Abstract
The aim of the present study was to develop a new in vitro method for evaluating the digestibility of commercial diets for dogs. First, in order to develop the in vitro method, the digestibility of four commercial diets for dogs was evaluated through several in vitro trials and results were compared with those that were retrieved from the literature. The in vitro method that was developed consists of two incubation phases, a first lasting 2h and taking place in the presence of pepsin, gastric lipase and HCl (gastric phase) and a second 4h one with pancreatin and bile salts (intestinal phase). Later, digestibility of 16 extruded diets for dogs was evaluated both in vivo with adult dogs and in vitro. There was a close linear relationship between in vivo total tract and in vitro dry matter digestibility (r2 = .81), whereas accuracy of crude protein digestibility using the in vitro method was lower (r2 = .51). Linear regression accuracy for ether extract and starch digestibility was low, but the digestibility results obtained with the in vitro method (95.3 and 98.7% for ether extract and starch, respectively) were very close to those from the in vivo trial (average digestibility of ether extract and starch was 94.8 and 99.1%, respectively). The present in vitro method has proved to be a relatively simple, quick procedure for predicting the digestibility of commercial diets for dogs. The utilisation of such a method may significantly reduce the need for in vivo digestion trials with dogs.
Introduction
Today’s pet food industry is growing rapidly, with pet owners demanding high-quality diets for their pets. Nutrient composition and digestibility are of crucial importance for the qualitative evaluation of pet food. Indeed, these aspects can provide indications as to the real availability of the different nutrients making up a diet, with significant implications in terms of an animal's health and well-being (Case et al. Citation2000). Although much attention is paid to nutritional quality, in the marketing of commercial food for dogs there is usually limited information on digestibility. The pet food industry traditionally uses a wide range of protein sources, both of animal and vegetable origin, including meat, bone and soybean meals. Variations in the quality of the ingredients as well as food processing methods directly affect the availability of nutrients, either positively or negatively (Zentek et al. Citation2004). The digestibility of dog foods has been evaluated in vivo using dogs housed in metabolic cages as experimental animals (Brambillasca et al. Citation2010; Dobenecker et al. Citation2010) or, in some cases, using fistulated dogs (Yamka et al. Citation2003a,Citationb; Barry et al. Citation2009; Faber et al. Citation2010; Hendriks et al. Citation2013). A comprehensive review of technologies that may be used to assess the digestibility of foods for dogs and cats has recently been published by de Godoy et al. (Citation2016). Methods based on the use of digestibility markers (inert and indigestible dietary components), included at low concentrations in the diet, are widely used [for example, chromic oxide (Guevara et al. Citation2008; Barry et al. Citation2009), yttrium oxide (Vhile et al. Citation2007) and celite (Scott & Boldaji Citation1997)]. Nowadays, the use of dogs as experimental animals is a source of great concern for most pet owners and many pet food producers would like to see a reduction in the need for in vivo trials with dogs. Moreover, European legislation for the protection of companion animals (European Directive Citation2010) such as dogs and cats is very strict and discourages the use of these species for experimental purposes, advocating research oriented toward the development, validation and implementation of alternative methods. For all these reasons, the pet food industry would benefit from the availability of a reliable in vitro method to estimate digestibility. The aim of the present study was to develop a new method for in vitro evaluation of the digestibility of commercial diets for dogs.
Materials and methods
The present study consisted of two phases, the first being the development of an in vitro method aimed at simulating the enzymatic digestion processes that take place in the canine gastrointestinal tract and the second being the validation of the method through parallel in vitro and in vivo trials.
Development of the in vitro method
Preliminary application of the method proposed by Vervaeke et al. (Citation1989). Four samples of commercial dog foods (three dry extruded and one wet canned) were used. Analyses of the diets (crude protein, crude fibre, ether extract, ash and starch) were performed according to AOAC standard methods (AOAC, Citation2000; Method 934.01 for dry matter; Method 954.01 for crude protein, Method 962.09 for crude fibre, Method 920.39 for ether extract, Method 942.05 for ash, Method 920.40 for starch). Prior to ether extraction, the samples were acid-hydrolyzed. The chemical composition of the diets is reported in Table .
