.
- Pigott CD. 1984. The flora and vegetation of Britain: ecology and conservation. New Phytol. 98:119–128.
- Pigott CD. 1989. Factors controlling the distribution of Tilia cordata at the northern limit of its geographical range. 4. Estimated age of the trees. New Phytol. 112:117–121.
- Pigott CD. 1991. Tilia cordata Miller - Biological flora of the British Isles. J Ecol. 79:1147–1207.
- Pigott CD. 2012. Lime-trees and basswoods: a biological monograph of the genus Tilia. Cambridge: Cambridge University Press.
- Pigott CD, Huntley JP. 1978. Factors controlling the distribution of Tilia cordata at the northern limits of its geographical range. New Phytol. 81(2):429–441.
- Pigott CD, Huntley JP. 1980. Factors controlling the distribution of Tilia cordata at the northern limits of its geographical range. II. History in north-west England. New Phytol. 84:145–164.
- Pigott CD, Huntley JP. 1981. Factors controlling the distribution of Tilia cordata at the northern limits of its geographical range III. Nature and causes of seed sterility. New Phytol. 87(4):817–839.
- Pigott CD, Pigott ME. 1969. Late-glacial and post-glacial deposits at Malham, Yorkshire. New Phytol. 62:317–334.
- Pigott CD, Walters SM. 1954. On the interpretation of the discontinuous distributions shown by certain British species of open habitats. J Ecol. 42:95–116.
- Pimm SL. 1991. The Balance of Nature? Ecological Issues in the conesrvation of species and communities. Chicago: University of Chicago Press.
- Piñero D, Martinez-Ramos M, Sarukhán J. 1984. A population model of Astrocaryum mexicanum and a sensitivity model of its finite rate of increase. J Ecol. 72:977–991.
- Pini R, Ravazzi C, Raiteri L, Guerreschi A, Castellano L, Comolli R. 2017. From pristine forests to high-altitude pastures: an ecological approach to prehistoric human impact on vegetation and landscapes in the western Italian Alps. J Ecol. 105(6):1580–1597.
- Piotti A, Leonarduzzi C, Postolache D, Bagnoli F, Spanu I, Brousseau L, Urbinati C, Leonardi S, Vendramin GG. 2017. Unexpected scenarios from Mediterranean refugial areas: disentangling complex demographic dynamics along the Apennine distribution of silver fir. J Biogeogr. 44(7):1547–1558.
- Piovesan G, Biondi F, Baliva M, Presutti Saba E, Calcagnile L, Quarta G, D’Elia M, De Vivo G, Schettino A, Di Filippo A. 2018. The oldest dated tree of Europe lives in the wild Pollino massif: Italus, a strip-bark Heldreich’s pine. Ecology. 99(7):1682–1684.
- Piperno DR. 2006. Quaternary environmental history and agricultural impact on vegetation in Central America. Ann Missouri Bot Garden. 93(2):274–296.
- Piperno DR. 2011. The origins of plant cultivation and domestication in the New World Tropics: patterns, process, and new developments. Curr Anthropol. 52(S4):S453–S470.
- Piperno DR, McMichael CH, Bush MB. 2015. Amazonia and the Anthropocene: What was the spatial extent and intensity of human landscape modification in the Amazon Basin at the end of prehistory? Holocene. 25(10):1588–1597.
- Pires MM, Guimarães PR, Galetti M, Jordano P. 2018. Pleistocene megafaunal extinctions and the functional loss of long-distance seed-dispersal services. Ecography. 41:153–163.
- Pirzamanbein B, Lindström J, Poska A, Sugita S, Trondman A-K, Fyfe R, Mazier F, Nielsen AB, Kaplan JO, Bjune AE et al. 2014. Creating spatially continuous maps of past land cover from point estimates: A new statistical approach applied to pollen data. Ecol Complexity. 20:127–141.
- Plotnick RE, Smith FA, Lyons SK. 2016. The fossil record of the sixth extinction. Ecol Lett. 19(5):546–553.
- Plumpton H, Whitney B, Mayle F. 2019. Ecosystem turnover in palaeoecological records: the sensitivity of pollen and phytolith proxies to detecting vegetation change in southwestern Amazonia. The Holocene. (in press). 0959683619862021
- Pokorný P, Chytrý M, Juřičková L, Sádlo J, Novák J, Ložek V. 2015. Mid-Holocene bottleneck for central European dry grasslands: Did steppe survive the forest optimum in northern Bohemia, Czech Republic? Holocene. 25(4):716–726.
- Poorter L, Bongers F, Aide TM, Almeyda Zambrano AM, Balvanera P, Becknell JM, Boukili V, Brancalion PHS, Broadbent EN, Chazdon RL et al. 2016. Biomass resilience of Neotropical secondary forests. Nature. 530:211.
- Porter SC. 1989. Some geological implications of average Quaternary glacial conditions. Quat Res. 32(3):245–261.
- Porto TJ, Carnaval AC, da Rocha PLB. 2012. Evaluating forest refugial models using species distribution models, model filling and inclusion: a case study with 14 Brazilian species. Divers Distrib. 19(3):330–340.
- Post E. 2013. Ecology of climate change. Princeton: Princeton University Press.
- Postigo-Mijarra JM, Morla C, Barrón E, Morales-Molino C, García S. 2010. Patterns of extinction and persistence of Arctotertiary flora in Iberia during the Quaternary. Rev Palaeobot Palynol. 162:416–426.
- Potter KA, Woods HA, Pincebourde S. 2013. Microclimate challenges in global change biology. Glob Chang Biol. 19:2932–2939.
- Powell JA, Zimmermann NE. 2004. Multiscale analysis of active seed dispersal contributes to resolving Reid’s paradox. Ecology. 85(2):490–506.
- Pratt S. 2018. Figure 2.2. In: Summers RW, editor. Abernethy forest. 30. Inverness: RSPB Scotland.
- Prebble M, Dowe JL. 2008. The late Quaternary decline and extinction of palms on oceanic Pacific islands. Quat Sci Rev. 27(27):2546–2567.
- Prebble M, Whitau R, Meyer J-Y, Sibley-Punnett L, Fallon S, Porch N. 2016. Abrupt late Pleistocene ecological and climate change on Tahiti (French Polynesia). J Biogeogr. 43:2438–2453.
- Prebble M, Wilmshurst JM. 2009. Detecting the initial impact of humans and introduced species on island environments in Remote Oceania using palaeoecology. Biol Invasions. 11(7):1529–1556.
- Preece RC, Bennett KD, Carter JR. 1986. The Quaternary palaeobotany of Inaccessible Island (Tristan da Cunha group). J Biogeogr. 13:1–33.
- Prentice IC. 1983. Postglacial climatic change: vegetation dynamics and the pollen record. Prog Phys Geog. 7(2):273–286.
- Prentice IC. 1985. Pollen representation, source area, and basin size – toward a unified theory of pollen analysis. Quat Res. 23:76–86.
- Prentice IC. 1986a. Forest-composition calibration of pollen data. In: Berglund BE, editor. Handbook of holocene palaeoecology and palaeohydrology. Chichester: J Wiley & Sons; p. 799–816.
- Prentice IC. 1986b. Vegetation responses to past climatic variation. Vegetatio. 67:131–141.
- Prentice IC. 1988a. Paleoecology and plant-population dynamics. Trends Ecol Evol. 3:343–345.
- Prentice IC. 1988b. Records of vegetation in time and space: the principles of pollen analysis. In: Huntley B, Webb T, editors. Vegetation history. Dordrecht: Kluwer Academic Publishers; p. 17–42.
- Prentice IC. 1992. Climate change and long-term vegetation dynamics. In: Glenn-Lewin DC, Peet RK, Veblen TT, editors. Plant succession: theory and prediction. London: Chapman and Hall; p. 293–339.
- Prentice IC, Bartlein PJ, Webb T. 1991. Vegetation and climate change in eastern North America since the last glacial maximum. Ecology. 72:2038–2056.
- Prentice IC, Cramer W, Harrison SP, Leemans R, Monserud RA, Solomon AM. 1992. A global biome model based on plant physiology and dominance, soil properties and climate. J Biogeogr. 19:117–134.
- Prentice IC, Guiot J, Huntley B, Jolly D, Cheddadi R. 1996. Reconstructing biomes from palaeoecological data: a general method and its application to European pollen data at 0 and 6 ka. Clim Dynam. 12:185–194.
- Prentice IC, Harrison SP, Bartlein PJ. 2011. Global vegetation and terrestrial carbon cycle changes after the last ice age. New Phytol. 189(4):988–998.
- Prentice IC, Harrison SP, Jolly D, Guiot J. 1998. The climate and biomes of Europe at 6000 yr BP: comparison of model simulations and pollen-based reconstructions. Quat Sci Rev. 17:659–668.
- Prentice IC, Helmisaari H. 1991. Silvics of north European trees: Compilation, comparison and implications for forest succession modelling. For Ecol Manage. 42:79–93.
- Prentice IC, Parsons RW. 1983. Maximum-likelihood linear calibration of pollen spectra in terms of forest composition. Biometrics. 39:1051–1057.
- Prentice IC, Webb T. 1986. Pollen percentages, tree abundances and the Fagerlind effect. J Quat Sci. 1:35–43.
- Preston CD. 2015. Derek Ratcliffe and the Atlantic bryophytes of Britain and Ireland. In: Thomspon DBA, Birks HH, Birks HJB, editors. Nature’s conscience - the life and legacy of Derek Ratcliffe. Peterborough: Langford Press; p. 123–148.
- Preston CD, Pearman DA, Hall AR. 2004. Archaeophytes in Britain. Bot J Linn Soc. 145:257–294.
- Price GJ, Louys J, Faith JT, Lorenzen ED, Westaway MC. 2018. Big data little help in megafauna mysteries. Nature. 558:23–25.
- Primack RB, Miao SL. 1991. Dispersal can limit local plant distribution. Conserv Bio. 6(4):513–519.
- Prins HHT, Gordon IJ, editors. 2014. Invasion biology and ecological theory: insights from a continent in transformation. Cambridge: Cambridge University Press.
- Provan J, Bennett KD. 2008. Phylogeographic insights into cryptic glacial refugia. Trends Ecol Evol. 23(10):564–571.
- Pulsford SA, Lindenmayer DB, Driscoll DA. 2016. A succession of theories: purging redundancy from disturbance theory. Biol Rev. 91(1):148–167.
- Pykälä J. 2017. Relation between extinction and assisted colonization of plants in the arctic-alpine and boreal regions. Conserv Bio. 31(3):524–530.
- Quammen D. 1996. The song of the Dodo: Island biogeography in an age of extinction. New York: Prentice Hall.
- Rackham O. 1992. Mixtures, mosaics and clones: the distribution of trees within European woods and forests. In: Cannell MGR, Malcolm DC, Robertson PA, editors. The ecology of mixed-species stands of trees. Oxford: Blackwell Scientific Publications; p. 1–20.
- Rackham O. 2003. Ancient Woodland: its history, vegetation and uses in England. 2nd ed. Dalbeattie: Castlepoint Press.
- Rackham O. 2006. Woodlands. London: Collins.
- Raczka MF, Bush MB, De Oliveira PE. 2017. The collapse of megafaunal populations in southeastern Brazil. Quat Res. 89(1):103–118.
- Raczka MF, Bush MB, Folcik AM, McMichael CH. 2016. Sporormiella as a tool for detecting the presence of large herbivores in the Neotropics. Biota Neotrop. 16 (1):e20150090.
- Radeloff VC, Williams JW, Bateman BL, Burke KD, Carter SK, Childress ES, Cromwell KJ, Gratton C, Hasley AO, Kraemer BM et al. 2015. The rise of novelty in ecosystems. Ecol App. 25(8):2051–2068.
- Rademaker K, Hodgins G, Moore K, Zarrillo S, Miller C, Bromley GRM, Leach P, Reid DA, Álvarez WY, Sandweiss DH. 2014. Paleoindian settlement of the high-altitude Peruvian Andes. Science. 346(6208):466–469.
- Ralska-Jasiewiczowa M. 1983. Isopollen maps for Poland: 0-11,000 years BP. New Phytol. 94:133–175.
- Ralska-Jasiewiczowa M, Latałowa M, Wasylikowa K, Tobolski K, Madeyska E, Wright HE, Turner C, editors. 2004. Late Glacial and Holocene history of vegetation in Poland based on Isopollen maps. Kraków: W. Szafer Institute of Botany, Polish Academy of Sciences.
- Ramstack Hobbs JM, Hobbs WO, Edlund MB, Zimmer KD, Theissen KM, Hoidal N, Domine LM, Hanson MA, Herwig BR, Cotner JB. 2016. The legacy of large regime shifts in shallow lakes. Ecol App. 26(8):2662–2676.
- Randsalu-Wendrup L, Conley DJ, Carstensen J, Fritz SC. 2016. Paleolimnological records of regime shifts in lakes in response to climate change and anthropogenic activities. J Paleolimnol. 56(1):1–14.
- Randsalu-Wendrup L, Conley DJ, Carstensen J, Hansson L-A, Brönmark C, Fritz SC, Choudhary P, Routh J, Hammarlund D. 2014. Combining limnology and palaeolimnology to investigate recent regime shifts in a shallow, eutrophic lake. J Paleolimnol. 51(3):437–448.
- Randsalu-Wendrup L, Conley DJ, Carstensen J, Snowball I, Jessen CA, Fritz SC. 2012. Ecological regime shifts in Lake Kälksjön, Sweden, in response to abrupt climate change around the 8.2 ka cooling event. Ecosystem 15(8):1336–1350.
- Rangel TF, Edwards NR, Holden PB, Diniz-Filho JAF, Gosling WD, Coelho MTP, Cassemiro FAS, Rahbek C, Colwell RK. 2018. Modeling the ecology and evolution of biodiversity: Biogeographical cradles, museums, and graves. Science. 361(6399):5452.
- Rapacciuolo G, Blois JL. 2019. Understanding ecological change across large spatial, temporal and taxonomic scales: integrating data and methods in light of theory. Ecography. doi:10.1111/ecog.04616.
- Rapacciuolo G, Roy DB, Gillings S, Fox R, Walker K, Purvis A. 2012. Climatic associations of British species distributions show good transferability in time but low predictive accuracy for range change. PLoS One. 7(7):e40212.
- Raper D, Bush MB. 2009. A test of Sporormiella representation as a predictor of megaherbivore presence and abundance. Quat Res. 71:490–496.
- Rasmussen P. 2005. Mid- to late-Holocene land-use change and lake development at Dallund Sø, Denmark: vegetation and land-use history inferred from pollen data. Holocene. 15(8):1116–1129.
- Rasmussen P, Bradshaw EG. 2005. Mid- to late-Holocene land-use change and lake development at Dallund Sø, Denmark: study aims, natural and cultural setting, chronology and soil erosion history. Holocene. 15(8):1105–1115.
- Ratajczak Z, Carpenter SR, Ives AR, Kucharik CJ, Ramiadantsoa T, Stegner MA, Williams JW, Zhang J, Turner MG. 2018. Abrupt change in ecological systems: Inference and diagnosis. Trends Ecol Evol. 33(7):513–526.
- Ratajczak Z, Nippert JB. 2012. Comment on “Global resilience of tropical forest and savanna to critical transitions”. Science. 336(6081):541.
- Ratcliffe DA. 1968. An ecological account of Atlantic bryophytes in British Isles. New Phytol. 67:365–439.
- Ratcliffe DA. 1971. Criteria for selection of nature reserves. Adv Sci. 27(134):294–296.
- Ratcliffe DA. 1976. Thoughts towards a philosophy of nature conservation. Biol Conserv. 9:45–53.
- Ratcliffe DA, editor 1977. A nature conservation review, volumes 1 and 2. Cambridge: Cambridge University Press.
- Ratcliffe DA. 1986. Selection of important areas for wildlife conservation in Great Britain: the Nature Conservancy Council’s approach. In: Usher MB, editor. Wildlife conservation evaluation. London: Chapman & Hall; p. 135–159.
- Ravazzi C, Donegana M, Vescovi E, Arpenti E, Caccianiga M, Kaltenrieder P, Londeix L, Marabini S, Mariani S, Pini R et al. 2006. A new Late-glacial site with Picea abies in the northern Apennine foothills: an exception to the model of glacial refugia of trees. Veg Hist Archaeobot. 15(4):357–371.
- RBG Kew. 2016. State of the World’s plants - 2016. Kew: Royal Botanic Gardens.
- Read DJ. 1993a. Mycorrhiza in plant communities. Mol Plant Pathol. 9:1–31.
- Read DJ. 1993b. Plant-microbe mutualism and community structure. In: Schulze E-D, Mooney HA, editors. Biodiversity and ecosystem function. Berlin: Springer; p. 181–209.
- Reczyńska K, Świerkosz K. 2017. Compositional changes in thermophilous oak forests in Poland over time: do they correspond to European trends? Appl Veg Sci. 20(2):293–303.
- Rehfeldt GE, Ying CC, Spittlehouse DL, Hamilton DA. 1999. Genetic responses to climate in Pinus contorta: niche breadth, climate change, and reforestation. Ecol Monogr. 69(3):375–407.
- Reid C. 1899. The Origin of the British Flora. London: Dulau & Co.
- Reid EM. 1920. A comparative review of Pliocene floras based on the study of fossil seeds. Q J Geol Soc London. 76:104–144.
