Abstract
The present study was conducted to investigate the effects of N-acetylcysteine (NAC) on antioxidant capacities and the expression of inflammatory cytokines in weaned piglets. In total, 15 litters, containing 150 14-day-old piglets, were divided, by litter, into suckling (control), weaning and NAC groups. Thus, there were five litters per group. Compared with the suckling group, the weaning group had increased (p < .05) malondialdehyde (MDA), nitric oxide (NO), hydrogen peroxide (H2O2) and interleukin (IL)-6 contents in their serum and pro-inflammatory cytokine IL-6 and IL-1β expression levels in the jejunum, while they had decreased superoxide dismutase (SOD) (p = .055) and glutathione peroxidase (GSH-Px) (p = .057) activities, as well as IL-4 (p < .05) concentrations in their serum and I-κBα expression (p < .05) in their jejunum. Dietary supplementation with NAC had improved (p < .05) antioxidant status in serum and increased (p < .05) levels of IL-4 but decreased (p < .05) mRNA expression levels and concentrations of IL-6 and IL-1β. Meanwhile, the I-κBα expression level in the jejunum of the NAC-treated group increased (p < .05) compared with the weaning group. In conclusion, weaning resulted in oxidative stress, and dietary supplementation with NAC could increase the antioxidant capacities, while decreasing the jejunal expression of inflammatory cytokines in weaned piglets.
Introduction
Weaning is a critical period in the life of piglets, characterised by changes in nutrition, environment and psychology (Niekamp et al. Citation2007). Early weaning causes a decrease in feed intake, nutrient absorption capacity and immunity in the piglets (McCracken et al. Citation1999). In the early-weaned piglets, the accumulation of reactive oxygen species (ROS), such as superoxide, hydroxyl radical and hydrogen peroxide (H2O2), would exceed the antioxidant capacities in vivo. Therefore, they could be vulnerable to oxidative stress (Pié et al. Citation2007; Zhu et al. Citation2012). Excessive ROS can be involved in triggering the expression of inflammatory cytokines that are closely associated with the gastrointestinal tract inflammatory response (Dröge Citation2002; Apel & Hirt Citation2004). Therefore, the increase in the antioxidant capacities and the regulation of inflammatory cytokine expression are essential for defending against oxidative stress in weaned piglets.
Glutathione (GSH) has a powerful capacity to scavenge free radicals, such as the ROS, in vivo. N-acetyl cysteine (NAC) is a thiol-containing compound and serves as a precursor for GSH synthesis (Johnson et al. Citation2006). Hou et al. (Citation2013) reported that oxidative stress is attenuated by dietary NAC supplementation in lipopolysaccharide (LPS)-challenged piglets. In a previous study of our group, NAC was considered a beneficial dietary supplement in the prevention of weaning stress (Zhu et al. Citation2013; Xu et al. Citation2014). Palacio et al. (Citation2011) reported that NAC also inhibits the expression of inflammatory cytokines in LPS-activated THP-1 macrophages under mild oxidative conditions. Additionally, NAC may alleviate the inflammatory response induced by LPS (Song et al. Citation2004). Volpin et al. (Citation2014) demonstrated that the inflammatory response was associated with the level of inflammatory cytokines. However, whether NAC could regulate the expression of inflammatory cytokines in the gastrointestinal tracts of newly weaned piglets is still unknown.
In the present study, we aimed to investigate the effects of NAC-supplemented diets on oxidative stress and the expression of inflammatory cytokines in weaned piglets. We also attempted to elucidate the possible relationship between the oxidative status and the expression of inflammatory cytokines in weaned piglets.
Materials and methods
Animals and experimental design
All the protocols used in these experiments were approved by the Guidelines for the Care and Use of Laboratory Animal established by Shanghai Jiao Tong University Institutional Animal Care and Use Committee.
