ABSTRACT
Background: The prevalence of non-alcoholic fatty liver disease (NAFLD) among Indian Asians in high-income countries is not well studied, but appears to be different from that for Western populations. Design: Cross-sectional study of subjects recruited through a community cardiovascular (CV) screening programme at two London Hindu temples from 2010–2012. NAFLD was diagnosed using the fatty liver index (FLI) and fibrosis stage through the BARD (Body Mass Index (BMI), Aspartate aminotransferase to Alanine aminotransferase ratio and Diabetes Mellitus) score. Results: 597 subjects were assessed; 306 (51%) female. Median (interquartile range) age and BMI were 49 (40.6–55.0) years and 26.4 (23.5–29.2) kg/m2, respectively. NAFLD was diagnosed in 184 (30.8%) cases, but 175 (29.3%) subjects could not be categorised. Overall, 117 (40.2%) men and 67 (21.9%) women had evidence of NAFLD (p < 0.001). In those with evidence of NAFLD, 142 (78.5%) had a BARD score suggestive of advanced fibrosis. Advanced fibrosis could be excluded in 5 (7.6%) women and 34 (29.6%) men (p < 0.001). Total cholesterol (TC), triglycerides (TG) and non-HDL (high-density lipoprotein cholesterol) were higher in the NAFLD group (p < 0.001), whereas HDL-C was lower (p < 0.001). Conclusion: There is evidence of a high prevalence of asymptomatic NAFLD, possibly in combination with advanced liver damage, among UK-based Gujarati Indians living in London. NAFLD is emerging as an independent risk factor for CV disease. Screening programmes should be developed in order to decrease liver and CV mortality and morbidity in these high-risk patients.
Introduction
Over the last two decades, non-alcoholic fatty liver disease (NAFLD) has become an increasingly diagnosed cause of liver disease particularly in Western countries [Citation1]. Depending on the population studied and the diagnostic method used, the estimated overall prevalence of NAFLD ranges from 6 to 35%, thus representing the commonest cause of hepatic disease [Citation2,3] but there are also studies mentioning NAFLD with a much higher prevalence (up to 100%) in populations with pre-existing metabolic conditions characterised by insulin resistance (IR) such as obesity, metabolic syndrome (MetS) or type 2 diabetes mellitus (T2DM) [Citation4,5]. NAFLD can result in non-alcoholic steatohepatitis which may progress over a variable period, in 25–40% patients to significant fibrosis, liver cirrhosis and hepatocellular carcinoma [Citation3,6]. Dyslipidaemia associated with NAFLD has been shown to increase cardiovascular (CV) risk; the possible mechanisms include oxidative stress, insulin resistance, inflammation, cytokines and endothelial abnormalities [Citation4,7]. In 2012, the UK Chief Medical Officer highlighted the rising tide of serious liver disease in the UK caused by NAFLD [Citation8].
NAFLD is recognised to be the main hepatic manifestation of the MetS and the principal risk factors for NAFLD are obesity, type 2 diabetes mellitus, insulin resistance, hyperglycaemia and hypertriglyceridaemia [Citation2]. NAFLD is also seen in individuals with normal body mass index (BMI) who do not necessarily have insulin resistance associated metabolic disorders [Citation9,10]. Age, gender and lifestyle measures also impact on the prevalence of NAFLD [Citation2,3,6] and a number of epidemiological studies clearly show an impact of ethnic origin with a suggestion in some studies that Asian Indians are at an increased risk for NAFLD [Citation10–Citation13]. Thus, liver biopsy and/or ultrasound-based studies on NAFLD conducted in India have reported a prevalence ranging from 9 to 32% [Citation9–Citation12] according to regional differences and social status [Citation10]. These numbers are similar to NAFLD prevalence reported in other countries [Citation3]. However, some studies suggest that Indians have higher grade histological abnormalities and a different clinicopathological profile compared with Caucasian, Hispanics or African-American subjects [Citation14,15]. Studies have suggested that Asian immigrants are at increased risk of NAFLD as compared with their western counterparts [Citation16,17].