- Reille M. 1975. Contribution pollenanalytique a l’histoire Tardiglaciaire et Holocène de la végétation de la Montagne Corse [PhD thesis]. France: L’Université d’Aix-Marseille.
- Reitalu T, Gerhold P, Poska A, Pärtel M, Väli V, Veski S. 2015. Novel insights into post-glacial vegetation change: functional and phylogenetic diversity in pollen records. J Veg Sci. 26:911–922.
- Reitalu T, Kunes P, Giesecke T. 2014. Closing the gap between plant ecology and Quaternary palaeoecology. J Veg Sci. 25:1188–1194.
- Rejmánek M. 1989. Invasibility of plant communities. In: Drake JA, di Castri F, Groves RH et al., editors, pp. 369-386. Biological invasions: a global perspective. Chichester: Wiley.
- Rejmánek M. 1999. Holocene invasions: finally the resolution ecologists were waiting for! Trends Ecol Evol. 14(1):8–10.
- Renberg I, Bigler C, Bindler R, Norberg M, Rydberg J, Segerström U. 2009. Environmental history: A piece in the puzzle for establishing plans for environmental management. J Environ Manage. 90(8):2794–2800.
- Reside AE, Welbergen JA, Phillips BL, Wardell-Johnson GW, Keppel G, Ferrier S, Williams SE, VanDerWal J. 2014. Characteristics of climate change refugia for Australian biodiversity. Austral Ecol. 39(8):887–897.
- Restrepo A, Colinvaux PA, Bush MB, Correa-Metrio A, Conroy J, Gardener MR, Jaramillo P, Steinitz-Kannan M, Overpeck J. 2012. Impacts of climate variability and human colonization on the vegetation of the Galápagos Islands. Ecology. 93(8):1853–1866.
- Reu B, Zaehle S, Bohn K, Pavlick R, Schmidtlein S, Williams JW, Kleidon A. 2014. Future no-analogue vegetation produced by no-analogue combinations of temperature and insolation. Global Ecol Biogeogr. 23(2):156–167.
- Rey F. 2017. Exploring eight millennia of climatic, vegetational and agricultural dynamics on the Swiss Plateau by using annually layered sedimentary time series [PhD thesis]. Bern: University of Bern.
- Rey F, Gobet E, Schwörer C, Wey O, Hafner A, Tinner W. 2019. Causes and mechanisms of synchronous succession trajectories in primeval Central European mixed Fagus sylvatica forests. J Ecol. 107(3):1392–1408.
- Rey F, Gobet E, van Leeuwen JFN, Gilli A, van Raden UJ, Hafner A, Wey O, Rhiner J, Schmocker D, Zünd J et al. 2017. Vegetational and agricultural dynamics at Burgäschisee (Swiss Plateau) recorded for 18,700 years by multi-proxy evidence from partly varved sediments. Veg Hist Archaeobot. 26(6):571–586.
- Rey F, Schwörer C, Gobet E, Colombaroli D, van Leeuwen JFN, Schleiss S, Tinner W. 2013. Climatic and human impacts on mountain vegetation at Lauenensee (Bernese Alps, Switzerland) during the last 14,000 years. Holocene. 23(10):1415–1427.
- Reyer CPO, Brouwers N, Rammig A, Brook BW, Epila J, Grant RF, Holmgren M, Langerwisch F, Leuzinger S, Lucht W et al. 2015a. Forest resilience and tipping points at different spatio‐temporal scales: approaches and challenges. J Ecol. 103(1):5–15.
- Reyer CPO, Rammig A, Brouwers N, Langerwisch F. 2015b. Forest resilience, tipping points and global change processes. J Ecol. 103(1):1–4.
- Rhemtulla JM, Mladenoff DJ. 2007. Why history matters in landscape ecology. Landsc Ecol. 22(1):1–3.
- Ricciardi A. 2007. Are modern biological invasions an unprecedented form of global change? Conserv Bio. 21(2):329–336.
- Ricciardi A, Blackburn TM, Carlton JT, Dick JTA, Hulme PE, Iacarella JC, Jeschke JM, Liebhold AM, Lockwood JL, MacIsaac HJ et al. 2017a. Invasion science: A horizon scan of emerging challenges and opportunities. Trends Ecol Evol. 32(6):464–474.
- Ricciardi A, Blackburn TM, Carlton JT, Dick JTA, Hulme PE, Iacarella JC, Jeschke JM, Liebhold AM, Lockwood JL, MacIsaac HJ et al. 2017b. Invasion science: Looking forward rather than revisiting old ground – A reply to Zenni et al. Trends Ecol Evol. 32(11):809–810.
- Richards K. 2017. Report on the conference to mark the Centenary (1916–2016) of pollen analysis and te legacy of Lennart von Post, Stockholm, November 24- 25th2016. AASP Palynol Soc Newsl. 50(1):21–24.
- Richards PW. 1952. The Tropical Rain Forest: an ecological study. 1st ed. Cambridge: Cambridge University Press.
- Richards PW. 1996. The Tropical Rain Forest: an ecological study. 2nd ed. Cambridge: Cambridge University Press.
- Richardson DM, Hellmann JJ, McLachlan JS, Sax DF, Schwartz MW, Gonzalez P, Brennan EJ, Camacho AE, Root TL, Sala OE et al. 2009. Multidimensional evaluation of managed relocation. Proc Natl Acad Sci USA. 106(24):9721–9724.
- Richardson DM, Pyšek P, Rejmánek M, Barbour MG, Panetta FD, West CJ. 2000. Naturalization and invasion of alien plants: concepts and definitions. Divers Distrib. 6(2):93–107.
- Richer S, Gearey B. 2017. From Rackham to REVEALS: Reflections on palaeoecological approaches to woodland and trees. Environ Archaeol.10.1080/14614103.14612017.11283765.
- Rick TC, Lockwood R. 2012. Integrating paleobiology, archaeology, and history to inform biological conservation. Conserv Bio. 27(1):45–54.
- Ricklefs RE. 2008. Disintegration of the ecological community. Am Nat. 172(6):741–750.
- Ricklefs RE. 2009. A brief response to Brooker et al.’s comment. Am Nat. 174(6):928–931.
- Rijsdijk KF, Hume JP, Bunnik F, Florens FBV, Baider C, Shapiro B, van der Plicht J, Janoo A, Griffiths O, van den Hoek Ostende LW et al. 2009. Mid-Holocene vertebrate bone Concentration-Lagerstätte on oceanic island Mauritius provides a window into the ecosystem of the dodo (Raphus cucullatus). Quat Sci Rev. 28(1):14–24.
- Rijsdijk KF, Hume JP, de Louw PGB, Meijer HJM, Janoo A, de Boer EJ, Steel L, de Vos J, van der Sluis LG, Hooghiemstra H et al. 2015. A review of the dodo and its ecosystem: insights from a vertebrate concentration Lagerstätte in Mauritius. J Vertebr Paleontol. 35(sup1):3–20.
- Rijsdijk KF, Zinke J, de Louw PGB, Hume JP, van der Plicht H, Hooghiemstra H, Meijer HJM, Vonhof HB, Porch N, Florens FBV et al. 2011. Mid-Holocene (4200 kyr BP) mass mortalities in Mauritius (Mascarenes): Insular vertebrates resilient to climatic extremes but vulnerable to human impact. Holocene. 21(8):1179–1194.
- Ritchie JC. 1976. The late-Quaternary vegetation of the western interior of Canada. Can J Bot. 53:381–396.
- Ritchie JC. 1984. Past and Present Vegetation of the Far Northwest of Canada. Toronto: University of Toronto Press.
- Ritchie JC. 1985. Late-Quaternary climatic and vegetational change in the lower MacKenzie Basin, northwest Canada. Ecology. 66(2):612–621.
- Ritchie JC. 1986. Climate change and vegetation response. Vegetatio. 67:65–74.
- Ritchie JC. 1991. Paleoecology: status and prospect. In: Shane LCK, Cushing EJ, editors. Quaternary landscapes. Minneapolis: University of Minnesota Press; p. 113–128.
- Ritchie JC. 1994. Holocene pollen spectra from Oyo, northwestern Sudan: problems of interpretation in a hyperarid environment. Holocene. 4(1):9–15.
- Ritchie JC. 1995. Current trends in studies of long-term plant community dynamics. New Phytol. 130:469–494.
- Ritchie JC. 2008. Postglacial Vegetation of Canada. Cambridge: Cambridge University Press.
- Ritchie JC, Eyles CH, Haynes CV. 1985. Sediment and pollen evidence for an early to mid-Holocene humid period in the eastern Sahara. Nature. 314:352.
- Ritchie JC, Haynes CV. 1987. Holocene vegetation zonation in the eastern Sahara. Nature. 330:645.
- Ritchie JC, Lichti-Federovich S. 1963. Contemporary pollen spectra in central Canada I. Atmospheric samples at Winnipeg, Manitoba. Pollen Spores. 5:95–114.
- Ritchie JC, MacDonald GM. 1986. The patterns of post-glacial spread of white spruce. J Biogeogr. 13:527–540.
- Rivas-Torres GF, Benítez FL, Rueda D, Sevilla C, Mena CF. 2018. A methodology for mapping native and invasive vegetation coverage in archipelagos: An example from the Galápagos Islands. Prog Phys Geogr Earth Environ. 42(1):83–111.
- Rivers M. 2017. Conservation assessment and understanding the threat on plant diversity. In: Blackmore S, Oldfield S, editors. Plant conservation science and practice. Cambridge: Cambridge University Press; p. 49–72.
- Roberts DR, Hamann A. 2012. Predicting potential climate change impacts with bioclimate envelope models: a palaeoecological perspective. Global Ecol Biogeogr. 21(2):121–133.
- Roberts DR, Hamann A. 2015. Glacial refugia and modern genetic diversity of 22 western North American tree species. Proc R Soc B Bio Sci. 282(1804).
- Roberts DR, Hamann A. 2016. Climate refugia and migration requirements in complex landscapes. Ecography. 39(12):1238–1246.
- Roberts L. 1989. How fast can trees migrate? Science. 243(4892):735–737.
- Roberts N. 2014. The Holocene - an environmental history. Third ed. Chichester: Wiley Blackwell.
- Roberts N, Fyfe RM, Woodbridge J, Gaillard M-J, Davis BAS, Kaplan JO, Marquer L, Mazier F, Nielsen AB, Sugita S et al. 2018. Europe’s lost forests: a pollen-based synthesis for the last 11,000 years. Sci Rep. 8(1):716.
- Roberts P. 2017. Forests of plenty: Ethnographic and archaeological rainforests as hotspots of human activity. Quatern Int. 448:1–4.
- Roberts P, Boivin N, Kaplan JO. 2018. Finding the anthropocene in tropical forests. Anthropocene. 23:5–16.
- Robin V, Nadeau M-J, Grootes PM, Bork H-R, Nelle O. 2016a. Too early and too northerly: evidence of temperate trees in northern central Europe during the Younger Dryas. New Phytol. 212(1):259–268.
- Robin V, Nadeau M-J, Grootes PM, Bork H-R, Nelle O. 2016b. Paleobotanical and climate data support the plausibility of temperate trees’ spread into central Europe during the Late Glacial. New Phytol. 212:19–21.
- Robinson BL. 1902. Flora of the Galápagos Islands. Proc Am Acad Arts Sci. 38(4):77–269.
- Robinson GS, Burney LP, Burney DA. 2005. Landscape paleoecology and megafaunal extinction in southeaastern New York State. Ecol Monogr. 75:295–315.
- Robinson M, De Souza JG, Maezumi SY, Cárdenas M, Pessenda L, Prufer K, Corteletti R, Scunderlick D, Mayle FE, De Blasis P et al. 2018. Uncoupling human and climate drivers of late Holocene vegetation change in southern Brazil. Sci Rep. 8(1):7800.
- Roche JR, Mitchell FJG, Waldren S. 2009. Plant community ecology of Pinus sylvestris, an extirpated species reintroduced to Ireland. Biodivers Conserv. 18 (8):2185.
- Rodwell JS, Pigott CD, Ratcliffe DA, Malloch AJC, Birks HJB, Proctor MCF, Shimwell DW, Huntley JP, Radford E, Wiggington MJ et al., editors. 1991. British Plant Communities 1. Woodlands and Scrub. Cambridge: Cambridge University Press.
- Romme WH, Everham EH, Frelich LE, Moritz MA, Sparks RE. 1998. Are large, infrequent disturbances qualitatively different from small, frequent disturbances? Ecosystem 1:524–534.
- Roosevelt AC, da Costa ML, Machado CL, Michab M, Mercier N, Valladas H, Feathers J, Barnett W, da Silveira MI, Henderson A et al. 1996. Paleoindian cave dwellers in the Amazon: the peopling of the Americas. Science. 272(5260):373–384.
- Rosenblad KC, Sax DF. 2017. A new framework for investigating biotic homogenization and exploring future trajectories: oceanic island plant and bird assemblages as a case study. Ecography. 40(9):1040–1049.
- Ross LC, Woodin SA, Hester AJ, Thomspon DBA, Birks HJB. 2012. Biotic homogenisation of upland vegetation: patterns and drivers at multiple spatial scales over five decades. J Veg Sci. 23:755–770.
- Ross NJ. 2011. Modern tree species composition reflects ancient Maya “forest gardens” in northwest Belize. Ecol App. 21(1):75–84.
- Ross NJ, Rangel TF. 2011. Ancient Maya agroforestry echoing through spatial relationships in the extant forest of NW Belize. Biotropica. 43(2):141–148.
- Rotherham ID, editor 2013. Trees, Forested Landscapes and Grazing Animals. London: Routledge.
- Roughgarden J. 2009. Is there a general theory of community ecology? Biol Philos. 24(4):521–529.
- Roxburgh SH, Shea K, Wilson JB. 2004. The intermediate disturbance hypothesis: Patch dynamics and mechanisms of species coexistence. Ecology. 85(2):359–371.
- Roy K, Valentine JW, Jablonski D, Kidwell SM. 1996. Scales of climatic variability and time averaging in Pleistocene biotas: implications for ecology and evolution. Trends Ecol Evol. 11(11):458–463.
- Rozas-Davila A, Valencia BG, Bush MB. 2016. The functional extinction of Andean megafauna. Ecology. 97(10):2533–2539.
- Ruddiman WF. 1990. Changes in climate and biota on geologic time scales. Trends Ecol Evol. 5(9):285–288.
- Ruddiman WF. 2003. The Anthropogenic Greenhouse Era began thousands of years ago. Clim Change. 61(3):261–293.
- Ruddiman WF. 2005. Plows, plagues, and petroleum. How humans took control of climate. Princeton and Oxford: Princeton University Press.
- Ruddiman WF. 2007. The early anthropogenic hypothesis: Challenges and responses. Rev Geophys. 45 (4):RG4001.
- Ruddiman WF. 2013a. Earth’s climate: past and future. 3rd ed. New York: WH Freeman.
- Ruddiman WF. 2013b. The Anthropocene. Annu Rev Earth Planet Sci. 41(4):4.1–4.28.
- Ruddiman WF, Ellis EC, Kaplan JO, Fuller DQ. 2015. Defining the epoch we live in. Science. 348:38–39.
- Rudolph K. 1930. Grundzüge der nacheiszeitlichen Waldgeschichte Mitteleuropas. Beih Bot Centralblatt. 47(2):111–176.
- Rueger BF, von Wallmenich TN. 1996. Human impact on the forests of Bermuda: the decline of endemic cedar and palmetto since 1609, recorded in the Holocene pollen record of Devonshire Marsh. J Paleolimnol. 16:59–66.
- Rule S, Brook BW, Haberle SG, Turney CSM, Kershaw AP, Johnson CN. 2012. The aftermath of megafaunal extinction: ecosystem transformation in the Pleistocene, Australia. Science. 335(6075):1483–1486.
- Rull V. 2004. An evaluation of the lost world and vertical displacement hypotheses in the Chimantá Massif, Venezuelan Guayana. Global Ecol Biogeogr. 13:141–148.
- Rull V. 2009. Microrefugia. J Biogeogr. 36:481–484.
- Rull V. 2010. On microrefugia and cryptic refugia. J Biogeogr. 37:1623–1625.
- Rull V. 2012. Community ecology: diversity and dynamics over time. Commun Ecol. 13(1):102–116.
- Rull V. 2014. Time continuum and true long-term ecology: from theory to practice. Front Ecol Evol. 2:75.
- Rull V. 2015. Long-term vegetation stability and the concept of potential natural vegetation in the Neotropics. J Veg Sci. 26(3):603–607.
- Rull V. 2016. Natural and anthropogenic drivers of cultural change on Easter Island: Review and new insights. Quat Sci Rev. 150:31–41.
- Rull V. 2019a. Human discovery and settlement of the remote Easter Island (SE Pacific). Quaternary. 2(2):15.
- Rull V. 2019b. Pristinity, degradation and landscaping: the three angles of human impact on islands. PeerJ Prepr. 7:e27775v27771.
- Rull V, Cañellas-Boltà N, Sáez A, Giralt S, Margalef O. 2010. Paleoecology of Easter Island: evidence and uncertainties. Earth Sci Rev. 99:50–60.
