ABSTRACT
More than ten years after the first biosimilars were authorized for use in the European Union, Belgium still experiences limited competition from biosimilars, as exemplified by low market shares. Achieving high biosimilar market shares is not necessarily a goal in itself, as cost savings are also realized by mandatory price reductions on originator medicines in Belgium. However, we believe that biosimilars play a role in ensuring the long-term sustainability of the off-patent biologicals market. It is therefore crucial to list what has been done and what is needed to support the Belgian government in establishing a policy framework for a competitive off-patent biologicals market. We provide a comprehensive overview of the Belgian biosimilar market, including existing hurdles for biosimilar use in Belgium. Based on these hurdles and supplemented with learnings from other European countries, we propose practical recommendations that can be implemented to overcome them. Several Belgian stakeholders had the opportunity to comment on these recommendations. Specifically, we suggest to evolve towards a long-term consistent, integrated policy framework via i) the creation of a proactive and transparent climate supporting a level playing field for both biosimilar and reference product, including public dissemination of how savings at the level of the Belgian healthcare system are used, ii) investment in educational activities, including raising awareness of societal responsibility, iii) enforcement of the practical implementation of public procurement law, and iv) the development of incentives for physicians, who are key stakeholders in the Belgian off-patent biologicals market.
1 Current biosimilar landscape in Belgium
In January [Citation1] 2020, 29 of the 54 biosimilars that received marketing authorization for the European Union were available and reimbursed in Belgium [Citation2,Citation3]. These 54 biosimilars include two biosimilars for bevacizumab that cannot yet be launched due to ongoing exclusivity rights protection on Avastin® [Citation4]. presents in which treatment setting these biosimilars are classified for use in non-hospitalised patients, i.e. ambulatory patients in the hospital and the retail setting. For ambulatory hospital patients, biosimilars were available and reimbursed for all active substances for which biosimilars could be marketed. In the retail setting, biosimilars were not available nor reimbursed for three of the nine reference products. The launch of a medicine in a country often depends on company strategies/choices. In July 2019, Sandoz retracted its filgrastim biosimilar (Zarzio®) from the Belgian market.
Table 1. Reimbursed biosimilars in Belgium, classified by treatment setting for use in non-hospitalised patients (as of January 2020) [Citation3]. Marketing authorization dates for the European Union are indicated between brackets [Citation2]. Molecules are sorted from oldest (on top) to newest biosimilar marketing authorization for the European Union. Please note that marketing authorization is not equal to access to treatment, since reimbursement and product launch usually occur later
In the context of financing and reimbursement, biological medicines, including biosimilars, are treated in the same way as other medicines and are financed by the Belgian reimbursement agency (National Institute for Health and Disability Insurance – NIHDI) via taxes, compulsory health insurance via a sickness fund of choice, and patient co-payments. For inpatients, the use of medicines is included in a lump-sum co-payment. For outpatients, including ambulant patients in the hospital (e.g, for infliximab), co-payment depends on different reimbursement categories. List prices and reimbursed prices are coordinated on a national level. Medicines used in the hospital setting (inpatients and ambulant patients) are procured by individual hospitals or hospital groups, which can keep the savings they negotiate or obtain via tendering (usually one-winner contracts). In this way, medicines with higher reimbursement prices are favoured, as higher absolute savings can be gained. Most biosimilars and reference products that are used in the hospital setting only, have therefore now the same list price.
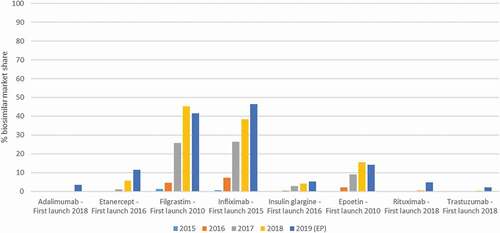
Market shares for most biosimilars in Belgium were in 2019 still below 20% (). Higher market shares were reached for filgrastim and infliximab biosimilars, 42% and 46%, respectively. Even with filgrastim being available in the retail setting, most of the volume was dispensed in the hospital setting (inpatients).
Figure 1. Biosimilar market shares in Belgium, based on volume (defined daily doses) versus the reference product, for the years 2015 to 2019. Data for 2019 are partly extrapolated (EP) based on 6 months of hospital data and 9 months of data for the retail setting. Data were provided by the Belgian reimbursement agency RIZIV/INAMI/NIHDI (Farmanet and DOC PH databases)
Over the last years, several policy measures related to biosimilars have been implemented to enhance competition in the Belgian off-patent biologicals market (). Although the first biosimilar was authorised in 2006, it is noteworthy that, apart from information on biosimilars on the website of the competent authority, a decade elapsed before the Belgian Ministry of Health and national authorities started to take action regarding biosimilars in 2016. Four years later, the market has evolved, however, the impact of these policy measures is still limited.
Table 2. Implemented policy measures related to biosimilars to enhance competition in the Belgian off-patent biologicals market
In addition to measures specifically aiming to enhance competition, a multitude of cost containment measures have also been introduced that affect off-patent biological products. These include the price reduction measure for ‘old medicines’ (−17% on the ex-factory price after 12 years of reimbursement; a revision to −19.75% is planned, but not yet implemented), the ‘volume cliff’ (up to −12.05% depending on the annual sales after 15 years of reimbursement, before 1 April 2019 this was fixed at −2.41%), and the price reduction measure for biological medicines (−15% after 18 years of reimbursement or in the first quarter following biosimilar availability and reimbursement for 2 months; following revision: −20%) [Citation5,Citation6]. If the measure for ‘old medicines’ and ‘volume cliff’ are not yet implemented when the entry of the biosimilar triggers the measure for biological medicines, all price reductions are enforced at once (up to 38% price reduction) [Citation6]. This is called the ‘bio cliff’ and was introduced on 1 April 2018. A revision of these cost containment measures was planned to be implemented on 1 April 2020, but has been postponed until further notice due to the outbreak of the coronavirus and has thus not been agreed on at the time of writing [Citation7]. This revision would also include that the introduction of the biosimilar will no longer be needed to trigger the ‘bio cliff’, and all these price reductions will be applied when the biological product is reimbursed for 12 years (‘old medicines cliff’). Another cost containment measure was introduced on 1 January 2017, and specified that, for biological medicines which have a biosimilar alternative, the reimbursement to the hospitals has been lowered with 10%, limiting reimbursement to only 90% of the amount invoiced by the hospital [Citation8]. With this measure the government wanted to stimulate hospitals to apply the law on public procurement. It is believed that this was an important incentive for hospitals. From 1 April 2019, reimbursement was further lowered from 90% to 85% [Citation9], and following revision, this reimbursement is likely to be reduced to 70%. Since 2012, biosimilars and their reference products can also be included in reimbursement category F, where a fixed reference price is set for reimbursement to the hospital and any additional price differences need to be covered by the hospital. This was the case for short and long acting epoetins in an attempt to level out price differences and corresponding advantages of the higher priced reference product in hospital procurement procedures. Some products (short and long acting epoetins and somatropin) are included in the prospective hospital budget for medicines since 2012, while these were previously invoiced based on actual consumption [Citation10]. Biosimilars are also part of quotas for prescribing low-cost medicines [Citation11]. However, as there are mandatory price decreases for the reference product as well as the biosimilar after entry of biosimilars, both reference and biosimilar products are defined as being low-cost medicines, hence limiting the impact of this measure on the uptake of biosimilars.
