Abstract
Background: Patients with Sjögren’s syndrome (SS) have a considerable higher risk of lymphoma development.
Objectives: To determine the incidence of lymphoma and the value of biomarkers to predict lymphoma development in patients with SS.
Methods: Clinical files of all patients with a presumed diagnosis of SS between 1991 and 2016 were retrospectively reviewed for the development of lymphoma. Biochemical data were plotted as a function of the relative time before and after the lymphoma diagnosis (for patients who developed lymphoma) or before the last available blood test (for patients who did not develop lymphoma). Correlations between several biochemical parameters and development of lymphoma were analyzed by logistic regression. In order to evaluate the evolution of cryoglobulins, a random effect model with random intercepts was used.
Results: Sixteen patients developed a lymphoma (prevalence 8.9%; median follow-up 6 years). Cryoglobulins were significantly higher in these patients (n = 16), when compared to the rest of patients (n = 164) without lymphoma (121 ± 250 versus 8 ± 24.9 mg/L for IgG; 231 ± 422 versus 13 ± 30 mg/L for IgM; 10 ± 20 versus 1 ± 4 mg/L for IgA in the cryoprecipitate). Cryoglobulin-levels were significantly more increasing (p-values for IgG = 0.0007; for IgM = 0.0123; and for IgA in the cryoprecipitate <0.0001) in the time period before the lymphoma diagnosis (patients with lymphoma) compared to the time period before the last available blood test (patients without lymphoma). Also low (i.e. under the detection limit) C3 (OR 13.9) or C4 (OR 7.1) levels, a progressively decreasing total complement activity (OR 6.6), progressively decreasing gammaglobulins (OR 13.4), a persistent detection of monoclonal bands (OR 14.6) on protein electrophoresis, a persistent low or decreasing serum IgG (OR 18), and decreasing IgM-serum levels (OR 17.7) were significantly associated with lymphoma.
Conclusion: Periodically follow-up of laboratory markers, such as cryogloblins, over time proved to be an accurate way to predict lymphoma.
Introduction
Sjögren’s syndrome (SS) is a chronic auto-immune disorder with a broad range of clinical manifestations, of which the development of lymphoproliferative diseases is the most severe.
The connection between SS and lymphoma was first reported by Talal and Bunim in 1964 [Citation1]. Subsequent studies showed that patients with SS have a 16–40 times higher risk of lymphoma, compared to the age-matched general population [Citation2–4]. Because lymphoma development is the main cause of decreased survival in SS patients [Citation5–10], it is important to designate laboratory, clinical and/or histopathological characteristics in order to identify patients who are at greater risk.
The presence of mixed cryoglobulinemia (MC) is commonly seen as a crossroad between autoimmune diseases and cancer. In SS patients, it is generally known to be the most reliable biochemical predictor of lymphoma development [Citation8,11–17] and inferior median survival [Citation8,13,15,18]. Also low complement C3 [Citation4,10,18] and C4 [Citation4,5,8,10,12,15–20] levels have been associated with lymphoma and overall higher mortality rates. The majority of SS patients display a diffuse hypergammaglobulinemia [Citation8,12] and this vigorous expansion makes them probably more susceptible to mutational events [Citation6,12]. Because monoclonality is correlated with Non-Hodgkin lymphoma (NHL) transition [Citation14,17], the presence of a monoclonal protein [Citation6,12,14,17,21–23] and urinary free light chains [Citation17,21] could identify patients with higher risk. Also low or decreasing gammaglobulins [Citation1,17] and high β2-microglobulin levels have been proposed as risk factors [Citation12,17]. Although serum IgG levels generally did not appear to be relevant [Citation4], low serum IgM levels [Citation12] were rarely associated with lymphoma. Some textbooks suggest a role of the disappearance of previously positive autoantibodies as a predictive biomarker for lymphoma development [Citation17]. Several other blood tests, such as anemia [Citation9] or a falling packed-cell volume [Citation6], leucopenia [Citation1,16], neutropenia [Citation8], lymphopenia [Citation1,9], CD4 + T-lymphocytopenia [Citation4,12,17], a high sedimentation rate [Citation9], the presence of anti-SSB/La antibodies [Citation16], and detection of monoclonal rheumatoid factor-associated crossreactive idiotypes (CRI) [Citation12,13] have also been mentioned as risk factors.
The most described clinical predictor of lymphoma development is an enlargement of the parotid gland [Citation3,6,12,16,19,22]. Also lymphadenopathy and splenomegaly [Citation1,3,6,8,9,12], the presence of skin vasculitis (i.e. palpable purpura and ulcers) [Citation1,4,9,12,15,19,22], peripheral neuropathy [Citation9,12] and fever [Citation9] have been associated with lymphoma.
Finally, aggressive histopathological features [Citation5,14,22,24] in labial gland biopsies indicate lymphoma development. Theander and colleges even stated that the negative predictive value of the absence of germinal center-like lesions in labial salivary gland biopsies achieved almost 99% [Citation4] and many authors agree that biopsies should be obtained more frequently as part of databases for prognostic purposes [Citation4,24–26].
However, a more specific biochemical characterization of patients is needed in order to detect patients at higher risk for lymphoma development. Some of the results of previously performed studies show severe discrepancy in significance. It is also assumable that blood tests in SS patients demonstrate different patterns over time (i.e. transient detection of cryoglobulins versus continuously increasing levels), while in most previous studies blood tests were only evaluated once at the first study visit instead of a more accurate longitudinal biochemical follow-up.