A total of 150 14-day-old piglets (Duroc × Landrace × Yorkshire) from 15 litters were randomly divided by litter to the suckling, the weaning and the NAC-treated groups, resulting in 5 litters per group. The piglets were kept with the sow in conventional farrowing pens and suckled until 21 days of age. From 14 to 25 days of age, piglets in the suckling and weaning groups had ad libitum access to the basal diet (Table), and the NAC-treated piglets were fed the basal diet supplemented with 500 mg/kg of NAC. The dose of NAC supplementation was chosen based on a study by Zhu et al. (Citation2013). Basal diets were prepared as crumbled. At 21 days of age, piglets in the weaning and NAC groups were weaned and moved from the farrowing pens to nursery pens without mixing the litters. The suckling piglets were retained in the farrowing pens to suckle until day 25 of the experiment. All of the piglets housed in the nursery pen had access to a one-sided stainless-steel feed spout and a nipple drinker. They were allowed free access to water throughout the 25-day experimental period. The room temperature was maintained at approximately 25 °C. Piglets were weighed at 21 days and 25 days of age, and the feed intakes were monitored per litter every day. These data were used to calculate the average daily gain (ADG) and the average daily feed intake (ADFI).
Table 1. Dietary composition, calculated energy and nutrient contents of the basal diet.
Sample collection
At 25 days of age, one piglet (close to the average body weight) per litter from each treatment, resulting in a total of five piglets per treatment, was selected and blood samples were collected from the anterior vena cava. Serum was separated by centrifugation at 3500 ×g for 15 min at 4 °C and stored at −20 °C. Then, the piglets were anaesthetised by intramuscular injection of sodium pentobarbital (40 mg/kg body weight), euthanised and gut samples were collected. The small intestine was carefully removed, and placed on ice. Pieces, approximately 2 cm in length, were resected from the middle portion of the jejunum and fixed in 4% neutral buffered formalin for histological assays. Other samples from the same intestinal segment were placed in 1.5 mL tubes, frozen in liquid N2 and stored at −70 °C for quantitative reverse transcription-PCR (qRT-PCR) analyses.
Determination of redox status in serum
The serum superoxide dismutase (SOD), glutathione peroxidase (GSH-Px) and catalase (CAT) activities, inhibition capacity of hydroxyl radicals (IHR), and malondialdehyde (MDA), H2O2, and nitric oxide (NO) contents were assayed by commercially available kits according to the manufacturer’s instructions (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). The SOD activity was determined by the percentage inhibition of nitro blue tetrazolium formation as measured by the absorbance at 505 nm on a spectrophotometer. The GSH-Px, CAT and IHR activities were measured by p-nitrobenzoic acid, molybdic acid and the Fenton reaction method at 412 nm, 405 nm and 550 nm, respectively. The contents of MDA, H2O2 and NO were analysed by thiobarbituric acid (TBA), molybdic acid and the NO reductase method at 532 nm, 405 nm and 550 nm, respectively (Thermo Scientific, Washington).
Histological observation of intestinal mucosa
Jejunum samples, fixed in 4% neutral buffered formalin for 48 h, were usually processed for paraffin embedding and the paraffin blocks were sectioned into 5 μm slices, and the slices were stained with haematoxylin and eosin for microscopy. Villus height, crypt depth and crypt width were measured at three well-orientated sampling sites for each variable from three sections per piglet, using the Diagnostic Insight visual analysis program (Olympus Co., Tokyo, Japan).
ELISA detection for inflammatory cytokines
Individual serum samples were thawed at 4 °C. The inflammatory cytokines interleukin-1β (IL-1β), IL-2, IL-4 and IL-6 in the serum were determined using specific ELISA kits according to the manufacturer’s instructions (R&D System, MN).