- Rull V, Connor SE, Elias RB. 2017c. Potential natural vegetation and pre-anthropic pollen records on the Azores Islands in a Macaronesian context. J Biogeogr. 44(11):2437–2440.
- Rull V, Lara A, Rubio-Inglés MJ, Giralt S, Goncalves V, Raposeiro P, Hernández A, Sánchez-López G, Vázquez-Loureiro D, Bao R et al. 2017a. Vegetation and landscape dynamics under natural and anthropogenic forcing on the Azores Islands: A 700-year pollen record from the São Miguel Island. Quat Sci Rev. 159:155–168.
- Rull V, Montoya E, Nogué S, Vegas-Vilarrúbia T, Safont E. 2013. Ecological palaeoecology in the neotropical Gran Sabana region: Long-term records of vegetation dynamics as a basis for ecological hypothesis testing. Perspect Plant Ecol Evol Syst. 15(6):338–359.
- Rull V, Montoya E, Seco I, Cañellas-Boltà N, Giralt S, Margalef O, Pla-Rabes S, D’Andrea W, Bradley R, Sáez A. 2018. CLAFS, a holistic climatic-ecological-anthropogenic hypothesis on Easter Island’s deforestation and cultural change: proposals and testing prospects. Front Ecol Evol. 6:32.
- Rull V, Nogué S. 2007. Potential migration routes and barriers for vascular plants of the Neotropical Guyana Highlands during the Quaternary. J Biogeogr. 34:1327–1341.
- Rull V, Vegas-Vilarrúbia T, Montoya E. 2016. The neotropical Gran Sabana region: Palaeoecology and conservation. Holocene. 26(7):1162–1167.
- Rull V, Vegas-Vilarrúbia T, Montoya E. 2017b. Paleoecology as a guide to landscape conservation and restoration in the neotropical Gran Sabana. PAGES Mag. 25(2):82–83.
- Ruosch M, Spahni R, Joos F, Henne PD, van der Knaap WO, Tinner W. 2016. Past and future evolution of Abies alba forests in Europe – comparison of a dynamic vegetation model with palaeo data and observations. Glob Chang Biol. 22(2):727–740.
- Rutherford D. 2017. Human colonization of Asia in the Late Pleistocene. Curr Anthropol. 58(S17):S371–S372.
- Sabatini FM, Burrascano S, Keeton WS, Levers C, Lindner M, Pötzschner F, Verkerk PJ, Bauhus J, Buchwald E, Chaskovsky O et al. 2018. Where are Europe’s last primary forests? Divers Distrib. doi:10.1111/ddi.12778.
- Saltré F, Rodríguez-Rey M, Brook BW, Johnson CN, Turney CSM, Alroy J, Cooper A, Beeton N, Bird MI, Fordham DA et al. 2016. Climate change not to blame for late Quaternary megafauna extinctions in Australia. Nat Commun. 7:10511.
- Saltré F, Saint Amant R, Gritti ES, Brewer S, Gaucherel C, Davis BAS, Chuine I. 2013. Climate or migration: what limited European beech post-glacial colonization? Global Ecol Biogeogr. 22:1217–1227.
- Samartin S, Heiri O, Kaltenrieder P, Kühl N, Tinner W. 2016. Reconstruction of full glacial environments and summer temperatures from Lago della Costa, a refugial site in Northern Italy. Quat Sci Rev. 143:107–119.
- Samartin S, Heiri O, Lotter AF, Tinner W. 2012. Climate warming and vegetation response after Heinrich event 1 (16 700–16 000 cal yr BP) in Europe south of the Alps. Clim Past. 8(6):1913–1927.
- Sandel B. 2019. Disequilibrium in trait-climate relationships of trees and birds. Front Ecol Evol. 7(138).
- Sanderson JG, Pimm SL. 2015. Patterns in nature. The analysis of species co-occurrences. Chicago: University of Chicago Press.
- Sandom CJ, Ejrnæs R, Hansen MDD, Svenning J-C. 2014b. High herbivore density associated with vegetation diversity in interglacial ecosystems. Proc Natl Acad Sci USA. 111(11):4162–4167.
- Sandom CJ, Faurby S, Sandel B, Svenning J-C. 2014a. Global late Quaternary megafauna extinctions linked to humans, not climate change. Proc R Soc B Bio Sci. 281(1787):3254.
- Sarmiento FO. 2002. Anthropogenic change in the landscape of Highland Ecuador. Geogr Rev. 92(2):213–234.
- Sauer CO. 1952. Agricultural origins and dispersals. New York: American Geographical Society.
- Saulnier-Talbot É. 2015. Overcoming the disconnect: are paleolomnologists doing enough to make their science accessible to aquatic managers and conservationists? Front Ecol Evol. 3:32.
- Saulnier-Talbot É. 2016. Paleolimnology as a tool to achieve environmental sustainability in the Anthropocene: an overview. Geosci. 6:26.
- Saupe EE, Qiao H, Hendricks JR, Portell RW, Hunter SJ, Soberón J, Lieberman BS. 2015. Niche breadth and geographic range size as determinants of species survival on geological time scales. Global Ecol Biogeogr. 24(10):1159–1169.
- Sauramo M. 1940. Suomen Iuonnen kehitys jääkaudesta nykyaikaan. Helsinki: Werner Söderstrom Oy.
- Sawada M, Viau AE, Vettoretti G, Peltier WR, Gajewski K. 2004. Comparison of North American pollen-based temperature and global lake-status with CCMA AGCMZ output at 6 ka. Quat Sci Rev. 23:225–244.
- Sax DF, Early R, Bellemare J. 2013. Niche syndromes, species extinction risks, and management under climate change. Trends Ecol Evol. 28(9):517–523.
- Sax DF, Gaines SD. 2008. Species invasions and extinction: The future of native biodiversity on islands. Proc Natl Acad Sci USA. 105(apl 1):11490–11497.
- Sax DF, Gaines SD, Brown JH. 2002. Species invasions exceed extinctions on islands worldwide: a comparative study of plants and birds. Am Nat. 160(6):766–783.
- Saxon E, Baker B, Hargrove W, Hoffman F, Zganjar C. 2005. Mapping environments at risk under different global climate change scenarios. Ecol Lett. 8(1):53–60.
- Sayer CD, Bennion H, Davidson TA, Burgess A, Clarke G, Hoare DJ, Frings P, Hatton-Ellis T. 2012. The application of palaeolimnology to evidence-based lake management and conservation: examples from UK lakes. Aquat Conserv. 22(2):165–180.
- Scheffer M. 2010. Foreseeing tipping points. Nature. 467:411–412.
- Scheffer M, Bascompte J, Brock WA, Brovkin V, Carpenter SR, Dakos V, Held H, van Nes EH, Rietkerk M, Sugihara G. 2009. Early-warning signals for critical transitions. Nature. 461:53–59.
- Scheffer M, Carpenter SR. 2003. Catastrophic regime shifts in ecosystems: linking theory to observation. Trends Ecol Evol. 18:648–650.
- Scheffer M, Carpenter SR, Dakos V, van Nes EH. 2015. Generic indicators of ecological resilience: inferring the chance of a critical transition. Annu Rev Ecol Evol Syst. 46(1):145–167.
- Scheffer M, Carpenter SR, Foley JA, Folke C, Walker B. 2001. Catastrophic shifts in ecosystems. Nature. 413:591–596.
- Scheffer M, Carpenter SR, Lenton TM, Bascompte J, Brock W, Dakos V, van de Koppel J, van de Leemput IA, Levin SA, van Nes EH et al. 2012. Anticipating critical transitions. Science. 338(6105):344–348.
- Scherrer D, Körner C. 2010. Infra-red thermometry of alpine landscapes challenges climatic warming projections. Glob Chang Biol. 16:2602–2613.
- Scherrer D, Körner C. 2011. Topographically controlled thermal-habitat differentiation buffers alpine plant diversity against climate warming. J Biogeogr. 38:406–416.
- Scherrer D, Schmid S, Körner C. 2011. Elevational species shifts in a warmer climate are overestimated when based on weather station data. Int J Biometeorol. 55(4):645–654.
- Schiferl JD, Bush MB, Silman MR, Urrego DH. 2017. Vegetation responses to late Holocene climate changes in an Andean forest. Quat Res. 89(1):60–74.
- Schimel DS, Asner GP, Moorcroft P. 2013. Observing changing ecological diversity in the Anthropocene. Front Ecol Environ. 11(3):129–137.
- Schlesinger WH. 2010. Translational ecology. Science. 329(5992):609.
- Schlesinger WH, Bernhardt ES. 2013. Biogeochemistry: an analysis of global change. 3rd ed. Cambridge, Massachusetts: Academic Press.
- Schluter D. 1984. A variance test for detecting species associations, with some example applications. Ecology. 65(3):998–1005.
- Schmid S, Genevest R, Gobet E, Suchan T, Sperisen C, Tinner W, Alvarez N. 2017. HyRAD-X, a versatile method combining exome capture and RAD sequencing to extract genomic information from ancient DNA. Methods Ecol Evol. 8(10):1374–1388.
- Schofield JE, Bunting MJ. 2005. Mid-Holocene presence of water chestnut (Trapa natans L.) in the meres of Holderness, East Yorkshire, UK. Holocene. 15:687–697.
- Schofield JE, Edwards KJ. 2011. Grazing impacts and woodland management in Eriksfjord: Betula, coprophilous fungi and the Norse settlement of Greenland. Veg Hist Archaeobot. 20:181–197.
- Schoonmaker PK, Foster DR. 1991. Some implications of paleoecology for contemporary ecology. Bot Rev. 57(3):204–245.
- Schulman E. 1954. Longevity under adversity in conifers. Science. 119(3091):396–399.
- Schupp EW, Jordano P, Gómez JM. 2010. Seed dispersal effectiveness revisited: a conceptual review. New Phytol. 188(2):333–353.
- Schwartz MW, Hellmann JJ, McLachlan JM, Sax DF, Borevitz JO, Brennan J, Camacho AE, Ceballos G, Clark JR, Doremus H et al. 2012. Managed relocation: integrating the scientific, regulatory, and ethical challenges. BioScience. 62(8):732–743.
- Schweiger AH, Beierkuhnlein C. 2017. The ecological legacy of 20th century acidification carried on by ecosystem engineers. Appl Veg Sci. 20(2):215–224.
- Schwörer C, Colombaroli D, Kaltenrieder P, Rey F, Tinner W. 2015. Early human impact (5000–3000 BC) affects mountain forest dynamics in the Alps. J Ecol. 103(2):281–295.
- Schwörer C, Gavin DG, Walker IR, Hu FS. 2017. Holocene tree line changes in the Canadian Cordillera are controlled by climate and topography. J Biogeogr. 44(5):1148–1159.
- Schwörer C, Henne PD, Tinner W. 2014. A model-data comparison of Holocene timberline changes in the Swiss Alps reveals past and future drivers of mountain forest dynamics. Glob Chang Biol. 20(5):1512–1526.
- Scott L. 1985. Palynological indications of the Quaternary vegetation history of Marion Island (sub-Antarctic). J Biogeogr. 12:413–431.
- Sears PB. 1964. The goals of paleoecological reconstruction. In: Smith ER, J.J. H, Schoenwetter J, editors. The reconstruction of past environments (Proceedings of the Fort Burgwin Conference on Paleoecology 1962). Taos (NM): Fort Burgwin Research Center; p.4–6.
- Seddon AWR, Cole L, Fletcher M, Morris J, Willis KJ, EcoRe3 working group. 2017. Measuring resistance, recovery and resilience in long-term ecological datasets. PAGES Mag. 25 (2):120.
- Seddon AWR, Froyd CA, Witkowski A, Willis KJ. 2014b. A quantitative framework for analysis of regime hifts in a Galápagos coastal lagoon. Ecology. 95(11):3046–3055.
- Seddon AWR, Macias Fauria M, Long PR, Benz D, Willis KJ. 2016. Sensitivity of global terrestrial ecosystems to climate variability. Nature. 531:229.
- Seddon AWR, Macias Fauria M, Willis KJ. 2015. Climate and abrupt vegetation change in Northern Europe since the last deglaciation. Holocene. 25(1):25–36.
- Seddon AWR, Mackay AW, Baker AG, Birks HJB, Breman E, Buck CE, Ellis EC, Froyd CA, Gill JL, Gillson L et al. 2014a. Looking forward through the past. Identification of fifty priority research questions in palaeoecology. J Ecol. 102:256–267.
- Seeley M, Goring S, Williams JW. 2019. Assessing the environmental and dispersal controls on Fagus grandifolia distributions in the Great Lakes region. J Biogeogr. 46(2):405–419.
- Segerström U, Bradshaw RHW, Hornberg G, Bohlin E. 1994. Disturbance history of a swamp forest refuge in northern Sweden. Biol Conserv. 68(2):189–196.
- Seidl R, Spies TA, Peterson DL, Stephens SL, Hicke JA. 2016. Searching for resilience: addressing the impacts of changing disturbance regimes on forest ecosystem services. J Appl Ecol. 53(1):120–129.
- Selleck G. 1960. The climax concept. Bot Rev. 26:534–545.
- Selling OH. 1946. Studies in Hawaiian pollen statistics part I: the spores of the Hawaiian Pteridophytes. Honolulu: Bernice P Bishop Museum Special Publication 37.
- Selling OH. 1947. Studies in Hawaiian pollen statistics part II: the pollens of the Hawaiian Phanerogams. Honolulu: Bernice P Bishop Museum Special Publication 38.
- Selling OH. 1948. Studies in Hawaiian pollen statistics part III: on the late quaternary history of the Hawaiian vegetation. Honolulu: Bernice P Bishop Museum Special Publication 39.
- Selling OH. 1949. Hawaiian pollen statistics. Sven Bot Tidskr. 43(1):1.
- Selling OH. 1951. A contribution to the history of the Hawaiian vegetation. Sven Bot Tidskr. 43(1):12–41.
- Selwood KE, Thomson JR, Clarke RH, McGeoch MA, Mac Nally R. 2015. Resistance and resilience of terrestrial birds in drying climates: do floodplains provide drought refugia? Global Ecol Biogeogr. 24(7):838–848.
- Seppä H, Alenius T, Bradshaw RHW, Giesecke T, Heikkilä M, Muukkonen P. 2009b. Invasion of Norway spruce (Picea abies) and the rise of the boreal ecosystem in Fennoscandia. J Ecol. 97(4):629–640.
- Seppä H, Alenius T, Muukkonen P, Giesecke T, Miller PA, Ojala AEK. 2009a. Calibrated pollen accumulation rates as a basis for quantitative tree biomass reconstructions. Holocene. 19:209–220.
- Seppä H, Bennett KD. 2003. Quaternary pollen analysis: recent progress in palaeoecology and palaeoclimatology. Prog Phys Geog. 27:548–579.
- Serebryanny LR. 1971. Dinamika rasprostraneniia nekotoryh drevesnyn porod na severo-zapade SSR v poslelednikovoe vremia. In: Neustadt MI, editor. Palinologiia golotsena. Moscow: Nauka; p. 17–31.
- Serebryanny LR. 1973. Post-glacial migration rates of tree species in the northwestern regions of the USSR: palynology and radiocarbon dating. In: Khotinsky NA, Koreneva EV, editors. Palynology: holocene and marine palynology. Moscow: Nauka; p. 14–18.
- Sernander R. 1890. Om förekomsten af subfossila stubbar på svenska insjöars bottem. Bot Notiser. 1890:10–20.
- Shaw H, Whyte I. 2013. Palaeoecological records of woodland history during recent centuries of grazing and management examples from Glen Affric, Scotland and Ribblesdale, North Yorkshire. In: Rotherham ID, editor. Trees, forested landscapes and grazing animals. London: Routledge; p. 223–241.
- Shaw RG, Etterson JR. 2012. Rapid climate change and the rate of adaptation: insight from experimental quantitative genetics. New Phytol. 195(4):752–765.
- Sheil D, Burslem DFRP. 2013. Defining and defending Connell’s intermediate disturbance hypothesis: a response to Fox. Trends Ecol Evol. 28(10):571–572.
- Shugart HH. 1990. Using ecosystem models to assess potential consequences of global climatic change. Trends Ecol Evol. 5(9):303–307.
- Shuman BN. 2012. Patterns, processes, and impacts of abrupt climate change in a warm world: the past 11,700 years. Wiley Interdiscip Rev Clim Change. 3(1):19–43.
- Shuman BN, Bartlein PJ, Logar N, Newby P, Webb T. 2002. Parallel climate and vegetation responses to the early Holocene collapse of the Laurentide Ice Sheet. Quat Sci Rev. 21(16):1793–1805.
- Shuman BN, Bravo J, Kaye JP, Lynch JA, Newby P, Webb T. 2001. Late Quaternary water-level variations and vegetation history at Crooked Pond, southeastern Massachusetts. Quat Res. 56(3):401–410.
- Shuman BN, Henderson AK, Plank C, Stefanova I, Ziegler SS. 2009. Woodland-to-forest transition during prolonged drought in Minnesota after ca. AD 1300. Ecology. 90:2792–2807.
- Shuman BN, Marsicek JP. 2016. The structure of Holocene climate change in mid-latitude North America. Quat Sci Rev. 141:38–51.
- Shuman BN, Newby P, Huang YS, Webb T. 2004. Evidence for the close climatic control of New England vegetation history. Ecology. 85(5):1297–1310.