Even with several national measures and initiatives to increase competition (see ), biosimilar uptake in Belgium remains limited () and this might indicate that the entry of biosimilars is not fully leveraged. We build on a report from the Belgian Health Care Knowledge Centre (KCE) on biosimilars and the work of Dylst et al. [Citation10,Citation12] from 2013 and 2014, respectively, and look again anno 2019 whether earlier identified barriers are still valid. In addition, we discuss potential reasons as to why biosimilar uptake in Belgium is still limited, and adopt learnings from biosimilar policies and practices in Europe to serve as input to guide policy decisions in Belgium and potentially increase competition in the Belgian off-patent biologicals market.
This perspective paper is informed by face-to-face discussions with various Belgian stakeholders to seek confirmation for the applicability of the factors identified by the research team as influencing market dynamics of originator biological and biosimilar products. The discussions offered as well the opportunity to Belgian stakeholders to add new suggestions for improving the current biosimilar policy framework. The interviewed stakeholders were also given the opportunity to comment on the draft paper. Via this process we wanted to ensure that practical recommendations are provided that allow for a certain consensus among stakeholders. Even though the number of interviewees (17) and selected sample (originator and biosimilar industry, hospital pharmacists, physicians, and authorities) might not guarantee general consensus, the authors put forward their own key takeaways to increase competition in the Belgian off-patent biologicals market.
2. Barriers to biosimilar uptake in Belgium
In 2013, the KCE report on barriers and opportunities for biosimilars in Belgium described the following main barriers to the market uptake of biosimilars in Belgium: a lack of knowledge on biosimilars that may be linked to the use of non-effective information dissemination channels in an attempt to inform stakeholders, a lack of confidence in the biosimilar pathway and services from biosimilar companies, loyalty to the innovator company and its value-added services, and large discounts offered on the reference product in the hospital setting [Citation10]. Similar barriers have been identified by Dylst et al. in 2014, i.e. a lack of confidence, concerns on interchangeability and substitution, and a hospital financing system that incentivizes the use of the originator product [Citation12]. Possible solutions to overcome these barriers are suggested, such as providing objective information to stakeholders, a reform of the hospital finance system, and the introduction of prescription quota for biosimilars.
Some of these earlier identified barriers seem to be partly overcome. The barrier of knowledge and trust in biosimilars is perceived not to be a key barrier anymore; even though there might still be a need for clear and neutral communication on biosimilars and further education. The position statement of the Belgian competent authority (2009, see ) is a step in the good direction, but is insufficient to influence physicians’ prescribing behaviour. In addition, although invited to the symposium organized by the competent authority in 2018, the presence of physicians as an important stakeholder was minimal compared to other stakeholders. A second barrier to biosimilar uptake, describing a hospital financing system that favours the higher-priced originator product, has been overcome, as almost all biosimilars and reference products that are used in the hospital setting have the same list price or reimbursement price, thereby providing a level playing field for tendering. However, although the barriers identified as key in 2013 and 2014 are now largely resolved and uptake remains still limited, this implies that other barriers and potentially new barriers play a role.
The climate in Belgium is historically innovator minded as a result of a large pharmaceutical R&D footprint (first place in R&D investments per inhabitant and third place in R&D employment per inhabitant, within the European Union [Citation28]). This is reflected in a strong lobby organisation from the innovator industry, politicians are careful not to take measures affecting employment in the pharmaceutical sector, and brand loyalty of physicians. A perception exists that rumours from the originator industry on immunogenicity and switching slowed down acceptance of biosimilars. This landscape might evolve with big pharma companies originally investing in innovator molecules entering the biosimilar market. In addition, as R&D efforts and production of medicines are for the global market these days, employment in the pharmaceutical sector will not only depend on market shares in Belgium. Furthermore, marketing efforts are traditionally lowered after patent expiry. So the will to safeguard the R&D footprint should not be a factor impeding competition in the off-patent biologicals market.
One structural barrier to biosimilar uptake seems to be that there are no tangible benefits and incentives for authorities, physicians, pharmacists, patients and hospitals to use biosimilars. The reimbursement agency realizes cost savings via mandatory price decreases on the originator product (although, without the entry of biosimilars, the reduced reimbursement to the hospitals will not be triggered). Physicians do not feel involved because the cost savings flow back to the reimbursement agency or hospital management and often not to the department or individual physician. Moreover, switching patients takes time for which physicians are not remunerated. Pharmacist remuneration and patient co-payments do not differ between biosimilar and reference product. Hospitals, on the other hand, have a financial incentive to tender in order to increase savings. However, originator companies are able to provide considerable discounts and can prolong the exclusivity contract just before patent expiry, avoiding the administrative burden of opening a tender.
In addition to these general barriers, setting-specific barriers can be identified.