A 96 microplate was precoated with specific antibodies, standard substances and samples, and then incubated at 37 °C. The secondary HRP-labelled antibody was added to the wells and then the plate was again incubated at 37 °C. After washing, a substrate solution was added to the wells for colorimetric detection. Finally, the reaction was stopped by the addition of the stop solution. Absorbances were read spectrophotometrically at 450 nm using a microtitre plate reader (Wellscan MK3, Lab Systems Dragon, Beijing, China).
qRT-PCR
The jejunal tissues were homogenised using TRIzol reagent (Invitrogen, Washington). The RNA was diluted in diethylpyrocarbonate-treated water and quantified by spectrophotometry (Nano Drop Lite, Thermo Scientific). An OD260/280 ratio between 1.8 and 2.0 was considered to indicate high quality RNA. After reverse transcription, the cDNA was synthesised as the PCR template. The PCR reaction was performed in a total volume of 20 μL using a Mastercycler ep Realplex Real-time Quantitative PCR system (Eppendorf, Germany) with SYBR Premix Ex Taq kits (Takara, Japan). The PCR programme was as follows: 95 °C for 30 s; followed by 40 cycles of 95 °C for 5 s, annealing at 60 °C for 30 s and extension at 72 °C for 15 s. Internal controls of β-actin and glyceraldehyde 3-phosphate dehydrogenase (GADPH) were used to normalise target-gene expression using the 2−ΔΔCt method (Schmittgen & Livak Citation2008), The primers designed for qRT-PCR are listed in Table , and agarose gel electrophoresis was used to verify the amplification products.
Table 2. Primer sequences used for qRT-PCR.
Statistical analyses
All the data are presented as means with standard errors. A one-way analysis of variance (ANOVA) was used to evaluate significant differences by the least-significant-difference (LSD) test, and p < .05 was considered statistically significant. All of the tests were performed with SPSS17.0 software (SPSS Inc., Chicago, IL).
Results
Growth performance
ADG and ADFI from 21 days to 25 days of age were significantly (p < .05) decreased in the weaning group compared with the suckling piglet. ADG and ADFI were increased (p = .055) in the piglets of the NAC-treated dietary group compared with the weaning group.
Status of antioxidant capacities
As shown in Table , the activities of SOD and GSH-Px in serum were decreased (p = .056 and p = .057, respectively) in the weaned piglets compared with the suckling piglets. The MDA content was significantly increased (p < .05) in the piglets of the weaning group compared with those of the suckling group. The SOD and GSH-Px activities in the serum of NAC-treated group were increased (p < .05) and the MDA content decreased (p < .05) compared with those of the weaned piglets.
Table 3. Serum antioxidant capacities in suckling, weaning and NAC-treated piglet groups.
Compared with the suckling piglets, the concentrations of NO and H2O2 in the weaning group increased (p = .056 and p = .058, respectively), and the IHR showed a clear reduction (p < .05) after weaning. The diet supplemented with NAC significantly reduced the concentrations of NO and H2O2 (p < .05), and significantly increased (p < .05) the IHR compared with the values in the weaning piglets.
Histology and histometry
Compared with in the suckling group, an oedema condition in the lamina propria was observed in the jejunal villi of the weaning group, as well as a partial destruction of villi (Figure , weaning). The intestinal villi were almost normal in their structure in the jejunum of the NAC-treated piglets compared with the weaning piglets (Figure , NAC-treated).
Figure 1. Morphology of jejunal villi in the suckling, weaning and NAC-treated piglet groups. The suckling group shows villi arrange neatly, whereas a partial villi destruction with an oedema condition are observed in the tunica mucosa of the weaning group. The NAC-treated group shows scarce signs of villi destruction.
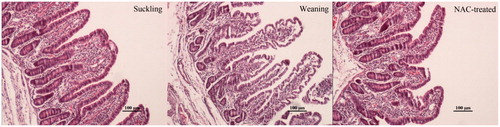
Compared with suckling piglets, the villi heights and widths decreased (p < .05), and the crypt depths increased (p < .05) in the jejunum of weaning piglets. The NAC-supplemented diet increased the villus height (p < .05) and width (p = .059), and reduced the crypt depth (p = .06) compared with the weaning group (Table ).
Table 4. Jejunal morphological measurement in the piglets of suckling, weaning and NAC-treated groups.
Serum levels of inflammatory cytokines
No significant difference was observed in the IL-1β concentration in the serum of the weaning group compared with the suckling group, but the concentration of IL-4 decreased (p < .05) and the IL-6 concentration increased (p < .05). Compared with the weaning group, the NAC-treated group had reduced (p < .05) IL-1β and IL-6 concentrations, and an increased (p < .05) IL-4 concentration (Table).