Table 1. Chemical composition (% on DM basis) of the four dog foods used during the development of the in vitro digestion method.
Samples of the dry extruded dog foods (Diets 1, 2 and 3) were first digested using the first two steps of the in vitro digestion technique proposed by Vervaeke et al. (Citation1989) for the prediction of the digestibility of diets and feedstuffs intended for pigs. This method can be briefly summarised as follows:
Sample preparation: samples of dry extruded dog foods were dried at 65 °C until constant weight and finely ground (<1 mm particle size).
Gastric digestion simulation: for each pet food sample, 400 mL of a pepsin-HCl solution (HCl 0.075N; 2 g/L pepsin from porcine gastric mucosa, 600–1800 units/mg, P7125, Sigma Aldrich Chemical, St. Louis, MO) was added in a 1 L bottle to 20 g of food. The bottles were incubated in a shaking waterbath at 39 °C for 4 h.
Small intestine digestion simulation: the pH level was adjusted to 7.5 with NaOH (1 N) and 400 mL of a pancreatin solution (10 g/L pancreatin in phosphate buffer; pancreatin from porcine pancreas, P1500, Sigma Aldrich) was added. The bottles were incubated in a shaking waterbath at 39 °C for 4 h.
Collection of the undigested fraction: after enzymatic digestion, the content of each bottle was centrifuged (3000 x g, 10 min, 4 °C), washed twice with distilled water, re-centrifuged (3000 x g, 5 min, 4 °C) and the residue was dried at 65 °C until constant weight.
The composition of the phosphate buffered solution used in the simulation of small intestine digestion was as follows: 3.72 g Na2HPO4, 3.92 g NaHCO3, 0.19 g NaCl, 0.23 g KCl, 0.12 g MgCl2, and 0.08 g CaCl2 in 1 L of distilled water (Martillotti et al. Citation1987). The pH of the phosphate buffer was adjusted to 7.5 with HCl 1N. There were three replicates for each diet tested.
Calculation and data analysis. In order to determine the dry matter digestibility of the food samples, the residue obtained from each bottle after the in vitro digestion was weighed and digestibility was calculated with the following equation:
The undigested fraction was analysed for crude protein, ether extract, crude fibre, starch and ash, according to AOAC standard methods (AOAC Citation2000). Nutrient digestibility was calculated with the following equation:
Digestibility data obtained with the in vitro technique were compared to digestibility data drawn from the literature (apparent total tract digestibility data obtained from feeding trials performed with adult dogs).
For the development of the in vitro method, it was decided to compare the results obtained in vitro with apparent total tract digestibility data from the literature, despite the fact that a comparison with ileal digestibility data would have been more appropriate. The main reasons for this decision were the fact that only a few ileal digestibility data for dogs can be found in the literature and, even more importantly, studies involving dogs for the measurement of ileal digestibility of diets are unacceptable nowadays for ethical reasons. In fact, in the second part of the present study, validation of the new in vitro method was performed by comparing in vitro results with apparent total tract digestibility data from a feeding trial with adult dogs.
Modifications to the method of Vervaeke et al. (Citation1989). As the digestibility coefficients of pet food samples obtained with the in vitro method proposed by Vervaeke et al. (Citation1989) greatly differed from those reported in the literature, the method was modified in order to achieve a more precise simulation of the canine digestive process.
In particular, during four in vitro trials, the following critical points were considered with the aim of improving the method proposed by Vervaeke et al. (Citation1989):
Different ratios (1:40 and 1:80; g/mL) between the amount of food and total volume of digestive solutions (pepsin-HCl solution + pancreatin solution) evaluated using one dry extruded diet for adult dogs (Diet 1).
Addition of gastric lipase (Rhizopus lipase, F-AP15, Amano Enzyme Inc., Japan) at different concentrations (1, 2 and 4 g/L) to the pepsin-HCl solution in combination with the addition of a non-ionic surfactant (Polyoxyethylene sorbitan monolaurate; Tween 20, Sigma Aldrich; added at a final concentration of 10 and 20 g/L) or bile salts (Cholic acid-Deoxycholic acid sodium salt mixture, 48305 Fluka BioChemika, Buchs, Switzerland; added at a final concentration of 10, 20 and 25 g/L) to the pancreatin solution using a wet canned food for adult dogs (Diet 4, chosen for its high fat content) as the experimental substrate.