- Shuman BN, Serravezza M. 2017. Patterns of hydroclimatic change in the Rocky Mountains and surrounding regions since the last glacial maximum. Quat Sci Rev. 173 (Supplement C):58–77.
- Shumilovskikh LS, Novenko E, Giesecke T, Reitalu T. 2017. Long‐term dynamics of the East European forest‐steppe ecotone. J Veg Sci. doi:10.1111/jvs.12585.
- Silander JA. 2002. The ultimate answer … is 42? J Biogeogr. 29:299–301.
- Silvertown J. 1985. History of a latitudinal diversity gradient: woody plants in Europe 13,000–1000 years B.P. J Biogeogr. 12(6):519–525.
- Simberloff D. 1980. A succession of paradigms in ecology: essentialism to materialism to probabilism. Synthese. 43:3–39.
- Simberloff D. 1986. The proximate cause of extinction. In: Raup DM, Jablonski D, editors. Patterns and processes in the history of life. Berlin: Springer; p. 259–276.
- Simberloff D. 2000. Global climate change and introduced species in United States forests. Sci Total Environ. 262(3):253–261.
- Simberloff D. 2004. Community ecology: is it time to move on? Am Nat. 163(6):787–799.
- Simberloff D. 2014. Biological invasions: What’s worth fighting and what can be won? Ecol Eng. 65:112–121.
- Simpson GG. 1952. Probabilities of dispersal in geologic time. Bull Am Mus Nat Hist N Y. 99:163–176.
- Simpson GL. 2012. Analogue methods in palaeolimnology. In: Birks HJB, Lotter AF, Juggins S et al., editors. Tracking environmental change using lake sediments volume 5: data handling and numerical techniques. Dordrecht: Springer; p. 495–555.
- Simpson GL, Birks HJB. 2012. Chapter 9, statistical learning in palaeolimnology. In: Birks HJB, Lotter AF, Juggins S et al., editors. Tracking environmental change using lake sediments volume 5: data handling and numerical techniques. Dordrecht: Springer; p. 249–327.
- Sinclair SJ, White MD, Newell GR. 2010. How useful are species distribution models for managing biodiversity under future climates? Ecol Soc. 15(1):a8.
- Singh G, Geissler EA. 1985. Late Cainozoic history of vegetation, fire, lake levels and climate at Lake George, New South Wales, Australia. Philos Trans R Soc B Bio Sci. 311:379–447.
- Sjölund MJ, González-Díaz P, Moreno-Villena JJ, Jump AS. 2017. Understanding the legacy of widespread population translocations on the post-glacial genetic structure of the European beech, Fagus sylvatica L. J Biogeogr. 44(11):2475–2487.
- Skellam JG. 1951. Random dispersal in theoretical populations. Biometrika. 38:196–218.
- Smith AG. 1965. Problems of inertia and threshold related to post-glacial habitata changes. Proc R Soc B Bio Sci 161:331–342.
- Smith D, Nayyar K, Schreve D, Thomas R, Whitehouse N. 2014. Can dung beetles from the palaeoecological and archaeological record indicate herd concentration and the identity of herbivores? Quatern Int. 341:119–130.
- Smith D, Whitehouse N, Bunting MJ, Chapman H. 2010. Can we characterise ‘openness’ in the Holocene palaeoenvironmental record? Modern analogue studies of insect faunas and pollen spectra from Dunham Massey deer park and Epping Forest, England. Holocene. 20(2):215–229.
- Smith FA, Elliott SM, Lyons SK. 2010. Methane emissions from extinct megafauna. Nat Geosci. 3:374–375.
- Smith FA, Elliott Smith RE, Lyons SK, Payne JL, Villaseñor A. 2019. The accelerating influence of humans on mammalian macroecological patterns over the late Quaternary. Quat Sci Rev. 211:1–16.
- Smith JMB. 1978. Dispersal and establishment of plants. In: Walker D, Guppy JC, editors. Biology and quaternary environments. Canberra: Australian Academy of Science; p. 207–223.
- Smol JP. 1992. Paleolimnology: an important tool for effective ecosystem management. J Aquat Ecosys Health. 1:49–58.
- Smol JP. 2008. Pollution of lakes and rivers: a paleoenvironmental perspective. 2nd ed. Oxford: Blackwell.
- Smol JP. 2017. Conservation biology and environmental change: a palaeolimnological perspective. In: Dietl GP, Flessa KW, editors. Conservation paleobiology science and practice. Chicago: University of Chicago Press; p. 31–43.
- Smol JP, Birks HJB, Last WM, editors. 2001. Tracking environmental change using lake sediments. Volume 3: terrestrial, algal, and siliceous indicators. Dordrecht: Kluwer Academic Publishers.
- Smol JP, Douglas MSV. 2007. Crossing the final ecological threshold in high Arctic ponds. Proc Natl Acad Sci USA. 104:12395–12397.
- Snell RC, Cowling SA. 2015. Consideration of dispersal processes and northern refugia can improve our understanding of past plant migration rates in North America. J Biogeogr. 42:1677–1688.
- Snell RS, Huth A, Nabel JEMS, Bocedi G, Travis JMJ, Gravel D, Bugmann H, Gutiérrez AG, Hickler T, Higgins SI et al. 2014. Using dynamic vegetation models to simulate plant range shifts. Ecography. 37(12):1184–1197.
- Soberón J. 2007. Grinnellian and Eltonian niches and geographic distribution of species. Ecol Lett. 10:1115–1123.
- Soepboer W, Sugita S, Lotter AF. 2010. Regional vegetation-cover changes on the Swiss Plateau during the past two millennia: A pollen-based reconstruction using the REVEALS model. Quat Sci Rev. 29(3):472–483.
- Somodi I, Molnár Z, Czúcz B, Bede-Fazekas Á, Bölöni J, Pásztor L, Laborczi A, Zimmermann NE. 2017. Implementation and application of multiple potential natural vegetation models – a case study of Hungary. J Veg Sci. 28(6):1260–1269.
- Somodi I, Molnár Z, Ewald J. 2012. Towards a more transparent use of the potential natural vegetation concept – an answer to Chiarucci et al. J Veg Sci. 23(3):590–595.
- Sousa WP. 1979. Experimental investigations of disturbance and ecological succession in a rocky intertidal algal community. Ecol Monogr. 49(3):227–254.
- Spanbauer TL, Allen CR, Angeler DG, Eason T, Fritz SC, Garmestani AS, Nash KL, Stone JR. 2014. Prolonged instability prior to a regime shift. PLoS One. 9(10):e108936.
- Spear RW, Davis MB, Shane LCK. 1994. Late Quaternary history of low- and mid-elevation vegetation in the White Mountains of New Hampshire. Ecol Monogr. 64(1):85–109.
- Stace CA, Crawley MJ. 2015. Alien Plants. London: Collins.
- Stachowicz-Rybka R. 2011. Flora and vegetation changes on the basis of plant macroremains analysis from an early Pleistocene lake of the Augustów Plain, NE Poland. Acta Palaeobot. 51:39–103.
- Stachowicz-Rybka R. 2015a. Environmental and climate changes reflected in the Domuraty 2 section (NE Poland) based on analysis of plant macroremains. Acta Palaeobot. 55(2):213.
- Stachowicz-Rybka R. 2015b. Record of environmental and climatic changes in middle Pleistocene sediments from Łuków (eastern Poland) on the basis of plant macroremains analysis. Acta Palaeobot. 55(1):68.
- Stachowicz-Rybka R. 2015c. Vegetation of the Ferdynandovian interglacial (MIS 13–15) based on plant macrofossils from a new profile of the stratotype site. Acta Palaeobot. 55(2):233.
- Stahle LN, Whitlock C, Haberle SG. 2016. A 17,000-year-long record of vegetation and fires from Cradle Mountain National Park, Tasmania. Front Ecol Evol. 4:82.
- Stambaugh MC, Varner JM, Jackson ST. 2017. Biogeography: an interweave of climate, fire, and humans. In: Kirkman K, Jacks S, editors. Ecological restoration of longleaf pine. Boca Raton: CRC Press; p. 17–37.
- Standish RJ, Hobbs RJ, Mayfield MM, Bestelmeyer BT, Suding KN, Battaglia LL, Eviner V, Hawkes CV, Temperton VM, Cramer VA et al. 2014. Resilience in ecology: abstraction, distraction, or where the action is? Biol Conserv. 177:43–51.
- Steadman DW. 2006. Extinction and biogeography of tropical pacific birds. Chicago: University of Chicago Press.
- Steenstrup JS. 1841. Geognostisk-geologisk Undersögelse af Skovmoserne Vidnesdam- og Lillemose i det Nordlige Sjælland, Ledsaget af Sammenlignende Bemærkninger, Hentede fra Danmarks Skov- Kjær- og Lyngmoser Lalmindelighed. Copenhagen: Gyldendahl.
- Steffen W, Crutzen P, McNeill JR. 2007. The Anthropocene: are humans now overwhelming the great forces of nature? Ambio. 36(8):614–621.
- Stegner MA, Ratajczak Z, Carpenter SR, Williams JW. 2019. Inferring critical transitions in paleoecological time series with irregular sampling and variable time-averaging. Quat Sci Rev. 207:49–63.
- Steinbauer MJ, Grytnes J-A, Jurasinski G, Kulonen A, Lenoir J, Pauli H, Rixen C, Winkler M, Bardy-Durchhalter M, Barni E et al. 2018. Accelerated increase in plant species richness on mountain summits is linked to warming. Nature. 556(7700):231–234.
- Stephens PA, Buskirk SW, Martínez del Rio C. 2007. Inference in ecology and evolution. Trends Ecol Evol. 22:192–197.
- Stephenson NL. 1999. Reference conditions for giant sequoia forest restoration: structure, process, and precision. Ecol App. 9(4):1253–1265.
- Stevens-Rumann CS, Kemp KB, Higuera PE, Harvey BJ, Rother MT, Donato DC, Morgan P, Veblen TT. 2018. Evidence for declining forest resilience to wildfires under climate change. Ecol Lett. 21(2):243–252.
- Stevenson J, Benson A, Athens JS, Kahn J, Kirch PV. 2017. Polynesian colonization and landscape changes on Mo’orea, French Polynesia: The Lake Temae pollen record. Holocene. 27(12):1963–1975.
- Stewart A. 1911. A botanical survey of the Galápagos Islands. Proc California Acad Sci. 4:7–288.
- Stewart A. 1915. Some observations concerning the botanical conditions on the Galápagos Islands. Trans Wisconsin Acad Sci Arts Lett. 18:272–340.
- Stewart JR, Lister AM, Barnes IM, Dalén L. 2010. Refugia revisited: individualistic responses of species in space and time. Proc R Soc B Bio Sci. 277:661–671.
- Steyermark JA, Dunsterville GCK. 1980. The lowland floral element on the summit of Cerro Guaiquinima and other cerros of the Guayana Highland of Venezuela. J Biogeogr. 7(3):285–303.
- Stivrins N, Buchan MS, Disbrey HR, Kuosmanen N, Latałowa M, Lempinen J, Muukkonen P, Słowiński M, Veski S, Seppä H. 2017. Widespread, episodic decline of alder (Alnus) during the medieval period in the boreal forest of Europe. J Quat Sci. 32(7):903–907.
- Stivrins N, Soininen J, Amon L, Fontana SL, Gryguc G, Heikkilä M, Heiri O, Kisielienė D, Reitalu T, Stančikaitė M et al. 2016. Biotic turnover rates during the Pleistocene-Holocene transition. Quat Sci Rev. 151:100–110.
- Stone R. 2010. Home, home outside the range? Science. 329(5999):1592–1594.
- Stralberg D, Carroll C, Pedlar JH, Wilsey CB, McKenney DW, Nielsen SE. 2018. Macrorefugia for North American trees and songbirds: Climatic limiting factors and multi‐scale topographic influences. Global Ecol Biogeogr. 27(6):690–703.
- Strandberg G, Kjellström E, Poska A, Wagner S, Gaillard M-J, Trondman A-K, Mauri A, Davis BAS, Kaplan JO, Birks HJB et al. 2014. Regional climate model simulations for Europe at 6 and 0.2 k BP: sensitivity to changes in anthropogenic deforestation. Clim Past. 10(2):661–680.
- Stroh PA, Leach SJ, August TA, Walker KJ, Pearman DA, Rumsey FJ, Harrower CA, Fay MF, Martin JP, Pankhurst T et al. 2014. A vascular plant red list for England. Bristol: Botanical Society of Britain and Ireland.
- Stroud JT, Bush MR, Ladd MC, Nowicki RJ, Shantz AA, Sweatman J. 2015. Is a community still a community? Reviewing definitions of key terms in community ecology. Ecol Evol. 5(21):4757–4765.
- Sublette Mosblech NA, Bush MB, van Woesik R. 2011. On metapopulations and microrefugia: palaeoecological insights. J Biogeogr. 38(3):419–429.
- Sublette Mosblech NA, Chepstow-Lusty A, Valencia BG, Bush MB. 2012. Anthropogenic control of late-Holocene landscapes in the Cuzco region, Peru. Holocene. 22(12):1361–1372.
- Suding KN, Higgs ES, Palmer MA, Callicott JB, Anderson CB, Baker ME, Giutrich JJ, Hondula KL, LaFevor MC, Larson BMH et al. 2015. Committing to ecological restoration. Science. 348(6235):638–640.
- Sugita S. 1993. A model of pollen source area for an entire lake surface. Quat Res. 39(2):239–244.
- Sugita S. 1994. Pollen representation of vegetation in Quaternary sediments: theory and method in patchy vegetation. J Ecol. 82:881–897.
- Sugita S. 1998. Modelling pollen representation of vegetation. Paläoklimaforschung. 27:1–16.
- Sugita S. 2007a. Theory of quantitative reconstruction of vegetation I: Pollen from large sites REVEALS regional vegetation composition. Holocene. 17:229–241.
- Sugita S. 2007b. Theory of quantitative reconstruction of vegetation II: All you need is LOVE. Holocene. 17(2):243–257.
- Summerhayes GR, Field JH, Shaw B, Gaffney D. 2017. The archaeology of forest exploitation and change in the tropics during the Pleistocene: The case of Northern Sahul (Pleistocene New Guinea). Quatern Int. 448:14–30.
- Summerhayes GR, Leavesley M, Fairbairn A, Mandui H, Field J, Ford A, Fullagar R. 2010. Human adaptation and plant use in highland New Guinea 49,000 to 44,000 years ago. Science. 330(6000):78–81.
- Sussman R. 2010. The Oldest Things in the World. Chicago: University of Chicago Press.
- Suwal MK. 2018. Spatial dynamics of species distributions in an anthropogenic landscape in the context of climate change [PhD thesis]. Bergen: University of Bergen.
- Svenning J-C. 2002. A review of natural vegetation openness in northwestern Europe. Biol Conserv. 104:133–148.
- Svenning J-C. 2003. Deterministic Plio-Pleistocene extinctions in the European cool-temperate tree flora. Ecol Lett. 6:646–653.
- Svenning J-C, Eiserhardt WL, Normand S, Ordonez A, Sandel B. 2015. The influence of paleoclimate on present-day patterns in biodiversity and ecosystems. Annu Rev Ecol Evol Syst. 46(1):551–572.
- Svenning J-C, Fløjgaard C, Marske KA, Nógues-Bravo D, Normand S. 2011. Applications of species distribution modeling to paleobiology. Quat Sci Rev. 30(21–22):2930–2947.
- Svenning J-C, Normand S, Kageyama M. 2008b. Glacial refugia of temperate trees in Europe: insights from species distribution modelling. J Ecol. 96:1117–1127.
- Svenning J-C, Normand S, Skov F. 2008a. Postglacial dispersal limitation of widespread forest plant species in nemoral Europe. Ecography. 31:316–326.
- Svenning J-C, Pedersen PBM, Donlan CJ, Erjnæs R, Faurby S, Galetti M, Hansen DM, Sandel B, Sandom CJ, Terborgh JW et al. 2016a. Science for a wilder Anthropocene: Synthesis and future directions for trophic rewilding research. Proc Natl Acad Sci USA. 113(4):898–906.
- Svenning J-C, Sandel B. 2013. Disequilibrium vegetation dynamics under future climate change. Am J Bot. 100(7):1266–1286.
- Svenning J-C, Skov F. 2004. Limited filling of the potential range in European tree species. Ecol Lett. 7(7):565–573.
- Svenning J-C, Skov F. 2007a. Ice age legacies in the geographical distribution of tree species richness in Europe. Global Ecol Biogeogr. 16(2):234–245.
- Svenning J-C, Skov F. 2007b. Could the tree diversity pattern in Europe be generated by postglacial dispersal limitation? Ecol Lett. 10:453–460.
- Swetnam TW, Allen CD, Betancourt JL. 1999. Applied historical ecology: using the past to manage the future. Ecol App. 9:1189–1206.
- Swetnam TW, Betancourt JL. 1990. Fire–Southern Oscillation relations in the southwestern United States. Science. 249:1017–1020.
- Szabó P, Hédl R. 2011. Advancing the integration of history and ecology for conservation. Conserv Bio. 25(4):680–687.
- Szabó P, Kuneš P, Svitavská Svobodová H, Švarcová MG, Křížová L, Suchánková S, Müllerová J, Hédl R. 2017. Using historical ecology to reassess the conservation status of coniferous forests in Central Europe. Conserv Bio. 31(1):150–160.
