Abstract
This study was conducted to evaluate the effect of microencapsulated organic acids and essential oils (MOE) on reproductive performance, nutrient digestibility, piglet diarrhoea score, and blood profiles in sows and suckling piglets. A total of 60 multiparous sows (Landrace × Yorkshire) with an average initial body weight (BW) of 249.47 ± 11. 62 kg were used and randomly subjected to three treatments such that 20 replicates per treatment were maintained in this trial. Dietary treatments consisted of a basal diet supplemented with 0, 500, 1000 mg MOE/kg respectively. The BW of sows increased (linear, p = .014) before farrowing; after farrowing and weaning showed both linear and quadratic (p = <.001) effects with the increase in the dose of MOE in the diet. Further, results showed that tendency of reduced (p = .063) BW loss and increased (linear, p = .046) digestibility of dry matter in sows fed with MOE in the diet. The back fat loss and blood profiles were unaffected (p > .05) both in the sows and suckling piglets by the dietary treatment. Dietary inclusion of increasing level of MOE in sows’ diet increased (p < .05) BW, average daily gain, faecal score of suckling piglets and reduced the number of diarrheal piglets. Therefore, the addition of MOE can be considered as safe and effective enhancement in both farrowing and lactating sows as it favours the growth, nutrient digestibility and helps in avoidance of diarrhoea among suckling piglets.
Introduction
Antibiotic residues among swine products that may lead to a possible risk of developing bacterial resistance to antibiotics in humans have raised concerns about using antibiotics as growth promoters in animal feed. This growing concern has led to the ban in the use of antibiotic as growth promoters by the EU since 2006 (Regulation 1983/2003/EC) and South Korea since 2011. This ban in Korea stimulated research in analysing alternatives to antibiotics as growth promoters (Yan et al. Citation2011). Organic acids (OA) and essential oils (EO) supplementation has shown a functional similarity to antibiotics, thus offering an interesting alternative to growth-promoting source in livestock nutrition. Previous reports indicated that OA and EO supplements in pig diets had beneficial effects on growth performance (Cho et al. Citation2006; Wang et al. Citation2009; Grilli et al. Citation2010; Gong et al. Citation2014), nutrient digestibility (Yan et al. Citation2010; Cho et al. Citation2014) and both of them are appropriate antibiotic alternatives in swine diets (Rota et al. Citation2004; Mroz Citation2005). Similar benefits generated by the inclusion of OA and EO to sows diets have been reported by other authors (Kis & Bilkei Citation2003; Allan & Bilkei Citation2005; Miller et al. Citation2009; Kluge et al. Citation2010; Ariza-Nieto et al. Citation2011). Langhout (Citation2000) suggested that a combination of OA and EO would be beneficial to work more in the later segments of the intestinal tract.
Additionally, microencapsulation is the envelopment of small solid particles, liquid droplets or gases in a coating (Thies Citation1987) and recently used to deliver substances into specific sites of the gastrointestinal tract and suggested efficiency in production performance in livestock industry that has led to economic profitability. Previous studies indicate microencapsulation would increase the effects of OA and EO (Burnell et al. Citation1988; Cho et al. Citation2014; Zhang et al. Citation2014; Mohammadi Gheisar et al. Citation2015; Upadhaya et al. Citation2015a,Citationb; Mohana Devi et al. Citation2016). The effects of micro-encapsulated organic acids and essential oils (MOE) in pigs and poultry have been variable because of different study conditions and differences in the type of MOE used as well as variation in the dose provided (Zhang et al. Citation2005; Upadhaya et al. Citation2014; Mohammadi Gheisar et al. Citation2015; Lee et al. Citation2015).
In our previous reports, the dietary supplementation of MOE had shown positive effects on the performances in poultry (Mohammadi Gheisar et al. Citation2015) and finishing pigs (Cho et al. Citation2014). The study on the effect of MOE supplementation in sows is limited. Thus, the present study was conducted to evaluate the effects of MOE on the reproductive performance, apparent total tract digestibility (ATTD) of nutrients in sows as well as to evaluate the growth performance, diarrhoea score, blood profiles in sows and their suckling piglets.