There is limited data on the prevalence of NAFLD in those of Indian Asian ethnicity living in the Western countries, and especially in the UK. The aim of this community-based study was to describe the prevalence of NAFLD among a cohort of first and second generation British Indians of Gujarati origin residing in North London.
Methods
Design, setting and subjects
This is a retrospective analysis on cross-sectional data routinely collected as part of a community cardiovascular risk programme conducted by the Healthy Hearts Team [Citation18]. The cardiovascular screening programme was carried out in two Hindu temples (BAPS, Shri Swaminarayan Mandir, Neasden, London NW10 8LD, UK and Shri Swaminarayan Temple, Willesden, London NW2 5RG, UK), situated in the borough of Brent in Greater London which has a 64% ethnic minority population and 18% are of Indian origin [Citation19]. The congregations of the temples (several thousand) were mostly Gujaratis. The uptake of screening in our programme was high and representative of single community [Citation18]. A detailed description of the cardiovascular programme and data collection has been previously published [Citation18].
Measurement of NAFLD and fibrosis stage
The prevalence of NAFLD was determined by the fatty liver index (FLI) [Citation20]. The mathematical equation on which this index is based includes the BMI, plasma gamma-glutamyl transferase (gGT) and triglyceride levels, as well as waist circumference measurements. FLI score <30 “rules out” NAFLD, while a score >60 “rules in” NAFLD. Subjects presenting a score of 30–60 are “unclassified”. In those subjects in whom NAFLD was diagnosed, the BARD index (BMI, AST/ALT ratio and Diabetes Mellitus index) was calculated to evaluate the fibrosis stage [Citation21]. A BARD score from between 0 and 1 is indicative of absence of significant fibrosis, while a score of 2–4 suggests advanced fibrosis. A negative predictive value of 96% was determined for this index [Citation21].
Statistical analysis
Descriptive and inferential statistical analyses were performed using SPSS 19.0 (IBM Corporation, Somers, NY, USA) and STATA 9.0 (Stata Corp LP, College Station, TX, USA).
Data were checked for normality using histogram and scatterplots. Categorical variables were evaluated using chi-square test or Fisher’s exact tests and continuous variables were compared using the Mann–Whitney U-test or the Student t-test, when applicable. The 95% confidence intervals (CI) were calculated for the prevalence of NAFLD and advanced fibrosis. All reported p values are two-sided and a p < 0.05 was deemed significant.
Results
Study population characteristics
Data from 597 sequentially recruited individuals from 2010 to 2012 were analysed; 306 (51.3%) were female. Three hundred and eighty-three (64.2%) subjects had a BMI of ≥25 kg/m2 and 479 (80.2%) a BMI of ≥23 kg/m2. The aspartate aminotransferase (AST)/alanine aminotransferase (ALT) ratio was >2 in 29 (4.9%) individuals and <1 in 181 (30.5%) subjects, respectively. The main characteristics of the study population are presented in . The presence and absence of DM form a part of the score so individuals with DM were not excluded.
Table 1. Characteristics of the study population (n = 597).
Only 6.5% (39 individuals) of the above cohort consumed alcohol. Of the 39 individuals who consumed alcohol, the majority (36) consumed 7 units or less of alcohol per week. Of the remaining, 2 had intakes of >21 units/week.
Prevalence of NAFLD and advanced fibrosis
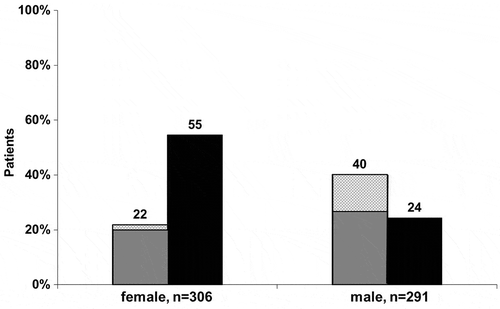
In the overall population, NAFLD was diagnosed in 30.8% (184/597), with 39.9% (238/597) screening negative. A further 29.3% (175/597) were unable to be categorised by applying the FLI. The prevalence of NAFLD according to the gender is shown in . Of those characterised as having NAFLD, 142 (78.5%; 95% CI: 71.7–84.2%) had a BARD score >1 suggestive of advanced fibrosis. Evidence for advanced fibrosis was detected in 61 (92.4%) of the females and 81 (70.4%) of the male individuals [odds ratio (95% CI): 5.12 (1.89–13.9); p = 0.001]. The corresponding figures for the subpopulation with elevated ALT levels (>19 IU/L for females and >31 IU/L for males) were: 12 (10.4%) for females and 34 (52.3%) for males [odds ratio 9.41; 95% CI: 4.36–20.4); p < 0.001]. The BARD index could not be calculated in 3 (1.6%) individuals.
