Abstract
The effects of dietary vitamin E supplementation on the susceptibility to lipid oxidation and colour of the Longissimus thoracis (LT) muscle aged in vacuum packaged conditions for 3 or 14 days were studied. For this purpose, Charolais cattle were fed on a diet providing daily 60 mg (control) or 5500 mg of vitamin E per animal (supplemented) for 30 and 60 days before slaughter. Dietary vitamin E supplementation increased the liver vitamin E content, but not in the LT muscle of treated animals. The vitamin supplementation for 30 and 60 days has shown non-consistent effects in reducing cholesterol oxidation products of vacuum-packed aged meat. However, the vitamin E supplementation for 60 days was effective on Lightness stability in LT muscle during vacuum-packed ageing. Overall, from the practical standpoint, this study suggests that supra-nutritional supplementation up to 60 days may not increase the vitamin E content of Charolais LT muscle giving little, if any, benefits on meat colour and cholesterol oxidation. However, the present study suggests that it would be interesting to determine in which extent specific oxy-sterols are related to the meat colour and whether colour parameters can be useful for predicting the formation of cholesterol oxidation products along the industrial meat production chain.
Introduction
Oxidation of lipids is a major deterioration reaction that often results in a significant loss of meat quality. In the last decade, the formation of cholesterol oxidation products (COPs) in meat and other food items containing high levels of cholesterol has attracted increased attention due to their undesirable implications in human health. COPs have been directly involved in the initiation and progression of major chronic diseases, including atherosclerosis and coronary heart disease (Sottero et al. Citation2009). One of the most important antioxidant molecules is vitamin E (α-tocopherol). This compound is often present in a tiny amount in grain-based diets for farm animals, but it is not a limiting factor in grazing systems (Yang et al. Citation2002). Supplementation of animal diets with vitamin E may rise the tocopherol content of muscles, leading to an improved nutritional value and shelf life of meat through the inhibition of lipid oxidation and loss of desirable colour during storage (O’Grady et al. Citation2001). Radical species produced during lipid oxidation may act directly to promote myoglobin oxidation and/or indirectly by damaging pigment reducing systems (Faustman et al. Citation2010). Arnold et al. (Citation1993) defined the relationship between muscle α-tocopherol (α-TCF) concentration and met-myoglobin percentage, concluding that it might depend on the muscle type. The α-TCF in muscles has also shown to inhibit COPs formation in meat during the refrigerated stage, even though the effects of dietary vitamin E on cholesterol oxidation appear to be muscle type dependent (Galvin et al. Citation2000). The aim of this study was to analyse the effects of two short periods (30 and 60 days) of supra-nutritional dietary vitamin E supplementation on COPs content as lipid oxidation indicators and colour meat of Charolais beef vacuum aged for 3 or 14 days.
Materials and methods
Animals, management and diets
The study was carried out in a fattening farm located in Veneto Region (Italy). The trial included 60 Charolais young bulls imported from France at an average age of 354 days (SD 35.8 days). Just arrived from France, animals were randomly selected to form three groups (20 animals each) weighing 460 ± 5, 459 ± 5 kg and 458 ± 5 kg LBW, respectively. The three experimental groups were housed in six feedlot pens (two pens/group) of 36 m2 each equipped with 3 m long feed bunks and drinking bowls and were raised for 167, 165 and 170 days, respectively. Throughout the study, the same total mixed ration (TMR) was supplied (Table ) once a day (9:00 a.m.) to appetite in order to ensure that no feed was wasted. Average feed intakes recorded for the first, second and third groups were 9.82, 9.81 and 9.85 kg DM/day, respectively. The TMRs were collected monthly and analysed for DM by drying in a forced air stream at 65 °C until constant weight, crude protein (CP) by Kjeldahl method and ether extract by Soxlhet method (AOAC Citation1990) and for NDF, ADF, ADL (Van Soest et al. Citation1991). Compared to the first experimental group (C) maintained on the basal diet throughout the study, the second (Vit30) and third group (Vit60) were supplemented with 5500 mg/animal/day (5500 IU) of dl-α-tocopheryl acetate for 30 and 60 days before slaughter, respectively. This specific supplementation strategy was conceived to provide animals with a higher supplementation of vitamin E than as usual but for two relatively short periods, with expected affordable costs allowing to be routinely implemented as on-farm practical feeding strategy.
