ABSTRACT
We describe a modified manual closed-chamber approach with detachable lid and vertically stackable chambers for sampling followed by simultaneous analysis of nitrous oxide (N2O) and methane (CH4) for measuring greenhouse gas flux from rice and upland cropping systems in peninsular India. A meta-analysis of leading internationally/regionally recommended approaches to monitor agricultural GHG emissions is presented to put our sampling choices (e.g., chamber design, sampling intensity, sample storage and analytical corrections) into perspective. Given our set-up, the sample retention capacity of polypropylene syringes and crimped glass vials with grey butyl-rubber septa was ∼6 hours and 10 days, respectively; and temperature correction of N2O and CH4 concentrations was essential but plant volume correction did not affect the flux rates substantially. Optimization of gas flow rates, pre-column sample retention period, oxygen venting and temperature/current were found to reduce run time from >14 to 7 min per sample and enhance sensitivity by 30–40% while improving analytical precision from 15–30% to < 2% relative standard deviation (RSD). We suggest an alternative to the linear interpolation approach of integrating the area under the N2O peak because linear interpolation can overestimate the cumulative seasonal N2O emissions by 50–100%, especially after fertilization and/or rain events.
Introduction
Current global population growth rate and consumption patterns require that agricultural production increase by ∼70% by 2050 to meet growing food demands, while adapting to a changing climate which is being significantly influenced by agricultural GHG (currently 10–12% of global emissions) [Citation1]. The majority of expected increases of agricultural production and GHG emissions in coming decades are in low- and middle-income countries, where smallholder farmers predominate [Citation2]. Crucially, the agricultural sector holds significant climate change mitigation potential through the reduction in the emission rates of major GHGs as well as enhanced sequestration of carbon in agricultural landscapes [Citation2]. Carefully selected and implemented sustainable farming practices have already been shown to lead to climate adaptation, enhanced crop yields, and decreased farm input costs [1,3 and references therein]. In addition to the direct economic benefit of sustainable farming practices at the farm level, there is a potential for smallholder farmers in developing countries to gain economic benefit from climate-smart (or low-carbon farming) practices, via quantification of reduction in GHG emission rates due to implementation of such practices at large scales and access to carbon markets [Citation4].
The IPCC provides an average default emission factor (EF) of 1% which implies that, on average, 1% of applied N is emitted as N2O from soil due to application of N in any form [Citation5]. These default EFs are estimated using a common set of activity data from many countries and do not take into account variability in soil and crop types, climate conditions and management regimes, and there is a large variation in EFs ranging from 0 to 10.8% [Citation6]. While the average IPCC EF can be utilized for generation of credits, it often results in a large amount of discounting and thus non-participation by small-scale farmers due to the lack of sufficient economic incentive. For the development of statistically robust regional EFs, which can provide better incentives, emission measurements must be conducted over multiple seasons for a particular crop in a given agro-ecological zone [Citation5]. Access to equipment and expertise necessary to accurately determine GHG emission reduction, however, is a significant challenge at small-scale farms for aggregators.
This study was conducted in partnership with the Fair Climate Network [Citation101], a pan-India coalition of NGOs that is promoting climate-smart (or low-carbon) agricultural practices across India, to meet the equipment and expertise needs of small-scale farmers. These farmers are actively adopting climate-smart farming practices across three Indian states for three different cropping systems (i.e., groundnut, rice and millet) in semi-arid peninsular India. In each of the study sites, we established a laboratory to monitor N2O and CH4 emissions within a 5–30 km radius of corresponding small-scale (< 1 acre) experimental farms that represented various baseline (i.e., mainstream) and alternate (or low-carbon) farming practices for specific crops in those regions.
Along with the sampling guidelines that are based on our experiments, we present a meta-analysis of six leading internationally and regionally recommended approaches to monitor GHG emissions from cropland soils to put our study in perspective. Our study does not replace but rather complements these existing recommendations, especially by focusing on sampling (e.g., transparent vs opaque chambers, stacked chambers for “in-row” placement, detachable lids, dead volume in chamber sampling lines) and data-processing issues (e.g., problematic integration of daily fluxes, relative importance of temperature and crop volume correction) that have not been discussed earlier. We also present in detail a pathway to enhance a gas chromatograph (GC)'s precision, which has direct influence on the minimum detection limit (MDL) of the whole methodology [Citation7]. The conceptual approach underlying GC optimization (e.g., oxygen venting/bypass, baseline stabilization, moisture backflush and adequate separation of CO2 from N2O) is systematically presented here, and it will orient new research groups and/or GC manufacturers, especially in the developing regions of the world, to precisely measure GHG emission fluxes.
Manual closed chamber-based air sampling, followed by analysis of N2O and CH4 in GCs with electron capture (ECD) and flame ionization (FID) detectors, is one of the most widely used and cost-effective methods to measure direct soil non-CO2 GHG emissions [Citation8–11]. This method has seen several modifications and is constantly being adapted by research groups for performing measurements on new soil/cropping systems [Citation11–13]. The Thermo Fisher Trace GC 600, as installed at our four labs, had poor precision of 15–30% relative standard deviation (RSD), poor sensitivity and a long run time of over 14 min per sample. In our experience, vendors of most GCs do not specifically certify their product for precise measurement of field samples which contain high percentages of oxygen and moisture, and it is not appropriate to use such GCs as supplied without ensuring <2–3% precision for both N2O and CH4. Also, some groups are trying to configure GCs that were originally acquired for other purposes, for example by adding a detector or new sample introduction line to be able to analyze GHGs, and need to optimize many parameters before the desired precision can be obtained. Here, we present GC optimization experiments conducted to achieve high precision (2–3% RSD), better sensitivity (30–40% increase) and a shorter run time (7 min per sample) to ensure that all field samples are processed precisely and within hours of sample collection.
Materials and method
Study sites
The studies were conducted across four experimental field sites located in three states of peninsular India. Each field site comprised of several small-scale farms (0.5–2.0 acres) used as experimental plots, and a GHG measurement laboratory. At each farm and for each crop, randomized block design was adopted to allocate replicate treatments to small replicate plots (200 to 400 m2) on participating farmers' land. Replicate plots were isolated from each other with 2–3-foot-high levees.