Table 5. Serum concentrations of inflammatory cytokines in piglets of the suckling, weaning and NAC-treated groups.
Gene expression of inflammatory cytokines andI-κBα
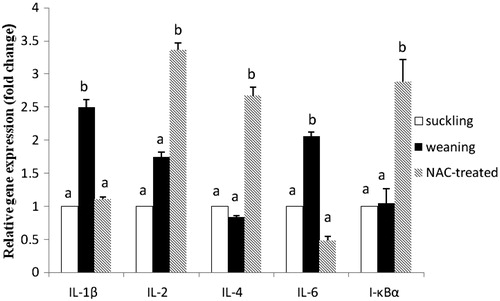
qRT-PCR results are shown in Figure . Significant increases in IL-1β (p < .05), IL-6 (p < .05) and IL-2 (p = .059) expression levels were observed in the weaned piglets compared with the suckling piglets, but a significant decrease (p = .058) in the IL-4 expression was observed. The I-κBα expression dramatically decreased (p < .05) in the weaned piglets. Compared with the weaning group, the IL-1β and IL-6 expression levels were obviously downregulated (p < .05), and the IL-2 and IL-4 expression levels were upregulated (p < .05) in the NAC-treated group. The NAC-treated group also had an elevated (p < .05) I-κBα expression level compared with the weaning group.
Figure 2. Jejunal relative gene expression of inflammatory cytokines and I-κB in piglets of the suckling, weaning and NAC-treated groups. Values are means ± SE (n = 5), with the SE indicated by vertical bars. Mean values with different lowercase letters were significantly different (p < .05). Gene expression levels in the weaning and NAC groups are presented as the multiples of the gene expression level in the suckling group, which was set as 1.0.
Discussion
During the weaning period, the reared piglets are usually challenged by serious stressors and intestinal inflammatory responses (Johnson et al. Citation2006). A previous study of our group had shown that weaning stress might induce free radical overproduction, which resulted in oxidative stress in piglets (Zhu et al. Citation2012). Some antioxidant enzymes are critical to protect cells and tissues against the damaging possibly exerted by free radicals and maintain proper cellular functions in vivo. For example, SOD can catalyse superoxide into H2O2, and CAT and GSH-Px can convert H2O2 into H2O. When the antioxidant enzyme systems fail to neutralise the free radicals, the cellular oxidative balance is disrupted (Halliwell Citation2007). In this study, decreases in the SOD and GSH-Px activities, and increases in the NO, H2O2 and lipid peroxide were observed in the weaning piglets. As the excessive generation of free radicals causes oxidative damage, an associated partial villi destruction in the intestine of weaning piglets was observed, which was in agreement with a previous study (Kenworthy Citation1976). These results suggested that the antioxidant capacities in vivo could not exceed the overproduction of free radicals linked to weaning, and thus oxidative stress occurred in the weaning piglets.
Studies reported that the free radicals (in particular, H2O2 and NO) might act as secondary messengers to activate the pro-inflammatory cytokines’ gene expression (Schulze-Osthoff et al. Citation1995; Guzik et al. Citation2003). The gene expression analysis results showed that the pro-inflammatory cytokines IL-1β and IL-6 (the mediators of inflammatory responses) were significantly increased, but the anti-inflammatory cytokine IL-4 was decreased in the intestines of weaning piglets. The upregulated gene expression levels of IL-1β and IL-6 were consistent with the results of Pie et al. (Citation2004) in 28-day-old weaned piglets. Also, Hou et al. (Citation2013) reported that the IL-6 concentration in plasma increased in LPS-challenged piglets, which was in agreement with our serum analysis results in the weaning group. Brzozowski et al. (Citation2003) reported that the balance between the pro- and anti-inflammatory cytokine expression levels and production was critical for the intestinal inflammatory responses. In addition, Murtaugh et al. (Citation1996) reported that a high level of pro-inflammatory cytokines could enhance the inflammatory response, and we observed an oedema condition in the lamina propria of the weaning group. Thus, free radicals induced by weaning may enhance the levels of pro-inflammatory cytokines, which increases the intestinal inflammatory response in the piglets.