Materials and methods
Ethical considerations
The protocol for these experiments was approved, and animals were cared for, according to the guidelines of the Animal Care and Use Committee of Dankook University, Cheonan, South Korea.
Source of microencapsulated organic acids and essential oils
In this study, commercially available MOE product (Aviplus-S®, VetAgroSpA™, 42100 Reggio Emilia, Italy) that contained citric acid (25%), sorbic acid (16.7%), thymol (1.7%) and vanillin (1.0%) was used.
Animals, housing and diets
A total of 60 multiparous sows (Landrace × Yorkshire, average parity 1.5, SD =0.41) and their litters were used in a 32 day (d) experiment. On d 108 of gestation, sows with an average weight of 249.47 ± 11. 62 kg were randomly allotted to three dietary treatments with 20 replicates per treatments based on parity number according to a randomised complete design. During gestation, sows were housed in individual stall that had partly slatted floors that consisted of a 0.80 m concrete solid floor and a 1.05 m concrete slatted floor. Approximately 10 d before the expected time of parturition, sows were moved to farrowing rooms, each with 2.20 × 1.60 m2 area. The concrete solid floor was equipped with floor heating. All rooms were equipped with auto-controlled heating and mechanical ventilation systems. Supplemental heat was provided for pigs using heat lamps. Piglets were weaned at day 25. Cross-fostering of piglets took place within two days of parturition and occurred only among sows of the same experimental treatment. The number of piglets per sow ranged from nine to 11 piglets and the numbers of weaned piglets were duly recorded. The care and treatment of the sows were according to animal welfare legislation.
The sows were fed with corn-soybean based diets (Table ) were formulated to meet or exceed the nutrient concentrations recommended by National Research Council (NRC Citation2012). The dietary treatments were as follows: Basal diet (CON), MOE1 (CON +0.05% MOE) and MOE2 (CON +0.10% MOE). From day 108 of gestation to parturition, sows were fed 2.5 kg/day of their respective experimental lactation diets. On the day of parturition, the sows were not offered feed. After farrowing, sows were fed their respective experimental lactation diets until weaning. During lactation, daily feed allowance was increased gradually until sows had ad libitum access to feed by week 2. All diets were provided in meal form twice daily. Care was taken that the sows and piglets had free access to drinking water ad libitum throughout the experimental period.
Table 1. Ingredient composition of the experimental diets (g/kg, as-fed).
Sampling and measurements
The body weights (BW) of the sows were recorded on day 108 of gestation, immediately after farrowing (d 0), week 1, 2, 3 and at weaning (d 25) to calculate BW loss during that trail period. The feed consumed during the gestation and lactation periods was recorded and used to calculate average daily feed intake (ADFI) of sows. The back-fat thickness (BFT) of the sows (6 cm off the midline at the 10th rib) was measured using a real-time ultrasound instrument (Piglot 105, SFK Technology, Herlev, Denmark) before farrowing, few hours after farrowing and at weaning to determine back-fat loss. Values from the two measurements were averaged to obtain a single BFT measurement (Wang et al. Citation2008). Detection of oestrus was conducted twice daily (08:00 and 16:00 h) from weaning onwards. A sow was considered to be in oestrus when exhibiting a standing response induced by a back pressure test when in the presence of a boar. Live piglets were weighed immediately after birth, d 7, 14, 21, and d 25 to calculate average daily gain (ADG). Number of piglets borne and weaned pigs was also recorded to calculate the survival rate. The incidence of diarrhoea in piglets was observed, and recorded three times a day throughout the study. In order to assess the severities of diarrhoea, faeces from piglet of each pen were scored during d 5 to 25 of lactation according to the method of Cho et al. (Citation2006). In brief, the scores were as follows: 1 = hard, dry pellets in a small, hard mass; 2 = hard, formed stool that remains firm and soft; 3 = soft, formed, and moist stool that retains its shape; 4 = soft, unformed stool that assumes the shape of the container; 5 = watery, liquid stool that can be poured. A cumulative diarrhoea score per diet and day was then assessed (Smiricky et al. Citation2002).
