Abstract
This study was conducted to investigate the effects of soybean antigen proteins on serum cytokine levels and claudin-1 distribution in the intestine of weaned piglets. Seventy piglets (24 d of age) were randomly divided into seven groups. Piglets in group A were fed a basal diet, in groups B, C and D were fed the basal diet supplemented with β-conglycinin; and in groups E, F, G were fed the basal diet supplemented with glycinin. Blood samples were collected and analysed for IFN-γ, IL-2, IL-4 and IL-6 levels on day 24, 26, 28 and 30. At the end of the trial, five piglets per group were sacrificed and the small intestine was collected to evaluate intestinal claudin-1 distribution and OD of the relative staining positivity. β-Conglycinin and glycinin decreased the growth performance in piglets by decreasing ADFI, ADG and increasing F/G. Serum levels of IL-2, IL-4 and IL-6 in β-conglycinin and glycinin groups ranged from 126.067 to 144.067 ng • L−1, 31.798 to 44.360 ng • L−1 and 36.360 to 40.482 ng • L−1 on day 24, 26 and 30. However, serum IFN-γ levels were negatively correlated with soybean antigen proteins supplementation levels ranged from 6.956 to 15.361 ng • L−1. The treatment groups had lower OD values of claudin-1 than those of the control group. The β-conglycinin groups had lower distribution and OD values than the glycinin groups at similar antigen protein supplementation levels. These results suggest that β-conglycinin and glycinin damage the intestinal mucosal immune barrier in weaned piglets.
Introduction
Soybean antigen proteins, present in raw soybeans and soybean products, are the main soybean allergens (Sun & Qin Citation2006) that affect mostly infants and young animals (Lallès et al. Citation1996; Christensen et al. Citation2003). These allergens not only pose a threat to human health, they also contribute to significant economic losses (Kou et al. Citation2014). β-Conglycinin and glycinin, which are the main soybean antigen proteins, reduce growth performance in piglets (Wang et al. Citation2012). Our previous study showed that soybean antigen protein could damage the intestinal mucosa, increase intestinal permeability and promote sIgA synthesis in weaned piglets (Wu et al. Citation2016). Studies focused on soybean antigen proteins have mainly evaluated processing and sensitisation methods (Li et al. Citation1990; Qin et al. Citation1996; Sun et al. Citation2008).
Studies have shown that the allergic reactions induced by soybean antigen proteins are mediated by IgE; however, other studies have reported that the allergic response is mediated by T cells (Sun et al. Citation2005). IL-2, IL-4, IL-6 and INF-γ, which are closely associated with both the humoral and cellular immune system, regulate the proliferation and differentiation of B cells, the secretion of immunoglobulins and the production of Th2 cells, monocytes and macrophages (Leonard et al. Citation1982). Serum IL-2 levels in pigs increase following the administration of soybean by gavage feeding (Yang et al. Citation2005). However, few studies have focused on the effects of β-conglycinin and glycinin on the serum cytokine levels of weaned piglets.
The intestinal tight junction protein claudin-1 plays an important role in the intestinal mucosal barrier (Luo Citation2011). Glucagon-like peptide-2 (GLP-2) protects the intestinal mucosal barrier by increasing the expression levels of claudin-1 mRNA in weaned piglets (Jiang et al. Citation2012). Claudin-1, which is an indicator of the presence of intestinal tight junctions, is involved in intercellular adhesion, mobility and permeability. Changes in claudin-1 increase intestinal permeability. To the best of our knowledge, few studies have evaluated the effects of soybean antigen protein on serum cytokine levels and claudin-1 protein. The purpose of this study was to assess the effects of β-conglycinin and glycinin on serum cytokine levels and claudin-1 distribution and expression in weaned piglets.
Materials and methods
The experimental use of animals and procedures followed were approved by the Anhui Agricultural University Animal Care Committee (Hefei, China).