- Szafer W. 1935. The significance of isopollen lines for the investigation of the geographic distribution of trees in the post-glacial period. Bull l’Acad Polonaise Sci B. 3:235–239.
- Szeicz JM, Haberle SG, Bennett KD. 2003. Dynamics of North Patagonian reainforests from fine-resolution pollen, charcoal and tree-ring analysis, Chonos Archipelago, southern Chile. Austral Ecol. 28:413–422.
- Taberlet P, Bonin A, Zinger L, Coissac E. 2018. Environmental DNA for biodiversity research and monitoring. Oxford: Oxford University Press.
- Tackenberg O. 2003. Modeling long-distance dispersal of plant diaspores by wind. Ecol Monogr. 73(2):173–189.
- Tackenberg O, Poschlod P, Bonn S. 2003. Assessment of wind diispersal potential in plant species. Ecol Monogr. 73(2):191–205.
- Tait C. 1794. An account of the peat-mosses of Kincardine and Flanders in Perthshire. Trans R SocEdinburgh 3:266–279.
- Tallantire PA. 1972. The regional spread of spruce (Picea abies) (L.) Karst. within Fennoscandia: a reassessment. Norw J Bot. 19:1–16.
- Talling JF. 1951. The element of chance in pond populations. Naturalist. 1951(Oct–Dec):157–170.
- Tallis JH. 1991. Plant community history. London: Chapman and Hall.
- Tang CQ, Ohsawa M. 2002. Tertiary relic deciduous forests on a humid subtropical mountain, Mt. Emei, Sichuan, China. Folia Geobot. 37:93–106.
- Taranu ZE, Carpenter SR, Frossard V, Jenny J-P, Thomas Z, Vermaire JC, Perga M-E. 2018. Can we detect ecosystem critical transitions and signals of changing resilience from paleo-ecological records? Ecosphere. 9(10):e02438.
- Tarasov P, Williams JW, Andreev A, Nakagawa T, Bezrukova E, Herzschuh U, Igareshi Y, Müller S, Werner K, Zheng Z. 2007. Satellite- and pollen-based quantitative woody cover reconstructions for northern Asia: Verification and application to late-Quaternary pollen data. Earth Planet Sci Lett. 264:284–298.
- Tausch RJ, Wigand PE, Burkhardt JW. 1993. Plant community thresholds, multiple steady states, and multiple successional pathways: legacy of the Quaternary? J Range Manag. 46(5):439–447.
- Taylor RH. 1979. How the Macquaire Island parakeet became extinct. N Z J Ecol 2:42–45.
- Tedersoo L, Laanisto L, Rahimlou S, Toussaint A, Hallikma T, Pärtel M. 2018. Global database of plants with root-symbiotic nitrogen fixation: NodDB. J Veg Sci. 29(3):560–568.
- Telford RJ, Chipperfield JD, Birks HH, Birks HJB. 2016. How foreign is the past? (Arising from Lyons et al. 2016). Nature. 538: E1–E2.
- Temple SA. 1977. Plant-animal mutualism: coevolution with dodo leads to near extinction of plant. Science. 197:885–886.
- Teodoridis V, Bruch AA, Vassio E, Martinetto E, Kvaček Z, Stuchlik L. 2017. Plio-Pleistocene floras of the Vildštejn Formation in the Cheb Basin, Czech Republic — A floristic and palaeoenvironmental review. Palaeogeogr Palaeoclimatol Palaeoecol. 467:166–190.
- Theuerkauf M, Bos JAA, Jahns S, Janke W, Kuparinen A, Stebich M, Joosten H. 2014. Corylus expansion and persistent openness in the early Holocene vegetation of northern central Europe. Quat Sci Rev. 90:183–198.
- Theuerkauf M, Couwenberg J. 2017. The extended downscaling approach: A new R-tool for pollen-based reconstruction of vegetation patterns. Holocene. 27(8):1252–1258.
- Theuerkauf M, Couwenberg J. 2018. ROPES Reveals Past Land Cover and PPEs From Single Pollen Records. Front Earth Sci. 6:art.14.
- Theuerkauf M, Couwenberg J, Kuparinen A, Liebscher V. 2016. A matter of dispersal: REVEALSinR introduces state-of-the-art dispersal models to quantitative vegetation reconstruction. Veg Hist Archaeobot. 25:541–553.
- Theuerkauf M, Dräger N, Kienel U, Kuparinen A, Brauer A. 2015. Effects of changes in land management practices on pollen productivity of open vegetation during the last century derived from varved lake sediments. Holocene. 25(5):733–744.
- Theuerkauf M, Joosten H. 2009. Substrate dependency of lateglacial forests in north-east Germany: untangling vegetation patterns, ecological amplitudes and pollen dispersal in the past by downscaling regional pollen. J Biogeogr. 36:942–953.
- Theuerkauf M, Joosten H. 2012. Younger Dryas cold stage vegetation patterns of central Europe - climate, soil and relief controls. Boreas. 41:391–407.
- Theuerkauf M, Kuparinen A, Joosten H. 2012. Pollen productivity estimates strongly depend on assumed pollen dispersal. Holocene. 23(1):14–24.
- Thom D, Rammer W, Seidl R. 2017. Disturbances catalyze the adaptation of forest ecosystems to changing climate conditions. Glob Chang Biol. 23(1):269–282.
- Thom D, Seidl R. 2016. Natural disturbance impacts on ecosystem services and biodiversity in temperate and boreal forests. Biol Rev. 91(3):760–781.
- Thomas CD. 2009. A speculative history of open-country species in Britain and northern Europe. Br Wildl. 20(5 Special Supplement):21–25.
- Thomas CD, Cameron A, Green RE, Bakkenes M, Beaumont LJ, Collingham YC, Erasmus BFN, de Siqueira MF, Grainger A, Hannah L et al. 2004. Extinction risk from climate change. Nature. 427(6970):145–148.
- Thomas CD, Palmer G. 2015. Non-native plants add to the British flora without negative consequences for native diversity. Proc Natl Acad Sci USA. 112(14):4387–4392.
- Thomas ZA. 2016. Using natural archives to detect climate and environmental tipping points in the Earth System. Quat Sci Rev. 152:60–71.
- Thompson AR, Jackson ST. 2013. The human influence. Moral responsibility for novel ecosystems. In: Sandler R, Basl J, editors. Designer biology: the ethics of intensively engineering biological and ecological systems. Lanham, Maryland: Lexington Books; p. 125–150.
- Thompson MSA, Brooks SJ, Sayer CD, Woodward G, Axmacher JC, Perkins DM, Gray C. 2017. Large woody debris “rewilding” rapidly restores biodiversity in riverine food webs. J Appl Ecol. 55:895–904.
- Thorley A. 1981. Pollen analytical evidence relating to the vegetation history of the chalk. J Biogeogr. 8:93–136.
- Thuiller W, Lavorel S, Araújo M, Sykes MT, Prentice IC. 2005. Climate change threats to plant diversity in Europe. Proc Natl Acad Sci USA. 102:8245–8250.
- Tian F, Cao XY, Dallmeyer A, Ni J, Zhao Y, Wang Y, Herzschuh U. 2016. Quantitative woody cover reconstructions from eastern continental Asia of the last 22 kyr reveal strong regional peculiarities. Quat Sci Rev. 137:33–44.
- Timpane-Padgham BL, Beechie T, Klinger T. 2017. A systematic review of ecological attributes that confer resilience to climate change in environmental restoration. PLoS One. 12(3):e0173812.
- Tingley MW, Beissinger SR. 2009. Detecting range shifts from historical species occurrences: new perspective on old data. Trends Ecol Evol. 24:625–633.
- Tinner W, Ammann B. 2005. Long-term responses of mountain ecosystems to environmental changes: resilience, adjustment, and vulnerability. In: Hüber UM, Bugmann HKM, Reasoner MA, editors. Global change and mountain regions. Dordrecht: Springer; p. 133–143.
- Tinner W, Colombaroli D, Heiri O, Henne PD, Steinacher M, Untenecker J, Vescovi E, Allen JRM, Carraro G, Conedera M et al. 2013. The past ecology of Abies alba provides new perspectives on future responses of silver fir forests to global warming. Ecol Monogr. 83(4):419–439.
- Tinner W, Conedera M, Ammann B, Lotter AF. 2005. Fire ecology north and south of the Alps since the last ice age. Holocene. 15(8):1214–1226.
- Tinner W, Hubschmid P, Wehrli B, Ammann B, Conedera M. 1999. Long-term forest fire ecology and dynamics in southern Switzerland. J Ecol. 87:273–289.
- Tinner W, Lotter AF. 2001. Central European vegetation response to abrupt climate change at 8.2 ka. Geology. 29(6):551–554.
- Tinner W, Lotter AF. 2006. Holocene expansions of Fagus sylvatica and Abies alba in central Europe: Where are we after eight decades of debate? Quat Sci Rev. 25:526–549.
- Tinner W, Lotter AF, Ammann B, Conedera M, Hubschmid P, van Leeuwen JFN, Wehrli M. 2003. Climatic change and contemporaneous land-use phases north and south of the Alps 2300 BC to 800 AD. Quat Sci Rev. 22(14):1447–1460.
- Tinner W, van Leeuwen JFN, Colombaroli D, Vescovi E, van der Knaap WO, Henne PD, Pasta S, D’Angelo S, La Mantia T. 2009. Holocene environmental and climatic changes at Gorgo Basso, a coastal lake in southern Sicily, Italy. Quat Sci Rev. 28:1498–1510.
- Titeux N, Henle K, Mihoub J-B, Regos A, Geijzendorffer IR, Cramer W, Verburg PH, Brotons L. 2017. Global scenarios for biodiversity need to better integrate climate and land use change. Divers Distrib. 23(11):1231–1234.
- Toledo VM. 1982. Pleistocene changes of vegetation in tropical Mexico. In: Prance GT, editor. Biological Diversification in the Tropics. New York: Columbia University Press; p. 93–111.
- Tollefsrud MM, Kissling R, Gugerli F, Johnsen Ø, Skrøppa T, Cheddadi R, van der Knaap WO, Latałowa M, Terhürne-Berson R, Litt T et al. 2008. Genetic consequences of glacial survival and postglacial colonization in Norway spruce: combined analysis of mitochondrial DNA and fossil pollen. Mol Ecol. 17:4134–4150.
- Tollefsrud MM, Sønstebø JH, Brochmann C, Johnsen Ø, Skrøppa T, Vendramin GG. 2009. Combined analysis of nuclear and mitochondrial markers privde new insight into the genetic structure of North European Picea abies. Heredity. 102:549–562.
- Tolonen K. 1983. Kuusen levinneisyy historiaa suomessa. Sorbifolia. 14:53–58.
- Tolonen K, Ruuhijärvi R. 1976. Standard pollen diagrams from the Salpausoelkä region of southern Finland. Ann Bot Fennici. 13:155–196.
- Tovar C, Breman E, Brncic TM, Harris DJ, Bailey R, Willis KJ. 2014. Influence of 1100 years of burning on the central African rainforest. Ecography. 37(11):1139–1148.
- Tovar C, Harris DJ, Breman E, Brncic TM, Willis KJ. 2019. Tropical monodominant forest resilience to climate change in Central Africa: A Gilbertiodendron dewevrei forest pollen record over the past 2,700 years. J Veg Sci.10.1111/jvs.12746.
- Tralau H. 1959. Extinct aquatic plants of Europe. Bot Notiser. 112(4):385–406.
- Tralau H. 1962. Najas tenuissima (A. Br.) Magnus during the Late-Cainozoic period in Europe. Bot Notiser. 115(4):421–428.
- Tralau H. 1963a. Asiatic Dicotyledonous affinities in the Cainozoic flora of Europe. Kungliga Sven Vetenskapsakademiens Handlingar. 9:7–87.
- Tralau H. 1963b. The recent and fossil distribution of some boreal and arctic montane plants in Europe. Ark Bot. 5:533–582.
- Travis JMJ. 2003. Climate change and habitat destruction: a deadly anthropogenic cocktail. Proc R Soc B Bio Sci. 270(1514):467–473.
- Tree I. 2018. Wilding - The Return of Nature to a British Farm. London: Picador.
- Treguboov SS. 1941. Les forêts vierges montagnardes des Alpes Dinariques, Massif de Klekovatcha-Guermetch: étude botanique et forestière. Montpellier: Causse, Graille et Castelnau.
- Tsuda Y, Chen J, Stocks M, Källman T, Sønstebø JH, Parducci L, Semerikov V, Sperisen C, Politov D, Ronkainen T et al. 2016. The extent and meaning of hybridization and introgression between Siberian spruce (Picea obovata) and Norway spruce (Picea abies): cryptic refugia as stepping stones to the west? Mol Ecol. 25:2773–2789.
- Tsukada M. 1981. Cryptomeria japonica D. Don I. Pollen dispersal and logistic forest expansion. Jpn J Ecol. 31:371–383.
- Tsukada M. 1982a. Pseudotsuga menziesii (Mirb.) Franco: its pollen dispersal and late Quaternary history in the Pacific Northwest. Jpn J Ecol. 32:159–187.
- Tsukada M. 1982b. Late-Quaternary development of the Fagus forest in the Japanese Archipelago. Jpn J Ecol. 32:113–118.
- Tsukada M. 1982c. Cryptomeria japonica: Glacial refugia and late-glacial and postglacial migration. Ecology. 63(4):1091–1105.
- Tsukada M. 1982d. Late-Quaternary shift of Fagus distribution. Bot Mag Tokyo. 95:203–217.
- Tsukada M. 1983. Late-Quaternary spruce decline and rise in Japan and Sakhalin. Bot Mag Tokyo. 96:127–133.
- Tsukada M, Sugita S. 1982. Late Quaternary dynamics of pollen influx at Mineral Lake, Washington. Bot Mag Tokyo. 95:401–418.
- Turner J. 1965. A contribution to the history of forest clearance. Proc R Soc B Bio Sci. 161:343–354.
- Turner MG. 2010. Disturbance and landscape dynamics in a changing world1. Ecology. 91(10):2833–2849.
- Turner MG, Baker WL, Peterson CJ, Peet RK. 1998. Factors influencing succession: lessons from large, infrequent natural disturbances. Ecosystems. 1:511–523.
- Turner MG, Dale VH. 1998. Comparing large, infrequent disturbances: what have we learned? Ecosystems. 1:493–496.
- Turner MG, Gardner RH, editors. 1991. Quantitative Methods in Landscape Ecology - The analysis and interpretation of landscape heterogeneity. New York: Springer.
- Turney CSM, Fogwill CJ, Lenton TM, Jones RT, von Gunten L, editors. 2016. Tipping Points. PAGES Mag. 24 (1):1–51.
- Turney CSM, Kershaw AP, Moss P, Bird MI, Fifield LK, Cresswell RG, Santos GM, Di Tada ML, Hausladen PA, Zhou Y. 2001. Redating the onset of burning at Lynch’s Crater (North Queensland): implications for human settlement in Australia. J Quat Sci. 16(8):767–771.
- Turvey ST. 2009a. In the shadow of the megafauna: prehistoric mammal and bird extinctions across the Holocene. In: Turvey ST, editor. Holocene extinctions. Oxford: Oxford University Press; p. 17–37.
- Turvey ST, editor 2009b. Holocene extinctions. Oxford: Oxford University Press.
- Tüxen R. 1956. Die heutige potentielle ntürliche Vegetation als Gegenstand der Vegetationskartierung. Angew Pflanzensoziol. 13:4–42.
- Tweiten MA, Calcote RR, Lynch EA, Hotchkiss SC, Schuurman GW. 2015. Geophysical features influence the climate change sensitivity of northern Wisconsin pine and oak forests. Ecol App. 25(7):1984–1996.
- Tweiten MA, Hotchkiss SC, Booth RK, Calcote RR, Lynch EA. 2009. The response of a jack pine forest to late-Holocene climate variability in northwestern Wisconsin. Holocene. 19:1049–1061.
- Tyrberg T. 2009. Holocene avian extinctions. In: Turvey ST, editor. Holocene extinctions. Oxford: Oxford University Press; p. 63–106.
- Tzedakis PC. 2007. Seven ambiguities in the Mediterranean palaeoenvironmental narrative. Quat Sci Rev. 26(17–18):2042–2066.
- Tzedakis PC, Bennett KD. 1995. Interglacial vegetation succession: a view from southern Europe. Quat Sci Rev. 14:967–982.
- Tzedakis PC, Channell JET, Hodell DA, Kleiven HF, Skinner LC. 2012. Determining the natural length of the current interglacial. Nat Geosci. 5(2):138–141.
- Tzedakis PC, Crucifix M, Mitsui T, Wolff EW. 2017. A simple rule to determine which insolation cycles lead to interglacials. Nature. 542(7642):427–432.
- Tzedakis PC, Emerson BC, Hewitt GM. 2013. Cryptic or mystic? Glacial tree refugia in northern Europe. Trends Ecol Evol. 28(12):696–704.
- Tzedakis PC, Hooghiemstra H, Pälike H. 2006. The last 1.35 million years at Tenaghi Philippon: revised chronostratigraphy and long-term vegetation trends. Quat Sci Rev. 25:3416–3430.