Table 1. Composition of the basal total mixed ration.
Slaughter and sampling procedure
Animals were slaughtered at commercial maturity for the Italian market in a private slaughterhouse (Verona, Italy) located 45 km from the farm. During the transport to the slaughterhouse, animals were managed in compliance with the Council Regulation No. 1/2005. From each of the three experimental groups, 10 animals (718 ± 27, 713 ± 27 and 706 ± 16 kg LBW for C, Vit30 and Vit60, respectively) were randomly selected for post-mortem sampling. Just after slaughtering, 20 g of liver tissue and 20 g of Longissimus thoracis (LT, 9th rib) muscle were removed, separately packed and stored frozen (−20 °C) until tocopherols determination. At 24 h after slaughtering, LT (10th–11th ribs) muscles were removed, vacuum-packed and aged at 4 °C for 3 (3d) or 14 days (14d). After ageing, the samples were unpacked and divided into two slices (2.5 cm thick); one slice was used to measure the meat colour; the second one was stored at −80 °C until analysis of the COPs content.
Reagents and standards
All chemicals were obtained from Sigma Chemical Co. (St Louis, MO). Super purity and gradient grade solvents for HPLC were obtained from Fluka Analytical (Milan, Italy). The vitamin integrator was kindly provided by Agristudio Nutrition SRL Bibbiano, RE, Italy.
Tocopherols determination
Tocopherols (α-, β-, γ- and δ-TCF, TCFs) in liver and LT samples were determined following the method proposed by Mestre Prate et al. (Citation2006). Neutralisation by adding 1 mL of H2SO4 (29% v/v) to enhance the recovery of δ-TCF after saponification with ethanolic KOH was the unique method’s modification. The HPLC analysis was performed using a SpectraSystem (Thermo Separation Product, Waltham, MA) equipped with a normal-phase silica column (Zorbax RX-Sil 4.6 mm ID, 250 mm, 5 μm; Agilent Technologies Inc., Santa Clara, CA) coupled to the corresponding 12.5 mm guard column. Samples and standards were injected (100 μL) in duplicate, and gradient elution was performed at 28 °C. The initial mobile phase n-hexane/isopropanol (99.5:0.5) was held for 1.5 min (flow = 1.6 mL/min), changed to 99:1 in 12.5 min (1.6 mL/min), changed to 99.5:0.5 in 0.5 min (2 mL/min) and then held at 99.5:0.5 for the last 2.5 min (2.0 mL/min) to allow for column equilibration. TCFs were detected using a SpectraSystem Fluorescence detector (Thermo Separation Product, Waltham, MA) set at λex 296 nm and λem 326 nm. In these conditions, α-, β, γ- and δ-TCF eluted at 4.4, 7.5, 8.1 and 10.5 min, respectively. The meat content of TCFs (μg/g ww) was estimated by external standard technique (calibration range: 0.02–1.0 μg/mL; R2 ≥ 0.996). Recoveries were estimated on spiked bovine liver (n = 4) and meat (n = 4) samples as described above. Mean recoveries of 83.8%, 96.5%, 98.0% and 104.5% were obtained for α-, β-, γ- and δ-TCF, respectively. The limits of detection (DIN Citation1994) were estimated to be 0.02 μg/g for all the TCFs.