Reagents
β-Conglycinin (purity of 95%) and glycinin (purity of 94%) were kindly donated by Professor Shuntang Guo from the Food Institute of China Agricultural University (patent number: 200,410,029,589.4, China). The primary claudin-1 antibody and goat anti-rabbit secondary antibody were purchased from Beijing Zhong Shan Jinqiao Biotechnology Development Co. Ltd (Beijing, China). Swine IL-2, IL-4, IL-6 and INF-γ Enzyme-Linked Immunosorbent Assay (ELISA) kits were obtained from Nanjing Senbeijia Biological (Nanjing, China).
Animals and diets
Seventy cross-bred [(Duroc × Landrace) × Yorkshire] weaned piglets (21 d of age) were selected from seven litters in the Anhui (China) Antai Agricultural Group. Ten piglets were kept in each pen, which was equipped with a feeder and a waterer to allow ad libitum access to feed and water. The piglets were housed in a mechanically ventilated nursery room with a lighting cycle of 12 h light and 12 h darkness, the temperature was maintained at 26–28 °C and the relative humidity was set at 50–60%.
During the experiment, the diet was provided (Table ) and the diet was formulated to meet or exceed nutrient requirements suggested by the National Research Council (NRC Citation1998).
Table 1. Ingredients and nutrient contents of experimental diets.
Growth performance
Feed consumption and BW of individual pigs were determined at weaning (24 d of age) and at the end of the trial (30 d of age) in order to calculate average daily gain (ADG), average daily feed intake (ADFI), and the feed:gain ratio (F/G).
Experimental design and samples collection
All the piglets were randomly divided by weight and gender into 7 groups, with 10 piglets per group. The piglets of the group A (control group) were fed a basal diet. The piglets of groups B, C and D were fed the basal diet supplemented with 500, 2000 and 5000 mg/kg body weight (BW) β-conglycinin, respectively. The piglets of groups E, F and G were fed the basal diet supplemented with 500, 2000 and 5000 mg/kg·BW glycinin, respectively. All piglets were weaned at 24 d of age and fed a soybean antigen protein from this day. The soybean antigen protein were supplemented into the diet during all the experimental period and lasted for 7 days.
Five milliliters of blood were collected from the anterior vena cava into test tubes containing no anticoagulants. Blood collection was performed from ten piglets between 7:00 and 9:00 AM on day 24, 26, 28 and 30. The blood samples were immediately centrifuged at 2000 g for 20 min at 4 °C; the resulting serum was stored at −80 °C.
At the end of the trial, five piglets from each group were randomly selected and slaughtered by an intracardiac injection of sodium pentobarbital at the dose of 50 mg/kg·BW followed by jugular exsanguination on d 31. The duodenum, proximal jejunum, middle jejunum, distal jejunum and ileum (each had a length of 6 cm) were collected and washed with physiological saline. Intestinal tissue sections were immediately fixed in 3–5 volumes of 4% paraformaldehyde solution for 24 h, dehydrated with gradient ethanol and embedded in paraffin. The paraffin sections were used for claudin-1 determination.
Determination of serum cytokines
The serum IL-2, IL-4, IL-6 and INF-γ levels were measured with commercially available kits (Nanjing Senbeijia Biological, Nanjing, China). The assays were performed at OD450 nm in an Automatic Microplate Reader (Multiskan MK3, Thermo Fisher Scientific Inc., Walthan, MA).
Determination of intestinal claudin-1 protein
The methods followed were in accordance with that reported by Wu et al. (Citation2016). The intestinal paraffin sections were de-waxed and dehydrated. The paraffin was subsequently incubated in the dark with 10% bovine serum albumin (BSA) for 10 min at 37 °C and with 50 to 100 μl primary antibodies (rabbit anti human, 1:50) overnight (Li et al. Citation2015). The paraffin was washed three times with PBS, pH 7.4 (5 min each time), incubated with 50 to 100 μl secondary antibody for 15 min at 37 °C and washed three times with PBS, pH 7.4 (5 min each time). Freshly prepared diaminobenzidine (DAB) working solution (50–100 μl) was incubated with the paraffin for 1 to 2 min. The paraffin was subsequently washed with ddH2O for 5–10 min, dyed with hematoxylin (HE) for 30 s, differentiated in 1% hydrochloric and alcohol, washed under running water for 3 min, dehydrated with alcohol and rinsed with xylene. The expression of claudin-1 protein was expressed as the average optical density in at least five areas and was observed under a fluorescence microscope (Olympus Optical Co., Ltd., Tokyo, Japan).
