Abstract
The objective was to study changes in nitrogen-saturated biodiesel irradiated by electron beam and to analyse them considering the influence of absorbed dose. Based on the obtained results, it can be concluded that irradiation in presented condition did not affect ester groups in fatty acid methyl esters (FAME) molecules, but slightly influenced on double bonds. After irradiation was observed relatively small change in concentration of methyl esters. Slightly influence can be explained by absorption of radiation by nitrogen present in samples. Therefore, the use of ionizing radiation to improve Rapeseed Methyl Esters (RME) properties can be used only in specific radiation dose range (total ester concentration must not be lower than 96.5 wt%). The reduction in the total concentration of methyl esters was lower than after irradiation of FAME samples saturated with water and air, which was presented in previous works.
Introduction
Biodiesel has obtained much importance in the past few decades as an alternative fuel in the diesel engines because of its unique properties as it is environment friendly, less toxic and biodegradable. However, biodiesel has higher lubricity than petro-diesel fuel because of no sulfur content, thereby enhancing engine longevity [Citation1]. The European Union requires the use of biofuels or their addition to fuels obtained from fossil sources due to environmental protection and the speed of climate change (use of a closed carbon cycle in nature). In order to obtain biofuels including biodiesel, newer and newer methods are being developed based on products of different generations [Citation2, Citation3]. According to the latest guidelines, vegetable and algal oils should be products for the production of biodiesel. During the production of renewable fuels, especially fatty acid methyl esters, a very important aspect is the biological life that can develop in them. For the production of this type of fuel, vegetable oils are used, the composition of which directly affects the physico-chemical properties of diesel fuels.
Among biofuels that can be classified into each of above groups there is biodiesel. Biodiesel is the name for a variety of ester-based oxygenated fuels (and also their blends with diesel fuel) for compression–ignition engines. Chemically, biodiesel consists of monoalkyl esters of long-chain fatty acids derived from renewable biolipids. Biodiesel (commonly know as FAME (fatty acid methyl esters)) is obtained in transesterification, which is the reaction of triglycerides with a short-chain alcohol, usually methanol or ethanol. Due to exchange of alkoxy groups, fatty acid alkyl esters are formed, as well as glycerol as a by-product [Citation4].
Biodiesel used as biofuel has a number of advantages (e.g. high flash point [Citation4], high biodegradability [Citation5] and low particle matter (PM), hydrocarbons (HC) and carbon monoxide emissions [Citation6]). Unfortunately, it also has disadvantages, which include, oxidation stability problems resulting in a negative effect on plastics and other engine elements [Citation7]. In addition to storage conditions on oxidation stability also has a biological life effect. Contributes it’s to worsen the parameters of this fuel and shortening the possible usability time. Proper sterilization would reduce the biological life in biodiesel and eliminate this factor that impairs fuel properties. In order to eliminate such factors, ionizing radiation, which is a known factor used in the sterilization of materials, can be successfully used. Gamma radiation and electron fluxes are the most effective radiation sources which can induce chemical reactions or initiate physical processes in materials [Citation8]. According to literature data radiation can effect on materials by energy absorption processes and induce change in its properties. Changes can be generated not only in aqueous solutions, but also directly in organic compounds and polymers [Citation8–16]. During acting of ionizing radiation on fuels, especially biofuels, it is very important to be aware of the influence on physico-chemical properties of the biofuel, in particular its composition. From industrial point of view, the best source of ionizing radiation is 60Co isotopes, which emits gamma-prime lines. In the case of laboratory applications, however, electron accelerators (e-beam) are most often used, which results from the possibility of switching off this radiation source [Citation17]. Both types of radiation sources can be used to induce changes in organic and inorganic materials. In organic materials (polymers and small organic molecules), radiation depends on individual bond sensitivities and the chemical make-up of the material [Citation8]. Radiation chemistry can involve bond cleavage and bond formation (cross-linking, leading to larger molecules) [Citation18, Citation19]. Radiation could induce detrimental and long-term polymer degradation, controlled curing of reactive resins initiated by e-beam, polymer modifications via radiation induced grafting reactions, surface modifications of biomaterials, organic waste processing (scission and breakdown of molecules in sludge and solution) and many others [Citation8–11, Citation18, Citation19].