Materials and methods
Study design and enrolment criteria
In this observational monocenter study, a database of 247 patients with possible SS was recorded. Medical charts (dated from the period of 1991 until 2016) were retrospectively analyzed. The following inclusion criteria were used: (1) fulfilling either the American-European Consensus Group (AECG) [Citation27] or/and American College of Rheumatology (ACR) [Citation7] classification criteria; or (2) fulfilling all three of the following criteria 2a–c: (2a) a sicca-syndrome (subjective and/or objective i.e. positive Schirmer’s test or Tear Break Up Time < 10 seconds); (2b) at least one objective biochemical (i.e. positive serum anti-SSA/Ro and/or anti-SSB/La or positive rheumatoid factor and ANA titer ≥ 1:320) or histopathological (labial salivary gland biopsy exhibiting focal lymphocytic sialadenitis with a focus score ≥ 1 focus/4 mm2 [Citation28]) characteristic of auto-immune activity; and (2c) absence of another possible explanation for either 2a or 2b. 47 patients did not meet these inclusion criteria and were excluded (see Appendix A). Also patients with less than two disease-specific hospital visits were excluded (N = 20). None of the remaining patients were forwarded to our tertiary center due to disease complications like the development of a lymphoma. In case of any clinical or biochemical suspicion for lymphoma, further investigations (including imaging studies or biopsies) were performed.
Demographics and clinical characteristics
All retained patients were divided into primary and secondary SS. Current age, age at diagnosis, age at start of follow-up (FU), start and end date of FU, the number of disease-specific hospital visits, and current health status were documented.
All medical histories were checked for lymphoproliferative diseases. In patients with lymphoma, additional information was documented: date of lymphoma diagnosis, anatomopathological features, affected organs, staging according Ann Arbor staging system, used treatment modalities, and the evolution of disease (progression or recurrence).
Biochemical characteristics
For the detection and quantification of cryoglobulins, blood samples were taken by venipuncture in pre-heated (to 37 °C) 10 mL serum tubes. Until the serum was separated, all tubes were maintained at 37 °C. The serum samples were transported at ambient temperature, and reheated to 37 °C before further processing [Citation29]. Aliquots of serum were allowed to precipitate at 4 °C for 7 days. These samples were washed three times with phosphate-buffered saline (PBS) at 4 °C and dissolved in PBS at 37 °C. This material was subjected to electrophoresis and identification of monoclonal proteins. Individual immunoglobulin levels (IgA, IgG, and IgM) were quantified with NOR-Partigen® reagents on radial immunodiffusion until 2004, Turbiquant® reagents on Turbitimer (Dade-Behring) until 2012, and thereafter on Immage (Beckman Coulter) [Citation29].
Antinuclear antibodies were detected by indirect immunofluorescence using HEp2000 cells. Anti-SSA and anti-SSB antibodies were identified by counterimmunoelectrophoresis until 2003 and by fluoroenzyme immunoassay (EliA, Thermo Fisher) after 2003.
Complement C3 and C4 and IgG, IgA, and IgM in serum was quantified by nephelometry (BN100, Siemens Dade Behring) with reagents from Dade Behring until December 1997, and thereafter with Immage (Beckman Coulter) with reagents from Beckman Coulter.
Serum protein electrophoresis was performed by sepharose cellulose polyacetate electrophoresis (Microzone System) (Gelman Sciences) until January 1998. From January 1998, capillary electrophoresis was performed (Paragon, Beckman Coulter until March 2006; thereafter Capillarys, Sebia) [Citation30,31].
Based on all available serological laboratory results, patients were categorized in biochemical groups with increasing suspicion of disease activity as follows:
| • | cryoglobulins (immunofixation and quantification of IgG, IgM, and IgA in the cryoprecipitate): (1) never detectable; (2) transiently increased levels; (3) always increased; and (4) progressively increasing; | ||||
| • | complement C3 and for C4: (1) normal values; (2) transiently too low; (3) always too low; and (4) descending or undetectable; | ||||
| • | total complement activity (TCA): (1) normal; (2) transiently increased; (3) always increased; (4) transiently decreased; and (5) always decreased; | ||||
| • | high β-globulin and high γ-globulin (protein electrophoresis): (1) normal; (2) transiently increased; (3) always increased; and (4) progressively increasing; | ||||
| • | low β-globulin and low γ-globulin (protein electrophoresis): (1) normal; (2) transiently decreased; (3) always decreased; and (4) progressively decreasing; | ||||
| • | presence of a monoclonal spike (protein electrophoresis): (1) never; (2) intermittent; and (3) permanent; | ||||
| • | high IgG and high IgM: (1) normal/decreased; (2) transiently increased; (3) always increased; and (4) progressively increasing; | ||||
| • | low IgG and low IgM: (1) normal/increased; (2) transiently decreased; (3) always decreased; and (4) progressively decreasing. | ||||
| • | ANA’s: (1) always negative; (2) always positive; (3) disappearance of previously positive ANA’s or anti-Ro/SSA or anti-La/SSB antibodies. | ||||
Statistical analysis
Patients were divided into patients with lymphoma and patients without lymphoma. Two different types of analyses were performed.
First, we performed a random effects model with random intercepts. The dates of performed blood tests were converted to relative time variables compared to the date of lymphoma detection (for patients with lymphoma) or compared to the date of the last performed blood tests (for patients who did not develop lymphoma). Consequently only patients with lymphoma could have positive time variables. Individual profiles of IgM-, IgG-, and IgA-cryoprecipitate levels with their corresponding time variable were plotted with R Studio®. Further descriptive statistics were carried out using SAS® 9.4 (Statistical Analysis Software). The skewed original data were transformed by the formula y = ln (cryoprecipitate + 1) and the transformed marginal regression lines (see Appendix C) were only analyzed for measurements before lymphoma diagnosis (i.e. time variables less than zero).