Statistical analysis
Data were expressed as mean ± SD. Statistical analysis was performed with the Statistical Package for the Social Sciences (SPSS) 17.0 software (SPSS, Inc., Chicago, IL). Differences among groups were assessed by ANOVA; multiple comparisons were performed by Duncan’s method. The models included in the ANOVA used the group as fixed factor and the fact that piglets used in four periods of different treatments was considered as animal effect. p < 0.05 was considered to be statistically significant. The images obtained from the immunohistochemistry experiments were analysed by the Image-Pro plus 6.0 Analysis Software (Media Cybernetics, China) to determine the average optical density (OD) values of intestinal claudin-1 expression.
Results
Growth performance
As shown in Table , β-conglycinin and glycinin have negative effects on growth performance. Compared with control group, ADFI had a decreased trend, especially in groups B (p < 0.05), C (p < 0.01), D (p < 0.01) and G (p < 0.05). Moreover, ADG in the groups C (p < 0.01), D (p < 0.01) and G (p < 0.01) were lower than that of the control group in the period of experiment. In the contrast, the F/G showed an increased trend, especially in groups C (p < 0.05) and D (p < 0.01). Collectively, supplementation of β-conglycinin and glycinin decreased the growth performance in piglets.
Table 2. Effects of soybean antigen proteins on growth performance in weaned piglets.
Serum IL-2 levels
The serum IL-2 levels were higher in groups C (p < 0.05), D (p < 0.01) and G (p < 0.01) than those of the control group on day 26. In addition, the serum IL-2 levels in the β-conglycinin and glycinin groups had an increasing trend, especially in groups C (p < 0.05), D (p < 0.01) and G (p < 0.01) on day 30 (Table ).
Table 3. Effects of soybean antigen proteins on serum IL-2 levels in weaned piglets (ng/l).
Serum IL-4 levels
Piglets of the β-conglycinin and glycinin groups had higher serum IL-4 levels than those of the control group, especially groups C (p < 0.05), D (p < 0.01) and G (p < 0.05) on day 26. At similar protein supplementation levels, group C (p < 0.05) had higher serum IL-4 levels than group F (Table ).
Table 4. Effects of soybean antigen proteins on serum IL-4 levels in weaned piglets (ng/l).
The treatment groups had higher serum IL-4 levels than those of the control group, especially in groups C (p < 0.05), D (p < 0.01) and group G (p < 0.05) on day 28.
The serum IL-4 levels in group D were higher than those of the control group (p < 0.05) on day 30.
Serum IL-6 levels
Piglets of the β-conglycinin and glycinin groups had higher serum IL-6 levels than those of the control group, especially in groups C (p < 0.05), D (p < 0.01), F (p < 0.05) and G (p < 0.05) on day 26 (Table ).
Table 5. Effects of soybean antigen proteins on serum IL-6 levels in weaned piglets (ng/l).
The serum IL-6 levels in the treatment groups were higher than those of the control group, especially in groups C (p < 0.05), D (p < 0.01) and G (p < 0.01) on day 28.
The β-conglycinin and glycinin groups had higher serum IL-6 levels than those of the control group, especially groups C (p < 0.01), D (p < 0.01), F and G (p < 0.01) on day 30.
Serum IFN-γ levels
All the β-conglycinin and glycinin groups had lower serum IFN-γ levels than those of the control group on day 26, especially in groups B (p < 0.01), C (p < 0.01), D (p < 0.01), E (p < 0.05), F (p < 0.01) and G (p < 0.01). At similar protein supplementation levels, the serum IFN-γ levels in group B were lower (p < 0.01) than in group E (Table ).