NAC plays a pivotal role in the antioxidant systems’ scavenging of ROS and the endogenous synthesis of GSH (Hou et al. Citation2013). As a result, it can restore the intracellular antioxidant enzyme activities, such as those of SOD and GSH-Px. In this study, the SOD and GSH-Px activities were increased in the NAC-treated group, which suggested that NAC was able to promote antioxidant capacities and prevent oxidative stress in weaning piglets. NAC also directly reacts with H2O2 and the hydroxyl radical, and protects cells from oxidative damage by the sulfhydryl group (Yi et al. Citation2014). Moreover, the NO, H2O2 and MDA contents in the serum were decreased and the IHR was increased in the NAC-treated piglets. These NAC properties contributed to the reduction of the free radical level in the NAC-treated piglets after weaning. In this study, increased villi heights and reduced crypt depths were observed in the NAC-treated group and were tentatively attributed to restoring the GSH level, which maintained the cell structure by neutralising excessive ROS. Thus, the NAC-containing diet may have a protective effect on oxidative damage and the morphological integrity of the intestinal mucosa.
In the NAC-treated group, the gene expression levels of the pro-inflammatory cytokines IL-1β and IL-6 were decreased in the intestines of piglets, probably because NAC scavenged free radicals and then inhibited the gene expression regulated by NO, H2O2 and other radicals (Bowie & O’Neill Citation2000). Qiu et al. (Citation2013) reported that the concentration of IL-6 in the plasma of the NAC group was significantly reduced after LPS injection, but the concentration of IL-1β showed no significant difference, perhaps because of the LPS dose. In this study, the concentrations of IL-6 and IL-1β in the serum were significantly reduced, and the IL-4 concentration was significantly increased in the NAC-treated group. As an anti-inflammatory cytokine, IL-4 could suppress the effects of pro-inflammatory cytokines, such as TNF-α, IL-6 and IL-1β (Schuerwegh et al. Citation2003). The high levels of anti-inflammatory cytokines may attenuate the inflammatory response. To some extent, the NAC-supplemented diet may reduce the susceptibility of the inflammatory response by changing the ROS level, which then modulates the inflammatory cytokines’ expression levels.
Free radicals induce the degradation of inhibitor-κB (IκB) and then activate nuclear factor kappa B (NF-κB) (O’Connell et al. Citation1998). NF-κB is a redox-sensitive transcription factor that induces the gene expression of inflammatory mediators. Normally, it is retained in an inactive form with its inhibitory subunit, IκB (Kyriakis et al. Citation1998). NO and H2O2 directly activate NF-κB by degrading or modifying IκB, which allows the active NF-κB to migrate into the nucleus and exert its transcriptional effect (Schreck et al. Citation1991). In this study, the overproduction of free radicals in weaning piglets resulted in the increasing IκB degradation, mRNA expression and pro-inflammatory cytokine production.
Henkel et al. (Citation1993) indicated that cells treated with antioxidants prevented the decay of IκB. Additionally, the activation of NF-κB induced by free radicals may be blocked by the antioxidant properties of NAC (Halliwell & Gutteridge Citation1990; Baeverle & Henkel Citation1994). In this study, the NAC-treated group raised intracellular GSH levels and eliminated the excessive ROS in the weaning piglets. As a result, the free radical level was reduced, and the gene expression of IκB in the NAC-treated piglets was threefold more than in the weaning piglets. Additionally, the inhibitory effects of NAC on the activation of NF-κB were strongly confirmed by the downregulation of the pro-inflammatory cytokines’ mRNA expression.
Conclusions
Weaning contributes to the oxidative stress and the decrease of antioxidant capacities in the piglets. Dietary supplementation with NAC could improve antioxidant capacities and decrease the inflammatory cytokines IL-1 and IL-6 expression levels in the intestines of weaned piglets. A possible mechanism of regulating intestinal inflammatory cytokine expression levels in weaning piglets is by increasing I-κBα and then inhibiting NF-κB. Further studies should be conducted to determine the NAC-specific mechanism modulating the inflammatory cytokines in weaning piglets.