Determination of cholesterol oxidation products
The determination of COPs was performed on total lipids according to Grau et al. (Citation2001). For the GC/MS analysis, extracted COPs were dried and re-dissolved in 200 μL of acetone containing 0.8 g/L BHT, with a subsequent addition of 90 mL of Sylon BTZ kit (Supelco, Bellefonte, PA) and incubated for 90 min at room temperature to complete the silanization. The GC/MS analyses were performed by an Agilent 6850A gas chromatograph coupled to a 5973N quadrupole mass selective detector (Agilent Technologies Inc., Santa Clara, CA). An Agilent HP-5MS fused-silica capillary column (30 m × 0.25 mm ID, film thickness 0.25 μm) was used for the GC separation. The injection mode was splitless at 280 °C. The column temperature program was 160 °C (1 min) to 280 °C at a rate of 20 °C/min and then held for 15 min. The carrier gas was helium at a constant flow of 1.0 mL/min. The spectra were obtained in electron impact mode at 70 eV ionisation energy with an ion source temperature of 280 °C and an ion source vacuum of 10−5 Torr. Analyses were performed both in total ion current and selected ion monitoring (SIM) mode. SIM analyses were performed by selecting the following representative ions: m/z 456 for 19-hydroxycholesterol (19-HC); m/z 474 for 7β − hydroxycholesterol (7β-HC); m/z 474 cholesterol-5β,6β-epoxide (5β,6β-epoxy); m/z 474 for cholesterol-5α,6α-epoxide (5α,6α-epoxy); m/z 472 for 7-ketocholesterol (7-keto). All samples were injected in duplicate. Linearity was verified for values up to 25 μg of each COP using 19-HC as an internal standard. COPs data were expressed as μg/g ww. All analyses were performed in triplicate.
Meat colour measurement
Meat colour was measured using a Minolta CM-3600d spectrophotometer (Minolta Co. Ltd., Osaka, Japan) after air exposure of the meat surface for 1 h at 4 °C. The data were acquired using the CIE L*a*b* coordinates using a D65 light source and a 7-mm diameter, generating readings of Lightness (L*), redness (a* value), yellowness (b* value), saturation (C value = (a*+b*)1/2) and Hue angle (H value = arctg b*/a*). Each data point was expressed as the mean of three measurements (Boccard et al. Citation1981). The myoglobin (Mb) content was estimated using K/S values, as reported by Hunt et al. (Citation1991), and because Minolta CM-3600d recorded wavelengths every 10 nm, we calculated data at 525 nm wavelength as a mean of 520 and 530 nm wavelengths.
Statistical analysis
Statistical analyses were performed using the mixed procedure of Statistic Analysis Systems Institute (SAS Institute, Citation2011), considering the diet (C, Vit30, Vit60) for the tocopherol content in liver and LT muscle with the following model: Yi = μ+Ai + ɛij, where μ is the general mean; Ai is the fixed effect of the ith diet; ɛ is the error term. The same statistic procedures were used analysing data on cholesterol and COPs concentrations, colour and oxymyoglobin (OMb) with the following model: Yijn = μ+Ai + Bj+ (A * B)ij + sn + ɛijnk, where μ is the mean; Ai is the fixed effect of the ith diet; Bj is the fixed effect of the jth ageing time; s is the random effect of the nth experimental subject; ɛ is the error term. Pearson’s product moment correlation (r) was used to assess the relationships between measured variables. Significance was declared for p < 0.05.
Results and discussion
Tocopherols in liver and LT muscle
In animals, the accumulation of TCFs has been reported to be both tissue- and organ-dependent, with liver and adrenal glands containing the highest quantities (Arnold et al. Citation1992). In this study, the dietary vitamin E supplementation for 30 and 60 days significantly increased the concentration of TCFs in liver (Table ). The muscle TCFs contents measured in treated animals increased in comparison to the controls but without reaching significance. Though partially, our results may be attributed to the high basal intake of the vitamin in the silage-based diets, as suggested by O’Grady et al. (Citation2001). In similar conditions, Nassu et al. (Citation2011) observed a wide overlapping in vitamin E content of beef samples from cattle supplemented daily with 350, 700 and 1400 IU α-tocopheryl acetate. These authors also pointed out that the effect of vitamin E supplementation may depend upon several factors, such as previous nutritional history, vitamin status of cattle at the start, stress level of animals as well as genetics, which is related to the ability of different animals to tolerate rearing conditions. In our experimental conditions, it cannot be excluded that variations in individual feed intake may have caused the high variable tissues accumulation of TCFs observed within each feeding group. The preponderance of α-TCF found both in the muscle and liver samples (>98%) in all the groups may be explained by the higher affinity shown by tocopherol-binding proteins for α-TCF (Arnold et al. Citation1993).
Table 2. Effects of vitamin E supplementation for 30 (Vit30) or 60 (Vit60) days before slaughter on vitamin E homologue content (μg/g ww) in the liver and LT muscle of Charolais cattle (means ± SD).
COPs in LT muscle
It has been demonstrated the presence of COPs in human blood and that they are potentially involved in the initiation and progression of major chronic diseases (Sottero et al. Citation2009). In comparison with the controls, we observed that the 7β-HC, the more toxic COP with 7β-keto, had a significant increase in the Vit30 group (+50%) but was lower in the Vit60 group. The inconsistency of the observed response to Vit E supplementation does not allow us to assert that supplementation with vitamin E for 60 days could be effective in maintaining this toxic oxysterol at low levels. As far as the ageing time effect, significant differences between 3 and 14 days were observed for the 5α,6α-epoxy and total COPs. As reported in the literature, the most predominant oxidised cholesterol form detected in animal products is 7-keto followed by α- and β-epoxycholesterol (Hur et al. Citation2007). Cholesterol α- and β-epoxides apparently originate from a double-oxidation mechanism via a ground-state dioxygen and a hydroperoxidation free radical-induction of cholesterol (Hur et al. Citation2007) that make them, therefore, secondary oxidation products. Surprisingly, in this study, the predominant occurrence of epoxides found in meat samples from all groups (Table ) may be indicative of undesired oxidation processes occurred probably during muscle handling (meat production phase and storage). Accordingly to the increase of epoxy-cholesterols, the total COPs/cholesterol ratio showed a significant increase in samples aged for 14 days (Table ). The increase of total COPs during meat ageing is related to an oxidation process depending on different factors such as meat manipulation and conservation conditions.
Table 3. Effects of vitamin E supplementation for 30 (Vit30) or 60 (Vit60) days before slaughter on COPs concentrations (μg/g ww) in Charolais LT samples vacuum aged for 3 or 14 days.
Meat colour
The results (Table ) show that neither supplementation strategy nor ageing time alone affected colour parameters with Lightness as the unique exception. In fact, regardless the feeding group, the L value after 14 days of ageing was higher (+7.72 p.p.) than the value measured at 3 days. Noteworthy, the diet × ageing time interaction was also significant. Partially in accordance with others’ findings (Boakye & Mittal Citation1996), Lightness of C and Vit30 meat samples increased during vacuum ageing but not in the Vit60 meat samples (Figure ). Negative correlation between Lightness and α-TCF or TCFs (r= −0.44, p < 0.05, respectively; data not shown) was found. These results may be regarded as an interesting effect of vitamin E supplementation especially taking into account that, in turn, total COPs, 7β-HC and 5β,6β-epoxy, were positively correlated (r = 0.65; r = 0.58 and r = 0.65, respectively; p < 0.05; data not shown) with L values at 3 days of ageing. Lipid oxidation and myoglobin oxidation in meat (that lead to discoloration) appear to be linked. Literature suggests that vitamin E can have a positive impact in preventing decreases of the a* values (correlated negatively with discoloration of meat) during meat storage (D’Agata et al. Citation2009). However, Eikelenboom et al. (Citation2000) did not find significant differences in a* values of vacuum aged (26 days) LT muscles from supplemented (2310 mg/day) and control (330 mg/day) cattle. As in the latter work, probably the basal level of vitamin E administered to animals in our trials was as high as needed to stabilise both a* and b* value during vacuum ageing up to 14 days. In supporting this hypothesis, Arnold et al. (Citation1992) stated that, for Longissimus muscle, a tissue level higher than 3.3 μg/g α-TCF should be adequate to stabilise the meat colour. Hue angle values (Table ) laid in the right side of the red-yellow region (i.e. 50–60°), and, interestingly, a marginal effect (p = 0.086) due to the diet × ageing time interaction was observed; in particular, at 14 days the Vit60 LT aged samples had a lower (p < 0.05) Hue angle compared with the controls (51.3 vs. 56.2; data not shown). At 14 days of ageing, the Hue angle was positively correlated with the content of 5β,6β-epoxy (r = −0.6; p < 0.05; data not shown), indicating that this meat colour parameter could be linked to specific cholesterol oxidation processes and most probably can capture subtle variation of meat colour more effectively than a* and/or b* can do. A highly significant ‘diet × ageing time’ interaction was observed for the OMb (Table ) but, more interestingly, OMb was statistically higher in Vit60 samples aged for 14 days than in the others (Figure ). This finding partially corroborates the hypothesis for this research that dietary vitamin E supplementation for a relatively short period to finishing cattle could delay lipid oxidationin muscle membranes and ferrous-to-ferric iron transaction in sarcoplasmic Mb. The linkage between lipid oxidation and myoglobin oxidation was confirmed by the negative correlation between, 5β,6β-epoxy, total COPs and OMb in muscles aged for 14 days (r = −0.63 and r = −0.52, respectively; p < 0.05; not shown). However, from examination of the literature, the relationship between meat colour and cholesterol oxidation in beef does not seem adequately investigated.
Figure 1. Lightness and oxymyoglobin content (%) determined in LT samples of different dietary treatment groups and at different ageing times. Mean values, gray bars: samples aged for 3 days; black bars: samples aged for 14 days. Different small letters (a, b) indicate significant differences between dietary treatments within the ageing time (p < 0.05). Asterisks (*) indicate significant differences between ageing times within the dietary treatment (p < 0.05).
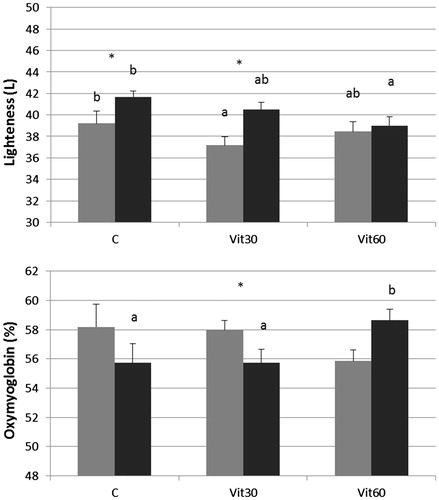
Table 4. Effects of vitamin E supplementation for 30 (Vit30) or 60 (Vit60) days before slaughter on colour parameters determined in Charolais LT samples vacuum aged for 3 or 14 days.
Conclusions
The individual capability of utilizing supplemented tocopherol has not allowed for the TCFs accumulation in the LT muscle that, most probably, gave inconsistent effects in preserving aged meat from COPs development. In this regard, the observed reduction of 7β-HC in animals submitted to the longest vitamin E treatment (60 days) needs further investigations to be performed using individual experimental units and, possibly, longer supplementation periods. The high vitamin E supplementation for 60 days was effective on meat Lightness stability during vacuum-packed ageing. In addition, the present study showed a correlation between some meat colour parameters and increased levels of both total COPs and some oxysterols such as β-epoxides and the toxic 7β-HC. Taken together, these results may be considered as a rationale justifying further research on medium-long lasting supra-nutritional administration of vitamin E in beef cattle. In addition, some findings of the present investigation suggest that it would be interesting to determine in which extent specific oxysterols are related to the meat colour and to understand whether the colour parameters can be useful for predicting the formation of COPs along the industrial meat production chain.
Funding information
This work was funded by the Ministry of Agricultural and Forestry Policies (MiPAF, Italy) through a Ministerial Decree DM 4809/7303/04 - Stand Beef project.
Acknowledgements
Agristudio s.r.l. in Reggio Emilia (Italy) for the formulation and manufacturing of the vitamin mix. Cooperativa Zootecnica Scaligera s.r.l. for their continued availability and support over the duration of this study.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- AOAC. 1990. Official methods of analysis. 15th ed. Arlington (VA): Association of Official Agricultural Chemistry.
- Arnold RN, Arp SC, Scheller KK, Williams SN, Schaefer DM. 1993. Tissue equilibration and subcellular distribution of vitamin E relative to myoglobin and lipidoxidation in displayed beef. J Anim Sci. 71:105–118.
- Arnold RN, Sheller KK, Arp SC, Williams SN, Buege SN, Schaefer DM. 1992. Effect of long- or short-term feeding of alpha-tocopheryl acetate to Holstein and cross bred beef steers on performance, carcass characteristics, and beef color stability. J Anim Sci. 70:3055–3065.
- Boakye K, Mittal GS. 1996. Changes in colour of beef M. longissimus dorsi muscle during ageing. Meat Sci. 42:347–354.
- Boccard R, Buchter L, Casteels E, Cosentino E, Dransfield E, Hood DE, Joseph RL, McDougall DB, Touraille C. 1981. Procedures for measuring meat quality characteristics in beef production experiments. Report of a Working group in the Commission of the European Communities (CEC) Beef Production Research Programme. Livest Prod Sci. 8:385–397.
- D’Agata M, Preziuso G, Russo C, Gatta D. 2009. Oxidant and antioxidant status: effects on shelf-life of meat from Limousine cattle fed with supplements of α-tocopherol. Ital J Anim Sci. 8:405–415.
- DIN. 1994. 32645 Norma – hemical analysis: decision limit, detection limit and etermination limit under repeatability conditions terms, ethods, evaluation. Deutsches Institut fur Normung ed. Berlin: Germany.
- Eikelenboom G, Hoving-Bolink AH, Kluitman I, Houben JH, Klont RE. 2000. Effect of dietary vitamin E supplementation on beef colour stability. Meat Sci. 54:17–22.
- Faustman C, Sun Q, Mancini R, Suman SP. 2010. Myoglobin and lipid oxidation interactions: mechanistic bases and control. Meat Sci. 86:86–94.
- Galvin K, Lynch AM, Kerry JP, Morissey PA, Buckley DJ. 2000. Effect of dietary vitamin E supplementation on cholesterol oxidation in vacuum packaged cooked beef steaks. Meat Sci. 55:7–11.
- Grau A, Codony R, Grimpa S, Baucells MD, Guardiola F. 2001. Cholesterol oxidation in frozen chicken meat: influence of dietary fat source, and α-tocopherol and ascorbic acid supplementation. Meat Sci. 57:197–208.
- Hunt MC, Acton JC, Benedict RC, Calkins CR, Cornforth DP, Jeremiah LE, Olson DG, Salm CP, Savell JW, Shivas SD, 1991. Guidelines for meat color evaluation Proceedings 44th Annual Reciprocal Meat Conference; Kansas State University, Manhattan, KS. p. 5.
- Hur SJ, Park GB, Joo ST. 2007. Formation of cholesterol oxidation products (COPs) in animal products. Food Contr. 18:939–947.
- Mestre Prate JA, Goncalves Quaesma MA, Branquinho Bessa RJ, Andrade Fontes CMG, Mateaus Alfaia CMP. 2006. Simultaneous HPLC quantification of total cholesterol, tocopherol and β-carotene in Barrosa-PDO veal. Food Chem. 9:469–477.
- Nassu RT, Dugan MER, Juárez M, Basarab JA, Baron VS, Aalhus JL. 2011. Effect of α-tocopherol tissue levels on beef quality. Animal. 5:2010–2018.
- O’Grady MN, Monahan FJ, Fallon RJ, Allen P. 2001. Effects of dietary supplementation with vitamin E and organic selenium on the oxidative stability of beef. J Anim Sci. 79:2827–2834.
- SAS. 2011. SAS/STAT 9.3 user’s guide. Cary, NC: SAS Institute Inc.
- Sottero B, Gamba P, Gargiulo S, Leonarduzzi G, Poli G. 2009. Cholesterol oxidation products and disease: an emerging topic of interest in medicinal chemistry. Curr Med Chem. 16:685–705.
- Van Soest PJ, Robertson B, Lewis BA. 1991. Methods for dietary fiber, neutral detergent fiber and non-starch polysaccharides in relation to animal nutrition. J Dairy Sci. 74:3583–3597.
- Yang A, Brewster MJ, Lanari MC, Tume RK. 2002. Effect of vitamin E supplementation on α-tocopherol and β-carotene concentrations in tissues from pasture- and grain-fed cattle. Meat Sci. 60:35–40.