Secondly, separate logistic regressions were performed to explore the relationship between lymphoma as a dependent dichotomous variable and the biochemical class variables (see biochemical groups in ‘Biochemical charateristics,’ ‘Materials And Methods’). Patients with only one positive cryoglobulin-result and patients with less than two available results of the other biochemical tests were not analyzed. The likelihood ratio chi-square test, as well as Score and Wald tests were used to explore the relationship between the categorical variables. Significance was determined by Fisher’s exact Test. A 2-sided p value < 0.05 was considered significant. Finally, also Odds Ratios (OR) with their 95% Confidence Interval (CI) were calculated.
Ethics
This study was approved by the Institutional Ethics Committee of the University Hospitals Leuven.
Results
Demographics
All patients (N = 180) were divided into primary (N = 144) and secondary (N = 36) SS. The median time of FU was 6 years (range from 1 month to 24 years). The majority (75%, N = 27) of secondary syndromes were considered to be secondary to Systemic Lupus Erythematosus (SLE). The remaining patients were equally divided and secondary to Rheumatoid Arthritis (RA), Systemic Sclerosis or CREST-syndrome (8.3% or N = 3 for each disease).
Of patients with primary SS (pSS) (N = 144), 30% (N = 43) fulfilled the AECG-classification criteria, 6% (N = 9) the ACR-criteria and 35% (N = 50) fulfilled both. The remaining patients lacked criteria testing because not all of the requisite tests were performed, yet were still diagnosed by a physician as having Sjögren’s and fulfilled the inlusion criteria. Six of fourteen pSS patients with a lymphoma (43%) fulfilled the AECG- and none the ACR-classification criteria. One of two patients with secondary SS (sSS) with lymphoma fulfilled the ACR-criteria. Two of sixteen lymphoma patients did not fulfill the criteria due to preceding lymphoma (before SS diagnosis) but would have been included otherwise (see Table ).
Table 1. Characteristics of SS patients with lymphoma.
In the overall study population, thirteen patients died (7%). Notably, five of them (38%) had a history of lymphoma: Marginal Zone lymphoma (MZL, N = 3), Hodgkin lymphoma (HL, N = 1) and diffuse large B-cell lymphoma (DLBCL, N = 1). The HL-patient died due to an overwhelming pneumonia secondary to sepsis after a leg amputation for severe SS vasculitis and ulcers. Five percent (N = 8) of non-lymphoma patients died. In those patients, causes of death were assumed to be secondary to an ovary carcinoma (N = 2), thrombotic aneurysm (N = 1), congestive cardiomyopathy without clear etiology (N = 1) or unknown (N = 4). Lymphoma development was correlated with a significant higher risk of death (p-value 0.0008; OR 8.9 with CI 2.5–31.7).
Lymphoproliferative disease
Of all 180 patients, sixteen developed a lymphoma (overall prevalence 8.9%). An overview of all patients with a lymphoma is given in Table . Two patients in whom the diagnosis and treatment of lymphoma preceded the diagnosis of SS, were also taken into account. In the other 14 patients, the median time of FU before lymphoma detection was 4.5 years (range 0 to 20 years with an average of 6.1 years). One patient was simultaneously diagnosed as having SS and lymphoma.
The prevalence of lymphoma was 9.7% (N = 14) in pSS and 5.6% (N = 2) in sSS, but this difference was not significant (p = 0.3892). The median age at lymphoma diagnosis was 59 years (range 21 to 70 years). The median age at SS diagnosis for lymphoma patients was 55 year, compared to 46 years in the overall study population. Non-Hodgkin type (NHL) was more common (88%), compared to HL. The predominant histological subtypes were MZL (44%; most commonly MALT-subtype: 38%) and DLBCL (38%). One patient developed both subtypes sequentially. Five of the sixteen included lymphoma patients (31%) died during a median FU of 6.5 years (range 0 to 32 years; average 8.4 years).
Cryoglobulins
The median number of cryoglobulin measurements per patient was 4. Individual profiles of all patients were plotted for the IgG, IgM, and IgA concentration in the cryoprecipitate (see Figures a, b and c, respectively). For each of the three tests, the slopes (i.e. increase in cryoglobulin values) were significantly different between patients with versus patients without lymphoma (0.024 versus −0.001 with a p-value = 0.0007 for IgG; 0.006 versus −0.006 with a p-value = 0.0123 for IgM; and 0.018 versus −0.000 with a p-value ≤ 0.0001 for IgA in the cryoprecipitate). Patients without lymphoma had slightly downwards (negative) slopes, although almost equal to the baseline 0 (i.e. there was no overall increase in cyoglobulins over time). On the other hand, patients who developed a lymphoma had a positive trend for each of the three immunoglobulin concentrations in the cryoprecipitate (i.e. overall the cryoglobulins tended to increase in the period before lymphoma diagnosis). Note that any transient increase in cryoglobulins (with levels subsequently descending back to zero before lymphoma diagnosis or before the last available blood test) does not result in a positive slope.
Figure 1a. Individual profile plots for serum cryoglobulin IgG-precipitate.
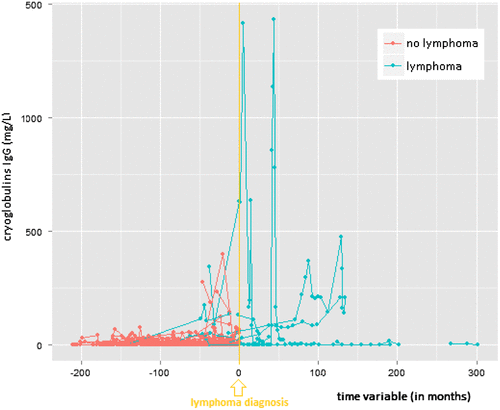
Figure 1b. Individual profile plots for serum cryoglobulin IgM-precipitate.