Ionizing radiation can be used for the preparation of microorganisms which can be applied for the synthesis of biofuels or the sterilization of materials for FAME production from algae [Citation20–24]. During this processes structure of fatty acid chain can be changed, which will cause change in biodiesel properties [Citation18, Citation19].
The aim of this work was to study changes in composition of nitrogen-saturated biodiesel irradiated by electron beam and to analyse them considering the influence of absorbed dose. The study investigated the protective effect of nitrogen on changes in the chemical composition of fatty acid methyl esters caused by ionizing radiation. The total concentration of methyl esters is one of the basic parameters in accordance with the EN 14214 standard.
Experimental
In the study, biodiesel from rapeseed (Rapeseed Methyl Esters, RME) produced in Poland was used. Its composition is shown in as a non-irradiated sample (0 kGy). All chemicals were used as received from distributors without further purification.
Table 2. Ester content in all tested RME samples.
The method concerning with pulse radiolysis experiments has been shown elsewhere [Citation18, Citation19]. Pulse radiolysis experiments were carried out with a high energy (6 MeV) 17 ns electron pulse generated from an ELU-6 linear electron accelerator. During the tests, the accelerator worked in a pulse mode with 20 Hz sweep. The current in the 4 µs pulse was 0.8 A, at 20 Hz (50 ms period), and the impulse duration was 4 µs, which gives 49.996 ms without electricity. Each pulse consisted of 7500 micro-pulses (50 ps each) [Citation18, Citation19].
Fricke dosimeter (Fe(II)/Fe(III)) adapted to work with high doses was used for the determination of dose absorbed per pulse. The dose delivered per pulse was equalled 680 Gy. Details of pulse radiolysis system are given elsewhere [Citation25, Citation26]. All experiments were carried out at room temperature after passing nitrogen through the sample for 30 min. For experiments, glass ampoules (according to DIN 58 377) were used. All ampoules had the same thickness of the walls, equipped with a ring to facilitate removal of the upper part of the ampoule. Calculated absorbed doses of all samples are shown in .
Table 1. Doses of radiation absorbed by samples.
After irradiation, visible spectra of the samples were recorded. In this study, spectrophotometer Hach DR 3900 was used. Composition of samples was examined with the use of gas chromatograph Agilent 7820A with Flame Ionization Detector (FID), according to PN-EN 14103 [Citation27]. All parameters were performed 2 weeks after irradiation. This time guarantees that the observed changes are a stationary state and there are no additional recombination changes in the obtained research samples [Citation18, Citation19].
Results and discussion
Vis spectroscopy
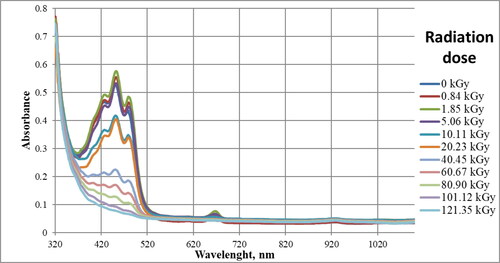
presents visible spectra of all samples. The ‘0 kGy’ label relates to non-irradiated reference sample.
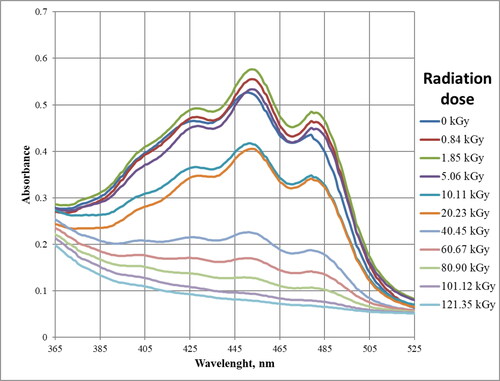
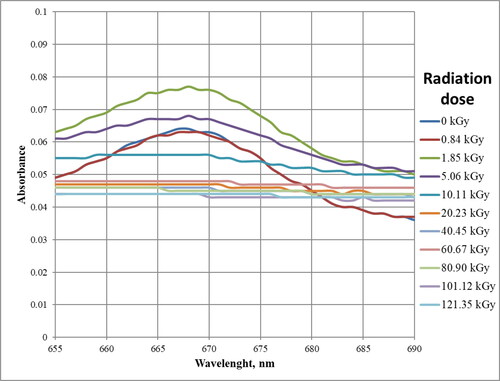
As can be seen in , changes that are possible to interpret occur in chromophores absorbing in the range 400–520 nm () and 655–680 nm (). Other ranges can be neglected or there are no observable changes.