- Tzedakis PC, Raynaud D, McManus JF, Berger A, Brovkin V, Kiefer T. 2009. Interglacial diversity. Nat Geosci. 2(11):751–755.
- Umbanhowar CE. 2004. Interaction of fire, climate and vegetation change at a large landscape scale in the Big Woods of Minnesota, USA. Holocene. 14(5):661–676.
- Umbanhowar CE, Camill P, Geiss CE, Teed R. 2006. Asymmetric vegetation responses to mid-Holocene aridity at the prairie–forest ecotone in south-central Minnesota. Quat Res. 66(1):53–66.
- Urban MA, Nelson DM, Street-Perrott FA, Verschuren D, Hu FS. 2015. A late-Quaternary perspective on atmospheric pCO2, climate, and fire as drivers of C4-grass abundance. Ecology. 96(3):642–653.
- Urban MC, Tewksbury JJ, Sheldon KS. 2012. On a collision course: competition and dispersal differences create no-analogue communities and cause extinctions during climate change. Proc R Soc B Bio Sci. 279(1735):2072–2080.
- Urrego DH, Bush MB, Silman MR, Correa-Metrio AY, Ledru M-P, Mayle FE, Paduano G, Valencia BG. 2009. Millennial-scale ecological changes in tropical South America since the Last Glacial Maximum. In: Vimeux F, Sylvestre F, Khodri M, editors. Past climate variability in South America and surrounding regions: from the last glacial maximum to the Holocene. Dordrecht: Springer Netherlands; p. 283–300.
- Urrego DH, Hooghiemstra H, Rama-Corredor O, Martrat B, Grimalt JO, Thompson L, Bush MB, González-Carranza Z, Hanselman J, Valencia B et al. 2016. Millennial-scale vegetation changes in the tropical Andes using ecological grouping and ordination methods. Clim Past. 12(3):697–711.
- Valencia BG, Bush MB, Coe AL, Orren E, Gosling WD. 2018. Polylepis woodland dynamics during the last 20,000 years. J Biogeogr. 45(5):1019–1030.
- Valencia BG, Matthews-Bird F, Urrego DH, Williams JJ, Gosling WD, Bush MB. 2016. Andean microrefugia: testing the Holocene to predict the Anthropocene. New Phytol. 212(2):510–522.
- Valsecchi V, Carraro G, Conedera M, Tinner W. 2010. Late-Holocene vegetation and land-use dynamics in the Southern Alps (Switzerland) as a basis for nature protection and forest management. Holocene. 20(4):483–495.
- Valsecchi V, Finsinger W, Tinner W, Ammann B. 2008. Testing the influence of climate, human impact and fire on the Holocene population expansion of Fagus sylvatica in the southern Prealps (Italy). Holocene. 18(4):603–614.
- van der Geer AAE, Lomolino MV, Lyras GA. 2017. ‘Island Life’ before man: biogeography of palaeo-insular mammals. J Biogeogr. 44:995–1006.
- van der Hammen T. 1974. The Pleistocene changes of vegetation and climate in tropical South America. J Biogeogr. 1(1):3–26.
- van der Hammen T, Correal Urrego G. 1978. Prehistoric man on the Sabana de Bogotá; data for an ecological prehistory. Palaeogeogr Palaeoclimatol Palaeoecol. 25(1–2):179–190.
- van der Hammen T, Hooghiemstra H. 2000. Neogene and Quaternary history of vegetation, climate, and plant diversity in Amazonia. Quat Sci Rev. 19(8):725–742.
- van der Hammen T, Wijmstra TA, Zagwijn WH. 1971. The floral record of the Late Cenozoic of Europe. In: Turekian KK, editor. The Late Cenozoic Glacial ages. New Haven: Yale University Press; p. 391–424.
- van der Knaap WO, van Leeuwen JFN. 1993. A recent pollen diagram from Antarctica (King George Island, South Shetland Islands). Holocene. 3(2):169–173.
- van der Leeuw S, Costanza R, Aulenbach S, Brewer S, Burek M, Cornell SE, Crumley C, Dearing JA, Downy C, Graumlich LJ et al. 2011. Toward an integrated history to guide the future. Ecol Soc. 16(4):a2.
- van der Sande MT, Gosling WD, Correa-Metrio A, Prado-Junior J, Poorter L, Oliveira RS, Mazzei L, Bush MB. 2019. A 7000-year history of changing plant trait composition in an Amazonian landscape; the role of humans and climate. Ecol Lett. 22(6):925–935.
- van der Veken S, Bellemare J, Verheyen K, Hermy M. 2007. Life-history traits are correlated with geographical distribution patterns of western European forest herb species. J Biogeogr. 34(10):1723–1735.
- van Geel B. 1986. Application of fungal and algal remains and other microfossils in palynological analyses. In: Berglund BE, editor. Handbook of holocene palaeoecology and palaeohydrology. Chichester: J Wiley & Sons; p. 497–505.
- van Geel B. 2001. Non-pollen palynomorphs. In: Smol JP, Birks HJB, Last WM, editors. Tracking environmental change using lake sediments volume 3: terrestrial, algal, and siliceous indicators. Dordrecht: Kluwer; p. 99–119.
- van Geel B, Andersen ST. 1988. Fossil ascospores of the parasitic fungus Usulina deusta in Eemian deposits in Denmark. Rev Palaeobot Palynol. 56:89–93.
- van Geel B, Aptroot A. 2006. Fossil ascomycetes in Quaternary deposits. Nova Hedwigia. 82(3–4):313–329.
- van Geel B, Coope GR, van der Hammen T. 1989. Palaeoecology and stratigraphy of the lateglacial type section at Usselo (The Netherlands). Rev Palaeobot Palynol. 60:25–129.
- van Geel B, Engels S, Martin-Puertas C, Brauer A. 2013. Ascospores of the parasitic fungus Kretzchmaria deusta as rainstorm indicators during a late Holocene beech-forest phase around lake Meerfelder Maar, Germany. J Paleolimnol. 50:33–40.
- van Geel B, Gelorini V, Lyaruu A, Aptroot A, Rucina S, Marchant R, Damsté JSS, Verschuren D. 2011. Diversity and ecology of tropical African fungal spores from a 25,000-year palaeoenvironmental record in southeastern Kenya. Rev Palaeobot Palynol. 164(3):174–190.
- van Geel B, Protopopov A, Protopopova V, Pavlov I, van der Plicht J, van Reenen GBA. 2017. Larix during the Mid-Pleniglacial (Greenland Interstadial 8) on Kotelny Island, northern Siberia. Boreas. 46(2):338–345.
- van Leeuwen JFN, Froyd CA, van der Knaap WO, Coffey EED, Tye A, Willis KJ. 2008. Fossil pollen as a guide to conservation in the Galápagos. Science. 322:1306.
- van Leeuwen JFN, Schafer H, van der Knaap WO, Rittenour TM, Björck S, Ammann B. 2005. Native or introduced? Fossil pollen and spores may say. An example from the Azores Islands. Neobiota. 6:27–34.
- van Nes EH, Holmgren M, Hirota M, Scheffer M. 2012. Response to comment on “Global resilience of tropical forest and savanna to critical transitions”. Science. 336(6081):541.
- van Woesik R. 2013. Quantifying uncertainty and resilience on coral reefs using a Bayesian approach. Environ Res Lett. 8(4):044051.
- van Zinderen Bakker EM. 1962. A late-glacial and post-glacial climatic correlation between East Africa and Europe. Nature. 194:201–203.
- van Zinderen Bakker EM. 1964. A pollen diagram from equatorial Africa. Geol Mijnbouw. 43:123–128.
- Vargot EV, Shcherbakov AV, Bolotova YV, Uotila P. 2016. Current distribution and conservation of Najas tenuissima (Hydocharitaceae). Nat Conserv Res. 1(3):2–10.
- Vaupell C. 1857. Bögens Indvandring i de Danske Skove. Copenhagen: C.A. Reitzels Bo & Arvinger.
- Vaupell C. 1863. De Danske Skove. Copenhagen: PG Philipsens Forlag.
- Vázquez-Rivera H, Currie DJ. 2015. Contemporaneous climate directly controls broad-scale patterns of woody plant diversity: a test by a natural experiment over 14,000 years. Global Ecol Biogeogr. 24(1):97–106.
- Vegas-Vilarrúbia T, Rull V, Montoya E, Safont E. 2011. Quaternary palaeoecology and nature conservation: a general review with examples from the neotropics. Quat Sci Rev. 30:2361–2388.
- Velichkevich FY. 1977. On the Likhvinian flora of Rubo on the West Divina River. Dokl Akad Nauk SSSR. 233:1158–1181.
- Velichkevich FY. 1982. The Pleistocene Floras of Glacial Areas of the East-European Plain. Minsk: Izdatel’stvo Nauka I Tekhnika.
- Velichkevich FY, Granoszewski W. 1996. Potamogeton sukaczevii Wieliczk. in the NeoPleistocene floras of Poland, Belarus and Lithuania. Acta Palaeobot. 36(1):97–105.
- Velichkevich FY, Lesiak MA. 1996. Fossil Potamogeton species of Mizerna. Acta Palaeobot. 36(1):79–95.
- Velichkevich FY, Mamakowa K. 1999. Taxonomic revision of the collections of plant macrofossils from some localities of Poland now referred to the Vistulian glaciation. Acta Palaeobot. 39(1):29–37.
- Velichkevich FY, Mamakowa K. 2003. Revision of plant macrofossils from the Mazovian interglacial locality Nowiny Żukowskie (SE Poland). Acta Palaeobot. 43(1):61–76.
- Velichkevich FY, Mamakowa K, Stuchlik L. 2004. Revision of some Mazovian interglacial macrofossil floras of Poland. Acta Palaeobot. 44(1):93–104.
- Velichkevich FY, Zastawniak E. 2003. The Pliocene flora of Kholmech, south-eastern Belarus and its correlation with other Pliocene floras of Europe. Acta Palaeobot. 43(2):137–259.
- Velichkevich FY, Zastawniak E. 2006. Pleistocene Vascular Plant Macrofossils of Central and Eastern Europe, I. Pteridophytes and Monocotyledons. Kraków: W Szafer Institute of Botany, Polish Academy of Sciences.
- Vellend M. 2010. Conceptual syntheses in community ecology. Q Rev Biol. 85:183–206.
- Vellend M. 2016. The theory of ecological communities. Princeton: Princeton University Press.
- Vellend M, Knight TM, Drake JM. 2006. Antagonistic effects of seed dispersal and herbivory on plant migration. Ecol Lett. 9(3):319–326.
- Vellend M, Myers JA, Gardescu S, Marks PL. 2003. Dispersal of Trillium seeds by deer: implications for long-distance migration of forest herbs. Ecology. 84(4):1067–1072.
- Vellend M, Srivastava DS, Anderson KM, Brown CD, Jankowski JE, Kleynhans EJ, Kraft NJB, Letaw AD, Macdonald AAM, Maclean JE et al. 2014. Assessing the relative importance of neutral stochasticity in ecological communities. Oikos. 123(12):1420–1430.
- Veloz SD, Williams JW, Blois JL, He F, Otto-Bliesner B, Liu Z. 2012. No-analog climates and shifting realized niches during the late Quaternary: implications for 21st-century predictions by species distribution models. Glob Chang Biol. 18(5):1698–1713.
- Vendramin GG, Degen B, Petit RJ, Anzidei M, Madaghiele A, Ziegenhagen B. 1999. High level of variation at Abies alba chrloroplast microsatellite loci in Europe. Mol Ecol. 8:1117–1126.
- Vera F. 1997. Metaforen voor de wildernis. Eik, hazelaar, rund en paard (Metaphors of wilderness. Oak, hazel, ox and horse) [PhD thesis]. Wageningen: Agricultural University of Wageningen.
- Vera F. 2000. Grazing Ecology and Forest History. Wallingford: CABI.f
- Verbesselt J, Umlauf N, Hirota M, Holmgren M, Van Nes EH, Herold M, Zeileis A, Scheffer M. 2016. Remotely sensed resilience of tropical forests. Nat Clim Chang. 6:1028.
- Vergeer P, Kunin WE. 2013. Adaptation at range margins: common garden trials and the performance of Arabidopsis lyrata across its northwestern European range. New Phytol. 197(3):989–1001.
- Vermaire JC, Taranu ZE, MacDonald GK, Velghe K, Bennett EM, Gregory-Eaves I. 2017. Extrinsic vs. intrinsic regimes shifts in shallow lakes: Long-term response of cyanobacterial blooms to historical catchment phosphorus loading and climate warming. Front Ecol Evol. 5:146.
- Verschuren D, Charman DJ. 2008. Latitudinal linkages in lat Holocene moisture-balance variation. In: Battarbee RW, Binney HA, editors. Natural climate variability and global warming: a holocene perspective. Oxford: Wiley-Blackwell; p. 188–231.
- Vescovi E, Ravazzi C, Arpenti E, Finsinger W, Pini R, Valsecchi V, Wick L, Ammann B, Tinner W. 2007. Interactions between climate and vegetation during the Lateglacial period as recorded by lake and mire sediment archives in Northern Italy and Southern Switzerland. Quat Sci Rev. 26(11):1650–1669.
- Vickers K, Bending J, Buckland PC, Edwards KJ, Hansen SS, Cook GT. 2005. Toftanes: The paleoecology of a Faroese landnám farm. Hum Ecol. 33(5):685–710.
- Vild O, Hédl R, Kopecký M, Szabó P, Suchánková S, Zouhar V. 2017. The paradox of long-term ungulate impact: increase of plant species richness in a temperate forest. Appl Veg Sci. 20(2):282–292.
- Villavicencio NA, Lindsey EL, Martin FM, Borrero LA, Moreno PI, Marshall CR, Barnosky AD. 2016. Combination of humans, climate, and vegetation change triggered Late Quaternary megafauna extinction in the Última Esperanza region, southern Patagonia, Chile. Ecography. 29:125–140.
- Virah-Sawmy M, Bonsall MB, Willis KJ. 2009b. ‘Tales of Symphonia’: extinction dynamics in response to past climate change in Madagascan rainforests. Biol Lett. 5:821–825.
- Virah-Sawmy M, Gillson L, Willis KJ. 2009a. How does spatial heterogeneity influence resilience to climatic change? Ecological dynamics in southeast Madagascar. Ecol Monogr. 79(4):557–574.
- Virah-Sawmy M, Willis KJ, Gillson L. 2010. Evidence for drought and forest declines during the recent megafaunal extinctions in Madagascar. J Biogeogr. 37:506–519.
- Visser ME, Both C. 2005. Shifts in phenology due to global climate change: the need for a yardstick. Proc R Soc B Bio Sci. 272(1581):2561–2569.
- Vitousek PM. 1988. Diversity and biological invasions of oceanic islands. In: Wilson EO, editor. Biodiversity. Washington DC: National Academy Press; p. 181–189.
- Vitousek PM, Ladefoged TN, Kirch PV, Hartshorn AS, Graves MW, Hotchkiss SC, Tuljapurkar S, Chadwick OA. 2004. Soils, agriculture, and society in precontact Hawai’i. Science. 304:1665–1669.
- Vitousek PM, Loope LL, Adsersen H, editors. 1995. Islands: Biological diversity and ecosystem function. Berlin: Springer.
- Vitousek PM, Mooney HA, Lubchenco J, Melillo JM. 1997. Human domination of Earth’s ecosystems. Science. 277:494–499.
- Volkov I, Banavar JR, Maritan A, Hubbell SP. 2004. Neutral theory (communications arising): The stability of forest biodiversity. Nature. 427:696.
- von Holle B, Delcourt HR, Simberloff D. 2003. The importance of biological inertia in plant community resistance to invasion. J Veg Sci. 14:425–432.
- von Post L. 1916. Mötet den 2 november 1916. Geologiska Föreningens i Stockholm Förhandlingar. 38:383–394.
- von Post L. 1918. Skogsträdpollen i sydsvenska torvmosselagerföljder. Förhandlingar ved 16 skandinavia naturforskermøte 1916. p. 433–465.
- von Post L. 1922. Sveriges Geologiska Undersöknings torvinventering och nägra av dess hittills vunna resultat. Sven Mosskulturföreningens Tidskr. 36:1–27.
- von Post L. 1924. Ur de sydsvenska skogarnas regionala historia under post-arktisk tid. Geologiska Föreningens i Stockholm Förhandlingar. 46:83–128.
- von Post L. 1926a. Einige Aufgaben der regionalen Moorforschung. Sveriges Geol Undersökning Ser C. 337:1–41.
- von Post L. 1929. Die Zeichenschrift der Pollenstatistik. Geologiska Föreningens i Stockholm Förhandlingar. 51(4):543–565.
- von Post L. 1930. Die postarktische Geschichte der europäischen Wälder nach den vorliegenden Pollendiagrammen. Medd från Stockholms Högskolas Geol Inst. 16:1–27.
- von Post L. 1946. The prospect for pollen analysis in the study of the Earth’s climatic history. New Phytol. 45(2):193–217.
- von Post L. 1967. Forest tree pollen in south Swedish peat bog deposits. Translated by M.B. Davis and K. Fægri with introduction by K. Fægri and J. Iversen. Pollen Spores. 9:375–401.
- Voosen P. 2018. Sticky glaciers slowed tempo of ice ages. Science. 361(6404):739.