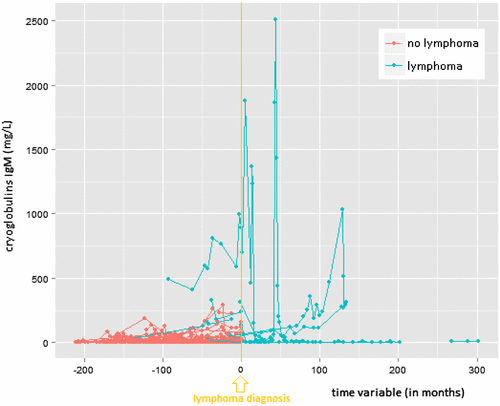
Figure 1c. Individual profile plots for serum cryoglobulin IgA-precipitate.
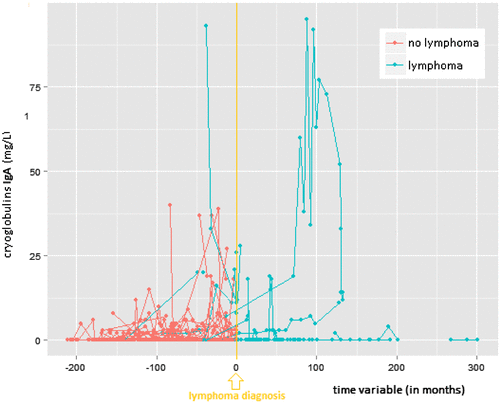
Mean values with standard deviation (±SD) for the IgG concentration in the cryoprecipitate were: 121 ± 250 mg/L (range 0 to 1430) versus 8 ± 24.9 mg/L (range 0 to 397) for patients with versus patients without lymphoma. Respective mean values (lymphoma versus no lymphoma) for the IgM and IgA concentration in the cryoprecipitates were: 231 ± 422 mg/L (range 0 to 2507) versus 13 ± 30 mg/L (range: 0 to 293), and 10 ± 20 mg/L (range 0 to 95) versus 1 ± 4 mg/L (range 0 to 40). The mean levels were significantly different between lymphoma and non-lymphoma groups for each of the three immunoglobulin concentrations in the cryoprecipitate (all p-values < 0.0001).
The distribution of different cryoglobulin groups and relative number of patients with lymphoma per group is shown in Table . When groups 2, 3, and 4 (see section ‘Biochemical characteristics’ in ‘Materials and methods’) were compared separately with logistic regressions, only patients in group 4 (i.e. progressively increasing cryoglobulins) had a significant higher risk for lymphoma development (p-value = 0.0001; OR 124.0 with CI 10.4- > 999.9) compared to group 1 (i.e. patients without cryoglobulinemia). Patients with a transient presence of cryoglobulins (i.e. group 2) did not have a significantly higher risk (p-value = 0.1168), and the p-value for a steady cryoglobulinemia (i.e. group 3) was borderline insignificant (p-value = 0.0604). When the same analysis was performed in only pSS-patients, also group 3 appeared to be significant (p-value = 0.0329; OR 8.6 with CI 1.2-61.5). Because all pSS patients in cryoglobulin group 4 developed lymphoma, the OR for group 4 could not be calculated. pSS-patients with a transient cryoglobulinemia (i.e. group 2) did not have a significant higher risk for lymphoma development (p-value = 0.5187).
Table 2. Distribution of different cryoglobulin groups (in %) and relative number of patients with lymphoma per group (in %).
Based on these findings, groups 3 and 4 were merged. Patients with a permanent or increasing (i.e. either group 3 or 4) cryoglobulinemia had a significantly higher risk for lymphoma development compared to patients without cryoglobulins for the overall study population (p-value = 0.0003; OR 16.9 with CI 3.7–77.3) as well as for the pSS-group separately (p-value = < 0.0001; OR 25.7 with CI 5.1–129.2).
In the overall study population, only group 4 was significantly associated with death (p-value 0.0053; OR 20.7 with CI 2.5–173.4). The p-value for group 2 was 0.2722 and for group 3 0.060. In pSS patients separately, also patients in group 3 (p-value 0.0329; OR 8.6 with CI 1.2–61.5) had a significant higher risk to die.
Hypocomplementemia
The distribution of patients in different C3- and C4-groups is given in Table . For both C3 (OR 13.9 with CI 1.7–114.0 and p-value = 0.0144) and C4 (OR 7.1 with CI 1.5–33.3 and p-value = 0.0137), only patients with either decreasing or persistent immeasurable low values (i.e. group 4) had a significant higher risk for lymphoma development compared to patients with normal complement levels. When pSS and sSS patients were evaluated separately, the same findings were confirmed for only pSS patients (OR 26.7 with CI 2.1–338.0 for C3; and 11.2 with CI 1.9–66.5 for C4). Patients with low, but measurable complement-levels (p-value = 0. 8970 and 0.5656 for C3 and C4 respectively) or patients with only intermittent finding of hypocomplementemia (p-values = 0.9600 and 0.6171, respectively) had no significant higher risk. Also a continuously decreased total complement activity (TCA) was a significant predictor for lymphoma development (p-value = 0.0176; OR 15.5 with CI 1.6–148.9), while a transient decreased TCA was not (p-value = 0.2681). Only decreasing or persistent immeasurable low C4 values (i.e. group 4) were significantly associated with death (p-value = 0.0152; OR 6.6 with CI 1.4–30.1).
Table 3. Distribution of different complement groups (in %).
Serum protein electrophoresis
The distribution of patients based on protein electrophoresis results are depicted in Table . Progressively decreasing gammaglobulins were a significant risk factor for lymphoma development in the overall population (p-value = 0.0137 and OR 13.4 with CI 1.7–105.4). A transient or persistent but steady hypogammaglobulinemia was not significantly correlated with lymphoma (p-values = 0.6422 and 0.2994, respectively). Also a persistent detection of monoclonality was a significant lymphoma predictor in the primary (p-value = 0.0003; OR 14.6 with CI 3.4–62.6) and overall group (p-value < 0.0001; OR 13.9 with CI 3.7–51.9), while a transient detection was not significant (p-value 0.0563 in pSS). No significant correlation between any of the hypergammaglobulinemia, hypobetaglobulinemia, or hyperbetaglobulinemia groups and lymphoma was found.