Table 6. Effects of soybean antigen proteins on serum IFN-γ levels in weaned piglets (ng/l).
The serum IFN-γ levels in the β-conglycinin and glycinin-treatment groups were lower in groups D (p < 0.01), F (p < 0.01) and G (p < 0.01) than those of in the control group on day 28. At similar protein supplementation levels, serum IFN-γ levels in group C were higher (p < 0.05) than in group F.
The serum IFN-γ levels in the treatment groups were lower than those of the control group, especially in groups C (p < 0.05), D (p < 0.01), F (p < 0.05) and G (p < 0.01) on day 30.
Distribution and expression of claudin-1 protein
There was significant staining in cell membranes of groups B, C and D (β-conglycinin groups) and groups E, F and G (glycinin groups); cell cytoplasm was stained blue. While the negative control group (group H) had blue-stained cytoplasm, there was no brown/yellow staining in this group (Figures ).
Figure 1. Immunohistochemistry of claudin-1 in duodenum of piglets (×400). Group A was control group and piglets in this group were fed with basal diet; Piglets in groups B, C and D were fed with basal diet + (500, 2000 and 5000 mg/kg·BW) β-conglycinin, respectively; Piglets in groups E, F and G were fed with basal diet + (500, 2000 and 5000 mg/kg·BW) glycinin, respectively; Group H was negative control. The average optical density (OD) value of claudin-1 expression was analysed by the Image-Pro plus 6.0 and as shown in Table .
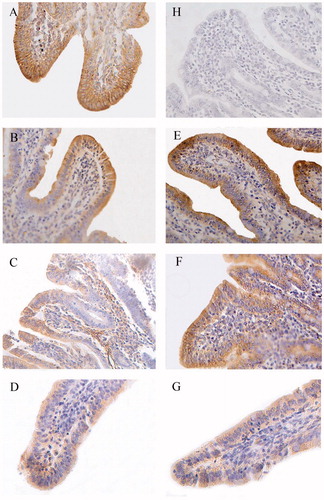
Figure 2. Immunohistochemistry of claudin-1 in proximal jejunum of piglets (×400). Group A was control group and piglets in this group were fed with basal diet; Piglets in groups B, C and D were fed with basal diet + (500, 2000 and 5000 mg/kg·BW) β-conglycinin, respectively; Piglets in groups E, F and G were fed with basal diet + (500, 2000 and 5000 mg/kg·BW) glycinin, respectively; Group H was negative control. The average optical density (OD) value of claudin-1 expression was analysed by the Image-Pro plus 6.0 and as shown in Table .
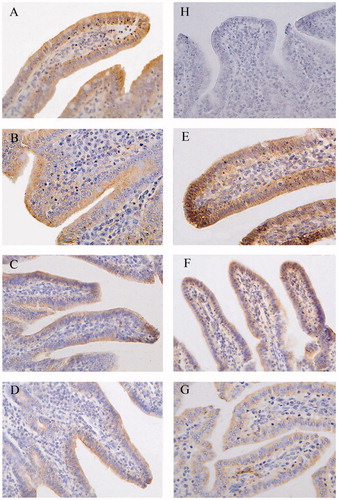
Figure 3. Immunohistochemistry of Claudin-1in middle jejunum of piglets (×400). Group A was control group and piglets in this group were fed with basal diet; Piglets in groups B, C and D were fed with basal diet + (500, 2000 and 5000 mg/kg·BW) β-conglycinin, respectively; Piglets in groups E, F and G were fed with basal diet + (500, 2000 and 5000 mg/kg·BW) glycinin, respectively; Group H was negative control. The average optical density (OD) value of claudin-1 expression was analysed by the Image-Pro plus 6.0 and as shown in Table .