2.1. Hospital market
A first hurdle for the use of biosimilars in the hospital setting is related to the opening of tenders. Several reasons might explain why hospitals do not open new contracts when a biosimilar enters the market: a) tenders require considerable time efforts from hospital pharmacists, b) hospitals might initially wait until more players can offer a bid, c) the innovator’s reach and possible benefits to hospitals can play a role in the lack of willingness to open a tender when the biosimilar enters, d) legally correct new exclusivity contracts with potentially substantial discounts are concluded just before biosimilars enter the market, and f) discounts are given on subcutaneous alternatives, limiting the need for a new contract on the intravenously administered biosimilar product.
To illustrate this, trastuzumab biosimilar Herzuma® launched in August 2018 (others in 2019), whereas the first notice of intent to tender was only published in March 2019 [Citation29]. Since then, five tenders have been awarded and two are ongoing. Based on information in the tender notices, these tenders cover only 19 of the 102 Belgian hospitals (general and university hospitals: 53 hospitals in Flanders, 12 in the Brussels-Capital Region, and 37 in Wallonia). Of these tender notices, only one was published for Flanders, covering four hospitals in the province of Flemish Brabant (ongoing). In the case of rituximab, biosimilar Truxima® first launched in July 2018, whereas the first notice of intent to tender was only published in October 2018. In the meantime, eight tenders have been awarded and two are ongoing. Based on buying groups, these tenders cover 38 of the 102 Belgian hospitals. Of these tender notices, again only one was published for Flanders, covering four hospitals in Flemish Brabant (ongoing). Infliximab biosimilars first launched in April 2015. The first notice of intent to tender was only published in October 2015. After more than four years, still, some hospitals have not tendered for infliximab. It is, however, unclear how many hospitals have not tendered, as for infliximab some hospitals appear to have joined existing tender contracts at a later point in time.
A second hurdle for the use of biosimilars in the hospital setting is the use of criteria other than price in some tenders that tend to favour the originator product, e.g. requests for scientific support (grants, conferences). This finding is in line with results from the KPMG report ‘Improving healthcare delivery in hospitals by optimized utilization of medicines’ of 2019 [Citation30]. Additionally, hospitals can request free goods via medical need programmes that can only exist for the originator product.
The opening of tenders and the correct use of tender criteria was addressed in two circular letters; from the government in 2016 and from the competent authority in 2019. Apart from these circular letters, in October 2019, the Belgian competition authority started an investigation on potential anti-competitive behaviour that restricts the use of biosimilars in the hospital setting [Citation31,Citation32].
Product-specific barriers can as well be identified for rituximab and pegfilgrastim. A patent litigation case related to patents for treatment of hematologic malignancies and of joint damage with rituximab and possible infringement by the entry of biosimilars in Belgium delayed access of Sandoz’s biosimilar up to September 2019 [Citation33], while the judgement on the invalidity of these two patents on a European level was decided in November 2017 and November 2018 (appeal proceedings are still pending), respectively [Citation34,Citation35]. In 2017, Mundipharma already lost a patent litigation case concerning possible infringement of their rituximab biosimilar. In the case of pegfilgrastim, the inclusion of biosimilar and originator pegfilgrastim in the same lot as lipegfilgrastim that has a higher reimbursement price, makes it almost impossible for pegfilgrastim products to win a tender, even by providing high discounts. It is currently studied how this barrier can be overcome.
2.2. Retail market
In the hospital setting, the use of biosimilars is driven by cost savings or potentially other criteria in the tender process and the switch is facilitated by clear formulary decisions and relatively small efforts to change IV bags. In the retail setting on the other hand, there is no such enforcement, as both the push and pull mechanism lack. After mandatory price cuts, the price difference between biosimilar and reference product is limited (). So why go through the burden of change for only a marginal additional cost saving, which does not provide any benefit to the physician? Moreover, different devices for subcutaneous administration complicate switching and ask for more time investment from physicians and pharmacists, while there are limited possibilities related to products dispensed in the retail setting to request support from companies. In addition, physicians do not appear to be price sensitive. Especially since price differences between reference and biosimilar product are much lower than between the reference product and newer treatment options. Also a personal financial award, as was implemented in 2019 for prescription of biosimilar adalimumab and etanercept, does not seem to considerably improve biosimilar uptake. Some physicians find it offending to be rewarded to prescribe a specific product, while others would like to see a more substantial remuneration.
Table 3. Price differences between biosimilar and reference product in the retail setting (as of January 2020) [Citation36]
3. Avenues and recommendations to increase competition in Belgium’s off-patent biologicals market
Biosimilars offer considerable advantages to healthcare systems. In general, the use of biosimilars leads to cost savings, price competition, increased patient access, improved cost effectiveness, incremental innovation, and might contribute to prevention of drug shortages [Citation10,Citation37]. Although there is probably not an access issue in Belgium, healthcare budgets under pressure are of considerable concern.
Based on the discussed barriers impeding a competitive off-patent biologicals market in Belgium and learnings from other European countries, we will propose several suggestions, which may or may not be compatible with each other, to change the Belgian off-patent biologicals market towards a more sustainable, competitive system.
There needs to be clarity on the objectives that wish to be attained in the Belgian off-patent biologicals market, and specifically on the role of biosimilars. This relates to the fact that biosimilars may generate savings by means of two mechanisms. First, biosimilars are less expensive due to lower R&D costs. Therefore, in Belgium, mandatory price decreases of up to 38% are imposed on the reference product following the entry of biosimilars to drive down the price of the reference product. A next step in further exploiting this approach is that these mandatory price decreases are applied to originator products once their exclusivity rights expire, irrespective of whether a biosimilar enters the Belgian market (as will be the case following revision of the current cost containment measures, up to −44%). This approach guarantees savings to the reimbursement agency, but does not provide an incentive to lower prices by more than the fixed reduction. Second, biosimilars may induce long-term price competition with reference products, generating additional savings. Based on the general economic relationship between volume and price of products, it can be hypothesised that prices of biosimilar and reference products will fall further when the volume of biosimilars increases. This approach, thus, requires measures supporting the use of biosimilars.
Whereas the government has previously implemented ad hoc measures, Belgium has to evolve towards a long-term consistent, integrated policy framework targeting all relevant stakeholders. We propose to rely on four pillars to build this framework: 1) the creation of a proactive and transparent climate supporting a level playing field; 2) investment in educational initiatives; 3) enforcement of the practical implementation of public procurement law; and 4) development of physician incentives.