Gas chromatograph
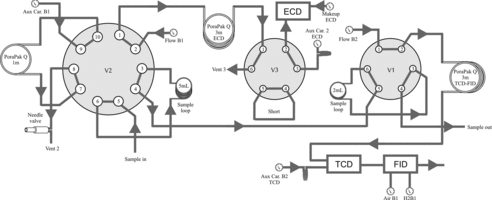
Trace GC 600 (Thermo-Fisher Scientific, USA) is a dual-channel, packed-column GC with ECD, FID and thermal conductivity detectors (TCD; see and Supplementary material, section 1 for GC description).
Figure 1. Schematic representation of a Thermo Fisher Trace 600 gas chromatograph. ECD: Electron dapture detector; FID: Flame ionization detector; TCD: Thermal conductivity detector; Aux Car.: Auxiliary carrier flow; V1, V2 and V3: two-position switching valves; B1, B2: Main carrier (nitrogen) flow identifiers; H2B1: Hydrogen flow; Air B1: Air flow.
When a sample was stored in the polypropylene syringe, the syringe was directly connected to the sample inlet using silicone tubing. However, when the samples were stored in pressurized vials, vials were connected to silicon tubing via a needle that immediately released the “excess” (pressurized) volume of gas in the vial into the GC inlet. The total volume of the sampling line was usually < 15 mL, and we purged ∼25 mL of sample into the sampling line to ensure that the sampling loops were completely filled with the sample.
Meta-analysis of existing sampling, storage and analysis recommendations
We compared the design features of all prominent internationally or regionally recommended guidelines, including those by the International Global Research Alliance (GRA), the United Nations-approved Clean Development Mechanism (CDM), the United States Department of Agriculture-approved GRACEnet, the Centre for International Forestry Research's (CIFOR) Carbon Benefit Project (CBP) and the Indian government's Indian Agricultural Research Institute (IARI), for collection and storage of samples, and analytical analysis [Citation12,Citation14–16]. We also included in our comparison a review by Rochette and Eriksen-Hamel [Citation17] that documents the wide variability in the design of chambers and sampling strategies, and ranks them on the basis of their reliability. The other published manuals (e.g., [Citation18]) are based, for the most part, on GRACEnet protocol [Citation7] and are not separately compared in this study (see and ).
Table 1. Comparison of GHG sampling chamber design and deployment recommendations.
Table 2. Comparison of sampling, storage, data processing and analytical recommendations.
Gas chromatograph optimization experiments
ECD channel settings (i.e., carrier gas flow rate, pre-column sample retention period, O2 venting period, detector temperature and current, and make-up gas flow rates) were optimized to achieve the highest possible sensitivity for N2O; efficiently retain moisture in the pre-column and later back-flush it; separate CO2 and N2O components in the samples; and vent O2 (also referred to as bypass O2). For FID, we optimized H2 and zero-air flow rates for better CH4 area response.
Flux rate calculation
Concentration (in ppm) at each time point was converted to a mass equivalent (in μg) using the ideal gas equation and correcting for temperature, chamber volume and pressure [Citation7,Citation10,Citation14] (See Supplementary material, section 2 for the equation).
The flux rates measured over the crop period were plotted against time (days after sowing or transplantation) to arrive at the cumulative seasonal emission. The linearity of increase in temperature-corrected concentration of GHGs over time was carefully monitored, and slopes with coefficient of determination (R2) values of less than 0.85 but above MDL (see below) [Citation11] were not included in cumulative seasonal emission calculations. The area under such plots was calculated by linear interpolation (i.e., by adding the areas of trapezoids formed by the daily flux rates). We compared the linear interpolation approach of calculating area under the curve with an “exponential peak fitting” approach, especially when no reliable measurements were available to mark either the rise or decline of an N2O peak in a plot after a known crucial event such as fertilization or rainfall. Whole-season emissions of N2O and CH4 were converted to CO2 equivalents using their 100-year global warming potentials of 298 and 34, respectively [Citation19].
Guidelines for field sampling and sample storage
To design an effective sampling approach for our tropical sites, we compared six leading international and regional GHG emission measurement guidelines ( and ). We found that none of the existing guidelines completely addressed the needs of tropical farms in India. For example, the GRA [Citation14] recommendations were found to be the most exhaustive, but they were not suitable for the “in-row” chamber deployment necessary for rice farms. The USDA's GRACEnet protocol did not address the problems associated with linear interpolation as well as venting [Citation7]. Here, we describe in detail our approach to the designs of chambers and base-frames, along with the effect of headspace temperature and chamber headspace volume corrections on measured flux rates.
Modified closed chamber design
In general, chambers should be deployed on anchors (also referred to as base-frames) to demarcate the sampling area [Citation7]. This approach based on base-frames is known to be less disturbing to soil biogeochemistry than non-anchored chamber designs, where soil can be disturbed as chambers are put in place and removed after each measurement [Citation14].
A water trough in the base-frame received the bottom of the chamber, and the base-frames remained in the soil throughout the cropping season. Base-frames were installed, with anchors pushed 5–8 cm deep into the soil depending on the hardness of the soils, >24 hours before the sampling. The sample on the day of sowing had to be collected only after 8–12 hours of base-frame installation because of the long time required for sowing in small-scale farms. Base-frames were removed only during events such as manure, fertilizer or pesticide applications, ploughing, intercultivation and weeding, and then re-installed in the same location immediately after the event. Usually, the complete depth of the anchors of the base-frames was pushed inside the soil and only the water trough remained above the soil level.
Different base-frame designs are appropriate for different crops. We prepared two base-frame designs (and corresponding chambers; see the section on “Lid design and access bridges”) of different footprints (area) and heights. Base-frames were fabricated in 1-mm mild steel (which is tough, contains carbon and is cheaper than other forms of steel), electroplated with zinc and treated with corrosion-resistant paints. A trough (5 cm wide and 5 cm deep) situated on top of the base-frame anchor received the bottom of the Perspex chamber.
Design 1 (see Supplementary material, section 3a) was used for sampling upland crops (groundnut and finger millet) and paddy nurseries; it demarcated an area of 0.09 m2 (30 × 30 cm) and had 5-cm-deep anchors. Design 2, with a larger basal area of 0.25 m2 (50 × 50 cm) and 8-cm anchors (12-cm corner legs), was used for sampling at rice paddies. GHG emission, especially CH4 from rice paddies, can occur in three ways: through diffusion from soil, bubble formation (ebullition) and through the plant/tillers [Citation21]. To capture emissions from the plants, it is necessary to place the chambers over the tillers “in rows” and not just “between rows,” and to ensure that the photosynthetic and other physiological activities remain normal during the sampling period [Citation7]. Therefore, Design 2, which has a larger footprint, was considered to be appropriate for rice cropping systems as it could cover 5–8 hills (bunches of rice tillers emerging together) as compared to Design 1, which could only cover 2–3 hills.