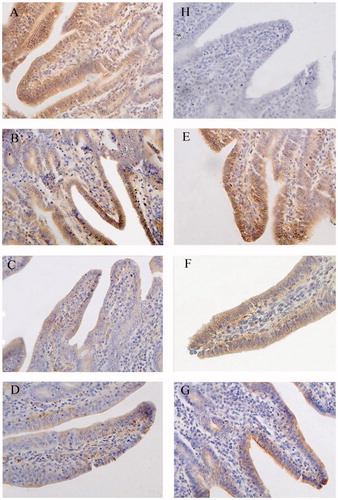
Figure 4. Immunohistochemistry of claudin-1 in distal jejunum of piglets (×400). Group A was control group and piglets in this group were fed with basal diet; Piglets in groups B, C and D were fed with basal diet + (500, 2000 and 5000 mg/kg·BW) β-conglycinin, respectively; Piglets in groups E, F and G were fed with basal diet + (500, 2000 and 5000 mg/kg·BW) glycinin, respectively; Group H was negative control. The average optical density (OD) value of claudin-1 expression was analysed by the Image-Pro plus 6.0 and as shown in Table .
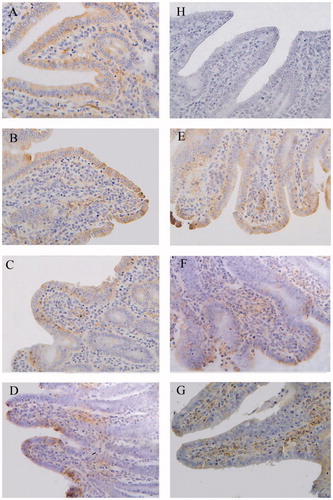
Figure 5. Immunohistochemistry of Claudin-1 in ileum of piglets (×400). Group A was control group and piglets in this group were fed with basal diet; Piglets in groups B, C and D were fed with basal diet + (500, 2000 and 5000 mg/kg·BW) β-conglycinin, respectively; Piglets in groups E, F and G were fed with basal diet + (500, 2000 and 5000 mg/kg·BW) glycinin, respectively; Group H was negative control. The average optical density (OD) value of claudin-1 expression was analysed by the Image-Pro plus 6.0 and as shown in Table .
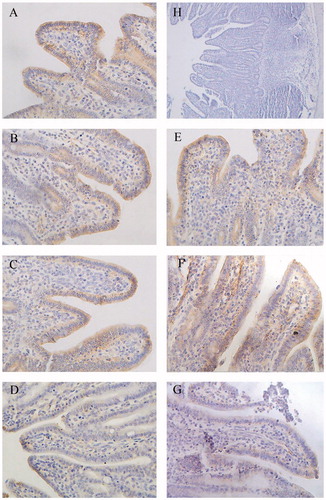
The distribution and OD values of claudin-1 in the duodenum were significantly lower in groups C (p < 0.05), D (p < 0.01) and G (p < 0.01) than those of the control group (Figure , Table ). The distribution and OD values of claudin-1 were lower in group D compared to groups B and C (p < 0.01 or p < 0.05) and in group G compared to groups E and F (p < 0.01). Compared with the group F, group C had lower OD values (p < 0.05) in the duodenum.
Table 7. The OD values of claudin-1 protein positive expression in different intestinal sections.
The distribution and OD values of claudin-1 in the proximal jejunum were significantly lower in groups C (p < 0.01), D (p < 0.01) and G (p < 0.01) than those of the control group (Figure , Table ). The distribution and OD values of claudin-1 were lower in groups D (p < 0.01) than groups B and C as well as lower in group G than the groups E (p < 0.01) and F (p < 0.01).
In the middle jejunum, groups C (p < 0.01), D (p < 0.01), F (p < 0.05) and G (p < 0.01) had lower distribution and OD values of claudin-1 (Figure , Table ) than those of the control group. The distribution and OD values of claudin-1 were significantly lower in group C compared with the groups B (p < 0.01) and D (p < 0.01) and in group D compared to groups B (p < 0.01). The distribution and OD values of claudin-1 protein were significantly lower in group G than in groups E and F (p < 0.01). Compared with the group F, the group C had lower OD values in the middle jejunum (p < 0.05).