To determine apparent total tract digestibility (ATTD) of dry matter (DM), gross energy (GE), and nitrogen (N), sows were fed diet containing chromic oxide (2 g/kg) as an indigestible marker for 5 d. Faecal grab sampling via rectal palpation was carried out from 10 randomly selected sows per treatment and stored at −20 °C until analysed. These faecal samples were then thawed and dried at 60 °C for 72 h, after which they were finely ground to a size that could pass through a 1-mm screen before being analysed for DM, GE, and N following the procedures by the Association of Official Analytical Chemists (AOAC Citation2007). Chromium was analysed via UV absorption spectrophotometry (Shimadzu UV-1201, Shimadzu, Kyoto, Japan) by following the method elucidated by Williams et al. (Citation1962).
Blood samples were collected via jugular venipuncture into K3EDTA vacuum tubes and clot activator vacuum tubes (Becton Dickinson Vacutainer Systems, Franklin Lakes, NJ) from 10 randomly selected sows per treatment and from two randomly selected piglets per sow at weaning. The WBC, RBC and lymphocyte counts in the whole blood were determined using an automatic blood analyser (ADVIA 120, Bayer Corp., Tarrytown, NY) according to the method described by Li and Kim (Citation2014).
Statistical analysis
Both sows and piglet performance data were analysed using the MIXED procedure (SAS Institute Inc., Cary, NC) for a randomised complete block design with each sow being used as the experimental unit. Parity effect was not significant, so it was not included in the model. Lactation length was used as a covariate for number of piglet survivability, weaning weight of sows and piglets, BW loss among sows, loss of ADFI and BFT, and ADG of piglets. Orthogonal polynomial contrasts were used to describe the linear and quadratic effects of increasing the concentration of MOE in the diet. Variability in the data was expressed as the pooled standard error of mean (SEM). A probability value of p ≤ .05 was considered as significant and trends were noted when .05 < p < .10.
Results
In the present study, significant differences were observed on the BW change in sows before farrowing (linear, p = .014), after farrowing (linear and quadratic, p = <.0001; .0008) and weanling (linear and quadratic, p = <.0001; .002) with tendency effects on ADFI during gestation (p = .095) and week 2 (p = .057) among treatment. However, there was tendency of reduction (p = .063) of body weight loss in sows during the lactating period with the increase in the level of MOE supplementation. However, there were no significant (p > .05) effects on BFT and weaning to oestrus interval (Table ) in sows fed MOE supplemented diet. The BW of piglets born to sows consuming MOE showed (linear and quadratic, p < .05) effects at d 7, 14, 21 and 25 (Table ). During the overall the experiment period and at d 22 to 25, piglets from sows fed MOE had higher ADG (linear, p = .002; 0.019, respectively) than CON diet. The ATTD of DM (linear, p = .046) and GE (trend-quadratic, p = .07) in sows fed the MOE diet were higher compared with those fed the CON at end of the experiment, but no significant difference in N digestibility (p > .05) in sows among the treatments (Table ).
Table 2. Effects of micro-encapsulated organic acids and essential oils (MOE) on growth performance traits in sowsTable Footnotea.
Table 3. Effects of micro-encapsulated organic acids and essential oils (MOE) on growth performance in pigletsTable Footnotea.
Table 4. Effects of micro-encapsulated organic acids and essential oils (MOE) on apparent total tract digestibility of nutrient in sowsTable Footnotea.
Dietary inclusion of MOE had no significant difference (p > .05) on blood profile in sows and piglets in this study. However, supplementation of the MOE diet compared to other treatments exhibited numerical increase in the WBC (109/L), RBC (1012/L), and lymphocytes (%) respectively in sows (average mean values: 11.12, 5.62, 31.9) and piglet’s (average mean values: 12.28, 6.25, 3.8) blood, but the differences (p > .05) were not statistically significant (data not shown).
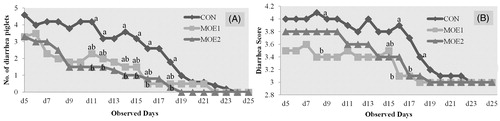
Dietary supplementation with MOE linearly decreased the number of diarrheal piglets at d 11, 12, 14, 15, 16, 18 (p = .007, .007, .035, .032, .042, .036, respectively) and increased faecal score at d 18 (p = .031) compared with those fed the CON and MOE1diet (Figure ).