Figure 1. Prevalence of non-alcoholic fatty liver disease (NAFLD) as determined by the fatty liver index (FLI).
Notes: Light bars: presence of NAFLD (FLI > 60); black bars: absence of NAFLD (FLI < 30); checked area: absence of advanced fibrosis, defined as BARD (Body Mass Index, AST/ALT ratio and Diabetes Mellitus-index 2–4), among those patients with NAFLD. In 72 (23.5%) of the female and 103 (35.4%) of the male subjects, presence or absence of NAFLD could not be characterised by FLI. The p-value for presence of NAFLD comparing male and female subjects was <0.001.
Lipid levels
We compared total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), triglycerides (TG) and non-HDL-C (TC–HDL-C) in individuals with and without NAFLD. TC, TG and non-HDL-C were higher (p < 0.001), whereas HDL-C was lower (p < 0.001) in the NAFLD group (). Within the NAFLD group, the individuals with advanced fibrosis using BARD scoring had lower levels of TC (p = 0.008), HDL-C (p = 0.403), TG (p = 0.160) and non-HDL-C (p = 0.029) ().
Table 2. Lipid levels in individuals with and without NAFLD.
Table 3. Lipid levels in individuals with and without fibrosis (as per BARD Score) in the NAFLD^ group.
Discussion
This study is the first to report the prevalence of NAFLD in a Gujarati Indian community setting in the UK. The high prevalence of NAFLD (ie 30.8%) we found among the Gujarati Indian population of North London is alarming and indicates a need for detailed characterisation and assessment for NAFLD in this population. This could allow intervention on potentially modifiable risk factors associated with the MetS and NAFLD. It should be noted that NAFLD is generally asymptomatic in the early stages [Citation4].
In this cohort, 15% of the subjects had a 10-year cardiovascular risk >20% [Citation18] and almost all participants (92%) had at least one risk factor for cardiovascular disease [Citation18] including obesity, MetS or DM [Citation18]. These factors have been identified as key risk factors for NAFLD both in Western populations and those living in India [Citation10–Citation12]. Lifestyle [Citation22] and dietary factors have also been shown to be important in subjects living in India with total calorie intake, per cent of carbohydrate and fat intake of NAFLD cases being significantly higher than controls in one study [Citation23]. Increased income was also associated with a greater prevalence of NAFLD in another Indian study [Citation10]. However, NAFLD is not limited to the prosperous sections of Indian society or only seen in those who are overweight [Citation10,13], although it is recognised that Asians have an increased body fat compared with Europeans even at the same BMI [Citation24]. In this context, a study involving almost 2000 subjects living in a rural region of East India reported that the only 25% of subjects with NAFLD had a BMI in the obese range but 39% had abdominal obesity which is associated with NAFLD [Citation10].
The prevalence of NAFLD described in this study is higher than that reported in previous studies conducted in populations living in India [Citation10–Citation13]. This may be the result of a combination of genetic predisposition among Indian Asians to develop NAFLD and a change in lifestyle associated with high calorie consumption and reduced physical activity in the Western world [Citation23,25–28].
Indian heritage comprised approximately 2.5% of the population in the UK in 2011 [Citation29]. In addition 2.6% are from Pakistan and Bangladesh, whose cardiovascular risk, risk for diabetes and MetS are shown to be higher than the people of Indian origin [Citation29]. Therefore, the future impact of NAFLD in terms of progressive liver disease is of public health concern for the UK.