Supplematal_1_.docx
Download MS Word (15.1 KB)Disclosure statement
The authors declare that they have no conflicts of interest.
References
- Apel K, Hirt H. 2004. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol. 55:373–399.
- Baeverle P, Henkel T. 1994. Function and activation of NF-kappa B in the immune system. Annu Rev Immunol. 12:141–179.
- Bowie A, O’Neill LAJ. 2000. Oxidative stress and nuclear factor-kappaB activation: a reassessment of the evidence in the light of recent discoveries. Biochem Pharmacol. 59:13–23.
- Brzozowski T, Ptak A, Kwiecien S, Pajdo R, Drozdowicz D, Pawlik M, Konturek SJ, Pawlik WW. 2003. Importance of nitric oxide (NO) and adenosine in the mechanism of gastric preconditioning induced by short ischemia. Rocz Akad Med Bialymst. 48:42–47.
- Chen ZW, Chien MS, Chang NY, Chen TH, Wu CM, Huang C, Lee WC, Hsuan SL. 2011. Mechanisms underlying Actinobacillus pleuropneumoniae exotoxin apxi induced expression of IL-1β, IL-8 and TNF-α in porcine alveolar macrophages. Vet Res. 81:2–4.
- De Groot J, Kruijt L, Scholten JW, Boersma WJ, Buist WG, Engel B, Van Reenen CG. 2005. Age, gender and litter-related variation in T-lymphocyte cytokine production in young pigs. Immunology. 115:495–505.
- Dröge W. 2002. Free radicals in the physiological control of cell function. Physiol Rev. 82:47–95.
- Guzik T, Korbut R, Adamek-Guzik T. 2003. Nitric oxide and superoxide in inflammation and immune regulation. J Physiol Pharmacol. 54:469–487.
- Halliwell B. 2007. Oxidative stress and cancer: have we moved forward? Biochem J. 401:1–11.
- Halliwell B, Gutteridge JM. 1990. The antioxidants of human extracellular fluids. Arch Biochem Biophys. 280:1–8.
- Henkel T, Machleidt T, Alkalay I, Krönke M, Ben-Neriah Y, Baeuerle PA. 1993. Rapid proteolysis of I kappa B-alpha is necessary for activation of transcription factor NF-kappa B. Nature. 365:182–185.
- Higashi N, Bang K, Gesser B, Lund M, Thestrup-Pedersen K. 2001. Cytokine expression of skin T-lymphocytes from patients with atopic dermatitis. Acta Derm Venereol. 81:3–7.
- Hou Y, Wang L, Yi D, Ding B, Yang Z, Li J, Chen X, Qiu Y, Wu G. 2013. N-acetylcysteine reduces inflammation in the small intestine by regulating redox, egf and TLR4 signalling. Amino Acids. 45:513–522.
- Johnson IR, Ball RO, Baracos VE, Field CJ. 2006. Glutamine supplementation influences immune development in the newly weaned piglet. Dev Comp Immunol. 30:1191–1202.
- Kenworthy R. 1976. Observations on the effects of weaning in the young pig. Clinical and histopathological studies of intestinal function and morphology. Res Vet Sci. 21:69–75.
- Kyriakis SC, Tzika ED, Lyras DN, Tsinas AC, Saoulidis K, Sarris K. 1998. Effect of an inactivated parapoxvirus based immunomodulator (baypamun) on post weaning diarrhoea syndrome and wasting pig syndrome of piglets. Res Vet Sci. 64:187–190.
- Lv Q, Zhang S, Zhao R. 2011. Transportation stress alters the expression of immunoregulatory cytokines in the porcine thymus. Vet J. 187:229–233.
- Murtaugh MP, Baarsch MJ, Zhou Y, Scamurra RW, Lin G. 1996. Inflammatory cytokines in animal health and disease. Vet Immunol Immunopathol. 54:45–55.