Table 4. Distribution of different protein electrophoresis groups (in %).
Serum immunoglobulins
Patients with persistent low or decreasing IgG serum levels had 18 times more chance to develop a lymphoma when compared to patients with normal IgG (CI 3.1–104.4; p-value = 0.0012). Patients with only transient decreased IgG levels did not have a significant higher risk (p-value = 0.9800). Also progressively decreasing IgM levels were associated with a significant higher lymphoma risk in the overall study population (p-value = 0.0007; OR 17.7 with CI 3.6–93.0) and pSS-group separately (p-value = 0.0051; OR 16.8 with CI 2.3–120.7). High serum IgG or IgM levels were not associated with lymphoma development.
Autoantibodies
In five of sixteen patients who developed a lymphoma, data were missing for an analysis: in 3 patients the lymphoma was diagnosed before the first ANA blood test and in 2 patients no autoantibodies were checked at the time of lymphoma diagnosis. In one of the remaining 11 lymphoma patients, the ANA’s were consistently not elevated (neither at the time of SS diagnosis nor at the time of lymphoma diagnosis). The remaining 10 patients who developed a lymphoma all had positive ANA’s (always anti-Ro/SSA or anti-La/SSB), but in none of them these antibodies disappeared during or shortly before/after the lymphoma diagnosis. Because of these observations, no further analysis was performed.
Discussion
The prevalence of lymphoma (8.9% with a median FU of 6 years) did not differ from earlier observations (4–11%) [Citation5–9]. Also the median age at which lymphoma was diagnosed (53 years) was in accordance with previous observations [Citation9,12], although our median time of FU before lymphoma diagnosis was slightly shorter (4.5 versus 7 years) [Citation9,24]. Congruent to the literature [Citation3,4,8,9,12,13,32,33], the most common subtypes were MZLs (44%) and DLBCLs (38%). In a multicenter European study of 33 malignant NHL in SS patients, a MZL was found most commonly (in 48.5%), being mostly extranodal (78.8%) and most often identified in the salivary glands (54.6%) [Citation9]. In our study, six of seven MZLs (86%) were identified in the salivary glands (i.e. MALT-subtype): five in the parotid and one in the submandibular gland. MALT lymphomas are relatively indolent [Citation32] and no difference in median survival for treated and untreated patients was found previously (median FU 6 years). In contrary, DLBCLs tend to be highly aggressive (median overall survival of only 1.8 years in SS patients) [Citation9,12]. Five of our sixteen lymphoma patients (31%) died during a median FU of 6.5 years: 50% of patients with MALT-lymphoma and 17% of DLBCL-patients during a median FU of 12.5 and 3 years, respectively.
In accordance to previous studies [Citation8,11–16], we were able to confirm that cryoglobulin levels were significantly higher in patients who developed a lymphoma compared to patients who did not develop lymphoma. Because we performed direct quantification of immunoglobulins in the cryoprecipitate in combination with agarose gel electrophoresis, we were able to quantify the levels of the immunoglobulins in the cryoprecipitate and false positive results due to contamination by serum proteins were avoided [Citation29]. Remarkably, only patients with a steady high or increasing cryoglobulinemia seemed to have a significant higher lymphoma risk (OR 17 in total study population; and OR 26 in pSS-patients), while patients with only a transient increase had no significant higher risk (p-value = 0.1168; and 0.5187 in pSS). These findings were strengthened by our findings that cryoglobulins significantly increased in patients before detection of a lymphoma, compared to patients without the development of a lymphoma. Patients with an increasing cryoglobulinemia also had a higher risk of death (OR 21) compared to those with a steady unaltered cryoglobulinemia (OR 9).
We noticed a remarkable higher prevalence of hypocomplementemia (22% of pSS-patients expressed low C3 values and 54% low C4) compared to other studies (12% and 12%, respectively) [Citation20]. In sSS patients, the prevalence was even higher: 45 and 61%, respectively. Previous studies showed that patients with type II or III cryoglobulinemia had decreased levels of C4 [Citation29]. We found that only decreasing or persistent immeasurable low C3 or C4 levels were significant predictors for lymphoma. Immeasurable C3 levels included a higher risk for lymphoma development than immeasurable C4 (OR 14 versus 7). In a long-term FU study, all patients with lymphoma-related death presented with either low C4 levels or palpable purpura at their first study visit [Citation19]. In our longitudinal study only a decreasing or persistent immeasurable low C4 was associated with death.
Monoclonal bands were seen in 19% of all SS patients, and these findings are comparable with those in other publications [Citation23]. We confirmed that especially a persistent detection of monoclonal bands on protein electrophoresis should raise attention for lymphoma development (OR 15 with CI 3.4–62.6). While low serum IgM levels have been already proposed to be a risk factor [Citation12], decreasing or low IgG levels had not yet been identified as significant so far [Citation4]. However, these results should be interpreted carefully, since long-term use of corticosteroids could be a major contributing factor in these results.
The disappearance of previously positive autoantibodies has been reported as a risk for lymphoma development in some textbooks [Citation17], however, there are few data in the literature validating this. In the patients with positive autoantibodies (always anti-Ro/SSA or anti-La/SSB) who developed a lymphoma in this study, these antibodies never disappeared during or shortly before/after the lymphoma diagnosis.
38% of patients who died had a lymphoma and patients with lymphoma (N = 5/16; 31%) had a significant higher risk of death than patients without lymphoma (N = 8/164; 5%) (p-value 0.0008; OR 8.9 with CI 2.5–31.7). In other studies, excess mortality for pSS was only found for lymphoproliferative malignancy [Citation10]. In a long-term FU study of 723 Greek pSS patients, 20% of death was attributable to lymphoma [Citation19].