- Waelbroeck C, Labeyrie L, Duplessy JC, Guiot J, Labracherie M, Leclaire H, Duprat J. 1998. Improving past sea surface temperature estimates based on planktonic fossil faunas. Paleoceanogr. 13(3):272–283.
- Wagner HH, Fortin M-J. 2005. Spatial analysis of landscapes: concepts and synthesis. Ecology. 86:1975–1985.
- Wagner S, Litt T, Sánchez-Goñi M-F, Petit RJ. 2015. History of Larix decidua Mill. (European larch) since 130 ka. Quat Sci Rev. 124:224–247.
- Wagner V, Chytrý M, Jiménez-Alfaro B, Pergl J, Hennekens S, Biurrun I, Knollová I, Berg C, Vassilev K, Rodwell JS et al. 2017. Alien plant invasions in European woodlands. Divers Distrib. 23(9):969–981.
- Wahl ER. 2004. A general framework for determining cutoff values to select pollen analogs with dissimilarity metrics in the modern analog technique. Rev Palaeobot Palynol. 128(3):263–280.
- Wake DB, Hadly EA, Ackerly DD. 2009. Biogeography, changing climates, and niche evolution. Proc Natl Acad Sci USA. 106(Suppl 2):19631–19636.
- Walker D. 1966. The late Quaternary history of the Cumberland lowland. Philos Trans R Soc B Bio Sci. 251:1–210.
- Walker D. 1972. Quantification in historical plant ecology. Proc Ecol Soc Aust. 6:91–104.
- Walker D. 1978. Envoi. In: Walker D, Guppy JC, editors. Biology and quaternary environments. Canberra: Australian Academy of Sciences; p. 259–264.
- Walker D. 1982a. Vegetation’s fourth dimension. New Phytol. 90(3):419–429.
- Walker D. 1982b. The development of resilience in burned vegetation. In: Newman EI, editor. Plant community as a working mechanism. Oxford: Blackwell Scientific Publications; p. 27–43.
- Walker D. 1989. Diversity and stability. In: Cherrett JM, Bradshaw AD, Goldsmith FB et al., editors. Ecological concepts. Oxford: Blackwell; p. 115–145.
- Walker D. 1990. Purpose and method in Quaternary palynology. Rev Palaeobot Palynol. 64(1–4):13–27.
- Walker D, Chen Y. 1987. Palynological light on tropical forest dynamics. Quat Sci Rev. 6:77–82.
- Walker D, Flenley JR. 1979. Late Quaternary vegetation history of the Enga Province of upland Papua New Guinea. Philos Trans R Soc B Bio Sci. 286:265–344.
- Wallace AR. 1881 ( reprinted 2017). Island life. New York: Cosimo.
- Waller LA. 2010. Bridging gaps between statistical and mathematical modeling in ecology. Ecology. 91:3500–3502.
- Waller MP, Early R. 2015. Vegetation dynamics from a coastal peatland: insights from combined plant macrofossil and pollen data. J Quat Sci. 30:779–789.
- Waller MP, Hamilton S. 2000. Vegetation history of the English chalklands: a mid-Holocene pollen sequence from the Caburn, East Sussex. J Quat Sci. 15:253–272.
- Walsh JC, Dicks LV, Sutherland WJ. 2015. The effect of scientific evidence on conservation practitioners’ management decisions. Conserv Bio. 29(1):88–98.
- Waltari E, Hijmans RJ, Peterson AT, Nyári AS, Perkins SL, Guralnick RP. 2007. Locating Pleistocene refugia: comparing phylogeographic and ecological niche model predictions. PLoS One. 2(7):e563.
- Wang L, Brook GA, Burney DA, Voarintsoa NRG, Liang F, Cheng H, Edwards RL. 2019. The African Humid Period, rapid climate change events, the timing of human colonization, and megafaunal extinctions in Madagascar during the Holocene: Evidence from a 2m Anjohibe Cave stalagmite. Quat Sci Rev. 210:136–153.
- Wang R, Dearing JA, Langdon PG, Zhang E, Yang X, Dakos V, Scheffer M. 2012. Flickering gives early warning signals of a critical transition to a eutrophic lake state. Nature. 492:419–422.
- Wang X, Edwards RL, Auler AS, Cheng H, Kong X, Wang Y, Cruz FW, Dorale JA, Chiang H-W. 2017. Hydroclimate changes across the Amazon lowlands over the past 45,000 years. Nature. 541(7636):204–207.
- Wang Y, Heintzman PD, Newsom L, Bigelow N, Wooller M, Shapiro B, Williams JW. 2017. The southern coastal Beringian land bridge: cryptic refugium or pseudorefugium for woody plants during the Last Glacial Maximum? J Biogeogr. 44(7):1559–1571.
- Wardle DA, Walker LR, Bardgett RD. 2004. Ecosystem properties and forest decline in contrasting long-term chronosequences. Science. 305(5683):509–513.
- Warren A, Goldsmith FB, editors. 1974. Conservation in Practice. London: J Wiley.
- Warren A, Goldsmith FB, editors. 1983. Conservation in Perspective. London: J Wiley.
- Warren DL. 2012. In defense of ‘niche modeling’. Trends Ecol Evol. 27(9):497–500.
- Warren DL, Cardillo M, Rosauer DF, Bolnick DI. 2014. Mistaking geography for biology: inferring processes from species distributions. Trends Ecol Evol. 29(10):572–580.
- Wasof S, Lenoir J, Gallet-Moron E, Jamoneau A, Brunet J, Cousins SAO, De Frenne P, Diekmann M, Hermy M, Kolb A et al. 2013. Ecological niche shifts of understorey plants along a latitudinal gradient of temperate forests in north-western Europe. Global Ecol Biogeogr. 22(10):1130–1140.
- Watt AS. 1919. On the causes of failure of natural regeneration in British oakwoods. J Ecol. 7:173–203.
- Watt AS. 1947. Pattern and process in plant communities. J Ecol. 35:1–22.
- Watt AS. 1964. The community and the individual. J Ecol. 52(Suppl.):203–211.
- Watts WA. 1967. Late-glacial plant macrofossils from Minnesota. In: Cushing EJ, Wright HE, editors. Quaternary paleoecology. New Haven (CT): Yale University Press; p. 89–97.
- Watts WA. 1970. The full-glacial vegetation of northwestern Georgia. Ecology. 51:17–33.
- Watts WA. 1971. The true identity of Micromenyanthes microsperma n. sp. Foss. New Phytol. 70:435–436.
- Watts WA. 1973. Rates of change and stability in vegetation in the perspective of long periods of time. In: Birks HJB, West RG, editors. Quaternary plant ecology. Oxford: Blackwell Scientific Publications; p. 195–206.
- Watts WA. 1977. The Late Devensian vegetation of Ireland. Philos Trans R Soc B Bio Sci. 280:273–293.
- Watts WA. 1978. Plant macrofossils and Quaternary palaeoecology. In: Walker D, Guppy JC, editors. Biology and Quaternary environments. Canberra: Australian Academy of Sciences; p. 53–67.
- Watts WA. 1980. The Late Quaternary vegetation history of the southeastern United States. Annu Rev Ecol Syst. 11:387–409.
- Watts WA. 1982. Response of biotic populations to rapid environmental and climatic changes. AMQUA Abstracts. American Quaternary Association; p. 19–21.
- Watts WA. 1984. The Holocene vegetation of the Burren, western Ireland. In: Haworth EY, Lund JWG, editors. Lake sediments and environmental history. Leicester: University of Leicester Press; p. 359–376.
- Watts WA. 1988. Europe. In: Huntley B, Webb T, editors. Vegetation history. Dordrecht: Kluwer; p. 155–192.
- Watts WA, Hansen BCS. 1986. Holocene climate and vegetation of Bermuda. Pollen Spores. 28(3–4):355–364.
- Watts WA, Hansen BCS, Grimm EC. 1992. Camel Lake - a 40000-yr record of vegetational and forest history from northwest Florida. Ecology. 73(3):1056–1066.
- Webb DA. 1954. Is the classification of plant communities either possible or desirable? Bot Tidsskr. 51:362–370.
- Webb DA. 1985. What are the criteria for presuming native status? Watsonia. 15:231–236.
- Webb SL. 1986. Potential role of passenger pigeons and other vertebrates in the rapid Holocene migrations of nut trees. Quat Res. 26:367–375.
- Webb SL. 1987. Beech range extension and vegetation history: pollen stratigraphy of two Wisconsin lakes. Ecology. 68:1993–2005.
- Webb SL, Dwyer M, Kaunzinger CK, Wyckoff PH. 2000. The myth of the resilient forest: case study of the invasive Norway maple Acer platanoides). Rhodora. 102(911):332–354.
- Webb T. 1981. The past 11000 years of vegetational change in eastern North America. BioScience. 31:501–506.
- Webb T. 1986. Is vegetation in equilibrium with climate? How to interpret Late-Quaternary pollen data. Vegetatio. 67:75–91.
- Webb T. 1987. The appearance and disappearance of major vegetational assemblages: Long-term vegetational dynamics in eastern North America. Vegetatio. 69:177–187.
- Webb T. 1988a. Eastern North America. In: Huntley B, Webb T, editors. Vegetation history. Dordrecht: Kluwer Academic Publishers; p. 385–414.
- Webb T. 1988b. Response to Bennett’s reply concerning the modelling of Holocene changes in beech populations. Rev Palaeobot Palynol. 56:362–364.
- Webb T, Anderson KH, Bartlein PJ, Webb RS. 1998. Late Quaternary climate change in eastern North America: a comparison of pollen-derived estimates with climate model results. Quat Sci Rev. 17:587–606.
- Webb T, Cushing EJ, Wright HE. 1983a. Holocene changes in the vegetation of the Midwest. In: Wright HE, editor. Late-Quaternary environments of the United States volume 2: the holocene. Minneapolis: University of Minnesota Press; p. 142–165.
- Webb T, Kutzbach JE. 1998. An introduction to ‘Late Quaternary climates: data syntheses and model experiments’. Quat Sci Rev. 17:465–471.
- Webb T, Richard PH, Mott RJ. 1983b. A mapped history of Holocene vegetation in southern Quebec. Syllogeus. 49:273–336.
- Webb T, Shuman B, Williams JW. 2003. Climatically forced vegetation dynamics in eastern North America during the late Quaternary Period. In: Gillespie AR, Porter SC, Atwater BF, editors. The Quaternary Period in the United States. Amsterdam: Elsevier; p. 459–478.
- Weckström J, Liao M, Yu G, Amsinck S, Kauppila T, Qin B, Zhu G, Sarvala J, Weckström K, Tarvainen M et al. 2015. Responses of aquatic ecosystems to environmental changes in Finland and China. Front Ecol Evol. 3:126.
- Weigelt P, Steinbauer MJ, Cabral JS, Kreft H. 2016. Late Quaternary climate change shapes island biodiversity. Nature. 532:99–102.
- Weiher E, Keddy P, editors. 1999. Ecological assembly rules: perspectives, advances, retreats. Cambridge: Cambridge University Press.
- Welker F, Duijim E, van der Gaag KJ, van Geel B, de Knijff P, van Leeuwen JFN, Mol D, Van der Plicht J, Raes N, Reumer J et al. 2014. Analysis of coprolites from the extinct mountain goat Myotragus balearicus. Quat Res. 81:106–116.
- Welten M. 1944. Pollenanalytische, stratigraphische und geochronologische Untersuchungen aus dem Faulenseemoos bei Spiez. Veröffentlichugen Geobotanisches Institut Rübel, Zürich. 21:1–201.
- Weppner KN, Pierce JL, Betancourt JL. 2013. Holocene fire occurrence and alluvial responses at the leading edge of pinyon-juniper migration in the Northern Great Basin, USA. Quat Res. 80:143–157.
- West K, Collins C, Kardailsky O, Kahn J, Hunt TL, Burley DV, Matisoo-Smith E. 2017. The Pacific rat race to Easter Island: tracking the prehistoric dispersal of Rattus exulans using ancient mitochondrial genomes. Front Ecol Evol. 5:52.
- West RG. 1964. Inter-relations of ecology and Quaternary palaeobotany. J Ecol. 52(Suppl):47–57.
- West RG. 1980. Pleistocene forest history in East Anglia. New Phytol. 85:571–622.
- Westaway MC, Olley J, Grün R. 2017. At least 17,000 years of coexistence: Modern humans and megafauna at the Willandra Lakes, South-Eastern Australia. Quat Sci Rev. 157:206–211.
- Westerling AL, Turner MG, Smithwick EAH, Romme WH, Ryan MG. 2011. Continued warming could transform Greater Yellowstone fire regimes by mid-21st century. Proc Natl Acad Sci USA. 108(32):13165–13170.
- Westman WE, Peet RK. 1982. Robert H. Whittaker (1920–1980): The man and his work. Vegetatio. 48:97–122.
- White JC, Penny D, Kealhofer L, Maloney BK. 2004. Vegetation changes from the late Pleistocene through the Holocene from three areas of archaeological significance in Thailand. Quatern Int. 113:111–132.
- White LJT. 2001. The African rain forest. In: Weber B, White LJT, Vedder A et al., editors. African rain forest ecology and conservation. New Haven: Yale University Press; p. 3–29.
- White PS. 1979. Pattern, process, and natural disturbance in vegetation. Bot Rev. 45:229–299.
- Whitehead DR. 1965. Palynology and Pleistocene phytogeography of unglaciated eastern North America. In: Wright HE, Frey DG, editors. The Quaternary of the United States. Princeton: Princeton University Press; p. 417–432.
- Whitehouse NJ, Smith D. 2010. How fragmented was the British Holocene wildwood? Perspectives on the “Vera” grazing debate from the fossil beetle record. Quat Sci Rev. 29(3):539–553.
- Whitfield J. 2002. Neutrality versus the niche. Nature. 417:480–481.
- Whitlock C. 2004. Forests, fires and climate. Nature. 432:28–29.
- Whitlock C, Colombaroli D, Conedera M, Tinner W. 2018. Land-use history as a guide for forest conservation and management. Conserv Bio. 32(1):84–97.
- Whitlock C, Higuera PE, McWethy DB, Briles CE. 2010. Paleoecological perspectives on fire ecoloy: revisiting the fire-regime concept. Open Ecol J. 3:6–23.
- Whitlock C, Shafer SL, Marlon JR. 2003. The role of climate and vegetation change in shaping past and present fire regimes in the northwestern US and implications for ecosystem management. For Ecol Manage. 178(1–2):5–21.
- Whitmore J, Gajewski K, Sawada M, Williams JW, Shuman PJ, Minchley T, Viau AE, Webb T, Shafer SL, Anderson P et al. 2005. Modern pollen data from North America and Greenland for multi-scale paleoenvironmental reconstructions. Quat Sci Rev. 24:1828–1848.
- Whitney BS, Cárdenas ML. 2017. Legacies of pre-Columbian land use on Latin American ecosystem composition and diversity: a case for paleoecology. PAGES Mag. 25(2):84–85.
- Whitney BS, Dickau R, Mayle FE, Soto JD, Iriarte J. 2013. Pre-Columbian landscape impact and agriculture in the Monumental Mound region of the Llanos de Moxos, lowland Bolivia. Quat Res. 80(2):207–217.
- Whitney BS, Smallman TL, Mitchard ETA, Carson JF, Mayle FE, Bunting MJ. 2019. Constraining pollen-based estimates of forest cover in the Amazon: A simulation approach. Holocene. 29(2):262–270.
- Whittaker RH. 1951. A criticism of the plant association and climatic climax concept. Northwest Sci. 25:7–31.
- Whittaker RH. 1953. A consideration of climax theory: the climax as a population and pattern. Ecol Monogr. 23(1):41–78.
- Whittaker RH. 1956. Vegetation of the Great Smoky Mountains. Ecol Monogr. 26(1):1–80.
- Whittaker RH. 1960. Vegetation of the Siskiyou Mountains, Oregon and California. Ecol Monogr. 30:279–338.
- Whittaker RH. 1967. Gradient analysis of vegetation*. Biol Rev. 42(2):207–264.
- Whittaker RH, Levin SA, Root RB. 1973. Niche, habitat, and ecoptype. Am Nat. 107:321–338.
- Whittaker RJ, Fernández-Palacios JM. 2007. Island biogeography: ecology, evolution, and conservation. 2nd ed. Oxford: Oxford University Press.
- Whittaker RJ, Fernández-Palacios JM, Matthews TJ, Borregaard MK, Triantis KA. 2017. Island biogeography: taking the long view of nature’s laboratories. Science. 357:885.
- Whittaker RJ, Triantis KA, Ladle RJ. 2008. A general dynamic theory of oceanic island biogeography. J Biogeogr. 35:977–994.
- Whittington G. 1994. Bruckenthalia spiculifolia (Salisb.) Reichenb. (Ericaceae) in the late Quaternary of western Europe. Quat Sci Rev. 13:761–765.
- Wiens JA. 1984. On understanding a non-equilibrium world: myth and reality in community patterns and processes. In: Simberloff D, Abele LG, Thistle AB, editors. Ecological communities: conceptual issues and the evidence. Princeton: Princeton University Press; p. 439–457.
- Wiens JA, Hayward GD, Safford HD, Giffen C, editors. 2012. Historical environmental variation in conservation and natural resource management. Chichester: Wiley-Blackwell.