Radiation in the wavelength range from 400 to 520 nm is absorbed mainly by double bond systems and by carotenoid pigments. They can include from several to over a dozen bonds, depending on the compound [Citation26].
shows three visible maxima with wavelengths of approximately 430, 450, and 480 nm. For samples that received the lowest radiation dose, that is, up to 5.056 kGy absorbance values are similar, this may be related to the viscosity of FAME and the low penetration of ionizing radiation. However, the higher the dose of absorbed radiation, the lower the absorbance value. This indicates the disappearance or changes in the area of double bonds, which are visible at the above wavelengths. RME in its composition contains methyl esters of higher fatty acids, which contain double bonds occurring in conjugated systems, that is, two or three bonds (methyl linolenate, methyl linolate) and small amount of carotenoid pigments. Absorption in this range occurs due to the transfer of electrons from bonding π to antibonding π* orbitals in conjugated systems of several double bonds [Citation26]. Unsaturated esters are probably responsible for the absorption, but it is also possible that pigments present in biodiesel are involved. Regardless of the actual explanation, it can be concluded that due to ionizing radiation, a reduction in the number of double bond systems occurs. Conjugated unsaturated bond systems exhibit absorption in visible light, for example, β-carotene containing 11 double bonds has the absorption maximum at 445 nm [Citation26].
In the range 655–690 nm presented in , there is one absorption maximum at the wavelength of 667 nm. At this length, there is a maximum absorbance for chlorophyll pigments. As can be seen from this figure, doses above 10 kGy caused the total degradation of these pigments.
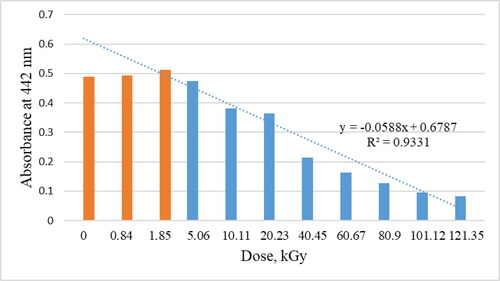
shows the change in absorbance at 442 nm depending on the radiation dose. As can be seen from this figure, a change in absorbance is not observed up to a dose of approximately 5 kGy. This indicates the lack of influence of ionizing radiation applied in the measurement conditions presented in the work on the double bonds system in fatty acid methyl esters and carotenoid pigments. Spectroscopic methods can be used only to preliminary study these changes on the basis changes of absorbance in the wavelength range characteristic of carotenoids dyes and chlorophyll dyes. However, the VIS spectroscopy results may be affected by the presence of carotenoids in biodiesel. The bonds can be destroyed or transformed due to reactions like saturation or polymerisation [Citation28, Citation29]. Similar processes probably occur in unsaturated esters molecules. The reduction of double bonds can cause changes in macroscopic parameters of biodiesel, like low-temperature properties [Citation30, Citation31]. Larger volumes of samples should be irradiated to examine the possible changes.
In the presented visible range, there is clear correlation between the absorption intensity and the dose absorbed by samples. With the increase in dose, the absorbance decreases, that is, the amount of carotenoids present in biodiesel is reduced due to the destruction or transformation of unsaturated bond systems [Citation32]. Similar phenomena may occur in biodiesel itself.
Based on previously published author's works, the studies did not take into account changes in IR spectra. Intensity and location of the band do not depend on the absorbed dose of radiation. This means that ionizing radiation does not affect the carbonyl group in the esters in tested biodiesel samples [Citation32, Citation33].