A higher prevalence of NAFLD in men than women was observed in this study, which is in keeping with previously published findings [Citation10,13,28,30].
Nevertheless, this study clearly shows that NAFLD is present in 30% of this population that are at risk of NAFLD due to the reported high prevalence of factors associated with the MetS [Citation18]. Furthermore, advanced fibrosis could only be excluded in as little as 8% of the female and 30% of the male population. Thus, more accurate diagnostic methods such as transient elastography [Citation31] or liver biopsy may be needed to further assess the prevalence of NAFLD and liver fibrosis in this population. However, a previous study found that an AST/ALT ratio <1 is associated with non-alcoholic steatohepatitis (NASH), while an AST/ALT ratio >2 is associated with alcoholic steatohepatitis [Citation32]. Approximately one-third of subjects in this study had an AST/ALT ratio <1 and only 5% showed an AST/ALT ratio >2, thus making alcohol consumption as a cause for fatty liver disease in this population unlikely [Citation32]. Furthermore, alcohol consumption is absent or minimal in this population for traditional religious reasons.
In addition to liver disease, patients with NAFLD have an increased mortality compared with the general population primarily related to CVD or malignancy [Citation33,34]. The pathogenesis of dyslipidaemia in NAFLD is likely related to imbalance between production/clearance of the lipoproteins like hepatic FFA (free fatty acid) from the circulation [Citation34,35]. This results in liver accumulation of TG predisposing to fatty liver. The FFAs and its derivatives are mainly responsible for lipotoxicity and the resultant liver injury [Citation35]. In the recent years, a better understanding of the pathogenesis has resulted in the discovery of medical therapies targeting various aspects of TG deposition and hepatocellular injury. Medications like peroxisome proliferator–activator receptors (PPARs), volixbat, are targeted to reduce hepatic fat accumulation and metabolic stress. Agents like vitamin E, pentoxifylline and PXS-4728A are aimed at reducing inflammation and injury associated with oxidative stress [Citation35]. With regard to lipid lowering agents, statins are safe in NAFLD (35–37). Statins not only improve dyslipidaemia but are also associated with a decreased risk of NASH and advanced fibrosis [Citation36,37]. Rosuvastatin monotherapy was associated with resolution of NASH, regression of MetS, reduction in plasma glucose and serum uric acid levels. These results suggest a decreased risk of vascular and liver morbidity and mortality-related NAFLD/NASH [Citation38]. There is an interest in the role of ezetimibe, not only in reducing cardiovascular risk but also in improvement of biochemical markers of NAFLD and reduction in hepatic steatosis [Citation39].
Combination drug therapy could be more effective in terms of NAFLD/NASH. Combining a potent statin (ie atorvastatin and rosuvastatin) with liraglutide has been suggested to maximise beneficial effects on liver and CVD morbidity and mortality in T2DM patients with NAFLD/NASH [Citation40].
Glucagon like peptide 1(GLP1) analogues and Sodium–glucose co-transporter 2 (SGLT2) inhibitors alone or in combination might have a beneficial effect on NASH progression This may improve CVD-related morbidity and mortality, as well as liver-related morbidity in patients with NASH [Citation41].
Drugs targeting gut microbial lipopolysaccharide (LPS) which has been implicated as one of the mechanism of liver injury for NAFLD are currently being evaluated in clinical trials [Citation35].
Although risk factors for progressive liver disease in the context of NAFLD are poorly characterised, there is no doubt that the MetS contributes to the progression in liver inflammation [Citation34]. Therefore, some of the measures above, particularly weight loss and diabetes control may well have a protective effect in terms of hepatic disease progression [Citation42,43].
The limitation of this community-based study is that only non-invasive algorithms, using a combination of blood biomarkers and anthropometric measures, were available to evaluate NAFLD and fibrosis staging. This limitation may have over or underestimated the true prevalence. Although the tests used in this study have been extensively validated [Citation20,21], approximately one-third of the population could not be categorised by means of the FLI, suggesting that the prevalence of NAFLD could be even higher in this population. Additionally, while the BARD score reliably excludes the presence of advanced fibrosis, its positive predictive value remains suboptimal [Citation21].