- Wiens JA, Moss MR, editors. 2005. Issues and perspectives in landscape ecology. Cambridge: Cambridge University Press.
- Wiens JJ. 2011. The niche, biogeography and species interactions. Philos Trans R Soc B Bio Sci. 366(1576):2336–2350.
- Wiens JJ. 2016. Climate-related local extinctions are already widespread among plant and animal species. PLoS Biol. 14(12):e20001104.
- Wiik E, Bennion H, Sayer C, Davidson T, Clarke S, McGowan S, Prentice S, Simpson GL, Stone L. 2015. The coming and going of a marl lake: multi-indicator palaeolimnology reveals abrupt ecological change and alternative views of reference conditions. Front Ecol Evol. 3:82.
- Wilkins DA. 1984. The Flandrian woods of Lewis (Scotland). J Ecol. 72:251–258.
- Wilkinson DM. 1997. Plant colonization: are wind dispersed seeds really dispersed by birds at larger spatial and temporal scales? J Biogeogr. 24:61–65.
- Williams JW. 2003. Variations in tree cover in North America since the last glacial maximum. Glob Planet Change. 35:1–23.
- Williams JW, Blois JL, Gill JL, Gonzales LM, Grimm EC, Ordonez A, Shuman B, Veloz SD. 2013a. Model systems for a no-analog future: species associations and climates during the last deglaciation. Ann N Y Acad Sci. 1297(1):29–43.
- Williams JW, Blois JL, Shuman BN. 2011a. Extrinsic and intrinsic forcing of abrupt ecological change: case studies from the late Quaternary. J Ecol. 99:664–677.
- Williams JW, Gonzales LM, Kaplan JO. 2008. Leaf area index for northern and eastern North America at the Last glacial Maximum - a data-model comparison. Global Ecol Biogeogr. 17:122–134.
- Williams JW, Grimm EC, Blois JL, Charles DF, Davis EB, Goring SJ, Graham RW, Smith AJ, Anderson M, Arroyo-Cabrales J et al. 2018. The Neotoma Paleoecology Database, a multiproxy, international, community-curated data resource. Quat Res. 89(1):156–177.
- Williams JW, Jackson ST. 2003. Palynological and AVHRR observations of modern vegetational gradients in eastern North America. Holocene. 13:485–497.
- Williams JW, Jackson ST. 2007. Novel climates, no-analog communities, and ecological surprises. Front Ecol Environ. 5:475–482.
- Williams JW, Jackson ST, Kutzbach JE. 2007. Projected distributions of novel and disappearing climates by 2100 AD. Proc Natl Acad Sci USA. 104(14):5735–5742.
- Williams JW, Kharouba HM, Veloz S, Vellend M, McLachlan JS, Liu Z, Otto-Bliesner B, He F. 2013b. The ice age ecologist: testing methods for reserve prioritization during the last global warming. Global Ecol Biogeogr. 22(3):289–301.
- Williams JW, Post DM, Cwynar LC, Lotter AF, Levesque AJ. 2002. Rapid and widespread vegetation responses to past climate change in the North Atlantic region. Geology. 30(11):971–974.
- Williams JW, Shuman B, Bartlein PJ. 2009. Rapid responses of the prairie–forest ecotone to early Holocene aridity in mid-continental North America. Glob Planet Change. 66(3):195–207.
- Williams JW, Shuman B, Bartlein PJ, Diffenbaugh NS, Webb T. 2010. Rapid, time-transgressive, and variable responses to early Holocene midcontinental drying in North America. Geology. 38(2):135–138.
- Williams JW, Shuman BN. 2008. Obtaining accurate and precise environmental reconstructions from the modern analog technique and North American surface pollen dataset. Quat Sci Rev. 27:669–687.
- Williams JW, Shuman BN, Webb T. 2001. Dissimilarity analyses of late-Quaternary vegetation and climate in eastern North America. Ecology. 82(12):3346–3362.
- Williams JW, Shuman BN, Webb T, Bartlein PJ, Leduc PL. 2004. Late-Quaternary vegetation dynamics in North America: scaling from taxa to biomes. Ecol Monogr. 74(2):309–334.
- Williams JW, Summers RL, Webb T. 1998. Applying plant functional types to construct biome maps from eastern North American pollen data: comparisons with model results. Quat Sci Rev. 17:607–627.
- Williams JW, Tarasov PE, Brewer S, Notaro M. 2011b. Late Quaternary variations in tree cover at the northern forest-tundra ecotone. J Geophys Res Biogeosci. 116:G01017.
- Williamson MH. 1996. Biological invasions. London: Chapman & Hall.
- Williamson MH. 1999. Invasions. Ecography. 22(1):5–12.
- Williamson MH, Fitter AH. 1996. The characters of successful invaders. Biol Conserv. 78:163–170.
- Willis KJ. 1993. How old is ancient woodland? Trends Ecol Evol. 8(12):427–428.
- Willis KJ. 1996. Where did all the flowers go? The fate of temperate European flora during glacial periods. Endeavour. 20(3):110–114.
- Willis KJ, editor 2017. State of the World’s Plants 2017. Kew: Royal Botanic Gardens.
- Willis KJ, Araujo MB, Bennett KD, Figueroa-Rangel BL, Froyd CA, Myers S. 2007b. How can a knowledge of the past help to conserve the future? Biodiversity conservation and the relevance of long-term ecological studies. Philos Trans R Soc B Bio Sci. 362:175–186.
- Willis KJ, Bailey RM, Bhagwat SA, Birks HJB. 2010a. Biodiversity baselines, thresholds, and resilience: testing predictions and assumptions using palaeoecological data. Trends Ecol Evol. 25:583–591.
- Willis KJ, Bennett KD, Bhagwat SA, Birks HJB. 2010b. 4°C and beyond: what did this mean for biodiversity in the past? Syst Biodivers. 8:1–7.
- Willis KJ, Bennett KD, Burrough SL, Macias-Fauria M, Tovar C. 2013. Determining the response of African biota to climate change: using the past to model the future. Philos Trans R Soc B Bio Sci. 368(1625):20120491.
- Willis KJ, Bhagwat SA. 2009. Biodiversity and climate change. Science. 326:806–807.
- Willis KJ, Bhagwat SA. 2010. Questions of importance to the conservation of global biological diversity: answers from the past. Clim Past. 6:759–769.
- Willis KJ, Bhagwat SA, Jeffers ES. 2012. Landscape planning for the future: using fossil records to map potential threats, opportunities and likely future developments for biodiversity and ecosystem services. PAGES News. 20(2):106.
- Willis KJ, Birks HJB. 2006. What is natural? The need for a long-term perspective in biodiversity conservation. Science. 314(5803):1261–1265.
- Willis KJ, Braun M, Sumegi P, Toth A. 1997. Does soil change cause vegetation change or vice versa? A temporal perspective from Hungary. Ecology. 78(3):740–750.
- Willis KJ, Gillson L, Brncic TM. 2004. How “virgin” is virgin rainforest? Science. 304(5669):402–403.
- Willis KJ, Gillson L, Brncic TM, Figueroa-Rangel BL. 2005. Providing baselines for biodiversity measurement. Trends Ecol Evol. 20(3):107–108.
- Willis KJ, Gillson L, Knapp S, editors. 2007a. Biodiversity hotspots through time: using the past to manage the future. Philos Trans R Soc B Bio Sci. 362:167–333.
- Willis KJ, Gillson L, Virah-Sawmy M. 2008. Nature or nurture: the ambiguity of C4 grasslands in Madagascar. J Biogeogr. 35(10):1741–1742.
- Willis KJ, Jeffers ES, Tovar C. 2018. What makes a terrestrial ecosystem resilient? Science. 359(6379):988–989.
- Willis KJ, Kleczkowski A, New M, Whittaker RJ. 2007c. Testing the impact of climate variability on European plant diversity: 320,000 years of water-energy dynamics and its long-term influence on plant taxonomic richness. Ecol Lett. 10:673–679.
- Willis KJ, MacDonald GM. 2011. Long-term ecological records and their relevance to climate change predictions for a warmer world. Annu Rev Ecol Evol Syst. 42:267–287.
- Willis KJ, McElwain JC. 2014. The evolution of plants. 2nd ed. Oxford: Oxford University Press.
- Willis KJ, Niklas KJ. 2004. The role of Quaternary environmental change in plant macroevolution: the exception or the rule? Philos Trans R Soc B Bio Sci. 359(1442):159–172.
- Willis KJ, Sumegi P, Braun M, Toth A. 1995. The Late Quaternary environmental history of Batorliget, NE Hungary. Palaeogeogr Palaeoclimatol Palaeoecol. 118(1–2):25–47.
- Willis KJ, van Andel TH. 2004. Trees or no trees? The environments of central and eastern Europe during the Last Glaciation. Quat Sci Rev. 23(23–24):2369–2387.
- Willner W, di Pietro R, Bergmeier E. 2009. Phytogeographical evidence for post-glacial dispersal limitation of European beech forest species. Ecography. 32:1011–1018.
- Wilmshurst JM, Anderson AJ, Higham TFG, Worthy TH. 2008. Dating the late prehistoric dispersal of Polynesians to New Zealand using the commensal Pacific rat. Proc Natl Acad Sci USA. 105(22):7676–7680.
- Wilmshurst JM, McGlone MS, Turney CSM. 2015. Long-term ecology resolves the timing, region of origin and process of establishment for a disputed alien tree. AoB Plants. 7:plv104.
- Wilmshurst JM, Moar NT, Wood JR, Bellingham PJ, Findlater AM, Robinson JJ, Stone C. 2014. Use of pollen and ancient DNA as conservation baselines for offshore islands in New Zealand. Conserv Bio. 28(1):202–212.
- Wilson JB. 1991. Does vegetation science exist? J Veg Sci. 2(3):289–290.
- Wilson JB, Agnew AD, Roxburgh SH. 2019. The nature of plant communities. Cambridge: Cambridge University Press.
- Wilson JRU, Dormontt EE, Prentis PJ, Lowe AJ, Richardson DM. 2009. Something in the way you move: dispersal pathways affect invasion success. Trends Ecol Evol. 24(3):136–144.
- Wimsatt WC. 2007. Re-Engineering Philosophy for Limited Beings. Cambridge (MA): Harvard University Press.
- Wingard GL, Bernhardt CE, Wachnicka AH. 2017. The role of paleoecology in restoration and resource management—The past as a guide to future decision-making: review and example from the Greater Everglades ecosystem, USA. Front Ecol Evol. 5:11.
- Wisz MS, Pottier J, Kissling WD, Pellissier L, Lenoir J, Damgaard CF, Dormann CF, Forchhammer MC, Grytnes J-A, Guisan A et al. 2013. The role of biotic interactions in shaping distributions and realised assemblages of species: implications for species distribution modelling. Biol Rev. 88(1):15–30.
- With KA. 2002. The landscape ecology of invasive spread. Conserv Bio. 16(5):1192–1203.
- Witmer MC, Cheke AS. 1991. The dodo and the tambalacoque tree: an obligate mutualism reconsidered. Oikos. 61(1):133–137.
- Wolkovitch EM, Cook BI, McLauchlan KK, Davies TJ. 2014. Temporal ecology in the Anthropocene. Ecol Lett. 17:1365–1379.
- Wood JR, Wilmshurst JM. 2013. Accumulation rates or percentages? How to quantify Sporormiella and other coprophilous fungal spores to detect late Quaternary megafaunal extinction events. Quat Sci Rev. 77:1–3.
- Wood JR, Wilmshurst JM. 2017. Changes in New Zealand forest plant communities following the prehistoric extinction of avian megaherbivores. J Veg Sci. 28(1):160–171.
- Wood JR, Wilmshurst JM, Turney CSM, Fogwill CJ. 2016. Palaeoecological signatures of vegetation change induced by herbivory regime shifts on subantarctic Enderby Island. Quat Sci Rev. 134:51–58.
- Wood JR, Wilmshurst JM, Worthy TH, Cooper A. 2011. Sporormiella as a proxy for non-mammalian herbivores in island ecosystems. Quat Sci Rev. 30:915–920.
- Woodbridge J, Fyfe RM, Roberts N. 2014. A comparison of remotely sensed and pollen-based approaches to mapping Europe’s land cover. J Biogeogr. 41(11):2080–2092.
- Woods KD, Davis MB. 1989. Paleoecology of range limits: beech in the Upper Peninsula of Michigan. Ecology. 70:681–696.
- Woolbright SA, Whitham TG, Gehring CA, Allan GJ, Bailey JK. 2014. Climate relicts and their associated communities as natural ecology and evolution laboratories. Trends Ecol Evol. 29(7):406–416.
- Wooller MJ, Saulnier-Talbot E, Potter BA, Belmecheri S, Bigelow N, Choy K, Cwynar LC, Davies K, Graham RW, Kurek J et al. 2018. A new terrestrial palaeoenvironmental record from the Bering Land Bridge and context for human dispersal. R Soc Open Sci. 5(6):180145.
- Worsley AT, Oldfield F. 1988. Palaeoecological studies of three lakes in the Highlands of Papua New Guinea. II. Vegetational history over the last 1600 years. J Ecol. 76:1–18.
- Worth JRP, Williamson GJ, Sakaguchi S, Nevill PG, Jordan GJ. 2014. Environmental niche modelling fails to predict Last Glacial Maximum refugia: niche shifts, microrefugia or incorrect palaeoclimate estimates? Global Ecol Biogeogr. 23(11):1186–1197.
- Wright HE. 1974. Landscape development, forest fires, and wilderness management. Science. 186(4163):487–495.
- Wright HE. 1984. Sensitivity and response time of natural systems to climatic change in the late Quaternary. Quat Sci Rev. 3:91–131.
- Wright HE, Kutzbach JE, Webb T, Ruddiman WF, Street-Perrott FA, Bartlein PJ, editors. 1993. Global climates since the last glacial maximum. Minneapolis: University of Minnesota Press.
- Wulf A. 2015. The invention of nature: the adventures of Alexander von Humboldt, the Lost Hero of Science. London: John Murray.
- Xhauflair H, Revel N, Vitales TJ, Callado JR, Tandang D, Gaillard C, Forestier H, Dizon E, Pawlik A. 2017. What plants might potentially have been used in the forests of prehistoric Southeast Asia? An insight from the resources used nowadays by local communities in the forested highlands of Palawan Island. Quatern Int. 448:169–189.
- Yasuhara M, Doi H, Wei C-L, Danovaro R, Myhre SE. 2016. Biodiversity–ecosystem functioning relationships in long-term time series and palaeoecological records: deep sea as a test bed. Philos Trans R Soc B Bio Sci. 371(1694):20150282.
- Yeung ACY, Richardson JS. 2016. Some conceptual and operational considerations when measuring ‘resilience’: a response to Hodgson et al. Trends Ecol Evol. 31(1):2–3.
- Yll E-I, Pérez-Obiol R, Juliá R. 1994. Vegetational change in the Balearic Islands (Spain) during the Holocene. Hist Biol. 9:83–89.
- Yll E-I, Perez-Obiol R, Pantaleon-Cano J, Roure JM. 1997. Palynological evidence for climatic change and human activity during the Holocene on Minorca (Balearic Islands). Quat Res. 48:339–347.
- Young AM, Higuera PE, Duffy PA, Hu FS. 2017. Climatic thresholds shape northern high-latitude fire regimes and imply vulnerability to future climate change. Ecography. 40(5):606–617.
- Young KR. 2014. Biogeography of the Anthropocene: novel species assemblages. Prog Phys Geog. 38(5):664–673.
- Zanon M, Davis BAS, Marquer L, Brewer S, Kaplan JO. 2018. European forest cover during the past 12,000 years: a palynological reconstruction based on modern analogs and remote sensing. Front Plant Sci. 9(253). 10.3389/fpls.2018.00253
- Zenni RD, Ziller SR, Pauchard A, Rodriguez-Cabal M, Nuñez MA. 2017. Invasion science in the developing world: A response to Ricciardi et al. Trends Ecol Evol. 32(11):807–808.
- Zhang Z, Leduc G, Sachs JP. 2014. El Niño evolution during the Holocene revealed by a biomarker rain gauge in the Galápagos Islands. Earth Planet Sci Lett. 404:420–434.
- Zhou JZ, Deng Y, Zhang P, Xue K, Liang Y, van Nostrand JD, Yang YF, He Z, Wu LY, Stahl DA et al. 2014. Stochasticity, succession, and environmental perturbations in a fluidic ecosystem. Proc Natl Acad Sci USA. 111(9):E836–E845.
- Zhu K, Woodall CW, Clark JS. 2012. Failure to migrate: lack of tree range expansion in response to climate change. Glob Chang Biol. 18(3):1042–1052.
- Ziegler AC. 2002. Hawaiian natural history, ecology, and evolution. Honolulu: University of Hawai’i.
- Zimmermann NE, Yoccoz NG, Edwards TC, Meier ES, Thuiller W, Guisan A, Schmatz DR, Pearman PB. 2009. Climatic extremes improve predictions of spatial patterns of tree species. Proc Natl Acad Sci USA. 106(Supplement 2):19723–19728.
- Zobel M, Davison J, Edwards ME, Brochmann C, Coissac E, Taberlet P, Willerslev E, Moora M. 2018. Ancient environmental DNA reveals shifts in dominant mutualisms during the late Quaternary. Nat Commun. 9(1):139.