- McCracken BA, Spurlock ME, Roos MA, Zuckermann FA, Gaskins HR. 1999. Weaning anorexia may contribute to local inflammation in the piglet small intestine. J Nutr. 129:613–619.
- Niekamp S, Sutherland M, Dahl G, Salak-Johnson J. 2007. Immune responses of piglets to weaning stress: impacts of photoperiod. J Anim Sci. 85:93–100.
- O’Connell MA, Bennett BL, Mercurio F, Manning AM, Mackman N. 1998. Role of IKK1 and IKK2 in lipopolysaccharide signaling in human monocytic cells. J Bio Chem. 273:30410–30414.
- Palacio J, Markert U, Martínez P. 2011. Anti-inflammatory properties of N-acetylcysteine on lipopolysaccharide-activated macrophages. Inflamm Res. 60:695–704.
- Pié S, Awati A, Vida S, Falluel I, Williams B, Oswald I. 2007. Effects of added fermentable carbohydrates in the diet on intestinal proinflammatory cytokine-specific mRNA content in weaning piglets. J Anim Sci. 85:673–683.
- Pie S, Lalles JP, Blazy F, Laffitte J, Seve B, Oswald IP. 2004. Weaning is associated with an upregulation of expression of inflammatory cytokines in the intestine of piglets. J Nutr. 134:641–647.
- Qiu Y, Zhang J, Liu Y, Ma H, Cao F, Xu J, Hou Y, Xu L. 2013. The combination effects of acetaminophen and N-acetylcysteine on cytokines production and NF-κB activation of lipopolysaccharide-challenged piglet mononuclear phagocytes in vitro and in vivo. Vet Immunol Immunop. 152:381–388.
- Schmittgen TD, Livak KJ. 2008. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 3:1101–1108.
- Schreck R, Rieber P, Baeuerle PA. 1991. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa b transcription factor and hiv-1. Embo J. 10:2247.
- Schuerwegh A, Dombrecht E, Stevens W, Van Offel J, Bridts C, De Clerck L. 2003. Influence of pro-inflammatory (IL-1 alpha, IL-6, TNF-alpha, IFN-gamma) and anti-inflammatory (IL-4) cytokines on chondrocyte function. Osteoarthr Cartilage. 11:681–687.
- Schulze-Osthoff K, Los M, Baeuerle PA. 1995. Redox signalling by transcription factors NF-kappa B and AP-1 in lymphocytes . Biochem Pharmacol. 50:735–741.
- Song M, Kellum JA, Kaldas H, Fink MP. 2004. Evidence that glutathione depletion is a mechanism responsible for the anti-inflammatory effects of ethyl pyruvate in cultured lipopolysaccharide-stimulated raw 264.7 cells. J Pharmacol Exp Ther. 308:307–316.
- Volpin G, Cohen M, Assaf M, Meir T, Katz R, Pollack S. 2014. Cytokine levels (IL-4, IL-6, IL-8 and TGF-β) as potential biomarkers of systemic inflammatory response in trauma patients. Int Orthop. 38:1303–1309.
- Xu C, Yang S, Zhu L, Cai X, Sheng Y, Zhu S, Xu J. 2014. Regulation of n-acetyl cysteine on gut redox status and major microbiota in weaned piglets. J Anim Sci. 92:1504–1511.
- Yi D, Hou Y, Wang L, Ding B, Yang Z, Li J, Long M, Liu Y, Wu G. 2014. Dietary N-acetylcysteine supplementation alleviates liver injury in lipopolysaccharide-challenged piglets. Br J Nutr. 111:46–54.
- Zhu L, Cai X, Guo Q, Chen X, Zhu S, Xu J. 2013. Effect of n-acetyl cysteine on enterocyte apoptosis and intracellular signalling pathways' response to oxidative stress in weaned piglets. Br J Nutr. 110:1938–1947.
- Zhu L, Zhao K, Chen X, Xu J. 2012. Impact of weaning and an antioxidant blend on intestinal barrier function and antioxidant status in pigs. J Anim Sci. 90:2581–2589.