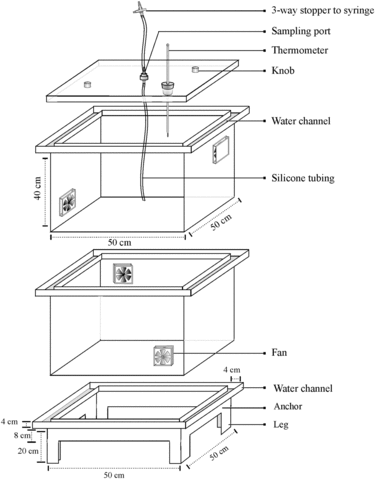
Our closed chambers were fabricated in 3-mm Perspex (plexiglass) which is inert to N2O [Citation7]. Each chamber was 40 cm tall and provided with a 2-cm-deep water trough on the top to receive either a detachable lid (see below) or another chamber's base.
In spite of better performance of opaque chambers with respect to change in temperature (See Supplementary material, section 4), we chose transparent chambers over opaque or reflective chambers so that rice tillers release GHGs under relatively normal physiological conditions. The chamber deployment period was limited to 30 min, which matches up with the recommendations of various other studies [Citation7,Citation17].
Since the ambient temperature in our study areas could get as high as 40°C and we could not use opaque/reflective chambers, we needed to ensure that the temperature increase over the chamber deployment period stayed within a range that can be tolerated by the plants, and did not create gradient of GHG concentration within the chamber's headspace. Rochette and Eriksen-Hamel [Citation17] note that to both ensure proper mixing of the chamber contents and minimize micro-climate effects, chamber height/deployment time should be ≥ 40 cm/hour. In order to be able to keep the chambers “in row” above the rice plant's height, we settled for an 80-cm height for a 30-min deployment. In order to achieve uniform mixing of the air before and during sampling, and to keep the headspace cooler, two 12-V (2500 rpm) fans (size 92 × 92 mm) were fitted on two opposite sidewalls [Citation22] (); one 8 cm from the top rim and the other 8 cm from the bottom rim (see also Supplementary material, section 3b, for dryland chamber assembly and sampling port design). We found that the GRACEnet recommendation to not use a fan () because fans cause pressure fluctuations was not helpful/relevant, because tropical higher temperatures increased headspace stratification.
For groundnut grown as a rainfed upland crop, the height of one chamber (40 cm) was sufficient to accommodate the crop almost throughout the cropping period. However, after 50 to 70 days of finger millet or rice growth, it was necessary to vertically stack two chambers to keep the plant from being bent (or getting distorted) during sampling. For fallow period sampling, one 40-cm-high chamber was used to keep the headspace volume low.
Lid design and access bridges
It has been recommended that the first sample (T = 0 min) should be given special attention [Citation14]. Ideally, the samples collected at time zero should have ambient air concentrations of N2O and CH4. In practice, we found that without proper mixing of the headspace air and prompt sampling after isolation of the headspace air from ambient air, the chambers accumulated GHGs (especially CH4 for rice), making the time-zero samples significantly above the corresponding ambient concentrations. To reduce such errors, we deployed and recommend two strategies: the use of detachable lids and access bridges.
Our detachable lids for manual chambers had the same footprint (length × breadth) as the Perspex chamber (or base-frame) footprint. A 12-mm-diameter hole in the center of the lid was used to fix a sampling port (Supplementary material, section 3b); another 18-mm hole was used to hold a rubber cork with a thermometer to measure headspace temperature. As opposed to existing recommendations (), the sampling port was lathe-fabricated with Perspex to minimize leaks that occur when rubber/silicone ports are used. The sampling port on the lid was connected to silicone tubing on both ends. The length of tubing inside the chamber was always half the total height of the Perspex chamber, to ensure that it reached the center of the chamber. The tubing outside the chamber was 40–50 cm long, long enough to connect with a Luer lock three-way stopper, which received a 50-mL gas-tight syringe during sampling. On each sampling day, chambers appropriate for the crop height/type were set up on the base-frames and sealed 10-15 min before the beginning of actual sampling. During this period of 10–15 min, fans allowed gentle mixing of the headspace with the ambient air. Then, the water channel on the top of the chamber (i.e., the trough that receives lids) was filled with water (150 mL for 30 × 30 cm and 250 mL for 50 × 50 cm chamber designs) to seal the gap between the lid and the chamber. The initial (or time zero) headspace sample was collected immediately after closing the lid.
In order to avoid disturbance of the redox conditions in the soil, and ebullience of methane bubbles, ladder-type “access bridges” made of wooden poles/planks were set up in paddy fields which enabled reaching the sampling points (which were up to 4 m away from bunds) without stepping into the flooded paddy plot. The bridges were set up on the day of transplantation, with one end of the bridge resting on the bund/levee and the other end just above the individual base-frame. The bridges were removed after harvest.
Sampling protocol
We used gas-tight syringes fitted with three-way Luer-lock stoppers to draw headspace samples. Samples were either stored in these gas-tight syringes or, as recommended [Citation22], transferred to glass vials sealed with grey butyl rubber septa (see the section on “Sample retention capacity of gas-tight syringes and vials,” below). Before withdrawing a (∼50 mL) homogenized headspace sample for storage and analysis, we recommend that the “dead-space” air in the extension tubing (3–5 mL depending on the length and volume of the tubing; see ), which can not homogenize with the chamber headspace, be withdrawn and purged out of the three-way stopper's side arm. We further withdrew samples at 10, 20 and 30 min after chamber deployment. When staff availability was less than the total number of deployed chambers, we utilized a systematic “time staggered” sampling approach such that replicate chambers were sampled within a gap of 1–2 min and exact time of sampling was recorded. We recorded headspace temperature and soil temperature changes over the deployment period. Samples, especially those stored in syringes, were transported to laboratory for analysis within 1.5 hours.
We recommend that the water level in the base-frame and the water channel that seals the chamber to the base-frame be noted on the sampling day for each channel, to calculate headspace volume (see Supplementary material, section 5). When chambers are placed “in rows,” crop volume should be estimated by the water displacement method (Supplementary material, section 6), and the volume of plant in each chamber should be subtracted from the headspace volume to correctly estimate the headspace volume.