Different concentrations of pancreatin (10 and 12.5 g/L of the phosphate buffer) and bile salts (at the final concentration of 20 and 25 g/L) evaluated using a wet canned food for adult dogs (Diet 4) as the experimental substrate.
Different durations (2 and 4 h) of the gastric digestive phase evaluated using one dry extruded (Diet 1) for adult dogs. The duration of the intestinal digestive phase was kept at 4 h.
Based on the digestibility results collected through the several trials described above, and after a comparison of these results with those that were retrieved from the literature, the following new method for in vitro evaluation of the digestibility of commercial diets for dogs was developed.
Sample preparation: each food sample is dried at 65 °C until constant weight and finely ground (<1 mm particle size).
Gastric digestion simulation: 10 g of food sample and 400 mL of a pepsin-HCl solution (HCl 0.075N; pepsin 2 g/L; P7125, Sigma Aldrich) containing gastric lipase (1 g/L; Rhizopus lipase, F-AP15, Amano Enzyme Inc., Japan) are incubated in a 1 L bottle in a shaking waterbath at 39 °C for 2 h.
Small intestine digestion simulation: first, the pH level is adjusted to 7.5 with NaOH (1 N). Then, 400 mL of a pancreatin solution [10 g/L of pancreatin (P1500, Sigma Aldrich) in the previously described phosphate buffered solution (Martillotti et al. Citation1987)] is added to each bottle. Immediately prior to the addition of the pancreatin solution, bile salts (Cholic acid-Deoxycholic acid sodium salt mixture, 48305, Fluka BioChemika, Buchs, Switzerland) are added to each bottle at a final concentration of 25 g/L. Finally, the bottles are placed in a shaking waterbath at 39 °C for 4 h.
Collection of the undigested fraction: after enzymatic digestion, the preparation is centrifuged (3000 x g, 10 min, 4 °C), washed with distilled water and re-centrifuged twice (3000 x g, 5 min, 4 °C). The undigested residue is dried at 65 °C until constant weight and later analysed for the determination of crude protein, ether extract, ash and starch, according to AOAC standard methods (AOAC Citation2000).
Validation of the in vitro method
In order to validate the new in vitro method, the digestibility of 16 dry extruded commercial diets for adult dogs was evaluated both in vitro and in vivo. A chemical analysis of the diets was performed according to AOAC methods (AOAC Citation2000); the results are reported in Table .
Table 2. Chemical composition (% on DM basis) of the 16 dry dog foods used for the validation of the in vitro method.
In vitro digestibility of diets was evaluated following the new method described above. All diets were analysed in triplicate.
In vivo trials. In vivo apparent total tract digestibility was evaluated using a total of 30 adult dogs (different breeds, individually housed in 12 m2 pens, in good health conditions, with an average body weight of 24.9 ± 6.4 kg). Four in vivo trials were conducted in order to evaluate digestibility of all experimental diets. The experimental protocol was reviewed and approved by the Ethical Committee of the University of Bologna.
Celite, a source of acid-insoluble ash, was included as a digestion marker at 15 g/kg in each tested diet.
Before the beginning of the trial, all dogs were treated against intestinal parasites (Drontal® Plus Flavour, Bayer, Germany). The dogs were randomly assigned to the different experimental groups (six animals for each diet) based on homogeneity of body weight. After a 5-day adaptation period (during which the dogs were progressively adapted to the experimental diet), the dogs received the experimental diet for 12 days. The dogs were fed once daily (at 08.00 h). The daily amount of food was calculated based on the dogs' maintenance energy requirements (estimated at 132 Kcal x Kg BW0.75; NRC, Citation2006) and the energy content of the diets, the latter being calculated using the modified Atwater factors for the canine species (3.5 kcal/g of crude protein and starch and 8.5 kcal/g of ether extract; Case et al. Citation2000). All dogs had free access to water and were allowed daily exercise outside of their pens. During the last five days, all faeces excreted by each dog were collected daily and immediately frozen. At the end of the trial, all faecal samples collected from each dog were mixed thoroughly and freeze-dried for the determination of moisture, protein, ether extract, starch and acid-insoluble ash.
Chemical analyses and calculation of digestibility coefficient. The concentration of protein, ether extract and starch in dietary undigested residues from in vitro digestion and faecal samples was determined according to AOAC standard methods (AOAC Citation2000). Prior to ether extraction, samples were acid-hydrolyzed. The moisture of faecal samples was likewise determined according to the AOAC method (AOAC Citation2000). The level of acid-insoluble ash (celite) in diets and faeces was determined using the method of Vogtmann et al. (Citation1975).
The calculation of the in vitro digestibility of all experimental diets was performed as described above.
The in vivo apparent total tract digestibility of dry matter (DM) of the experimental diets was calculated using the following equation:
The apparent digestibility of crude protein, ether extract and starch was calculated using the following equation:
Statistical analysis of results
During the development of the in vitro method, it was decided to compare the results obtained in vitro with data drawn from the literature. However, no statistical analysis was conducted.
Linear regression was performed to evaluate the accuracy of the relationships between the digestibility coefficients of commercial foods evaluated both in vitro and in vivo (GraphPad Prism 3.0, GraphPad Software, San Diego, CA).
Results and discussion
Development of the in vitro method
Preliminary application of the method proposed by Vervaeke et al. (Citation1989). The results obtained from the digestion of three dry extruded diets for adult dogs using the method proposed by Vervaeke et al. (Citation1989) are shown in Table .
Table 3. In vitro digestibility (mean values ± SD) of three dry extruded diets for adult dogs as determined using the method proposed by Vervaeke et al. (Citation1989) and literature data.
The average mean DM digestibility of foods processed with the method of Vervaeke et al. (Citation1989) was 70.4%, far below the average in vivo digestibility (82.2%) that can be derived from the data reported in the literature. More specifically, when the method proposed by Vervaeke et al. (Citation1989) was used, lipid digestibility showed to be very low (the average in vitro digestibility of lipids was only 36%, whereas the average fat in vivo digestibility derivable from the literature is 94.3%) and was the main factor that affected total DM digestibility. It has to be underlined that Vervaeke et al. (Citation1989) proposed their method to determine the digestibility of diets intended for pigs, which usually contain much lower concentrations of lipids than diets for dogs (in this case, the lipid content of the dry extruded diets ranged from 11.2 to 16.0% on a DM basis). In particular, the Vervaeke method does not call for the utilisation of bile salts, which represent essential factors in lipid digestion. Conversely, protein and starch digestibility data were consistent with those reported in the literature. Based on these results, it was decided to modify the Vervaeke method to adapt it to dogs’ digestion characteristics.
Modifications to the method of Vervaeke et al.
Ratio between the weight of food samples and volume of digestive solution. The ratio between the amount of food and total volume of digestive solutions (pepsin-HCl solution + pancreatin solution) in the original method proposed by Vervaeke et al. (Citation1989) was 1:40 (20 g of food in 800 mL). As this ratio influences the quantity of enzymes that are available to digest the food substrate, the effect of different food/digestive solution ratios (1:40 and 1:80) was evaluated. In this case, only DM digestibility was evaluated.
Average DM digestibility was 66.5 ± 5.79 and 70.9 ± 3.79 with the utilisation of 1:40 and 1:80 solution ratios, respectively. Despite the fact that no statistically significant difference was observed among digestibility values obtained with different food/digestive solution ratios, the utilisation of the 1:80 ratio led to digestibility values that were numerically closer to in vivo digestibility data than can be obtained from the literature (82.2 ± 4.91). For this reason, it was decided to use the 1:80 ratio in the further development of the in vitro method.
Utilisation of gastric lipase and emulsifiers. In order to further improve lipid digestion, the effects of adding gastric lipase to the pepsin-HCl solution and two different types of emulsifiers (Polysorbate 20 and bile salts) to the pancreatin solution were studied. For this purpose, it was decided to use the wet canned food (Diet 4) in view of its high fat content (30.3% on a DM basis).
The presence of lipase in dog gastric juice was confirmed by Carriere et al. (Citation1991), who were able to extract it from canine gastric tissue. The role played by bile salts in the digestion of lipids has long been recognised in all domestic animals, including dogs. In fact, Playoust et al. (Citation1965) found that ileal resection caused steatorrhoea in dogs due to reduced reabsorption of bile acids. Polysorbate 20 is an amphipathic, non-ionic surfactant composed of fatty acid esters of polyoxyethylene sorbitan (Kerwin Citation2008) and is used in many applications, including food and biotechnical applications. It was decided to evaluate polysorbate 20 as a possible alternative to bile salts, mainly because it is less expensive than the latter.
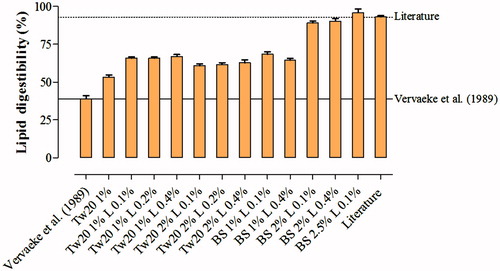
The addition of gastric lipase and bile salts (at both concentrations) improved lipid digestibility and enabled data more consistent with those reported in the literature to be obtained (Figure ). In contrast, the utilisation of polysorbate 20 only partially improved lipid digestion and failed to reach digestibility coefficients similar to those retrieved from the literature. Based on these results, it was decided to use gastric lipase at 1 g/L during the gastric digestive phase in the further development of the in vitro method while the use of bile salts at a final concentration of 20 and 25 g/L was later evaluated in combination with different concentrations of pancreatin.
Figure 1. In vitro lipid digestibility of a sample of wet canned food for adult dogs as determined using different combinations of gastric lipase and emulsifiers and literature data. The dotted line indicates the mean of results drawn from the literature (Dust et al. Citation2005; Yamka et al. Citation2006; Vhile et al. Citation2007; Kempe and Saastamoinen, Citation2007; Guevara et al. Citation2008; Barry et al. Citation2009; Dobenecker et al. Citation2010; Brambillasca et al. Citation2010; Hendriks et al. Citation2013; Menniti et al. Citation2014) while the solid line indicates results obtained with the technique proposed by Vervaeke et al. (Citation1989) with no addition of gastric lipase and emulsifiers. BS: Bile salts (cholic acid-deoxycholic acid sodium salt mixture); Tw20: Polysorbate 20 (polyoxyethylene sorbitan monolaurate; Tween 20, Sigma Aldrich); L: Gastric lipase.
Concentration of pancreatin and bile salts. An additional in vitro study was subsequently conducted to evaluate the effect of different concentrations of pancreatin (10 and 12.5 g/L of the phosphate buffer) and bile salts (at a final concentration of 20 and 25 g/L).
Among the combinations tested, compared with data from the literature, the utilisation of bile salts at 25 g/L led to a better estimation of lipid digestibility than when bile salts were used at 20 g/L (Table ). Conversely, increasing pancreatin concentrations from 10 to 12.5 g/L did not affect lipid digestibility. Based on these results, it was decided to use pancreatin at 10 g/L in association with bile salts at 25 g/L in the further development of the in vitro method.
Table 4. In vitro digestibility (mean values ± SD) of a wet canned food for adult dogs as determined using different concentrations of pancreatin (g/L of phosphate buffer) and bile salts (g/L of total volume of digestive solutions) and literature data.
Duration of the gastric phase. The last step in the development of the in vitro method consisted in the evaluation of different durations (2 and 4 h) of the gastric digestive phase, while the duration of the intestinal digestive phase was kept at 4 h.
As shown in Table , the duration of the gastric phase had no significant effect on digestibility. Consequently, it was decided to reduce the duration of the gastric phase from four to two hours.
Table 5. In vitro digestibility (mean values ± SD) of a dry extruded food for adult dogs as determined using different durations of the gastric digestive phase and literature data.
Validation of the in vitro method
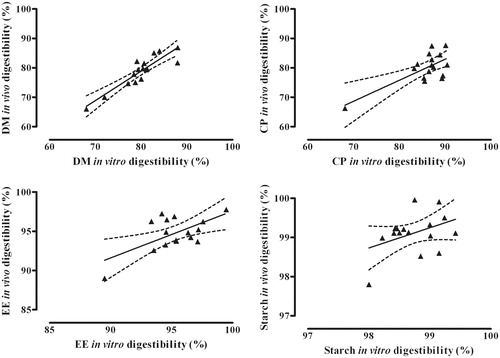
The relationships between digestibility of dry matter, crude protein, ether extract and starch determined in vivo and using the new in vitro method are shown in Figure .
Figure 2. Relationship between digestibility of dry matter (DM), crude protein (CP), ether extract (EE) and starch of 16 dry extruded pet food samples determined in vivo (n = 5) and in vitro (n = 3). In vivo DM digestibility (%) = −1.15 + 1.00 ± 0.13 in vitro DM digestibility (%); r2 = .810; p < .001. In vivo CP digestibility (%) = 18.7 + 0.71 ± 0.19 in vitro CP digestibility (%); r2 = .510; p < .01. In vivo EE digestibility (%) = 37.8 + 0.60 ± 0.20 in vitro EE digestibility (%); r2 = .383; p < .05 In vivo starch digestibility (%) = 48.2 + 0.52 ± 0.31 in vitro starch digestibility (%); r2 = .161; p > .05.
The following linear regressions were obtained and used in order to define the relationship between in vitro and in vivo digestibility results.
in vivo DM digestibility (%) = −1.15 + 1.00 ± 0.13 in vitro DM digestibility (%); r2 = .810; p < .001
in vivo CP digestibility (%) = 18.7 + 0.71 ± 0.19 in vitro CP digestibility (%); r2 = .510; p < .01
in vivo EE digestibility (%) = 37.8 + 0.60 ± 0.20 in vitro EE digestibility (%); r2 = .383; p < .05
in vivo starch digestibility (%) = 48.2 + 0.52 ± 0.31 in vitro starch digestibility (%); r2 = .161; p > .05
The correlation between in vitro and in vivo digestibility of crude protein was not as accurate (r2 = .51) as the one found for DM digestibility. Average protein digestibility was 86.0% in vitro and 80.1% in vivo, and the average absolute difference between in vitro and in vivo results for CP digestibility was 6.0% ± 3.9 (mean ± SD). The discrepancy between in vitro and in vivo results was presumably caused by the fact that faeces do not contain only protein of dietary origin, but also bacteria and other endogenous protein sources (mainly sloughed off intestinal cells and digestive enzymes), leading to an underestimation of protein digestibility (Crampton & Rutherford Citation1954). The presence of dietary fibre (Burrows et al. Citation1982) and protein digestibility are the factors that most affect the digestibility of a commercial food for dogs, because the digestibility of starch, if it has been properly cooked, and fat is in general very high (Twomey et al. Citation2002; Vhile et al. Citation2007). Protein digestibility of a commercial pet food may be negatively affected by the utilisation of poor protein sources (Cramer et al. Citation2007) and an excessive heat treatment (Opstvedt et al. Citation1984). Undigested protein reaches the animal hindgut, where it may be fermented by proteolytic bacteria, resulting in the production of ammonia and other toxic compounds (including biogenic amines; Russell et al. Citation1983), leading to increased intestinal absorption of ammonia, faecal odour (Macfarlane et al. Citation1986) and higher incidence of intestinal diseases (Ramakrishna et al. Citation1991). For these reasons, a precise evaluation of protein digestibility is critical for characterising a pet food's nutritional value.
The correlation between in vitro and in vivo digestibility of ether extract and starch was low (r2 = .38 for ether extract and 0.16 for starch). With only one exception, for all diets tested, the digestibility of ether extract was comprised between 93 and 99% in vitro and between 93 and 98% in vivo. Similarly, for all diets tested, the digestibility of starch was comprised between 98 and 99% in vitro and between 98 and 100% in vivo. The low variability that characterised the ether extract and starch digestibility of the tested diets seems to be the main reason for the low correlations that were found. In fact, digestibility results obtained with the in vitro method (95.3 and 98.7% for ether extract and starch, respectively) were very close to those from the in vivo trial (the average digestibility of ether extract and starch was 94.8 and 99.1%, respectively) as shown by the low average absolute differences observed between in vitro and in vivo results, which were 1.8% ± 0.9 and 0.6% ± 0.3 (mean ± SD) for EE and starch, respectively. However, the equations that we have proposed to estimate in vivo digestibility of fat and starch fractions of a pet food from in vitro digestibility data may not be accurate in the case of pet food samples characterised by a low digestibility of these nutrients.
Conclusions
In conclusion, the in vitro method that was developed during the present study is a relatively simple, quick procedure for predicting the digestibility of commercial diets for dogs. The utilisation of such a method may significantly reduce the need for in vivo digestion trials with dogs.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- AOAC. 2000. Official methods of analysis. 17th ed. Gaithersburg (MD): AOAC.
- Barry KA, Hernot DC, Middelbos IS, Francis C, Dunsford B, Swanson KS, Fahey GC Jr. 2009. Low-level fructan supplementation of dogs enhances nutrient digestion and modifies stool metabolite concentrations, but does not alter faecal microbiota populations. J Anim Sci. 87:3244–3252.
- Brambillasca S, Purtscher F, Britos A, Repetto JL, Cajarville C. 2010. Digestibility, fecal characteristics, and plasma glucose and urea in dogs fed a commercial dog food once or three times daily. Canadian Vet J. 51:190.
- Burrows CF, Kronfeld DS, Banta CA, Merritt AM. 1982. Effects of fiber on digestibility and transit time in dogs. J Nutr. 112:1726–1732.
- Carriere F, Moreau H, Raphel V, Laugier R, Benicourt C, Junien JL, Verger R. 1991. Purification and biochemical characterization of dog gastric lipase. Eur J Biochem. 202:75–83.
- Case LP, Carey DP, Hirakawa DA, Daristotle L. 2000. Canine and feline nutrition. 2nd ed. St. Louis (MO): Mosby.
- Cramer KR, Greenwood MW, Moritz JS, Beyer RS, Parsons CM. 2007. Protein quality of various raw and rendered by-product meals commonly incorporated into companion animal diets. J Anim Sci. 85:3285–3293.
- Crampton EW, Rutherford BE. 1954. Apparent digestibility of dietary protein as a function of protein level. J Nutr. 54:445–451.
- European Directive. 2010. Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes. Official J Eur Union. 276:33–79.
- Dobenecker B, Frank V, Kienzle E. 2010. High calcium intake differentially inhibits nutrient and energy digestibility in two different breeds of growing dogs. J Anim Physiol Anim Nutr. 94:109–114.
- Dust JM, Grieshop CM, Parsons CM, Karr-Lilienthal LK, Schasteen CS, Quigley JD, Merchen NR, Fahey GC. 2005. Chemical composition, protein quality, palatability, and digestibility of alternative protein sources for dogs. J Anim Sci. 83:2414–2422.
- Faber TA, Bechtel PJ, Hernot DC, Parsons CM, Swanson KS, Smiley S, Fahey GC Jr. 2010. Protein digestibility evaluations of meat and fish substrates using laboratory, avian, and ileally cannulated dog assays. J Anim Sci. 88:1421–1432.
- de Godoy MRC, Hervera M, Swanson KS, Fahey Jr GC. 2016. Innovations in canine and feline nutrition: Technologies for food and nutrition assessment. Annu Rev Anim Biosci. 4:311–313.
- Guevara MA, Bauer LL, Abbas CA, Beery KE, Holzgraefe DP, Cecava MJ, Fahey JGC. 2008. Chemical composition, in vitro fermentation characteristics, and in vivo digestibility responses by dogs to select corn fibres. J Agr Food Chem. 56:1619–1626.
- Hendriks WH, Thomas DG, Bosch G, Fahey Jr GC. 2013. Comparison of ileal and total tract nutrient digestibility of dry dog foods. J Anim Sci. 91:3807–3814.
- Hervera M, Baucells MD, Blanch F, Castrillo C. 2007. Prediction of digestible energy content of extruded dog food by in vitro analyses. J Anim Physiol Anim Nutr (Berl). 91:205–209.
- Kempe R, Saastamoinen M. 2007. Effect of linseed cake supplementation on digestibility and faecal and haematological parameters in dogs. J Anim Physiol Anim Nutr (Berl). 91:319–325.