Conclusion
Our data suggest a very high prevalence of NAFLD among the Gujarati Indian community living in North London, with evidence of significant liver damage. Screening programmes are urgently needed to fully characterise and address this important health issue to prevent future morbidity and mortality, not only from the associated cardiovascular disease but also from severe liver disease.
Competing interests
DRN has received grants from Pfizer (Pfizer Foundation award 2008), Solvay, Merck Sharp & Dohme and Astra Zeneca for this service (see funding below). DRN has advisory board membership for Merck Sharp & Dohme, Sanofi and Amgen. DRN is a speaker for Merck Sharp & Dohme, Sanofi and Amgen. KN is the recipient of a Miguel Servet research grant from the Instituto de Salud Carlos III (grant number CP13/00187) and received lecture fees from Merck. SB have served on Advisory Boards and been on the speaker bureau for Merck Sharp & Dohme. AR has received unrestricted grant from Gilead. AJ, JO and DN, have no competing interests to disclose.
Funding
The study was financially supported by the Pfizer Foundation award 2008, Pfizer UK. We also received unrestricted educational grants from MSD, Solvay and AstraZeneca. The design and conduct of the service and the analysis, interpretation and presentation of the data were solely the responsibility of the authors. DRN has received grants from Pfizer (Pfizer Foundation award 2008), Solvay, Merck Sharp & Dohme, and Astra Zeneca for this service (see funding below). DRN has advisory board membership for Merck Sharp & Dohme, Sanofi and Amgen. DRN is a speaker for Merck Sharp & Dohme, Sanofi and Amgen. KN is the recipient of a Miguel Servet research grant from the Instituto de Salud Carlos III [grant number CP13/00187] and received lecture fees from Merck. SB have served on Advisory Boards and been on the speaker bureau for Merck Sharp & Dohme. AR has received unrestricted grant from Gilead.
Ethical approval
The ethical approval was obtained from NHS Health Research authority Research Ethics Committee (REC reference:13/NW0070) and this was stated in the paper in the ethical approval section.
Guarantor
DRN
Contributors
DRN developed the proposal for the outreach clinic and is the lead for the project. KN, SB and AJ planned the analysis, drafted the paper, SB, AR, JO and DRN edited the paper. DRN, AJ, DN, were responsible for the implementation of the study and acquisition of data. All the authors gave their intellectual input towards the preparation of manuscript and approved the final version.
Disclosure statement
No potential conflict of interest was reported by the authors.
Acknowledgements
We thank the staff of BAPS Swaminarayan Mandir, Neasden, for their support.
References
- Younossi ZM, Blissett D, Blissett R, et al. The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology. 2016;64:1577–1586.10.1002/hep.28785
- Byrne CD, Targher G. NAFLD: a multisystem disease. J Hepatol. 2015;62:S47–S64.10.1016/j.jhep.2014.12.012
- Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274–285.10.1111/apt.2011.34.issue-3
- Katsiki N, Mikhailidis DP, Mantzoros CS. Non-alcoholic fatty liver disease and dyslipidemia: an update. Metabolism. 2016;65:1109–1123.
- Athyros VG, Tziomalos K, Katsiki N, et al. Cardiovascular risk across the histological spectrum and the clinical manifestations of non-alcoholic fatty liver disease: an update. World J Gastroenterol. 2015;21:6820–6834.
- Farrel GC, Larter CZ. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology. 2006;43:S99–S112.10.1002/(ISSN)1527-3350
- Nseir W, Shalta A, Marmor A, et al. Mechanisms linking nonalcoholic fatty liver disease with coronary artery disease. Dig Dis Sci. 2011;56:3439–3449.10.1007/s10620-011-1767-y
- Davies SC. Annual report of the chief medical officer volume one 2011: on the state of the public’s health. London: Department of Health; 2012. Available from: Http://webarchive.nationalarchives.gov.uk/20130107105354/http://www.dh.gov.uk/health/2012/11/cmo-annual-report/
- Margariti E, Deutsch E, Manolakopoulos S, et al. Non-alcoholic fatty liver disease may develop in individuals with normal body mass index. Ann Gastroenterol. 2012;25:45–51.