In the distal jejunum, the distribution and OD values of claudin-1 were significantly lower in groups C (p < 0.01), D (p < 0.01), F (p < 0.05) and G (p < 0.01) than those of the control group (Figure , Table ). The distribution and OD values of claudin-1 were significantly lower in group D than groups B (p < 0.01) and C (p < 0.05) as well as lower in group G than groups E (p < 0.01) and F (p < 0.01).
In the ileum, the distribution and OD values of claudin-1 were lower in groups D (p < 0.01) and G (p < 0.01) than those of the control group (Figure , Table ). The distribution and OD values of claudin-1 in group D were significantly lower than groups B (p < 0.01) and C (p < 0.01) as well as lower in group G than groups E (p < 0.01) and F (p < 0.01).
Discussion
β-Conglycinin- and glycinin-induced allergic reactions in piglets, thereby contributing to intestinal mucosa damage, diarrhoea and reduced growth performance (Hampson & Kidder Citation1986; Wang et al. Citation2012) . In the present study, β-conglycinin and glycinin showed growth suppression by decreasing the ADFI and ADG. Cytokines play important roles in the immune response (Raymond & Wilkie Citation2004). Soybean antigen proteins affect both the humoral and cellular immune system, reduce chyme residence in the gut, affect the intestinal barrier and contribute to malabsorption and diarrhoea in piglets (Lallès et al. Citation1993). β-Conglycinin and its subunits increase serum levels of IgE and IgG1 and histamine concentrations in the gut (Guo et al. Citation2007). A study reported that soybean antigen proteins damage the mesenteric lymph nodes and inhibit lymphocyte proliferation (Dreau et al. Citation1994), while another study reported that soybean antigen proteins do not induce allergic reactions when injected subcutaneously in weaned piglets (Li et al. Citation1991). The difference in the results may be attributed to differences in the experimental methods. In this study, the serum levels of IL-2, IL-4 and IL-6 increased with increasing supplementation levels of β-conglycinin and glycinin, in agreement with a previous report (Liu Citation2008). Collectively, the results revealed that soybean antigen proteins promote cytokine production, inflammatory cell proliferation and intestinal mucosa damage.
INF-γ plays a distinct role in humoral and cellular immune responses (Yang Citation1996). IFN-γ is involved in T-cell proliferation and in the differentiation of CD4+ T cells into Th1 cells. In this study, IFN-γ levels were significantly reduced with increasing protein supplementation levels, in contrast to the results obtained with serum levels of IL-2, IL-4 and IL-6. IFN-γ plays different roles compared to IL-2, IL-4 and IL-6 in the allergic reaction induced by β-conglycinin and glycinin. Similar results have been reported by other researchers (Zhu et al. Citation2011), suggesting that the intestinal immune function of piglets is damaged following soybean antigen protein supplementation. Furthermore, with increasing soybean antigen protein levels, the intestinal mucosa was significantly damaged.
Tight junctions, which are on the apical side of epithelial cells, connect adjacent cells and close cellular gaps. Tight junctions allow a water–salt balance and maintain the polarity of epithelial cells. Claudin-1 is one of the most important tight junction proteins, which plays significant roles in the intestinal nutrient absorption, water–salt balance and as a barrier to pathogens and large molecules. Claudin-1 interacts with other tight junction proteins to adjust the permeability of intestinal epithelial cells and is an important indicator of the intestinal permeability (Yang et al. Citation2005). In weaned piglets with Escherichia coli-induced diarrhoea, the expression levels of claudin-1 was significantly decreased in the ileum and colon (Xia Citation2010). In this study, diarrhoea appeared in the β-conglycinin- and glycinin-supplemented groups. Additionally, claudin-1 expression levels were lower in the treatment groups than in the control group, suggesting that the intestinal tight junctions of the weaned piglets were damaged. Furthermore, the OD values of claudin-1 decreased in proportion to the supplementation levels of β-conglycinin and glycinin; however, there was no significant reduction in OD values in groups supplemented with 500 mg/kg·BW β-conglycinin or glycinin. These results revealed that β-conglycinin- and glycinin-induced intestinal damage were similar to the previous findings (Han et al. Citation2013). In addition, the OD values of claudin-1 were lower in the β-conglycinin groups than in the glycinin groups, indicating a higher allergenicity of β-conglycinin. Based on these results, we speculate that the soybean antigen proteins, especially β-conglycinin, contributed to intestinal damage in piglets.
Conclusions
The present study indicated that the supplementation of β-conglycinin and glycinin caused humoral and cellular immune responses in weaned piglets by increasing the serum IL-2, IL-4 and IL-6 levels and reducing the serum IFN-γ levels. The β-conglycinin and glycinin damaged the intestinal mucosal barrier of piglets, ultimately led to the damage of intestine, by reducing the relative expression levels of tight junction protein claudin-1.
Funding information
This study was jointly supported by the National Natural Science Foundation of China [No. 31472250 and 31502136] and the National ‘Fu Min Qiang Xian’ Program of China [No. 2012-745].
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Christensen HR, Bruun SW, Frøkiaer H. 2003. Antigenic specificity of serum antibodies in mice fed soy protein. Int Arch Allergy Immunol. 132:58–67.
- Dreau D, Lalles JP, Philouze-Rome V, Toullec R, Salmon H. 1994. Local and systemic immune responses to soybean protein ingestion in early-weaned pigs. J Anim Sci. 72:2090–2098.
- Guo P, Piao X, Cao Y, Ou D, Li DF. 2007. Recombinant soybean protein β-conglycinin α-subunit expression and induced hypersensitivity reaction in rats. Int Arch Allergy Imm. 145:102–110.
- Hampson DJ, Kidder DE. 1986. Influence of creep feeding and weaning on brush border enzyme activities in the piglet small intestine. Res Vet Sci. 40:24–31.
- Han R, Zhao Y, Pan L, Wang Y, Qin GX. 2013. Effects of glycinin on occludin mRNA expression in piglets intestinal epithelial cells. Chinese J Anim Vet Sci. 44:1258–1262.
- Jiang Y, Jia G, Chen XL, Li H, Wang KN. 2012. Effects of glucagon-like peptide-2 supplementation on expression of intestinal epithelial tight junction protein related genes in weaned piglets in vitro. Chinese J Anim Nut. 24:1785–1792.
- Kou YN, Ma LY, Luo Y, Wang XC, Wu JJ. 2014. Establishment of an indirect enzyme-linked immune sorbent assay for detection of serum antibody of soybean antigen protein in piglets. J Northwest A & F Univ. 42:17–22.
- Lallès JP, Dreau D, Salmon H, Toullec R. 1996. Identification of soyabean allergens and immune mechanisms of dietary sensitivities in preruminant calves. Res Vet Sci. 60:111–116.
- Lallès JP, Salmon H, Bakker NPM, Tolman GH. 1993. Effects of dietary antigens on health, performance and immune system of calves and piglets. In: van der Poel AFB, Huisman J, Saini HS editors. Recent advances of research in antinutritional factors in legume seeds. Wageningen (The Netherlands): Wageningen Pers. pp. 253–270.
- Leonard WJ, Depper JM, Uchiyama TK, Smith A, Waldmann TA, Greene WC. 1982. A monoclonal antibody that appears to recognize the receptor for human T-cell growth factor; partial characterization of the receptor. Nature. 300:267–269.
- Li WJ, Dong HM, Zhao HJ, Song Jf, Tang HX, Yao LH, Liu LY, Tong WC, Zou MC, Zou F, et al. 2015. 1,25-Dihydroxyvitamin D3 prevents toluene diisocyanate-induced airway epithelial barrier disruption. Int J Mol Med. 36:263–270.