Figure 1. Effects of micro-encapsulated organic acids and essential oils (MOE) on the number of diarrhoea piglets (A) and their diarrhoea scores (B). CON - Basal diet; MOE1 - CON +0.05% MOE; MOE2 - CON +0.10% MOE; a,b,abMeans in the same day with different superscript differ significantly (p < .05).
Discussion
Proper nutritional administration of sows during gestation and lactation is significant for achieving and maintaining adequate sow productivity, longevity and litter performance (Kuller et al. Citation2004; Oliviero et al. Citation2009, Begum et al. Citation2015; Hossain et al. Citation2015). In the present study, it was hypothesised that feeding gestating and lactating sows a diet supplemented with MOE would confer beneficial effects to the sow by enhancing BWG, nutrient digestibility, manipulation of intestinal microbiota, and that such benefits would be passed on to the suckling piglets, as previously reported (Ilsley et al. Citation2003; Matysiak et al. Citation2012; Rossi and Soares Citation2013; Chengquan et al. Citation2015).
In this study, there were significant effects on growth performance in piglets accordance with Ariza-Nieto et al. (Citation2011) and Ramanau et al. (Citation2004) who reported that dietary supplementation of EO to sows improves the growth pattern of suckling piglets. Similarly, Cho et al. (Citation2014) reported that the addition of 0.05% MOE in diet of pigs improved growth performance and 3 kg/ton Aviplus® added to weaned piglet diets increased ADG (from d 15 to d 41) and at the end of study (Grilli et al. Citation2010). Dietary MOE supplemented sows were significantly effective on BW change and the relevant result of effect was tendency to reduction of body weight loss in lactation period, which result consistent with Mohana Devi et al. (Citation2016) reported that the effect of dietary supplement with protected organic acids on reproductive performance in sows. This perhaps is a consequence of improved intake and utilisation of food supplements with no relationship with sow back fat loss during lactation as suggested with previous reports (Ilsley et al. Citation2003; Matysiak et al. Citation2012; Kingori Citation2012; Chengquan et al. Citation2015; Hossain et al. Citation2015).
Our results are in agreement with previous experiments that showed feeding a diet supplemented with protected OA blends, improved ADG, feed efficiency and did not affect ADFI (Upadhaya et al. Citation2014, Citation2015b). It has been reported that the addition of single or blends of OA may have a negative effect on the palatability of the diet, thus decreasing ADFI (Walsh et al. Citation2007). This study showed that losses of backfat and weaning to oestrus interval during experimental period showed no effects in the MOE-treated sows. Likewise, Ilsley et al. (Citation2003) and Mohana Devi et al. (Citation2016) found that had no significant effect on the loss of backfat during lactation among sows.
In the present study, DM digestibility was increased with MOE supplementation which was consistent with the findings by Kluge et al. (Citation2010) and Mohana Devi et al. (Citation2016) reported that the inclusion of 2% benzoic acid and protected organic acid blends respectively in the diet of lactating sows improved the digestibility of nutrients. Similarly, Bolduan et al. (Citation1988), Jongbloed & Jongbloed (Citation1996) found that dietary supplementation of OA increased the ATTD of DM, but not in organic matter. Nevertheless, there was no significant influence on the ATTD of N and GE by MOE in this study. Similarly, Radecki et al. (Citation1988) reported that apparent N digestibility was not affected by citric acid supplementation. Yan et al. (Citation2011) determined that herb extracts containing thyme could not improve the ATTD of N, and GE. Our previous study Cho et al. (Citation2014) reported that N digestibility was not affected by MOE in finishing pigs.
Moreover, study by Zhao and Lacasse (Citation2008) indicated that higher apparent digestibility can improve the synthesis of milk and consequent milk yield, leading to improvement in piglet performance. In the study the inclusion MOE in the sows diet enhanced piglet performance and resulted in a higher BWG and ADG during the suckling period and weaning, which can usually indicate a better quantity and/or quality of colostrum and milk, as they are major determinants of litter performance (Hossain et al. Citation2015). However, in the current study colostrum and milk composition were not determined to provide a better explanation of the experiential positive piglet response Therefore, this should be an important consideration in future studies to explicate the effects of MOE in sow’s milk on litter performance.