3.1. The creation of a proactive and transparent climate supporting a level playing field
Policy and decision makers need to foster a proactive and transparent climate that creates a level playing field for all off-patent biologicals, including biosimilars, with a view to inducing long-term competition. This requires that a number of actions are implemented prior to and following biosimilar market entry.
3.1.1. Prior to biosimilar market entry
1. In order to stimulate biosimilars to enter the Belgian market and to encourage reference products to lower their price, biosimilars and low-cost originator products could be exempted from the clawback tax (i.e. a tax calculated on sales in case the pharmaceutical budget is exceeded).
2. There is a need for horizon scanning to predict when the patent and other exclusivity rights related to originator biologicals will expire, and when and how many biosimilars are likely to be granted regulatory approval by the European Medicines Agency, and which are likely to enter the Belgian market. Updates on patent expiry dates published by the Generics and Biosimilars Initiative (GaBI) Journal [Citation38] and information on medicines under evaluation at the European Medicines Agency [Citation39] can serve as a guide.
3. Prior to the expiry of patents and other exclusivity rights on a specific biological product, views of all relevant stakeholders regarding the use of that off-patent biological and its biosimilars should be aligned through a collaborative, multi-stakeholder approach. The government and other authorities should increasingly involve scientific medical associations as, via these associations important key opinion leaders can be reached. With limited price differences in the retail setting, the attitude of key opinion leaders can greatly influence originator/biosimilar market dynamics, as was the case for etanercept in Sweden [Citation40]. In addition, hospital pharmacist associations can play an important role in the hospital setting, and working with the local hospital management can be a way to target physicians. Also, patient organisations can be more involved to anticipate potential nocebo effects and changes in treatment adherence. Furthermore, it should be explored how the different sickness funds can contribute, e.g. by providing mirror-information on prescribing behaviour.
4. The Belgian competent authority should assign a dedicated spokesperson to deal with queries from stakeholders regarding regulatory aspects of biosimilars. At the moment, the potential role of the competent authority in education and dissemination of information is underused. Intensified collaboration with physician associations and the reimbursement agency is needed to avoid duplication of work.
5. The new ‘programme manager biological and biosimilar medicines’ appointed by the reimbursement agency needs to act as a key opinion leader. He needs to be proactive and visible by participating in seminars and act as the contact person for different stakeholders.
6. The development of a protocol describing a managed switching programme can help to build trust in biosimilars and assists physicians in their efforts. In the Netherlands, the hospital pharmacists together with the medical specialists have built a Biosmilar Toolbox, which contains a collection of best practices for biosmilar implementation. In Scotland, the National Health Service (NHS) shared case studies for infliximab and etanercept on the switch from originator to biosimilar on their website to encourage the different regions to learn from this experience [Citation41]. National managed switch letters exist in Scotland that can be adapted to local needs.
7. Competition seems to be more present in the hospital setting than in the retail setting. A change in treatment setting for some products from retail to hospital could increase competition and cost savings. In the Netherlands, TNF-alpha inhibitors such as etanercept and adalimumab are for this reason included in the hospital budget since 2012 [Citation42]. On the other hand, in Sweden a managed entry agreement with rebates for subcutaneously administered TNF-alpha inhibitors is used to stimulate competitive pricing in the retail setting [Citation40]. Following the agreement, recommendations are made available that inform physicians which product is the most cost-effective to prescribe.
3.1.2. Following biosimilar market entry
8. Scientific medical associations need to update (clinical) guidelines when biosimilars enter the Belgian market and take into account the price evolution of biological therapies. Based on such an exercise, they could rank products and offer guidance to physicians on what is the most cost-effective option to prescribe, be it a low-cost reference product or a biosimilar. This approach could be tested in disease areas such as rheumatology, where for most patients it does not matter with which biological they start and where a change in product is acceptable. In addition, the proposed ranking should create cost-consciousness on the increase in cost per patient per year of new products, such as for example JAK inhibitors, versus the added therapeutic value these treatments supposedly have.
9. The reimbursement agency needs to revise the reimbursement conditions of all products in a therapeutic class following market entry of a specific biosimilar product with a view to account for the evolution in cost-effectiveness of these products as a result of price changes of the biosimilar and reference biological. This is particularly relevant for product classes with next-generation products such as G-CSF, where the market entry of filgrastim biosimilars and associated price decreases affect the cost-effectiveness of pegfilgrastim (and its biosimilars) and lipegfilgrastim.
10. The reimbursement agency could regularly publish aggregated data on biosimilar uptake per product in the hospital and retail setting on a website and make a critical appraisal of the situation to create more transparency. In addition, report publicly on the savings generated from mandatory price reductions and the entry of biosimilars, where these savings go, and how these benefit the healthcare budget and care for patients.
3.2. Investment in educational activities
Although the knowledge about and trust in biosimilars might not be considered a key barrier anymore, further efforts to increase awareness and acceptance might be needed and can be relatively easy implemented. Educational initiatives regarding biological medicines, including biosimilars, should target both future and active healthcare professionals. However, although education is necessary before implementing other measures, educational initiatives alone will likely be insufficient to drive changes in prescribing behaviour.
1. Education needs to start early and should include future healthcare professionals. Therefore, it is proposed to include more information on biological medicines, including biosimilars, in the curriculum of (bio-)medical and pharmacy students. Also, an increased focus on societal responsibility and rational prescribing of medicines might pay off on the long term. However, due to only small price differences, this will have limited impact on biosimilar uptake on the short term. This initiative can be implemented by urging universities to include courses in the training of medical students on how marketing authorization of medicines works, health economic aspects of first line and second line therapy and the difference in cost when changing to second line. The reimbursement agency needs to support these courses and be involved in teaching.
2. Efforts to inform and communicate with physicians and pharmacists should use proven effective channels like guidelines, information letters from hospital management, and communication from medical associations. Education of physicians could be linked to accreditation. Previous research has suggested that there is no lack of available information and has stressed the importance of an active communication strategy tailored to reach the target audience [Citation43].