- Li DF, Nelssen JL, Reddy PG, Blecha F, Hancock JD, Allee GL, Goodband RD, Klemm RD. 1990. Transient hypersensitivity to soybean meal in the early-weaned pig. J Anim Sci. 68:1790–1799.
- Li DF, Nelssen JL, Reddy PG, Blecha F, Klemm RD, Giesting DW, Hancock JD, Allee GL, Goodband RD. 1991. Measuring suitability of soybean products for early-weaned pigs with immunological criteria. J Anim Sci. 69:3299–3307.
- Li GR, Li JJ, Tan B, Wang J, Kong XF, Guan GP, Li FN, Yin YL. 2015. Characterization and regulation of the amino acid transporter SNAT2 in the small intestine of piglets. PLoS One. 10:1–15.
- Liu X. 2008. Research on mechanism of allergic reactions caused by soybean glycinin and β-conglycinin in Balb/c mice. Hangzhou: Zhe Jiang University.
- Luo HZQQ. 2011. Roles of tight junction protein claudins phosphorylation in gastrointestinal barrier function. Chin J Gastroenterol. 16:438–441.
- NRC. 1998. Nutrient requirements of swine. 10th ed. Washington (DC, USA): National Academic Press.
- Qin GX, Ter Elst ER, Bosch MW, Van der Poel AFB. 1996. Thermal processing of whole soya beans: studies on the inactivation of antinutritional factors and effects on ileal digestibility in piglets. Anim Feed Sci Tech. 57:313–324.
- Raymond CR, Wilkie BN. 2004. Th-1/Th-2 type cytokine profiles of pig T-cells cultured with antigen-treated monocyte-derived dendritic cells. Vaccine. 22:1016–1023.
- Sun P, Li DF, Li Z, Dong B. 2008. Effects of glycinin on IgE-mediated increase of mast cell numbers and histamine release in the small intestine. J Nutr Biochem. 19:627–633.
- Sun P, Qin GX. 2006. Effects of steam-processing on the content and immunogenicity of purified soybean antigens. Chinese J Vet Med Sci. 26:551–554.
- Sun ZW, Qin GX, Lou Y. 2005. Soybean antigens and its influence on piglets and calves. Acta Zoonutrimenta Sinica. 17:20–24.
- Wang XC, Xu SL, Wang Y, Chen L, Yin C, Feng SB, Wu JJ. 2012. Effects of soybean protein antigen on growth performance, intestine digestive enzyme activity and histamine levels in weaning piglets. Chin J Vet Sci. 32:619–623.
- Wu JJ, Cao CM, Ren DD, Zhang Y, Kou YN, Ma LY, Feng SB, Li Y, Wang XC. 2016. Effects of soybean antigen proteins on intestinal permeability, 5-hydroxytryptamine levels and secretory IgA distribution in the intestine of weaned piglets, Ital. J Anim Sci. 15:174–180.
- Xia T. 2010. Expression and significance of claudin-1 in the gastrointestinal tract of diarrhea piglets infected with the E. Coli. Degree Master, Huazhong Agricultural University, China.
- Yang HC. 1996. Animal Immunology. 1st Rev. ed. Beijing, China: China Agricultural University Press.
- Yang R, Harada T, Li J, Uchiyama T, Han Y, Englert JA, Fink MP. 2005. Bile modulates intestinal epithelial barrier function via an extracellular signal related kinase 1/2 dependent mechanism. Intens Care Med. 31:709–717.
- Yang XJ, Zuo WY, Chen WH, Zou SX. 2005. Effects of hydrolysates from soybean with pepsin on immunity in rats. Chin J Vet Sci. 36:348–351.
- Zhu C, Jiang ZY, Zheng CY, Wang SK, Wang L. 2011. Soybean antigenic protein:composition and allergy mechanisms. Chinese J Anim Nutr. 23:2053–2063.