- Das K, Das K, Mukherjee PS, et al. Nonobese population in a developing country has a high prevalence of nonalcoholic fatty liver and significant liver disease. Hepatology. 2010;51:1593–1602.10.1002/hep.23567
- Amarapurkar D, Kamani P, Patel N, et al. Prevalence of non-alcoholic fatty liver disease: population based study. Ann Hepatol. 2007;6:161–163.
- Bajaj S, Nigam P, Luthra A, et al. A case-control study on insulin resistance, metabolic co-variates & prediction score in non-alcoholic fatty liver disease. Indian J Med Res. 2009;129:285–292.
- Mohan V, Farooq S, Deepa M, et al. Prevalence of non-alcoholic fatty liver disease in urban south Indians in relation to different grades of glucose intolerance and metabolic syndrome. Diabetes Res Clin Pract. 2009;84:84–91.10.1016/j.diabres.2008.11.039
- Mohanty SR, Troy TN, Huo D, et al. Influence of ethnicity on histological differences in non-alcoholic fatty liver disease. J Hepatol. 2009;50:797–804.10.1016/j.jhep.2008.11.017
- Duseja A, Das A, Das R, et al. The clinicopathological profile of Indian patients with nonalcoholic fatty liver disease (NAFLD) is different from that in the West. Dig Dis Sci. 2007;52:2368–2374.10.1007/s10620-006-9136-y
- Weston SR, Leyden W, Murphy R, et al. Racial and ethnic distribution of nonalcoholic fatty liver in persons with newly diagnosed chronic liver disease. Hepatology. 2005;41:372–379.10.1002/(ISSN)1527-3350
- Tabibian JH, Lazo M, Durazo FA, et al. Nonalcoholic fatty liver disease across ethno-racial groups: do Asian-American adults represent a new at-risk population? J Gastroenterol Hepatol. 2011;26:501–509.10.1111/j.1440-1746.2010.06443.x
- Rao N, Eastwood SV, Jain A, et al. Cardiovascular risk assessment of south Asians in a religious setting: a feasibility study. Int J Clin Pract. 2012;66:262–269.10.1111/j.1742-1241.2011.02773.x
- Brent Diversity Profile. 2014. [cited Nov 2016]. Available from: https://intelligence.brent.gov.uk/BrentDocuments/Brent%20Diversity%20Profile.pdf0
- Bedogni G, Bellentani S, Miglioli L, et al. The fatty liver index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006;6:44.10.1186/1471-230X-6-33
- Harrison SA, Oliver D, Arnold HL, et al. Development and validation of a simple NAFLD clinical scoring system for identifying patients without advanced disease. Gut. 2008;57:1441–1447.10.1136/gut.2007.146019
- Madan K, Batra Y, Gupta SD, et al. Non-alcoholic fatty liver disease may not be a severe disease at presentation among Asian Indians. World J Gastroenterol. 2006;12:3400–3405.10.3748/wjg.v12.i21.3400
- Sathiaraj E, Chutke M, Reddy MY, et al. A case-control study on nutritional risk factors in non-alcoholic fatty liver disease in Indian population. Eur J Clin Nutr. 2011;65:533–537.10.1038/ejcn.2011.3
- Deurenberg P, Deurenberg-Yap M, Guricci S. Asians are different from Caucasians and from each other in their body mass index/body fat per cent relationship. Obesity Rev. 2002;3:141–146.10.1046/j.1467-789X.2002.00065.x
- Gupta AC, Misra R, Sakhuja P, et al. Association of adiponectin gene functional polymorphisms (-11377C/G and +45T/G) with nonalcoholic fatty liver disease. Gene. 2012;496:63–67.10.1016/j.gene.2011.12.023
- Bhatt SP, Nigam P, Misra A, et al. SREBP-2 1784 G/C genotype is associated with non-alcoholic fatty liver disease in north Indians. Dis Markers. 2011;31:371–377.10.1155/2011/950102