- Kerwin BA. 2008. Polysorbates 20 and 80 used in the formulation of protein biotherapeutics: structure and degradation pathways. J Pharm Sci. 97:2924–2935.
- Macfarlane GT, Cummings JH, Allison C. 1986. Protein degradation by human intestinal bacteria. J Gen Microbiol. 132:1647–1656.
- Martillotti F, Antongiovanni M, Rizzi L, Santi E, Bittante G. 1987. Metodi di analisi per la valutazione degli alimenti d’impiego zootecnico. Quaderni Metodologici N. 8, IPRA-CNR. 6–7.
- Menniti MF, Davenport GM, Shoveller AK, Cant JP, Osborne VR. 2014. Effect of graded inclusion of dietary soybean meal on nutrient digestibility, health, and metabolic indices of adult dogs. J Anim Sci. 92:2094–2104.
- National Research Council. 2006. Nutrient requirements of dogs and cats. Washington (DC): The National Academies Press.
- Opstvedt J, Miller R, Hardy RW, Spinelli J. 1984. Heat-induced changes in sulfhydryl groups and disulphide bonds in fish protein and their effect on protein and amino acid digestibility in rainbow trout (Salmo gairdneri). J Agr Food Chem. 32:929–935.
- Playoust MR, Lack L, Weiner IM. 1965. Effect of intestinal resection on bile salt absorption in dogs. Am J Physiol. 208:363–369.
- Ramakrishna BS, Roberts-Thomas IC, Pannall PR, Roediger WE. 1991. Impaired sulphation of phenol by the colonic mucosa in quiescent and active colitis. Gut. 32:46–49.
- Russell JB, Sniffen CJ, Van Soest PJ. 1983. Effect of carbohydrate limitation on degradation and utilization of casein by mixed rumen bacteria. J Dairy Sci. 66:763–775.
- Savoie L. 1994. Digestion and absorption of food: usefulness and limitations of in vitro models. Can J Physiol Pharm. 74:407–414.
- Scott TA, Boldaji F. 1997. Comparison of inert markers [chromic oxide or insoluble ash (Celite)] for determining apparent metabolizable energy of wheat- or barley-based broiler diets with or without enzymes. Poult Sci. 76:594–598.
- Twomey LN, Pethick DW, Rowe JB, Choct M, Pluske JR, Brown W, Laviste MC. 2002. The use of sorghum and corn as alternatives to rice in dog foods. J Nutr. 132:1704S–1705S.
- Vervaeke IJ, Dierick NA, Demeyer DL, Decuypere JA. 1989. Approach to the energetic importance of fibre digestion in pigs. II. An experimental approach to hindgut digestion. Anim Feed Sci Tech. 23:169–194.
- Vhile SG, Skrede A, Ahlstrøm Ø, Hove K. 2007. Yttrium oxide (Y2O3) as an inert marker in digestibility studies with dogs, blue foxes and mink fed diets containing different protein sources. J Anim Physiol Anim Nutr. 91:381–389.
- Vogtmann HP, Frirter P, Prabuck AL. 1975. A new method of determining metabolisability of energy and digestibility of fatty acids in broiler diets. Br Poult Sci. 16:531–534.
- Yamka RM, Harmon DL, Schoenherr WD, Khoo C, Gross KL, Davidson SJ, Joshi DK. 2006. In vivo measurement of flatulence and nutrient digestibility in dogs fed poultry by-product meal, conventional soybean meal, and low-oligosaccharide low-phytate soybean meal. Am J Vet Res. 67:88–94.
- Yamka RM, Jamikorn U, True AD, Harmon DL. 2003a. Evaluation of low-ash poultry meal as a protein source in canine foods. J Anim Sci. 81:2279–2284.
- Yamka RM, Jamikorn U, True AD, Harmon DL. 2003b. Evaluation of soyabean meal as a protein source in canine foods. Anim Feed Sci Tech. 109:121–132.
- Zentek J, Fricke S, Hewincker-Trautwein M, Ehinger B, Amstberg G, Baums C. 2004. Dietary protein source and manufacturing processes affect macronutrient digestibility, faecal consistency and presence of faecal Clostridium perfringens in adult dogs. J Nutr. 134:2158–2161.