In contrary to other clinical studies, we did not necessarily exclude patients if they did not fulfill the classification criteria. Of our patients who did not meet the classification criteria, 86% had at least a combination of a sicca-syndrome and characteristic SSA or SSB-antibodies. Most authors agree that patients with this combination can be reliable diagnosed with SS [Citation6]. Notably, other authors published that excess mortality due to lymphoma was found only in patients who fulfilled the AECG-criteria (with 484 patients and a median FU of 7 years [Citation10] and with 507 patients and a median FU of 8 years [Citation4]). However, in our study only 43% of pSS and 50% of sSS patients with lymphoma fulfilled at least one of the ACR- or AECG-criteria. These findings suggest that, also in clinical studies, caution should be taken with excluding patients based on classification criteria. Patients with milder and asymptomatic forms of the syndrome (i.e. in early stage of disease) could be falsely misdiagnosed [Citation6,27]. At least in clinical settings, classification criteria should be used as a supportive tool and cannot be considered the golden standard for diagnosis [Citation7,27].
Strength and weakness
Although many other authors only evaluated primary SS-patients, the differential diagnosis between different auto-immune modalities can be challenging in clinical settings. In our study, two patients with sSS developed a lymphoma, and therefore, we suggest to evaluate those patients as well in future studies.
We also attempted to mimic daily clinical setting as good as possible by not obligatory using classification criteria for inclusion. This prevented exclusion of several patients who developed a lymphoma during FU. On the other hand, not using validated criteria for patient selection increases the risk of misclassification and selection bias.
We characterized different biochemical patterns over time (i.e. longitudinally), opposed to the static evaluations in several other studies. However, a considerable amount of data were missing for analysis and a substantial amount of patients could not be evaluated due to the availability of less than two blood tests. Due to the relatively small number of patients, no significant results could be obtained in several analyses (in particular in the sSS-group with only 34 patients).
Conclusion
Lymphoma development is the major cause of decreased survival in patients with SS and also occurs in patients with secondary SS. In this retrospective monocenter cohort study of 180 primary and secondary Sjögren’s patients, 16 lymphomas (prevalence 8.9%) were found (median FU of 6 years). Patients who developed a lymphoma had significantly higher levels of cryoglobulins than those who did not. Patients with a chronic cryoglobulinemia had a 17 times higher risk for lymphoma development (OR 26 in pSS-patients only), while a transient increase in cryoglobulins was no significant predictor. Especially (rapidly) increasing cryoglobulin levels should raise suspicion, since cryoglobulins increased significantly more in patients before detection of a lymphoma, compared to patients with no lymphoma development. Occurrence of lymphoma in SS-patients could also be predicted by the presence of decreasing or persistent immeasurable low C3- or C4-levels, a continuously decreased TCA, progressively decreasing gammaglobulins or persistent detection of monoclonal bands on protein electrophoresis, persistent low or decreasing serum IgG, and decreasing IgM serum levels. A repeatedly clinical and biochemical assessment including these laboratory tests is therefore recommended in all patient with SS. A disappearance of previously positive autoantibodies (anti-Ro/SSA or anti-La/SSB) was not seen in any of the patients who developed a lymphoma.
Keypoints
| • | Lymphoma development is the major cause of decreased survival in patients with Sjögren’s syndrome. | ||||
| • | 16 of 180 SS patients were diagnosed with a lymphoma (prevalence = 8.9%) at a median follow-up of 6 years | ||||
| • | Patients who developed a lymphoma had significantly higher levels of cryoglobulins than those who did not. | ||||
| • | Especially (rapidly) increasing cryoglobulin levels should raise suspicion, while a transient increase in cryoglobulins was no significant predictor. Also the evaluation of complement, gammaglobulins, monoclonal paraproteinemia, and serum IgG and IgM levels can be useful in lymphoma prediction. Because the evaluation of trends seem to be more predictive than single lab results, a repeatedly biochemical assessment is recommended in all patients with SS. | ||||
Conflicts of interest
No potential conflict of interest was reported by the authors.
Funding Sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
| List of abbreviations | ||
| SS | = | Sjögren’s syndrome |
| MC | = | mixed cryoglobulinemia |
| AECG | = | American-European consensus group |
| ACR | = | American College of Rheumatology |
| anti-SSA/Ro | = | anti-Sjögren’s syndrome A antibodies |
| anti-SSB/La | = | anti-Sjögren’s syndrome B antibodies |
| ANA | = | antinuclear antibodies (also known as ANF = antinuclear factor) |
| FU | = | follow-up |
| PBS | = | phosphate-buffered saline |
| Ig | = | Immunoglobulin |
| SAS | = | statistical analysis software |
| OR | = | odds ratio |
| CI | = | 95% confidence interval |
| SLE | = | systemic lupus erythematosus |
| RA | = | rheumatoid arthritis |
| CREST | = | calcinosis, raynaud phenomenon, esophageal dysmotility, sclerodactyly, and telangiectasia |
| pSS | = | primary Sjögren’s syndrome |
| sSS | = | secondary Sjögren’s syndrome |
| l.l. | = | lymphoplasmacytic lymphoma |
| MZL | = | marginal zone lymphoma |
| nMZL | = | nodal marginal zone lymphoma |
| HL | = | Hodgkin lymphoma |
| DLBCL | = | diffuse large B-cell lymphoma |
| NHL | = | non-Hodgkin lymphoma |
| MALT | = | mucosa-associated lymphoid tissue |
| SD | = | standard deviation |
| TCA | = | total complement activity |
Acknowledgements
We would like to thank An Carbonez and Alexandra Likhareva for their excellent statistical advice.