Sample retention capacity of gas tight syringes and vials
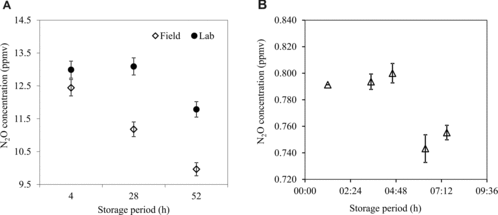
Various international and regional guidelines provide different recommendations with respect to sample storage (see ), and these differences reflect varying levels of confidence in the ability of polypropylene gas-tight syringes to store nitrous oxide (from a few hours [Citation22] to up to 48 hours [Citation7]). We tested the ability of polypropylene syringes to retain N2O over the course of a long (52 hours) and a short (8 hours) experiment. About 20 syringes were filled with an N2O standard (∼12.75 ppmv) at one of our field sampling sites and transported to the laboratory for analysis. Five replicate samples were analyzed at regular intervals of time over 52 and 8 hours in two separate experiments. The results of the longer duration experiment (A) implied that the syringes were leaking N2O continuously. The shorter duration experiment (B) with a lower concentration of N2O standard (∼0.8 ppmv) showed that the samples were stable for up to 4 hours but lost 4–6.5% of the N2O by 6 hours. We note that when gas samples were collected and also stored in the laboratory, without any temperature or other changes that are involved with bringing the samples from the field to the lab, the syringes could retain N2O for about 24 hours (A).
Sample retention capacity of glass vials with grey butyl rubber septa
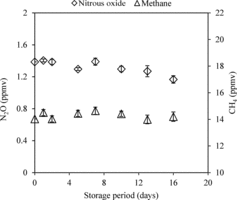
To store the samples when samples could not be analyzed on the same day (i.e., within 6 hours; see above), we used pre-evacuated glass vials (30 mL) with thick grey butyl rubber septa sealed with aluminum crimps. Vials were evacuated under laboratory conditions > 30 times their volume on a vacuum manifold (see Supplementary material, section 7) a few hours before being transported to the field for sample collection. We found that these pre-evacuated vials could hold vacuum for at least 5 hours. Vials were filled to 2–3 times their volume with samples or standard gases in the field. To test the sample retention period, pre-evacuated vials were filled with a known volume and concentration of a standard gas mixture. Over the following 14-day period, four replicate vials were periodically analyzed for the retrievable sample volume and concentrations of CH4 and N2O. We found that the vials could reproduce the starting N2O and CH4 concentrations for at least 10 days (). As indicated by previous studies [Citation7,Citation17], septa made of natural rubber could not retain samples for more than a day, and butyl rubber septa from different vendors have different sample retention capacity (data not shown). Once again, our study highlights the need to test the sample retention capability of any new set-up (syringes or vials) before implementing it on a large scale.
Optimization of analytical methodology
The following sections present our results of the experiments conducted to optimize the GC performance. Several published studies [Citation23–25] provide general guidelines for enhancing ECD performance, but we found detailed discussion of the need for, principles of and process of optimization of N2O analysis lacking. The effects of different variables on the area response to ambient concentrations of N2O and CH4, signal noise, analytical precision and chromatogram properties are discussed below. The sections below describe the experiments conducted to optimize the ECD channel, and are followed by a discussion on optimization of the FID channel. We note that parts of our approach and conclusions with respect to analytical optimization might require the involvement of GC manufacturers' technical teams, especially for automated and pre-configured GC systems where the user does not have an easy pathway to adjust flow rates and/or timing of events.
Main carrier gas flow rate
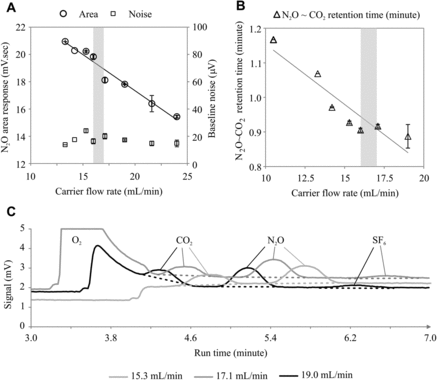
At each lab location, we tested the effect of main carrier gas flow rates ranging from 13 to 24 mL min–1 to identify optimum flow rate with respect to N2O area response and signal noise (A), and the separation of O2, CO2 and N2O components in the ECD channel (B). The difference between the retention times of CO2 and N2O was used as an indicator of component separation, with an increase in this difference indicating improved separation of CO2 and N2O. Below a flow rate of ∼13 mL min–1, the area response was poor (data not shown), probably because of insufficient carrier gas flow through the column. As the flow rates were increased, we found that the carrier gas flow rate was inversely related to the N2O area response (A) as expected for a concentration-based detector [Citation26,Citation27]. Noise levels remained relatively constant and did not follow any particular trend with changes in carrier gas flow rate (A). At most of our labs, a flow rate between 16 and 17 mL min–1 was found to be optimum for the GCs based on a reasonable N2O area response, a clear separation of CO2 from N2O, and the detection of a small peak which is likely to be a halogenated trace gas (e.g., SF6) [29] indicating complete elution (and detection) of sample components (C). We recommend choosing a flow rate of carrier gas which is high enough to purge out all of the gaseous components in the sample within a short period of time, but also low enough to get the components clearly separated in the column.
Figure 5. Effect of main carrier gas flow rate on performance of GC. Primary ordinate represents Electron Capture Detector's N2O area response and secondary ordinate is signal noise (A); function of retention time difference of N2O and CO2 peak to carrier gas flow rate. Gray vertical bands in both plots show the range of optimum flow rate (B). The overlaid chromatograms show the effect of three different carrier gas flow rates on peak separation and peak shapes (C). Error bars represent ± 1 SE.
Moisture retention and oxygen venting system
The exposure to ambient air components such as H2O, O2 and CO2 reduces the column life or the detector's response to N2O [Citation26,Citation29–31]. Analysis of a large number of field samples having 21% (volume/volume) oxygen and also high moisture content tends to saturate the detector (i.e., the sensitivity of the ECD to N2O declines). When the decline in sensitivity or precision cannot be corrected by regular (daily or weekly) conditioning, such saturation is likely due to the exposure of the ECD to moisture and oxygen because of sub-optimal oxygen venting and moisture back-flush mechanisms. Whenever possible, after setting the optimum main carrier gas flow rates, we recommend optimizing the GC settings to minimize the exposure of the main column and/or ECD to O2, CO2 and moisture in the samples.