- Kanth VV, Sasikala M, Rao PN, et al. Pooled genetic analysis in ultrasound measured non-alcoholic fatty liver disease in Indian subjects: a pilot study. World J Hepatol. 2014;6:435–442.10.4254/wjh.v6.i6.435
- Chatterjee A, Basu A, Chowdhury A, et al. Comparative analyses of genetic risk prediction methods reveal extreme diversity of genetic predisposition to nonalcoholic fatty liver disease (NAFLD) among ethnic populations of India. J Genet. 2015;94:105–113.10.1007/s12041-015-0494-0
- Office National Statistics [ Online]. Population of United Kingdom: by ethnic group 2011. [cited 2016 Dec 21]. Available from: http://webarchive.nationalarchives.gov.uk/20160105160709/http://www.ons.gov.uk/ons/resources/figure1_tcm77-290598.png
- Singh SP, Kar SK, Panigrahi MK, et al. Profile of patients with incidentally detected nonalcoholic fatty liver disease (IDNAFLD) in coastal eastern India. Trop Gastroenterol. 2013;34:144–152.10.7869/tg
- Lédinghen V, Vergniol J, Foucher J, et al. Non-invasive diagnosis of liver steatosis using controlled attenuation parameter (CAP) and transient elastography. Liver Int. 2012;32:911–918.10.1111/liv.2012.32.issue-6
- Sorbi D, Boynton J, Lindor KD. The ratio of aspartate aminotransferase to alanine aminotransferase: potential value in differentiating nonalcoholic steatohepatitis from alcoholic liver disease. Am J Gastroenterol. 1999;94:1018–1022.10.1111/ajg.1999.94.issue-4
- Fargion S, Porzio M, Fracanzani AL. Nonalcoholic fatty liver disease and vascular disease: state-of-the-art. World J Gastroenterol. 2014;20:13306–13324.10.3748/wjg.v20.i37.13306
- Chatrath H, Vuppalanchi R, Chalasani N. Dyslipidemia in patients with nonalcoholic fatty liver disease. Semin Liver Dis. 2012;32:22–29.
- Rotman Y, Sanyal AJ. Current and upcoming pharmacotherapy for non-alcoholic fatty liver disease. Gut. 2017;66:180–190.10.1136/gutjnl-2016-312431
- Pastori D, Polimeni L, Baratta F, et al. The efficacy and safety of statins for the treatment of non-alcoholic fatty liver disease. Dig Liver Dis. 2015;47:4–11.10.1016/j.dld.2014.07.170
- Dongiovanni P, Petta S, Mannisto V, et al. Statin use and non-alcoholic steatohepatitis in at risk individuals. J Hepatol. 2015;63:705–712.10.1016/j.jhep.2015.05.006
- Kargiotis K, Athyros VG, Giouleme O, et al. Resolution of non-alcoholic steatohepatitis by rosuvastatin monotherapy in patients with metabolic syndrome. World J Gastroenterol. 2015;21:7860–7868.10.3748/wjg.v21.i25.7860
- Simon TG, Corey KE, Chung RT, et al. Cardiovascular risk reduction in patients with nonalcoholic fatty liver disease: the potential role of ezetimibe. Dig Dis Sci. 2016; 61:3425–3435.
- Katsiki N, Athyros VG, Mikhailidis DP. Non-alcoholic fatty liver disease in patients with type 2 diabetes mellitus: effects of statins and antidiabetic drugs. J Diabetes Complications. 2016 Dec 29. [ Epub ahead of print].
- Athyros VG, Katsiki N, Karagiannis A. Editorial: can glucagon like peptide 1 (GLP1) agonists or sodium-glucose co-transporter 2 (SGLT2) inhibitors ameliorate non-alcoholic steatohepatitis in people with or without diabetes? Curr Vasc Pharmacol. 2016;14:494–497.10.2174/1570161114666160909161811
- Hazlehurst JM, Woods C, Marjot T, et al. Non-alcoholic fatty liver disease and diabetes. Metabolism. 2016;65:1096–1108.10.1016/j.metabol.2016.01.001
- Ryu S, Chang Y, Jung HS, et al. Relationship of sitting time and physical activity with non-alcoholic fatty liver. J Hepatol. 2015;63:1229–1237.