In the present study, no effects were observed on the blood characteristics of either lactating sows or suckling piglets by supplementation of MOE similar to our previous studies that reported no effects on blood profiles in farrowing sows (Mohana Devi et al. Citation2016) and broilers (Mohammadi Gheisar et al. Citation2015). Blood lymphocyte, WBC, and RBC counts when compared with control gradually showed an increase but without any significant difference in this study. The gastrointestinal system and the associated lymphatic system have the largest immunological capacity; therefore, the concentration of lymphocytes and WBC response to the immune state were also determined. However, the type and doses of OA and EO used, as well as the differences in environmental factors, feed characteristics and age of animals used in this study, might explain these inconsistent results (Zhang et al. Citation2005).
In this study, the diarrheal scores were much higher in CON than the other treatments. The incidence of diarrhoea among piglets reduced in suckler sows whose diet was supplemented with 0.10% MOE among treatments. Ariza-Nieto et al. (Citation2011) reported that dietary supplementation of EO to sows reduced the fat percentage in milk; increase the growth pattern of suckling pigs. The reason for enhanced growth performance traits by OA and EO may be transferred into suckling piglets through breast milk, which can improve piglet resistance to diarrhoea. Grilli et al. (Citation2010) reported that microencapsulation can slow down the release of these extracts through the intestine, in order to enable them to reach the large intestine where they may be much more effective in inducing diarrhoea. Kyriakis et al. (Citation1998) suggested that essential origanum was effective at controlling post weaning diarrhoea syndrome, which is consistent with the results of this study.
Conclusions
The microencapsulated mixture containing citric acid, sorbic acid, thymol and vanillin, when supplemented to sows’ diet showed partial beneficial effects on the performance of sows and piglets. Therefore, supplementing sows with MOE in diets may improve growth performance traits and reduce the effects of diarrhoea in sucking piglets. The biological growth potential of suckling piglets through the dietary supplementation of MOE in sows is much greater than that achieved in current conventional systems.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Allan P, Bilkei G. 2005. Oregano improves reproductive performance of sows. Theriogenology. 63:716–721.
- Ariza-Nieto C, Bandrick M, Baidoo SK, Anil L, Molitor TW, Hathaway MR. 2011. Effect of dietary supplementation of oregano essential oils to sows on colostrum and milk composition, growth pattern and immune status of suckling pigs. J Anim Sci. 89:1079–1089.
- AOAC. 2007. Official methods of analysis. 18th ed. Gaithersburg (MD): Association of Official Analytical Chemists.
- Begum M, Li HL, Hossain MM, Kim IH. 2015. Dietary bromelain-C.3.4.22.32 supplementation improves performance and gut health in sows and piglets. Livest Sci. 180:177–182.
- Bolduan G, Jung H, Schneider R, Block J, Klenke B. 1988. Influence of propionic and formic acids on piglets. J Anim Physiol Anim Nutr. 59:72–78.
- Burnell TW, Cromwell GL, Staly TS. 1988. Effects of dried whey and copper sulfate on the growth responses to organic acid in diets for weanling pigs. J Anim Sci. 66:1100–1108.
- Chengquan T, Hongkui W, Haiqing S, Jiangtao A, Guang L, Siwen J, Jian P. 2015. Effects of dietary supplementation of oregano essential oil to sows on oxidative stress status, lactation feed intake of sows, and piglet performance. BioMed Res Int. 2015:525218. doi: 10.1155/2015/525218.
- Cho JH, Chen YJ, Min BJ, Kim HJ, Kwon OS, Shon KS, Kim IH, Kim SJ, Asamer A. 2006. Effects of Essential oils supplementation on growth performance, IgG concentration and faecal noxious gas concentration of weaned pigs. Asian-Austr J Anim Sci. 19:80–85.
- Cho JH, Song MH, Kim IH. 2014. Effect of microencapsulated blends of organic acids and essential oils supplementation on growth performance and nutrient digestibility in finishing pigs. Rev Colomb Cienc. 27:264–272.
- Gong J, Yin F, Hou R, Yin Y. 2014. Review: Chinese herbs as alternatives to antibiotics in feed for swine and poultry production: Potential and challenges in application. Can J Anim Sci. 94:223–241.