References
- Talal N, Bunim JJ. The development of malignant lymphoma in the course of Sjögren’s syndrome. Am J Med. 1964;36:529–540.10.1016/0002-9343(64)90101-9
- Zintzaras E, Voulgarelis M, Moutsopoulos H. The risk of lymphoma development in autoimmune disease: a meta-analysis. Arch Intern Med. 2005;165:2337–2344.10.1001/archinte.165.20.2337
- Kassan SS, Thomas TL, Moutsopoulos HM, et al. Increased risk of lymphoma in Sicca syndrome. Ann Intern Med. 1978;89:888–892.10.7326/0003-4819-89-6-888
- Theander E, Henriksson G, Ljunberg O, et al. Lymphoma and other malignancies in primary Sjögren’s syndrome: a cohort study on cancer incidence and lymphoma predictors. Ann Rheum Dis. 2006;65:796–803.10.1136/ard.2005.041186
- Tzioufas AG, Kapsogeorgou EK, Moutsopoulos HM. Pathogenesis of Sjögren’s syndrome: what we know and what we should learn. J Autoimmun. 2012;39:4–8.10.1016/j.jaut.2012.01.002
- Fox RI. Sjögren’s syndrome. Lancet. 2005;366:321–331.10.1016/S0140-6736(05)66990-5
- Shiboski SC, Shiboski CH, Criswell LA, et al. American College of Rheumatology classification criteria for Sjögren’s syndrome: a data-driven, expert consensus approach in the Sjögren’s International Collaborative Clinical Alliance Cohort. Arthritis Care Res. 2012;64:475–487.10.1002/acr.21591
- Baimpa E, Dahabreh IJ, Voulgarelis M, et al. Hematologic manifestations and predictors of lymphoma in primary Sjögren syndrome: clinical and pathophysiologic aspects. Medicine (Baltimore). 2009;88:284–293.10.1097/MD.0b013e3181b76ab5
- Voulgarelis M, Dafni UG, Isenberg DA, et al. Malignant lymphoma in primary Sjögren’s syndrome: a multicenter, retrospective, clinical study by the European Concerted Action on Sjögren’s syndrome. Arthritis Rheum. 1999;42:1765–1772.10.1002/(ISSN)1529-0131
- Theander E, Manthorpe LT, Jacobsson LT. Mortality and cause of death in primary Sjögren’s syndrome: a prospective cohort study. Arthritis Rheum. 2004;50:1262–1269.10.1002/art.20176
- Ramos-Casals M, Tzioufas AG, Font J. Primary Sjögren’s syndrome: new clinical and therapeutic concepts. Ann Rheum Dis. 2005;64:347–354.
- Voulgarelis M, Skopouli FN. Clinical, immunologic, and molecular factors predicting lymphoma development in Sjögren’s syndrome patients. Clin Rev Allergy Immunol. 2007;32:265–274.10.1007/s12016-007-8001-x
- Tzioufas AG, Boumba DS, Skopouli FN, et al. Mixed monoclonal cryoglobulinemia and monoclonal rheumatoid factor cross-reactive idiotypes as predictive factors for the development of lymphoma in primary Sjögren’s syndrome. Arthritis Rheum. 1996;39:767–772.10.1002/(ISSN)1529-0131
- Moutsopoulos HM, Tzioufas AG, Bai MK, et al. Papadimitriou. Association of serum IgM kappa monoclonality in patients with Sjögren’s syndrome with an increased proportion of kappa positive plasma cells infiltrating the labial minor salivary glands. Ann Rheum Dis. 1990;49:929–931.10.1136/ard.49.11.929
- Skopouli FN, Dafni U, Ioannidis JP, et al. Clinical evolution, and morbidity and mortality of primary Sjögren’s syndrome: an aid to the diagnosis of malignant lymphoma. Ann Rheum Dis. 2000;45:210–219.
- Quartuccio L, Isola M, Baldini C, et al. Biomarkers of lymphoma in Sjögren’s syndrome and evaluation of the lymphoma risk in prelymphomatous conditions: Results of a multicenter study. J Autoimmun. 2014;51:75–80.10.1016/j.jaut.2013.10.002
- Bijlsma JW, Burmester G, da Silva JA, et al, editors. EULAR compendium on rheumatic diseases. London: BMJ Publishing Group; 2009. p. 322, §biological predictive factors.