Composition of samples (esters content)
In order to accurately investigate changes in the composition of irradiated RME samples, gas chromatography was used. Measurements were made in accordance with the requirements of PN-EN 14103 [Citation27]. Determined content of all esters present in biodiesel samples is shown in . The number accompanying ‘C’ letter represents the total quantity of carbon atoms in the ester chain, an additional number indicates the amount of unsaturated bonds.
As in the case of previously published studies for pure FAME samples [Citation33] and FAME samples after water saturation [Citation32], esters which concentrations decreases most are those which content in FAME is the highest. The radiation interacts with the main constituents of biodiesel (the so-called matrix of the sample), that is why esters at low concentrations are not affected [Citation32, Citation33].
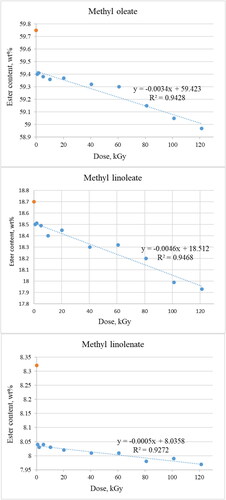
Electron radiation mostly influences on methyl oleate, methyl linoleate and methyl linolenate, which contain a different number of unsaturated double bonds. Changes in concentration of these esters are shown in .
The most distinct difference in content of methyl oleate, as well as methyl linoleate and methyl linolenate is observed between the non-irradiated and 0.84 kGy sample. Further changes are not so significant considering the rate of increase in dose. Ester content and radiation dose are very well linearly correlated if the first measurement (non-irradiated sample) is excluded. The coefficient of determination (R2) values are 0.94 for methyl oleate and methyl linoleate and 0.93 for methyl linolenate.
Determination of ester content with the use of gas chromatography seem to correspond with spectroscopic results and suggests that double bonds may undergo fragmentation, polymerisation or saturation with different radical existing in sample [Citation35, Citation36].
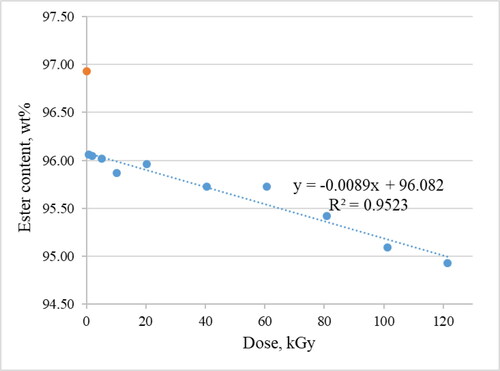
Changes in total ester content are shown in . The concentration decreases from 96.93 wt% in non-irradiated sample to 94.93 wt% in a 121.35 kGy sample. As in the case of C18 esters described above, the most significant decrease occurs from non-irradiated sample to 0.84 kGy sample. Also, the dependence on dose is clearly linear (R2 value is 0.95) if the non-irradiated sample is excluded.
Possible explanation of relatively small decrease in ester content may be that water present in samples absorbs most of the radiation and its radiolysis occurs. However, even the smallest dose causes biodiesel not to meet requirement of PN-EN 14214 which is 96.5 wt% [Citation34].
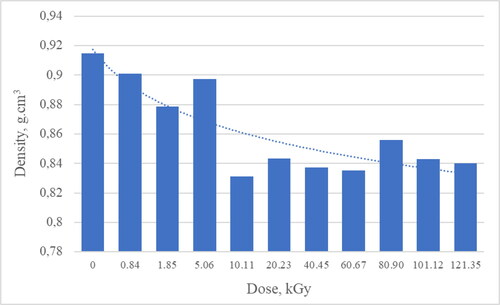
Density testing of RME samples subjected to radiolysis was performed at room temperature (20 °C). The results are summarized in . Based on the results obtained, it can be seen that to a dose of about 10 kGy the density decreases to 0.8312 g/cm3 from 0.9147 g/cm3 for non-irradiated RME. After reaching this dose, the density reaches an average value of 0.8408 ± 0.0080 g/cm3. Based on the results, it can be stated that the effect of ionizing radiation up to a dose of 5.06 kGy does not significantly change the density and its value is in the range of EN ISO 14214 of 0.86–0.90 g/cm3.
The use of ionizing radiation as sterilizing agent before transesterification process of vegetal or algal oils could be useful [Citation37–39]. However, sterilization should be carried out in a specific dose range, due to the possible change in the fat profile in the substrate, that is, vegetable oils. This kind of elimination of biological life in biodiesel allows for their removal with much lower doses of radiation [Citation40], instead of using biocides, that is, compounds that remove biological life from fuels.
Dendrogram analysis
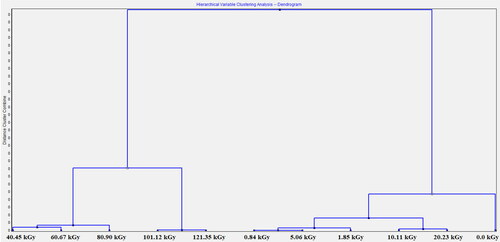
Banding patterns generated for the data from analysis described in this article were used for cluster analysis and the creation of a dendrogram (). The dendrogram was performed using the TANAGRA 1.4.50 data mining research software [Citation41]. The dendrogram was created using unweighted pair groupings of a similarity coefficient (SAB) matrix, and consistently resulted in the groupings shown in . Based on this analysis, the samples were divided into two clusters. Each group corresponds to different changes in sample composition. In the first group, two subgroups were observed, which were radiated with low radiation dose (from 0.84 to 5.06 kGy) and medium radiation dose (from 10.11 to 20.23 kGy). Based on this analysis, non-irradiated sample was similar to samples radiated with low radiation dose. The second group consists of samples that have absorbed high doses of radiation. A sample exposed to the highest radiation dose may be considered as a separate group, but due to analogous variations such as 40–120 kGy dose samples, it was classified in the same group. Cluster analysis confirms observations resulting from direct analysis of data obtained from VIS and GC studies. It is a possibility, that for low doses (up to about 20 kGy), statistical effects of radiation from RME saturated by nitrogen are observed, while for high doses (from about 20 kGy) processes that can occur is mainly polymerisation and/or degradation of methyl esters of higher fatty acids. Similar observations after electron beam irradiation biodiesel samples without any additives [Citation19] and water saturated biodiesel [Citation18] was observed.
Principal components analysis
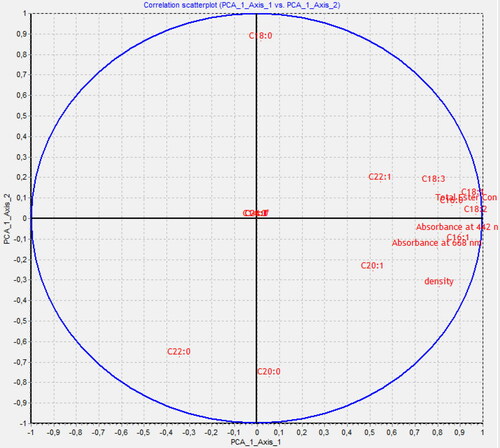
Principal components analysis (PCA) was performed based on all measured concentrations of fatty acids methyl esters and the absorption maxima for carotenoids and chlorophyll dyes in the VIS range in FAME samples treated with ionizing radiation. According to the PCA analysis, the three main components contain 82.93% of the information. PCA1 (55.41%) and PCA2 (15.00%) as shown in the figure cover as much as 70.41% of the information contained in the measurement data. Based on this information, a PCA analysis can be applied to the description of the examined dataset.
The purpose of the analysis was to identify the relationship between the change in absorbance and the concentration of methyl esters. As presented in the graph shown in , changes in absorbance in the VIS range are not related to changes in saturated ester concentrations such as C14:0, C18:0, C22:0 and C24:0. Similar as was described by Grabowski et al. [Citation18, Citation19], changes in absorbance correlate with changes in esters containing unsaturated bonds (double and triple), that is, they confirm the effects of ionizing radiation with polyunsaturated fatty acids (PUFAs). These correlations confirm the presence of all wavelengths and ester concentrations on the right side of the graph in . Additionally, unsaturated methyl esters as main components of FAME also impact on density of the measured samples.
Conclusions
Based on conducted research, it can be concluded that ionizing radiation causes changes in composition of rapeseed methyl esters. Analysis in ultraviolet–visible range indicates that electron beam mainly influences on unsaturated bonds in ester chains. Spectroscopic methods can be used only to preliminary study these changes on the basis changes of absorbance in the wavelength range characteristic of carotenoids dyes and chlorophyll dyes. Carotenoids, containing conjugated double bond systems, are very sensitive to ionizing radiation.
Esters most affected by electron radiation are methyl oleate, methyl linoleate and methyl linolenate. These are compounds which concentration in tested biodiesel is the highest. The most distinct change in content is in each case observed between the non-irradiated sample and the sample irradiated with 0.84 kGy. Further decrease is always linear with very good coefficient of determination (R2), which is 0.93 or 0.95. The results of total ester content are similar, with analogous R2 (0.95). Total ester content decreases from 96.93 wt% in non-irradiated sample to 94.93 wt% in a 121.35 kGy sample. Unfortunately, even the least irradiated sample does not meet the requirement of PN-EN 14214 concerning the ester content (96.5 wt%). Therefore, the use of ionizing radiation to improve RME properties can be used only in specific radiation dose range. The reduction in the total concentration of methyl esters was lower than after irradiation of FAME samples saturated with water and air, which was presented in previous works [Citation18, Citation19].
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Dehkhoda AM, West AH, Ellis N. Biochar based solid acid catalyst for biodiesel production. Appl Catal A. 2010;382(2):197–204.
- Lee KO, Chung KH. Method for producing biofuel using electron beam. US Patent 20080313954 A1.
- Lee RA, Lavoie JM. From first- to third-generation biofuels: challenges of producing a commodity from a biomass of increasing complexity. Animal Front. 2013;3(2):6–11.
- Demirbas A. Biodiesel – a realistic fuel alternative for diesel engines. London: Springer; 2008.
- Blin J, Volle G, Girard P, et al. Biodegradability of biomass pyrolisis oils: comparison to conventional petroleum fuels and alternative fuels in current use. Fuel. 2007;86(17–18):2679–2686.
- Wang WG, Lyons DW, Clark NN, et al. Emissions from nine heavy trucks fueled by diesel and biodiesel blend without engine modification. Environ Sci Technol. 2000;34(6):933–939.
- Pullen J, Saeed K. An overview of biodiesel oxidation stability. Renew Sust Energ Rev. 2012;16(8):5924–5950.
- Leathers L, Celina M, Chianelli R, et al. Systems Analysis and Futuristic Designs of Advanced Biofuel Factory Concepts Sandia Report – SAND2007-6872. 2007.
- Dole M. The radiation chemistry of macromolecules. New York: Academic Press; 1973.
- Kochetkov NK, Kudryashov Li, Chlenov Ma. Radiation chemistry of carbohydrates. Oxford: Pergamon Press; 1979.
- Schnabel W. Polymers and electromagnetic radiation – fundamentals and practical applications. Weinheim: Wiley-VCH; 2014.
- Woods RJ, Pikaev AK. Applied radiation chemistry: radiation processing. Canada: John Wiley & Sons; 1994.
- Clegg DW, Collyer AA. Irradiation effects on polymers. New York: Elsevier Science Publishing; 1991.
- Clough RL, Shalaby SW. Radiation effects on polymers. Washington, D.C: ACS Publications; 1991.
- Clough RL, Shalaby SW. Irradiation of polymers: fundamentals and technological applications. Washington, D.C: ACS Publications; 1996.
- Clough RL. High-energy radiation and polymers: a review of commercial processes and emerging applications. Nucl Instrum Methods Phys Res B. 2001;185(1–4):8–33.
- Kroh J. Selected issues of radiation chemistry. Warsaw: PWN; 1986. (in Polish)
- Grabowski P, Jarosiński P, Szajerski P, et al. Influence of electron beam irradiation on water-saturated biodiesel. J Radioanal Nucl Chem. 2018;318(2):1401–1408.
- Grabowski P, Tomkielski D, Szajerski P, et al. Changes of biodiesel composition after electron beam irradiation. J Radioanal Nucl Chem. 2019;319(3):727–736.
- Driscoll MS, Stipanovic AJ, Cheng K, et al. Ionizing radiation and a wood-based biorefinery. Radiat Phys Chem. 2014;94:217–220.
- Zhang H, Wang W, Wang H, et al. Effect of e-beam irradiation and microwave heating on the fatty acid composition and volatile compound profile of grass carp surimi. Radiat Phys Chem. 2017;130:436–441.
- Sundar S, Bergey NS, Salamanca-Cardona L, et al. Electron beam pretreatment of switchgrass to enhance enzymatic hydrolysis to produce sugars for biofuels. Carbohydr Polym. 2014;100:195–201.
- Kovacs E, Keresztes A. Effect of gamma and UV-B/C radiation on plant cells. Micron. 2002;33(2):199–210.
- Joe MH, Kim JY, Lim S, et al. Microalgal lipid production using the hydrolysates of rice straw pretreated with gamma irradiation and alkali solution. Biotechnol Biofuels. 2015;8:125.
- Karolczak S, Hodyr K, Łubis R, et al. Pulse radiolysis system based on ELU-6E LINAC. J Radioanal Nucl Chem. 1986;101(2):177–188.
- Czerwinska M, Sikora A, Szajerski P, et al. Mechanistic aspects of alloxan diabetogenic activity: a key role of keto-enol inversion of dialuric acid on ionization. J Phys Chem A. 2006;110(22):7272–7278.
- PN-EN 14103 fat and oil derivatives – Fatty Acid Methyl Esters (FAME) – determination of ester and linolenic acid methyl ester contents.
- Galanakis CM. Extraction of carotenoids and applications. Carotenoids: properties, processing and applications. London: Elsevier; 2020. p. 259–288.
- Koike ACR, Araujo ES, Almeida-Muradian AB, et al. Analysis of carotenoids in edible flowers of Dianthus chinensis processed by ionizing radiation. In: International Nuclear Atlantic Conference, Santos, SP, Brazil: 2007–2014; 2019.
- Knothe G, van Gerpen J, Krahl J. The biodiesel handbook. USA: AOCS; 2005.
- Knothe G. Improving biodiesel fuel properties by modifying fatty ester composition. Energy Environ Sci. 2009;2(7):759–766.
- Kumar S. Spectroscopy of organic compounds. India: National Science Digital Library at NISCAIR; 2006.
- Pinnola A, Staleva-Musto H, Capaldi S, et al. Electron transfer between carotenoid and chlorophyll contributes to quenching in the LHCSR1 protein from Physcomitrella patens. Biochim Biophys Acta. 2016;1857(12):1870–1878.
- PN-EN 14214 Liquid petroleum products – fatty acid methyl esters (FAME) for use in diesel engines and heating applications – requirements and test methods.
- IAEA. Controlling of degradation effects in radiation processing of Polymers. Vienna: IAEA; 2009.
- Navarro R, Burillo G, Adem E, et al. Effect of ionizing radiation on the chemical structure and the physical properties of polycaprolactones of different molecular weight. Polymers. 2018;10(4):397.
- Kleinova A, Cvengrosova Z, Rimarcik J, et al. Biofuels from algae. Procedia Eng. 2012;42:231–238.
- Parejo Calvo WA, Duarte CL, Machado LDB, et al. Electron beam accelerators—trends in radiation processing technology for industrial and environmental applications in Latin America and the Caribbean. Radiat Phys Chem. 2012;81(8):1276–1281.
- Jairurob P, Phalakornkule C, Na-Udom A, et al. Reactive extraction of after-stripping sterilized palm fruit to biodiesel. Fuel. 2013;107:282–289.
- ISO 11137-2:2013. Sterilization of health care products – radiation – Part 2: Establishing the sterilization dose.
- Rakotomalala R. TANAGRA: a free software for research and academic purposes. Proc EGC 2005 RNTI-E-3. 2005;2:697–702. (in French)