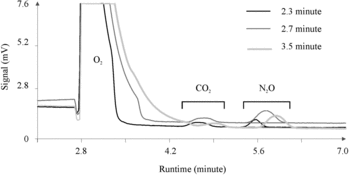
In the analytical sequence configured in our GCs (see Supplementary material, section 1, for the four stages of the analytical events described in this section), the sample gets purged from the sample loop into a 1-m pre-column at ∼0.34 min. The pre-column retention period (Stage 2; Supplementary material, section 1) is the time between the start of this purge and the beginning of the reversal of flow through the pre-column to back-flush H2O retained in the pre-column. The separation of N2O from other sample components is further completed in the 3-m main column (Stage 3; Supplementary material, section 1). The pre-column retention period, therefore, should be long enough to allow all sample components except moisture to be transferred to the main column. We found that if the pre-column retention period was too short (e.g., 2.3 min in ), the N2O peak areas were sub-optimal owing to incomplete elution of sample components from the pre-column, and the small peak (likely to be a halogenated trace gas like SF6) was never detected. We found that a pre-column retention period of 2.5–3.0 min was optimum for the columns and carrier gas flow rates in our GCs (e.g., 2.7 min in ). If this period was too long (e.g., 3.5 min), the water vapor entered the main column and interfered with the separation and detection of N2O. At a long pre-column retention period (3.5 min), component separation was inefficient and CO2 and N2O peaks appeared on a long O2 tail (see also C, 19 mL min–1).
Figure 6. Effect of pre-column retention period on component separation without complete venting of oxygen and CO2.
In our systems, the end of the third configuration coincided with the end of O2 venting period (Stage 3; Supplementary material, section 1). The moisture back-flush continues till the end of analytical events (∼7 min; Stage 4; Supplementary material, section 1). We found that venting of O2 (also called O2 bypass) increased the ECD response to N2O drastically, by about 40% (from 18 mV.sec without O2 venting to about 26 mV.sec after venting) and also led to a better precision of <5–7% RSD as compared to 15–30% before venting. We found that baseline signals were relatively flat for O2-vented chromatograms compared to the baseline signals for partly O2-vented chromatograms (data not shown). O2 venting also improved the performance of integration software with respect to N2O peaks (and led to minimization of the bias involved in manual integration). Due to the optimization of the length of the pre-column retention period and the O2 venting period, the detection period (i.e., the period when main column flow is in line with ECD: fourth configuration, Supplementary material, section 1) was automatically optimized such that CO2 was mostly vented out and the detector was primarily exposed to the analyte of interest (i.e., N2O). Long-term analysis of sample components without completely venting O2 resulted in a worsening of ECD performance, apparently because the ECD became saturated and required replacement.
Auxiliary carrier gas flow rate
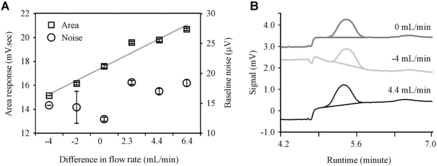
In our GCs, the auxiliary carrier line maintains the flow of N2 through the ECD until the flow of the main carrier gas is diverted into ECD for N2O detection (i.e., the beginning of Stage 4, Supplementary material, section 1). We found that if the flow rates of the auxiliary N2 and main carrier N2 lines were not similar, the ECD's performance and sensitivity were negatively affected (). A lower auxiliary carrier gas flow rate led to a lower area response and the baseline signal showed a negative drift, whereas a higher auxiliary carrier gas flow rate showed positive signal drift and area response (A). When auxiliary N2 and main carrier N2 flow rates were the same, signal baselines were flat and the N2O peak was symmetric (B). It was also observed that the integration software was very poor in handling problems with integrating peaks on drifting baselines compared to peaks on flat baselines. This imbalance did not have a systematic impact on baseline noise and/or retention times (A). The effect of the flow rate of make-up gas, and the temperature and current of the ECD, are discussed in the Supplementary material, section 8.
Figure 7. Effect of difference between main carrier and auxiliary carrier gas flow rates on GC performance. Difference in flow rate equals auxiliary carrier gas flow rate minus main carrier gas flow rate. Effect of varying differences in flow rates on area response and noise (7A) and baseline and peak shapes (7B) are shown here.
H2 and air flow rates for FID
FID is a mass flow sensitive detector [Citation26]. Though there are no direct studies reporting the effect of H2 or O2 flow rate on FID response for CH4 analysis, one study [Citation32] reported an increase in the response of the detector to an increase in oxyhydrogen (mixture of oxygen and hydrogen) flow.
We studied the effect of H2 and zero-air flow rates on CH4 area response in the FID in two separate experiments, while keeping the main carrier N2 flow rate at the optimum rate identified for the ECD channel (see the section on “Main carrier gas flow rate,” above). The main carrier gas flow rate was also found to affect the CH4 area response (data not shown), but since both ECD and FID channels had one common pressure controller for the main carrier gas flow, we optimized the main carrier rates for ECD performance.
First, the H2 flow rate was varied from 30 to 45 mL min–1, keeping N2 and zero air flow rates constant at 14.3 and 117 mL min–1, respectively. To identify the optimum H2 flow rate for a range of CH4 concentration expected in the samples, standard gas mixtures (with 1.527 ppmv and 14.941 ppmv CH4 concentration certified for 2% analytical precision) were used. In addition to these two standard concentrations, the effect of H2 flow rates on the ambient concentration of CH4 was also tested. Different GCs had different responses to changing flow rates.
In general, a sustained FID flame could not be maintained at H2 flow rates lower than ∼30 mL min–1, and for all three samples, an increase in H2 flow rate led to a decrease in the CH4 area response (A). In general, we set the H2 flow rate at a slightly higher value than 31 mL min–1.
Figure 8. CH4 area response in FID as a function of H2 and zero air flow rates with main carrier gas flow constant at 14.3 mL min−1. Zero air was kept constant at 117 mL min−1 for H2 flow rate optimization (A), and H2 flow rate was set to 31.2 mL min−1 during zero air flow rate optimization experiments (B).
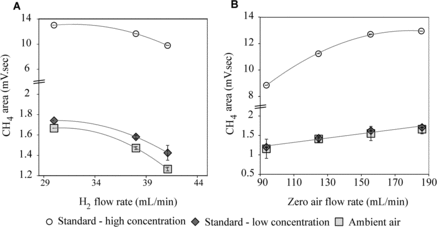
After optimization of the H2 flow rate, zero-air flow rate was optimized for each instrument. In the example shown in B, zero-air flow rates were varied from 70 to 115 mL min−1, with constant carrier gas and hydrogen gas flow rates of 16.3 mL min−1 and 31.2 mL min−1, respectively. An increase in zero-air flow rate was found to increase the CH4 area response (B). The highest recorded response was ∼100–110 mL min−1 for both ambient and high concentration standards. The detector flame went off beyond 110 mL min−1.