3. It is best to have educational activities initiated by an independent organisation like the government, competent authority or scientific medical associations. Support from the government to an independent expert group that can set up educational initiatives and talk to hospitals and patient organizations could be a solution. In the Netherlands, the ‘Initiatiefgroep Biosimilars Nederland’ (IBN) offers via the ‘Biosimilars op maat’ project e-learning modules and on-site training for hospitals by a group of experts [Citation44,Citation45]. Their expertise and material could be leveraged and adapted for the Belgian market.
4. The KCE report of 2013 reports that medical scientific literature and medical conferences are the main sources of information regarding biosimilars for physicians [Citation10]. However, pharmaceutical companies can play an important role as they have good access to physicians via sales representatives and the organisation of symposia. It is important that information given on symposia is neutral and not linked to the marketing of a specific product. However, increased marketing efforts by companies for biosimilars, like for any other new product, require investments and this might be reflected in a lower level of discounts that can be offered.
3.3. Enforcement of the practical implementation of public procurement law
When correctly applied, tenders create competition between biosimilar and reference product in the hospital setting. The implementation of tender procedures in Belgium can be improved by several initiatives.
1. In order to prioritize tenders for pharmaceuticals, it is important to implement horizon scanning. A special unit should be established to support hospitals with information on upcoming loss of exclusivities of top-selling molecules and the entry of biosimilars in Belgium. In Denmark and the United Kingdom (UK), discussions often start years before patent expiry and possible entry of biosimilars in order to align stakeholders. New contracts should also take into account future entry of products and the length of the contract should be set accordingly.
2. The tender process could be simplified to reduce workload for hospitals and industry. A template can be made available with criteria to be considered, while criteria other than price can be taken into account to allow for product differentiation and incremental innovation. Guidance to hospitals can be provided on how to set up a tender unit and educate personnel.
3. Adherence to laws on public procurement is key to ensure an equal level playing field and induce competition. A close follow-up and appropriate enforcement measures are needed to guarantee the timely opening of tenders after the entry of biosimilars and the adoption of appropriate criteria in the tender process. In this respect, the Belgian Competition Authority needs to conduct audits of hospital tenders for biologicals, including biosimilars, and needs to follow up hospitals' adherence with laws on public procurement.
4. In order to reduce workload for hospitals and industry, tenders could be organised on a larger scale, for example via hospital networks or on a national level. Organising the tender process on a national level would have the advantage that one single expert unit can ensure a smooth process supported by adequate preparation. In addition, savings can go directly to the healthcare system. When tendering on a national level, multiple winners should be selected to ensure plurality of supply and the length of the contract could be limited to a maximum of two years to stimulate market dynamics. To divide the market among players, different ideas can be proposed. The NHS England experimented with a ‘managed market share tender approach’ for the public procurement of adalimumab in 2018 [Citation46]. Depending on the competitiveness of the bid, the company has access to a greater or smaller part of the market, which is divided in 11 regions. A second option is to work with a ranking like in Norway and to recommend products based on the first year treatment cost for each indication [Citation47]. In order to make tendering on a national level feasible for hospitals, the hospital financing system needs to be reformed to address the resulting shortfall in income from procurement savings for hospitals. In addition, savings accruing to the reimbursement agency can be partially used to remunerate physicians and nurses for their time investment in switching patients through gainsharing arrangements.
3.4. Development of physician incentives
As a key stakeholder affecting the long-term sustainability of the biologicals market, physicians should be motivated and appropriately incentivized to prescribe rationally. It is specifically important that incentives are precisely formulated and that the benefit to physicians is clear. This does not mean that the benefit should go to individual physicians, but rather to support the hospital or hospital department. In addition, physician incentives should allow freedom of prescribing. Several actions can be proposed to increase physician engagement towards the use of biosimilars.
1. Position statements from medical associations or scientific associations on biosimilars and switching can help to build trust among physicians and give them confidence to prescribe biosimilars. Physicians are more likely to accept information and guidance from key opinion leaders and peers than from authorities. These position statements also need to be updated over time in concordance with evolving scientific insights into biologicals, including biosimilars. The Belgian inflammatory bowel disease research and development group (BIRD) and the Belgian Royal Society of Rheumatology published a cautious position statement in 2015 [Citation48,Citation49]. The BIRD group published new, more proactive statements for 2017 and 2019 [Citation50,Citation51]. It is unclear if and how the existing position statements have influenced prescribing behaviour in a positive or negative way.
2. The implementation of guidelines could provide advice to physicians to prescribe the most cost-effective product, without enforcement. This is a practice that tends to work well in other countries and is established in e.g. Sweden, Norway, Denmark, and the UK. In Germany and Austria, health insurance funds (‘sickness funds’) provide as well information to physicians to guide the rational prescribing of medicines. However, it will take time for physicians to adapt to this new system. One step further would be to develop an indicator for how well physicians follow prescribing guidelines or for when physicians prescribe the most cost-effective product.
3. Although quota for prescribing low-cost medicines have already been implemented in Belgium, there is scope to further fine-tune this measure by, for instance, moving away from a target for low-cost medicines to a target for the lowest-cost medicine in the retail setting; or by calculating a separate target for lowest-cost biological medicines (excluding chemical medicines). The implementation of a target agreement should be accompanied by follow-up on adherence and a reward or punishment system. In Germany, target agreements are used for many medical products, including biosimilars. These prescription targets are set for each region in Germany and can differ per molecule and per specialisation.
4. Gainsharing, where part of the savings or benefits from using biosimilars flow back to the hospital department and are transparent for prescribing physicians, can be a strong incentive for the use of biosimilars. This can especially work well for products used in the retail setting, where, in contrast to the hospital setting, the use is not driven by formulary decisions. Potential savings should be used to create tangible benefits rather than just providing a financial reward. The government could invest in study nurses to help support physicians as they will need to allocate more time to patients that will switch from the reference product to the biosimilar. In Scotland, the ‘invest to save’ approach was recognised and additional staffing resources were arranged to manage the switch from originator to biosimilar etanercept. In Denmark, more nurses were present during the first weeks of the tender agreement for biosimilar adalimumab to help switch patients. This resulted in more than 90% of patients being switched to biosimilar adalimumab in three weeks’ time [Citation52]. The Belgian government could also invest in applications to improve patient flow and educational material, like the national managed switch letters in Scotland [Citation41].
5. When a benefit is more visible, it might encourage physicians to change prescribing behaviour. For this, the communication via newsletters that outline what was realised with the savings from a certain percentage of biosimilar prescription can motivate physicians. Especially when part of these savings have been used to make investments in their therapeutic area.
6. When cost-effective, a broader reimbursement (indications, posology, medical tests) or change in treatment line following price decreases for an active substance can offer more options for physicians to prescribe biologicals and improve patient care. These are two factors that physicians find important.
7. The use of an electronic prescribing system offers opportunities to support rational prescribing. Prescribing software should be linked to price lists and show for each product price information to increase price awareness among physicians. The most cost-effective product can be presented first in the ranking.
4. Conclusions
The current biosimilar landscape in Belgium is characterised by low biosimilar uptake and the implementation of several ad hoc policy measures. Instead, the government should foster a competitive climate and establish a level playing field for both biosimilar and reference product. Even though high biosimilar market shares are not a goal in itself, but rather the generation of cost savings by leveraging competition from biosimilars, we question whether Belgium has maximized the savings potential at the moment, even with a multitude of cost containment measures. To fully realise the potential of biosimilar competition, we advocate that all stakeholders develop a long-term coherent, integrated policy framework that is built on the following four pillars: i) the creation of a proactive and transparent climate supporting a level playing field for both biosimilar and reference product, including public dissemination of how savings at the level of the Belgian healthcare system are used, ii) investment in educational activities, including raising awareness of societal responsibilities, iii) enforcement of the practical implementation of public procurement law, and iv) development of physician incentives. Further work is needed to translate each of these four pillars into specific policy measures to be implemented, in particular with respect to physician incentives and prescribing behaviour.
Authors’ contributions
IH, AV, and EM developed the idea for and were involved in the design of this study. EM was involved in data collection and drafted the initial version of the manuscript. IH and AV critically reviewed the manuscript. All authors read and approved the final manuscript.
Acknowledgments
This manuscript is supported by KU Leuven and the Fund on Market Analysis of Biologics and Biosimilars following Loss of Exclusivity (MABEL).
Disclosure statement
AV is involved in consulting, advisory work and speaking engagements for a number of companies, a.o. AbbVie, Accord, Amgen, Biogen, EGA (now Medicines for Europe), Pfizer/Hospira, Mundipharma, Roche, Sandoz.
IH and EM declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Memorandum. Strombeek-Bever: Medaxes; 2019.
- Download medicine data | European Medicines Agency: European Medicines Agency; [cited 2020 Feb]. Available from: https://www.ema.europa.eu/en/medicines/download-medicine-data.
- BCFI | Startpagina: Belgisch Centrum voor Farmacotherapeutische Informatie; [ cited 2020 Jan]. Available from: https://www.bcfi.be/nl/start.
- Moorkens E, Vulto A, Huys I An overview of patents on therapeutic monoclonal antibodies in Europe: are they a hurdle to biosimilar market entry? [Unpublished]. 2020.
- De prijsdalingsmaatregel voor de “oude geneesmiddelen” en de “volumecliff” (The price decrease measure for “old medicines” and the “volume cliff”): RIZIV/INAMI/NIHDI; 2019; [cited 2020 Jan] Available from: https://www.inami.fgov.be/nl/themas/kost-terugbetaling/door-ziekenfonds/geneesmiddel-gezondheidsproduct/terugbetalen/specialiteiten/Paginas/prijsdalingsmaatregel-oude-geneesmiddelen.aspx.
- De prijsdalingsmaatregel voor de biologische geneesmiddelen (The price decrease measure for biological medicines): RIZIV/INAMI/NIHDI; 2019; [cited 2020 Jan]. Available from: https://www.inami.fgov.be/nl/themas/kost-terugbetaling/door-ziekenfonds/geneesmiddel-gezondheidsproduct/terugbetalen/specialiteiten/Paginas/prijsdalingsmaatregel-biologische-geneesmiddelen.aspx.
- Prijsdalingen en lijst goedkoopsten [Price decreases and list of low-cost medicines]: Brabants Apothekers Forum (BAF); 2020; [ cited 04]. Available from: https://baf.be/diensten/tarifering-helpdesk/prijsdalingen-en-lijst-goedkoopsten.
- Omzendbrief actieplan biosimilars (Circular letter action plan biosimilars): RIZIV/INAMI/NIHDI; 2016; [cited 2019 Nov]. Available from: https://www.inami.fgov.be/SiteCollectionDocuments/omzendbrief_actieplan_biosimilar.pdf.
- Terugbetaling van geneesmiddelen: wat is gewijzigd sinds 1 april 2019 (Reimbursement of medicines: changes from 1 April 2019): RIZIV/INAMI/NIHDI; 2019; [ cited Nov]. Available from: https://www.inami.fgov.be/nl/professionals/andere-professionals/farmaceutische-industrie/Paginas/terugbetaling-geneesmiddelen-01042019.aspx#Daling_tot_85%_voor_de_facturatie_van_bepaalde_geneesmiddelen_in_het_ziekenhuis.
- Lepage-Nefkens I, Gerkens S, Vinck I, et al. Barriers and opportunities for the uptake of biosimilar medicines in Belgium. Health Services Research (HSR) Brussels: belgian Health Care Knowledge Centre (KCE); 2013. Report No.: KCE Report 199. D/2013/10.273/13 Contract No.: KCE Report 199. D/2013/10.273/13.
- ‘Goedkoop voorschrijven’ (Low-cost prescribing): RIZIV/INAMI/NIHDI; 2018; [cited Nov 2019]. Available from: https://www.riziv.fgov.be/nl/professionals/individuelezorgverleners/artsen/verzorging/Paginas/goedkoop-voorschrijven-20150101.aspx#.WTh9WdwlHIU.
- Dylst P, Vulto A, Simoens S. Barriers to the uptake of biosimilars and possible solutions: a Belgian case study. Pharmacoeconomics. 2014;32(7):681–691.
- Biosimilars: FAGG/AFMPS/FAMHP; 2009; [cited 2019 Nov]. Available from: https://www.famhp.be/en/human_use/medicines/medicines/MA_procedures/types/Biosimilars.
- Switch and substitution of biological medicinal products: FAGG/AFMPS/FAMHP; 2009; [cited 2019 Nov]. Available from: https://www.famhp.be/en/switch_and_substitution_of_biological_medicinal_products.
- Convenant “Doorstart voor biosimilaire geneesmiddelen in België” (Convenant on the use of biosimilar medicines in Belgium): RIZIV/INAMI/NIHDI; 2018; [cited 2019 Nov]. Available from: https://www.inami.fgov.be/nl/themas/kost-terugbetaling/door-ziekenfonds/geneesmiddel-gezondheidsproduct/geneesmiddel-voorschrijven/Paginas/biosimilaire-geneesmiddelen.aspx.
- Biosimilaire geneesmiddelen: aanzienlijke besparingen voor het gezondheidszorgsysteem (Biosimilar medicines: significant savings for the healthcare system): RIZIV/INAMI/NIHDI; 2018; [ cited 2020 Jan]. Available from: https://www.inami.fgov.be/nl/themas/kost-terugbetaling/door-ziekenfonds/geneesmiddel-gezondheidsproduct/geneesmiddel-voorschrijven/Paginas/biosimilaire-geneesmiddelen-belangrijk-besparingen.aspx.
- Omzendbrief Opname van biosimilaire geneesmiddelen in het therapeutisch formularium (TF) door het Medisch-Farmaceutisch Comite (MFC) in uitvoering van de wetgeving op de overheidsopdrachten (Circular letter on the use of biosimilars in the therapeutic protocol of the Medical-Pharmaceutical Committee for the implementation of the law on public procurement): RIZIV/INAMI/NIHDI; 2016; [cited 2019 Nov]. Available from: https://www.inami.fgov.be/SiteCollectionDocuments/omzendbrief_biosimilaire_geneesmiddelen_ziekenhuis.pdf.
- Archief presentaties (Archived presentations): FAGG/AFMPS/FAMHP; 2018; [cited 2019 Nov]. Available from: https://www.fagg.be/nl/2018_archief_presentaties.
- Nieuwe campagne om patiënten te informeren over biologische geneesmiddelen en om het voorschrijven van biosimilaire geneesmiddelen aan te moedigen. (New campaign to inform patients about biological medicines and encourage the prescription of biosimilars.): FAGG/AFMPS/FAMHP; 2018; [cited 2019 Nov]. Available from: https://www.fagg.be/nl/news/nieuwe_campagne_om_patienten_te_informeren_over_biologische_geneesmiddelen_en_om_het.
- Informatiecampagne om patiënten te informeren over biologische geneesmiddelen en om het voorschrijven van biosimilaire geneesmiddelen aan te moedigen. (Information campaign to inform patients about biological medicines and to encourage the prescription of biosimilars.): RIZIV/INAMI/NIHDI; 2018; [cited 2019 Nov]. Available from: https://www.riziv.fgov.be/nl/pers/Paginas/biosimilaire-geneesmiddelen.aspx.
- Biologische en biosimilaire geneesmiddelen (Biological and biosimilar medicines): FAGG/AFMPS/FAMHP; 2018; [cited 2019 Nov]. Available from: https://www.faggcampagnes.be/nl/biologische-en-biosimilaire-geneesmiddelen.
- Koninklijk besluit tot vaststelling van de procedures, termijnen en voorwaarden inzake de tegemoetkoming van de verplichte verzekering voor geneeskundige verzorging en uitkeringen in de kosten van farmaceutische specialiteiten (Royal Decree laying down the procedures, terms and conditions for the reimbursement of the mandatory insurance for medical care and in the costs of pharmaceutical specialties): Belgisch Staatsblad; 2018; [cited 2019 Nov]. Available from: http://www.ejustice.just.fgov.be/cgi_loi/change_lg.pl?language=nl&la=N&cn=2018020122&table_name=wet.
- Biosimilaire geneesmiddelen: incentive voor het voorschrijven van biosimilaire geneesmiddelen buiten het ziekenhuis (Biosimilars: incentive to prescribe biosimilars in the outpatient setting): RIZIV/INAMI/NIHDI; 2019; [cited 2019 Nov]. Available from: https://www.inami.fgov.be/nl/themas/kost-terugbetaling/door-ziekenfonds/geneesmiddel-gezondheidsproduct/geneesmiddel-voorschrijven/Paginas/biosimilaire-geneesmiddelen-buiten-ziekenhuis.aspx.
- Biosimilars newsletter September 2019: Medaxes; 2019; [cited 2019 Nov]. Available from: https://www.medaxes.be/nl/actualiteit/biosimilars-newsletter.
- Omzendbrief nr. 646: FAGG/AFMPS/FAMHP; 2019; [cited 2019 Nov]. Available from: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=1&ved=2ahUKEwiIotPep43mAhUQblAKHYC2DH0QFjAAegQIBRAC&url=https%3A%2F%2Fwww.fagg.be%2Fsites%2Fdefault%2Ffiles%2Fcontent%2FDC-CT%2F20190625151700.pdf&usg=AOvVaw3vQ8Lp4w3QZCWtvAmVFHNq.
- Advies van de commissie voor de overheidsopdrachten betreft verenigbaarheid van premies of voordelen als gunningscriterium (Advice of the commission for public procurement concerning compatibility of premiums or benefits as an award criterion): Publicprocurement.be; 2019; [cited 2019 Nov]. Available from: https://www.publicprocurement.be/nl/documenten/verenigbaarheid-van-premies-voordelen-als-gunningscriterium.
- De begroting van de verzekering voor geneeskundige verzorging in 2020 (The budget for the insurance of medical care in 2020): RIZIV/INAMI/NIHDI; 2019; [cited 2020 Jan]. Available from: https://www.riziv.fgov.be/nl/nieuws/Paginas/begroting-geneeskundige-verzorging-20191122.aspx.
- Belgium: an innovative pharma valley in Europe Brussels: pharma.be; 2019; [cited 2020 Feb]. Available from: https://pharma.be/nl/component/attachments/?task=download&id=580:Broch.
- TED Tenders Electronic Daily: European Union; 2020; [cited 2020 Jan]. Available from: https://ted.europa.eu/TED/browse/browseByMap.do.