- Grilli E, Messina MR, Tedeschi M, Piva A. 2010. Feeding a microencapsulated blend of organic acids and nature identical compounds to weaning pigs improved growth performance and intestinal metabolism. Livest Sci. 133:173–175.
- Hossain MM, Begum M, Nyachoti CM, Hancock JD, Kim IH. 2015. Dietary fenugreek seed extract improves performance and reduces faecal E. coli counts and faecal gas emission in lactating sows and suckling piglets. Can J Anim Sci. 95:561–568.
- Ilsley SE, Miller HM, Greathead HMR, Kamel C. 2003. Plant extracts as supplements for lactating sows: effects on piglet performance, sow food intake and diet digestibility. Anim Sci. 77:247–254.
- Jongbloed AW, Jongbloed R. 1996. The effect of organic acids in diets for growing pigs on enhancement of microbial phytase efficacy. ID-DLO Report No. 96009. Lelystad (Netherlands): Institute for Animal Science and Health.
- Kingori A. 2012. Sow lactation: colostrum and milk yield: a review. J Anim Sci Adv. 2:525–533.
- Kis RK, Bilkei G. 2003. Effect of a phytogenic feed additive on weaning-to-oestrus interval and farrowing rate in sows. J. Swine Health Prod. 11:296–299.
- Kluge H, Broz J, Eder K. 2010. Effect of dietary benzoic acid on urinary pH and nutrient digestibility in lactating sows. Livest Sci. 134:119–121.
- Kuller WI, Soede NM, van Beers-Schreurs HMG, Langendijk P, Taverne MAM, Verheijden JHM, Kemp B. 2004. Intermittent suckling: effects on piglet and sow performance before and after weaning. J Anim Sci. 82:405–413.
- Kyriakis SC, Sarris K, Lekkas S, Tsinas AC, Giannakopoulos C, Alexopoulos C, Saoulidis K. 1998. Control of post weaning diarroea syndrome of piglets by infeed application of origanum essential oils. Proc 15th IPVS Congress, Birmingham, England; 3:218.
- Langhout P. 2000. New additives for broiler chickens. World Poult. 16:22–27.
- Lee SI, Kim HS, Kim IH. 2015. Microencapsulated organic acid blend with MCFAs can be used as an alternative to antibiotics for laying hens. Turk J Vet Anim Sci. 39:520–527.
- Li J, Kim IH. 2014. Effects of Saccharomyces cerevisiae cell wall extract and poplar propolis ethanol extract supplementation on growth performance, digestibility, blood profile, fecal microbiota and fecal noxious gas emissions in growing pigs. Anim Sci J. 85:698–705.
- Matysiak BE, Jacyno M, Kawecka A, Pietruszka KA. 2012. The effect of plant extracts fed before farrowing and during lactation on sow and piglet performance. S Afr J Anim Sci. 42:15–21.
- Miller JA, Laurenz JC, Rounsavall JW, Burdick NC, Neher FJ. 2009. Enhancing feed intake by the sow during lactation using BIOMIN®PEP. In: Steiner T, editor. Phytogenics in animal nutrition: natural concepts to optimise gut health and performance. Chicago: Nottingham University Press. p. 87–96.
- Mohammadi Gheisar M, Hosseindoust A, Kim IH. 2015. Evaluating the effect of microencapsulated blends of organic acids and essential oils in broiler chickens diet. J Appl Poult Res. 24:511–519.
- Mohana Devi S, Lee KY, Kim IH. 2016. Analysis of the effect of dietary protected organic acid blend on lactating sows and their piglets. R Bras Zootec. 45:39–47.
- Mroz Z. 2005. Organic acids as potential alternatives to antibiotic growth promoters for pigs. Proc Banff Pork Semin. 16:169–182.
- NRC. 2012. Nutrient requirements of swine. 11th ed. Washington (DC): National Research Council Academy Press.
- Oliviero C, Kokkonen T, Heinonen M, Sankari S, Peltoniemi O. 2009. Feeding sows with high fibre diet around farrowing and early lactation: impact on intestinal activity, energy balance related parameters and litter performance. Res Vet Sci. 86:314–319.
- Radecki SV, Juhland MR, Miller ER. 1988. Fumaric and citric acids as feed additives in starter pig diets: effect on performance and nutrient balance. J Anim Sci. 66:2598–2605.
- Ramanau A, Kluge H, Spilke J, Eder K. 2004. Supplementation of sows with L-carnitine during pregnancy and lactation improves growth of the piglets during the suckling period through increased milk production. J Nutr. 134:86–92.
- Rossi CAR, Soares M. 2013. Production index of the sows fed diets supplemented in the parturition and lactation with essential oils of oregano and rosemary: evaluation of litters. Ciência Rural. 43(11). doi: 10.1590/S0103-84782013001100025.
- Rota C, Carraminana JJ, Burillo J, Herrera A. 2004. In vitro antimicrobial activity of essential oils from aromatic plants against selected food borne pathogens. J Food Prot. 67:1252–1256.
- Smiricky MR, Grieshop CM, Albin DM, Wubben JE, Gabert VM, Fahey GC. 2002. The influence of soy oligosaccharides on apparent and true ileal amino acid digestibilities and fecal consistency in growing pigs. J Anim Sci. 80:2433–2441.
- Thies C. 1987. Microencapsulation. In: Mark HF, Bikales NM, Overberger CG, et al. editors. Encyclopaedia of polymer science and engineering. New York: John Wiley & Sons. p. 724–745.
- Upadhaya SD, Lee KY, Kim IH. 2014. Influence of protected organic acid blends and diets with different nutrient densities on growth performance, nutrient digestibility and faecal noxious gas emission in growing pigs. Vet Med-Czech. 59:491–497.
- Upadhaya SD, Lee KY, Kim IH. 2015a. Effect of protected organic acid blends on growth performance, nutrient digestibility and faecal micro flora in growing pigs. J Appl Anim Res. 44:238–242.
- Upadhaya SD, Lee KY, Kim IH. 2015b. Protected Organic Acid Blends as an Alternative to Antibiotics in Finishing Pigs. Asian-australas. J Anim Sci. 27:1600–1607.
- Walsh MC, Sholly DM, Hinson RB, Saddoris KL, Sutton AL, Tadcliffe JS, Odgaard R, Murphy J, Richert BT. 2007. Effects of water and diet acidification with and without antibiotics on weanling pig growth and microbial shedding. J Anim Sci. 85:1799–1808.
- Wang JP, Yoo JS, Lee JH, Jang HD, Kim HJ, Shin SO, Seong SI, Kim IH. 2009. Effects of phenyllactic acid on growth performance, nutrient digestibility, microbial shedding, and blood profile in pigs. J Anim Sci. 87:3235–3243.
- Wang Q, Kim HJ, Cho JH, Chen YJ, Yoo JS, Min BJ, Wang Y, Kim IH. 2008. Effects of phytogenic substances on growth performance, digestibility of nutrients, faecal noxious gas content, blood and milk characteristics and reproduction in sows and litter performance. J Anim Feed Sci. 17:50–60.
- Williams CH, Davidand DJ, Iismaa O. 1962. The determination of chromic oxide in faeces samples by atomic absorption spectrophotometry. J Agri Sci. 59:381–385.
- Yan L, Mengand QW, Kim IH. 2011. The effect of an herb extract mixture on growth performance, nutrient digestibility, blood characteristics and faecal noxious gas content in growing pigs. Livest Sci. 141:143–147.
- Yan L, Wang JP, Kim HJ, Meng QW, Ao X, Hongand SM, Kim IH. 2010. Influence of essential oil supplementation and diets with different nutrient densities on growth performance, nutrient digestibility, blood characteristics, meat quality and faecal noxious gas content in grower-finisher pigs. Livest Sci. 128:15–122.
- Zhang KY, Yan F, Keen CA, Waldroup PW. 2005. Evaluation of microencapsulated essential oils and organic acids in diets for broiler chickens. Int J Poult Sci. 4:612–619.
- Zhang Y, Gong J, Yu H, Guo Q, Defelice C, Hernandez M, Yin Y, Wang Q. 2014. Alginate-whey protein dry powder optimized for target delivery of essential oils to the intestine of chickens. Poult Sci. 93:2514–2525.
- Zhao X, Lacasse P. 2008. Mammary tissue damage during bovine mastitis: causes and control. J Anim Sci. 86:57–65.