Factors affecting accurate seasonal flux calculation
A systematic record of GHG analysis related variables (see Supplementary material, section 9, for a spreadsheet used to record parameters that could have affected GHG flux calculation) shows that it is crucial to monitor and improve some parameters that have a significant effect on the eventual estimation of the GHG mitigation potential of small-scale low-carbon farming projects [see also 14, table 3.2].
Daily precision of gas chromatograph and range of standards
At an instrumental precision of 2% RSD, a constant temperature of 25°C and a box volume of 100 L, MDL was about 18 µg N2O h−1 m−2 and 37 µg CH4 h−1 m−2 [Citation7,Citation11]. Depending on the exact fluctuation in temperature during deployment, every 1% increase in RSD resulted in an increase in N2O MDL of 9–12 µg h−1 m−2. Thus, the precision of the GC should not increase beyond 2–3%, because poorer precision compromises the ability to measure small changes in GHG concentration with time. None of the existing recommendations () have highlighted the importance of ensuring <2% RSD before starting GHG measurements, likely because most well-established labs have much better RSDs. As noted earlier [Citation14], accuracy and appropriate ranges of GHG standards are very important. Several labs in developing countries employ only one GHG standard for GC calibration, and this is not an acceptable option in our opinion.
Temperature and volume corrections
In arid and semi-arid parts of the world, where maximum daily temperatures can be as high as 45°C and chamber temperatures routinely increase by up to 10°C over the course of a half-hour sampling period, we highly recommend applying a temperature correction to calculate the chamber's headspace volume at any given time point and, therefore, hourly flux rates (see supplementary section 2). At an instrumental precision of 1% RSD and at emission rates close to MDL levels, a 1°C increase in chamber temperature at time 30 min versus 0 min caused ∼10–15% underestimation in the measured N2O flux rate. Under a similar scenario, a 5°C increase underestimated the flux rate by up to 70%.
When chambers are placed “in rows,” it is essential to rule out the effect of plant volume on effective headspace volume. We found that given the ratio of volumes (maximum possible crop volume/the chamber volume) used in our study, the crop volume correction did not change the flux rates substantially, and this effect was always less than 2–2.5% of the GHG flux rate (see Supplementary material section 5 for headspace volume calculation, and Supplementary material section 6 for crop volume estimation).
Sampling intensity, spatio-temporal variation and estimation of seasonal emissions
In our study, as is usual for manual chamber studies, the hourly flux rates measured during a 30–45-min sampling period were assumed to be the mean flux for the entire day. Wherever possible, this assumption should be evaluated in the future, through continuous deployment of automated chambers that can help measure the diurnal variation of fluxes. We note that UNFCCC's CDM methodology [Citation102] would clearly not estimate the seasonal flux correctly, because it assumes the flux measured during a single hour to be constant not just for 24 hours but throughout the “gap period.” We also recommend discussions offered by the GRA and GRACEnet protocols on pros and cons of Q10 corrections [Citation7,Citation14]. In order to capture spatial variation in fluxes, we recommend at least three replicates per treatment per soil type. In the absence of replicate management areas, the GRACEnet recommends the use of at least two chambers per treatment [Citation7].
In addition to the regular once per week sample collection, we recommend intensive sampling around events such as sowing, transplantation, weeding, manure, fertilizer and pesticide application, irrigation and drainage, in line with the recommendations of GRA [Citation14]. Whenever possible, sampling should be conducted 1 day before any of the above events, and after such events, sampling should be conducted for 3–4 consecutive days until N2O, or CH4 in case of rice paddy emissions, recedes to background levels (20–30 µg h−1 m−2 N2O-N and 40 µg CH4 h−1 m−2). Given these recommendations, we found that for upland crops, sampling was necessary on at least 40% of days in a cropping period to capture the dynamicity of the N2O fluxes. For rice paddies, sampling intensity needed to be higher (up to 60–80% of the 90–100 days of a typical rice-growth period after transplantation) because the temporal variability of both CH4 and N2O fluxes needs to be captured. Some studies [e.g., 34], have suggested weekly sampling to be rigorous enough for rice paddies, but for landscapes where water level fluctuations are high (e.g., because of alternate wetting and drying regimes or very sandy soils), weekly sampling will not capture the temporal variation in the flux of both CH4 and N2O. Based on our experience in 2011–2012 across four regions in India, we note that improving sampling intensity and analysis frequency from 40 to 80 days in a cropping season increases the annual recurring cost only by 10–15%. The one-time capital investment needed to set up a GHG measurement is US$ 38,000 ± 5000, with an annual recurring cost of ∼US$ 16,500 ± 1500 (see Supplementary material, sections 10a and 10b).
During the periods when sampling frequency is high and data from consecutive days are available without a large “gap,” and/or when the hourly/daily emission rates do not include any N2O or CH4 emission peaks, linear interpolation (i.e., trapezoidal method) for integrating the area under curve is appropriate. However, when sampling frequency is lower, linear interpolation can result in both substantial over- and under-estimation of cumulative seasonal GHG emission (especially for N2O, which exhibits much higher temporal variation than CH4). The underestimation occurs when the spikes in N2O, which usually occur following fertilization and/or rainfall or drainage, are not captured at all by the field sampling. The overestimation, which is more common, occurs when either the rise or the decline of the N2O peak is not fully captured by the field data [Citation14].
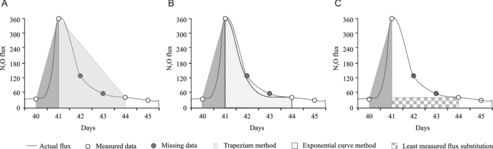
N2O emissions generally exhibit peaking behavior, and the peak flux decay is usually exponential [Citation34,Citation35], which has already led to concern over the use of the linear interpolation/trapezium method [Citation36]. Even when the sampling frequency is adequate in general (>40% of the crop growth days), it is possible that no (reliable) samples are available at a few critical times (e.g., right before or after an N2O emission peak). To deal with such rare cases, therefore, we considered the following strategy: when the decline/decay of an N2O emission peak with a height beyond 10 times the MDL (> 200 μg h−1 m−2) was not captured by field measurements, the spikes were decayed to MDL levels (or the available measured data) by adopting a best-fit exponential equation for each spike. When possible, the number of days needed for an emission spike to “come down” to MDL levels was derived from other measured peaks for the same crop and replicate treatment. While this strategy is far from perfect, and is subjective, we think it might be more reasonable than linear interpolation for N2O peaks. In general, we found that linear interpolation (A) overestimated the flux by 50–100% as compared to exponential decay of the peak value and a “least possible emission approach” where a constant value, which was equal to the least measured flux rate immediately before or after the gap period, was presumed for all days in the “gap period.” The extent of overestimation depended on the length of the gap period and the height of the peak. Of course, the “least possible emission” approach underestimated the emissions (see C) as compared to the “exponential curve method.”
Figure 9. Effect of integration approaches on cumulative emissions. Hypothetical N2O flux over 40–45 days after sowing of a hypothetical crop where no samples were available for analysis on two crucial “gap” days (42 and 43) integration using (A) trapezoid and (B) exponential curve fit method and (C) “least measured flux” substitution approaches.
Conclusions
In conclusion, we have offered detailed considerations for the design of sampling chambers suitable for measuring GHG fluxes from upland rainfed crops and rice paddies on tropical semi-arid smallholder farms. We have also provided guidelines for optimizing N2O and CH4 analysis to be able to precisely process a large number of field samples every day. Detailed infrastructural and financial requirements for setting up a lab (Supplementary material, section 10), general templates for recording lab safety (e.g., cylinder pressure(s) and leak-check) information, GC conditioning and baseline data (Supplementary material, sections 1 and 11) and systematic recommendations and flux calculation sheets (Supplementary material, section 9 and 12) provided in the paper will be able to assist new research/NGO groups in establishing labs in other parts of the world to record field and lab data necessary for safely and reliably calculating GHG emission fluxes, and/or to quantify the GHG mitigation potential of alternative farming practices.
Supplementary Material
Download MS Word (1.3 MB)Field sampling log sheet for paddy
Download PDF (47.4 KB)Lab Records 1-7
Download MS Word (129.5 KB)GHG flux calculation sheet with example data
Download MS Excel (245.5 KB)GHG flux calculation sheet with example data
Download PDF (183.8 KB)Acknowledgements
This work would have been impossible without the constant cooperation of small-scale farmers in conducting field sampling on their farms for several years. We would also like to thank Drs. Steven Hamburg, V.R. Ramakrishna Parama, Indu K. Murthy, S. Padmanabha and N.H. Ravindranath for their critical comments, and the directors of the participating NGOs (Dr. M. Reddy, Accion Fraterna Ecology Centre, Andhra Pradesh), M. Philomena (Social Animation Centre for Rural Education and Development, Karnataka), D. Anandaraj (Palmyrah Workers Development Society, Tamil Nadu) and D. Athiyaman (Director, Bharath Environment Seva Team, Tamil Nadu) for their practical support and advice on the ground. This work was supported by EDF and ICCO Cooperation.
Supplemental data for this article as well as a Spanish language translation can be accessed here: http://dx.doi.org/10.1080/17583004.2015.1082233.
References
- Porter JR, Xie L, Challinor A et al. Food security and food production systems. In: Climate Change 2014: Impacts, Adaptation, and Vulnerability. Part A: Global and Sectoral Aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, Field, CB, VR Barros, DJ Dokken, KJ Mach, MD Mastrandrea, TE Bilir, M Chatterjee, KL Ebi, YO Estrada, RC Genova, B Girma, ES Kissel, AN Levy, S MacCracken, PR Mastrandrea, and LL White (eds.). IPCC, 1–82 (2014). Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA, pp. 485–533.
- Smith P, Bustamante M, Ahammad H et al. Agriculture, forestry and other land use (AFOLU). In: Working Group III – Mitigation of Climate Change. IPCC. In: Contribution of Working Group III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change [Edenhofer, O., R. Pichs-Madruga, Y. Sokona, E. Farahani, S. Kadner, K. Seyboth, A. Adler, I. Baum, S. Brunner, P. Eickemeier, B. Kriemann, J. Savolainen, S. Schlömer, C. von Stechow, T. Zwickel and J.C. Minx (eds.)]. Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA Link to the report: http://www.ipcc.ch/pdf/assessment-report/ar5/wg3/ipcc_wg3_ar5_chapter11.pdf
- Tilman D, Balzer C, Hill J, Befort BL. Global food demand and the sustainable intensification of agriculture. Proc. Nat. Acad. Sci. USA 108(50), 20260–20264 (2011).
- Milne E, Neufeldt H, Rosenstock T et al. Methods for the quantification of GHG emissions at the landscape level for developing countries in smallholder contexts. Environ. Res. Lett. 8(1), 1–6 (2013).
- De Klein C, Novoa RSA, Ogle S et al. N2O emissions from managed soils, and CO2 emissions from lime and urea application. In: 2006 IPCC Guidelines for National Greenhouse Gas Inventories. IPCC, 1–54 (2006). Report Editors: Simon Eggleston, Leandro Buendia, Kyoko Miwa, Todd Ngara and Kiyoto Tanabe. Institute for Global Environmental Strategies 2108–11, Kamiyamaguchi, Hayama, Kanagawa, Japan, 240–0115.
- Stehfest E, Bouwman L. N2O and NO emission from agricultural fields and soils under natural vegetation: summarizing available measurement data and modeling of global annual emissions. Nutr. Cycl. Agroecosyst. 74(3), 207–228 (2006).
- Parkin TB, Venterea RT. Chamber-based trace gas flux measurements. In: USDA–ARS GRACEnet Project Protocols. 1–39 (2010). Editor: R.F. Follett, http://www.ars.usda.gov/SP2UserFiles/Program/212/Chapter%203.%20GRACEnet%20Trace%20Gas%20Sampling%20Protocols.pdf.
- Hutchinson GL, Livingston GP, Healy RW, Striegl RG. Chamber measurement of surface-atmosphere trace gas exchange: numerical evaluation of dependence on soil, interfacial layer, and source/sink properties for impermeable atmospheric mixing processes. J. Geophys. Res. 105, 8865–8875 (2000).
- Matthias AD, Blackmer AM, Bremner JM. A simple chamber technique for field measurement of emissions of nitrous oxide from soils. J. Environ. Qual. 9(2), 251–256 (1980).
- Mosier AR, Hutchinson GL. Nitrous oxide emissions from cropped fields. J. Environ. Qual. 10(2), 169–173 (1981).
- Parkin TB, Venterea RT, Hargreaves SK. Calculating the detection limits of chamber-based soil greenhouse gas flux measurements. J. Environ. Qual. 41, 705–715 (2012).
- Pathak H. Greenhouse gas emission from agriculture, Indian Agricultural Research Institute. In: Carbon Management in Agriculture for Mitigating Greenhouse Effect. Editors: A.K. Singh, S.V. Ngachan, G.C. Munda, K.P. Mohapatra, B.U. Choudhury, Anup Das, Ch. Srinivasa Rao, D.P. Patel, D.J. Rajkhowa, Ramkrushna, G.I. and A.S. Panwar. ICAR Research Complex for NEH Region, Umiam-793 103, Meghalaya, India. (2012). pp 377.
- West JM, Julius SH, Kareiva P et al. US natural resources and climate change: concepts and approaches for management adaptation. Environ. Manage. 44(6), 1001–1021 (2009).
- De Klein C, Harvey M. Nitrous Oxide Chamber Methodology Guidelines. Workshop report, Global Research Alliance, 8–27 (2012). Ministry for Primary Industries Pastoral House, Wellington, New Zealand.
- Parkin BT, Venterea R. Chapter 3: chamber-based trace gas flux measurements. In: Sampling Protocols. 3–1 to 3–39 (2010). Editor: R.F. Follett. Available at: www.ars.usda.gov/research/GRACEnet
- Verchot L, Thiongo M, Anyango E, Mutuo P, Abwanda S. Field and Laboratory Protocols: Non-CO2 GHG Measurements, Inorganic-N Measurements, Soil Water Content Measurements. A report prepared by Centre For International Forestry Research as part of a sub-contract with International Centre For Research in Agroforestry (2010). http://cbp.carbon2markets.org/cbp/protocols/nonco2.pdf
- Rochette P, Eriksen-Hamel NS. Chamber measurements of soil nitrous oxide flux: are absolute values reliable? Soil Sci. Soc. Am. J. 72(2), 331–342 (2008).
- Sapkota TB, Rai M, Singh LK et al. Greenhouse Gas Measurement From Smallholder Production Systems: Guidelines for Static Chamber Method. Consultative Group for International Agricultural Research (CGIAR), New Delhi, India, 18 (2014).
- Myhre G, Shindell D, Bréon FM et al. Anthropogenic and natural radiative forcing. In: Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, 659–740 (2013). [Stocker, T.F., D. Qin, G.-K. Plattner, M. Tignor, S.K. Allen, J. Boschung, A. Nauels, Y. Xia, V. Bex and P.M. Midgley (eds.)]. Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA.
- IPCC. Methane emissions from rice cultivation: flooded rice fields. In: Revised 1996 IPCC Guidelines for National Greenhouse Gas Inventories: Reference Manual. Houghton JT, Meira Filho LG, Lim B et al. (Eds). UK Meteorological Office, 53–75 (1996). http://www.ipcc-nggip.iges.or.jp/public/gl/invs6c.html
- Datta A, Das S, Manjunath KR, Adhya TK. Comparison of two methods for the estimation of greenhouse gas flux from rice ecosystems in India. Greenh. Gas Meas. Manage. 2(1), 43–49 (2012).
- Rochette P, Bertrand N. Soil air sample storage and handling using polypropylene syringes and glass vials. Can. J. Soil Sci. 83, 631–637 (2003).
- Takeuchi M. Studies on characteristics of electron capture responses. V. Estimation of electron attachment mechanisms from carrier gas flow-rate dependence of electron capture coefficients. Bull. Chem. Soc. Jpn. 54(8), 2466–2469 (1981).
- Devaux P, Guiochon G. Variations of the response of the electron capture detector with carrier gas flow-rate. J. Chromatogr. Sci. 7(9), 561–564 (1969).
- Kristenson EM, Korytár P, Danielsson C et al. Evaluation of modulators and electron-capture detectors for comprehensive two-dimensional GC of halogenated organic compounds. J. Chromatogr. A. 1019(1–2), 65–77 (2003).
- Grob RL, Barry EF. Modern Practice of Gas Chromatography. (4th Edition). Wiley Interscience, NJ, USA (2004).
- Rotocki P, Drozdowicz B. Influence of the carrier gas flow-rate on the pulsed electron-capture detector. J. Chromatogr. A. 446, 329–337 (1988).
- van der Laan S, Neubert REM, Meijer HAJ. A single gas chromatograph for accurate atmospheric mixing ratio measurements of CO2, CH4, N2O, SF6 and CO. Atmos. Meas. Tech. Discuss. 2(3), 1321–1349 (2009).
- Zheng X, Mei B, Wang Y et al. Quantification of N2O fluxes from soil–plant systems may be biased by the applied gas chromatograph methodology. Plant Soil. 311(1–2), 211–234 (2008).
- Wang Y, Wang Y, Ling H. A new carrier gas type for accurate measurement of N2O by GC–ECD. Adv. in Atmos. Sci. 27(6), 1322–1330 (2010).
- Van De Wiel HJ, Tommassen P. Effect of oxygen on electron capture detection. J. Chromatogr. A. 71(1), 1–7 (1972).
- Zimmermann S, Krippner P, Vogel A. Miniaturized flame ionization detector for gas chromatography. Sens. Actuators. 83, 285–289 (2002).
- Sander BO, Wassmann R. Common practices for manual greenhouse gas sampling in rice production: a literature study on sampling modalities of the closed chamber method. Greenh. Gas Meas. Manage. 4(1), 1–13 (2014).
- Christiansen JR, Korhonen JFJ, Juszczak R, Giebels M, Pihlatie M. Assessing the effects of chamber placement, manual sampling and headspace mixing on CH4 fluxes in a laboratory experiment. Plant Soil 343(1–2), 171–185 (2011).
- Pedersen AR, Petersen SO, Schelde K. A comprehensive approach to soil–atmosphere trace-gas flux estimation with static chambers. Eur. J. Soil Sci. 61(6), 888–902 (2010).
- Purves RD. Optimum numerical integration methods for estimation of area-under-the-curve (AUC) and area-under-the-moment-curve (AUMC). J. Pharmacokinet. Biopharm. 20(3), 211–226 (1992).
Website
- FCN. Fair Climate Network website. (2014). www.fairclimate.com
- UNFCCC. United Nations Framework Convention on Climate Change. 2015. Methane emission reduction by adjusted water management practice in rice cultivation. CDM Executive Board approved small-scale methodology “AMS-III.AU” https://cdm.unfccc.int/methodologies/DB/D6MRRHNNU5RUHJXWKHN87IUXW5F5N0