- Improving healthcare delivery in hospitals by optimized utilization of medicines - A study into 8 European countries. KPMG Advisory N.V. Commissioned by Medicines for Europe. 2019.
- Broens B. Huiszoekingen in de farmasector (Searches in the pharmaceutical sector): De Tijd; 2019; [cited 2020 Jan]. Available from: https://www.tijd.be/ondernemen/farma-biotech/huiszoekingen-in-de-farmasector/10169639.html.
- Ook ziekenhuizen in vizier mededingingsautoriteit (Also hospitals targeted by the competition authority): De Specialist; 2019; [cited 2020 Jan]. Available from: https://www.despecialist.eu/nl/nieuws/ook-ziekenhuizen-in-vizier-mededingingsautoriteit.html.
- Nederlandstalige Ondernemingsrechtbank Brussel - Vonnis (Dutch Brussels enterprise court -judgement): IEFbe; 2019; [cited 2020 Jan] Available from: http://ie-forum.be/documents/ecli/5dbc3f24-a054-4bd2-83bf-2a97c35ff8c2.pdf.
- EP2055313 - Treatment of hematologic malignancies associated with circulating tumor cells using chimeric anti-CD20 antibody European Patent Register: European Patent Office; 2020; [cited 2020 Jan]. Available from: https://register.epo.org/application?number=EP09002504&tab=main.
- EP1951304 - METHOD FOR TREATING JOINT DAMAGE European Patent Register: European Patent Office; 2020. [cited 2020 Jan]. Available from: https://register.epo.org/application?number=EP06837634&tab=main.
- BCFI - start: Belgisch Centrum voor Farmacotherapeutische Informatie (BCFI); 2020; [cited 2020 Jan]. Available from: https://www.bcfi.be/nl/start.
- Dutta B, Huys I, Vulto AG, et al. Identifying key benefits in European off-patent biologics and biosimilar markets: it is not only about price! BioDrugs. 2019. DOI:https://doi.org/10.1007/s40259-019-00395-w
- Shina S, Derbyshire M. Patent expiry dates for biologicals: 2018 update. GaBI J. 2019;8(1):24–31.
- Medicines under evaluation: European Medicines Agency; 2020; [cited 2020 Feb]. Available from: https://www.ema.europa.eu/en/medicines/medicines-under-evaluation.
- Moorkens E, Simoens S, Troein P, et al. Different policy measures and practices between Swedish counties influence market dynamics: part 2-biosimilar and originator etanercept in the outpatient setting. BioDrugs. 2019;33(3):299–306.
- Biosimilar medicines: case studies: NHS Scotland; 2016; [cited 2019 Oct]. Available from: http://www.healthcareimprovementscotland.org/our_work/technologies_and_medicines/adtc_resources/biosimilar_meds_case_studies.aspx.
- Grotere rol poliklinische apotheek bij TNF-alfaremmers [Greater role for hospital pharmacy for outpatients for TNF-alpha inhibitors]: Stichting Farmaceutische Kengetallen; 2013; [cited 2020 Jan]. Available from: https://www.sfk.nl/publicaties/PW/2013/grotere-rol-poliklinische-apotheek-bij-tnf-alfaremmers.
- Moorkens E, Vulto A, Huys I. Biosimilars. Regulatory frameworks for marketing authorisation of biosimilars: where do we go from here? Eur Pharm Law Rev. 2018;2(3):149–154.
- Het Biosimilars Nederland/IVM project: Biosimilars op Maat (BOM) [The Biosimilars Nederland/IVM project: A taillored approach to biosimilars]: Biosimilars Nederland; 2019; [cited 2020 Jan]. Available from: https://www.biosimilars-nederland.nl/het-biosimilars-nederland-ivm-project-biosimilars-op-maat-bom/.
- Biosimilars Op Maat [A tailored approach to biosimilars]: Instituut Verantwoord Medicijngebruik; 2020; [cited 2020 Jan]. Available from: https://www.medicijngebruik.nl/projecten/biosimilars-op-maat.
- Commissioning intentions: adalimumab: NHS England; 2018; [cited 2020 Jan]. Available from: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=12&cad=rja&uact=8&ved=2ahUKEwjf28S3mK7nAhXFnFwKHYFECYsQFjALegQIBBAB&url=https%3A%2F%2Fwww.sps.nhs.uk%2Fwp-content%2Fuploads%2F2018%2F09%2F20180925-Contractual-Commissioning-Intentions-Adalimumab_corporate-template.pdf&usg=AOvVaw3_UBUjJ57R5TQKGZQjV7e7.
- Mack A. Norway, biosimilars in different funding systems: what works? GaBI J. 2015;4(2):90–92.
- Vermeire S, Louis E, Dewit O, et al. Clinical and scientific aspects related to biosimilars in inflammatory bowel diseases (IBD): position document of the Belgian IBD Research & Development Group (BIRD). Acta Gastroenterol Belg. 2015;78(1):26–29.
- Westhovens R. Biosimilars in Rheumatic Diseases. Position of the Royal Belgian Society of Rheumatology: Reumanet; 2015; [cited 2020 Jan]. Available from: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=2&cad=rja&uact=8&ved=2ahUKEwj74fCFuq7nAhXsTxUIHbGJBo8QFjABegQIBhAB&url=https%3A%2F%2Fwww.reumanet.be%2Fsites%2Fdefault%2Ffiles%2FBiosimilars%2520KBVRSRBR%2520viewpoint.pdf&usg=AOvVaw2SxSM6qazfQJPQ7NQy4MHb.
- Franchimont D, Ferrante M, Louis E, et al. Belgian IBD research group (BIRD) position statement 2017 on the use of biosimilars in inflammatory bowel diseases (IBD). Acta Gastroenterol Belg. 2018;81(1):49–53.
- Somers M, Bossuyt P, Ferrante M, et al. For B. Belgian IBD Research Group (BIRD) position statement 2019 on the use of adalimumab biosimilars in inflammatory bowel diseases. J Crohns Colitis. 2019. DOI:https://doi.org/10.1093/ecco-jcc/jjz209
- New international record for switch to biosimilar: amgros; 2019; [cited 2020 Jan]. Available from: https://amgros.dk/en/knowledge-and-analyses/articles/new-international-record-for-switch-to-biosimilar/.