- Brito-Zeron P, Ramos-Casals M, Bove A, et al. Predicting adverse outcomes in primary Sjögren’s syndrome: identification of prognostic factors. Rheumatology (Oxford). 2007;46:1359–1362.10.1093/rheumatology/kem079
- Ioannidis JP, Vassiliou VA, Moutsopoulos HM. Long term risk of mortality and lymphoproliferative disease and predictive classification of primary Sjögren’s syndrome. Arthritis Rheum. 2002;46:741–747.10.1002/art.10221
- Ramos-Casals M, Brito-Zerón P, Yagüe J, et al. Hypocomplementaemia as an immunological marker of morbidity and mortality in patients with primary Sjögren’s syndrome. Rheumatology (Oxford). 2005;44:89–94.10.1093/rheumatology/keh407
- Walters MT, Stevenson FK, Herbert A, et al. Urinary monoclonal free light chains in primary Sjögren’s syndrome: an aid to the diagnosis of malignant lymphoma. Ann Rheum Dis. 1986;45:210–219.10.1136/ard.45.3.210
- De Vita S, Boiocchi M, Sorrentino D, et al. Characterization of prelymphomatous stages of B cell lymphoproliferation in Sjögren’s syndrome. Arthritis Rheum. 1997;40:318–331.10.1002/(ISSN)1529-0131
- Brito-Zerón P, Ramos-Casals M, Nardi N, et al. Circulating monoclonal immunoglobulins in Sjögren syndrome: prevalence and clinical significance in 237 patients. Medicine (Baltimore). 2005;84:90–97.10.1097/01.md.0000157398.37679.47
- Theander E, Vasaitis L, Baecklund E, et al. Lymphoid organization in labial salivary gland biopsies is a possible predictor for the development of malignant lymphoma in primary Sjögren’s syndrome. Ann Rheum Dis. 2011;70:1363–1368.10.1136/ard.2010.144782
- Fox RI. The importance of minor salivary gland biopsy in prediction of lymphoma in Sjögren’s syndrome: should we be obtaining more information about prognosis from minor salivary gland samples? Ann Rheum Dis. 2011;70:1351–1353.10.1136/ard.2011.152751
- Zulman J, Jaffe R, Talal N. Evidence that the malignant lymphoma of Sjögren's syndrome is a monoclonal B-cell neoplasm. N engl J Med. 1978;299:1215–1220.10.1056/NEJM197811302992204
- Vitali C, Bombardieri S, Jonsson R, et al. Classification criteria for Sjögren’s syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis. 2002;61:554–558.10.1136/ard.61.6.554
- Daniels TE, Cox D, Shiboski CH, et al. Lanfranchi H, et al, for the Sjögren’s International Collaborative Clinical Alliance Research Groups. Associations between salivary gland histopathologic diagnoses and phenotypic features of Sjögren’s syndrome among 1,726 registry participants. Arthritis Rheum. 2011;63:2021–2030.10.1002/art.v63.7
- Vermeersch P, Gijbels K, Knockaert D, et al. Establishment of reference values for immunoglobulins in the cryoprecipitate. Clin Immunol. 2008 Nov;129(2):360–364.10.1016/j.clim.2008.07.012
- Bossuyt X, Schiettekatte G, Bogaerts A, et al. Serum protein electrophoresis by CZE 2000 clinical capillary electrophoresis system. Clin Chem. 1998 Apr;44(4):749–759.
- Bossuyt X, Lissoir B, Mariën G, et al. Automated serum protein electrophoresis by Capillarys. Clin Chem Lab Med. 2003 May;41(5):704–710.
- Bahler DW, Miklos JA, Swerdlow SH. Ongoing Ig gene hypermutation in salivary gland mucosa-associated lymphoid tissue-type lymphomas. Blood. 1997;89:3335–3344.
- Royer B, Cazals-Hatem D, Sibilia J, et al. Lymphomas in patients with Sjögren’s syndrome are marginal zone B-cell neoplasms, arise in diverse extranodal and nodal sites, and are not associated with viruses. Blood. 1997;90:766–775.
Appendix A: Excluded patients
The original database contained 247 patients with possible SS.
67 patients were excluded and 180 patients were included in this study.
Reasons of exclusion:
| • | less than two disease-specific hospital visits (N = 20) | ||||
| • | symptoms related to HIV-infection (N = 3) | ||||
| • | sicca-syndrome or salivary gland swelling or parotitis-like complaints, with insufficient evidence for an underlying auto-immune disease (N = 26) | ||||
| • | sicca-symptoms most probably related to the use of anticholinergic drugs (N = 3) | ||||
| • | xerophtalmia most probably related to Graves orbitopathy (N = 1) | ||||
| • | symptoms most probably related to sarcoïdosis (N = 1) | ||||
| • | preference for diagnosis of another auto-immune disease, with insufficient evidence for the presence of secondary SS (CREST N = 2, PMR N = 1, RA N = 1, SLE N = 7, Systemic Sclerosis N = 1, GPA N = 1) (total N = 13) | ||||
Appendix B: Ranges of laboratory parameters
Normal laboratory values according to university hospitals leuven.
Cryoglobulins:
| • | cryoprecipitate IgG: <10 mg/L. | ||||
| • | cryoprecipitate IgM: <20 mg/L. | ||||
| • | cryoprecipitate IgA: <2 mg/L. | ||||
Complement:
| • | C3: 0.79–1.52 g/L. | ||||
| • | C4: 0.16–0.38 g/L. | ||||
| • | Total complement activity: between 70 and 140% | ||||
Protein electrophoresis:
| • | betaglobulin: 6.0–9.4 g/L. | ||||
| • | gammaglobulin: 8–13.59 g/L. | ||||
Immunoglobulins:
| • | IgG: 7.51–15.6 g/L. | ||||
| • | IgM: 0.46–3.04 g/L. | ||||
Appendix C. Analysis of cryoglobulins
The following transformed marginal regression lines were calculated for ‘diff_month < 0’:
IgG: Y (lymfoma = 0) = 0.9 ± 0.001*diff_month; and Y (lymfoma = 1) = 3.6 + 0.024*diff_month;
IgM: Y (lymfoma = 0) = 0.9 ± 0.006*diff_month; and Y (lymfoma = 1) = 4.3 + 0.006*diff_month;
IgA: Y (lymfoma = 0) = 0.2 + -0.000*diff_month; and Y (lymfoma = 1) = 1.7 + 0.018*diff_month.
There is a significant interaction of diff_month*lymphoma for all of the three blood tests.
Example of original data:
Example of transformed data:
We used Y (=ln(bloodtest + 1)) for the transformation.
Yij = the j-th (transformed) measurement of the i-th patient at moment diff_month tj.
Yij = β1 + bi + β2tj + ɛij, in the group lymphoma = 0
Yij = β3 + bi + β4tj + ɛij, in the group lymphoma = 1
Interpretation:
1.
| (1) | There is a significant interaction between diff_month*lymphoma. H0: β2 = β4 is rejected. | ||||
| (2) | The two slopes are not equal (p-value = 0.0199). | ||||
2. The marginal model is:
E(Yij) = β1 + β2tj, group lymphoma = 0
E(Yij) = β3 + β4tj, in group lymphoma = 1
3. De β coeff is estimated:
